Abstract
Purpose
Legumes form root nodules to gain fixed nitrogen from rhizobia and can also access nitrogen in soil. Data suggest that plants might discriminate among these sources to optimize growth, but recognition of symbiotically fixed nitrogen and its regulation remain poorly understood.
Methods
A greenhouse inoculation study manipulated the molecular form and concentration of nitrogen available using two Lotus japonicus genotypes and the nitrogen-fixing symbiont, Mesorhizobium loti. Plants were supplied with sole organic and inorganic nitrogen sources to simulate forms that plants might receive from symbiotic nitrogen fixation or from the soil. Host benefit from and regulation of symbiosis was investigated by quantifying symbiotic trait variation and isotopic analysis of nitrogen fixation.
Results
Host growth varied in response to fertilization with alanine, aspartic acid, ammonium, and nitrate, suggesting differences in catabolism efficiency. Net benefits of nodulation were reduced or eliminated under all forms of extrinsic fertilization. However, even when symbiosis imposed significant costs, hosts did not reduce investment into nodulation or nitrogen fixation when exposed to aspartic acid, unlike with other nitrogen sources.
Conclusions
L. japonicus can adaptively downregulate investment into symbiosis in the presence of some but not all nitrogen sources. Failure to downregulate any aspect of symbiosis in the presence of aspartic acid suggests that it might be jamming the main signal used by L. japonicus to detect nitrogen fixation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In nutritional mutualisms, plant hosts gain key nutrients from microbes that would otherwise be growth-limiting and unreliable if accessed only from soil. Plants that associate with mycorrhizal fungi can gain substantial amounts of phosphorus, promoting plant growth and expanding the ecological niche of the host species (Corrales et al. 2016; Gerz et al. 2018; Simard et al. 2012). In parallel, plants form root nodulating symbioses with bacteria that can provide host plants with a substantial portion of their nitrogen budget (Ngom et al. 2016; Regus et al. 2017a) also expanding the host species range (Simonsen et al. 2017). But the net fitness effect of these associations can vary from highly beneficial to harmful (Hoeksema et al. 2010; Sachs and Simms 2008; Sachs et al. 2018). Variation in the services that hosts receive can be driven by the intrinsic capacities of the microbe and host to provide or receive nutritional services, respectively (i.e., genotypic variation: Gano-Cohen et al. 2020; Wendlandt et al. 2019), availability of nutrients in the soil environment, which can reduce the net benefit of interacting with microbes (i.e., environmental variation; Batstone et al. 2020; Regus et al. 2017a, b; Porter and Simms 2014), and interactions among these factors (i.e., GxE; Heath 2010). To maximize the benefits and minimize the costs of investing into nutritional mutualisms, host plants must exhibit traits to regulate microbial infection depending on the net benefit that the microbe offers in the environmental context (West et al. 2002).
Plants in the legume family (Fabaceae) associate with diverse rhizobia, proteobacteria that instigate nodule formation on host roots and fix nitrogen within plant tissues in exchange for photosynthates (Sawada et al. 2003). To initiate this interaction, legumes release host-specific flavonoids into the soil, and in response, compatible rhizobia secrete Nod factors that cause a suite of changes on host roots that instigate nodule development (Ferguson et al. 2018). During root nodule organogenesis, hosts appear to exhibit little or no ability to detect a rhizobia strain’s capacity to fix nitrogen, as rhizobia mutants without nitrogen-fixation function instigate nodules with similar frequency as do near-isogenic strains that can fix nitrogen (Amarger 1981; Champion et al. 1992; Hahn and Studer 1986; Quides et al. 2021; Westhoek et al. 2017). However, within days after nodule organogenesis, legumes begin to differentially invest in rhizobia that vary in symbiotic nitrogen fixation, tending to produce more numerous and larger nodules with nitrogen fixing rhizobia (Quides et al. 2017; Regus et al. 2015) and reducing rhizobial proliferation within nodules that do not fix nitrogen (Kiers et al. 2003; Oono et al. 2011; Regus et al. 2017b; Sachs et al. 2010).
Like other nutritional mutualisms, legume hosts can access nitrogen from multiple sources, including from nodulating rhizobia and from the soil (Lin et al. 2021). Availability of substantial nitrogen in the soil can render rhizobia superfluous, reducing the net benefits of nodulation to zero (Hussain et al. 1999; Regus et al. 2017a). Importantly, host support of nodulation and nitrogen fixation by rhizobia involves metabolic costs that must be outweighed by the benefits of the mutualism (Sachs et al. 2018; Sulieman and Tran 2012). To minimize costs, many legumes can reduce or halt nodulation in the presence of extrinsic nitrogen (Lin et al. 2021; Streeter 1988), however this pattern is far from universal, with variation among nitrogen sources, concentrations, and host species, including cases where nitrogen fertilization is associated with increased nodulation (Gan et al. 2004; Heath et al. 2010; Regus et al. 2017a). Moreover, some legumes form smaller nodules when nitrogen is available in the soil (Kiers et al. 2006; Regus et al. 2015). When legumes are exposed to mixed inoculations of nitrogen-fixing and non-fixing rhizobia, the host preferentially punishes rhizobial genotypes that fail to fix nitrogen, and these host ‘sanctions’ are not altered by the availability of soil nitrogen (Kiers et al. 2006; Regus et al. 2014). This host capacity to differentiate nitrogen-fixing and non-fixing rhizobia in the presence of extrinsic nitrogen suggests that hosts can differentiate biologically fixed nitrogen (i.e., from the bacteria) from extrinsic forms of reactive nitrogen (i.e., from the soil). How the host legumes recognize different sources of nitrogen remains poorly understood.
