Abstract
Background and aims
ABA plays an important role in modulating stomatal response to drought and elevated atmospheric CO2 (e [CO2]). This study aimed to investigate the effect of e[CO2] on the response of leaf gas exchange and plant water relations of barley and tomato plants with different endogenous ABA levels to progressive soil drying.
Methods
Barley and tomato plants were grown in ambient (a[CO2], 400 ppm) and e[CO2] (800 ppm) and subjected to progressive drought stress. Wild type (WT) genotypes (Steptoe barley and AC tomato) and their ABA-deficient mutants (Az34 barley and flacca) were examined.
Results
e[CO2] sensitized the photosynthetic decline with soil drying. Soil-drying induced stomatal closure was affected by [CO2] in WT genotypes, where e[CO2] sensitized stomatal closure in barley but retarded it in tomato, whereas such effects were absent in mutants. Compared to a[CO2], e[CO2] maintained leaf water potential and improved turgor pressure except in the flacca mutant. For the WT genotypes, the stomata became less sensitive to an increase in leaf ABA concentration ([ABA]leaf) under e[CO2] than a[CO2]; while for both mutants, the stomata was predominately controlled by leaf turgor and not an increase in [ABA]leaf during soil drying.
Conclusion
Endogenous ABA level played an important role in modulating the effect of e[CO2] on stomatal response to soil drying. These findings improve our understanding of the mechanisms of stomatal control in monocot and dicot species responding to a future drier and CO2-enriched environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The opening and closure of a stomatal pore under different environmental conditions are controlled by the deformation and turgor of guard cells (Schroeder et al. 2001). Depolarization of the guard cell membrane potential induces stomatal closure at elevated atmospheric CO2 concentration (e[CO2]) (Ainsworth and Rogers 2007). Besides, abscisic acid (ABA) has been suggested to play a role in inducing stomatal closure under e[CO2] (Chater et al. 2015; Tazoe and Santrucek 2015; Engineer et al. 2016). However, to date it remains largely elusive about the relative significance of chemical signal (i.e., ABA) and hydraulic signal (i.e., leaf turgor) in modulating stomatal response to e[CO2], and further investigations are needed.
It is widely accepted that decreased stomatal conductance (gs) under drought stress is attributed to the partial stomatal closure induced by root-to-shoot chemical signaling (mainly xylem sap ABA concentration, [ABA]xylem) at mild drought (Davies and Zhang 1991; Liu et al. 2005; Yan et al. 2017). Earlier study has revealed that ABA could be synthesized in the root and transported to leaf where triggers a decrease in stomatal aperture and causes lowered transpiration rate while maintaining plant water status during progressive soil drying (Liu et al. 2003; Wilkinson and Davies 2002). Nonetheless, a study indicated that the application of external pressure caused a short term decrease in cell volume, and induced rapid ABA biosynthesis predominantly in the leaf, not in other tissues of angiosperms (Zhang et al. 2018). Likewise, some evidence supports the dominance of foliar ABA biosynthesis during drought stress (McAdam et al. 2016), as the carotenoid precursors for ABA in leaf are most abundant (Manzi et al. 2015). A recent study also documented that ABA appears to be transported predominantly from shoot to root, but a root-derived signal triggers ABA biosynthesis in the leaf (Takahashi et al. 2018).
Soil water deficit has a stronger effect on gs as compared to e[CO2], and a larger reduction in gs is caused under drought associated with e[CO2] growth environment (Leakey et al. 2006). Some studies have suggested that e[CO2] could alleviate the negative effects of drought by suppressing gs and transpiration rate, hereby maintaining a high leaf water potential (Tausz-Posch et al. 2015). However, recent evidence revealed that impaired stomatal control in response to drought stress was observed in plants grown under e[CO2] (Haworth et al. 2016). During progressive soil drying, the gs of e[CO2] plant had a delayed response to soil water deficit as compared with that of ambient CO2 (a[CO2]) plant (Yan et al. 2017). Furthermore, the gs reduction in a[CO2] tomato leaf was mostly induced by an increased [ABA]xylem at moderate soil water deficit; while the gs was primarily regulated by leaf turgor pressure at e[CO2] (Yan et al. 2017). Similarly, Liu et al. (2019) found that e[CO2] retarded the response of leaf gas exchange to progressive soil drying, and declined gs in a[CO2] tomato could be controlled by both leaf ABA concentration ([ABA]leaf) and [ABA]xylem, whereas under e[CO2], the gs response was ABA-independent at moderate drought stress. Nevertheless, whether both chemical and hydraulic signals are involved in the gs regulation under drought stress and e[CO2] environment still remains largely elusive.
In plant species, there are generally two morphological types of guard cell, either dumb-bell shape arranged parallel along the leaf longitudinally in monocots or kidney shape randomly distributed in dicots (Meidner and Mansfield 1968). Such difference in morphological feature of stomata could induce disparate physiological response to e[CO2] during progressive soil drying, and the underlying mechanisms on gs regulation could be different between monocot and dicot plants (Bunce 2004).
Therefore, the objective of this study was to investigate the effects of e[CO2] on response of leaf gas exchange and plant water relations in barley and tomato plants to progressive soil drying. For each species, two genotypes differing in endogenous ABA level were examined. The Az34 barley and flacca are ABA-deficient mutants and isogenic to Steptoe barley and AC tomato, respectively. Both mutants are impaired in the oxidation of ABA-aldehyde to ABA precursor and have reduced ABA concentrations (Sagi et al. 2002; Sharp et al. 2000; Walker-Simmons et al. 1989). The plants were grown in two atmospheric [CO2] (400 and 800 ppm) environments and subjected to progressive drought stress by withholding irrigation from the pots. Leaf gas exchange rates, plant water relations, and leaf ABA concentrations were determined during progressive soil drying. It was hypothesized that: 1) e[CO2] would modulate the response of leaf gas exchange and plant water relation differently in barley (monocot) and tomato (dicot) plants to progressive soil drying; and 2) ABA would be involved in mediating the stomatal response to drought stress and e[CO2] in the two species.
