Abstract
Aims
Here, we assess the differential impact of drought on root carbon metabolism in the widely cultivated alfalfa (Medicago sativa, Ms) and the model legume Medicago truncatula (Mt). Understanding how carbon allocation is regulated under drought stress conditions is a central issue to improving alfalfa productivity under future climate change scenarios.
Methods
Alfalfa and Medicago truncatula were compared under water deficit conditions. Root carbon metabolism of the taproot and fibrous roots was analysed. M. truncatula drought tolerance variability was compared to that of alfalfa using six accessions of the Medicago Hapmap project. The prominent taproot is much less developed in M. truncatula than in alfalfa with the former exhibiting an extensive fibrous root system.
Results
In both examined Medicago species the taproot contained the major pools of soluble protein, sucrose and pinitol, whereas the major pools of hexoses and carbon metabolism enzymes appeared to be in the fibrous roots. Under water-deficit conditions, the response of M. sativa strongly differed from that of M. truncatula at the root level.
Conclusions
Water deficit conditions differentially modulate the root carbon metabolism of M. sativa and M. truncatula. Mt maintained a more active carbon metabolism in the fibRs, as sucrose, myo-inositol and pinitol accumulated to cope with the water deficit (WD). Conversely, the root system of Ms did not accumulate cyclitols and carbon metabolism was more severely affected under water deficit conditions. This differentially exerted control may determine the drought response of these two close relatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is the main adverse environmental factor that affects crop productivity (Sinclair 2011; Lipiec et al. 2013). Climate change is predicted to aggravate the intensity and distribution of drought worldwide (Lobell et al. 2008; Dai 2011). Therefore, it is very important to select and develop tolerant crop varieties adapted to drought under future scenarios, as crop production needs to double over the next 50 years to meet food supply requirements for an increasing world population (Rothstein 2007; Foley et al. 2011).
The model plant barrel medic Medicago truncatula (Mt) is a cool-season legume originating from the Mediterranean basin and is now cultivated as an annual forage crop (~32 Mha) in several regions in the world, especially in Australia (Michaud 1988). In addition, this species is largely employed as a model legume for molecular studies (Barker et al. 1990; Young and Udvardi 2009). One of the most valuable resources generated for Mt is the Medicago Hapmap Project. Developed by an international consortium involving institutions in the USA and France, the project has sequenced 384 inbred lines spanning the range of Medicago diversity using Illumina next-generation technology (Stanton-geddes et al. 2013). Due to the Mediterranean-wide origin of the collection, a region experiencing annual cycles of drought, the M. truncatula Hapmap accessions provide a rich source of drought tolerance variability (Yoder et al. 2014). In this context, 220 drought-adaptive traits of the Hapmap collection were characterized by in vitro leaf dehydration assays using 25% polyethylene glycol (PEG) 8000, revealing significant differences among accessions (Kang et al. 2015).
Medicago sativa (Ms) is a common forage legume worldwide and is economically important in the temperate European region. Alfalfa may be grown as an irrigated or rainfed forage crop depending on the region, although as a rainfed crop, it faces environmental constraints and is marginally economical. Although alfalfa is a close relative of Mt, there are significant shoot and root anatomical differences between them, which may determine their differential drought tolerances (Castañeda et al. 2019). Regarding their underground organs, alfalfa has a large taproot (Radovic et al. 2009; Araújo et al. 2015; Quan et al. 2016), while Mt is defined by a well-developed fibrous root system (Zhang et al. 2014; Castañeda et al. 2019).
Drought tolerance mechanisms have been explored in different alfalfa varieties with a major focus on shoot strategies (Kang et al. 2011; Zhang et al. 2019) with attention given to the roots only recently (Soba et al. 2019; Zhang et al. 2019). However, the root system is essential for crop productivity (Paez-Garcia et al. 2015) and is the first organ to sense water deficit (WD) and, as such, has an essential signalling role. Tian et al. (2014) propose that differential growth dynamics between primary and lateral roots are crucial for plants to adapt to ever-changing environmental conditions. Lynch and Brown (2012) distinguished between taproots (tapRs) or primary roots, which are those that grow from the embryo directly downwards, and fibrous roots (fibRs) or secondary roots, which are formed post-embryonically, developing from the pericycle.
In situations where water is a limiting factor, metabolites that participate in energy production and growth are transported from shoots to roots (Gargallo-Garriga et al. 2014). Although effects on photosynthesis have received most of the attention (Chaves et al. 2009), it should be noted that upon the progressive imposition of WD stress, this process is affected long after cell growth (Hsiao and Acevedo 1974; Taiz and Zeiger 2010). At the whole-plant level, numerous studies have shown that, compared with aboveground organs, root systems show an earlier and more active response to drought (Gargallo-Garriga et al. 2014). Recently, modulation of carbon metabolism has been shown to markedly disturb the metabolism and development of Arabidopsis roots (Pignocchi et al. 2020). Castañeda et al. (2019) showed that carbon metabolism was differentially modulated in the different parts of the root system of Mt under WD conditions, sparking a new hypothesis about the role of root metabolism as a mechanism of drought tolerance. Roots use sucrose, which may be catabolized by the enzymes sucrose synthase (SuSy) and alkaline invertase (INV), as the main form of carbon transport (Koch 1996; Ciereszko and Kleczkowski 2002). SuSy is a cytosolic reversible glycosyltransferase that degrades sucrose into UDP-glucose and fructose in the presence of UPD, controlling the partitioning of sucrose into various metabolic, structural, and storage pools within plant cells (Zrenner et al. 1995; Dejardin et al. 1997; Sturm 1999; Haigler 2001). Conversely, INV irreversibly hydrolyses sucrose into glucose and fructose (Winter and Huber 2000) and is involved in sucrose catabolism in nitrogen-fixing root nodules (Morell and Copeland 1984; Barratt et al. 2009; Ruan et al. 2010). Sucrose metabolism has been extensively analysed in the last decade, but the regulation of carbon reallocation is still not fully understood and is a central issue to improve plant productivity under abiotic stress such as drought (Ruan 2014).
Although the root is the first organ to sense drought, the role of root carbon metabolism on carbon partitioning at the whole plant level has been scarcely explored in forage plants. We hypothesize that root carbon metabolism plays a role in the drought tolerance of Medicago forage species. For this purpose, we first explore Mt drought tolerance variability in a collection of accessions of the Hapmap Project. Second, we discuss the existing differences between Ms and Mt in root system morphology and explore carbon metabolism in the root systems of both Medicago species exposed to moderate water deficit conditions.
