Abstract
Aims
R2R3-type MYB transcription factors are associated with diverse developmental processes and responses to abiotic stresses. However, there is limited information regarding drought-responsive R2R3-MYB in a widespread subtropical tree species, Schima superba. Hence, the purpose of this study was to identify and functionally characterize the role of SsMYB113 in S. superba under drought stress.
Results
SsMYB113, a novel R2R3-MYB transcription factor that was targeted to the nucleus in Arabidopsis thaliana protoplasts, functioned as a transcriptional activator during in vitro and in vivo assays. SsMYB113 transcript was abundant in the leaves of six-month-old S. superba, and was significantly up-regulated by PEG-simulated drought stress and abscisic acid (ABA). Overexpression of SsMYB113 in A. thaliana seedlings led to enhanced tolerance to drought stress and facilitated flavonoid biosynthesis and ABA accumulation, including of corresponding biosynthetic genes, particularly SsCHS and SsNCED. Furthermore, SsMYB113 was shown to bind directly to the promoters of SsCHS and SsNCED using Y1H and a dual-LUC assay, thus activating their expression. In addition, in SsMYB113-overexpressing lines, proline, water content, superoxide dismutase, and peroxidase activities increased, while malondialdehyde, electrolyte leakage, and the rate of superoxide production decreased, suggesting the explicit role of SsMYB113 in conferring drought tolerance.
Conclusions
Drought-responsive SsMYB113 functioned as a positive regulator by participating in flavonoid and ABA biosynthesis, thereby enhancing drought stress tolerance in indigenous fast-growing S. superba.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schima superba Gardner & Champ. (Theaceae), which is widespread across most regions of subtropical China, is an indispensable dominant species in evergreen broad-leaved subtropical forests of southern China with, as one example, an approximate distribution of 1236 hm2 in Guangdong Province (Han et al. 2016; Zhao et al. 2018). S. superba is a precious timber tree species with excellent wood quality, resistance to drought, fire, and salt, and is shade-tolerant (Li et al. 2016). Since S. superba has an irreplaceable function in plant community constitution and succession, natural regeneration of S. superba seedlings is a top priority of forest vegetation succession and ecological restoration (Han et al. 2016; Li et al. 2010). However, the viability of feral S. superba populations does not exceed 11% at the seedling developmental stage (Hu et al. 2007) due to various restrictive physiological and environmental factors, especially insufficient light. Drought may play an indispensable role in seedling survival and forest regeneration. For instance, there are two consistently dry seasons, from July to August and October to February, in Gutianshan forest dynamics (Han et al. 2016). Consequently, greater research focus on S. superba is needed to cope with intermittent or frequent droughts.
To adapt to drought stressors, plants have evolved an inherent mechanism to retain high water content (WC) and to accumulate various hormones and osmo-protectants, such as proline, flavonoids, abscisic acid (ABA), and soluble sugar, thereby mitigating drought stress (Wasilewska et al. 2008; Xiong et al. 2006; Zhu 2016). Flavonoids not only alter plant pigmentation, but also latently provide protection against various abiotic stresses, such as drought, salt, and cold (Butelli et al. 2012; Nakabayashi and Saito 2015; Wang et al. 2016). Simultaneously, multiple signaling cascades modulate the transcript levels of stress-related genes that enhance a plant’s resistance to drought, either directly or indirectly (Gupta et al. 2020). Over the past decade, transcription factors (TFs), including v-myb myeloblastosis viral oncogene homolog (MYB), NAM, ATAF1/2, CUC1/2 (NAC), basic helix-loop-helix (bHLH), APETALA2/ethylene-responsive factor (AP2/ERF), and WRKY, have played a decisive function in orchestrating stress-induced hormone signals and functional gene expression, and are essential in plants’ responses to abiotic stresses (Baldoni et al. 2015; Guo et al. 2021; Jiang et al. 2017; Licausi et al. 2013; Puranik et al. 2012). Depending on the amount of adjacent MYB domain repeats, the plant MYB family has been clustered into four subfamilies, 1R-, 4R-, R1R2R3-, and R2R3-MYB, which have been identified at the genome-wide level in various plant species, such as Arabidopsis thaliana (Dubos et al. 2010; Stracke et al. 2001), Populus trichocarpa (Fang et al. 2020), and Solanum lycopersicum (Millard et al. 2019). Among them, the R2R3-MYB family is the largest subfamily of MYB TFs, which were characterized through knockdown or overexpression approaches, and have been shown to participate in plants’ response to drought stress (Baldoni et al. 2015; Stracke et al. 2001). For instance, P. trichocarpa PtrMYB94 was shown to coordinate with ABA signaling to enhance drought tolerance (Fang et al. 2020). When a Triticum aestivum R2R3-MYB gene TaMpc1-D4 was silenced, this positively modulated drought tolerance by activating the activities of antioxidant enzymes and up-regulating antioxidant-related genes (Li et al. 2020). However, the regulatory role of R2R3-MYB in S. superba is unknown.
Additionally, R2R3-MYB TFs have played a prominent role in the biosynthesis of flavonoids (Baldoni et al. 2015; Hichri et al. 2011; Millard et al. 2019). Several MYB TFs act as activators and promote the production of flavonoids, such as A. thaliana AtMYB12 (Wang et al. 2016), Brassica oleracea BoMYB2 (Chiu and Li 2012), P. trichocarpa PtrMYB94 (Fang et al. 2020), Solanum tuberosum AN1, MYBA1, and MYB113 (Liu et al. 2016). In contrast, several MYB TFs act as inhibitors and suppress the production of flavonoids, including T. aestivum TaMpc1-D4 (Li et al. 2020) and Brassica rapa BrMYB4 (Zhang et al. 2014). BrMYB4 is a negative transcriptional regulator that mediated cinnamate 4-hydroxylase (BrC4H) gene expression, thereby suppressing the accumulation of phenylpropanoids and anthocyanins (Zhang et al. 2014). Overexpression of AtMYB12 in A. thaliana promoted the production of total flavonoids and activated the expression of flavonoid biosynthetic genes such as chalcone synthase (CHS), thereby enhancing tolerance to salt and drought stresses (Wang et al. 2016). SmMYB113 from Solanum melongena enhanced the expression of CBF-regulated genes (SmCBF1, SmCBF2 and SmCBF3) in response to low temperature, and bound to the promoter of SmCHS and SmDFR to increase their expression, thereby facilitating anthocyanin-related flavonoid accumulation (Zhou et al. 2020). Intriguingly, the biosynthesis of flavonoids can be mediated by abiotic stress signals (Nakabayashi and Saito 2015), as well as the drought stress-induced accumulation of ABA and proline (Gupta et al. 2020; Wasilewska et al. 2008; Zhu 2016). Although the drought-induced biosynthesis of flavonoids is well documented, little is known about how drought affects the accumulation of flavonoids in S. superba.
In this study, a novel R2R3-MYB TF, SsMYB113, was cloned from S. superba leaves and functionally characterized as a positive modulator when plants were subjected to drought stress. Bioinformatic properties, subcellular localization, tissue-specific expression profiles, and phylogenetic trees of SsMYB113 in S. superba were investigated. Transgenic A. thaliana seedlings constitutively expressing SsMYB113 significantly promoted the accumulation of flavonoids and ABA, and also upregulated the expression of their corresponding biosynthetic genes, leading to enhanced drought tolerance. Furthermore, SsMYB113 regulated the biosynthesis of flavonoids and ABA by binding to the promoters of SsCHS and SsNCED, respectively, thereby activating the expression of these two genes. Taken together, our findings shed light on the response of S. superba to drought stress as a consequence of the modulated accumulation of ABA and flavonoids by SsMYB113.