The metabolic pathways by which rhizobia transfer nitrogen to the host provide clues about host discrimination among different sources of nitrogen. Early work demonstrated that ammonium (NH4+) is an immediate product of nitrogen fixation (Klucas 1974) and later work suggested that it moves from the bacteroids to the plant cytoplasm via an NH4+ transporter (Tyerman et al. 1995) and or via ammonia diffusion (NH3) (White et al. 2007). However, studies where nitrogen was isotopically labelled found that amino acids were the predominant compounds being transferred to hosts (Meeks et al. 1978; Ohyama and Kumazawa 1980). In parallel, cell fractionation and reverse genetics generated evidence that aspartic acid and or alanine were transferred from bacteroids to the plant cytoplasm (Appels and Haaker 1991; Rastogi and Watson 1991; Rosendahl et al. 1992). Researchers knocked out amino-acid transporters in Rhizobium, and showed that although the mutants could fix nitrogen efficiently, the plants received negligible amounts of nitrogen, indicating that amino acid transfer is a required step for the host to receive fixed nitrogen (Lodwig et al. 2003). More recently, genome scale metabolic modeling in Sinorhizobium suggested the potential flexibility of these systems, indicating that amino acid export is the main mechanism of nitrogen transport from bacteroids to the host cytoplasm, but that ammonia transport occurs when oxygen is enriched and or when certain amino acid metabolic pathways are knocked out (diCenzo et al. 2020; Pfau et al. 2018).
Here, we experimentally manipulated the molecular form and concentration of extrinsic nitrogen supply to Lotus japonicus to investigate how the host plant modulated investment into symbiosis with its root nodulating symbiont, Mesorhizobium loti. Two host lines were tested, L. japonicus MG-20 (Kawaguchi 2000) and its near-isogenic hypernodulating mutant har1-7 (Krusell et al. 2002; Nishimura et al. 2002), allowing us to examine how different degrees of control over nodulation affects how hosts regulate investment into rhizobia. Hosts were fertilized with inorganic forms of nitrogen that can simulate nitrogen rich soils, including potassium nitrate or ammonium sulfate (Galloway et al. 2004), as well as organic forms of nitrogen, including alanine or aspartic acid, predicted to be excreted in planta by rhizobia (Lodwig et al. 2003; Rastogi and Watson 1991; Waters et al. 1998). For each source of nitrogen, four different concentrations were used, based upon previous estimates of host growth saturation (Regus et al. 2017a). The goals of the experiment were to i) assess the capacity of L. japonicus to catabolize diverse nitrogen sources, ii) test whether host plants differentially respond to sources of nitrogen that could simulate extrinsic enrichment versus symbiotic services, and iii) investigate regulation of symbiotic nitrogen fixation under different extrinsic nitrogen sources to study the response to costs imposed by the nitrogen fixation pathway.
Materials and methods
Biological materials
The wildtype L. japonicus ecotype ‘Miyakojima’ MG-20 has an early flowering phenotype and effective nodulation with M. loti strain MAFF303099 (MAFF) (Kawaguchi 2000). The hypernodulating mutant har1-7 was derived from MG-20 by ethylmethane sulfonate mutagenesis resulting in a nonsense mutation in the leucine rich repeat domain of the HAR1 gene. The mutant has an impaired ability to autoregulate nodule development, and in some conditions can form up to 2–5 × more nodules compared to MG-20 (Magori et al. 2009). The hypernodulating phenotype has also been reported alongside inhibition of plant growth and deterioration of overall plant vitality as well as nitrate tolerance for HAR1 mutants (among eight or more available) and orthologs in other legume species (Carroll et al 1985; Magori et al. 2009; Schnabel et al 2005; Wopereis et al. 2000). MG-20 and har1-7 were inbred at the University of California Riverside to generate additional seeds following published protocols (Quides et al. 2017).
Greenhouse experiment
Seeds were surface sterilized in bleach (5% sodium hypochlorite), rinsed in sterile water, nick scarified, germinated in sterile water in the dark, and planted under axenic conditions in bleach sterilized Ray-Leach SC10 containers (Stuewe & Sons, Corvallis, OR, USA) using an equal mix of coarse and fine calcined clay that was autoclave-sterilized (Pro League, Quickdry; Turface Athletics, Buffalo Grove, Illinois, USA).
MAFF was plated from frozen stocks onto agar plates with a modified arabinose gluconate medium (MAG) and cells were resuspended in sterile ddH2O at a concentration of 1 × 108 cells ml−1 (Sachs et al. 2009). Plants were inoculated on 03/31/2020 with 5 ml of MAFF (5 × 108 cells) or 5 ml sterile ddH2O as a control. All plants were supplemented weekly with Broughton and Dilworth nitrogen-free nutrient solution, with sources of boron, calcium, copper, iron, magnesium, potassium, phosphorus, sodium, sulphur, and zinc (Somasegaran and Hoben 1994). For plants that received nitrogen fertilization, sources and concentrations of dissolved nitrogen were added to the above micronutrient solution, depending on the treatment.
Nitrogen sources included potassium nitrate (KNO3), ammonium sulfate ((NH4)2SO4), alanine (C3H7NO2), or aspartic acid (C4H7NO4). Solutions were adjusted to a pH of ~ 6.7 and were titrated so that the nitrogen moiety available to the host was equimolar among the different molecular forms of nitrogen. Matching concentration levels were defined as 0%, 25% (7.4 mM), 50% (14 mM) and 100% (29 mM) for each nitrogen source with the 50% level matching ‘Growth Saturating Nitrogen’ as defined by Regus and colleagues on the related plant species, Acmispon strigosus (formerly L. strigosus; i.e., 0.5 g l−1 KNO3, Regus et al. 2014; Table S1).
Plants were randomized into seven blocks in the greenhouse (Figure S1). Each block consisted of one replicate per host genotype (MG-20, har1-7), inoculation treatment (MAFF, water), nitrogen source (ammonium sulphate, potassium nitrate, alanine, aspartic acid) and concentration (0%, 25%, 50%, 100%), resulting in 64 plants per block, and 448 in total. Fertilization started immediately after inoculation, with increasing volume as the plants grew, starting with 2 ml in week one, and increasing by 1 ml per week until a maximum of 5 ml was reached in week four until the completion of the experiment. Fertilization occurred after watering to minimize leaching.
Harvest was initiated six weeks post inoculation and lasted for ten days, beginning on 05/8/2020. Plants were removed from pots, roots were washed free of soil, and dissected into root, shoot, and nodule portions. Nodules were counted and photographed. Roots, shoots, and nodules were weighed for biomass after being oven dried (≥ 3 days, 60 °C).