Materials and methods
Plant material and growth conditions
M. sativa seeds of the commercial variety Sitel were used. M. truncatula seeds were obtained through the M. truncatula germplasm request service of the Hapmap Project (http://www.medicagohapmap.org/hapmap/germplasm). Kang et al. characterized 220 drought-adaptive traits in the Medicago truncatula Hapmap Project collection. These researchers performed in vitro leaf dehydration assays using 25% polyethylene glycol (PEG) 8000, revealing significant differences among accessions (Kang et al. 2015). We selected six M. truncatula (Mt) accessions based on the abovementioned PEG assays exhibiting from 2 to 41% leaf biomass reduction (Mt. IDRA (2%), Mt. HM267 (10.4%), Mt. HM290 (15%), Mt. HM268 (20.2%), Mt. HM287 (30%) and Mt. HM307 (41.4%). First, progressive water deficit conditions were applied to late-vegetative-stage (8-week-old) M. truncatula accessions to evaluate the tolerance to water deficit among them and in relation to that of M. sativa plants. Based on this screening, the Mt. HM307 accession was selected for further comparisons with M. sativa, since they reported a similar response to WD in terms of growth and time required to reach −1.8 MPa water potential values.
Seeds were scarified with 98% sulfuric acid for 7 min, washed several times and then sterilized with 3.5% sodium hypochlorite for 90 s. After that, seeds were washed again approximately 10 times, soaked in water and shaked for 3 h. When the seeds were hydrated, they were transferred to 7% agar plates at 4 °C for one day in the dark and then incubated at 20 °C for two days. After germination, seeds were sown in 1-L pots containing a mixture of perlite:vermiculite (2:5, v/v) under controlled environmental conditions (14 h photoperiod; 400 μmol m−2 s−1 light intensity; 22 °C/16 °C day/night temperature; 60 to 70% relative humidity). Plants were irrigated to field capacity 3 times a week with Evans nutrient solution containing (values in mg L−1) the following: MgSO4·7H2O (493), K2SO4 (279), K2HPO4 (145), CaCl2 (56), KH2PO4 (23), EDTA-Fe (17), H3BO3 (1.43), CaSO4·2H2O (1.03), MnSO4·7H2O (0.77), ZnSO4·7H2O (0.22), CoCl2·6H2O (0.12), CuSO4·5H2O (0.08), and NaMoO4·2H2O (0.05); (Evans, 1981). This nutrient solution was supplemented with 5 mM NH4NO3.
Eight-week-old plants were randomly separated into 2 treatments, control and water deficit (WD), containing 4 biological replicates each. Controls (C) were irrigated daily with Evans nutrient solution to field capacity, and WD was imposed by withholding water. The hydric status of water-deprived and well-watered plants was monitored daily by measuring the water potential of the leaves of different plants. Water-stressed and control plants were harvested simultaneously when the desired water potential of the leaves was reached (≈ −1.8 MPa). The harvesting period lasted for 7 to 19 days if we considered all Mt species (Fig. 1) and for 7 days if we considered the Ms and Mt. HM307 species comparison (Figs. 2, 3, 4 and 5). The whole tapRs and fibRs were harvested, immediately frozen in liquid nitrogen and stored at −80 °C for various analyses. A tissue aliquot of each root type was used for dry weight (DW) determinations after drying for 48 h at 70 °C. Water content was calculated using the following equation: WC (%) = (FW-DW) /(FW*100) where FW represents the fresh weight (FW).
(a) Days until wilt in water deficit-treated plants (defined at −1.8 MPa). (b) Shoot and (c) fibR dry weight biomasses in control and water deficit-treated plants in the Hapmap Project accession experiment. Bars represent the mean ± SE (n = 4). Different letters indicate differences according to Duncan’s test (P ≤ 0.05). Significant differences between the control and water deficit treatments are indicated with an asterisk (Student’s t test; p < 0.05)
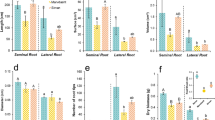
Dry weight (DW) biomass and water content of (a,c) taproots and (b,d) fibrous roots of Medicago sativa (Ms) and Medicago truncatula HM307 (Mt. HM307) under control (C) and water deficit (WD) conditions. Control and WD-treated plants were harvested when WD-treated plants reached MD conditions (Ψw, −1.8 MPa). Bars represent the mean ± SE (n = 4). Different letters indicate differences according to Duncan’s test (P ≤ 0.05). (e) Visual appearance of Medicago sativa and Medicago truncatula taproots (tapR) and fibrous roots (fibR) in control conditions
Concentrations of (a-b) sucrose, (c-d) glucose and (e-f) fructose contents (μmol g DW−1) of Medicago sativa (Ms) and Medicago truncatula HM307 (Mt. HM307) taproots and fibrous roots under control and moderate water deficit conditions. Control and WD-treated plants were harvested when WD-treated plants reached MD conditions (Ψw, −1.8 MPa). Bars represent the mean ± SE (n = 4). Different letters indicate differences according to Duncan’s test (P ≤ 0.05). WD*S indicates the interactive effect between the water deficit treatment and species
Changes in (a-b) myo-inositol and (c-d) pinitol contents (μmol g DW−1) of Medicago sativa (Ms) and Medicago truncatula HM307 (Mt. HM307) taproots and fibrous roots under control and moderate conditions. Control and WD-treated plants were harvested when WD-treated plants reached MD conditions (Ψw, −1.8 MPa). Bars represent the mean ± SE (n = 4). Different letters indicate differences according to Duncan’s test (P ≤ 0.05). WD*S indicates the interactive effect between the water deficit treatment and species
Total soluble protein of the (a) taproots and (b) fibrous roots of Medicago sativa (Ms) and Medicago truncatula HM307 (Mt. HM307) under control and moderate water deficit conditions. Control and WD-treated plants were harvested when WD-treated plants reached MD conditions (Ψw, −1.8 MPa). Carbon metabolism-related enzymatic activities (measured as nmol NADH min−1 μg prot−1) of UDP-SuSy (c-d), INV (e-f), and G6PDH (g-h) measured in the taproots and fibrous roots of Medicago sativa and Medicago truncatula HM307 under control and moderate water deficit conditions. Bars represent the mean ± SE (n = 4). Different letters indicate differences according to Duncan’s test (P ≤ 0.05). UDP-SuSy, UDP-glucose synthase; INV, alkaline invertase; G6PDH, glucose-6-phosphate dehydrogenase. WD*O indicates the interactive effect of the organ and water deficit treatment for each species defined in brackets
Plant water status
Leaf water potential was monitored daily from the 5th day of the water stress period in the first fully expanded leaf of three randomly selected plants at 10:00 h using a pressure chamber (Scholander et al. 1965). Control plants showed a leaf Ψw value of −1.16 ± 0.06 for Ms and − 1.20 ± 0.15 for Mt. HM307 and were harvested randomly during the WD experiment. Moderate water deficit (MD)-stressed plants were collected when the leaf Ψw reached a value of −1.92 ± 0.06 for Ms and − 1.8 ± 0.09 for Mt. HM307. Water stress was monitored by daily measurements of stomatal conductance (mmol m−2 s−1) using a delta AP4 cyclic porometer. Plant transpiration (T) was measured gravimetrically daily.
