Abstract
Background
Masson pine (Pinus massoniana) plantations are one of the most common forestry plantations in southern China. A high proportion of these plantations is managed as monoculture forests. Productivity in these plantations often declines as they age due to stand retrogression. In these plantations, fertilization is a key practice to prevent stand retrogression. Understanding how plants respond to nutrient fertilization at the tree and stand-level is crucial for evaluating the effects of fertilization and devising nutrient management strategies to prevent stand retrogression and to maintain plantation productivity.
Methods
To determine the effects of nutrient fertilization on plant growth and nutrient stoichiometry, we conducted a nitrogen and phosphorus fertilization experiment in an 8-year old Masson pine plantation in subtropical Hubei Province, China. Plant growth and nutrient stoichiometry responses to fertilization were determined over 12 months.
Results
Tree growth in height and trunk diameter and resorption efficiency of both N and P responded positively to N and/or P fertilization. Soil total P concentration was low (0.32 mg/g). Leaf N:P ratio was high and increased (>20) in N fertilization but decreased (<9) in P fertilization.
Conclusions
Our results show that Masson pine plantations of the study area are severely P limited, with low soil P concentration, high leaf N:P ratio, and positive growth responses to P fertilization. As the most common forestry plantations in subtropical and tropical China, P limitation is likely to be a widespread problem facing the Masson pine plantation industry. Thus nutrient fertilization of Masson pine plantations in comparable contexts should focus on P management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Masson pine (Pinus massoniana) plantations are one of the most common forestry plantations in southern China, comprising 14 Mha (Forestry Commission of China 2014). A substantial part of these plantations is managed as monoculture forests using intensive silviculture (Department of Forest Resource Management 2010; Forestry Commission of China 2014). Productivity in these plantations often declines as they age (Huang et al. 2013; Liu et al. 2021; Ni et al. 2021), a phenomenon that may develop in secondary growth forests or in plantation forests, and is commonly referred to as stand retrogression; apart from a decline in plant productivity, stand retrogression is also characterized by declines in the rate of litter decomposition and in the amounts of soil nutrients such as nitrogen and phosphorus (Peltzer et al. 2010).
Stand retrogression often develops in Masson pine monospecific rotation plantations, where Masson pine is dominating and forms a closed canopy. Understory plant diversity is low and devoid of any nitrogen-fixing species (Ali et al. 2019; Yan et al. 2019), thus the status of N will not improve as it would in natural forests in which N is supplemented by biological N-fixation and from the decomposing litter of relatively high N content (Ni et al. 2021; Yu et al. 2021). Litter decompositions in these plantations are slow due to the poor quality litter and reduced diversity of the soil microbial community (Zeng et al. 2018; He et al. 2020a; He et al. 2020b). In addition, logging coupled with the lack of understory vegetation cover often lead to soil erosion. As a result, soil nutrients such as N, P, and K in many of the Masson pine plantations are depleted by plant growth and subsequent logging, leaching, or erosion, leading to decreased productivity especially in replanted plantations with the same or other conifer species of the original plantation species (Huang et al. 2013; Chen et al. 2015).
In these plantations, fertilization is a key practice to prevent stand retrogression and to maintain the sustainability of plantation productivity. Understanding plant responses to changes in nutrient status at the tree and stand level is crucial for evaluating the effects of fertilization on timber production at the tree level and the productivity at the stand level.
At the tree level, plants may respond to N, P, and K fertilization in a scenario termed multiple nutrient limitation (Harpole et al. 2011; Wright et al. 2011) or as independent nutrient co-limitation (Kaspari et al. 2017) in which N, P, and K all enhance plant growth when supplemented but by different mechanisms. Meta-analyses of studies on N and P fertilization demonstrate that N and P tend to co-limit plant growth in both natural plant communities and in plantation forests (Elser et al. 2007; LeBauer and Treseder 2008).
Further, nutrient fertilization can affect plant nutrient resorption efficiency (i.e. the difference between the concentration of a given nutrient in green versus fully senesced tissue relative to the amount in the green tissue) (Killingbeck 1996; Elser et al. 2000). Plant nutrient resorption is a key process for plants to recycle nutrients from tissues during senescence and before abscission. These nutrients are translocated within plant from senesced tissues such as leaves to point of new growth and reused, thus reducing the need of plants for nutrient absorption from the soil. A global meta-analysis shows N fertilization reduces plant N resorption efficiency for many of the plant species studied (Yuan and Chen 2015), but increases the resorption efficiency for other elements such as P (Barros et al. 2021; Lu et al. 2021). At the tree level, N fertilization can affect P via two mechanisms. First, N fertilization increases P resorption, as plants increase P conservation during the transition from N limitation to P limitation (Vitousek et al. 2010; Reed et al. 2012). Second, it can increase soil P availability and therefore increase P absorption by enhancing extracellular phosphatase enzyme activity (Marklein and Houlton 2012).
Nutrient fertilization can also affect plant nutrient stoichiometry in plant tissues and in the litter (.i.e. the ratio of different nutrient elements such as C:N and N:P) (Elser et al. 2000). The addition of N can significantly increase plant tissue N content, thus increasing the plant N:P ratio (Jia et al. 2010; Hedwall et al. 2017). Alternatively, P fertilization generally increases plant tissue P content and reduces the plant N:P ratio (Jia et al. 2010; Hedwall et al. 2017; Lu et al. 2021).
