Abstract
Background and aims
It is known that the single and combined use of phosphate-solubilizing bacteria (PSB) and silicon (Si) have the potential to improve the uptake of phosphorus (P) by plants in calcareous soils. However, it was unclear which form of Si in soil would have the most profound effects on the uptake of P by wheat plant inoculated with PSB. Here we investigated the effect of Si fertilizer on chemical forms of Si and P uptake by wheat plant inoculated with PSB in a calcareous soil. Determining different forms of Si in calcareous soils with a low P supply is essential to better understand the capacity of these forms to supply wheat plant with P in the presence of PSB.
Methods
A pot trial in a completely randomized design with factorial arrangement in 3 repetitions under greenhouse conditions was adopted to investigate the effect of Si fertilizer alone or in combination with PSB on the uptake of P and Si by wheat plant grown on a calcareous soil with low available P. Experimental treatments included: Si factor at four levels of 0, 150, 300, and 600 mg Si kg−1 from silicic acid source and PSB strains factor at three levels of B0 (control), Pseudomonas sp. FA1, and Bacillus simplex UT1. The impacts of Si levels and PSB on shoot and root dry weight and the wheat shoot uptake of Si and P were measured. Also, the chemical forms of Si in wheat rhizosphere and non-rhizosphere soil and the regression models of the variables were studied to better understand the mechanisms of this process.
Results
With increasing the levels of Si, the plant available Si with the lowest level, adsorbed Si, and amorphous Si with the highest level in both the rhizosphere and non-rhizosphere soil increased. In addition, Si fertilization-mediated increase at level of the soil Si fractions was intensified in the presence of PSB strains. The highest plant available Si (75.50 mg Si kg−1 soil) was obtained from the treatment of 600 mg Si kg−1 soil in the presence of Pseudomonas sp. FA1. The combined application of Si and PSB strains also increased the wheat shoot dry weight by 3.5 times compared to the control treatments. The use of Si alone at level of 300 mg Si kg−1 also increased the wheat shoot content of P by 2.3 times compared to the control treatment. However, the combined application of Pseudomonas sp. FA1 and Si at level of 600 mg Si kg−1 increased the wheat shoot content of P by 4 times compared to the control treatment. According to the correlations among the studied parameters, in addition to the expected positive correlation between plant available Si of wheat rhizosphere soil and the measured parameters, a positive and significant correlation between adsorbed Si of wheat rhizosphere soil and the shoot uptake of Si (r2 = 0.84, P < 0.01) and the shoot uptake of P (r2 = 0.58, P < 0.05) was also observed in this study.
Conclusions
The information on the distribution of different forms of Si and the availability of P following the combined use of PSB strains and Si in this study (e.g., the role of rhizosphere adsorbed Si in increasing the wheat shoot uptake of P) may help in better management of P-fertilization in calcareous soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential nutrient of the plant and plays a main role in plant growth and development. Phosphorus in plants is the second essential nutrient after nitrogen that is required for various biochemical processes and completing the reproductive growth of the plant. On average, soils contain an average of 400 to 1000 mg kg−1 of total P, of which only 1.00–2.50% is available to plants for uptake (Chen et al. 2008). Phosphorus is found in soil in both mineral and organic forms. Most of the organic P (20 to 80%) has been found to be inert (Abdi et al. 2014). Mineral P in soils may become unavailable through fixation or adsorption. For example, mineral P may become unavailable because of precipitation reactions with cations such as Ca-P and Mg-P in alkaline soil or Fe–P and Al-P in acidic soil (Yadav et al. 2017; Bayer et al. 2001). As a result, the concentration of mineral P in the soil solution is rarely more than 0.10 mg kg−1. In most calcareous soils, which are most abundant in arid and semi-arid zones of the world such as Iran, the available P concentration is usually less than the critical level (10–12 mg P kg−1) and a large amount of chemical P fertilizers is added to the arable lands annually to supply the P needed by crops (Golmohammad et al. 2016). In addition, it has been reported that only 15–20% of the applied P to calcareous soils becomes available to plants (Sharif et al. 2002). However, the use of large amounts of the fertilizers in calcareous soils leads to the accumulation of P in the soil and causes environmental pollution as well as nutrient imbalance in these soils (Jalali and Sajadi Tabar 2011). It means that it is necessary to study how to optimize better management of P fertilization in calcareous soils (the soils with a high concentration of Ca and an alkaline pH).
It has been well proven that phosphate-solubilizing bacteria (PSB) can improve P supply to plants by organic P mineralization and dissolution of insoluble mineral phosphates (Sharma et al. 2013). Decrease in soil pH, production of organic acids, and phosphatases are the main mechanisms of PSB in improving P availability to plants (Sharma et al. 2013; Etesami 2020). The use of PSB can increase the availability of P to plant in calcareous soils and improve P uptake by the plant and as a result increase crop yield (Ghorchiani et al. 2018; Shirmohammadi et al. 2020). Many studies have reported the use of PSB as an alternative to chemical P fertilizers (Beheshti et al. 2021; Alori et al. 2017; Etesami 2020; Kaur and Reddy 2015). According to previous studies, the bacteria of the genus Pseudomonas and Bacillus are the most potent insoluble phosphate solubilizers (Sharma et al. 2013; Etesami 2020; Shirmohammadi et al. 2020).
Application of silicon (Si) is also known as an ecologically compatible and environmentally amicable way to improve the level of plant-available P in agro-ecosystems, alleviate environmental stresses, and stimulate plant growth (Etesami and Jeong 2020, 2018; Etesami et al. 2021). Silicon has been shown to improve plant growth under low P conditions by various mechanisms (e.g., by improvement of exudation of organic acids, up-regulation of P transporters in P-deficient plant roots, and weakening the ability of P to bind the surface of iron/aluminum-oxides, and consequently enhancing P bioavailability)(Etesami et al. 2021; Kostic et al. 2017; Koski-Vähälä et al. 2001).
Although Si is one of the major soil constituents, which is naturally available to plants in soil, the natural soil Si depletion, cultivation of Si high-accumulator plants, and removal of the residues of these plants can reduce the pool of plant available Si (Li et al. 2020a). This, thus, points out that Si fertilizers are encouraged to boost the cultivation safety of plants and crop productivity (Epstein 2009; Li and Delvaux 2019). The primary source of Si for plant uptake is the reserve of weatherable lithogenic silicates (Alexandre et al. 1997; Henriet et al. 2008). Their dissolution delivers monosilicic acid (plant available Si), aluminum, iron and other solutes, which may form pedogenic alumino-silicates, aluminum and iron oxides, which mostly accumulate in highly weathered soils with advanced desilication and Si depletion. These pedogenic aluminosilicates can be in turn dissolved depending on monosilicic acid activity (Rai and Kittrick 1989).
The largest proportion of amorphous silica in most soils is represented by bio-opal (Richard Drees et al. 1989). Amorphous silica consists mainly of biogenic silica, produced by plants as phytoliths (monosilicic acid polymerized in transpiration sites of plant shoots) (Jones and Handreck 1965), with a variable contribution of a non-crystalline inorganic fraction, such as Si included in iron oxides/hydroxides and Si in inorganic alumino-silica coatings (Saccone et al. 2007). It is known that the amorphous biogenic Si (phytolith) returns to soil within plant residues (Li and Delvaux 2019), readily dissolves at common soil solution pH (Fraysse et al. 2009), replenishes dissolved Si pool (Li et al. 2020a), and acts as a major source of dissolved Si in soil solution (Matichenkov and Bocharnikova 2001). For example, in a study, Alexandre et al. (1997) estimated that Si release from bio-opal is two to three times as great as that from silicate weathering. In a resent study, Li et al. (2020a) also demonstrated that with increasing soil weathering stage, the biological Si feedback loop takes over the mineral contribution to plant available Si. This implicates a high turnover of this fraction.
Due to the physical and chemical properties of soils in different regions, Si can be observed as a variety of chemical compounds in the soil (e.g., plant available Si, Si adsorbed or precipitated with iron and aluminum oxides, and Si as a component of crystalline or amorphous silicate minerals) (Haynes 2014; Crusciol et al. 2018). Despite the abundance of Si in the Earth's crust, most of its forms are not available by plants. Amounts of Si in soils depend on parent material and soil type, reflecting pedogenic processes at pedon and landscape scale (Georgiadis et al. 2013). Monosilicic acid in soil solution is part of a dynamic system; its amount depends on soil properties such as mineral composition, adsorption and desorption effects, water balance, temperature, various organic compounds, and biochemical activity (Milnes and Twidale 1983), which are different in various soils. For example, organic compounds such as low molecular weight organic acids contribute to the weathering of soil minerals through acidification and complexation and increase silica solubility (Bennett et al. 2001). Adsorption of monosilicic acid in various soils also depends on soil reaction, soil composition and the specific surface of sorbents and increases with increasing pH and specific surface area of the soil particles (Gehlen and Van Raaphorst 2002; Georgiadis et al. 2013). For example, pedogenic oxides and hydroxides that are abundant in soils (Schwertmann and Taylor 1989) play an important role in adsorption, occlusion and release of silicic acid in soils (Cornelis et al. 2011). The amplitude of plant Si uptake and mineralomass depends on plant species, soil weathering stage, pH and buffer capacity (Li et al. 2019).