Materials and methods
Experimental setup
Pot experiments were conducted in climate-controlled greenhouses at Taastrup campus, University of Copenhagen, Denmark (55°67 N, 12°30 E). The seeds of isogenic barley (Hordeum vulgare) Steptoe (wild type, WT) and its respective ABA-deficient mutant (Az34 barley) were sown on 20th December 2017; and the seeds of isogenic tomato (Solanum lycopersicum) (WT, cv. Ailsa Craig) and its respective ABA-deficient mutant (flacca) were sown on 7th February 2018. The ABA-deficient mutants were unable to produce as much ABA as the WT genotype in response to soil drying (Holbrook et al. 2002; Martin-Vertedor and Dodd 2011). Both barley and tomato plants were grown in 4 L pots filled with 2.6 kg of peat material (Plugg-och Såjord-Dry matter ca.110 kg m−3, organic matter >95%, pH 5.5-6.5 and EC 1.5-2.5 mS cm−1). Four weeks after sowing, perlite was covered on the soil surface to minimize evaporation and fertilizers as NH4NO3 (2.8 g) and H2KPO4 (3.5 g) per pot were added together with irrigation water to avoid any nutrient deficiency.
From sowing, the plants were grown in two greenhouse cells with CO2 concentration of 400 ppm (ambient CO2, a[CO2]) and 800 ppm (elevated CO2, e[CO2]), respectively. The desired [CO2] in the cell was sustained by pure CO2 emission from a bottled tank, released in one point and distributed evenly by internal ventilation. The [CO2] in the cells was monitored every 6 s by a CO2 Transmitter Series GMT220 (Vaisala Group, Helsinki, Finland). The average daily CO2 concentration ([CO2]) in each cell during experiment are shown in Fig. 1. The climate conditions in two glasshouse cells were set at: 20/16 ± 2 °C day/night air temperature, 60 ± 2% relative humidity, 16 h photoperiod and > 500 μmol m−2 s−1 photosynthetic active radiation (PAR) supplied by sunlight plus LDE lamps. The vapour pressure deficit (VPD) in the greenhouse cells was maintained at 0.8-1 kPa.
After seedling establishment, the pots were constantly irrigated to 90% of pot holding capacity. In WT barley and Az34 barley, the soil drying treatment started at 29th January 2018. In WT tomato and flacca, the soil drying treatment started at 6th March and 15th March 2018, respectively. In each cell and genotype, four plants were well irrigated as control plants, the others (20 barley and 20 tomato plants) were subjected to progressive soil drying by withholding irrigation from pots until the gs decreased to ca. 10% of the control plants. During progressive soil drying, the drought-stressed plants were harvested five times at different soil water status; and for each genotype at each harvest, four plants were harvested.
Measurements
Soil water status
Soil water content was measured daily by weighing the pots with an Analytical Balance (Sartorius Model QA35EDE-S) at 15:30 h and expressed as the fraction of transpirable soil water (FTSW). The daily value of FTSW was estimated as ratio between transpirable soil water amount that still remained in pots and total transpirable soil water amount (TTSW). TTSW was defined as the difference of pot weight between 100% water holding capacity (i.e., 4.5 kg) and when gs of the drought-stressed plant decreased to ca. 10% of the control plant (i.e., 2.5 kg). Then FTSW was calculated as:
where WTn is the pot weight on a given date, WTf is pot weight at the time when gs of drought plant was 10% of control plant (i.e. 2.5 kg). Changes of FTSW during the experimental period in each cell and genotype are presented in Fig. 2.
Trends of fraction of transpirable soil water (FTSW) in the pots of WT barley and tomato, its ABA deficient mutant Az34 barley and flacca grown under ambient (400 ppm) and elevated (800 ppm) atmospheric CO2 concentrations during progressive soil drying. Error bars indicate standard error of the means (SE) (n = 4)
Leaf gas exchange measurement
During the progressive soil drying, leaf gas exchange rates, including net photosynthetic rate (An, μmol m−2 s−1) and stomatal conductance (gs, mol m−2 s−1) were measured daily on flag leaves for barley plants and upper canopy fully expanded leaves for tomato plants between 9:00 to 12:00 h with a portable photosynthetic system (LiCor-6400XT, LI-Cor, NE, USA). Measurements were performed on one leaf per plant at 20 °C cuvette temperature and 1500 μmol m−2 s−1 photosynthetic active radiation (PAR), and [CO2] of 400 ppm for a[CO2] and 800 ppm for e[CO2] growth environment, respectively.
Plant water relations
Midday leaf water potential (Ψl) was measured on flag leaves in barley and young fully expanded leaf in tomato (one leaflet per plant, four plants per genotype in each cell), respectively, using a scholander-type pressure chamber (Soil Moisture Equipment Corp., Santa Barbara, CA, USA). After measuring Ψl, the leaf was immediately separated into two pieces, packed in aluminum foil and frozen in liquid nitrogen. The leaf samples were then stored at −80 °C for determination of leaf osmotic potential (Ψπ) and leaf ABA concentration ([ABA]leaf). Ψπ was measured with a psychrometer (C-52 sample chamber, Wescor Crop, Logan, UT, USA) connected to a microvoltmeter (HR-33 T, Wescor, Logan, UT, USA) at 20 ± 1 °C. Leaf turgor pressure (Ψp) was calculated as the difference between Ψl and Ψπ.