Here we present a study that assessed the responses of trees immediately after N and P fertilization in an 8-year old Masson pine plantation of Taizishan Forest Farm in subtropical Hubei, China. The Masson pine plantations of Taizishan Forest Farm are in their second or third rotation since first planted in the 1950s and the productivity of these plantations is now relatively low (Ali et al. 2019; Yan et al. 2019). Stand retrogression with reduced amounts of soil N and P (Table 1) may be responsible for this reduced productivity. Records of the Taizishan Forest Farm show that N and P fertilization can improve plantation productivity (unpublished data). Thus these plantations are ideal sites for studying how trees growing under low amounts of nutrients will respond to nutrient fertilization. Our objectives were (1) to assess how N and P fertilization influenced tree growth, (2) to evaluate the interactive effects of multiple nutrient fertilization on nutrient stoichiometry in leaves and litter and on nutrient resorption efficiency at the stand level in subtropical China, where plant growth is often restrained by multiple nutrients, and (3) to apply the knowledge gained to devise better nutrient management strategy of Masson pine plantations. We hypothesized that the Masson pine plantation of Taizishan Forest Farm is multiple nutrient-limited with the expectation that N and P fertilization will increase tree growth. Further, we expected that N fertilization would lower N resorption efficiency, and increase P resorption efficiency or vice versa, creating positive synergetic effects when fertilized simultaneously with both N and P. Understanding how Masson pine trees respond to N and P fertilization will be useful for plantation managers in their efforts to address stand retrogression and reduced productivity of the Masson pine plantations.
Materials and methods
Site description
This study was conducted in an 8-year old Masson pine plantation on Taizishan Forest Farm (112°53′20′′ E, 30°53′45′′ N; 40-467 m asl) in subtropical Hubei Province, China. The area is characterized by low mountains and hills. The long-term annual average precipitation for the area is 1059 mm and the annual average temperature is 16.5 °C. Compared to the long-term average, the study year (2019) was a dry and hot year, with a yearly rainfall of 937 mm and a mean annual temperature of 16.8 °C (S1). The site was planted with Masson pine in 2010. Prior to the plantation, the site was cleared of vegetation. A second round of clearing was carried out in 2012-13. The planting density was 2 × 1.5 m. In December 2018, the average tree height was 7.6 m and the average DBH was 13.2 cm. Prior to the fertilization experiment, soil samples were collected and soil pH, organic carbon (TC), total nitrogen (TN), and total phosphorus (TP) were determined to provide the baseline data of the study site. The soil is yellow-brown loam and slightly acidic with pH of 5.5 ± 0.8 (mean ± se). Soil TC, TN, and TP were 14.1 ± 0.41 (mg/g), 1.32 ± 0.04 (mg/g) and 0.32 ± 0.01 (mg/g), respectively (Table 1). The experimental plots were set up and fertilized in December 2018.Fertilization treatments.
We used three fertilization treatments plus control: control, no nutrient addition; N+, N addition at 100 kg N/ha; P+. P addition at 50 kg P/ha; and NP+, N and P addition at 100 kg N/ha and 50 kg P/ha in our study. In November 2018, we demarcated 12 (20 × 20 m) plots at the selected site. Each plot was randomly assigned a fertilization treatment or control, each replicated three times, giving a total of 12 treatment plots. To minimize the topographic effect on nutrient movement, all plots were located on relatively flat terrain (slope < 15°) with a minimum space of 20 m between plots.
Fertilizers, urea (CH4N2O), and superphosphate (Ca(H2PO4)2·H2O) were applied in December 2018. For fertilizer application, we adopted a furrow method that has been commonly in use on Tazishan Forest Farm. Briefly, a furrow (~50 cm wide and 20 cm deep) running along the line of planted trees was dug at approximately 1 m away from the tree trunk on the upper side of the slope, fertilizer was then spread evenly in the furrow, and immediately covered with soil.
Tree growth measurements
To quantify the influences of fertilization on tree growth, we measured tree height (H) and stem diameter at breast height (DBH; 1.30 m) of all trees within the treated plots in December 2018 (before fertilization) and December 2019 (at the end of the experiment). H was measured by a stick ruler and infrared altimeter. DBH was measured with Vernier calipers.
Foliar and litter sample collection
To explore how nutrient stoichiometric relationships in foliar and litter respond to nutrient fertilization, we collected leaves at 3-month intervals (December 2018, March, June, September and December, 2019). Litter samples were collected at half-year intervals (December 2018, June and December 2019). At each sampling time, five trees were arbitrarily selected within each plot. Leaves (~200 g fresh weight) from the top third of the tree crown were collected in four directions (due south, east, north and west). Leaf samples from the same plot were bulked to make a composite sample for the plot. As Masson pine is an evergreen species, leaves of different ages co-exist. Response to fertilization of these leaves may vary with age. As fresh leaves are most sensitive to fertilization, we sampled the fresh growth for leaf N and P content determination. The age of these leaves varied from a few months to up to 1 year, depending on sampling time. As we were primarily interested in the overall response of leaf nutrient resorption to fertilization, we averaged the nutrient resorption efficiency of the four sampling times. Litter samples were collected using nylon litter-trap. Three nylon net traps (1 × 1 m) were randomly placed in each plot to collect litter sample. The traps were set up 2 weeks before sample collection at each sampling time to ensure the freshness of the litter. Fresh leaf and litter samples were brought back to laboratory within 48 h and oven-dried at 80 °C to constant weight. After drying, the samples were crushed in a plant grinder, passed through a 150-μm sieve, placed in zip-seal plastic bags and refrigerated at 4 °C until used for nutrient analysis.
Soil sample collection
Soil samples were collected at 3-month intervals (December 2018, March, June, September and December 2019) as for foliar sample. In each plot, to minimize the spatial heterogeneity, we collected five subsamples, one at each corner and one in the middle of the plot. A soil auger was used to collect soil of 0-30 cm in depth or until bedrock was reached, whichever was shallower. The subsamples from each plot was mixed to make a composite sample for the plot. Care was taken to avoid sampling directly in the middle of the fertilizing furrow and from the same locations in subsequent samplings. Soil samples were brought back to laboratory within 48 h. The samples were air dried in the laboratory. A 5-mm sieve was used to remove macrofauna, large roots, litter residues, and small stones. The soil was then crushed and sieved with a 150-μm sieve, placed in zip-seal plastic bags and refrigerated at 4 °C until used for nutrient analysis.