In addition to the physical and chemical properties of soils, the biological properties of soils such as microorganisms (bacteria and fungi) are also known to affect Si-release from litter or minerals. Some microorganisms have developed mechanisms for Si-storage (biomineralization) in their bodies (Sommer et al. 2006) and converting silicate forms to available forms for plants (Etesami et al. 2021). For example, in a previous study, the use of plant growth-promoting-bacteria (PGPB) positively affected the available, adsorbed, and amorphous fractions of Si under water-deficit stress conditions (Valizadeh-rad et al. 2021). It has also been reported that Si also interacts with the growth of beneficial soil microorganisms and alters their structure and diversity (Song et al. 2021a, b).
Recently, the study on interaction of Si and PGPB in the combination has been proposed as a novel research field (Etesami 2018; Etesami and Adl 2020a). In our previous studies, it was found that PSB and Si can synergistically improve P uptake by wheat (Rezakhani et al. 2019) and sorghum (Rezakhani et al. 2020) fertilized with soluble or insoluble P source under controlled conditions (soilless). However, these studies did not determine which form of Si would have the greatest effect on P uptake and improving growth indices of the plant under soil conditions. In addition, so far, no documentary study has been conducted on the effect of chemical forms of Si on P availability in the presence of PSB in a calcareous soil. It is also known that PSB can also affect the availability of Si and increase the uptake of this element by plants (Etesami et al. 2021). Information on the status of Si and the determination of its most important form(s) in increasing the availability and uptake of P by the plant in calcareous soils is of special importance and PSB can be effective in this regard. Due to the different solubility of various forms of Si, the determination of their frequency and distribution will provide more knowledge of the ability of plants to use more soil Si. Quantification of Si pools in soils is needed for improving our understanding of biogeochemical processes involving Si in the plant–soil–water system which has represented a black box in global Si-cycling models so far (Georgiadis et al. 2013). Such quantification requires a sequential extraction method for the various Si fractions in soils. Some studies have been done to identify the different forms of Si to determine the relationship between plant uptake of Si with soil Si by using different extractors (Babu et al. 2016; Crusciol et al. 2018; Georgiadis et al. 2013, 2017). However, none of these extractants have been practicable in all types of soils because the contents of Si extracted vary considerably depending on the solution used in the extraction process (Snyder 2001; Babu et al. 2016). Keeping above in view, the objective of this study was to investigate the effect of Si fertilizer and PSB strains on various chemical forms of Si (plant available Si, adsorbed Si, and amorphous Si), determined by modifying the current method of Si extraction (Georgiadis et al. 2017), the shoot uptake of P and Si, and biomass of wheat plant, as a major cereal crop in many countries, grown on a calcareous soil with low available P under greenhouse conditions.
Materials and methods
Sampling and analysis of soil
Due to the need for a soil with low available P (lower than the critical level, 12 mg kg−1) in this experiment, a calcareous soil was sampled at a depth of 0–30 cm from the research farm of Soil and Water Research Institute (SWRI), located in Karaj, Iran. After being air-dried and passed through two-mm sieve, the soil samples were analyzed according to standardized procedures and used in pots. The soil had a clay loam texture (31% sand, 44% silt, and 25% clay); total P (Bowman 1988; Murphy and Riley 1962), 1186 mg kg−1; organic C (Walkley and Black 1934), 0.60%; pH (Haluschak 2006), 7.80; electrical conductivity (Haluschak 2006), 1.03 dS m−1; moisture content at field capacity (FC), 22.70%; available P (Olsen-P) (Olsen and Sommers 1982), 5.50 mg kg−1; available K, 258 mg kg−1 (Swift and Sparks 1996); micronutrients (Swift and Sparks 1996), e.g., DTPA-extractable Fe, 4.51 mg kg−1; DTPA-extractable Cu, 1.12 mg kg−1; DTPA-extractable Zn, 0.93 mg kg−1; and DTPA-extractable Mn, 8.05 mg kg−1; available Si (Narayanaswamy and Prakash 2009), 34.22 mg kg−1; cation exchange capacity (Haluschak 2006), 14.52 cmolc Kg−1; and calcium carbonate equivalent (Loeppert and Suarez 1996), 10.96%.
Experimental set up and treatments
To study the impact of various concentrations of Si and PSB strains on chemical forms of Si and growth parameters of wheat plant (cv., Sirvan), an experiment was carried out in a completely randomized design in factorial arrangement in 3 replications under greenhouse conditions. Experimental treatments included: (i) phosphate-solubilizing bacterial strain factor at 3 levels: non-inoculated seedlings (B0), Bacillus simplex UT1-inoculated seedlings, and Pseudomonas sp. FA1-inoculated seedlings; and (ii) silicon factor at 4 levels, soil non-fertilized with silicic acid (H4SiO4; 96.113 g mol−1), soil fertilized with 150 mg Si kg−1 soil as silicic acid, soil fertilized with 300 mg Si kg−1 soil as silicic acid, and soil fertilized with 600 mg Si kg−1 soil as silicic acid. A total of 12 various treatments (3 × 4) were obtained from combination of various factors aforesaid. A total of 36 pots (12 × 3) were used in this experiment. This experiment was also performed twice under greenhouse conditions. Colonization assay, plant growth promoting traits, and how to prepare inoculants for PSB strains were well described in our previous study (Rezakhani et al. 2019). Strains UT1 and FA1 solubilized tricalcium phosphate (220 and 239.68 mg P L−1, respectively) and rock phosphate (94.90 and 164 mg P L−1, respectively) (Rezakhani et al. 2019). Since different plant species have indicated various needs for Si addition (Etesami et al. 2020), different Si levels were used in this study to obtain the optimized content of Si. In addition, the Si levels used here were equivalent to those extensively used in other studies (Rezakhani et al. 2020, 2019; Bahari et al. 2012).
In this study, rhizobag system was used to separate the rhizosphere soil from non-rhizosphere soil in pots. Each experimental-unit consisted of plastic-pots (15.5 cm in diameter and 14 cm high) with two holes for drainage containing 3 kg of soil passed through a 4 mm-mesh sieve. Silicon treatments were added to the potting soil about 28 days before planting. The pots were incubated at 25 °C to balance soil biological and chemical reactions for four weeks. During the incubation period, the soil moisture of the pots was maintained with distilled water at FC as determined by gravimetric water content by weight. The seeds of wheat were obtained from Seed and Plant Breeding Research Institute (SPBRI), Karaj, Iran. Surface-disinfected seeds were germinated on agar-water (0.8%) plates in the dark for 48 at 28 °C. Five healthy-germinated same-sized seeds were planted in each pot and later thinned to three uniform seedlings per pot. These seedlings were then inoculated with 1 mL of the suspensions of PSB strains (5 × 108 cells mL−1) according to the experimental design. Greenhouse conditions included: temperature, 25 °C; light intensity, 20,000 lx; and relative humidity content, 70%. To ensure that the greenhouse conditions are the same, the treatments and repetitions related to each treatment were alternated to eliminate possible errors due to differences in transpiration, temperature, evaporation, light, and other factors as much as possible. Daily irrigation with Hoagland nutrient solution (Hoagland and Arnon 1950) and distilled water was done by weight to provide 80% moisture of FC. Potassium dihydrogen phosphate (KH2PO4) as a source of P was removed from the nutrient solution and the amount of K removed was supplied by potassium sulfate.
Measurement of plant biomass
Before entering the reproductive stage (60 days of growth period), wheat plants were cut from the crown. After rinsing with distilled water, they were dried in a ventilated oven at 70 °C for 48 h and then their dry weight was measured. In addition, the roots of wheat were carefully removed from the pot and gently separated from the soil by rinsing with water and then washed with distilled water, dried at 70 °C for 48 h and weighed.
Measurement of shoot Si and P concentration
The concentration of Si in the shoot (0.2 g ground samples) digested with 2 mL of 50% H2O2 in 100 mL polyethylene tubes (previously rinsed with 0.1 M NaOH and demineralized water) was determined using colorimetric amino-molybdate blue color method (Elliott and Snyder 1991) by a UV visible spectrophotometer (Shimdzu, Spectronic 100, Japan) at 650 nm. Dry-ashing method and the digestion with hydrochloric acid were used to determine shoot P concentration (Waling et al. 1989). Extraction was carried out based on the method described by Ryan et al. (2001). Phosphorus concentration in the extract was read by spectrophotometer (Novaspec II-England) at 430 nm. Wheat shoot uptake of Si and P was calculated by multiplying the Si and P concentration of wheat shoot by the shoot dry weight, respectively.
