Abstract
Background and aims
Amazonia comprises a mosaic of ecosystems that harbor high biodiversity. Knowledge about fungal diversity and ecology in this region remains very limited. Here, we examine soil fungal communities in forests of the Colombian Amazonia and their relationship to important edaphic variables.
Methods
Fungal communities were studied in terra-firme forests dominated by arbuscular mycorrhizal (AM) trees, terra-firme forests with the ectomycorrhizal (EcM) tree Pseudomonotes tropenbosii (Dipterocarpaceae), and white sand forests (WSF) with the EcM host plant genera Dicymbe and Aldina (Fabaceae). Fungal community composition was determined through 454-pyrosequencing of the ITS2 region of ribosomal DNA. We established the impact of the type of forest and edaphic parameters in structuring the fungal communities.
Results
We found a high diversity of fungi with 2,507 OTUs occurring in the soil samples studied. Carbon content and pH were the main edaphic factors contributing to structure the fungal community across all forests. Fungal community composition differs among terra-firme plots and WSF, while it was similar among AM and EcM-dominated areas in terra-firme. Our results revealed an important EcM fungal diversity in terra-firme AM-forests, where some EcM plants such as the ones in the genera Coccoloba and Neea occur scattered within an AM-matrix.
Conclusions
This is a first approximation to understand the ecology of soil fungal communities in forests of the Colombian Amazonia. We found that fungal soil communities have a spatial variation related to forest type (terra-firme and WSF), soil pH, and soil carbon content. Due to the strong correlation between vegetation and soil fertility in Amazonia, it is difficult to understand the effects of those factors to the fungal communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amazonia has the largest and most continuous tropical forest coverage in the world (Hoorn and Wesselingh 2010) and comprises different types of forest composed of complex and diverse microhabitats that harbor a high diversity of plants and animals (Gentry 1988; Hoorn et al. 2010; Ter Steege et al. 2010). The high plant biodiversity of such forests has been explained mostly by its paleoclimatic history that has resulted in important differences in soil fertility, and by the rainfall seasonality (Duivenvoorden and Duque 2010; Jiménez et al. 2014; Quesada et al. 2009, 2012; Ter Steege et al. 2006, 2010; Ter Steege et al. These forests also play a significant role in the global carbon balance and regulation of the global climate. Together with soils and biomass, the Amazon basin region contains approximately 10% of the global terrestrial carbon pool (Ter Steege et al. 2010). Nonetheless, soil fertility in Amazonia is low and forest maintenance depends on litter nutrient cycling and carbon allocation (Fearnside 2005; Jiménez et al. 2014; Quesada et al. 2012). A closed nutrient cycle mediated by fungi could explain how plant biomass is maintained despite low levels of plant-available nutrients in soil (Averill et al. 2014; Bunn et al. 2019; Lindahl and Tunlind 2015). Moreover, it has been found that the special guild such as plant pathogens and EcM fungi may influence the diversity and abundance of trees in the tropical rainforest (Averill et al. 2014; Comita et al. 2010; Corrales et al. 2016; Frąc et al. 2018). On the other hand, plant diversity is considered an important factor in the structuring of the soil fungal communities due to the symbiosis with ectomycorrhizal fungi (EcM) fungi and the diversity of compounds for the saprotroph community (McGuire et al. 2012). Although plant and animal ecology are relatively well studied in Amazonia and the number of scientific studies on tropical fungi has increased, information about the fungal diversity, the community structure at the local and the regional scales, and plant-soil-fungal interactions remains very limited.
Soil fungi play key roles in ecosystems, driving nutrient cycling as decomposers, regulating species composition as pathogens and providing mutualistic benefits as symbionts (Comita et al. 2010; Tedersoo et al. 2014). Mycorrhizae are a mutualistic association between soil fungi and roots that occurs in about 80 to 90% of the plant species (Smith and Read 2008). In this association, mycorrhizal fungi improve growth, nutrient and water uptake, and resistance to pathogens and heavy metals of the plant. In return, the plant provides the fungus with carbon derived from its photosynthetic activity (Smith and Read 2008; van der Heijden et al. 2015). The most common types of mycorrhizas are arbuscular mycorrhizal fungi (AM) and ectomycorrhizal fungi (EcM). The EcM association was considered rare in the tropics, compared with the AM fungi. However, recent studies showed that EcM fungi are more common in the tropics than previously considered. In neotropical ecosystems, including those from Central and South America and the Caribbean, 60 species of EcM hosts have been reported (Corrales et al. 2018). Indeed, the plant families Fabaceae (subfamilies Caesalpinioideae and Papilionoideae) and Dipterocarpaceae are predominantly EcM associated. Previous data indicated that plant diversity in neotropical ecosystems varies between forests dominated by plants that host arbuscular mycorrhizal fungi (AM) versus forests with plants that host EcM fungi (Roy et al. 2016, 2017). The EcM-habit is also one of the mechanisms that explain monodominance or codominance of EcM host trees in tropical ecosystems (Fukami et al. 2017; McGuire 2007). Some studies suggested that tropical trees might rely on EcM-mediated nitrogen (N) acquisition, particularly those occurring in monodominant forests with high soil organic matter and low N availability (Mayor et al. 2015). For example, in forests dominated by Oreomunnea mexicana (Juglandaceae) in Panama, EcM fungi may slow the cycling of soil N by decreasing the available N of the soil for other plants, while EcM fungi obtain the N directly from organic sources (Corrales et al. 2016). These processes are important in carbon and nutrient cycles and carbon sequestration in forests and may affect the nutrient balance and favor EcM plants (McGuire et al. 2010; Van der Heijden et al. 2015).