Foliar, litter and soil total nitrogen and total phosphorus laboratory analysis
TN and TP for leaves, litter or soil were determined by the Kjeldahl digestion method and the molybdenum-antimony colorimetric method, respectively. In brief, a subsample of 2.5 g leaves, litter or soil were placed in a digestion tube, mixed with 50 ml 2 M H2SO4, shaken for 0.5 h and left to stand overnight at 4 °C. Total nitrogen (TN) and total phosphorus (TP) for leaf, litter or soil were then determined by using the Kjeldahl digestion procedure (Bremner and Tabatabai 1972; Allen 1989). For TP determination, a subsample of 5 g leaves, litter or soil was placed in a digestion tube, mixed with 50 ml 2 M H2SO4, shaken for 0.5 h and the supernatant was then used for determining P by the molybdenum-antimony colorimetric method (Allen 1989).
Data processing and analysis
Tree growth in H and DBH was calculated as the difference between measurements in December 2018 and 2019 (Eqs. 1 and 2). Changes in TN of leaves and litter under various fertilization treatments were calculated as the differences between the mean values over the experiment period and that in December 2018 (Eqs. 3 and 4). Changes in TP of leaves and litter under various fertilization treatments were calculated the same as TN. Nutrient resorption efficiency (%) were calculated as the difference in N or P concentration of fresh leaves and litter divided by N or P concentration of the fresh leaves (Eq. 5 and 6).
Where, H is tree height, DBH is tree stem diameter at 1.30 m height, TNleaf is total nitrogen concentration in leaves, TPleaf is total phosphorus concentration in leaves, TNlitter is total nitrogen concentration in litter, TPlitter is total phosphorus concentration in litter, NRE is the N resorption efficiency and PRE is P the resorption efficiency. NRE and PRE were calculated for June and December 2019 separately and then averaged. The averages were used for subsequent graphing and analyses.
In our study, changes in TNleaf, TNlitter, TPleaf, and TPlitter were calculated as the difference between the averages of the four sampling times (two for litter) and the December 2018 measurements (Eqs. 3 and 4). Subsequently, our calculations of NRE and PRE (Eqs. 5 and 6) did not capture the maximum changes in these parameters. Rather, our calculations reflected the average responses for the duration of the sampling. For plantation managers, these changes in NRE and PRE in response to fertilization are meaningful in terms of nutrient management and plantation productivity. This approach for NRE and PRE calculations is similar to those adopted by Killingbeck (1996) and Sun et al. (2016).
To take into consideration of the mass loss of the senesced leaves, we have also calculated the NREm and PREm ((Eqs. 7 and 8) and NRPm and PRPm ((Eqs. 9 and 10).
Where, NREm and PREm is the N and P resorption efficiency after taking the mass loss of the senesced leaves into consideration for N and P, respectively; MLCF, mass loss correction factor, which is set to 0.745 for conifers (Vergutz et al. 2012); NRP, N resorption proficiency; PRP, P resorption proficiency.
While closely related, the nutrient resorption efficiency is defined as the percent reduction between nutrient concentrations in green and senesced leaves and the nutrient resorption proficiency is defined as the nutrient concentration in senesced leaves (Killingbeck 1996) or litter in the present study. The nutrient resorption efficiency was determined and analyzed using the method of Killingbeck (1996). To take the mass loss of senesced leaves into consideration, we have also calculated the nutrient resorption efficiency using the MLCF suggested by Vergutz et al. (2012). The nutrient resorption proficiency was calculated as the mass-based nutrient concentration in the litter, taking the mass loss of senesced leaves into consideration.
Due to a range of measurement units used for these variables, effect size and general linearized models were used to compare their responses to fertilization treatments. The size of the response were measured as Cohen’s d effect size (Cohen 1988). The responses were calculated as differences between averages of March, June, September and December 2019 measurements (or June and December 2019 for litter) for each treatment and the measurement of December 2018 expressed as Cohen’s effect sizes in standard deviations. Cohen’s d effect size standardizes the direction and magnitude of each of the treatment effects relative to the control by dividing the average of the treatment and the control with the pooled standard deviation of each. We define d > |1.0| as large effect where the mean effect of treatment is at the 84th percentile of the control. Similar analytical approach has been adapted by Kaspari and colleagues (Kaspari et al. 2017). The statistical P value was derived from a factorial generalized linear model (GLIMMIX procedure in SAS V9.4, SAS 2009) matched to the normal distribution of our datasets, and the PDIFF option to compare least square means of a treatment versus the control, and between treatments. Significant threshold for our analysis was set to 0.05.
Results
Effect of fertilization on growth of tree height and diameter
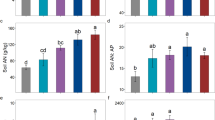
Both growth in H and in DBH responded positively to fertilization treatment (Fig. 1a, b). Growth in H was about 2 standard deviation (SD) (or 0.33 m) more in the three fertilized treatments than in the control (GLM P’s < 0.0001). Among the three fertilization treatments, differences in H growth were not statistically significant (GLM P > 0.74). Growth in DBH was 1.7 SD (or 0.38 cm) and 1.4 SD (or 0.32 cm) more in the NP+ and P+ treatments, respectively, than in the control (GLM P < 0.05). Growth in DBH in the N+ treatment was not significantly different from that of the control (GLM P = 0.17) (Fig. 1b). Among the three fertilizing treatments, differences in DBH growth were not statistically significant (GLM P > 0.20).