Determination of soluble sugars
Ethanol extraction was performed for soluble sugar determination (Gálvez et al. 2005). Frozen aliquots of root tissue (100 mg FW) were extracted three times in 1.5 mL 80% (v/v) boiling ethanol for 30 s and once more at room temperature. All the collected supernatants were dried in a Turbovap® LV Evaporator (Zymark, Hopkinton, MA, USA) at 40 °C and 1.2 bar. After total evaporation the dry residue was resuspended in 1 mL deionized water in two steps, with ultrasonication for 10 min between both steps. After that, 2 mL of the suspension was centrifuged for 10 min (2300 g, 4 °C) and the supernatants were stored at −20 °C for future determinations. Sucrose, fructose and glucose were determined by ionic chromatography using a 940 Professional IC Vario Metrohm system (Metrosep Carb2 guard and Metrosep Carb2 150/4.0 Metrohm columns; 0.5 ml/min; 30 °C; 300 mM NaOH, 1 mM sodium acetate).
Determination of total soluble protein and enzymatic activities
Aliquots of frozen tapRs and fibRs (≈0.35 g FW) were homogenized into a fine powder with liquid nitrogen. Extraction buffer (50 mM MOPS pH 7.5, 0.1% (v/v) Triton X-100, 10 mM MgCl2, 1 mM EDTA, 20 mM KCl, 10 mM DTT β-mercaptoethanol, 2.5% PVPP, 2 mM PMSF and protease inhibition cocktail tablet) was added, and samples were centrifuged at 24,000 g (4 °C, 30 min). The protein content was determined in the crude extract per Bradford (Bradford 1976) using BSA as the protein standard.
The crude extract was desalted through a BioGel P-6 DG desalting gel (BioRad) equilibrated with desalting buffer (250 mM MOPS pH 7.5, 50 mM MgCl2, 100 mM KCl) to determine the activity of UDP-sucrose synthase (SuSy, EC 2.4.1.13), alkaline invertase (INV, EC 3.2.1.26) and glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49). SuSy and INV were assayed per Gonzalez et al. (1998), whereas G6PDH was determined per Gibon et al. (2004). All the enzyme activities were assayed spectrophotometrically at 30 °C and 340 nm for 10 min in their respective assay media. Enzymatic activities were expressed on a protein basis.
Statistical analysis
The results were examined using two-factor analysis of variance (P ≤ 0.05) and Duncan’s multiple range test. Student’s t test was employed when a single comparison was needed. All data are shown as the mean ± standard error of n = 4–5 independent measurements. Each figure legend shows the different biological replicates used in each analysis. Two-way ANOVA was performed to analyse the interactions of the accessions (Ms, Mt. HM307) and organs (tapRs and fibRs) with the WD treatment. The ANOVA results are presented in Supplementary Table 1.
Results
The WD effect on shoot and root dry weight and the days until wilt were determined to confirm the differential tolerance of Hapmap M. truncatula accessions and M. sativa under MD conditions (Ψw ≈ −1.8 MPa) (Fig. 1). On one side, Mt. IDRA, which exhibited higher tolerance in the in vitro PEG assays (2% leaf DW reduction), reached MD conditions 11.80 ± 1.40 days after irrigation was withheld. On the other side, Mt. HM307, which exhibited lower tolerance in the in vitro PEG assays (40% leaf DW reduction), reached MD conditions 7.00 ± 0.01 days after irrigation was withheld (Fig. 1a). However, regarding the shoot biomass reduction induced by the WD conditions, Mt. IDRA was markedly affected by WD stress while Mt. HM307 maintained biomass production at control levels (Fig. 1b). The Mt accessions exhibiting an intermediate response to water deficit in the in vitro PEG assays (Mt. HM287, Mt. HM268, Mt. HM290, Mt. HM267) and, showed a variable response in biomass production and days until wilt, which did not correlate with the tolerance range established by Kang et al. (2015). The response of Ms was similar to that of Mt. HM307, as it reported 7.75 ± 1.06 days to reach MD conditions and stable shoot biomass under WD conditions (Fig. 1b). Therefore, the Mt. HM307 accession was employed for further comparative studies with Ms.
Transpiration (T) and stomatal conductance (gs) were monitored throughout the time-course study. Figure S1 represents the reduction of both parameters in the WD-treated plants compared to the controls (percentage) during the first four days of WD treatment. Stomatal conductance decreased progressively in both species along with the establishment of WD stress during the initial days of the treatment. On day 2, Mt. HM307 showed a substantial reduction in gs and T, while the Ms plants maintained mostly open stomata and were only significantly affected after 3 days (Fig. S1). During the following days of the WD time-course, T and gs significantly declined in both Ms and Mt. HM307 species without any significant differences among them (Fig. S1).
As mentioned above, MD-stressed plants entailed a decline of approximately 0.6–0.8 MPa with respect to control plants. Days to reach this WD level were monitored and similar values were observed between species i.e., periods of 7.75 ± 1.05 for Ms and 7.00 ± 0.01 days for Mt. HM307 (Fig. 1a).