Materials and methods
Plant materials and stress treatments
Six-month-old S. superba plants (Fig. S1) were purchased from Guangzhou Ruijing Landscape Design Co., Ltd. (Guangzhou, China), and recovered in sandy-clay soil and turfy soil (3:1, v/v) under natural conditions. Average temperature and annual rainfall were 23.1 °C and 1820.5 mm, respectively, information that was simultaneously collected from the Guangzhou Meteorological Bureau (http://www.tqyb.com.cn/en/). For the ABA treatment, leaves of six-month-old S. superba plants were sprayed with exogenous 100 μM ABA (Sigma-Aldrich, St. Louis., MO, USA) in 0.02% ethanol as the solvent (Baek et al. 2018). Simultaneously, the control group was sprayed with an equal amount of tap water. For the drought treatment, six-month-old S. superba plants were watered with aqueous 20% (v/v) polyethylene glycol 6000 (PEG 6000; Sigma-Aldrich) with an osmotic potential of -0.53 MPa. while S. superba plants of the same age that were watered with tap water served as the control (Tang et al. 2019). Leaves of S. superba from all treatments and controls were sampled after 0, 3, 6, 12, and 24 h of treatment, immersed immediately in liquid nitrogen, then stored at -80 °C until needed.
Arabidopsis thaliana Col-0 seedlings, which were used to assess the subcellular localization of SsMYB113, were cultivated in a growth cabinet with controlled conditions, as follows: a 16-h photoperiod, 100 µmol photons m−2 s−1, 70% relative humidity, and 22 ± 1 °C. A. thaliana Col-0 plants in the initial flowering period (approximately four weeks old) were selected for genetic transformation with the S. superba SsMYB113 gene. For the drought treatment, two-week-old transgenic A. thaliana plants overexpressing SsMYB113 and wild-type (WT) seedlings that were initially well-watered with Hyponex (Murakami Bussan, Tokyo, Japan) and subsequently placed in the above growth conditions, were not provided water for 14 days until they began to wilt slightly, using a methodology described previously (Zhao et al. 2016). In a parallel controlled experiment, the control group was irrigated with 100 mL of tap water in each pot every three days. Leaves from treatment and control plants were harvested, immersed immediately in liquid nitrogen, then stored at -80 °C. All experiments were conducted as three replicates per treatment, and each treatment consisted of three S. superba plants.
Molecular cloning and bioinformatic analysis of SsMYB113
Nested-PCR amplification of the S. superba SsMYB113 gene was conducted in a 50-μL reaction mixture including 2 μL of 5 ng μL−1 cDNA produced from S. superba leaves, 2 μL of 10 μM L−1 forward primer, 2 μL of 10 μM L−1 reverse primer, 25 μL of 2 × Hieff® PCR Master Mix (Yeasen, Shanghai, China), and 19 μL of deionized water. Thermocycling was conducted as follows: 5 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C, and then constant 72 °C for 10 min. PCR products were electrophoresed and purified with the HiPure Gel Pure Micro Kit (Magen, Guangzhou, China).
The coding sequences, corresponding protein, isoelectric point, and molecular weight of SsMYB113 were investigated with the ExPasy server (https://www.expasy.org/). The secondary structure of SsMYB113 was forecast using the SOPM tool (http://npsa-pbil.ibcp.fr/). The three-dimensional structure of SsMYB113 was visualized using the SWISS-MODEL website (https://swissmodel.expasy.org/). The subcellular localization of SsMYB113 was predicted on the pLoc-mPlant server (http://www.jci-bioinfo.cn/pLoc-mPlant/).
A comparison of SsMYB113 with reported homologous MYB113 proteins (Table S2) was possible by alignment with Clustal X 2.0 (www.clustal.org/), and phylogenetically investigated using a neighbor-joining tree generated in MEGA 7.0 (www.mega.com/). Additionally, a total of 126 A. thaliana R2R3-MYB sequences, as previously published by Dubos et al. (2010), were obtained from the TAIR database (www.arabidopsis.org/), and were clustered with SsMYB113 using both Clustal X 2.0 and MEGA 7.0, with 1000 bootstrap replicates.
Total RNA extraction and RT-qPCR analysis
Total RNA from six-month-old S. superba leaves during the vegetative growth period was extracted with the Quick RNA Isolation Kit (Huayueyang, Beijing, China) based on the manufacturer’s protocol, as reported previously (Yu et al. 2021). Afterwards, cDNA from S. superba leaves from both 20% (v/v) PEG 6000 and 100 μM ABA treatments at different time points, as well as from stems and roots, was acquired with the Reverse Transcription Kit (Promega, Madison, WI, USA). The RT-qPCR procedure was performed with the LightCycler® 480 Instrument (Roche Diagnostics, Mannheim, Germany) as published previously (Yu et al. 2021). The house-keeping gene (SsACTIN), which was determined to be the optimum reference gene in S. superba (Yang et al. 2021), was used to detect the relative expression levels of genes with the 2−ΔΔCT protocol (Livak and Schmittgen 2001). Similarly, A. thaliana ubiquitin 10 (UBQ10, At4g05320) gene was used as the house-keeping gene to detect the relative expression levels of genes involved in the biosynthesis of flavonoids (Fig. S2) and ABA (Fig. S3). The gene-specific primers that were used are indicated in Table S1.
Overexpression of SsMYB113 in A. thaliana seedlings
The 774-bp coding sequences of SsMYB113 without a termination codon (TAA) were introduced into pCAMBIA 1302 (CAMBIA, Canberra, Australia) to construct the recombinant pCAMBIA 1302-SsMYB113 vector with the In-Fusion® HD Cloning Kit (Takara, Dalian, China). The verified recombinant plasmid harboring SsMYB113 was integrated into Agrobacterium tumefaciens EHA105 (Weidi Biotechnology Co., Shanghai, China) and subsequently used for the genetic transformation of four-week-old A. thaliana Col-0 with the A. tumefaciens-mediated floral dip protocol (Clough and Bent 1998), as published previously (Yu et al. 2017). Positive transformants cultivated in half-strength Murashige and Skoog medium (Murashige and Skoog 1962) supplemented with 50 μg mL−1 hygromycin B (Sigma-Aldrich), 3% (w/v) sucrose, and 0.8% (w/v) agar at pH 5.8 were identified using RT-qPCR. Two homozygous lines overexpressing SsMYB113, OE1 and OE2, were selected based on the significant elongation of cotyledons and roots, and utilized in follow-up assays.
Measurement of water content and the content of total flavonoids and ABA in A. thaliana seedlings
Water content (WC) was monitored in WT and transgenic A. thaliana plants (OE1 and OE2) using an electronic analytical balance (Shanghai Minqiao Precision Scientific Instrument Co., Shanghai, China), as described previously (Zhang et al. 2021), and calculated according to the formula WC (%) = (W0-Wt)/W0 × 100, where W0 indicates the initial weight and Wt indicates the weight after placing plants at ambient temperature (25 °C) for 0, 3, 6, 9, and 12 h. There were at least three samples for each time point.
The amount of total flavonoids in WT and transgenic A. thaliana plants (OE1 and OE2) was spectrophotometrically determined with a UV-6000 spectrophotometer (Shanghai Metash Instruments Co., Shanghai, China) using a colorimetric procedure, as performed previously (Yu et al. 2018), and expressed as mg of rutin (Sigma-Aldrich) equivalents per gram of fresh weight (FW).
Fresh leaves of WT and transgenic A. thaliana plants (OE1 and OE2) were ground into separate homogenates with precooled 80% methanol and centrifuged at 4 °C and 5000 × g for 10 min. The supernatants were collected and ABA content was quantified with the enzyme-linked immunosorbent assay (ELISA) according to the protocol provided by Agdia Inc. (Elkhart, IN, USA). All tests were performed in triplicate from three independent seedlings, and expressed as ng g−1 on a FW basis.