Leaf ẟ15N assays
Leaf 15 N ‘atom per cent difference’, a measure of nitrogen fixation (Regus et al. 2014), was estimated as the percentage of 15 N atoms over total nitrogen in each sample (Unkovich et al. 2008). Both L. japonicus genotypes were analyzed with 0% or 100% of each of the four nitrogen sources tested for a total of 188 plants. Individual leaves of each plant were oven dried, powdered using steel bead beaters at 14,000 rpm, and transferred into individual tin capsules for isotopic analysis at the UC Davis Stable Isotope Facility. The δ15N of each sample was calculated by comparing 15 N abundance expressed as parts per thousand, relative to atmospheric N2. These values were used to compare among inoculated and uninoculated plants in each of the 0% and 100% nitrogen treatments following the formula:
When plants incorporate fixed nitrogen, leaf tissues exhibit a decrease in δ15N relative to uninfected plants due to isotopic fractionation by rhizobia. Absolute values of δ15N were used so that increased positive values indicates enhanced symbiotic nitrogen fixation.
Data analysis
Linear mixed models (LMMs) were used to analyze experimental treatment effects on growth, nodulation, and nitrogen fixation. Host genotype, nitrogen source, and nitrogen concentration were all treated as fixed effects, blocks within the greenhouse layout were treated as a random effect, and harvest date was used as a covariate (i.e., days post inoculation until harvest) in the models. All statistical models were fitted with the R package lme4 (Bates et al. 2015). We tested the significance of fixed effects of each model described above with marginal likelihood ratio tests using the Anova function in the car package (Fox and Weisberg 2019). Post hoc tests were conducted to identify differences among genotypes, inoculum, nitrogen sources and concentrations using the lsmeans package (Lenth 2016). Response variables were transformed if necessary to improve normality. Analyses were performed using The R project for Statistical Computing version 3.6.1
Investment into symbiosis was calculated by dividing the dry nodule biomass of each plant over the total biomass (Ortiz-Barbosa et al. 2022). Host relative growth was calculated as a percentage, by subtracting the total biomass values (i.e., shoot, root, and nodules) of inoculated plants from the mean mass of their uninoculated controls and multiplying by 100 (Regus et al. 2015). Growth saturating nitrogen was defined as the lowest concentration of a nitrogen source that maximizes L. japonicus growth in the absence of rhizobial infection and was determined using independent t-tests among nitrogen concentrations (Regus et al. 2014).
Results
Inoculation and fertilizer effects
In hosts that received no extrinsic nitrogen source, inoculated plants had higher biomass compared to controls (7.26 mg ± 0.39; 6.17 mg ± 0.42; F1,99 = 3.99; p = 0.04; Table S2). The L. japonicus genotype har1-7 formed ~ 42% more nodules compared to MG-20 (har1-7, 38.71 ± 4.14; MG-20, 27.32 ± 3.56; F1,45 = 7.92; p = 0.007; Table S3) but there were no significant differences in biomass between genotypes (har1-7, 6.77 mg ± 0.45; MG-20, 6.66 ± 0.37; F1,98 = 0.18; p = 0.66; Table S2). Additionally, there were no significant differences between the MG-20 and har1-7 hosts for mean nodule biomass, total nodule biomass, root to shoot ratio, investment into symbiosis, or 15 N difference in the absence of extrinsic nitrogen (Tables S2, S3).
In uninoculated hosts, all four nitrogen sources significantly enhanced host growth, indicating that L. japonicus has efficient pathways to catabolize each of them (Fig. 1; Tables S4, S5). For the organic sources of nitrogen, alanine and aspartic acid, each increasing concentration significantly enhanced L. japonicus growth relative to the lower levels, indicating that growth saturating concentrations of these organic nitrogen sources were not reached. For the inorganic forms of nitrogen, the 50% treatment was found to be the growth saturating concentration for potassium nitrate and ammonium sulfate, with no significant increase in host biomass with the addition of more fertilizer (Fig. 1).
Effects of different nitrogen sources on the growth of aposymbiotic plants. Plant biomass (a) and root to shoot ratio (b) in uninoculated plants is shown with four nitrogen source treatments (i.e., alanine, AL; aspartic acid, AA; potassium nitrate, PN; ammonium sulphate, AS), each with three different fertilization levels (i.e., 25%, 50%, 100%). Connecting letter reports indicate significant differences within each nitrogen source tested, with the no nitrogen source controls combined for all blocks (NNS). Points represent individual plant replicates. The lower and upper hinges correspond to the first and third quartiles. The upper whiskers extend from the hinge to the largest value no further than 1.5 inter-quartile range. Data were combined for the MG-20 and har1-7 host genotypes
Plants fertilized with organic nitrogen sources grew to a lower level of total biomass on average compared to the plants receiving the inorganic nitrogen sources (organic, 78.87 mg ± 5.05; inorganic, 91.34 mg ± 3.53; Fig. 1). Additionally, plants inoculated with the organic nitrogen sources produced higher root to shoot ratios on average compared to plants inoculated with the inorganic nitrogen sources, independently of the nitrogen concentration tested (organic, 0.59 ± 0.01; inorganic, 0.34 ± 0.01; Fig. 1). These data suggest enhanced catabolism efficiency with inorganic nitrogen sources relative to the organic forms of nitrogen.
Effects of fertilization on plant regulation of symbiosis traits
There were no effects of plant genotype for host relative growth, total biomass, or root to shoot ratio (Tables 1,2). The only significant host genotype effect was for nodule number (F1,133 = 4.27; p = 0.04; Table 1), where MG-20 produced fewer nodules (84.83 ± 7.52) compared to the hypernodulating mutant, har1-7 (90.4 ± 4.89). Moreover, there were subtle genotypic differences in how the hosts regulated nodule number and size (Table S6). In previous work, differences between har1 and wildtype depended on which har1 mutant was used and the environment (i.e., growth chamber, greenhouse, etc.,) in which plants were assessed (Buzas and Gresshoff 2007; Kawaguchi et al. 2002; Magori et al. 2009; Nishimura et al. 2002; Schauser et al. 1998; Szczyglowski et al. 1998; Wopereis et al. 2000). Plant trait results are reported separately for each genotype (Table S6), but data were combined for all factors that showed no effect of plant genotype.