Measurement of chemical forms of Si in rhizosphere and non-rhizosphere soil
In this assay, plant available Si, adsorbed Si, and amorphous Si of the soil after harvesting wheat plant, both soil inside rhizobag (rhizosphere soil) and soil outside rhizobag (non-rhizosphere soil), were determined. To measure these forms, we modified the method proposed by Georgiadis et al. (2017), which is proper for acidic soils, for calcareous soil in this study. For this purpose, first the extract solutions for plant available Si and adsorbed Si and amorphous Si were prepared according to Korndörfer et al. (1999) and Breuer and Herrmann (1999), respectively, then these forms were measured by sequential extraction method. To prepare the samples, the soil was passed through a 2-mm sieve after drying. Two g of the soil was poured into 50-mL centrifuge tube. At the first step, 20 mL of 0.01 M calcium chloride was added to the tube. The resulting suspension was shaken with an electric shaker at 180 rpm for 60 min and then centrifuged at 3000 rpm for 5 min. The obtained mixture was immediately filtered and the content of plant available Si in the resulting solution was read by using ICP (Perkin Elmer ICP-Optima 2100 DV). At the second step, 20 mL of 0.5 M acetic acid extractor was added to the centrifuge tube at the first step. The resulting suspension was shaken with an electric shaker at 180 rpm for 60 min and then centrifuged at 3000 rpm for 5 min. The obtained mixture was immediately filtered and the content of adsorbed Si in the resulting solution was read by using ICP (Perkin Elmer ICP-Optima 2100 DV). At the third stage, 40 mL of 0.5 M sodium carbonate extractor was added to the centrifuge tube at the second step. The resulting suspension was shaken with an electric shaker at 180 rpm for 16 h and then centrifuged at 3000 rpm for 5 min. The obtained mixture was immediately filtered and the content of amorphous Si in the resulting solution was read by using ICP (Perkin Elmer ICP-Optima 2100 DV).
Statistical analysis
All statistical calculations (two-way analysis of variance) were analyzed using SAS software (v.9.4). By using Shapiro–Wilk test for normality, and Levene ́s test for homogeneity of variances, the assumptions for analysis of variance were checked. Tukey’s multiple comparison test was used at the level of 5% probability to detect the means that were significantly different from each other. Backward test was also used for regression equations and the correlation was analyzed with the Pearson test (two-tailed) at P ≤ 0.05.
Results
Morphological responses of wheat to PSB and Si
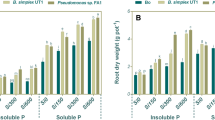
Interactions between various levels of Si and PSB strains on shoot and root dry weight of wheat plant were significant (P ≤ 0.05) (Table S1). The dry weights of shoot and root for wheat plants treated with Si and inoculated with PSB strains were greater than those grown in the absence of these treatments (Fig. 1). Both Si and PSB strains indicated a role in improving wheat plant root and shoot biomass. However, the ability of the PSB strains to improve shoot and root dry weight of wheat plant varied depending on the concentration of Si applied. The highest amount of the shoot dry weight (5.0 g pot−1) and root dry weight (2.6 g pot−1) was recorded in the plants treated with 300 mg Si kg−1 and 600 mg Si kg−1 and inoculated with Pseudomonas sp. FA1, respectively (Fig. 1A and B). In general, the combined use of Si and PSB, especially Pseudomonas sp. FA1, led to the greatest enhancement of wheat plant shoot and root dry weight under calcareous soil conditions.
Dual effects of various treatments (silicon and PSB strains) on shoot dry weight (a) and root dry weight (b) of wheat plant grown on a calcareous soil under greenhouse conditions for 60 days. Means ± SD (n = 3) followed by the same letters are not significantly different according to Tukey’s multiple range test at P < 0.05
Nutritional responses of wheat to PSB and Si
Interactions between various concentrations of Si and phosphate-solubilizing bacterial strains on Si and P uptake by wheat plant were significant (P ≤ 0.01) (Table S1). With increasing Si levels, the wheat shoot uptake of Si increased and this effect was intensified in the presence of PSB. The highest wheat shoot uptake of Si (77 mg pot−1) was obtained from the wheats treated with Si at level of 600 mg Si kg−1 in the presence of B. simplex UT1 (Fig. 2A). The highest amount of wheat shoot uptake of P (17.3 mg pot−1) was obtained from the treatment of 600 mg Si kg−1 + B. simplex UT1 (Fig. 2B), although there was no significant difference between this treatment and treatments of 600 mg kg−1 + Pseudomonas sp. FA1 and 300 mg Si kg−1 + bacterial strain FA1. With enhancing Si levels, the wheat shoot uptake of P increased in all treatments on a regular basis. However, with increasing application of Si more than the level of 300 mg Si kg−1, the wheat shoot uptake of P decreased in the treatments of no bacterial inoculation. Inoculation of PSB strains increased P uptake at all levels of Si compared to the control treatment (no bacterial inoculation). According to Fig. 2A, there was a synergistic effect between Si and P, so that with increasing Si concentration, P uptake by wheat plant increased and this impact was intensified in the presence of phosphate solubilizing-bacterial strains (probably due to enhanced availability of P by the PSB strains).
Dual effects of various treatments (silicon and PSB strains) on shoot Si uptake (A) and shoot P uptake (B) of wheat plant grown on a calcareous soil under greenhouse conditions for 60 days. Means ± SD (n = 3) followed by the same letters are not significantly different according to Tukey’s multiple range test at P < 0.05
Effect of treatments on chemical forms of soil Si
Interactions between various levels of Si and PSB strains on plant available Si, adsorbed Si, and amorphous Si in rhizosphere soil were not significant. But these interactions were significant only on plant available Si in non-rhizosphere soil (Table S1). As the level of Si increased to 600 mg Si kg−1 level (main effects of Si treatment), the plant available Si, adsorbed Si, and amorphous Si in the rhizosphere and non-rhizosphere soil also increased (Data not shown). According to the results (Fig. 3), with increasing Si levels, the plant available Si in non-rhizosphere soil also increased. The highest plant available Si (75.50 mg Si kg−1 soil) was obtained from the treatment of 600 mg Si kg−1 soil in the presence of Pseudomonas sp. FA1.
In both rhizosphere and non-rhizosphere soil, the highest level of Si fraction was observed in amorphous Si form and there was also a significant difference between the level of this Si form and the level of plant available Si and adsorbed Si (Fig. 4A). With increasing Si levels, the level of soil Si fractions increased significantly (Fig. 4B). The highest level of chemical forms of Si, in both rhizosphere and non-rhizosphere soil of wheat, was assigned to soil amorphous Si and followed in order: amorphous Si > adsorbed Si > plant available Si. In addition, no significant difference was observed between the rhizosphere and non-rhizosphere soil Si fractions. Due to the low concentration of plant available Si in the control treatment (no application of Si) in the rhizosphere soil and also the stability of Si, it seems that the amount of Si harvested by the wheat plant was more than the availability of Si in rhizosphere soil. However, with the addition of each level of Si, the amount of Si in the rhizosphere soil solution was at an almost constant level (Fig. 4B).
With increasing Si levels, the level of soil Si fractions increased with or without bacterial inoculation. However, this increase did not show a significant difference between the use of bacterial strains and control (no use of bacterial strain) and this result was observed in all three components of the Si fractions. The highest level of plant available, adsorbed and amorphous Si was observed at the level of 600 mg Si kg−1 soil (Fig. 5A). With increasing levels of Si in the non-rhizosphere soil, the level of soil Si fractions also increased with or without bacterial inoculation. There was a significant difference in the plant available Si fraction between bacterial treatment and control (no bacterial application) at the levels of 300 and 600 mg Si kg−1 (Fig. 5B).