Leaf ABA concentration
Leaf sample was grounded into fine powder, 27-33 mg per sample was weighed and added into a 1.5 ml Eppendorf tube. The ABA was extracted with 1.0 ml milli-Q water on a shaker at 4 °C over the night. The extracts were centrifuged at 14,000 g and 0.7 ml supernatants were collected for [ABA]leaf analysis. [ABA]leaf was determined by enzyme-linked immunosorbent assay (ELISA) using the protocol of Asch (2000).
Data analysis and statistics
The responses of An, gs, Ψl, Ψπ and Ψp to soil drying were described by a linear-plateau model (Faralli et al. 2019):
where y means An, gs, Ψl, Ψπ or Ψp, and yinitial means An max, gs max or Ψl max, Ψπ max or Ψp max, respectively; C was the FTSW threshold at which y started to diverge from yinitial for An, gs, Ψl, Ψπ or Ψp (denoted as CA, Cg, Cl, Cπ or Cp, respectively). The parameters y and C were estimated by PROC NLIN of PC SAS 9.4 (SAS Institute Inc., Cary, NC, USA, 2002-2012) and coefficient of determination (r2) was calculated. Statistical comparison of each parameter obtained from the linear-plateau regression between [CO2] treatments or genotypes within each species was performed by t-test using MedCalc statistical software 19.0.7.
The relationships between gs and [ABA]leaf/Ψl/Ψp were evaluated by linear regressions. r2 of the regression lines were calculated and statistical difference on the slopes of regression lines between a[CO2] and e[CO2] was performed by analysis of covariance (ANCOVA, FTSW as covariate).
Results
Leaf gas exchange rates
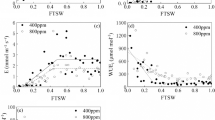
Before imposing drought stress, the net photosynthetic rate (An) of WT and Az34 barley at e[CO2] was 73.0 and 52.3% greater than those at a[CO2], respectively. In WT barley, An under e[CO2] began to decrease at a higher FTSW threshold (CA) than that under a[CO2] (i.e., 0.67 vs 0.36) during the progressive soil drying (Fig. 3a; Tables 1 and 2). While in Az34 barley, there was no notable difference in CA between the two CO2 treatments (P = 0.123) (Fig. 3b; Tables 1 and 2). The An max was similar between WT barley and Az34 barley under both a[CO2] and e[CO2] environment; whilst the CA of WT barley were higher than that of Az34 barley under a[CO2] (i.e., 0.36 vs 0.26) and e[CO2] (i.e., 0.67 vs 0.35), respectively (Fig. 3a, b; Tables 1 and 2).
Changes of net photosynthetic rate (An) and stomatal conductance (gs) of WT barley (n = 36) and its ABA deficient mutant Az34 barley (n = 40) grown under ambient (400 ppm) and elevated (800 ppm) atmospheric CO2 concentrations during progressive soil drying. Closed circles indicate plants at 400 ppm CO2 concentration, open circles indicate plants at 800 ppm CO2 concentration
Before imposing drought stress, WT and Az34 barley grown under e[CO2] had 40.0 and 23.8% lower stomatal conductance (gs) than those grown under a[CO2], respectively. In WT barley, gs under e[CO2] started to decline at a significant higher FTSW threshold (Cg) than that under a[CO2] (i.e., 0.50 vs 0.37) during progressive soil drying (Fig. 3c; Tables 1 and 2). Whereas in Az34 barley, there was no significant difference in Cg between the two CO2 treatments (P = 0.766) (Fig. 3d; Tables 1 and 2). The gs max of WT barley was 16.7 and 34.4% lower than that of Az34 barley under a[CO2] and e[CO2], respectively. While, the Cg was similar between WT barley and Az34 barley under both a[CO2] and e[CO2] environment (Fig. 3c, d; Tables 1 and 2).
Before imposing drought stress, the An max of WT tomato and flacca plants grown at e[CO2] were 55.1 and 19.0% greater than those grown at a[CO2], respectively. Compared to flacca, the An max of WT tomato was 29.4 and 7.9% lower under a[CO2] and e[CO2], respectively. During progressive soil drying, CA of WT tomato and flacca at e[CO2] were greater than that at a[CO2] (i.e., 0.38 vs 0.28 and 0.33 vs 0.21, respectively) (Fig. 4a, b; Tables 1 and 2). Compared to flacca, the CA of WT tomato was higher at a[CO2] (i.e., 0.28 vs 0.21), whereas it was similar between the two genotypes at e[CO2] (Fig. 4a, b; Tables 1 and 2).
Changes of net photosynthetic rate (An) and stomatal conductance (gs) of WT tomato (n = 48) and its ABA deficient mutant flacca (n = 32) grown under ambient (400 ppm) and elevated (800 ppm) atmospheric CO2 concentrations during progressive soil drying. The y-axis range for WT tomato gs was from 0 to 1.0, and flacca gs was from 0 to 2.0. Closed circles indicate plants at 400 ppm CO2 concentration, open circles indicate plants at 800 ppm CO2 concentration
Before imposing drought stress, gs max of WT tomato grown under e[CO2] was 12.5% lower than those grown under a[CO2]. Compared to flacca, the gs max of WT tomato was 60.4 and 63.1% lower under a[CO2] and e[CO2], respectively. During progressive soil drying, Cg of WT tomato was significantly lower when grown at e[CO2] than those grown under a[CO2] (i.e., 0.51 vs 0.62) (Fig. 4c; Tables 1 and 2). While in flacca, there was no notable difference in gs max and Cg between the two CO2 treatments (Fig. 4d; Tables 1 and 2). In addition, the Cg of WT tomato was higher than that of flacca under a[CO2] and e[CO2] (i.e., 0.62 vs 0.34 and 0.51 vs 0.29, respectively) (Fig. 4c, d; Tables 1 and 2).