In inoculated host plants, increasing concentrations of nitrogen fertilization were associated with significantly enhanced plant growth for each individual nitrogen source tested (p = 0.001; Table S7; Table S8). However, the inoculated plants in almost all cases had significantly lower plant biomass than the uninoculated controls, indicating that nodulation imposed a net cost on plants that were already receiving substantial benefit from chemical fertilization (Fig. 2; Table S7).
Effects of inoculation under different fertilization treatments. Total plant biomass is compared between inoculated and control plants under the four nitrogen source treatments, each with three different fertilization levels (i.e., 25%, 50%, 100%) and plants without nitrogen source added (No Nitrogen Source NNS). Connecting letter reports show significant differences between inoculated and control plants within each nitrogen source and concentration tested. Points represent individual plant replicates. The lower and upper hinges correspond to the first and third quartiles. The upper whiskers extend from the hinge to the largest value no further than 1.5 inter-quartile range. Data were combined for the MG-20 and har1-7 host genotypes
For all four nitrogen sources, the root to shoot ratio was higher for uninoculated plants compared to inoculated ones, indicating that nodulation reduced the root biomass (p ≤ 0.001; Table S7). Nitrogen fertilization consistently increased raw nodule counts in all four nitrogen sources (Table S9, Table S10), inconsistent with recent work on L. japonicus that used a different host genotype (Gifu) and employed continuous fertilization (Lin et al. 2021). However, consistent with previous work (Lin et al. 2021; Regus et al. 2015), increasing nitrogen concentrations were associated with significant reduction in investment into symbiosis, hence that a smaller proportion of plant tissue was made up of nodules (Table S10; Fig. 3).
Mean nodule biomass under different fertilization treatments. Mean nodule biomass is compared between each of the three different fertilization levels (i.e., 25%, 50%, 100%) of the four nitrogen sources (i.e., alanine, AL; aspartic acid, AA; potassium nitrate, PN; ammonium sulphate, AS). Connecting letter reports indicate significant differences within each nitrogen source. Points represent individual plant replicates. The lower and upper hinges correspond to the first and third quartiles. The upper whiskers extend from the hinge to the largest value no further than 1.5 inter-quartile range. Data were combined for the MG-20 and har1-7 host genotypes
For mean nodule biomass, host response varied among the nitrogen sources. For the organic sources, mean nodule biomass did not vary among nitrogen concentrations. For ammonium sulfate, the mean nodule mass was reduced over 37% at the highest nitrogen concentration, with nodule mass at the 100% fertilizer concentration (0.05 mg ± 0.008) being significantly smaller than under 25% (0.08 mg ± 0.008; t25 = -3.6; p = 0.003) or 50% (0.08 mg ± 0.004; t26 = -3.4; p = 0.004). A similar reduction in mean nodule mass, but with greater variance, was uncovered for potassium nitrate (Fig. 3), but was not significant (Table S10).
Nitrogen fixation under different nitrogen sources
Plants that were inoculated with rhizobia, but did not receive extrinsic nitrogen, exhibited absolute values of δ15N (3.77 ‰ ± 0.21) that were significantly higher than uninoculated controls (0.68 ‰ ± 0.38; Table S11), consistent with substantial incorporation of symbiotically fixed nitrogen (Regus et al. 2014). Among inoculated plants, significant reductions in nitrogen fixation (i.e., lower absolute values of δ15N) were uncovered when plants were fertilized at the 100% level with alanine (t66 = -3.5; p = 0.01), ammonium sulfate (t68.9 = -3.64; p = 0.01) and potassium nitrate (t65.2 = -4.74; p = 0.0003), but not for aspartic acid (t67.4 = -0.26; p = 1; Table 3; Table S11; Fig. 4). Nitrogen fixation was reduced the most with potassium nitrate fertilization compared to both alanine (t67.5 = -4.14, p = 0.0023) and ammonium sulfate (t68.1 = -3.54, p = 0.01; Table S11).
15 N difference of inoculated plants under different fertilization treatments. Absolute values of 15 N difference are indicated for inoculated plants that received no nitrogen source (NNS) or were fertilized at the 100% concentration with one of the four nitrogen sources (i.e., alanine, AL; aspartic acid, AA; potassium nitrate, PN; ammonium sulphate, AS). Connecting letter reports show significant differences among the treatments. Points represent individual plant replicates. The lower and upper hinges correspond to the first and third quartiles. The upper whiskers extend from the hinge to the largest value no further than 1.5 inter-quartile range. Data were combined for the MG-20 and har1-7 host genotypes
Discussion
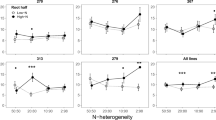
A major goal of research is to uncover the mechanisms that legumes use to optimize benefits from rhizobia, including the sanctioning of poorly fixing strains, and adjusted investment into nodulation when sufficient extrinsic nitrogen is available. Here, we found that under some fertilization regimes plants downregulate investment into nodule growth when exposed to inorganic fertilization, as was previously shown for the related host A. strigosus, and also for soybeans (Kiers et al. 2006; Regus et al. 2015; Fig. 3). Our data indicated that nitrogen fixation was downregulated in the presence of some but not all nitrogen sources (i.e., not aspartic acid). These results suggest that L. japonicus varies in its response in terms of mechanism, reducing investment into nodule size or nitrogen fixation, dependent on the form of extrinsic nitrogen available and its concentration (Fig. 5). The differences could be driven by variation in the catabolic costs of different nitrogen sources, nitrogen metabolism and amino acid cycling between the plant and the rhizobia (Pfau et al. 2018), or as we hypothesize, that the legume would be unable to differentiate some extrinsic sources of nitrogen from biologically fixed nitrogen (i.e., aspartic acid).