Correlation analysis
The results of linear multivariate regression analysis between the measured indices in wheat and the chemical forms of Si in the soil are shown in Table 1. According to regression models, shoot and root dry weight, shoot P concentration, and shoot P and Si uptake were affected by the adsorbed Si in the wheat rhizosphere soil and had an increasing effect on these variables. However, the amount of shoot Si concentration was affected by the plant available Si in the wheat rhizosphere soil. Silicon fractions also had no significant correlation with shoot dry weight but had a positive and significant correlation with root dry weight (Table 2). Silicon fractions had a positive and significant correlation with the shoot and root uptake of Si as well as with root uptake of P. However, they did not have a significant correlation with the shoot uptake of P, except for the adsorbed Si in the rhizosphere soil, which had a significant correlation (P < 0.05). The plant available Si of rhizospheric soil had a positive and significant correlation with the Si fractions of rhizosphere and non-rhizosphere soil and had the highest correlation with the amorphous Si of non-rhizosphere soil. The adsorbed Si of rhizospheric soil had a positive and significant correlation with the Si fractions of rhizosphere and non-rhizosphere soil and had the highest correlation with the adsorbed Si of non-rhizosphere soil (Table 2). The amorphous Si of rhizosphere soil had a positive and significant correlation with the Si fractions of rhizosphere and non-rhizosphere soil and had the highest correlation with the plant available Si of non-rhizosphere soil. In general, according to the correlations among the studied traits (Table 2), it can be concluded that with increasing the plant available Si and adsorbed Si in the rhizosphere and non-rhizosphere soil, the root dry weight, shoot and root uptake of Si and also the root uptake of P increase. In addition, with increasing the adsorbed Si of rhizosphere soil, the shoot uptake of P also increases.
Discussion
Effect of Si and PSB on wheat biomass
The potential of Si at a lower level and PSB at a wider level in improving P uptake by various plants under P poor conditions has been studied in previous studies (Etesami and Jeong 2018; Sharma et al. 2013). Recently the combined use of Si and PSB has been proposed as a practical strategy for boosting P use efficiency in sustainable agriculture (Etesami et al. 2021; Etesami 2018). Some known mechanisms by which PSB and Si can improve the uptake of P by plants have been well reviewed (Etesami et al. 2021). In the research, we also indicated that the combined use of Si and phosphate solubilizing bacterial strains could synergistically improve the uptake of P and Si by wheat plant and as a result increase plant biomass. Various levels of Si used in this study improved wheat biomass (root and shoot dry weight) in the presence and absence of the PSB. In previous studies, Si application to soil depleted of bioavailable Si also increased the plant height and shoot dry weight in plants (Yan et al. 2018; Guntzer et al. 2012). For example, it was reported that the application of Si from the source of potassium silicate (Ranjbar et al. 2019) and monosilicic acid (Kowalska et al. 2020) increased the biomass of wheat shoot.
Roots of plants provide essential functions including the uptake of water and nutrients for plant growth. An increase in the root’s surface area provides supplementary exposed sites for the uptake of diffusible ions (Barber 1995). It has been found that Si increases not only root growth (positive changes in morphological traits such as diameter, root dry bulk, area, volume, and total and main root lengths) but also the shoot dry weight of plants (Etesami and Jeong 2018; Kim et al. 2014). According to a previous study (Hattori et al. 2005), the effect of Si stimulation on plant root growth may be due to the increase in root length resulting from the increased ability of the cell wall to expand in the growth area. Increased root growth caused by Si supplement has also been reported in some studies (Etesami and Jeong 2018; Wang et al. 2015; Hameed et al. 2013). Therefore, this increase in wheat biomass in the presence of Si might be correlated with the Si-mediated increase of root system (i.e., modifications of root morphology) and as a consequence the greater absorption of nutrients (e.g., P) by the wheat plants (an increase in shoot dry weight). We also found a significant correlation between plant available Si and root uptake of Si (r2 = 0.90, P < 0.01), root uptake of Si and root dry weight (r2 = 0.86, P < 0.01), root dry weight and root uptake of P (r2 = 0.95, P < 0.01), and root dry weight and shoot dry weight (r2 = 0.83, P < 0.01) (Table 2).
Increase in root and shoot growth due to PGPB inoculation has also been reported in previous research (Etesami and Maheshwari 2018; Glick 2012). Increased shoot and root dry weight by the PSB used in this study can be attributed to the solubilization of phosphate, the production of bacterial IAA and 1-aminociclopropane-1-carboxylase (ACC) deaminase (e.g., by an increase in plant root system and greater uptake of nutrients by wheat plant) (Etesami and Maheshwari 2018). According to our previous study, strains UT1 and FA1 produced ACC deaminase (380 and 294 nmol α–ketobutyrate mg−1 h−1, respectively) and IAA (10.72 and 24.70 μg mL−1, respectively) (Rezakhani et al. 2019). It is known that PSB such as Pseudomonas and Bacillus can also improve the plant growth by producing IAA and ACC deaminase, which are mostly involved in improved plant root system and as a result greater amounts of water and nutrient (e.g., Si and P) uptake by the wheat plant (Karimzadeh et al. 2020; Sharma et al. 2013). It has also been reported that the use of PSB can also promote the growth of plant in other way such as by speeding up seed germination, improving seedling emergence, and improving root morphology (Lugtenberg et al. 2002). In a recent study, the application of PSB such as Pseudomonas baetica and Pseudomonas helmanticensis also improved P nutrition of wheat plants under P-deficit conditions and as result increased the root and shoot dry weight of the plants (Shirmohammadi et al. 2020). It is known that PSB can also increase the availability of Si to plants by the same mechanisms used for P availability (Etesami et al. 2021). For example, in the study of Valizadeh-rad et al. (2021), the use of PGPB (Pseudomonas sp. and Bacillus sp.) increased plant available Si to wheat and canola treated with Si nano-particles (Si-NPs) under water-deficit stress. We also found a significant correlation between root dry weight and root uptake of P (r2 = 0.95, P < 0.01), root dry weight and root uptake of Si (r2 = 0.86, P < 0.01), and shoot dry weight and shoot uptake of P (r2 = 0.96, P < 0.01) (Table 2). In this study, the effect of PSB on wheat plant biomass was dependent on the concentration of Si (Fig. 1A). The reason for the less effect of the Pseudomonas sp. FA1 on the wheat shoot dry weight at higher levels of Si (600 mg kg−1 Si) is unclear and needs to be further perused.
Effect of Si and PSB on shoot uptake of Si
There are numerous reports of substantial yield responses following applications of Si to crops when grown on Si-depleted soils (Berthelsen et al. 2003; Li and Delvaux 2019). According to Fig. 2A, as the concentration of Si increased, the shoot uptake of Si also increased, and this effect was intensified in the presence of PSB, Pseudomonas sp. FA1 and B. simplex UT1 (probably due to increased availability of Si by the PSB). The effect of Pseudomonas sp. FA1 and B. simplex UT1 on Si uptake by wheat plant was also not similar, and their difference in the availability of Si did not follow the special trend. At the concentration higher than 300 mg kg−1 Si, increase in shoot uptake of Si in Pseudomonas sp. FA1 bacterial treatment was lower than B. simplex UT1 bacterial treatment. However, at lower concentrations of Si (150 and 300 mg kg−1 Si), the effect of Pseudomonas sp. FA1 on shoot uptake of Si was greater than that of B. simplex UT1. Some of the possible reasons for the difference were mentioned above (e.g., their different ability to increase Si availability in soil). In the study of Valizadeh-rad et al. (2021), Pseudomonas sp. compared to Bacillus sp. had greater effect on plant available Si in rhizosphere soil of canola. The researchers attributed the difference to the different ability of these bacteria to dissolve silicate minerals.
Increase in the uptake of Si by wheat plant in this study can be attributed to increasing the availability of Si in the soil and improving the root system, which stimulates the plant to absorb the greater amount of Si from the soil (Pati et al. 2016). In a previous review study, it was reported that PSB can also increase the availability of Si to plants through similar mechanisms involved in P solubilization (Etesami et al. 2021). The results of a study (El-Leboudi et al. 2019) in a clay soil showed that following increased application of Si levels from 0 to 400 mg Si kg−1, plant available Si (up to 168 mg Si kg−1) also increased. In the study of these researchers, with increasing application of Si levels, the Si concentration of wheat shoot, from 3.5 mg g−1 in the control treatment to 7.3 mg g−1 in the treatment 400 mg Si kg−1, increased. We also found a significant correlation between root dry weight and plant available Si (r2 = 0.74, P < 0.01), plant available Si and root uptake of Si (r2 = 0.90, P < 0.01), plant available Si and shoot uptake of Si (r2 = 0.86, p < 0.01), root dry weight and root uptake of Si (r2 = 0.90, P < 0.01), and shoot dry weight and shoot uptake of Si (r2 = 0.84, P < 0.01) (Table 2).