Growth in tree height (a) and diameter at breast height (b) in response to fertilization treatments. N+, nitrogen addition; NP+, nitrogen and phosphorus addition; and P+, phosphorus addition. Responses to fertilization treatments were measured as Cohen’s effect sizes in standard deviations from control (Cohen 1988). Values are differences between the December 2019 measurement for each treatment and that of December 2018 expressed as Cohen’s effect sizes in standard deviations. Least square of mean differences at P < 0.05 indicated by asterisk in two ways. When treatments differ from control, asterisks are on the baseline; when they differ from each other, asterisks are on the dotted line connecting the two treatments
Effect of fertilization on leaf and litter N and P concentration
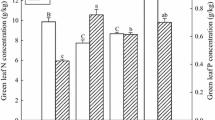
Leaf and litter TN and TP varied across the measurement period and peaked in June with the exception of leaf TP in the P+ addition treatment, which peaked in September (Fig. 2). Compared to the controls, leaf and litter TN increased by 1-2 SD (or 1.77-3.53 mg/g) in the N+ and NP+ treatments (GLM P < 0.001, Fig. 3a, b). In the P+ treatment, leaf TN was the same as the control (GLM P = 0.99, Fig. 3a), but litter TN decreased by 0.96 mg/g (GLM P < 0.01, d = 0.6 SD, Fig. 3b). Also, leaf and litter TN differed among the three fertilizing treatments. Leaf TN was the highest in the N+ treatment, followed by NP+, and the lowest in the P+ treatment. Litter TN had similar pattern among treatments to that of leaf TN, it was the highest in the N+ treatment, and the lowest in the P+ treatment.
Changes in leaf and litter total nitrogen (TN) and total phosphorous (TP) in response to fertilization treatments. N+, N addition; NP+, N and P addition; and P+, P addition . a Leaf TN; b litter TN; c soil TN d leaf TP; e litter TP. f soil TP. Values are differences between averages of the four sampling times (two sampling times for litter) for each treatment and the measurement of December 2018 expressed as Cohen’s effect sizes in standard deviations (Cohen 1988). Responses to fertilization treatments were measured as Cohen’s effect sizes from control, see Fig. 2 for details on significance indicators
Leaf and litter TP increased by about 2 SD (or 0.79 mg/g) from the control in the P+ and NP+ treatments (GLM P < 0.001) but was the same as the control in the N+ treatment (GLM P > 0.09). (Fig. 3d, e). Leaf TP were about 2 SD (or 0.79 mg/g) more in the NP+ and P+ treatments than in the N+ treatment (GLM P = < 0.001, Fig. 3d), litter TP were about 1.5 SD (or 0.32 mg/g) more in NP+ and P+ than in the N+ treatment (GLM P < 0.001, Fig. 3e).
Effect of fertilization on soil N and P concentration
Soil TN and TP were relatively stable across the measurement period (Fig. 2c, f) with the exception of soil TP in the P+ treatment, which peaked in March 2019 and then decreased (Fig. 2f). However, soil TN and TP varied between fertilization treatments. Compared to the controls, soil TN decreased by 1.1 and 1.6 SD (or 1.77 and 3.53 mg/g), in the NP+ and P+ treatments, respectively, (GLM P < 0.001), but increased by 0.7 SD in the N+ treatment (GLM P = 0.07) (Fig. 3c). Soil TP increased by 1.4 and 1.1 SD (or 1.77 and 3.53 mg/g) in the NP+ and P+ treatments, respectively (GLM P’s < 0.001), but decreased by 0.9 SD in the N+ treatment (GLM P < 0.01) (Fig. 3f).
Effect of fertilization on leaf N and P resorption efficiency
Leaf N or P resorption efficiency (NRE or PRE) refers to the difference between the amount of N or P in leaf versus litter relative to the amount in leaves. Both NRE and PRE increased with fertilization except PRE in the N+ treatment (Fig. 4). NRE was 1.0-2.4 SD (or 9.8-19.7%) higher in fertilization treatments than in the controls (GLM P < 0.01), NRE was 1.4 SD (or 13.8%) more in the P+ treatment than in the NP+ treatment (GLM P < 0.05) (Fig. 4a). PRE was 1.7-2.1 SD (or 27.5-33.9%) more in the NP+ and P+ treatments than in the controls and in the N+ treatment (GLM P < 0.001) (Fig. 4b).
Leaf N and P resorption efficiency changes in response to fertilization treatments. N+, N addition; NP+, N and P addition; and P+, P addition. a NRE, leaf N resorption efficiency; b PRE, leaf P resorption efficiency. Values are differences between averages of the four sampling times (two sampling times for litter) for each treatment and the measurement of December 2018 expressed as Cohen’s effect sizes in standard deviations (Cohen 1988). Responses to fertilization treatments were measured as Cohen’s effect sizes from control, see Fig. 2 for details on significance indicators
Effect of fertilization on N:P ratio in leaves and litter
Leaf and litter N:P ratios varied between the fertilization treatments and the control (Fig. 5). In leaves, N:P ratio in the N+ treatment increased to 27 which was greater than that in the control (GLM P < 0.05, d = 0.7 SD). In contrast, N:P ratios decreased to 8.4 and 5.6 in the NP+ and P+ treatments, respectively, which were less than that in the controls (NP+, GLM P < 0.001, d = −1.4 SD; P+, P < 0.001, d = −1.6 SD). Litter N:P ratios among the three fertilization treatments showed a similar pattern to that in leaves, they increased to 22 in the N+ treatment and decreased to 10 and 6.5 in the NP+ and P+ treatments, respectively. The treatment effects on N:P ratio were statistically significant in the NP+ and P+ treatments but not in the N+ treatment (Fig. 5b). Also, N:P ratios differed significantly between the three fertilization treatments.