Under MD, the shoot biomass of Ms and Mt. HM307 was unaltered (Fig. 1). In the roots, although an increasing trend was observed in both Ms and Mt. HM307, no significant difference was detected between the control and MD treatments in any root type (Fig. 2a, b). The fibR:tapR DW ratio was significantly higher in Mt. HM307 (12.21 ± 0.83) than in Ms (3.94 ± 0.77). The relevance of the Ms tapR is well known while this part of the root is less developed in Mt, with fibRs being the main organ type (Fig. 2e).
Water content was significantly affected in all the organs examined with a similar pattern in the two species (Fig. 2c, d). However, tapRs maintained a higher water content under MD (close to 50%), while fibRs showed a water content decline of approximately 30% in both species (Fig. 2c, d). Two-way ANOVA showed a significant interaction between WD treatment and organs in Ms (Supplementary Table 1).
Important differences were observed when comparing the tapRs with the fibRs in terms of the concentration of neutral sugars in well-watered plants. Regardless of the species, the sucrose concentration was up to four times higher in tapRs than in fibRs, while conversely, the glucose and fructose concentrations tended to be higher in fibRs (Fig. 3). MD stress induced the accumulation of sucrose in the roots, but a differential pattern was observed in Ms compared to that in Mt. HM307. In Ms, sucrose accumulation occurred mainly in the tapRs where the concentration increased 100% in MD plants compared to controls. Conversely, in Mt. HM307, the main accumulation occurred in the fibRs, where the concentration of sucrose in WD-treated plants was triple the values of the controls (Fig. 3a, b). Two-way ANOVA indicated a significant interaction between the WD treatment and species in terms of the sucrose concentration of fibRs (Supplementary Table 1). For hexoses, glucose and fructose were significantly accumulated in the fibRs and tapRs of Mt. HM307, respectively.
The concentrations of polyalcohols in roots of well-watered plants tended to be higher in the tapRs than in fibRs, with the major difference being the pinitol concentration (Fig. 4c, d). The concentration of pinitol in Ms and Mt. HM307 tapRs was similar, but it significantly increased in the fibRs of Mt. HM307 under WD conditions. Mt. HM307 showed a significant accumulation of myo-inositol and pinitol in the fibRs, whereas Ms did not show any response. Two-way ANOVA showed a significant interaction between WD treatment and species in terms of myo-inositol and pinitol at the fibR level (Supplementary Table 1). Soluble protein was usually higher in the tapRs than in the fibRs, with this difference particularly marked for Ms, with a value 12 mg g DW−1 (Fig. 5a, b). Mt. HM307 showed a lower soluble protein content in the tapRs at approximately 8 mg g DW−1 than did Ms, but even so, it was higher than that of the fibRs (Fig. 5a, b). MD only modulated the total soluble protein content in the Ms roots, with significant differences only at the tapR level. Conversely, the root soluble protein content of Mt. HM307 was completely unaltered under MD stress (Fig. 5a, b).
With regard to carbohydrate metabolization at the root level, Ms and Mt. HM307, showed similar sucrose-degrading activities in the tapRs, except for UDP-SuSy, which significantly decreased in Mt. HM307 (Fig. 5c, e). In the fibRs, sucrose degradation by UDP-SuSy significantly decreased in both Ms and Mt. HM307 (Fig. 5d). Conversely, INV activity increased significantly in Ms fibRs (Fig. 5f). Furthermore, INV levels in the fibRs were significantly higher than those in the tapRs under control conditions (Fig. 5e, f); therefore, ANOVA showed a significant interaction between WD and organs in both Ms and Mt. HM307 (Supplementary Table 1). G6PDH, a crucial enzyme in the pentose phosphate pathway, was significantly higher in Ms fibRs under control conditions (Fig. 5g, h). Thus, G6PDH significantly decreased in Ms fibRs under MD conditions (Fig. 5h), showing a significant interaction between the WD treatment and organs (Supplementary Table 1).
Discussion
Alfalfa (Medicago sativa L.) is a major perennial forage legume crop of agronomical importance in temperate regions and is widely grown in arid and semiarid regions. Although compared to other crops, alfalfa exhibits a substantial drought avoidance strategy linked to its capacity to explore deep soil layers, crop productivity is still affected by drought (Quan et al. 2016; Huang et al. 2018). M. truncatula (Mt) species far exceed M. sativa (Ms) in terms of drought tolerance capacity, being naturally present in the Mediterranean basin and cultivated annually in several regions worldwide (Aubert et al. 2006; Phan et al. 2007). The M. truncatula Hapmap project provides a rich source of drought tolerance variability (Yoder et al. 2014) that needs to be explored. Based on PEG in vitro assays, Kang et al. (2015) established the drought tolerance of 220 accessions of the Hapmap Project. We selected six of these accessions with differential drought tolerances to test their response to progressively applied and moderate WD conditions in late vegetative stage plants to simulate field conditions. Our results showed a lack of correlation with the in vitro studies performed by Kang et al. (2015) which may be related to the assay system employed in each study. Therefore, Mt. IDRA, which was unaffected under in vitro PEG assays, exhibited a 50% reduction in shoot biomass when moderate WD was applied progressively at the late vegetative stage in a growth chamber (Fig. 1b). Conversely, Mt. HM307, which was characterized as nontolerant based on in vitro PEG studies (Kang et al. 2015), maintained stable biomass production under moderate WD conditions (Fig. 1b). In addition, Mt. HM307 and Ms exhibited a similar WD response pattern at the physiological level (Figs. S1, 1 and 2). Based on this screening, we compared the WD response of root metabolism in Mt. HM307 and Ms to explore the differential strategies of these closely related forage legumes at the root system level.
Ms is a perennial forage legume and, therefore, possesses a deep taproot with limited fibrous roots (Humphries and Auricht 2001; Radovic et al. 2009; Araújo et al. 2015; Quan et al. 2016). Conversely, Mt. HM307 develops branched tapRs in the upper part of the root system with abundant fibRs that explore the surrounding soil (Zhang et al. 2014; Castañeda et al. 2019). These size differences are observable in the present comparison, wherein Ms showed significantly higher tapRs, and Mt. HM307 showed more fibRs biomass (Fig. 2a, b, e). MD conditions did not significantly promote root growth, but a slight increase was observed in both species and root types (Fig. 2a, b). Although an increase in the root elongation rate has been widely described in response to drought stress (Chaves et al. 2003; Kang et al. 2011; Araújo et al. 2015; Zhang et al. 2015; Quan et al. 2016), it must be noted that a moderate water deficit over a short time period was assayed in the present study.