Effect of Si and PSB on shoot uptake of P
In this study, wheat shoot uptake of P increased by increasing the level of Si to the level of 300 mg Si kg−1 in the absence of bacterial strains (Fig. 2B). In previous studies, with increasing Si levels, an increase in the amount of P absorbed by plant (Tavakkoli et al. 2011; Alovisi et al. 2014; Song et al. 2021b; Schaller et al. 2019) and in crop growth and yield (Carey and Fulweiler 2016; Li et al. 2019) was also observed. The results of El-Leboudi et al. (2019) showed that the application of Si increased the P availability to wheat, which is consistent with the results of this study. According to a recent study, combined Si-P fertilization also substantially influenced Si and P biocycling in the soil–plant system (Li et al. 2020b). Some of the known mechanisms by which Si improves the availability of P and thus its uptake by wheat plants are by (i) up-regulating the expression of transporter genes for inorganic P uptake; (ii) increasing exudation of malate and citrate; (iii) weakening the ability of P to bind the surface of iron/aluminum-oxides, and consequently enhancing P bioavailability; and (iv) altering root morphology. Root morphological alterations increase the soil volume available to the root, improve the root’s absorptive surface area and root exudates, and finally increase the absorption of nutrients such as Si and P (and other nutrients) (Etesami et al. 2021; Kostic et al. 2017; Koski-Vähälä et al. 2001). For example, it was found that Si application beneficially altered the root morphology of alfalfa seedlings, which enhanced the uptake ability of the roots to nutrients and moisture, and significantly increased root dry weight (Liu et al. 2018). These researchers also reported that Si-mediated increase of P uptake was due to increased root growth and hence enhanced P availability of soil, which eventually led to improving the efficiency of phosphate fertilizer application.
In this study, the reason for the decrease in the lower uptake of shoot P at a concentration of 600 mg Si kg−1 compared to 300 mg Si kg−1 is unclear and needs to be further perused. In some previous studies (Mali et al. 2008; Shamshiripour et al. 2021), the high concentrations of Si also decreased root length and root dry weight of Si-treated plants. However, in this study the higher concentration of Si (600 mg Si kg−1) also caused the highest shoot uptake of P in the presence of PSB strains. In general, in this study, phosphate-solubilizing bacterial strains increased P uptake by wheat plant in all Si concentrations. Phosphorous uptake by crop can be improved by enhancing P solubility in soil solution and/or decreasing P fixation in soil. PSB-mediated increase in P uptake by the wheat in this study can be attributed to the ability of these bacteria to solubilize insoluble phosphates (e.g., rock phosphate and tricalcium phosphate) as confirmed in our previous studies (Rezakhani et al. 2019, 2020). It is well known that PSB present in rhizosphere soil play an important function in determining the impact of rhizosphere on the availability and uptake of P by wheat plant (Shirmohammadi et al. 2020; Karimzadeh et al. 2020) through many process such as a decrease in the pH of the soil by producing organic and mineral acids (e.g., through complexation of carboxylic and hydroxyl groups to metal cations such as Al, Fe, and Ca), alkaline phosphatases, phytohormones and H+ protonation, which causes dissolution since the dissolution of silicates and apatites is largely dependent on pH (Wang et al. 2016), anion exchange (e.g., exchange of organic compounds and sorbed P), chelation and siderophores production (Etesami 2020).
The PSB strains used in this study also showed different ability to improve shoot uptake of P by wheat plant (e.g., higher ability of Pseudomonas sp. FA1 vs. B. simplex UT1) (Fig. 2B), which can be due to the different abilities of these strains to solubilize phosphate insoluble sources. As assayed in our previous study, the amount of P obtained from the solubilization of tricalcium phosphate and rock phosphate by B. simplex UT1 and Pseudomonas sp. FA1 was 220 and 94.90 mg L−1 and 239.68 and 164 mg L−1, respectively (Rezakhani et al. 2019). The genera Pseudomonas and Bacillus are also known as the active genera of PSB involved in this conversion (Sharma et al. 2013). According to Alzoubi and Gaibore (2012), the use of PSB such as Bacillus increased the availability of P to plant by about 30%. Also, the results of a previous study (Elhaissoufi et al. 2020) showed that the use of PSB increased the availability of P in the rhizosphere of wheat plant. In this study, the combined application of the various levels of Si (150, 300, and 600 mg kg−1 Si) in the presence of B. simplex UT1 and Pseudomonas sp. FA1 could improve the wheat shoot P concentration (data not shown) to a sufficient level (> 0.3%) (Fischer 1992). In a previous study by Mahmood et al. (2016), the dual application of Si and PGPB also showed an additional effect on improving the growth of mung bean. It is also known that Si can affect bacteria including PSB (Etesami et al. 2021). For example, in previous studies, Rangaraj et al. (2014) and Shamshiripour et al. (2021) reported the positive effect of Si from various sources on beneficial soil microbial population including PSB, nitrogen-fixing bacteria, silicate-solubilizing bacteria as well as microbial biomass carbon and nitrogen in the rhizosphere of plants. Recently, it has been also observed that soil microorganisms drive Si and P release from soil minerals (Brucker et al. 2020). In a previous study, for bacteria at the genus level, Pseudomonas abundance significantly increased with Si fertilizer (Song et al. 2021b). According to the researchers, the reason for the greater effect of Pseudomonas sp. FA1 than B. simplex UT1 on increasing plant available Si (Fig. 3) may be due to Si-induced abundance of Pseudomonas sp. FA1 in wheat non-rhizosphere soil. Furthermore, many Pseudomonas and Bacillus species including PSB strains used in this study have the ability to produce IAA, synthesize ACC deaminase, and solubilize P (Etesami and Maheshwari 2018). Thus, this might be the main mechanism by which Si fertilizer enhanced plant growth and increased soil available P (synergistic effects between Si and PSB on improving P availability).
Effect of Si and PSB on chemical forms of Si
Research concerning the effect of soil microbes and their interaction with Si fertilizers on chemical forms of Si in soils, especially in calcareous soils, is still limited. In this study, our results showed an increase in the levels of available, adsorbed, and amorphous fractions of Si with increasing the level of Si in both rhizosphere and non-rhizosphere soil (Fig. 5). These results are in agreement with the results of Valizadeh-rad et al. (2021), who noted that the application of Si (100 mg kg−1 Si-NPs, 200 mg kg−1 Si-NPs, and 200 mg kg−1 potassium silicate) to soil under cultivation of canola also increased available and adsorbed fractions of Si in rhizosphere soil of this plant. In this study, it was found that amorphous Si in the soil was higher than other Si forms. The results of Georgiadis et al. (2017) and Valizadeh-rad et al. (2021) also showed that the highest amount of Si obtained from sequential extraction was allocated to amorphous Si (more than 5.6% of total Si) while plant available Si was the lowest amount of Si form (about 0.01% of total Si), which might be released into the soil solution by dissolving iron and manganese oxides. Higher levels of Si in amorphous fraction (between 280.45 and 15,611.86 mg kg−1 soil, with its highest values in fine textured soils) in soil were also reported by Morsy (2008). In a previous study (Danilova et al. 2010), the amounts of Si in the available and adsorbed fractions were also very small in the profiles of three studied soils. In this study, Si from minerogenic amorphous silica and organically bound silicon made up the highest proportion of extractable silicon in the soils analyzed in this study (Danilova et al. 2010). However, in a previous study (A Argeaa et al. 2016), the amounts of Si in the available and adsorbed fractions were the higher than the other fractions (e.g., the amounts of amorphous Si were the lower in all soil samples in this study). It seems that the nature of parent materials in various soils can explain the various Si levels in the soils (A Argeaa et al. 2016).
Dissolved (plant available Si) Si concentration in the soil solution can be lowered by plant uptake (Riotte et al. 2018), uptake by silica-shelled microorganisms (Puppe et al. 2014) and Si adsorption to mineral surfaces (Hiemstra et al. 2007). Soil particles can adsorb dissolved silicic acid from the soil solution. Silica can also be included in sesquioxides or bound to organic matter (Danilova et al. 2010). The soil parameters that control the pool of plant available Si are still poorly documented (Caubet et al. 2020), particularly in calcareous soils. The biological properties of soils such as microorganisms (bacteria and fungi) are also known to affect Si-release from litter or minerals (Bennett et al. 2001). A recent study indicated that soil microbes could interact with Si mobility, especially soil bioavailable Si (Song et al. 2021a). In the present study, PSB strains increased the level of available Si fraction in wheat non-rhizosphere soil compared to the control (no bacterial treatment). But this increase was significant only at higher concentrations of Si (300 and 600 mg Si kg−1) compared to the controls (Fig. 5B). These findings indicate that PSB positively interact with the mobility of Si and P under higher levels of Si. In the study of Valizadeh-rad et al. (2021), PGPB significantly (P ≤ 0.01) also increased the available and adsorbed fractions of Si in the soil under canola-wheat cultivation compared to the control. These authors attributed the PGPB-mediated increase in the Si fractions to the ability of PGPB to dissolve silicate minerals. It has been reported that biogenic silicate weathering by PSB can be caused by the same mechanisms as the weathering of phosphate minerals (Brucker et al. 2020; Uroz et al. 2009).