Plant water relations
Before imposing drought stress, the leaf water potential (Ψl) was similar between the two CO2 growth environments for both WT and Az34 barley (Fig. 5a, b; Tables 1 and 2). In WT barley, there was no difference in Cl between the two CO2 treatments during progressive soil drying. While in Az34 barley, Ψl under e[CO2] began to decrease linearly at a lower Cl than that under a[CO2] (i.e., 0.30 vs 0.49) during progressive soil drying (Fig. 5b; Tables 1 and 2). The Ψl max and Cl of WT barley were both similar to those of Az34 barley at a[CO2]; whilst at e[CO2], WT barley had higher Ψl max (i.e., −0.49 vs − 0.67 MPa) and Cl (i.e., 0.41 vs 0.30) than those of Az34 barley, respectively (Fig. 5a, b; Tables 1 and 2).
Changes of leaf water potential (Ψl), osmotic potential (Ψπ) and turgor pressure (Ψp) of WT barley (n = 20) and its ABA deficient mutant Az34 barley (n = 20) grown under ambient (400 ppm) and elevated (800 ppm) atmospheric CO2 concentrations during progressive soil drying. Closed circles indicate plants at 400 ppm CO2 concentration, open circles indicate plants at 800 ppm CO2 concentration
Before imposing drought stress, there was no notable difference in leaf osmotic potential (Ψπ) of WT barley between the two CO2 environments (P = 0.362) (Fig. 5c; Tables 1 and 2). While for Az34 barley grown under e[CO2], Ψπ max was 0.16 MPa lower than that grown under a[CO2] (Fig. 5d; Tables 1 and 2). For WT and Az34 barley, Ψπ max under e[CO2] started to decline at significantly lower FTSW threshold (Cπ) than those under a[CO2] (i.e., 0.32 vs 0.45 and 0.20 vs 0.48, respectively) during the progressive soil drying (Fig. 5c, d; Tables 1 and 2). The Ψπ max and Cπ of WT barley were both similar to those of Az34 barley under a[CO2]; while at e[CO2], WT barley had higher Ψπ max (i.e., −1.04 vs − 1.25 MPa) and Cπ (i.e., 0.32 vs 0.20) than those of Az34 barley, respectively (Fig. 5c, d; Tables 1 and 2).
Before imposing drought stress, the leaf turgor pressure (Ψp max) in both WT and Az34 barley at e[CO2] was 33.3 and 25.0%, respectively, higher than those at a[CO2] (Fig. 5e, f; Tables 1 and 2). In WT barley, there was no significant difference in FTSW threshold (Cp) of Ψp between the two CO2 treatments; while in Az34 barley, Ψp max under e[CO2] began to decline at a lower Cp than that under a[CO2] (i.e., 0.34 vs 0.49) during progressive soil drying (Fig. 5e, f; Tables 1 and 2). The Ψp max and Cp of WT barley were both similar to those of Az34 barley under a[CO2]; at e[CO2], the Ψp max was similar between WT barley and Az34 barley, while Cp of WT barley was greater than that of Az34 barley (i.e., 0.61 vs 0.34) (Fig. 5e, f; Tables 1 and 2).
Before imposing drought stress, the Ψl was similar between the two CO2 environments in both WT tomato and flacca (Fig. 6a, b; Tables 1 and 2). In WT tomato, Ψl under e[CO2] started to decline at a lower Cl than that under a[CO2] (i.e., 0.26 vs 0.34) during progressive soil drying; whereas in flacca, there was no notable difference in Cl between the two CO2 treatments (P = 0.620) (Fig. 6a, b; Tables 1 and 2). The Ψl max of WT tomato was 0.38 and 0.44 MPa higher than that of flacca at a[CO2] and e[CO2], respectively. There was no significant difference in Cl between WT tomato and flacca under a[CO2] (P = 0.347); whereas at e[CO2], Cl of WT tomato was lower than that of flacca (i.e., 0.26 vs 0.37) (Fig. 6a, b; Tables 1 and 2).
Changes of leaf water potential (Ψl), osmotic potential (Ψπ) and turgor pressure (Ψp) of WT tomato (n = 20) and its ABA deficient mutant flacca (n = 20) grown under ambient (400 ppm) and elevated (800 ppm) atmospheric CO2 concentrations during progressive soil drying. Closed circles indicate plants at 400 ppm CO2 concentration, open circles indicate plants at 800 ppm CO2 concentration
Before imposing drought stress, the Ψπ was similar between the two CO2 environments in both WT tomato and flacca. Likewise, in both WT tomato and flacca, FTSW threshold of Ψπ (Cπ) was similar between the two CO2 treatments during progressive soil drying (Fig. 6c, d; Tables 1 and 2). The Ψπ of WT tomato was 0.19 and 0.19 MPa greater than that o f flacca under a[CO2] and e[CO2], respectively; whilst the Cπ was similar between WT tomato and flacca at each [CO2] treatment (Fig. 6c, d; Tables 1 and 2).