The tapRs of Ms have been extensively studied and are described as the main storage organ containing the most important soluble nitrogen pools (Erice et al. 2007). The annual life cycle of Mt. HM307 may explain why these tapRs are only 50% of the size of those of alfalfa (Fig. 2a) and have much less soluble protein (Fig. 5a). Although taproot proteins are known to be used for the regrowth of aerial parts under optimal conditions, drought induced a marked reduction in this nitrogen reserve in alfalfa (Fig. 5a; Erice et al. 2007) being unaffected in Mt, which supports the higher drought tolerance of this latter species. Overall, a comparison between the different root types has not been undertaken in alfalfa, and studies on forage legumes are generally scarce. Concerning the different root types of Mt, Castañeda et al. (2019) remarked that tapRs showed a higher resilience than fibRs towards water deficit stress, reporting a key role of this primary root in carbon partitioning. Our data reported similar differences in WC in the tapRs and fibRs of both species under WD conditions (Fig. 2c, d). TapRs of Ms and Mt. HM307 was less affected than fibRs in terms of the WC, showing values of approximately 50% in the tapRs that decreased to values of approximately 30% in the fibRs (Fig. 2c, d). Several factors may play a role in this response, as the higher surface to volume ratio of the fibRs favours the dehydration of this tissue under soil water restrictions. On the one hand, the thickness of the root cortex and the suberization characteristics of the exodermis determine the tissue hydraulic conductivity (Rieger and Litvin 1999) favouring water retention by tapRs compared to fibRs. In addition, the dispersion of fibRs in the soil exposes them to dehydrating conditions, creating a protective environment around the main tapR (Ye et al. 2018). This differential resilience throughout the root system together with the particular anatomy of alfalfa compared to that of Mt. HM307, makes it particularly important to examine how primary root metabolism is modulated by WD stress. Maize genotypes with reduced cortex thickness showed improved yield and growth under water stress, opening new strategies in breeding to improve the drought tolerance of cereals (Chimungu et al. 2014).
Figure 6 summarizes the distribution of carbon metabolites and enzymes between the tapRs and fibRs in Ms and Mt. HM307. In agreement with Castañeda et al. (2019), tapRs contain the main pool of sucrose, whereas hexoses such as glucose and fructose are mainly located in fibRs (Fig. 6), suggesting a high rate of sucrose metabolization in this root type. Overall, this pattern is in agreement with the distribution of carbon metabolism enzyme activities, which were mostly concentrated in fibRs. In root tissue, sucrose is rapidly hydrolysed by invertase into hexoses or cleaved by SuSy into UDP-glucose and fructose to power and support the growth of the roots (Ruan 2014). Although, the role of sucrose cleavage has been traditionally assigned to SuSy, emerging evidence indicates that INV is required for root growth and cell development (Barratt et al. 2009; Welham et al. 2009). Indeed, comparing the Medicago species, the hexose content seems to be well correlated with INV and G6PDH enzyme activities in the fibRs. In the A17 Mt accession, Castañeda et al. (2019) linked the high sucrose content of tapRs to the higher SuSy activity in this root type. However, SuSy seems to be equally distributed among tapRs and fibRs in alfalfa as it was in Mt. HM307 in the present study, suggesting a similar role in carbon partitioning for both root types.
Distribution of enzymatic activities, and metabolite and protein contents between both root types. Bars represent the log2 of the taproot:fibrous root ratio for each parameter under control conditions. Those parameters more abundant in the taproots (taproots:fibrous root ratio higher than 1.5) were marked as positive, and those more abundant in the fibrous roots (taproots:fibrous root ratio lower than −1.5) were marked as negative. Medicago sativa (Ms) and Medicago truncatula HM307 (Mt. HM307) are labelled with blue and orange, respectively. Control and WD-treated plants were harvested when WD-treated plants reached MD conditions (Ψw, −1.8 MPa). Asterisks indicate significant differences for a given parameter between the values of the taproots and the fibrous roots (Student’s t test, P ≤ 0.05)
Under WD conditions, the carbon metabolism of the root system significantly differed among the studied species (Fig. 7). Ms accumulated sucrose mainly at the tapRs whereas Mt. HM307 restricted its accumulation to fibRs (Fig. 3b). In other genotypes of Mt, Castañeda et al. (2019) observed sucrose accumulation in both root types, and linked this to a blockage of SuSy activity in both organs. In addition, the increase in INV activity observed in the fibRs of Ms may suggest that carbon metabolism of this species is more affected than that of Mt (Fig. 5f). Indeed, a maintenance role has been assigned to INV in those cases when the activities of other sucrose metabolizing enzymes, such as SuSy, are low (Winter and Huber 2000; Barratt et al. 2009). Additionally, cytosolic invertases have been demonstrated to play a role in stress responses involved in reactive oxygen species homeostasis (Xiang et al. 2011). On the other hand, the decrease in G6PDH, a key enzyme of the pentose phosphate pathway, suggests that the carbon supply to fibRs in Ms is affected at these downstream steps of catabolism (Fig. 5h). In Mt. HM307, carbon metabolism decreased markedly, as represented by the SuSy activity level in the whole root system, but sucrose accumulation was limited to fibRs (Fig. 5c, d, 3a, b). In nitrogen-fixing legumes, the presence of nodules may provoke a sink effect modulating carbon allocation in the whole root system and explaining the better performance under water deficit stress observed in different species (Lodeiro et al. 2000; Frechilla et al. 2000; Kirova et al. 2008). Carbon metabolism has been shown to be rapidly blocked in nodules (Gonzalez et al. 1998; Gálvez et al. 2005) and carbon competition between roots and nodules may determine root development under water deficit stress. Indeed, the carbon metabolism of alfalfa nodules seems to be less affected by drought than that in other legumes (Naya et al. 2007), which may limit carbon availability for the root system.