Changes in leaf and litter N:P ratios in response to fertilization treatments. N+, N addition; NP+, N and P addition; and P+, P addition. a leaf N:P ratio; b litter N:P ratio. Values are differences between averages of June and December 2019 measurements for each treatment and measurement of December 2018 expressed as Cohen’s effect sizes in standard deviations (Cohen 1988). Responses to fertilization treatments were measured as Cohen’s effect sizes from control, see Fig. 2 for details on significance indicators
Discussion
Leaf nitrogen and phosphorus concentration
Our results showed that leaf TN and TP of Masson pine were 5.38 and 0.24 mg/g, respectively (Table 1), which are significantly lower than the national averages for coniferous species of China (11.7 and 1.06 mg/g) (Han et al. 2005) and the global averages for the genus Pinus (11.5 and 1.21 mg/g) (Reich and Oleksyn 2004). The extremely low amounts of soil TN and TP at the study site (Table 1) may have been the primary contributor to the observed low leaf TN and TP. The site is a part of the rotational plantation of Chinese fir (Cunninghamia lanceolata) which was harvested in 2008 and replanted with Masson pines in 2010. Logging and movement of heavy machinery associated with logging and replanting have created large patches of bare ground, coupled with high rainfall in the region exacerbating N and P losses through leaching and erosion. Soil TN and TP were 1.32 and 0.32 mg/g, respectively for the site, placing it at the lowest soil TP concentration category of China (<0.35 mg/g) (Fig. 4, Han et al. 2005).
The growth phase of the Masson pines may be the second contributor to the observed low leaf TN and TP. According to the growth rate theory (Elser et al. 2000; Elser et al. 2003), for a given species, tissue nutrient concentration, N and P in particular, is closely coupled with its growth rate. In the rapidly growing phase, there is an increased allocation of resources to P-rich ribosomal RNA, as rapid protein synthesis by ribosomes is required to support fast growth, resulting in high tissue P concentration. In subtropical China where the present study is conducted, Masson pine trees do not reach a rapid growth phase until they are 13-20 years old (Liu et al. 2016). Leaf TN and TP concentrations remained low at the early phase of development, plateaued between 13 and 20 years and remained high thereafter, coinciding with the maximum growth rate of the species (Liu et al. 2016). The Masson pines of the present study were 8 years old and were probably still in the slow-growing phase, thus the low leaf TN and TP recorded in the present study.
Effect of N and P fertilization on leaf nutrient concentration, resorption efficiency and resorption proficiency
Plant leaf TN and TP concentration responded positively to the fertilization treatments, increasing by 1-77% for leaf TN and by 40-400% for leaf TP (Table 2). These results are consistent with the general consensus that nutrient concentration in plant tissues is tightly coupled with soil nutrient supply (Elser et al. 2000). The coupling of TN and TP concentration between leaf and soil has been reported both at the community level and at the species level. At the community level, high atmospheric N deposition is responsible for the higher plant N concentration in urban area than in rural areas, but in rural area, P fertilization increases soil P and plant P concentration (Elser et al. 2007; Penuelas et al. 2012). At the species level, N fertilization increased plant TN concentration from 18 to 65% in Chinese fir (Xu and Timmer 1999) and 24% in slash pine (Pinus elliottii) (Chen et al. 2010). Similarly, P fertilization increased tissue TP by 20% in slash pine (Hu et al. 2011) and by up to 800% in Sitka spruce (Picea sitchensis) (Proe and Millard 1995).
Although Masson pine leaf TN and TP concentration generally paralleled changes of soil nutrient concentration following fertilization treatments, there were two exceptions: (1) leaf TN concentration did not change in the P+ treatment (Fig. 3a) whereas soil TN decreased (Fig. 3c) and (2) leaf TP concentration did not change in the N+ treatment (Fig. 3d) whereas soil TP concentration decreased (Fig. 3f). This decoupling of nutrient concentration between leaf and soil has been reported in fertilization studies for other species (Vitousek et al. 2010; Reed et al. 2012) but the underlying mechanisms remain unclear. We suggest two possible causes of the decoupling. First, N addition enhances plant extracellular phosphatase enzyme activity thus increase soil P availability (Marklein and Houlton 2012) although soil TP concentration was lower. A second possible cause is that the higher NRE in the P+ treatment and the higher PRE in the N+ treatment (Fig. 4) may have compensated for the effects of the lower soil TN or TP, as a higher proportion of N or P was translocated from senesced leaves to the growing leaves.
In our study, leaf N and P resorption efficiency, NRE and PRE, responded positively to the three fertilization treatments (Fig. 4). These results differ from findings of studies on other coniferous species (Chen et al. 2015; Liu et al. 2021). The enhanced growth after fertilization may have contributed to the higher N and P resorption efficiency. Contrary to our expectations, we did not find a decrease in NRE in the N+ treatment or a decrease in PRE in the P+ treatment as reported for other species (Yuan and Chen 2015; Lu et al. 2021). The uncorrected resorption efficiency of 11-45% for Masson pine recorded in the present study (Table 3) is substantially lower than the global average for conifers (56% for N and 68% for P; Vergutz et al. 2012). These differences may have been affected by the method NRE and PRE were determined. After correcting for mass loss of the senesced leaves, the resorption efficiency increased to 43-54% for N and 35-59% for P (Table 3). The patterns of NRE and PRE, however, did not change between the fertilization treatments after accounting for correction for mass loss of the senesced leaves.