Assay of the activities of antioxidative enzymes, and non-enzymatic antioxidants in A. thaliana seedlings
Fresh leaves of transgenic A. thaliana seedlings overexpressing SsMYB113 (0.5 g), as well as their corresponding control groups, were ground with 5 mL of pre-cooled aqueous phosphate buffer (0.05 M, pH 7.8) containing 1% polyvinyl pyrrolidone (PVP40; Sigma-Aldrich). The supernatants were obtained after centrifugation at 12,000 × g for 20 min at 4 °C. The activities of antioxidative enzymes, as well as of non-enzymatic antioxidants, were immediately quantified.
Superoxide dismutase (SOD, E.C. 1.15.1.1) activity was detected by monitoring the inhibition of nitro-blue tetrazolium via photochemical reduction, as described previously (Zhang et al. 2021), and expressed as U g−1 min−1 on a FW basis.
Peroxidase (POD; E.C. 1.11.1.7) activity was spectrophotometrically determined at 470 nm by converting guaiacol to tetra-guaiacol equivalents following the method of Wang et al. (2016). Briefly, the crude enzyme extract of OE1 and OE2, as well as their corresponding control groups, was separately integrated into a 3-mL reaction system harboring 50 mM phosphate buffer (pH 6.0), 10 mM H2O2, and 20 mM guaiacol. The increase in absorbance was monitored every 0.5 min after the addition of H2O2 for 3 min. POD activity was expressed as U g−1 min−1 on a FW basis.
Malondialdehyde (MDA) content in two-week-old seedlings of transgenic A. thaliana overexpressing SsMYB113 (OE1 and OE2), as well as their corresponding control groups, was measured following the method of Zhang et al. (2021), and expressed as nmol g−1 on a FW basis. Electrolyte leakage between transgenic A. thaliana seedlings overexpressing SsMYB113 and WT exposed to drought treatment were detected according to the method of Yu et al. (2019).
The superoxide (O2•–) production rate was spectrophotometrically monitored based on the inhibition of O2•–-driven reactions with a UV-6000 spectrophotometer (Shanghai Metash Instruments Co.) at 530 nm using the Superoxide Detection Assay kit (Solarbio, Beijing, China) that directly monitored the production of reactive oxygen species (ROS). The O2•– production rate in WT and transgenic two-week-old A. thaliana seedlings overexpressing SsMYB113 (OE1 and OE2) was detected and expressed as nmol g−1 min−1 on a FW basis.
Proline content was spectrophotometrically determined with a UV-6000 spectrophotometer (Shanghai Metash Instruments Co.) at 520 nm on the basis of the reaction between L-proline and ninhydrin following a published method (Zhang et al. 2021). Proline content in non-transgenic and transgenic A. thaliana seedlings overexpressing SsMYB113 was quantified against the standard curve of L-proline, and expressed as μg g−1 on a FW basis.
De novo transcriptome analysis of S. superba seedlings in response to drought stress
S. superba transcriptome large-scale sequencing data (Han et al. 2016) that was summited to NCBI (https://www.ncbi.nlm.nih.gov/) by the Institute of Botany, Chinese Academy of Sciences, under accession no. PRJNA308885, was downloaded. Drought treatment (DT1-3; SRR3107137, SRR3107138, SRR3107140) and control (CK1-3; SRR3107140, SRR3107140, SRR3107140) data were re-analyzed using BMK Cloud (www.biocloud.net/) strictly following the company’s instructions. Transcript levels of genes involved in the biosynthesis of flavonoids and ABA were analyzed by Pearson’s correlation, and visualized with the TBtools Toolkit (Chen et al. 2020) and Cytoscape software (https://cytoscape.org/).
Subcellular localization assay
The 774-bp coding sequences without a termination codon (TAA) of SsMYB113 were introduced into pSAT6-eYFP-N1 to generate the pSAT6-SsMYB113-eYFP fusion construct with the In-Fusion Cloning Kit (Takara). The construct was transiently integrated into the protoplasts of 4-week-old A. thaliana Col-0, as previously described (Yu et al. 2021). After incubation for 16 h at 22 °C in the dark, fluorescent signals in infected protoplasts were visualized with a Leica TCS SP8 STED 3 × microscope (Leica Camera AG, Solms, Germany).
Transcriptional activity assay in yeast cells
The 774-bp coding sequences without a termination codon (TAA) of SsMYB113 were introduced into pGBKT7 (Clontech, Palo Alto, CA, USA) harboring the GAL4 DNA-binding domain to generate recombinant pGBKT7-SsMYB113. The negative control (pGBKT7) and pGBKT7-SsMYB113 were separately transformed into AH109 (Weidi Biotechnology Co.). Transcriptional activity of SsMYB113 was investigated by assessing growth performance on double synthetic dropout (SD; Clontech) plates without tryptophan (-Trp; Clontech), or tryptophan, histidine, and adenine (-Trp-His-Ade; Clontech).
Yeast one-hybrid (Y1H) screen
The 774-bp coding sequences without a termination codon (TAA) of SsMYB113 were introduced into pJG4-5 (Clontech) to construct recombinant pJG4-5-SsMYB113. The 1154-bp promoter regions of SsCHS (SsCHSp; Fig. S4) and the 956-bp promoter regions of SsNCED (SsNCEDp; Fig. S5) were inserted into pLACzi (Clontech). Thereafter, pJG4-5-SsMYB113 was co-transformed together with either recombinant pLACzi-SsCHSp or pLACzi-SsNCEDp into competent EGY48 cells (Weidi Biotechnology Co.), which were grown on SD plates without tryptophan and uracil (-Trp-Ura; Clontech) at 29 °C for 3 d. Blue spots, along with the negative control (white or transparent spots) (pLACzi + pJG4-5-SsMYB113), were selected and dotted on SD-Trp-Ura plates with additional 80 mg L−1 of filter-sterilized 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal; Sigma-Aldrich), at 29 °C for 3 d, as previously described (Yu et al. 2021).
Dual-luciferase (dual-LUC) assay
To determine the binding transcriptional activities of SsMYB113 on the promoter regions of SsCHS and SsNCED, 774-bp coding sequences without the termination codon (TAA) of SsMYB113 were inserted into pGreenII 62-SK (Clontech) as the effector, whereas the promoter regions of SsCHS and SsNCED were inserted individually into pGreenII 0800-LUC (Clontech), as the reporter, following the protocols described in Yu et al. (2021). The resulting effector and reporter recombinants were transiently co-infiltrated into tobacco (Nicotiana benthamiana) leaves with Agrobacterium tumefaciens GV3101 strain (pSoup-p19; Weidi Biotechnology Co.). The activities of LUC and REN luciferase were detected after 48 h of infiltration with the Dual-Luciferase® Reporter Assay Kit (Promega). Transcriptional activation activity of SsMYB113 SsCHS and SsNCED promoters was assessed by measuring the LUC to REN ratio. At least six replicates were employed for each combination.
Statistical analysis
All experimental data, represented as the mean ± standard deviation (SD) of three independent assays in triplicate replicates, were subject to statistical analysis using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Statistical differences between treatments and their corresponding control were evaluated using the Student’s t-test (p < 0.05, p < 0.01) while statistical differences between harvested tissues (roots, stems, and leaves), and different time points exposed to 100 μM ABA or 20% (v/v) PEG 6000 were evaluated using Duncan’s multiple range test (p < 0.05). The correlation between transcript levels of SsMYB113 and other genes was calculated using Pearson’s correlation coefficient (R2) at p < 0.01.