Before imposing drought stress, Ψp max of WT tomato grown under e[CO2] had 29.6% higher than that at a[CO2]. During progressive soil drying, the FTSW threshold at which Ψp max (Cp) of WT tomato started to decline was higher at e[CO2] than at a[CO2] (i.e., 0.35 vs 0.27) (Fig. 6e; Tables 1 and 2). While in flacca, both Ψp max and Cp were similar between the two CO2 treatments (Fig. 6f; Tables 1 and 2). The Ψp max of WT tomato was 2.0 and 2.9 times greater than that of flacca under a[CO2] and e[CO2], respectively. The Cp of WT tomato was lower than that of flacca under a[CO2] (i.e., 0.18 vs 0.31); whereas at e[CO2], there was no significant difference in Cp between WT tomato and flacca (P = 0.951) (Fig. 6e, f; Tables 1 and 2).
Leaf ABA concentration
In each CO2 environment, leaf ABA concentration ([ABA]leaf) increased exponentially with declining of FTSW in both WT genotypes, but not in ABA deficient mutants. (Fig. 7a, b). In WT barley, only under severe drought stress (i.e. FTSW <0.3), [ABA]leaf of e[CO2] plant tended to be higher than that of a[CO2] plant (Fig. 7a). While in WT tomato, [ABA]leaf under e[CO2] was greater compared to that under a[CO2] during the progressive soil drying (P = 0.001, ANCOVA) (Fig. 7b). In both ABA deficient mutants, the [ABA]leaf remained lower than those in the hydrated corresponding WT genotypes and were similar between the two CO2 treatments (Fig. 7a, b).
Trends of leaf ABA concentration ([ABA]leaf) of WT barley and its ABA deficient mutant Az34 barley, WT tomato and its ABA deficient mutant flacca grown under ambient (400 ppm) and elevated (800 ppm) atmospheric CO2 concentrations, respectively during progressive soil drying. Error bars indicate stand error of the means (SE) (n = 4)
Relationships of chemical and hydraulic signals with stomatal conductance during progressive soil drying
At moderate soil water deficits (i.e. FTSW >0.3), for both WT genotypes, gs decreased linearly with increasing [ABA]leaf (Figs. 8a and 9a). The output of ANCOVA reveals that [CO2] had significant effect on the slope for the regression of gs to [ABA]leaf, and gs for plants grown at a[CO2] was more sensitive to increasing [ABA]leaf compared to that grown at e[CO2] as gs was initially higher under a[CO2] (Figs. 8a and 9a). However, the relationships of gs to [ABA]leaf were similar for both ABA deficient mutants under the two CO2 environments (ANCOVA output: P = 0.58 for Az34 barley and P = 0.34 for flacca); Thus, only one regression line of both [CO2] treatments was made for each of the ABA deficient mutants (Figs. 8d and 9d).
Relationships between stomatal conductance (gs) and leaf ABA concentration ([ABA]leaf), gs and leaf water potential (Ψl), gs and turgor pressure (Ψp) of WT barley and its ABA deficient mutant Az34 barley grown under ambient (400 ppm) and (800 ppm) atmospheric CO2 concentrations during progressive soil drying. Closed circles indicate plants at 400 ppm CO2 concentration, open circles indicate plants at 800 ppm CO2 concentration. Error bars indicate standard error of the means (SE) (n = 4). *, ** and *** indicate the regression lines were statistically significantly at P < 0.05, P < 0.01 and P < 0.001 level, respectively (ANCOVA). Slope with P value indicates significant difference between the slopes of the regression lines for a[CO2] and e[CO2] treatments
Relationships between stomatal conductance (gs) and leaf ABA concentration ([ABA]leaf), gs and leaf water potential (Ψl), gs and turgor pressure (Ψp) of WT tomato and its ABA deficient mutant flacca grown under ambient (400 ppm) and (800 ppm) atmospheric CO2 concentrations during progressive soil drying. Closed circles indicate plants at 400 ppm CO2 concentration, open circles indicate plants at 800 ppm CO2 concentration. Error bars indicate standard error of the means (SE) (n = 4). *, ** and *** indicate the regression lines were statistically significantly at P < 0.05, P < 0.01 and P < 0.001 level, respectively (ANCOVA). Slope with P values indicates significant difference between the slopes of the regression lines of a[CO2] and e[CO2] treatments
The gs decreased linearly with decreasing Ψl in barley and tomato plants under each [CO2] environments (Figs. 8b,e and 9b, e). The output of ANCOVA shows that the slopes of the regressions of gs to Ψl were similar between the two [CO2] treatments in both barley and tomato genotypes (P = 0.05 and P = 0.28 for WT barley and Az34 barley, respectively, and P = 0.79 and P = 0.57 for WT tomato and flacca, respectively). Therefore, only one regression line of the two [CO2] treatments was made for each of the genotypes (Figs. 8b,e and 9b, e).
The gs decreased linearly with decreasing ΨP in barley and tomato plants under both CO2 environments except WT tomato grown at a[CO2] (Figs. 8c, f and 9c, f). The output of ANCOVA shows that [CO2] had significant effect on the slope of the regression lines of gs to ΨP in WT barley being that gs of a[CO2] plants was more sensitive to increasing ΨP than that of e[CO2]. For both ABA deficient mutants, no difference in the slopes of the regression lines was found (i.e., P = 0.07 for Az34 barley and P = 0.22 for flacca, respectively) (Figs. 8c, f and 9f). Therefore, only one regression line of both [CO2] treatments was made for each of the ABA deficient mutants (Figs. 8f and 9f).