Plant nutrient resorption efficiency has been reported to be negatively correlated with soil P concentration. For instance, in P-rich site, P resorption efficiency is lowered with high proportion of inorganic P remaining in senescent leaves (Ostertag 2010). However, P resorption efficiency reached 82% in sites with severe P deficiency (Lambers et al. 2015). In the Taizishan area, as soil P concentration is low (Table 1) and is P deficient (see discussion below), therefore a higher P resorption efficiency would be expected than that recorded in the present study. Tree age and colonization of mycorrhiza may be two of the major causes of this abnormality (or disparity). The Masson pines in the present study were 8 years old and N and P resorption efficiency of Masson pine has been reported to be the lowest in trees less than 10 years old and increases with tree age (Liu et al. 2016). In addition, Masson pine is capable of growing in soil of low nutrient availability, a characteristics that depends primarily on colonization of mycorrhizal fungi (Zhang et al. 2017). It is possible that mycorrhizal fungi colonization of Masson pine allows it to absorb nutrients more efficiently from soil, thus weakening its reliance on internal nutrient cycling. Further research is warranted to clarify the disparity between the low P resorption rate of Masson pine and the low P concentration of the soil.
Similar to the resorption efficiency, in the present study, the leaf N and P resorption proficiency increased under the three fertilization treatments with the exception of N resorption proficiency under the P+ treatment (Table 3). These results differ from the findings of Wang et al. for Chinese fir in subtropical China (Wang et al. 2019) but are consistent with the findings of Tsujii et al. (2017). It has been suggested that the increase in P resorption proficiency under P fertilization may be caused by the increase in recalcitrant P compounds concentration in the senesced leaf (Tsujii et al. 2017).
Phosphorus deficiency of Masson pine plantation
Globally, soil P concentration is low in tropical and subtropical ecosystems due to the leaching associated with high rainfall (Han et al. 2005). As a result, most tropical and subtropical forest ecosystems are deficient in P (Reich and Oleksyn 2004; Han et al. 2005; Vitousek et al. 2010). The deficiency of P is further exacerbated by the atmospheric N deposition which has increased over the past few decades due to growth of global population, industrialization, and agricultural intensification; the atmospheric N deposition in subtropical regions is estimated at 20–30 kg/ha/year, which is approximately 100-time higher than that of P deposition (Zhu et al. 2016). Our results show that N and P fertilization, either alone or in combinations, enhance Masson pine tree growth in height and/or in diameter (DBH) (Fig. 1), thus support the hypothesis that Masson pine plantation of Taizishan Forest Farm is N and P limited, a scenario called independent co-limitation (Kaspari et al. 2017) where the addition of either N or P or both enhances tree growth.. The low soil TP concentration (Table 1) is further evidence that the plantation maybe P limited, although low soil P alone cannot be used as the indicator of plant P limitation. Some species, such as those in the southwest of Western Australia, thrive in the soil with low P content. The extensive mycorrhizas in these species have been suggested to be pivotal in phosphorus acquisition in severely phosphorus-impoverished soils (Lambers et al. 2015). The third line of evidence supporting the P deficiency postulation is the extremely high values of the leaf and litter N:P ratios (Table 2) recorded for the site. Prior to the fertilization treatment, leaf N:P ratio was higher than 20. It was reduced to less than 9 in the two P fertilization treatments (P+ and NP+), but remained above 20 in the N+ addition treatment. The leaf N:P ratio has been suggested as a predictor of whether plants are limited by either or both nutrients. In earlier studies, a ratio lower than 14 has been suggested to indicate nitrogen limitation and above 16 phosphorus limitation (Koerselman and Meuleman 1996). In between these limits the plants may be limited by either or both of these factors. However, in light of the large intra- and interspecies variation reported in many studies, a larger interval of co-limitation where ratios below 10 are to indicate nitrogen limitation and ratios above 20 to indicate phosphorus limitation has been proposed (reviewed by Hedwall et al. 2017).
The enhanced DBH growth with P fertilization (P+ and NP+ treatments; Fig. 1b) highlights the importance of P management to productivity of the Masson pine plantations. As Masson pine plantations are managed primarily for timber production, tree log diameter is an important criterion for timber grading and any improvement in DBH growth is of economic significance.
In our experiment, the observed tree-growth responses may be potentially affected by the Ca contained in the superphosphate. At the fertilization rate of 50 kg P/ha, the amount of contained Ca equals c. 35 kg/ha. However, we consider any potential effect of this contained Ca on tree growth to be small if any for two reasons. First, as the soil in the study area is only slightly acidic, any small changes in soil acidity on tree growth would be minimal. Second, lime, or CaCO3, has been used to increase the pH in acidic soil. To have any meaningful effect on soil pH, lime is usually applied at the rate of 1-8 tons/ha (Jiang et al. 2020; Kalkhoran et al. 2020); the contained Ca is up to several hundred-fold of the rate used in the present study.
Our study focused on the response of Masson pines to N and P fertilization for the first 12 months. The effects of N fertilization last for 1 to a few years (Nakaji et al. 2001; Hogberg et al. 2006) whereas the effects of P fertilization usually last even longer (Yuan and Chen 2015; Kou et al. 2017; Wang et al. 2019). The N and P fertilization will continue to affect the growth of Masson pine trees and their nutrient stoichiometry beyond the one-year period reported in the present study.