(a) Graphical overview of the water deficit response at the different root systems in Medicago sativa, Mt.HM307 and Mt.IDRA. (b) A general overview of carbon metabolism in the root. INV, alkaline invertase; F6P, fructose-6-phosphate; F1,6P2, fructose-1,6-biphosphate; G1P, glucose-1-phosphate; G6P, glucose-6-phosphate; G6PDH, glucose-6-phosphate dehydrogenase; RFOs, raffinose family oligosaccharides; Susy, sucrose synthase; UDP, uridine diphosphate; UDPG, UDP-glucose; UDPGPP, UDP-glucose pyrophosphorylase; 2-OG, 2-oxoglutarate. ETC, Electron Transport Chain; TCA, Tricarboxylic acid cycle
D-pinitol and its precursor, myo-inositol, are natural compounds commonly found in most plants (Al-Suod et al. 2017), although legumes are the major natural source (Smith and Phillips 1982; Lahuta et al. 2018). These compounds play a particularly important role in cell functioning, osmoregulation and antioxidation (Loewus and Murthy 2000; Al-Suod et al. 2017). Thus, myo-inositol-derived galactinol and raffinose family oligosaccharides (RFOs), can also function as antioxidants or stress-signalling response molecules that are important in stress tolerance (Valluru and Van den Ende 2011). Indeed, it is known that myo-inositol, sucrose and RFOs, are intimately connected in a regulatory circuit under sugar starvation (Valluru and Van den Ende 2011). This sugar regulatory complex may help plants face different stresses when sugars are limited, contributing to plant growth and homeostasis (Valluru and Van den Ende 2011). In Medicago roots, tapRs are the main reservoir of myo-inositol, and particularly pinitol (Figs. 4, 6). In addition, pinitol was found to be a good drought marker in Mt. HM307, accumulating significantly in the fibRs but remaining stable in alfalfa (Fig. 4d, 7). The differences in the activation of cyclitol metabolism in the roots between Ms and Mt. HM307 may be explained by the nature of the species, as they are cultivated and wild species, respectively. Although in this study no relevant differences in drought tolerance were observed among Mt. HM307 and Ms at moderate water stress levels, the accumulation of myo-inositol and pinitol in the fibRs of Mt. HM307 under WD conditions may represent one of the mechanisms acquired over time by Mt that make this species more WD tolerant than Ms under more severe stress conditions.
Conclusions
The M. truncatula Hapmap project provides a rich source of drought tolerance variability that needs to be explored. The progressive water deficit applied under field-like growth conditions in the present study shows the complexity of the drought tolerance variability exhibited in the M. truncatula Hapmap accessions.
Carbon metabolism is finely modulated in Medicago species with sucrose catabolizing enzymes controlling carbon partitioning within the root system. This control is differentially exerted in alfalfa and Mt at the taproot and fibrous root levels (Fig. 7); therefore, it may determine the drought responses of these two close relatives. Mt maintained a more active carbon metabolism in the fibRs, as sucrose, myo-inositol and pinitol accumulated in response to the WD. Conversely, the root system of Ms did not accumulate cyclitols and carbon metabolism was more severely affected under water deficit conditions.
Abbreviations
- C:
-
control
- DW:
-
dry weight
- G6PDH:
-
glucose-6-phosphate dehydrogenase
- fibRs:
-
fibrous roots
- FW:
-
fresh weight
- g s :
-
stomatal conductance
- INV:
-
alkaline invertase
- MD:
-
moderate deficit
- Ms :
-
Medicago sativa
- Mt :
-
Medicago truncatula
- SuSy:
-
sucrose synthase
- T:
-
transpiration
- tapRs:
-
taproots
- WC:
-
water content
- WD:
-
water deficit
References
Al-Suod H, Ligor M, Rațiu IA et al (2017) A window on cyclitols: characterization and analytics of inositols. Phytochem Lett 20:507–519. https://doi.org/10.1016/j.phytol.2016.12.009
Araújo SS, Beebe S, Crespi M, Delbreil B, González EM, Gruber V, Lejeune-Henaut I, Link W, Monteros MJ, Prats E, Rao I, Vadez V, Patto MCV (2015) Abiotic stress responses in legumes: strategies used to cope with environmental challenges. CRC Crit Rev Plant Sci 34:237–280. https://doi.org/10.1080/07352689.2014.898450
Aubert G, Morin J, Jacquin F, Loridon K, Quillet MC, Petit A, Rameau C, Lejeune-Hénaut I, Huguet T, Burstin J (2006) Functional mapping in pea, as an aid to the candidate gene selection and for investigating synteny with the model legume Medicago truncatula. Theor Appl Genet 112:1024–1041. https://doi.org/10.1007/s00122-005-0205-y
Barker DG, Bianchl S, Blondon F et al (1990) Medicago truncatula, a model plant for studying the molecular genetics of the rhizobium-legume symbiosis. Plant Mol Biol Rep 8:40–49. https://doi.org/10.1007/BF02668879
Barratt DHP, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM (2009) Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. PNAS 106:13124–13129. https://doi.org/10.1073/pnas.0900689106
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Castañeda V, de la Peña M, Azcárate L, Aranjuelo I, Gonzalez EM (2019) Functional analysis of the taproot and fibrous roots of Medicago truncatula: sucrose and proline catabolism primary response to water deficit. Agric Water Manag 216:473–483. https://doi.org/10.1016/j.agwat.2018.07.018
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought — from genes to the whole plant. Funct Plant Biol 30:239–264. https://doi.org/10.1071/fp02076
Chimungu JG, Brown KM, Lynch JP (2014) Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiol 166:1943–1955. https://doi.org/10.1104/pp.114.249037
Ciereszko I, Kleczkowski LA (2002) Glucose and mannose regulate the expression of a major sucrose synthase gene in Arabidopsis via hexokinase-dependent mechanisms. Plant Physiol Biochem 40:907–911. https://doi.org/10.1016/S0981-9428(02)01452-3
Dai A (2011) Drought under global warming: a review. Wiley Interdiscip Rev Clim Chang 2:45–65. https://doi.org/10.1002/wcc.81