Discussion
There is common consensus that e[CO2] decreases leaf gs in angiosperms (i.e., Wei et al. 2018). Likewise, in this study except flacca, most of the plants grown at e[CO2] had lower gs max compared to those grown at a[CO2] (Figs. 3c, d and 4c, d; Tables 1 and 2). Besides, in accordance with previous studies (Yan et al. 2017; Liu et al. 2019), here we found that e[CO2] increased net photosynthetic rate (An) under well-watered or moderate drought stress, and the enhancement of An max was observed in all plants grown at e[CO2]. In addition, more pronounced increase of An max was observed in barley as compared to tomato as An max was lower in barley relative to tomato at a[CO2] (Figs. 3a, b and 4a, b; Tables 1 and 2). Thereby, those together lead to an improved water use efficiency at leaf scale in all plants under e[CO2] environment.
As illustrated in Fig. 7, when FTSW greater than 0.3, [ABA]leaf of e[CO2] WT barley plant was similar to that of a[CO2] plant, and it became higher under severe drought stress (e.g., when FTSW <0.3) (Fig. 7a). In WT tomato plant, [ABA]leaf under e[CO2] was generally greater than that under a[CO2] during progressive soil drying (Fig. 7b). Earlier studies have reported that e[CO2]-induced stomatal closure was mediated by endogenous ABA (Chater et al. 2015; Tazoe and Santrucek 2015). In the absence of decreased leaf water status at e[CO2], the higher [ABA]leaf in e[CO2] plants might be resulted from stimulated root growth at e[CO2] (Wullschleger et al. 2002) as the enhanced root biomass could have stimulated root-to-shoot ABA signaling and further increasing foliar ABA concentration (Martin-Vertedor and Dodd 2011). Consistent with the finding by Li et al. (2016), here the decrease in gs max of WT tomato could be mainly ascribed to higher leaf ABA concentration under e[CO2], but the effect was absence in ABA-deficient flacca as the gs max was unaffected by [CO2] growth environments (Fig. 4c, d; Tables 1 and 2). Whereas, the e[CO2]-induced reduction of gs max in barley was probably not related to an increase of [ABA]leaf and most likely ABA-independent as the gs max reduction was found in both WT genotype and ABA-deficient mutant (Fig. 3c, d; Tables 1 and 2). Thus, it is plausible that putative differences exist between barley (monocot) and tomato (dicot) plants in the response of gs to e[CO2] environment.
In the present study, soil water status in pot was expressed as the fraction of transpirable soil water (FTSW) and linear plateau model was used to evaluate the response of leaf gas exchange to progressive soil drying. With the progression of soil drying, e[CO2] sensitized gs decrease in WT barley (Fig. 3c; Tables 1 and 2), while this was reverse in WT tomato where e[CO2] retarded the reduction of gs (Fig. 3c; Tables 1 and 2), affirming our earlier findings that gs became less sensitive to soil drying in tomato plants grown at e[CO2] than grown at a[CO2] (Yan et al. 2017; Liu et al. 2019). Furthermore, An of all plants grown at e[CO2] were more sensitive to soil drying than those grown at a[CO2] (Fig. 3a, b and 4a, b; Tables 1 and 2). In WT barley, the earlier reduction in An during soil drying could be a result of earlier decrease in gs under e[CO2] (Kusumi et al. 2012) (Fig. 3; Tables 1 and 2). However, this was not the case for WT tomato, as gs decreased later at e[CO2] than a[CO2] (Fig. 3c; Tables 1 and 2). Hereby, the earlier reduction in An of WT tomato during soil drying under e[CO2] was not due to an earlier closure of stomata, other factors could be involved. Opposite to the WT genotypes, the sensitivity of gs to progressive soil drying for both ABA-deficient mutants was unaffected by the [CO2] growth environment (Figs. 3c, d and 4c, d; Tables 1 and 2). Therefore, it is obvious that endogenous ABA level could have been involved in modulating the gs response to soil drying when plants grown under e[CO2].
Previous evidence has demonstrated that ABA-induced stomatal closure in tomato could increase Ψl, indicating the dependence of Ψl on leaf gs (Chaves et al. 2016; Dodd et al. 2009). In addition, ABA-deficient mutants often had lower Ψl than WT genotypes as described previously for barley (Martin-Vertedor and Dodd 2011; Mulholland et al. 1996) and tomato (Fambrini et al. 1995; Jones et al. 1987; Sharp et al. 2000). In agreement with this, here the greater gs max of both ABA-deficit mutants could lead to lower Ψl max as compared to WT genotypes except barley plant at a[CO2] (Figs. 5a, b and 6a, b; Tables 1 and 2), although the stomata was closed as Ψl declined in each genotype and [CO2] environment (Figs. 8b, e and 9b, e). This relationship could be resulted from the obvious decline in both gs and Ψl during severe soil drying. The isohydric plants are able to keep constant Ψl by lowering gs in response to soil drying, whereas anisohydric plants could decrease Ψl while maintaining gs (Tardieu and Simonneau 1998). In the current study, the barley and tomato plants grown under e[CO2] environment tended to delay the decline in Ψl during progressive soil drying as compared to those grown at a[CO2] (Figs. 5a, b and 6a, b; Tables 1 and 2). Thus, they tended towards isohydric in response to drought.