In this study, one of the interesting results of this study was the lack of significant effect of PSB strains in improving the available fraction of Si in wheat rhizosphere soil compared to controls (no bacterial treatment) (Fig. 5A). It is known that plant available Si concentration in soil solution is affected by Si sorption/desorption (Haynes and Zhou 2020). It is well documented that Si adsorption also increases with soil pH (Miles et al. 2014). Therefore, the lower the pH (the higher the presence of protons) is, the higher the chance to desorb Si (Meunier et al. 2018). Since wheat rhizosphere soil has a lower pH (due to the release of root exudates, microbial activity, etc.) compared to wheat non-rhizosphere soil and this causes greater release of Si from the surfaces of soil material into soil solution. This rhizosphere deposits-mediated increase in Si availability seems to be a possible reason for the ineffectiveness of PSB strains in increasing the plant available fraction of Si in wheat rhizosphere soil compared to the controls (Fig. 5A). Although the statement needs further study, it is well documented that the lower the availability of nutrients in soil is, the higher the microbial solubilization of nutrients in soil (Etesami and Adl 2020b). In addition, improved wheat plant growth can induce rhizospheric mineral weathering through silicate dissolution (Hinsinger 1998) that this can also be another reason for the ineffectiveness of PSB strains in increasing the plant available fraction of Si in wheat rhizosphere soil compared to the controls (Fig. 5A).
According to previous studies (Miles et al. 2014; Meunier et al. 2018; Valizadeh-rad et al. 2021), the availability of soil Si to plant during the growing season can be a function of the solubility and amounts of all or of certain fractions of the Si present in the soil especially adsorbed Si fraction. In this study, we also found a significant correlation between plant available Si and adsorbed Si of wheat rhizosphere soil (r2 = 0.96, P < 0.01) and between plant available Si and adsorbed Si of wheat non-rhizosphere soil (r2 = 0.88, P < 0.01) (Table 2). A Argeaa et al. (2016) also found a positive correlation between the availability of soil Si to plant and adsorbed Si (R2 = 0.702) in calcareous soils.
In general, the results of this study, in confirmation of the results of our previous studies done under controlled conditions (Rezakhani et al. 2019, 2020), showed that the combined use of Si (adsorbed Si and plant available Si of non-rhizosphere and rhizosphere soil) and PSB can synergistically increase the uptake of P by the wheat plant (root and shoot) and thus lead to an increase in wheat biomass in calcareous soil conditions. In addition, it was found that the bacteria of the genus Pseudomonas and Bacillus used in this study have the potential to be used in biofertilizers because their plant growth-promoting effects were constant both under soilless conditions (Rezakhani et al. 2019, 2020) and the soil conditions used in this study. They were able to improve plant growth and supply P for wheat plant under P-deficiency conditions. The information on the distribution of different forms of Si and the availability of P following the combined use of PSB strains and Si in this study may help in better management of P fertilization in calcareous soils.
Conclusions
In the research, Si fertilization alone and in combination with the PSB strains, Pseudomonas sp. FA1 and B. simplex UT1, increased available, adsorbed, and amorphous fractions of Si in both wheat non-rhizosphere and rhizosphere soil. However, the beneficial effect of the PSB strains on plant available Si in non-rhizosphere and rhizosphere soil varied. These PSB strains showed a significant effect on improving the available fraction of Si in wheat non-rhizosphere soil compared to controls (no bacterial treatment). The dual application of Si at all levels and PSB strains increased the shoot uptake of Si and P and wheat biomass compared to control and the use of Si and PSB alone. In addition to the expected positive correlation between the plant available Si of rhizosphere soil and the measured parameters, a positive and significant correlation between adsorbed Si of wheat rhizosphere soil and the shoot uptake of P and Si (and shoot dry weight) was also observed in this study. These close relationships between extractable Si levels and P uptake by plant point to the importance of applying Si fertilizer to calcareous soils in regulating P supply for crop uptake. In general, this study concludes that supplying Si source to calcareous soils has a positive relationship with Si bioavailability, increasing the uptake of P by wheat plant inoculated with PSB to improve the growth of this plant under available P-poor conditions. However, their interaction mechanism requires further investigation in future. Nevertheless, this study provides valuable information regarding better management of P fertilization in calcareous soils.
References
Abdi D, Cade-Menun BJ, Ziadi N, Parent LÉ (2014) Long-term impact of tillage practices and phosphorus fertilization on soil phosphorus forms as determined by 31P nuclear magnetic resonance spectroscopy. J Environ Qual 43(4):1431–1441
Alexandre A, Meunier J-D, Colin F, Koud J-M (1997) Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim Cosmochim Acta 61(3):677–682
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971. https://doi.org/10.3389/fmicb.2017.00971
Alovisi AMT, Furtini Neto AE, Carneiro LF, Curi N, Alovisi AA (2014) Silicon-phosphorus interactions in soils cultivated with bean plants. Acta Sci Agron 36(1):79–86
Alzoubi MM, Gaibore M (2012) The effect of phosphate solubilizing bacteria and organic fertilization on availability of Syrian rock phosphate and increase of triple superphosphate efficiency. World J Agric Sci 8(5):473–478
Argeaa HA, Nasseem MG, Mahmoud HA, Hussein MA (2016) Assessment of silicon status in calcareous soils of Banger Elsokkar Region, Egypt. Alex Sci Exch J 37(January-March):45–53
Babu T, Tubana B, Paye W, Kanke Y, Datnoff L (2016) Establishing soil silicon test procedure and critical silicon level for rice in Louisiana soils. Commun Soil Sci Plant Anal 47(12):1578–1597
Bahari SS, Pirdashti H, Yaghoubian Y (2012) The effects of nitrogen and silicon biofertilizers on powdery mildew disease, physiological parameters and yield of wheat (Triticum aestivum L.).
Barber SA (1995) Soil nutrient bioavailability: a mechanistic approach. Wiley, New York
Bayer C, Martin-Neto L, Mielniczuk J, Pillon CN, Sangoi L (2001) Changes in soil organic matter fractions under subtropical no-till cropping systems. Soil Sci Soc Am J 65(5):1473–1478
Beheshti M, Alikhani HA, Pourbabaee AA, Etesami H, Asadi Rahmani H, Norouzi M (2021) Periphytic biofilm and rice rhizosphere phosphate-solubilizing bacteria and fungi: A possible use for activating occluded P in periphytic biofilms in paddy fields. Rhizosphere 19:100395. https://doi.org/10.1016/j.rhisph.2021.100395
Bennett PC, Rogers JR, Choi WJ, Hiebert FK (2001) Silicates, silicate weathering, and microbial ecology. Geomicrobiol J 18(1):3–19
Berthelsen S, Noble AD, Kingston G, Hurney A, Rudd A, Garside A (2003) Improving yield and ccs in sugarcane through the application of silicon based amendments. Final Report, Sugar Research and Development Corporation Project CLW009
Bowman RA (1988) A rapid method to determine total phosphorus in soils. Soil Sci Soc Am J 52(5):1301–1304
Breuer J, Herrmann L (1999) Eignung der Extraktion mit Natriumbikarbonat für die Charakterisierung von bodenbildendenProzessen. Mitteilungen Der Deutschen Bodenkundlichen Gesellschaft 91:1375–1378
Brucker E, Kernchen S, Spohn M (2020) Release of phosphorus and silicon from minerals by soil microorganisms depends on the availability of organic carbon. Soil Biol Biochem 143:107737
Carey JC, Fulweiler RW (2016) Human appropriation of biogenic silicon–the increasing role of agriculture. Funct Ecol 30(8):1331–1339
Caubet M, Cornu S, Saby NPA, Meunier JD (2020) Agriculture increases the bioavailability of silicon, a beneficial element for crop, in temperate soils. Sci Rep 10(1):1–11