Summary of results for phenotypic trait expression from four different traits measured. In blue are traits where the plants invested into symbiosis whereas the red represents total biomass, a trait that measures the benefit from symbiosis. Results showed downregulation of investment and nitrogen fixation as nitrogen concentrations increased for three out of the four nitrogen sources tested
Some legume host species can reduce nodulation when fertilized with nitrogen (Lin et al. 2021; Streeter 1988). However, the relationship between fertilization and nodule count is complex, and exceptions can be instructive. In the present study, nodulation was never suppressed under the fertilization regimes tested, as has been shown for other legumes (Habinshuti et al. 2021; Streeter 1988). Diverse empirical outcomes require cautious interpretation, as it can be difficult to compare among varied experimental conditions, nitrogen concentrations, forms of nitrogen, and delivery methods. For instance, in soybean, Glycine max, nodulation can vary under different fertilization regimes, and low concentrations of nitrate (i.e., 1 mM) can enhance nodule count, as well as nodule dry weight and total N2 fixation per plant (Gan et al. 2004). In the same study, a significant suppression in nodule number, nodule dry weight and nitrogen fixed per plant was observed under higher concentrations of nitrate and ammonium (i.e., 10 mM). Additional evidence from G. max, barrel medic (Medicago truncatula), and A. strigosus shows that root nodulation can be accelerated by low concentrations of nitrogen and significantly suppressed by higher concentrations (Heath et al. 2010; Regus et al. 2017a; Xia et al. 2017). In A. strigosus, a species within the same tribe as L. japonicus, plants exhibited reduced nodulation at high nitrogen concentrations, but these patterns were only observed at 6-10x the nitrogen concentrations used here, levels that were also associated with toxicity (Regus et al. 2017a). Given this diversity of outcomes, a pluralistic view is needed, one that legume traits to regulate symbiosis can vary among plant species with divergent evolutionary histories and be differentially impacted by the soil environmental context.
In some scenarios, legumes can downregulate or shut down the costly process of nitrogen fixation as extrinsic nitrogen becomes sufficient in the soil (Gan et al. 2004; Lin et al. 2021; Reinprecht et al. 2020). Our data showed that as concentration of extrinsic nitrogen increased, L. japonicus reduced investment into symbiosis by producing smaller nodules in some cases or by reducing or fully shutting down nitrogen fixation from rhizobia. In contrast, for aspartic acid, plants did not respond by reducing nodulation or nitrogen fixation as nutrient concentrations increased, which suggests that L. japonicus did not differentiate it as an extrinsic nitrogen source that would allow symbiotic nitrogen fixation to be shut down (Fig. 4). These data implicate aspartic acid as the signal by the host to detect symbiont cooperation, similar to what has been predicted on other systems (Lodwig et al. 2003; Rastogi and Watson 1991). Moreover, these results are consistent with the hypothesis that aspartic acid is the predominant source of nitrogen being excreted from the bacteroids of M. loti to the cytoplasm of L. japonicus. Given that rhizobia are predicted to shuttle aspartate to hosts, fertilization with this nitrogen source might abrogate the hosts capacity to adaptively regulate nodule size and nitrogen fixation.
Under the experimental conditions here, the wild-type MG20 and the mutant har1-7 responded similarly to the different nitrogen sources and concentrations tested. The HAR1 gene regulates nodule and root meristems through long distance signaling from shoot to root, and it has been shown that the mutation of this gene can cause tolerance to high nitrate concentrations and stunted roots and shoots (Magori et al. 2009; Oka-Kira et al. 2005). However, there are over eight different HAR1 mutants that have been tested, expressing different phenotypic traits under variable experimental conditions (Kawaguchi et al. 2002; Magori et al. 2009; Nishimura et al. 2002; Wopereis et al. 2000). In our results, neither the wild-type nor the mutant har1-7 were inhibited by increased nitrate concentrations and their roots and shoots appeared to be healthy unlike what has been reported for har1-1 mutants (Wopereis et al. 2000).
Mutualistic interactions are defined by the net fitness benefits that interspecific partners receive from association. But a focus on net benefits can obscure marginal costs that individuals must pay to participate in an interaction (Bull and Rice 1991), costs that can be exposed in a context specific manner (Hoeksema and Bruna 2015). A meta-analysis of plant mycorrhizal associations, where net effects to hosts can range from positive to negative, found that plant responses to infection are context dependent and are most positive when plants are phosphorus limited rather than nitrogen limited, which relates to the service that these fungi are delivering to their hosts (Hoeksema et al. 2010). Our work here suggests that L. japonicus can suffer a net cost from symbiosis, dependent on soil nutrient conditions. Unlike the widespread perception that host plants can invariably reduce nodulation to optimize growth, our data suggest that legumes cannot always mitigate costs associated with nodulation and nitrogen fixation (Fig. 2) (Sachs et al. 2018).
Our experiments demonstrate that L. japonicus can grow efficiently under an array of sole nitrogen sources, including both organic and inorganic forms in the absence of root nodulation (Fig. 1), as well as with biologically fixed nitrogen, when no extrinsic source of nitrogen is available. Organic sources appear more costly for L. japonicus to catabolize given that plants grew less with organic nitrogen even when sources were equimolar for the nitrogen moiety (Fig. 1). Examining catabolic cost differentials, and their metabolic bases, could be one way to improve fertilizer formulations, as these data suggest that some nitrogen sources might promote host growth more than others. Moreover, enhanced nitrogen availability can decrease plant allocation into roots as more resources available mean less effort to acquire them (Agren and Franklin 2003). This is consistent with what we see with inorganic nitrogen sources which according to our results provide more benefits and trigger plant adjustment of root to shoot ratios (Fig. 1).
In agricultural settings, growers might often add rhizobial inoculants to their crops, but enhancing root nodulation might decrease host growth significantly in fertilized soils. However, other nutrients in the fertilizer as well as the molecular form of nitrogen could potentially influence the outcome. A study using plants with rhizobia on fertilized and unfertilized conditions found that the fertilized soil provided the legume with higher mutualistic benefits, showing that short term fertilizer application is sufficient to alter rhizobial population and communities, and is potentially a strong selective agent acting on natural populations (Simonsen et al. 2015). Conversely, in urbanized areas, eutrophication and nitrogen deposition can decrease the abundance of rhizobium nodules, as plant hosts primarily acquired nitrogen from the soil rather than from nitrogen fixation (Murray-Stoker and Johnson 2021). Similarly, long term fertilization can lead to a reduction in the net benefits that legume hosts gain from nitrogen fixation (Weese et al. 2015). Thus, while reduced investment in symbiosis can be an adaptive response by host legumes in the short term, it can lead to the destabilization, or potential loss of mutualism if these conditions persist.