Conclusion
In conclusion, our results show that Masson pine plantations of the Taizishan area are severely P limited, with low soil total phosphorus concentration (0.32 mg/g), high leaf N:P ratio (>20) and positive growth responses to P fertilization treatments. As the most common forestry plantations in subtropical and tropical China, an area known for low soil P concentration, P limitation is likely to be a widespread problem facing the Masson pine plantation industry. In addition, P fertilization enhanced growth both in tree height and in tree DBH, but N fertilization enhanced growth in tree height only. These results highlight the importance of P supply in Masson pine plantations. As Masson pine plantations are managed primarily for timber production, any improvement in DBH growth will be beneficial for plantation productivity. Thus nutrient fertilization of Masson pine plantations should focus on P management.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ali A, Ahmad A, Akhtar K, Teng MJ, Zeng WS, Yan ZG, Zhou ZX (2019) Patterns of biomass, carbon, and soil properties in Masson pine (Pinus massoniana lamb) plantations with different stand ages and management practices. Forests 10
Allen SE (1989) Chemical analysis of ecological materials. Blackwell Scientific Publications, Oxford
Barros IB, Cacalcante VS, Moulin AS, da Silva IR, de Barros NF, Vergutz L, Valadares SV (2021) Integrating forest residue and mineral fertilization: effects on nutrient acquisition, nutrient use efficiency and growth of eucalypt plants. For Ecol Manag 496
Bremner JM, Tabatabai MA (1972) Use of an ammonia electrode for determination of ammonium in Kjeldahl analysis of soils. Commun Soil Sci Plant Anal 3:159–165
Chen FS, Fahey TJ, Yu MY, Gan L (2010) Key nitrogen cycling processes in pine plantations along a short urban-rural gradient in Nanchang, China. For Ecol Manag 259:477–486
Chen FS, Niklas KJ, Liu Y, Fang XM, Wan SZ, Wang H (2015) Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol 35:1106–1117
Cohen J (1988) Statisitical power analysis for the behavioral sciences, Second edn. Hillsdale, New Jersey, USA
Department of Forest Resource Management (2010) China's forest resource status and dynamic change-based on analysis of the seventh national forest resource inventory results. For Econ 7:42e48
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LJ (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner RW (2003) Growth rate-stoichiometry couplings in diverse biota. Ecol Lett 6:936–943
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Forestry Commission of China (2014) Perspective of China forestry. China Forest Press, Beijing
Han WX, Fang JY, Guo DL, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 168:377–385
Harpole WS, Ngai JT, Cleland EE, Seabloom EW, Borer ET, Bracken MES, Elser JJ, Gruner DS, Hillebrand H, Shurin JB, Smith JE (2011) Nutrient co-limitation of primary producer communities. Ecol Lett 14:852–862
He W, Lei L, Ma Z, Teng M, Wang P, Yan Z, Huang Z, Zeng L, Xiao W (2020a) Nonadditive effects of decomposing mixed foliar litter on the release of several metallic elements in a Pinus massoniana Lamb forest. Ann For Sci:77
He W, Xu X, Zhang C, Ma Z, Xu J, Ten M, Yan Z, Wang B, Wang P (2020b) Understory vegetation removal reduces the incidence of non-additive mass loss during leaf litter decomposition in a subtropical Pinus massoniana plantation. Plant Soil 446:529–541
Hedwall PO, Bergh J, Brunet J (2017) Phosphorus and nitrogen co-limitation of forest ground vegetation under elevated anthropogenic nitrogen deposition. Oecologia 185:317–326
Hogberg P, Fan HB, Quist M, Binkley D, Tamm CO (2006) Tree growth and soil acidification in response to 30 years of experimental nitrogen loading on boreal forest. Glob Chang Biol 12:489–499
Hu XF, Chen FS, Nagle G, Fang YT, Yu MQ (2011) Soil phosphorus fractions and tree phosphorus resorption in pine forests along an urban-to-rural gradient in Nanchang, China. Plant Soil 346:97–106
Huang WJ, Liu JX, Wang YP, Zhou GY, Han TF, Li Y (2013) Increasing phosphorus limitation along three successional forests in southern China. Plant Soil 364:181–191
Jia SX, Wang ZQ, Li XP, Sun Y, Zhang XP, Liang AZ (2010) N fertilization affects on soil respiration, microbial biomass and root respiration in Larix gmelinii and Fraxinus mandshurica plantations in China. Plant Soil 333:325–336
Jiang C, Shen J, Cui Q, Yan Y, Liu Y, Zu C (2020) Optimal lime application rates for ameliorating acidic soils and improving the yield and quality of tobacco leaves. Appl Ecol Environ Res 18:5411–5423
Kalkhoran SS, Pannell D, Thamo T, Polyakov M, White B (2020) Optimal lime rates for soil acidity mitigation: impacts of crop choice and nitrogen fertiliser in Western Australia. Crop Pasture Sci 71:36–46
Kaspari M, Roeder KA, Benson B, Weiser MD, Sanders NJ (2017) Sodium co-limits and catalyzes macronutrients in a prairie food web. Ecology 98:315–320
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727
Kou L, Wang HM, Gao WL, Chen WW, Yang H, Li SG (2017) Nitrogen addition regulates tradeoff between root capture and foliar resorption of nitrogen and phosphorus in a subtropical pine plantation. Trees-Structure and Function 31:77–91
Lambers H, Martinoia E, Renton M (2015) Plant adaptations to severely phosphorus-impoverished soils. Curr Opin Plant Biol 25:23–31
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Liu JT, Gu ZJ, Shao HB, Zhou F, Peng SY (2016) N-P stoichiometry in soil and leaves of Pinus massoniana forest at different stand ages in the subtropical soil erosion area of China. Environ Earth Sci 75
Liu C, Kang H, Wang B, Zhang L, Yi Y, Zheng J, Hu X, Yu J (2021) Studies on low-function plantations in the Middle Basin of Yangtz River. Northeast Forestry University Press, Haerbin, China, China