Several studies have shown that e[CO2] enhanced An and solutes accumulation, thereby contributing to the lower Ψπ and higher Ψp, further improving leaf turgor (Mamatha et al. 2015; Yan et al. 2017). Consistent with this, in this study, compared to a[CO2] plants, the e[CO2] plants showed a tendency of lower Ψπ max (although only significant in Az34 barley) and notable higher Ψp max except flacca. However, it should be noted that e[CO2] delayed the Ψπ response to progressive soil drying in barley, not in tomato, and Ψp response to progressive soil drying combined with [CO2] environment between barley and tomato was different (Figs. 5c–f and 6c–f; Tables 1 and 2). Moreover, in both [CO2] growth environments, the Ψp max of Az34 barley was similar to that of WT barley (Fig. 5e, f; Tables 1 and 2), while, the Ψp max of flacca was much lower than that of WT tomato (Fig. 6e, f; Tables 1 and 2). This was probably attributed to the contrasting leaf anatomy and stomatal morphology between dicot and monocot species, indicating that hydraulic properties in response to soil drying under disparate [CO2] growth environment would be species-dependent. However, it should be notable that the linear-plateau model used in this study might have wrongly estimated the FTSW thresholds at which the leaf water relation parameters started to decline from their maximal values due to the insufficient data points. Further studies with more frequent measurements of leaf water relation characteristics during soil drying should be conducted to verify these results.
It is widely recognized that endogenous ABA level plays an important role in stomatal regulation in response to drought stress (Wilkinson and Davies 2002; Yan et al. 2017). Here, the gs decreased linearly with the increase of [ABA]leaf for both WT genotypes (Figs. 8a and 9a), while such relationships between gs and [ABA]leaf in both ABA-deficit mutants were not evident (Figs. 8d and 9d), implying that endogenous leaf ABA level was involved in the regulation of stomatal aperture and this regulation was species-independent. There was little available information about the effect of e[CO2] on the sensitivity of stomata to ABA signaling when plants exposed to drying soil. Gray et al. (2016) reported that e[CO2] increased the sensitivity of soybean gs to [ABA]xylem under drought stress in a multi-year study. On the contrary, Liu et al. (2019) found that ABA was less important in inducing gs reduction at moderate drought stress under e[CO2], and Yan et al. (2017) observed that e[CO2] plants possessed lowered sensitivity of gs to [ABA]xylem. Similarly, in the present study, the gs of both WT genotypes grown at e[CO2] become less sensitive to [ABA]leaf (Figs. 8a and 9a), implying that other signal rather than ABA was more essential for controlling gs during mild drought stress. Yan et al. (2017) showed that the gs of e[CO2] tomato was positively correlated with Ψp. In accordance with this, here the gs of WT genotypes as well as their ABA-deficient mutants revealed positive correlations with Ψp under both [CO2] environments except WT tomato grown under a[CO2] (Figs. 8c, f and 9c, f). The lack of correlation between gs and Ψp in WT tomato grown under a[CO2] agrees with earlier findings from the root pressurization experiments showing that soil-drying induced stomatal closure even leaf turgor was maintained (Holbrook et al. 2002), which further emphasized the significance of chemical signalling (i.e., ABA) in inducing stomatal closure. On the other hand, our results indicated that Ψp and not ABA could have acted as a major factor inducing stomatal closure for the ABA-deficient mutants.
Conclusions
In this experiment, e[CO2] sensitized photosynthetic decline with soil moisture deficit in most genotypes. Soil-drying induced stomatal closure was affected by [CO2] in wild type genotypes but not in ABA-deficient mutants; e[CO2] sensitized the stomata response in barely whilst delayed it in tomato. In all genotypes, e[CO2] sustained leaf water potential and caused notable higher turgor pressure except flacca as compared to a[CO2]. In both wild type genotypes, The stomata become less sensitive to endogenous ABA at e[CO2] than a[CO2], whereas for the mutants, the stomata was predominately controlled by leaf turgor and not ABA during soil drying. These findings provide some novel insights into the mechanism of stomatal control in monocot and dicot plants response to drought stress under CO2-enriched environment.
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Asch F (2000) Determination of abscisic acid by indirect enzyme linked immuno sorbent assay (ELISA). Technical Report. Laboratory for Agrohydrology and Bioclimatology, Department of Agricultural Science, The Royal Veterinary and Agricultural University, Taastrup, Denmark
Bunce JA (2004) Carbon dioxide effects on stomatal responses to the environment and water use by crops under field conditions. Oecologia 140:1–10
Chater C, Peng K, Movahedi M, Dunn JA, Walker HJ, Liang YK, McLachlan DH, Casson S, Isner JC, Wilson I, Neill SJ (2015) Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr Biol 25:2709–2716
Chaves MM, Costa JM, Zarrouk O, Pinheiro C, Lopes CM, Pereira JS (2016) Controlling stomatal aperture in semi-arid regions–the dilemma of saving water or being cool? Plant Sci 251:54–64
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Dodd IC, Theobald JC, Richer SK, Davies WJ (2009) Partial phenotypic reversion of ABA-deficient flacca tomato (Solanum lycopersicum) scions by a wild-type rootstock: normalizing shoot ethylene relations promotes leaf area but does not diminish whole plant transpiration rate. J Exp Bot 60:4029–4039