References
Appels MA, Haaker H (1991) Glutamate oxaloacetate transaminase in pea root nodules. Plant Physiol 95:740–747
Agren G, Franklin O (2003) Root: shoot ratios, optimization and nitrogen productivity. Ann Bot 92:795–800. https://doi.org/10.1093/aob/mcg203
Amarger N (1981) Competition for nodule formation between effective and ineffective strains of Rhizobium meliloti. Soil Biol Biochem 13:475–480. https://doi.org/10.1016/0038-0717(81)90037-7
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed effects models using lme4. J Stat Softw 67:48
Batstone RT, Peters MAE, Simonsen AK, Stinchcombe JR, Frederickson ME (2020) Environmental variation impacts trait expression and selection in the legume-rhizobium symbiosis. Am J Bot 107(2):195–208. https://doi.org/10.1002/ajb2.1432
Bull JJ, Rice WR (1991) Distinguishing mechanism for the evolution of co-operation. J Theor Biol 149(1):63–74. https://doi.org/10.1016/S0022-5193(05)80072-4
Buzas DM, Gresshoff PM (2007) Short and long-distance control of root development by LjHAR1 during the juvenile stage of Lotus japonicus. J Plant Physiol 164:452–459
Carroll BJ, McNeil DL, Gresshoff PM (1985) A supernodulation and nitrate-tolerant symbiotic (nts) soybean mutant. Plant Physiol 78:34–40
Champion RA, Mathis JN, Israel DW, Hunt PG (1992) Response of soybean to inoculation with efficient and inefficient Bradyrhizobium japonicum variants. Crop Sci 32:457–463. https://doi.org/10.2135/cropsci1992.0011183X003200020034x
Corrales A, Mangan SA, Turner BL, Dalling JW (2016) An ectomycorrhizal nitrogen economy facilitates monodominance in a neotropical forest. Ecol Lett 19:383–392. https://doi.org/10.1111/ele.12570
Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM (2018) Legume nodulation: The host controls the party. Plant, Cell Environ 42(1):41–51. https://doi.org/10.1111/pce.13348
Fox J, Weisberg S (2019) An R companion to applied regression. Sage, Thousand Oaks
Galloway JN, Dentener DG, Capone EW, Boyer RW, Howarth S, Seitzinger P, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70(2):153–226. https://doi.org/10.1007/s10533-004-0370-0
Gan Y, Stulen I, Keulen HV, Kuiper PJC (2004) Low concentrations of nitrate and ammonium stimulate nodulation and N2 fixation while inhibiting specific nodulation (nodule DW g-1 root dry weight) and specific N2 fixation (N2 fixed g-1 root dry weight) in soybean. Plant Soil 258:281–292. https://doi.org/10.1023/B:PLSO.0000016558.32575.17
Gano-Cohen KA, Wendlant CE, Moussawi KA, Stokes PJ, Quides KW, Weisberg AJ, Chang JF, Sachs JL (2020) Recurrent mutualism breakdown events in a legume rhizobia metapopulation. Proc R Soc B 287:20192549. https://doi.org/10.1098/rspb.2019.2549
Gerz M, Bueno CG, Ozinga WA, Zobel M, Moora M (2018) Niche determination and expansion of plant species are associated with mycorrhizal symbiosis. J Ecol 106:254–264. https://doi.org/10.1111/1365-2745.12873
Habinshuti SJ, Maseko ST, Dakora FD (2021) Inhibition of N2 fixation by N fertilization of common bean (Phaseolus vulgaris L.) plants grown on fields of farmers in the eastern cape of South Africa, measured Using 15N natural abundance and tissue ureide analysis. Frontiers in Agronomy. 3:692933. https://doi.org/10.3389/fagro.2021.692933
Hahn M, Studer D (1986) Competitiveness of a nif− Bradyrhizobium japonicum mutant against the wild-type strain. FEMS Microbiol Lett 33:143–148. https://doi.org/10.1111/j.1574-6968.1986.tb01228.x
Heath KD (2010) Intergenomic epistasis and coevolutionary constrains in plants and rhizobia. Evolution 64–5:1446–1458. https://doi.org/10.1111/j.1558-5646.2009.00913.x
Heath KD, Stock AJ, Stinchcombe JR (2010) Mutualism variation in the nodulation response to nitrate. J Evol Biol 23(11):2494–2500. https://doi.org/10.1111/j.1420-9101.2010.02092.x
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407. https://doi.org/10.1111/j.1461-0248.2009.01430.x
Hoeksema JD, Bruna EM (2015) Context-dependent Outcomes of Mutualistic Interactions. In: Bronstein JL (ed) Mutualism. Oxford University Press, pp 181–202
Hussain AKM, Jiang Q, Broughton WJ, Gresshoff PM (1999) Lotus japonicus nodulates and fixes nitrogen with the broad host range Rhizobium sp. NGR234. Plant Cell Physiol 40(8):894–899. https://doi.org/10.1093/oxfordjournals.pcp.a029619
Kawaguchi M (2000) Lotus japonicus ‘Miyakojima’ MG-20: An early-flowering accession suitable for indoor handling. J Plant Res 113:507–509. https://doi.org/10.1007/PL00013961
Kawaguchi M, Imaizumi-Anraku H, Koiwa H, Niwa S, Ikuta A, Syono K, Akao S (2002) Root, root hair, and symbiotic mutants of the model legume Lotus japonicus. Molec Plant Microbe Interact 15(1):17–26
Kiers T, Rousseau RA, West SA, Denison RF (2003) Host Sanctions and the legume-rhizobium mutualism. Nature 425:78–81. https://doi.org/10.1038/nature01931
Kiers ET, Rousseau RA, Denison RF (2006) Measured sanctions: legumes hosts detect quantitative variation in rhizobium cooperation and punish accordingly. Evol Ecol Res 8:1077–1086
Klucas RV (1974) Studies on soybean nodule senescence. Plant Physiol 54:612–616
Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, Pajuelo E, Sandal N, Stougaard J (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420:422–426. https://doi.org/10.1038/nature01207
Lenth, R. V. (2016). Least-Squares Means: The R Package lsmeans. J Stat Software, 69(1):1–33. https://doi.org/10.18637/jss.v069.i01
Lin J, Roswanjaya YP, Kohlen W, Stougaard J, Reid D (2021) Nitrate restricts nodule organogenesis through inhibition of cytokinin biosynthesis in Lotus japonicus. Nat Commun 12:6544. https://doi.org/10.1038/s41467-021-26820-9
Lodwig EM, Hosie AHF, Bourdès A, Findlay K, Allaway D, Karunakaran R, Downie DA, Poole PS (2003) Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature 422:722–726. https://doi.org/10.1038/nature01527
Magori S, Oka-Kira E, Shibata S, Umehara Y, Kouchi H, Hase Y, Tanaka A, Sato S, Tabata S, Kawaguchi M (2009) TOO MUCH LOVE, a root regulator associated with the long-distance control of nodulation in Lotus japonicus. Molec Plant Microbe Interact 22(3):259–268. https://doi.org/10.1094/MPMI-22-3-0259
Meeks JC, Wolk CP, Schilling N, Shaffer PW (1978) Initial organic products of fixation of [13N]dinitrogen by root nodules of soybean (Glycine max). Plant Physiol 61:980–983
Murray-Stoker D, Johnson MTJ (2021) Ecological consequences of urbanization on a legume-rhizobia mutualism. Oikos 130:1750–1761. https://doi.org/10.1111/oik.08341
Ngom M, Gray K, Diagne N, Oshone R, Fardoux J, Gherbi H, Hocher V, Svistoonoff S, Laplaze L, Tisa LS, Sy MO, Champion A (2016) Symbiotic performance of diverse Frankia strains on salt-stressed Casuarina glauca and Casuarina equisetifolia plants. Front Plant Sci 7:1331. https://doi.org/10.3389/fpls.2016.01331
Nishimura R, Hayashi M, Wu G, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, Harada K, Kawaguchi M (2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420:426–429. https://doi.org/10.1038/nature01231
Ohyama T, Kumazawa K (1980) Nitrogen assimilation in soybean nodules. Soil Sci Plant Nutr 26(2):205–213
Oka-Kira E, Tateno K, Miura K, Haga T, Hayashi M, Harada K, Sato S, Tabata S, Shikazono N, Tanaka A, Watanabe Y, Fukuhara I, Nagata T, Kawaguchi M (2005) Klavier (klv), a novel hypernodulation mutant of Lotus japonicus affected in vascular tissue organization and floral induction. Plant J 44:505–515. https://doi.org/10.1111/j.1365-313X.2005.02543.x
Oono R, Anderson CG, Denison RF (2011) Failure to fix nitrogen by non-reproductive symbiotic rhizobia triggers host sanctions that reduce fitness of their reproductive clonemates. Proc R Soc 278:2698–2705. https://doi.org/10.1098/rspb.2010.2193
Ortiz-Barbosa GS, Torres-Martinez L, Manci A, Neal S, Soubra T, Khairi F, Trinh J, Cardenas P, Sachs JL (2022) No disruption of rhizobial symbiosis during early stages of cowpea domestication. Evolution 76(3):496–511. https://doi.org/10.1111/evo.14424
Pfau T, Christian N, Masakapalli SK, Sweetlove LJ, Poolman MG, Ebenhoh O (2018) The interwined metabolism during symbiotic nitrogen fixation elucidated by metabolic modelling. Sci Rep 8:12504. https://doi.org/10.1038/s41598-018-30884-x
Porter SS, Simms EL (2014) Selection for cheating across disparate environments in the legume-rhizobium mutualism. Ecol Lett 17:1121–1129. https://doi.org/10.1111/ele.12318
Quides KW, Stomackin GM, Lee H, Chang JH, Sachs JL (2017) Lotus japonicus alters in planta fitness of Mesorhizobium loti dependent on symbiotic nitrogen fixation. PLoS ONE. 12(9):e0185568. https://doi.org/10.1371/journal.pone.0185568
Quides KW, Salaheldine F, Jariwala R, Sachs JL (2021) Dysregulation of host-control causes interspecific conflict over host investment into symbiotic organs. Evolution. https://doi.org/10.1111/evo.14173
Rastogi VP, Watson RJ (1991) Aspartate aminotransferase activity is required for aspartate catabolism and symbiotic nitrogen fixation in Rhizobium meliloti. J Bacteriol 2879–2887. https://doi.org/10.1128/jb.173.9.2879-2887.1991
Regus JU, Gano KA, Hollowell AC, Sachs JL (2014) Efficiency of partner choice and sanctions in Lotus is not altered by nitrogen fertilization. Proc R Soc B 281:20132587. https://doi.org/10.1098/rspb.2013.2587
Regus JU, Gano KA, Hollowell AC, Sofish V, Sachs JL (2015) Lotus hosts delimit the mutualism-parasitism continuum of Bradyrhizobium. J Evol Biol 28:447–456. https://doi.org/10.1111/jeb.12579
Regus JU, Wendlandt CE, Bantay RM, Gano-Cohen KA, Gleason NJ, Hollowell AC, O’Neill MR, Shahin KK, Sachs JL (2017a) Nitrogen deposition decreases the benefits of symbiosis in a native legume. Plant Soil 2017(414):159–170. https://doi.org/10.1007/s11104-016-3114-8
Regus JU, Quides KW, O’Neill MR, Suzuki R, Savory EA, Chang JH, Sachs JL (2017b) Cell autonomous sanctions in legumes target ineffective rhizobia in nodules with mixed infections. Am J Bot 104(9):1–14. https://doi.org/10.3732/ajb.1700165
Reinprecht Y, Schram L, Marsolais F, Smith TH, Hill B, Pauls KP (2020) Effects of nitrogen application on nitrogen fixation in common bean production. Front Plant Sci 11:1172. https://doi.org/10.3389/fpls.2020.01172
Rosendahl L, Dilworth MJ, Glenn AR (1992) Exchange of metabolites across the peribacteroid membrane in pea root nodules. J Plant Physiol. 139: 635–638.