Results
Identification and bioinformatics analysis of SsMYB113
Based on the reported S. superba transcriptome exposed to drought stress (Han et al. 2016), 141 genes were annotated as R2R3-MYB. Two genes, c63040 and c71380, were clustered into the S6 subfamily of R2R3-MYB (Fig. S6), which regulates the biosynthesis of flavonoids (Gonzalez et al. 2008). Moreover, the c71380 gene exhibited higher expression under drought treatment than the control (Fig. S7), and was thus selected as the candidate gene in this study, and defined as SsMYB113. SsMYB113 was cloned and identified from the leaves of six-week-old S. superba seedlings. Sequence analysis demonstrated that SsMYB113 harbored a full-length coding sequence of 777 bp (accession no. MZ131646), encoding a peptide of 258 amino acids with a predicted molecular weight of 29.47 kDa and an isoelectric point of 8.84. The secondary structure of SsMYB113 consisted of 32.17% α-helices, 8.53% β-turns, 14.73% extended strands, and 44.57% random coils (Fig. S8A). The tertiary structure of SsMYB113 was assessed and visualized (Fig. S8B). SsMYB113 possessed two conserved MYB-like DNA-binding domains at the 10–57 and 63–108 amino acid positions (Fig. 1A). It thus belongs to the R2R3-type MYB superfamily. A phylogenetic analysis suggested that SsMYB113 is a member of the R2R3-type MYB S6 subgroup (Fig. S9), and was homologous to A. thaliana AtMYB113, which is a known R2R3-type MYB that regulates the biosynthesis of flavonoids (Gonzalez et al. 2008). Moreover, in a phylogenetic analysis of MYB113 with its reported homologous proteins in other plants (Table S2), SsMYB113 was evolutionarily closer to Fragaria ananassa FaMYB10 and Pyrus communis PcMYB10 with an evolutionary distance of 0.538 and 0.576, respectively (Fig. 1B). In addition, a highly conserved motif [R/K]Px[P/A/R]xx[F/Y] in a flavonoids-regulating R2R3-type MYB TF (Hichri et al. 2011), was observed in SsMYB113 at the C-terminal (Fig. 1A).
Sequence alignment and phylogenetic analysis of SsMYB113. A Multiple alignments of SsMYB113 and its reported homologue proteins in other plants. Black, 100% sequence homology; dark gray, > 75% sequence homology; light grey, > 50% sequence homology. Red points indicate a conserved motif [A/S/G]NDV for anthocyanin-promoting R2R3-type MYB. Blue points indicate a conserved motif [R/K]Px[P/A/R]xx[F/Y] for flavonoids-regulating R2R3-type MYB. B Phylogenetic analysis of SsMYB113 with its reported homologous proteins in other plants. The neighbor-joining tree was constructed using MEGA 7 with 1000 bootstrap replicates. Reported homologous proteins are shown in Table S2. MYB, v-myb myeloblastosis viral oncogene homolog
Nuclear-targeted SsMYB113 functions as a positive regulator
Based on the pLoc-mPlant server, SsMYB113 was considered to be localized in the nucleus. The experimental results indicate that the fluorescence of the SsMYB113-YFP fused protein was specifically localized in the nucleus, suggesting that SsMYB113 is a nuclear-targeted protein (Fig. 2A-C).
Subcellular localization and transcriptional activity of SsMYB113. A-C Subcellular localization of SsMYB113 in Arabidopsis thaliana protoplasts. The SsMYB113-YFP fusion protein with yellow fluorescence is localized in the nucleus. Red fluorescence represents chloroplast auto-fluorescence. D Trans-activation of SsMYB113 in vitro. The 777-bp coding sequences of SsMYB113 were inserted into pGBKT7 to generate pGBKT7-SsMYB113 (BD-SsMYB113). Transformed yeast AH109 cells were cultured on SD plates without tryptophan (-Trp) or without tryptophan, histidine, and adenine (-Trp-His-Ade) for 3 d at 29 °C. pGBKT7 was used as the negative control. E Schematic representation of constructs used in the dual-LUC reporter assay. LUC, firefly luciferase; REN, renilla luciferase; SD, synthetic dextrose. F Trans-activation of SsMYB113 in vivo. The LUC/REN value of pBD (negative control), defined as the calibrator, was set at 1. Data are expressed as the mean ± standard deviation of six individual biological replicates. Asterisks indicate significant differences between pBD and pBD-SsMYB113 at p < 0.01 based on the Student’s t-test
To investigate whether SsMYB113 displays trans-activation activity, Y1H and the dual-LUC system were employed. Both BD and BD-SsMYB113 grew normally on SD-Trp selective medium, but only BD-SsMYB113 exhibited GAL4 activity and survived on SD-Trp-His-Ade selective medium (Fig. 2D). The dual-LUC system, which was used to understand trans-activation activity in vivo, indicated an obvious activation of the relative LUC/REN value (4.64-fold) in pBD-SsMYB113, relative to pBD (Fig. 2E), suggesting that SsMYB113 is a positive regulator that exhibits trans-activation activity.
Expression profiles of SsMYB113 in S. superba
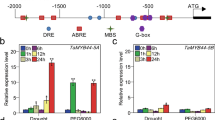
To understand the organ-specific expression profiles of SsMYB113, RT-qPCR was employed to examine its transcription levels in the roots, stems, and leaves of six-month-old S. superba. SsMYB113 was expressed in all the tested organs, but compared with roots and stems, the highest expression was in leaves (Fig. 3B). To estimate the potential role of SsMYB113 under adverse environments, the relative abundance of SsMYB113 transcript was analyzed in the leaves of six-month-old S. superba plants subjected to drought stress and exogenous ABA (100 μM) treatment. Compared to the treatment at 0 h, the levels of SsMYB113 transcription increased significantly (p < 0.05) after treatment with 20% PEG 6000 at 3 h (2.18-fold) and 6 h (3.13-fold), but exhibited a significant decrease at 24 h (Fig. 3C). Following ABA treatment, the levels of SsMYB113 transcript were significantly higher at 3, 6, 12 and 24 h than at 0 h, with an obvious increase (4.02-fold) at 12 h (Fig. 3D). These findings suggest that the abundance of SsMYB113 transcript was up-regulated by both PEG 6000 and ABA (p < 0.05).
Expression profiles of SsMYB113. A The Schima superba plant that was tested. B Transcript levels of SsMYB113 in roots, stems, and leaves of a six-month-old S. superba plant. C Transcript levels of SsMYB113 in S. superba leaves after treatment with 20% PEG 6000 at different time points. D Transcript levels of SsMYB113 in S. superba leaves after treatment with 100 μM ABA at different time points. Relative expression is expressed as the mean ± standard deviation of three independent assays, carried out in triplicate for each. Different lowercase letters above bars represent significant differences among different treatments at p < 0.05 according to Duncan's multiple range test. All transcript abundance was normalized to the SsACTIN transcript levels and carried out following the 2−ΔΔCT method (Livak and Schmittgen 2001). ABA, abscisic acid; PEG 6000, polyethylene glycol 6000
Overexpression of SsMYB113 in A. thaliana seedlings promotes the production of flavonoids
To establish the role of SsMYB113 in the response to drought stress, SsMYB113 was introduced into WT A. thaliana. Two positive transformants (OE1 and OE2) were identified and selected for subsequent analysis (Fig. 4A). Total flavonoid content was significantly higher in OE1 (1.61-fold) and OE2 (1.60-fold) compared to WT under normal (unstressed) conditions (Fig. 4B). The expression levels of genes associated with flavonoid biosynthesis, including AtANS, At4CL, AtC4H, AtCHI, AtCHS, AtDFR, AtF3H, AtF3′H, AtF3′5′H, AtFLS, AtPAL, and AtUFGT, were measured between WT and the two overexpression lines (OE1 and OE2). Aside from AtC4H, AtF3′H, AtF3′5′H, and AtUFGT, eight genes (AtANS, At4CL, AtCHI, AtCHS, AtDFR, AtF3H, AtFLS, and AtPAL) that participate in flavonoid biosynthesis showed an obvious increase (1.22- to 6.11-fold) in the overexpressed SsMYB113 A. thaliana seedlings, especially AtCHS, compared with WT (Fig. 5). Interestingly, in addition to AtF3′H and AtF3′5′H, other genes (AtANS, At4CL, AtC4H, AtCHI, AtCHS, AtDFR, AtF3H, AtFLS, AtPAL, and AtUFGT) were obviously up-regulated (1.33- to 29.37-fold) more than in WT in the overexpressed SsMYB113 A. thaliana seedlings under drought stress (Fig. 5), suggesting that overexpression of SsMYB113 in A. thaliana seedlings promotes the production of flavonoids.