Dejardin A, Rochat C, Maugenest S, Boutin J-P (1997) Purification, characterization and physiological role of sucrose synthase in the pea seed coat (Pisum sativum L.). Planta 201:128–137. https://doi.org/10.1007/bf01007697
Erice G, Irigoyen JJ, Sánchez-Díaz M, Avice JC, Ourry A (2007) Effect of drought, elevated CO2 and temperature on accumulation of N and vegetative storage proteins (VSP) in taproot of nodulated alfalfa before and after cutting. Plant Sci 172:903–912. https://doi.org/10.1016/j.plantsci.2006.12.013
Evans HJ (1981) Symbiotic nitrogen fixation in legume nodules. In TC Moore, ed, Research Experiences in Plant Physiology. Springer-Verlag, New York, pp 294–310
Foley JA, Ramankutty N, Brauman KA et al (2011) Solutions for a cultivated planet. https://doi.org/10.1038/nature10452
Frechilla S, Gonzalez EM, Royuela M et al (2000) Source of nitrogen nutrition (nitrogen fixation or nitrate assimilation) is a major factor involved in pea response to moderate water stress. J Plant Physiol 157:609–617. https://doi.org/10.1016/S0176-1617(00)80003-6
Gálvez L, González EM, Arrese-Igor C (2005) Evidence for carbon flux shortage and strong carbon/nitrogen interactions in pea nodules at early stages of water stress. J Exp Bot 56:2551–2561. https://doi.org/10.1093/jxb/eri249
Gargallo-Garriga A, Sardans J, Pérez-Trujillo M, Rivas-Ubach A, Oravec M, Vecerova K, Urban O, Jentsch A, Kreyling J, Beierkuhnlein C, Parella T, Peñuelas J (2014) Opposite metabolic responses of shoots and roots to drought. Sci Rep 4:1–7. https://doi.org/10.1038/srep06829
Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M (2004) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16:3304–3325. https://doi.org/10.1105/tpc.104.025973
Gonzalez EM, Aparicio-Tejo PM, Gordon AJ, Minchin FR, Royuela M, Arrese-Igor C (1998) Water-deficit effects on carbon and nitrogen metabolism of pea nodules. J Exp Bot 49:1705–1714. https://doi.org/10.1093/jxb/49.327.1705
Haigler C (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47:29–51. https://doi.org/10.1023/A:1010615027986
Hsiao TC, Acevedo E (1974) Plant responses to water deficits, water use efficiency, and drought resistance. Ag Meteorogical 14:59–84
Huang Z, Liu Y, Cui Z, Fang Y, He H, Liu BR, Wu GL (2018) Soil water storage deficit of alfalfa (Medicago sativa) grasslands along ages in arid area (China). F Crop Res 221:1–6. https://doi.org/10.1016/j.fcr.2018.02.013
Humphries AW, Auricht GC (2001) Breeding lucerne for Australia’s southern dryland cropping environments. Aust J Agric Res 52:153–169. https://doi.org/10.1071/AR99171
Kang Y, Han Y, Torres-Jerez I, Wang M, Tang Y, Monteros M, Udvardi M (2011) System responses to long-term drought and re-watering of two contrasting alfalfa varieties. Plant J 68:871–889. https://doi.org/10.1111/j.1365-313X.2011.04738.x
Kang Y, Sakiroglu M, Krom N, Stanton-Geddes J, Wang M, Lee YC, Young ND, Udvardi M (2015) Genome-wide association of drought-related and biomass traits with HapMap SNPs in Medicago truncatula. Plant Cell Environ 38:1997–2011. https://doi.org/10.1111/pce.12520
Kirova E, Tzvetkova N, Vaseva I, Ignatov G (2008) Photosynthetic responses of nitrate-fed and nitrogen-fixing soybeans to progressive water stress. J Plant Nutr 31:445–458. https://doi.org/10.1080/01904160801894988
Koch KE (1996) Carbohydrate-modulated gene expression in plants. https://doi.org/10.1146/annurev.arplant.47.1.509
Lahuta LB, Ciak M, Rybiński W, Bocianowski J, Börner A (2018) Diversity of the composition and content of soluble carbohydrates in seeds of the genus Vicia (Leguminosae). Genet Resour Crop Evol 65:541–554. https://doi.org/10.1007/s10722-017-0552-y
Lipiec J, Doussan C, Nosalewicz A (2013) Effect of drought and heat stresses on plant growth and yield: a review. 2015–2020. https://doi.org/10.2478/intag-2013-0017
Lobell D, Burke M, Tebaldi C, et al (2008) Prioritizing climate change adaptation needs for food security in 2030. 319:607–611. https://doi.org/10.1126/science.1151194
Lodeiro AR, González P, Hernández A et al (2000) Comparison of drought tolerance in nitrogen-fixing and inorganic nitrogen-grown common beans. Plant Sci 154:31–41. https://doi.org/10.1016/S0168-9452(99)00246-0
Loewus FA, Murthy PPN (2000) Myo-inositol metabolism in plants. Plant Sci 150:1–19. https://doi.org/10.1016/S0168-9452(99)00150-8
Lynch JP, Brown KM (2012) New roots for agriculture: exploiting the root phenome. Philos Trans R Soc B Biol Sci 367:1598–1604. https://doi.org/10.1098/rstb.2011.0243
Michaud R (1988) World distribution and historical development. In: Hanson A (ed) Alfalfa and alfalfa improvement, vol. 29. American society of agronomy. Madison, Wisconsin, pp. 259–291
Morell M, Copeland L (1984) Enzymes of sucrose breakdown in soybean nodules. Plant Physiol 74:1030–1034. https://doi.org/10.1104/pp.74.4.1030
Naya L, Ladrera R, Ramos J, González EM, Arrese-Igor C, Minchin FR, Becana M (2007) The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol 144:1104–1114. https://doi.org/10.1104/pp.107.099648
Paez-Garcia A, Motes CM, Scheible WR, Chen R, Blancaflor E, Monteros M (2015) Root traits and phenotyping strategies for plant improvement. Plants 4:334–355. https://doi.org/10.3390/plants4020334
Phan HTT, Ellwood SR, Hane JK, Ford R, Materne M, Oliver RP (2007) Extensive macrosynteny between Medicago truncatula and Lens culinaris ssp. culinaris. Theor Appl Genet 114:549–558. https://doi.org/10.1007/s00122-006-0455-3
Pignocchi C, Ivakov A, Feil R, Trick M, Pike M, Wang TL, Lunn JE, Smith AM (2020) Restriction of cytosolic sucrose hydrolysis profoundly alters development, metabolism, and gene expression in Arabidopsis roots. J Exp Bot 72:1850–1863. https://doi.org/10.1093/jxb/eraa581
Quan W, Liu X, Wang H, Chan Z (2016) Comparative physiological and transcriptional analyses of two contrasting drought tolerant alfalfa varieties. Front Plant Sci 6:1–16. https://doi.org/10.3389/fpls.2015.01256
Radovic J, Sokolovic D, Markovic J (2009) Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol Anim Husb 25:465–475. https://doi.org/10.2298/bah0906465r