Lu XT, Hou SL, Reed S, Yin JX, Hu YY, Wei HW, Zhang ZW, Yang GJ, Liu ZY, Han XG (2021) Nitrogen enrichment reduces nitrogen and phosphorus resorption through changes to species resorption and plant community composition. Ecosystems 24:602–612
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704
Nakaji T, Fukami M, Dokiya Y, Izuta T (2001) Effects of high nitrogen load on growth, photosynthesis and nutrient status of Cryptomeria japonica and Pinus densiflora seedlings. Trees-Structure and Function 15:453–461
Ni X, Lin C, Chen G, Xie J, Yang Z, Liu X, Xiong D, Xu C, Yue K, Wu* F, Yang Y (2021) Decline in nutrient inputs from litterfall following forest plantation in subtropical China. For Ecol Mang 496. https://doi.org/10.1016/j.foreco.2020.118855
Ostertag R (2010) Foliar nitrogen and phosphorus accumulation responses after fertilization: an example from nutrient-limited Hawaiian forests. Plant Soil 334:85–98
Peltzer DA, Wardle DA, Allison VJ, Baisden TW, Bardgett RD, Chadwick OA, Condron LM, Parfitt RL, Porder S, Richardson SJ, Turner BL, Vitousek PM, Walker J, Walker LR (2010) Causes and consequences of ecosystem retrogression. Geochim Cosmochim Acta 74:A802–A802
Penuelas J, Sardans J, Rivas-Ubach A, Janssens IA (2012) The human-induced imbalance between C, N and P in Earth's life system. Glob Chang Biol 18:3–6
Proe MF, Millard P (1995) Effect of P supply upon seasonal growth and internal cycling of P in Sitka spruce (Picea sitchensis (bong) Carr) seedlings. Plant Soil 168:313–317
Reed SC, Townsend AR, Davidson EA, Cleveland CC (2012) Stoichiometric patterns in foliar nutrient resorption across multiple scales. New Phytol 196:173–180
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci U S A 101:11001–11006
Sun ZZ, Liu LL, Peng SS, Penuelas J, Zeng H, Piao SL (2016) Age-related modulation of the nitrogen resorption efficiency response to growth requirements and soil nitrogen availability in a temperate pine plantation. Ecosystems 19:698–709
Tsujii Y, Onoda Y, Kitayama K (2017) Phosphorus and nitrogen resorption from different chemical fractions in senescing leaves of tropical tree species on mount Kinabalu, Borneo. Oecologia 185:171–180
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Wang FC, Fang XM, Wang GG, Mao R, Lin XF, Wang HM, Chen FS (2019) Effects of nutrient addition on foliar phosphorus fractions and their resorption in different-aged leaves of Chinese fir in subtropical China. Plant Soil 443:41–54
Wright SJ, Yavitt JB, Wurzburger N, Turner BL, Tanner EV, Sayer EJ, Santiago LS, Kaspari M, Hedin LO, Harms KE, Garcia MN, Corre MD (2011) Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 92:1616–1625
Xu XJ, Timmer VR (1999) Growth and nitrogen nutrition of Chinese fir seedlings exposed to nutrient loading and fertilization. Plant Soil 216:83–91
Yan ZG, Zhou QH, Teng MJ, Ji H, Zhang JL, He W, Ye YM, Wang B, Wang PC (2019) High planting density and leaf area index of masson pine forest reduce crown transmittance of photosynthetically active radiation. Glob Ecol Conserv 20
Yu Z, Zhou GY, Liu L, Manzoni S, Ciais P, Goll D, Penuelas J, Sardans J, Wang WT, Zhu J, Li L, Yan JH, Liu JX, Tang XL (2021) Natural forests promote phosphorus retention in soil. Glob Chang Biol
Yuan ZY, Chen HYH (2015) Negative effects of fertilization on plant nutrient resorption. Ecology 96:373–380
Zeng LX, He W, Teng MJ, Luo X, Yan ZG, Huang ZL, Zhou ZX, Wang PC, Xiao WF (2018) Effects of mixed leaf litter from predominant afforestation tree species on decomposition rates in the three gorges reservoir, China. Sci Total Environ 639:679–686
Zhang T, Wen XP, Ding GJ (2017) Ectomycorrhizal symbiosis enhances tolerance to low phosphorous through expression of phosphate transporter genes in masson pine (Pinus massoniana). Acta Physiol Plant 39:101
Zhu JX, Wang QF, He NP, Smith MD, Elser JJ, Du JQ, Yuan GF, Yu GR, Yu Q (2016) Imbalanced atmospheric nitrogen and phosphorus depositions in China: implications for nutrient limitation. J Geophys Res Biogeosci 121:1605–1616
Acknowledgements
We would like to thank Taizishan Forest Farm for providing access and logistical support for the field fertilization experiment. Special thanks to Yiyuan Li, Licheng Liu, Xionghui Qi, Lihao Song and Qinghui Zhou for field and laboratory work assistance.
Funding
This study was funded by Chinese Ministry of Science and Technology (2017YFD0600304) and The Chinese Ministry of Education through Fundamental Research Funds for the Central Universities (2662020YLPY022 & 2662018QD059).
Author information
Authors and Affiliations
Contributions
Zhaogui Yan: conceptualization, funding acquisition, manuscript drafting; Ben Wang: methodology, data curation and analysis, manuscript drafting, funding acquisition; Jihao Chen: data analysis, field sampling, manuscript drafting; Guan Huang: methodology, field sampling, laboratory chemical analysis; Shuai Zhao: methodology, data analysis, editing; Fangwei Dong: methodology, data analysis, editing; Yangyang Zhang: methodology; data analysis, editing; Wei He: conceptualization, methodology, editing; Pengcheng Wang: conceptualization, funding acquisition, review. All authors read and approve the submission to Plant and Soil.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Pre-submission review
The manuscript was pre-reviewed by Prof. Ian T. Riley (a consulting editor with Plant and Soil, formerly of the University of Adelaide). Email: ian.t.riley@uni.sydney.edu.au.
Additional information
Responsible Editor: Timothy J. Fahey.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 92 kb)
Rights and permissions
About this article
Cite this article
Wang, B., Chen, J., Huang, G. et al. Growth and nutrient stoichiometry responses to N and P fertilization of 8-year old Masson pines (Pinus massoniana) in subtropical China. Plant Soil 477, 343–356 (2022). https://doi.org/10.1007/s11104-022-05424-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05424-2