Overexpression of SsMYB113 in A. thaliana seedlings. A Determination of SsMYB113 gene expression in control (WT) and SsMYB113-overexpressing plants (OE1 and OE2). Total flavonoid content (B), ABA content (C) and WC (D) in WT, OE1 and OE2 treated with 20% PEG 6000 for 2 weeks. Total flavonoid, ABA, and WC contents are expressed as the mean ± standard deviation of three independent assays, carried out in triplicate for each. Asterisks indicate significant differences between control (WT) and transgenic plants (OE1 or OE2) at p < 0.01 based on the Student’s t-test. ABA, abscisic acid; OE, overexpression; PEG 6000, polyethylene glycol 6000; WC, water content; WT, wild-type
Transcription level of flavonoid biosynthetic genes in overexpression SsMYB113 plants. Relative expression is shown as the mean ± standard deviation of three independent assays, carried out in triplicate for each. Asterisks indicate significant differences between control (WT) and transgenic plants (OE1 or OE2) based on the Student’s t-test (*p < 0.05, **p < 0.01). ANS, anthocyanin synthase; 4CL, 4-coumaroyl CoA ligase; C4H, cinnamate 4-hydroxylase; CHI, chalcone isomerase; CHS, chalcone synthase; DFR, dihydroflavanol 4-reductase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′5′ hydroxylase; FLS, flavonol synthase; OE, overexpression; PAL, phenylalanine ammonia lyase; UFGT, UDP glucose-flavonoid 3-O-glycosyltranferase; WT, wild-type
Overexpression of SsMYB113 in A. thaliana seedlings stimulates the accumulation of ABA
There were significant differences in stress-responsive ABA levels between WT and transgenic plants (OE1 and OE2), and levels were significantly higher in OE1 (3.99-fold) and OE2 (4.13-fold) than in WT plants (Fig. 4C). In addition, the transcript abundance of genes associated with ABA biosynthesis, including AtAAO, AtABA2, AtABA4, AtNCED, AtZEP1, and AtZEP2, displayed no significant differences between WT and transgenic lines (OE1 and OE2) in the normal treatment. However, there was wide variation (2.20- to 9.85-fold) in expression levels of ABA biosynthetic genes between WT and the two transgenic lines under drought conditions (Fig. 6), suggesting that overexpression of SsMYB113 in A. thaliana seedlings can stimulate the accumulation of ABA.
Transcription level of ABA biosynthetic genes in overexpression SsMYB113 plants. Relative expression is shown as the mean ± standard deviation of three independent assays, carried out in triplicate for each. Asterisks indicate significant differences between control (WT) and transgenic plants (OE1 or OE2) at p < 0.01 based on the Student’s t-test. AAO, aldehyde oxidase; ABA, abscisic acid; ABA2/SDR, short-chain dehydrogenases/reductases; ABA4/NSY, neoxanthin synthase; NCED, 9-cis-epoxycarotenoid dioxygenases; OE, overexpression; WT, wild-type; ZEP, zeaxanthin epoxidase
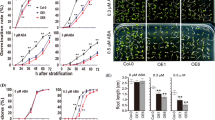
Overexpression of SsMYB113 in A. thaliana seedlings modulates ROS generation
Plants encounter an armory of biotic and abiotic stimuli, in extreme cases resulting in the excessive accumulation of ROS, which cause oxidative damage and ultimately obstruct plant growth and development (Mittler 2002). Under normal conditions, O2•– production rate, MDA content, and electrolyte leakage, which are indicators of cell membrane damage, as well as proline content, were not statistically significant between WT plants and OE lines (Fig. 7). After drought treatment, O2•– production rate, MDA content, and electrolyte leakage in OE1 and OE2 decreased significantly (with an average reduction of 48.21%, 49.39%, and 35.98%, respectively), while proline content increased significantly (1.77- and 1.62-fold in OE1 and OE2, respectively), compared with WT plants (Fig. 7). Two ROS-scavenging antioxidant enzymes, SOD and POD, presented an equivalent ratio between WT plants and OE lines in the absence of stress, but exhibited an obvious and significant enhancement of average activity (1.42-fold for SOD; 1.46-fold for POD), compared to WT plants after exposure to drought stress (Fig. 7). Additionally, WC in OE plants was significantly higher than in WT plants (with an average increase of 9.19% at 3 h to 41.01% at 12 h; Fig. 4D), suggesting that overexpression of SsMYB113 enhanced drought tolerance in transgenic plants. These results suggest that overexpression of SsMYB113 activated these two ROS-scavenging enzymes and alleviated oxidative damage in transgenic A. thaliana seedlings.
Determination of superoxide (O2•–) production rate, MDA content, electrolyte leakage, proline content, SOD activity, and POD activity. O2•– production rate (A), MDA content (B), electrolyte leakage (C), proline content (D), SOD activity (E), and POD activity (F) in WT plants, and OE1 and OE2 lines treated with 20% PEG 6000 for 2 weeks. O2•– production rate, MDA content, electrolyte leakage, proline content, and SOD and POD activities are expressed as the mean ± standard deviation of three independent assays, carried out in triplicate for each. Asterisks indicate significant differences between control (WT) and transgenic plants (OE1 or OE2) at p < 0.01 based on the Student’s t-test. MDA, malondialdehyde; OE, overexpression; PEG 6000, polyethylene glycol 6000; POD, peroxidase; SOD, superoxide dismutase; WT, wild-type
Transcriptome analysis of differential expression of flavonoid and ABA biosynthetic genes under drought stress in S. superba seedlings
A reported S. superba transcriptome (SRX1531873; Han et al. 2016) was de novo re-evaluated by BMK Cloud, showing the differential transcript abundance of flavonoid and ABA biosynthetic genes between the drought treatment and control. Among them, the average expression levels of SsCHS and SsNCED were significantly up-regulated (1.71- and 13.07-fold, respectively) in the drought treatment compared to the control (Fig. 8). This trend coincided with the increased expression of SsMYB113 subjected to drought stress, showing a very strong correlation between SsMYB113 and SsCHS (Pearson’s R2 = 0.98), as well as between SsMYB113 and SsNCED (Pearson’s R2 = 0.97) (Fig. S10). Taken together, under drought stress, SsMYB113 was synergistically associated with the upregulation of flavonoid biosynthesis and the ABA signaling pathway.