Chen Z, Ma S, Liu LL (2008) Studies on phosphorus solubilizing activity of a strain of phosphobacteria isolated from chestnut type soil in China. Biores Technol 99(14):6702–6707
Cornelis JT, Delvaux B, Georg RB, Lucas Y, Ranger J, Opfergelt S (2011) Tracing the origin of dissolved silicon transferred from various soil-plant systems towards rivers: a review. Biogeosciences 8(1):89–112
Crusciol CAC, de Arruda DP, Fernandes AM, Antonangelo JA, Alleoni LRF, Nascimento CACd, Rossato OB, McCray JM (2018) Methods and extractants to evaluate silicon availability for sugarcane. Sci Rep 8(1):916. https://doi.org/10.1038/s41598-018-19240-1
Danilova A, Sauer D, Hermann L, Breuer J, Zarei M, Stahr K (2010) Development of a method for sequential extraction of Si-pools from soils. In: 19th world congress of soil science, soil solutions for a changing world, Brisbane, Australia, 1st-6th august. pp 31–34
El-Leboudi AE-S, El-Sebaay A-ES, Abd-Elrahman SH, El-Etr WM, Saad HY (2019) Effect of Silicon and Phosphorus Additions and Their Interactions on Wheat Plants Grown on a Clay Soil. Asian Soil Res J 1–10
Elhaissoufi W, Khourchi S, Ibnyasser A, Ghoulam C, Rchiad Z, Zeroual Y, Lyamlouli K, Bargaz A (2020) Phosphate solubilizing rhizobacteria could have a stronger influence on wheat root traits and aboveground physiology than rhizosphere P solubilization. Front Plant Sci 11:979
Elliott CL, Snyder GH (1991) Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J Agric Food Chem 39(6):1118–1119
Epstein E (2009) Silicon: its manifold roles in plants. Ann Appl Biol 155(2):155–160
Etesami H (2018) Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agr Ecosyst Environ 253:98–112
Etesami H (2020) enhanced phosphorus fertilizer use efficiency with microorganisms. In: Nutrient dynamics for sustainable crop production. Springer, pp 215–245
Etesami H, Jeong BR (2018) Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol Environ Saf 147:881–896. https://doi.org/10.1016/j.ecoenv.2017.09.063
Etesami H, Adl SM (2020a) Can interaction between silicon and non–rhizobial bacteria help in improving nodulation and nitrogen fixation in salinity–stressed legumes? A review. Rhizosphere 15:100229. https://doi.org/10.1016/j.rhisph.2020.100229
Etesami H, Adl SM (2020b) Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. Phyto-Microbiome in stress regulation, pp 147–203
Etesami H, Maheshwari DK (2018) Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol Environ Saf 156:225–246. https://doi.org/10.1016/j.ecoenv.2018.03.013
Etesami H, Jeong BR (2020) Importance of silicon in fruit nutrition: Agronomic and physiological implications. In: Fruit Crops. Elsevier, pp 255–277
Etesami H, Jeong BR, Glick BR (2021) Contribution of arbuscular mycorrhizal fungi, phosphate-solubilizing bacteria, and silicon to P uptake by plant. Front Plant Sci 12(1355):699618. https://doi.org/10.3389/fpls.2021.699618
Etesami H, Jeong BR, Rizwan M (2020) The use of silicon in stressed agriculture management: action mechanisms and future prospects. Metalloids in plants: advances and future prospects, pp 381–431
Fischer G (1992) Nutritional disorders of plants-development. Visual and Analytical Diagnosis, New York
Fraysse F, Pokrovsky OS, Schott J, Meunier J-D (2009) Surface chemistry and reactivity of plant phytoliths in aqueous solutions. Chem Geol 258(3–4):197–206
Gehlen M, Van Raaphorst W (2002) The role of adsorption–desorption surface reactions in controlling interstitial Si (OH) 4 concentrations and enhancing Si (OH) 4 turn-over in shallow shelf seas. Cont Shelf Res 22(10):1529–1547
Georgiadis A, Sauer D, Herrmann L, Breuer J, Zarei M, Stahr K (2013) Development of a method for sequential Si extraction from soils. Geoderma 209:251–261
Georgiadis A, Rinklebe J, Straubinger M, Rennert T (2017) Silicon fractionation in Mollic Fluvisols along the Central Elbe River, Germany. CATENA 153:100–105
Ghorchiani M, Etesami H, Alikhani HA (2018) Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agr Ecosyst Environ 258:59–70
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012
Golmohammad H, Ramezanpour H, Rezapour S (2016) Study on some soil properties as affected by different slope position and aspect in mountainous landform with different parent materials in Masouleh. Water Soil Sci 26(2–2):53–66
Guntzer F, Keller C, Meunier J-D (2012) Benefits of plant silicon for crops: a review. Agron Sustain Dev 32(1):201–213. https://doi.org/10.1007/s13593-011-0039-8
Haluschak P (2006) Laboratory methods of soil analysis. Canada-Manitoba soil survey, pp 3–133
Hameed A, Sheikh MA, Jamil A, Basra SMA (2013) Seed priming with sodium silicate enhances seed germination and seedling growth in wheat (Triticum aestivum L.) under water deficit stress induced by polyethylene glycol. Pak J Life Soc Sci 11(1):19–24
Hattori T, Inanaga S, Araki H, An P, Morita S, Luxová M, Lux A (2005) Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol Plant 123(4):459–466. https://doi.org/10.1111/j.1399-3054.2005.00481.x
Haynes RJ (2014) A contemporary overview of silicon availability in agricultural soils. J Plant Nutr Soil Sci 177(6):831–844
Haynes RJ, Zhou Y-F (2020) Silicate sorption and desorption by a Si-deficient soil–Effects of pH and period of contact. Geoderma 365:114204
Henriet C, De Jaeger N, Dorel M, Opfergelt S, Delvaux B (2008) The reserve of weatherable primary silicates impacts the accumulation of biogenic silicon in volcanic ash soils. Biogeochemistry 90(2):209–223
Hiemstra T, Barnett MO, van Riemsdijk WH (2007) Interaction of silicic acid with goethite. J Colloid Interface Sci 310(1):8–17
Hinsinger P (1998) How do plant roots acquire mineral nutrients? Chemical processes involved in the rhizosphere. Adv Agron 64:225–266
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular California agricultural experiment station 347 (2nd edit)
Jalali M, Sajadi Tabar S (2011) Chemical fractionation of phosphorus in calcareous soils of Hamedan, western Iran under different land use. J Plant Nutr Soil Sci 174(4):523–531
Jones LHP, Handreck KA (1965) Studies of silica in the oat plant. Plant Soil 23(1):79–96
Karimzadeh J, Alikhani HA, Etesami H, Pourbabaei AA (2020) Improved Phosphorus Uptake by Wheat Plant (Triticum aestivum L.) with rhizosphere fluorescent pseudomonads strains under water-deficit stress. J Plant Growth Regul 1–17
Kaur G, Reddy MS (2015) Effects of Phosphate-Solubilizing Bacteria, Rock Phosphate and Chemical Fertilizers on Maize-Wheat Cropping Cycle and Economics. Pedosphere 25(3):428–437. https://doi.org/10.1016/S1002-0160(15)30010-2
Kim YH, Khan AL, Waqas M, Shim JK, Kim DH, Lee KY, Lee IJ (2014) Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J Plant Growth Regul 33(2):137–149
Korndörfer GH, Coelho NM, Snyder GH, Mizutani CT (1999) Avaliação de métodos de extração de silício em solos cultivados com arroz de sequeiro. Rev Bras Ciênc Solo 23:101–106
Koski-Vähälä J, Hartikainen H, Tallberg P (2001) Phosphorus mobilization from various sediment pools in response to increased pH and silicate concentration. J Environ Qual 30(2):546–552
Kostic L, Nikolic N, Bosnic D, Samardzic J, Nikolic M (2017) Silicon increases phosphorus (P) uptake by wheat under low P acid soil conditions. Plant Soil 419(1):447–455
Kowalska J, Tyburski J, Jakubowska M, Krzymińska J (2020) Effect of different forms of silicon on growth of spring wheat cultivated in organic farming system. Silicon:1–7
Li Z, Delvaux B (2019) Phytolith-rich biochar: a potential Si fertilizer in desilicated soils. Gcb Bioenergy 11(11):1264–1282
Li Z, Unzué-Belmonte D, Cornelis J-T, Vander Linden C, Struyf E, Ronsse F, Delvaux B (2019) Effects of phytolithic rice-straw biochar, soil buffering capacity and pH on silicon bioavailability. Plant Soil 438(1):187–203
Li Z, Cornelis J-T, Vander Linden C, Van Ranst E, Delvaux B (2020a) Neoformed aluminosilicate and phytogenic silica are competitive sinks in the silicon soil–plant cycle. Geoderma 368:114308
Li Z, Guo F, Cornelis J-T, Song Z, Wang X, Delvaux B (2020b) Combined silicon-phosphorus fertilization affects the biomass and phytolith stock of rice plants. Front Plant Sci 11:67
Liu D, Liu M, Liu X-L, Cheng X-G, Liang Z-W (2018) Silicon Priming Created an Enhanced Tolerance in Alfalfa (Medicago sativa L.) Seedlings in Response to High Alkaline Stress. Frontiers in Plant Science 9 (716). https://doi.org/10.3389/fpls.2018.00716