Rieger M, Litvin P (1999) Root system hydraulic conductivity in species with contrasting root anatomy. J Exp Bot 50:201–209. https://doi.org/10.1093/jxb/50.331.201
Rothstein SJ (2007) Returning to our roots: Making plant biology research relevant to future challenges in agriculture 19:2695–2699. https://doi.org/10.1105/tpc.107.053074
Ruan Y, Jin Y, Yang Y et al (2010) Sugar input , metabolism , and signaling mediated by invertase: Roles in development , yield potential , and response to drought and heat. Mol Plant 3:942–955. https://doi.org/10.1093/mp/ssq044
Ruan YL (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65:33–67. https://doi.org/10.1146/annurev-arplant-050213-040251
Scholander PF, Bradstreet ED, Hemmingsen EA, Hammel HT (1965) Sap pressure in vascular plants: negative hydrostatic pressure can be measured in plants. Science 148:339–346. https://doi.org/10.1126/science.148.3668.339
Sinclair TR (2011) Challenges in breeding for yield increase for drought. Trends Plant Sci 16:289–293. https://doi.org/10.1016/j.tplants.2011.02.008
Smith AE, Phillips DV (1982) Influence of sequential prolonged periods of dark and light on pinitol concentration in clover and soybean tissue. Physiol Plant 54:31–33. https://doi.org/10.1111/j.1399-3054.1982.tb00572.x
Soba D, Zhou B, Arrese-Igor C, Munné-Bosch S, Aranjuelo I (2019) Physiological, hormonal and metabolic responses of two alfalfa cultivars with contrasting responses to drought. Int J Mol Sci 20. https://doi.org/10.3390/ijms20205099
Stanton-geddes J, Paape T, Epstein B, et al (2013) Candidate genes and genetic architecture of symbiotic and agronomic traits revealed by whole-genome , Sequence-Based Association Genetics in Medicago truncatula 8:1–9. https://doi.org/10.1371/journal.pone.0065688
Sturm A (1999) Invertases: primary structures, functions and roles in plant development and sucrose partitioning. Plant Physiol 121:1–7. https://doi.org/10.1104/pp.121.1.1
Taiz L, Zeiger E (2010) Plant physiology. Sinauer Associates
Tian H, De Smet I, Ding Z (2014) Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci 19:426–431. https://doi.org/10.1016/j.tplants.2014.01.007
Valluru R, Van den Ende W (2011) Myo-inositol and beyond - emerging networks under stress. Plant Sci 181:387–400. https://doi.org/10.1016/j.plantsci.2011.07.009
Welham T, Pike J, Horst I, Flemetakis E, Katinakis P, Kaneko T, Sato S, Tabata S, Perry J, Parniske M, Wang TL (2009) A cytosolic invertase is required for normal growth and cell development in the model legume, Lotus japonicus. J Exp Bot 60:3353–3365. https://doi.org/10.1093/jxb/erp169
Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol 35:253–289. https://doi.org/10.1080/10409230008984165
Xiang L, Li Y, Rolland F, van den Ende W (2011) Neutral invertase, hexokinase and mitochondrial ROS homeostasis: emerging links between sugar metabolism, sugar signaling and ascorbate synthesis. Plant Signal Behav 6:1567–1573. https://doi.org/10.4161/psb.6.10.17036
Ye H, Roorkiwal M, Valliyodan B, Zhou L, Chen P, Varshney RK, Nguyen HT (2018) Genetic diversity of root system architecture in response to drought stress in grain legumes. J Exp Bot 69:3267–3277. https://doi.org/10.1093/jxb/ery082
Yoder JB, Stanton-Geddes J, Zhou P, Briskine R, Young ND, Tiffin P (2014) Genomic signature of adaptation to climate in Medicago truncatula. Genetics 196:1263–1275. https://doi.org/10.1534/genetics.113.159319
Young ND, Udvardi M (2009) Translating Medicago truncatula genomics to crop legumes. Curr Opin Plant Biol 12:193–201. https://doi.org/10.1016/J.PBI.2008.11.005
Zhang C, Shi S, Liu Z, Yang F, Yin G (2019) Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J Plant Physiol 232:226–240. https://doi.org/10.1016/J.JPLPH.2018.10.023
Zhang JY, Cruz De Carvalho MH, Torres-Jerez I et al (2014) Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ 37:2553–2576. https://doi.org/10.1111/pce.12328
Zhang T, Yu L-X, Zheng P et al (2015) Identification of loci associated with drought resistance traits in heterozygous autotetraploid alfalfa (Medicago sativa L.) using genome-wide association studies with genotyping by sequencing. PLoS one 10:e0138931. https://doi.org/10.1371/journal.pone.0138931
Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U (1995) Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J 7:97–107. https://doi.org/10.1046/j.1365-313x.1995.07010097.x
Acknowledgements
Andres Echeverria was supported by a predoctoral fellowship from the Government of Navarra. We would like to thank Dr. Gustavo Garijo for technical assistance and the seed bank of the Medicago truncatula Hapmap Project consortium for supplying seeds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Ian Dodd.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Fig S1
(a) Percentage of transpiration (T) and (b) stomatal conductance (gs) of WD-treated plants, expressed as a percentage of the control levels in Medicago sativa (Ms) and Medicago truncatula HM307 (Mt. HM307) during the first 4 days of WD treatment which lasted for 7 days. Bars represent the mean ± SE (n = 4). The transpiration of control plants on day 1 was 97.16 ± 3.68 and 102.42 ± 5.60 g of water per g shoot DW for Ms and Mt. HM307, respectively. The stomatal conductance of control plants on day 1 was 0.34 ± 0.03 and 0.32 ± 0.02 nmol m−2 s−1 for Ms and Mt. HM307, respectively. An asterisk indicates significant differences between the control and water deficit treatments (Student’s t test, P ≤ 0.05). Different letters indicate significant differences between varieties and days 1–4 into the water deficit treatment according to Duncan’s test (P ≤ 0.05) (PDF 11 kb)
Supplementary Table 1
(DOCX 125 kb)
Rights and permissions
About this article
Cite this article
Echeverria, A., Gonzalez, E.M. Root system of Medicago sativa and Medicago truncatula: drought effects on carbon metabolism. Plant Soil 463, 249–263 (2021). https://doi.org/10.1007/s11104-021-04912-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04912-1