Transcriptomic analysis of differential transcript abundance of flavonoid and ABA biosynthetic genes in S. superba seedlings under drought stress. Heat map of the log2-transformation of the expression values generated by the TBtools Toolkit (Chen et al. 2020). Red and blue in the color scale represent high and low transcript abundance, respectively
SsMYB113 activates the transcription of SsCHS and SsNCED genes
Since overexpression of SsMYB113 notably activated the expression of flavonoid and ABA biosynthetic genes, especially CHS (29.37-fold) and NCED (9.85-fold), the Y1H and dual-LUC assays were employed to investigate the transcriptional role of SsMYB113 in response to drought stress. As illustrated in Fig. 9A, three MYB binding sites (MBSs) were observed in the 1154-bp promoter regions of the SsCHS gene, and one MBS was found in the 956-bp promoter region of the SsNCED gene. Co-transformed yeast cells (pLacZi-SsCHSp and pLacZi-SsNCEDp, with pJG4-5-SsMYB113, respectively) grew on SD/-Ura/-Trp + X-Gal medium while the corresponding control cells (pLacZi + pJG4-5, pLacZi + pJG4-5-SsMYB113, pLacZi-SsCHSp + pJG4-5, and pLacZi-SsNCEDp + pJG4-5) were unable to grow on this medium (Fig. 9B). More importantly, transcriptional activation of SsMYB113 in SsCHSp (3.03-fold) and SsNCEDp (2.91-fold) was observed, relative to the control group. After treatment with 100 μM of exogenously applied ABA, the relative LUC/REN ratio of SsMYB113 + SsNCEDp increased obviously (6.46-fold) compared to the MOCK (i.e., treatment without ABA) (Fig. 9C). Accordingly, SsMYB113 was capable of binding directly to the promoter region of SsCHS and SsNCED genes, activating their transcript levels and resulting in the accumulation of flavonoids and ABA, thus contributing to enhanced tolerance to drought stress in S. superba.
SsMYB113 directly binds to the promoter of SsCHS and SsNCED, and stimulated the expression of SsCHS and SsNCED. A Diagrams of SsCHS and SsNCED promoter fragments. Solid circles indicates the MBS sequence 5'-CAACTG-3'. B SsMYB113 binds to the promoter of SsCHS and SsNCED harboring the MBS domain via a yeast one-hybrid screen. The interaction is indicated by the ability of yeast cells to grow on a synthetic dropout (SD) medium without uracil and tryptophan (-Ura-Trp), but containing X-gal, for 3 d at 29 °C. Empty pLACzi and pJG4-5-DobHLH4 were used as negative controls. C SsMYB113 activated the expression of SsCHS and SsNCED in the 100 μM ABA treatment, as assessed by the dual-LUC assay. The ratio of LUC/REN of the empty vector was used as a calibrator (set as 1). Relative LUC/REN ratio of the control, SsMYB113 + SsCHSp, and SsMYB113 + SsNCEDp is expressed as the mean ± standard deviation of three independent assays, carried out in triplicate for each. Asterisks indicate significant differences between the control (WT) and transgenic plants (OE1 or OE2) at p < 0.01 based on the Student’s t-test. ABA, abscisic acid; LUC, firefly luciferase; REN, renilla luciferase; WT, wild-type; X-gal, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside
Discussion
Considerable evidence has accumulated that demonstrates that R2R3-MYB family members make a valuable contribution towards the response of plants to various abiotic stresses, such as drought, salt, and cold (Baldoni et al. 2015; Li et al. 2015; Millard et al. 2019; Wang et al. 2016). Despite this, most information of enhanced stress resistance for the majority of R2R3-MYB members is available for model plants, such as A. thaliana and P. trichocarpa (Stracke et al. 2001; Dubos et al. 2010; Fang et al. 2020). In the present study, we functionally characterized a R2R3-MYB TF, SsMYB113, from S. superba. SsMYB113 harbored two signature R2R3-type MYB motifs (Fig. 1A) as well as two conserved motifs ([A/S/G]NDV and [R/K]Px[P/A/R]xx[F/Y]) found in a flavonoids-regulating R2R3-type MYB (Hichri et al. 2011). Furthermore, SsMYB113 was grouped into the same subfamily as its A. thaliana homologue, AtMYB113 (Fig. S9), which regulates flavonoid biosynthesis (Gonzalez et al. 2008), suggesting that SsMYB113 may be a flavonoid-specific R2R3-MYB regulator.
Our results indicate that SsMYB113 is a nuclear-targeted protein that functions as a positive regulator (Fig. 2). Similarly, several reported R2R3-MYB activators, such as B. oleracea BoMYB2 (Chiu and Li 2012), P. trichocarpa PtrMYB94 (Fang et al. 2020), and S. tuberosum StMYB113 (Liu et al. 2016), all displayed an obvious up-regulation of transcript levels of flavonoid biosynthetic genes. Flavonoids enhance tolerance to abiotic stresses, resulting from their cells’ ability to detoxify ROS during abiotic stress (Agati et al. 2012). Overexpression of SsMYB113 obviously increased total flavonoid content in OE1 and OE2 lines more than in WT plants (Fig. 4B), consistent with reported R2R3-MYBs, such as A. thaliana AtMYB12 (Wang et al. 2016) and S. lycopersicum SlMYB14 (Li et al. 2021). Concurrently, a significant increase in transcript levels of flavonoid biosynthetic genes (AtANS, At4CL, AtCHI, AtCHS, AtDFR, AtF3H, AtFLS, and AtPAL) was observed in OE1 and OE2 lines compared with WT plants (Fig. 5). Furthermore, SsMYB113 bound directly to the promoter sequences of SsCHS, resulting in activated expression of the SsCHS gene (Fig. 9). Taken together, SsMYB113 has been shown to regulate the transcript levels of flavonoid biosynthetic genes, especially SsCHS, leading to the accumulation of flavonoids. The enhanced accumulation of flavonoids in transgenic lines may enhance their antioxidant ability, thereby improving their environmental stress tolerance to drought, salt, and cold (Gupta et al. 2020; Pourcel et al. 2013).
SsMYB113 exhibited the highest transcript levels in the leaves of six-month-old S. superba plants (Fig. 3B), and SsMYB113 was up-regulated by PEG-induced drought stress (Fig. 3C) as well as by the stress signal molecule ABA (Fig. 3D), suggesting that SsMYB113 is a drought stress-responsive gene. Earlier transcriptome data (Han et al. 2016) revealed a significant increase in the transcript levels of SsMYB113 under drought stress in one-year-old S. superba seedlings than in normal conditions. In addition, in our study, the endogenous level of ABA was markedly enhanced in transgenic A. thaliana seedlings overexpressing SsMYB113 (Fig. 4C). ABA is a pivotal mediator of plants’ responses to drought stress by triggering the activation of ABA biosynthetic genes, as well as by modulating the transcript levels of ABA-dependent drought-responsive genes, thereby strengthening drought stress resistance (Shokat et al. 2021; Xiong et al. 2006; Zhu 2016). As expected, overexpression of SsMYB113 in A. thaliana seedlings dramatically up-regulated the transcript levels of ABA biosynthetic genes (AtAAO, AtABA2, AtABA4, AtNCED, AtZEP1, and AtZEP2), especially the maximum expression of AtNCED (Fig. 6). Notably, SsMYB113 was capable of binding to the promoter sequences of SsNCED, thereby activating the expression of the SsNCED gene (Fig. 9). Based on these observations, ABA-inducible SsMYB113, which is involved in the ABA signaling pathway, may be instrumental to drought tolerance.