Sachs JL, Simms EL (2008) The origins of uncooperative rhizobia. Oikos 117:961–964. https://doi.org/10.1111/j.2008.0030-1299.16606.x
Sachs JL, Kembel SW, Lau AH, Simms EL (2009) In situ phylogenetic structure and diversity of wild Bradyrhizobium communities. Appl Environ Microbiol 75:4727–4735. https://doi.org/10.1128/AEM.00667-09
Sachs JL, Russel JE, Lii YE, Black KC, Lopez G, Patil AS (2010) Host control over infection and proliferation of a cheater symbiont. J Evol Biol 23:1919–1927. https://doi.org/10.1111/j.1420-9101.2010.02056.x
Sachs JL, Quides KW, Wendlant CE (2018) Legumes versus rhizobia: a model for ongoing conflict in symbiosis. New Phytol 219:1199–1206. https://doi.org/10.1111/nph.15222
Sawada H, Kuykendall LD, Young JM (2003) Changing concepts in the systematics of bacterial nitrogen-fixing legume symbionts. J Gen Appl Microbiol 49:155–179. https://doi.org/10.2323/jgam.49.155
Schauser L, Handberg K, Sandal N, Stiller J, Thykjaer T, Pajuelo E, Nielsen A, Stougaard J (1998) Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol Gen Genet 159:414–423
Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J (2005) The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol 58:809–822
Simard SW, Beiler KJ, Bingham MA, Deslippe JR, Philip LJ, Teste FP (2012) Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol Rev 39–60. https://doi.org/10.1016/j.fbr.2012.01.001
Simonsen AK, Han S, Rekret P, Rentschler CS, Heath KD, Stinchcombe JR (2015) Short-term fertilizer application alters phenotypic traits of symbiotic nitrogen fixing bacteria. PeerJ 3:e1291. https://doi.org/10.7717/peerj.1291
Simonsen AK, Dinnage R, Barrett LG, Prober SM, Thrall PH (2017) Symbiosis limits establishment of legumes outside their native range at a global scale. Nat Commun 8:14790. https://doi.org/10.1038/ncomms14790
Somasegaran P, Hoben HJ (1994) Handbook for rhizobia: methods in legume rhizobium technology. Springer Verlag, New York, p 450
Streeter J (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci 7(1):1–23. https://doi.org/10.1080/07352688809382257
Sulieman S, Tran LP (2012) Asparagine: an amide of particular disctinction in the regulation of symbiotic nitrogen fixation of legumes. Crit Rev Biotechnol 1–19. https://doi.org/10.3109/07388551.2012.695770
Szczyglowski K, Shaw RS, Wopereis J, Copeland S, Hamburger D, Kasiborski B, Dazzo FB, de Bruijn FJ (1998) Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Molecular Plant Microbe Interactions 11(7):684–697
Tyerman SD, Whitehead LF, Day DA (1995) A channel-like transporter for NH4+ on the symbiotic interface of N2-fixing plants. Nature 378:629–632
Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey B, Giller K, Alves B, Chalk P (2008) Measuring Plant-associated Nitrogen Fixation in Agricultural Systems. Australian Centre for International Agricultural Research (ACIAR), Canberra
Waters JK, Hughes BL II, Purcell LC, Gerhardt KO, Mawhinney TP, Emerich DW (1998) Alanine, not ammonia, is excreted from N2-fixing soybean nodule bacterioids. Proc Natl Acad Sci 95:12038–12042. https://doi.org/10.1073/pnas.95.20.12038
Weese DJ, Heath KD, Dentinger BTM, Lau JA (2015) Long-term nitrogen addition causes the evolution of less cooperative mutualists. Evolution 69(3):631–642. https://doi.org/10.1111/evo.12594
Wendlandt CE, Regus JU, Gano-Cohen KA, Hollowell AC, Quides KW, Lyu JY, Adinata ES, Sachs JL (2019) Host investment into symbiosis varies among genotypes of the legume Acmispon strigosus, but host sanctions are uniform. New Phytol 221:446–458. https://doi.org/10.1111/nph.15378
West SA, Kiers ET, Simms EL, Denison RF (2002) Sanctions and mutualism stability: why do rhizobia fix nitrogen. Proc R Soc London B 269:685–694. https://doi.org/10.1098/rspb.2001.1878
Westhoek A, Field E, Rehling F, Mulley G, Webb I, Poole PS, Turnbull LA (2017) Policing the legume-Rhizobium symbiosis: a critical test of partner choice. Sci Rep 7:1419. https://doi.org/10.1038/s41598-017-01634-2
White J, Prell J, James EK, Poole P (2007) Nutrient sharing between symbionts. Plant Physiol 144:604–614. https://doi.org/10.1104/pp.107.097741
Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, De Bruijn FJ, Stougaard J, Szczyglowski K (2000) Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23(1):97–114
Xia X, Ma C, Dong S, Xu Y, Gong Z (2017) Effects of nitrogen concentrations on nodulation and nitrogenase activity in dual root systems of soybean plants. Soil Sci Plant Nutr 63(5):470–482. https://doi.org/10.1080/00380768.2017.1370960
Acknowledgements
We thank LegumeBase at the University of Miyazaki Japan, from which we acquired MG-20 seeds. We thank Masayoshi Kawaguchi at the National Institute for Basic Biology, Okazaki, Aichi, Japan, for providing the har1-7 mutant seeds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11104_2022_5762_MOESM1_ESM.csv
Supplementary file1 Experimental setup and plants during harvest. (A) Experimental set up in the greenhouse (B) har1-7 plant inoculated with rhizobia and fertilized with potassium nitrate (100%) (C) MG20 plant inoculated with rhizobia and fertilized with potassium nitrate (100%). (CSV 76.0 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortiz-Barbosa, G.S., Torres-Martínez, L., Rothschild, J. et al. Lotus japonicus regulates root nodulation and nitrogen fixation dependent on the molecular form of nitrogen fertilizer. Plant Soil 483, 533–545 (2023). https://doi.org/10.1007/s11104-022-05762-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05762-1