Loeppert RH, Suarez DL (1996) Carbonate and gypsum. Methods of soil analysis Part 3, pp 437–474
Lugtenberg BJJ, Chin-A-Woeng TFC, Bloemberg GV (2002) Microbe–plant interactions: principles and mechanisms. Antonie Van Leeuwenhoek 81(1):373–383
Mahmood S, Daur I, Al-Solaimani SG, Ahmad S, Madkour MH, Yasir M, Hirt H, Ali S, Ali Z (2016) Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front Plant Sci 7:876
Mali M, Aery, Naresh C (2008) Silicon effects on nodule growth, dry-matter production, and mineral nutrition of cowpea (Vigna unguiculata). J Plant Nutr Soil Sci 171(6):835–840
Matichenkov VV, Bocharnikova EA (2001) The relationship between silicon and soil physical and chemical properties. In: Studies in plant science, vol 8. Elsevier, pp 209–219
Meunier J-D, Sandhya K, Prakash NB, Borschneck D, Dussouillez P (2018) pH as a proxy for estimating plant-available Si? A case study in rice fields in Karnataka (South India). Plant Soil 432(1):143–155
Miles N, Manson AD, Rhodes R, van Antwerpen R, Weigel A (2014) Extractable silicon in soils of the South African sugar industry and relationships with crop uptake. Commun Soil Sci Plant Anal 45(22):2949–2958
Milnes AR, Twidale CR (1983) An overview of silicification in Cainozoic landscapes of arid central and southern Australia. Soil Research 21(4):387–410
Morsy MA (2008) Silicon in agriculture conference. Wild Coast Sun, KwaZulu-Natal, South Africa, 26–31 October
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Narayanaswamy C, Prakash NB (2009) Calibration and categorization of plant available silicon in rice soils of South India. J Plant Nutr 32(8):1237–1254
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (ed) Methods of Soil Analysis Part 2 Chemical and Microbiological Properties. American Society of Agronomy, Soil Science Society of America, Madison, pp 403–430
Pati S, Pal B, Badole S, Hazra GC, Mandal B (2016) Effect of silicon fertilization on growth, yield, and nutrient uptake of rice. Commun Soil Sci Plant Anal 47(3):284–290
Puppe D, Kaczorek D, Wanner M, Sommer M (2014) Dynamics and drivers of the protozoic Si pool along a 10-year chronosequence of initial ecosystem states. Ecol Eng 70:477–482
Rai D, Kittrick JA (1989) Mineral equilibria and the soil system. Minerals in soil environments 1, pp 161–198
Rangaraj S, Gopalu K, Rathinam Y, Periasamy P, Venkatachalam R, Narayanasamy K (2014) Effect of silica nanoparticles on microbial biomass and silica availability in maize rhizosphere. Biotechnol Appl Biochem 61(6):668–675
Ranjbar SS, Motesharezadeh B, Moshiri F, Hosseini HM, Alikhani HA (2019) Silicon utilization efficiency of different wheat cultivars in a calcareous soil. Silicon 11(4):2159–2168
Rezakhani L, Motesharezadeh B, Tehrani MM, Etesami H, Mirseyed Hosseini H (2019) Phosphate–solubilizing bacteria and silicon synergistically augment phosphorus (P) uptake by wheat (Triticum aestivum L.) plant fertilized with soluble or insoluble P source. Ecotoxicol Environ Saf 173:504–513. https://doi.org/10.1016/j.ecoenv.2019.02.060
Rezakhani L, Motesharezadeh B, Tehrani MM, Etesami H, Mirseyed Hosseini H (2020) Effect of Silicon and Phosphate-Solubilizing Bacteria on Improved Phosphorus (P) Uptake Is Not Specific to Insoluble P-Fertilized Sorghum (Sorghum bicolor L.) Plants. J Plant Growth Regul 39(1):239–253. https://doi.org/10.1007/s00344-019-09978-x
Richard Drees L, Wilding LP, Smeck NE, Senkayi AL (1989) Silica in soils: quartz and disordered silica polymorphs. Minerals in Soil Environments 1, pp 913–974
Riotte J, Meunier J-D, Zambardi T, Audry S, Barboni D, Anupama K, Prasad S, Chmeleff J, Poitrasson F, Sekhar M (2018) Processes controlling silicon isotopic fractionation in a forested tropical watershed: Mule Hole Critical Zone Observatory (Southern India). Geochim Cosmochim Acta 228:301–319
Ryan J, Estefan G, Rashid A (2001) Soil and plant analysis laboratory manual. ICARDA
Saccone L, Conley DJ, Koning E, Sauer D, Sommer M, Kaczorek D, Blecker SW, Kelly EF (2007) Assessing the extraction and quantification of amorphous silica in soils of forest and grassland ecosystems. Eur J Soil Sci 58(6):1446–1459
Schaller J, Faucherre S, Joss H, Obst M, Goeckede M, Planer-Friedrich B, Peiffer S, Gilfedder B, Elberling B (2019) Silicon increases the phosphorus availability of Arctic soils. Sci Rep 9(1):1–11
Schwertmann U, Taylor RM (1989) Iron oxides. Minerals in soil environments 1, pp 379–438
Shamshiripour M, Motesharezadeh B, Rahmani HA, Alikhani HA, Etesami H (2021) Optimal concentrations of silicon enhance the growth of soybean (Glycine Max L.) cultivars by improving nodulation, root system architecture, and soil biological properties. Silicon 1–13
Sharif M, Khattak RA, Sarir MS (2002) Effect of different levels of lignitic coal derived humic acid on growth of maize plants. Commun Soil Sci Plant Anal 33(19–20):3567–3580
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2(1):587. https://doi.org/10.1186/2193-1801-2-587
Shirmohammadi E, Alikhani HA, Pourbabaei AA, Etesami H (2020) Improved phosphorus (P) uptake and yield of rainfed wheat fed with P fertilizer by drought-tolerant phosphate-solubilizing fluorescent pseudomonads strains: a field study in drylands. J Soil Sci Plant Nutr 20(4):2195–2211
Snyder GH (2001) Methods for silicon analysis in plants, soils, and fertilizers. In: Studies in plant science, vol 8. Elsevier, pp 185–196
Sommer M, Kaczorek D, Kuzyakov Y, Breuer J (2006) Silicon pools and fluxes in soils and landscapes—a review. J Plant Nutr Soil Sci 169(3):310–329
Song A, Li Z, Liao Y, Liang Y, Wang E, Wang S, Li X, Bi J, Si Z, Lu Y (2021a) Soil bacterial communities interact with silicon fraction transformation and promote rice yield after long-term straw return. Soil Ecol Lett 1–14
Song A, Li Z, Wang E, Xu D, Wang S, Bi J, Wang H, Jeyakumar P, Li Z, Fan F (2021) Supplying silicon alters microbial community and reduces soil cadmium bioavailability to promote health wheat growth and yield. Sci Total Environ 796:148797
Swift RS, Sparks DL (1996) Methods of soil analysis: Part 3. Chemical methods. Soil Science Society of America Book Series 5, pp 1018–1020
Tavakkoli E, Lyons G, English P, Guppy CN (2011) Silicon nutrition of rice is affected by soil pH, weathering and silicon fertilisation. J Plant Nutr Soil Sci 174(3):437–446
Uroz S, Calvaruso C, Turpault M-P, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol 17(8):378–387
Valizadeh-rad K, Motesharezadeh B, Alikhani HA, Jalali M (2021) Direct and residual effects of water deficit stress, different sources of silicon and plant-growth promoting bacteria on silicon fractions in the soil. Silicon 1–13
Waling I, Van Vark W, Houba VJG, Van der Lee JJ (1989) Soil and plant analysis, a series of syllabi: Part 7. Plant analysis procedures. Wageningen Agriculture University
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Wang S, Liu P, Chen D, Yin L, Li H, Deng X (2015) Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front Plant Sci 6:759
Wang D, Xie Y, Jaisi DP, Jin Y (2016) Effects of low-molecular-weight organic acids on the dissolution of hydroxyapatite nanoparticles. Environ Sci Nano 3(4):768–779
Yadav H, Fatima R, Sharma A, Mathur S (2017) Enhancement of applicability of rock phosphate in alkaline soils by organic compost. Appl Soil Ecol 113:80–85
Yan G-c, Nikolic M, Ye M-j, Xiao Z-x, Liang Y-c (2018) Silicon acquisition and accumulation in plant and its significance for agriculture. J Integr Agric 17(10):2138–2150
Acknowledgements
We are grateful for the financial support of the University of Tehran for doing this study.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally to this study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Responsible Editor: Christopher Guppy.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rezakhani, L., Motesharezadeh, B., Tehrani, M.M. et al. The effect of silicon fertilization and phosphate-solubilizing bacteria on chemical forms of silicon and phosphorus uptake by wheat plant in a calcareous soil. Plant Soil 477, 259–280 (2022). https://doi.org/10.1007/s11104-021-05274-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05274-4