Engineer CB, Hashimoto-Sugimoto M, Negi J, Israelsson-Nordström M, Azoulay-Shemer T, Rappel WJ, Iba K, Schroeder JI (2016) CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci 21:16–30
Fambrini M, Vernieri P, Toncelli ML, Rossi VD, Pugliesi C (1995) Characterization of a wilty sunflower (L.) mutant. J Exp Bot 46(5):525–530
Faralli M, Williams KS, Han J, Corke FM, Doonan JH, Kettlewell PS (2019) Water-saving traits can protect wheat grain number under progressive soil drying at the meiotic stage: a phenotyping approach. J Plant Growth Regul:1–12
Gray SB, Dermody O, Klein SP, Locke AM, Mcgrath JM, Paul RE, Rosenthal DM, Ruiz-Vera UM, Siebers MH, Strellner R, Ainsworth EA, Bernacchi C, Long SP, Ort DR, Leakey ADB (2016) Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat Plants 2:16132
Haworth M, Killi D, Materassi A, Raschi A, Centritto M (2016) Impaired stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated [CO2]. Front Plant Sci 7:1568
Holbrook NM, Shashidhar VR, James RA, Munns R (2002) Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J Exp Bot 53:1503–1514
Jones HG, Sharp CS, Higgs KH (1987) Growth and water relations of wilty mutants of tomato (Lycopersicon esculentum Mill.). J Exp Bot 38:1848–1856
Kusumi K, Hirotsuka S, Kumamaru T, Iba K (2012) Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J Exp Bot 63:5635–5644
Leakey AD, Bernacchi CJ, Ort DR, Long SP (2006) Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open-air conditions. Plant Cell Environ 29:1794–1800
Li XN, Tan D-X, Jiang D, Liu FL (2016) Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J Pineal Res 61:328–339
Liu FL, Jensen CR, Andersen MN (2003) Hydraulic and chemical signals in the control of leaf expansion and stomatal conductance in soybean exposed to drought stress. Funct Plant Biol 30:65–73
Liu FL, Andersen MN, Jacobsen SE, Jensen CR (2005) Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environ Exp Bot 54:33–40
Liu J, Hu TT, Fang L, Peng XY, Liu FL (2019) CO2 elevation modulates the response of leaf gas exchange to progressive soil drying in tomato plants. Agric For Meteorol 268:181–188
Mamatha H, Srinivasa Rao NK, Vijayalakshmi T (2015) Physiological responses of tomato (Lycopersicon esculentum mill) cv. Arka Ashish to elevated atmospheric CO2 under water limiting conditions. Indian J Agric Res 49:299–307
Manzi M, Lado J, Rodrigo MJ, Zacarías L, Arbona V, Gómez-Cadenas A (2015) Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant Cell Physiol 56:2457–2466
Martin-Vertedor AI, Dodd IC (2011) Root-to-shoot signalling when soil moisture is heterogeneous: increasing the proportion of root biomass in drying soil inhibits leaf growth and increases leaf abscisic acid concentration. Plant Cell Environ 34:1164–1175
McAdam SAM, Manzi M, Ross JJ, Brodribb TJ, Gómez-Cadenas A (2016) Uprooting an abscisic acid paradigm: shoots are the primary source. Plant Signal Behav 11:e1169359
Meidner H, Mansfield TA (1968) Physiology of stomata. Bot Gaz 46:62–63
Mulholland BJ, Black CR, Taylor IB, Roberts JA, Lenton JR (1996) Effect of soil compaction on barley (Hordeum vulgare L.) growth: I. possible role for ABA as a root-sourced chemical signal. J Exp Bot 47:539–549
Sagi M, Scazzocchio C, Fluhr R (2002) The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. Plant J 31:305–317
Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Biol 52:627–658
Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F (2000) Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J Exp Bot 51:1575–1584
Takahashi F, Suzuki T, Osakabe Y, Betsuyaku S, Kondo Y, Dohmae N, Fukuda H, Yamaguchi-Shinozaki K, Shinozaki K (2018) A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556:235–238
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot 49:419–432
Tausz-Posch S, Dempsey RW, Seneweera S, Norton RM, Fitzgerald G, Tausz M (2015) Does a freely tillering wheat cultivar benefit more from elevated CO2 than a restricted tillering cultivar in a water-limited environment? Eur J Agron 64:21–28
Tazoe Y, Santrucek J (2015) Superimposed behaviour of gm under ABA-induced stomata closing and low CO2. Plant Cell Environ 38:385–387
Walker-Simmons M, Kudrna DA, Warner RL (1989) Reduced accumulation of ABA during water-stress in a molybdenum cofactor mutant of barley. Plant Physiol 90:728–733
Wei ZH, Du TS, Li XN, Fang L, Liu FL (2018) Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agric Water Manag 202:174–182
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Wullschleger SD, Tschaplinski TJ, Norby RJ (2002) Plant water relations at elevated CO2-implications for water-limited environments. Plant Cell Environ 25:319–331
Yan F, Li X, Liu FL (2017) ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO2 concentration. Environ Exp Bot 139:99–104
Zhang F-P, Sussmilch F, Nichols DS, Cardoso AA, Brodribb TJ, McAdam SAM (2018) Leaves, not roots or floral tissue, are the main site of rapid external pressure-induced ABA biosynthesis in angiosperms. J Exp Bot 69:1261–1267
Acknowledgements
This work was partly supported by the Fundamental Research Funds for the Central Universities (2452018063) and National Natural Science Foundation of China (51909220). The technical assistance by Rene Hvidberg Petersen, Britta Garly Henriksen and Lene Korsholm Jørgensen was gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Janusz J. Zwiazek.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenhua Wei and Liang Fang are co-first author
Rights and permissions
About this article
Cite this article
Wei, Z., Fang, L., Li, X. et al. Effects of elevated atmospheric CO2 on leaf gas exchange response to progressive drought in barley and tomato plants with different endogenous ABA levels. Plant Soil 447, 431–446 (2020). https://doi.org/10.1007/s11104-019-04393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04393-3