Increasing evidence has demonstrated that various abiotic stresses, particularly drought stress, exacerbate the accumulation of ROS such as O2•– and H2O2 (Choudhury et al. 2017; Mittler 2002), thereby enhancing ROS-scavenging antioxidant enzymes, which offer protection to a plant against oxidative damage (Qi et al. 2018). Compared to the control group, PEG-inducible drought treatment resulted in the generation of ROS, eventually leading to increased O2•– production, MDA content, and electrolyte leakage (Fig. 7). Intriguingly, overexpression of SsMYB113 in A. thaliana seedlings substantially alleviated the O2•– production rate, MDA content, and electrolyte leakage (Fig. 7) while simultaneously increasing the activity of ROS-scavenging enzymes, SOD and POD (Fig. 7). Hence, transcriptional regulation of SsMYB113 may be necessary for the adaptation of S. superba to abiotic stresses. These findings are consistent with those from previous studies that extensively examined R2R3-MYB TFs, including A. thaliana AtMYB12, which enhanced salt and drought tolerance (Wang et al. 2016), S. tuberosum StMYB113, which increased drought tolerance (Liu et al. 2016), and P. trichocarpa PtrMYB94, which improved drought tolerance (Fang et al. 2020), demonstrating their essential roles in responding to abiotic stresses. Proline is a ROS scavenger in response to environmental stresses, stabilizes subcellular structures and promotes cell recovery from damage (Per et al. 2017). Proline accumulation and WC significantly accumulated in transgenic SsMYB113-overexpressing A. thaliana seedlings (Fig. 4D; Fig. 7D), in turn stimulating the ROS scavenging antioxidant-based defense machinery. Furthermore, the accumulation of proline upon exposure to drought or ABA treatment serves the dual purpose of supplying a compatible solute for osmotic adaptation in SsMYB113-overexpressing leaves and partially preceding stomatal closure (Bharath et al. 2021). ABA not only induces stomatal closure but also inhibits stomatal opening, helps plant defense responses, and is beneficial to osmotic adaptation under drought stress by increasing WC and accumulating proline (Verslues and Sharma 2010). This suggests that SsMYB113 positively controls S. superba drought tolerance by increasing WC through osmotic adjustment via the accumulation of proline.
Combined with the existing results, SsMYB113 functions in the accumulation of flavonoids and enhanced ABA levels, protecting S. superba from abiotic stress-induced damage by enhancing ROS detoxification, improving the retention of WC, and reducing ROS production, ultimately enhancing PEG-induced drought tolerance (Fig. 10).
A simple model depicting how SsMYB113 responds to drought stress in S. superba. SsMYB113, which is significantly up-regulated as a result of drought stress, contributes to the induced increase of total flavonoid content and ABA content, resulting from the up-regulated transcript levels of biosynthetic genes involved in the production of flavonoids and ABA. The ROS scavenger is activated, thereby relieving membrane peroxidation. Ultimately, ROS decreases accordingly, while WC increases significantly, leading to enhanced drought tolerance. ABA, abscisic acid; ROS, reactive oxygen species; WC, water content
Conclusion
S. superba SsMYB113 is a nucleus-localized transcriptional activator that is modulated by PEG-induced drought stress and exogenous ABA. Overexpression of SsMYB113 in A. thaliana seedlings exhibited an increase in flavonoid accumulation and ABA hypersensitivity, as well as high WC and low levels of ROS. In parallel experiments, SsMYB113 overexpression obviously up-regulated genes associated with the biosynthesis of flavonoids and ABA. Furthermore, SsMYB113 was able to bind directly to the promoter sequences of SsCHS and SsNCED, thereby activating their expression. Consequently, our findings reveal a novel drought-responsive R2R3-MYB, which can be utilized to increase flavonoid content and enhance drought stress tolerance in S. superba.
References
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76. https://doi.org/10.1016/j.plantsci.2012.07.014
Baek W, Lim CW, Lee SC (2018) A DEAD-box RNA helicase, RH8, is critical for regulation of ABA signalling and the drought stress response via inhibition of PP2CA activity. Plant Cell Environ 41:1593–1604. https://doi.org/10.1111/pce.13200
Baldoni E, Genga A, Cominelli E (2015) Plant MYB transcription factors: their role in drought response mechanisms. Int J Mol Sci 16:15811–15851. https://doi.org/10.3390/ijms160715811
Bharath P, Gahir S, Raghavendra AS (2021) Abscisic acid-induced stomatal closure: an important component of plant defense against abiotic and biotic stress. Front Plant Sci 12:615114. https://doi.org/10.3389/fpls.2021.615114
Butelli E, Licciardello C, Zhang Y, Liu JJ, Mackay S, Bailey P, Reforgiato-Recupero G, Martin C (2012) Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 24:1242–1255. https://doi.org/10.1105/tpc.111.095232
Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202. https://doi.org/10.1016/j.molp.2020.06.009
Chiu LW, Li L (2012) Characterization of the regulatory network of BoMYB2 in controlling anthocyanin biosynthesis in purple cauliflower. Planta 236:1153–1164. https://doi.org/10.1007/s00425-012-1665-3
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867. https://doi.org/10.1111/tpj.13299
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15:573–581. https://doi.org/10.1016/j.tplants.2010.06.005
Fang Q, Wang XQ, Wang HY, Tang XW, Liu C, Yin H, Ye SL, Jiang YZ, Duan YJ, Luo KM (2020) The poplar R2R3 MYB transcription factor PtrMYB94 coordinates with abscisic acid signaling to improve drought tolerance in plants. Tree Physiol 40:46–59. https://doi.org/10.1093/treephys/tpz113
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/MYB transcriptional complex in Arabidopsis seedlings. Plant J 53:814–827. https://doi.org/10.1111/j.1365-313X.2007.03373.x
Guo JR, Sun BX, He HR, Zhang YF, Tian HY, Wang BS (2021) Current understanding of bHLH transcription factors in plant abiotic stress tolerance. Int J Mol Sci 22:4921. https://doi.org/10.3390/ijms22094921
Gupta A, Rico-Medina A, Caño-Delgado AI (2020) The physiology of plant responses to drought. Science 368:266–269. https://doi.org/10.1126/science.aaz7614
Han BC, Wei W, Mi XC, Ma KP (2016) De novo sequencing and comparative analysis of Schima superba seedlings to explore the response to drought stress. PLoS ONE 11:e0166975. https://doi.org/10.1371/journal.pone.0166975
Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V (2011) Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot 62:2465–2483. https://doi.org/10.1093/jxb/erq442
Hu XS, Hong W, Wu CZ, Hong T, Fan HL, Song P (2007) Analysis of the life table of natural population of Schima superba. Guihaia 27:469–474. https://doi.org/10.1016/S1872-2075(07)60055-7
Jiang JJ, Ma SH, Ye NH, Jiang M, Cao JS, Zhang JH (2017) WRKY transcription factors in plant responses to stresses. J Integr Plant Biol 59:86–101. https://doi.org/10.1111/jipb.12513
Li L, Chen JH, Ren HB, Mi XC, Yu MJ, Yang B (2010) Spatial patterns of Castanopsis eyrei and Schima superba in mid-subtropical broad-leaved evergreen forest in Gutianshan National Reserve. China Chin J Plant Ecol 34:241–252. https://doi.org/10.3773/j.issn.1005-264x.2010.03.001
Li C, Ng CKY, Fan LM (2015) MYB transcription factors, active players in abiotic stress signaling. Environ Exp Bot 114:80–91. https://doi.org/10.1016/j.envexpbot.2014.06.014
Li XR, Tang Y, Li HL, Luo W, Zhou CJ, Zhang LX, Lv JY (2020) A wheat R2R3 MYB gene TaMpc1-D4 negatively regulates drought tolerance in transgenic Arabidopsis and wheat. Plant Sci 299:110613. https://doi.org/10.1016/j.plantsci.2020.110613
Li ZY, Dai PF, Wang YH, Li T, Webb AA, Wang YH, Li ZH, Kou TJ, Shi GA, Zhang BC (2016) Effects of liming on health and growth of young Schima superba trees under canopy of a Pinus massoniana stand damaged by soil acidification in Chongqing, China. New for 47:801–813. https://doi.org/10.1007/s11056-016-9545-5
Li Z, Peng R, Yao Q (2021) SlMYB14 promotes flavonoids accumulation and confers higher tolerance to 2,4,6-trichlorophenol in tomato. Plant Sci 303:110796. https://doi.org/10.1016/j.plantsci.2020.110796
Licausi F, Ohme-Takagi M, Perata P (2013) APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol 199:639–649. https://doi.org/10.1111/nph.12291
Liu YH, Wang KL, Espley RV, Wang L, Yang HY, Yu B, Dare A, Varkonyi-Gasic E, Wang J, Zhang JL, Wang D, Allan AC (2016) Functional diversification of the potato R2R3 MYB anthocyanin activators AN1, MYBA1, and MYB113 and their interaction with basic helix-loop-helix cofactors. J Exp Bot 67:2159–2176. https://doi.org/10.1093/jxb/erw014
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MIR, Anjum NA (2017) Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem 115:126–140. https://doi.org/10.1016/j.plaphy.2017.03.018
Millard PS, Kragelund BB, Burow M (2019) R2R3 MYB transcription factors - functions outside the DNA-binding domain. Trends Plant Sci 24:934–946. https://doi.org/10.1016/j.tplants.2019.07.003
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/s1360-1385(02)02312-9
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nakabayashi R, Saito K (2015) Integrated metabolomics for abiotic stress responses in plants. Curr Opin Plant Biol 24:10–16. https://doi.org/10.1016/j.pbi.2015.01.003
Pourcel L, Irani NG, Koo AJ, Bohorquez-Restrepo A, Howe GA, Grotewold E (2013) A chemical complementation approach reveals genes and interactions of flavonoids with other pathways. Plant J 74:383–397. https://doi.org/10.1111/tpj.12129
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17:369–381. https://doi.org/10.1016/j.tplants.2012.02.004
Qi J, Song CP, Wang B, Zhou J, Kangasjärvi J, Zhu JK, Gong Z (2018) Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol 60:805–826. https://doi.org/10.1111/jipb.12654
Shokat S, Großkinsky DK, Liu F (2021) Impact of elevated CO2 on two contrasting wheat genotypes exposed to intermediate drought stress at anthesis. J Agron Crop Sci 207:20–33. https://doi.org/10.1111/jac.12442
Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4:447–456. https://doi.org/10.1016/s1369-5266(00)00199-0
Tang D, Wei F, Qin S, Khan A, Kashif MH, Zhou R (2019) Polyethylene glycol induced drought stress strongly influences seed germination, root morphology and cytoplasm of different kenaf genotypes. Ind Crop Prod 137:180–186. https://doi.org/10.1016/j.indcrop.2019.01.019
Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 8:e0140. https://doi.org/10.1199/tab.0140
Wang FB, Kong WL, Wong G, Fu LF, Peng RH, Li ZJ, Yao QH (2016) AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol Genet Genomics 291:1545–1559. https://doi.org/10.1007/s00438-016-1203-2
Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, Frei dit Frey N, Leung J (2008) An update on abscisic acid signaling in plants and more. Mol Plant 1:198–217. https://doi.org/10.1093/mp/ssm022
Xiong L, Wang RG, Mao G, Koczan JM (2006) Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol 142:1065–1074. https://doi.org/10.1104/pp.106.084632
Yang ZY, Zhang R, Zhou ZC (2021) Identification and validation of reference genes for gene expression analysis in Schima superba. Genes 12:732. https://doi.org/10.3390/genes12050732
Yu Z, He C, Teixeira da Silva JA, Zhang G, Dong W, Luo J, Duan J (2017) Molecular cloning and functional analysis of DoUGE related to water-soluble polysaccharides from Dendrobium officinale with enhanced abiotic stress tolerance. Plant Cell Tiss Org 131:579–599. https://doi.org/10.1007/s11240-017-1308-2
Yu Z, He C, Teixeira da Silva JA, Luo J, Yang Z, Duan J (2018) The GDP-mannose transporter gene (DoGMT) from Dendrobium officinaleis critical for mannan biosynthesis in plant growth and development. Plant Sci 277:43–54. https://doi.org/10.1016/j.plantsci.2018.07.021
Yu ZM, Yang ZY, Teixeira da Silva JA, Luo JP, Duan J (2019) Influence of low temperature on physiology and bioactivity of postharvest Dendrobium officinale stems. Postharvest Biol Tech 148:97–106. https://doi.org/10.1016/j.postharvbio.2018.10.014
Yu ZM, Zhang GH, Teixeira da Silva JA, Zhao CH, Duan J (2021) The methyl jasmonate-responsive transcription factor DobHLH4 promotes DoTPS10, which is involved in linalool biosynthesis in Dendrobium officinale during floral development. Plant Sci 309:110952. https://doi.org/10.1016/j.plantsci.2021.110952
Zhao X, Yang XW, Pei SQ, He G, Wang XY, Tang Q, Jia CL, Lu Y, Hu RB, Zhou GK (2016) The Miscanthus NAC transcription factor MlNAC9 enhances abiotic stress tolerance in transgenic Arabidopsis. Gene 586:158–169. https://doi.org/10.1016/j.gene.2016.04.028
Zhao XW, Ouyang L, Zhao P, Zhang CF (2018) Effects of size and microclimate on whole-tree water use and hydraulic regulation in Schima superba trees. PeerJ 6:e5164. https://doi.org/10.7717/peerj.5164
Zhang GH, Yu ZM, Teixeira da Silva JA, Wen DZ (2021) Identification of aquaporin members in Acacia auriculiformis and functional characterization of AaPIP1-2 involved in drought stress. Environ Exp Bot 185:104425. https://doi.org/10.1016/j.envexpbot.2021.104425
Zhang LL, Wang Y, Sun M, Wang J, Kawabata S, Li YH (2014) BrMYB4, a suppressor of genes for phenylpropanoid and anthocyanin biosynthesis, is down-regulated by UV-B but not by pigment-inducing sunlight in turnip cv. Tsuda. Plant Cell Physiol 55:2092–2101. https://doi.org/10.1093/pcp/pcu137
Zhou L, He YJ, Li J, Liu Y, Chen HY (2020) CBFs function in anthocyanin biosynthesis by interacting with MYB113 in eggplant (Solanum melongena L.). Plant Cell Physiol 61(2):416–426. https://doi.org/10.1093/pcp/pcz209
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324. https://doi.org/10.1016/j.cell.2016.08.029
Acknowledgements
We thank Guanshen Liu (Biomarker Technologies, Beijing, China) for providing technical assistance. This research was supported by the China Postdoctoral Science Foundation (2019M663148).
Author information
Authors and Affiliations
Contributions
Guihua Zhang: Conceptualization, Data curation, Writing-original draft preparation, Writing-review & editing, Funding acquisition. Zhenming Yu: Conceptualization, Supervision, Data curation, Software, Methodology, Validation, Writing-original draft preparation, Writing-review & editing. Bo Yao: Investigation. Jaime A. Teixeira da Silva: Formal analysis, Validation, Writing-original draft preparation, Writing-review & editing. Dazhi Wen: Project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Ricardo Aroca.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, G., Yu, Z., Yao, B. et al. SsMYB113, a Schima superba MYB transcription factor, regulates the accumulation of flavonoids and functions in drought stress tolerance by modulating ROS generation. Plant Soil 478, 427–444 (2022). https://doi.org/10.1007/s11104-022-05466-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05466-6