Recent advances in molecular approaches, such as high throughput sequencing, and the presence of curated molecular databases containing fungal DNA sequences, provide a strong tool to study the composition of fungal communities (Bálint et al. 2016). Microorganisms, including fungi, were long assumed to be cosmopolitan based on massive spore production and long-range dispersal capabilities (Baas-Becking 1934). However, current molecular-based studies suggest that fungi have restricted distributions and distinct biogeographic patterns (Bahram et al. 2015, 2018; Burns et al. 2015). The soil fungal richness and community composition from local to global levels are influence by climatic factors such as mean annual precipitation, followed by edaphic factors (pH, N, calcium or phosphorous), plant community, and spatial patterns (Corrales et al. 2017; Geml et al. 2014; Peay et al. 2013; Talbot et al. 2014; Tedersoo et al. 2014). In forest ecosystem, fungal diversity and community composition and structure have been reported closely related with edaphic variables such as pH and soil nutrients, and plant diversity (Lucheta et al. 2016; Peay et al. 2013; Rousk et al. 2010).
In Amazonia there are few studies on soil fungi ecology despite the high biodiversity of fungi that occurs within this region (Grupe et al. 2016; Houbraken et al. 2011; López-Quintero et al. 2012, 2013; Lucheta et al. 2016, 2017; McGuire et al. 2012; Moyersoen 2006; Mueller et al. 2014, 2016; Paula et al. 2014; Peay et al. 2013; Tedersoo et al. 2010c; Vasco-Palacios et al. 2018; Yilmaz et al. 2016). Plant diversity and soil factors such as pH nitrogen, phosphorous, and calcium have been found correlating with soil fungal diversity and fungal community structure in natural forests in Amazonia (López-Quintero et al. 2012; Lucheta et al. 2016; Tedersoo et al. 2014). Local-scale variation of soils plays an important role in influencing plant community structure (Duivenvoorden and Duque 2010; Ter Steege et al. 2010). Such changes also influence the community structure of soil fungi from natural forests, that present a high degree of spatial variation related to forest type (Mueller et al. 2014, 2016; Peay et al. 2013). Similar patterns occur with special guilds, such as parasitic and mycorrhizal fungi that depend on the tree composition (Peay et al. 2013). On the other hand, in disturbed areas, the fungal soil communities correlate with the plant community composition rather than with soil properties (Mueller et al. 2014). However, in anthropogenic and non-anthropogenic dark earth soil in Brazil, a type of soil rich in nutrients due to high levels of carbon, the fungal communities were shaped by soil pH, calcium, phosphorous, and aluminum saturation (Lucheta et al. 2016).
In this study, we examined the community structure and diversity of soil fungi from three types of forests in the Colombian Amazonian region. We hypothesized that 1) communities of soil fungi do exhibit similar composition and functional structure among the two terra-firme forests studied, due to similarities in plant composition and edaphic factors; 2) the EcM fungal community composition differs between forests with a high abundance of AM plant hosts and forests with a high abundance of EcM plant hosts (forests with P. tropenbosii and forests with Dicymbe and Aldina); 3) there is a positive relationship between fungal community composition and edaphic factors such as pH, nitrogen and phosphorous. To evaluate the differences of the fungal communities in these three forest types that show differences in the abundance of AM and EcM plant hosts we performed metabarcoding using high-throughput DNA sequencing. Demonstrating a potential link between the type of forests, presence of EcM plant hosts, and the diversity and structure of the fungal community with respect to the edaphic variables is a first step in establishing the role of fungi in influencing Amazonian forest structure.
Materials and methods
Study sites and sampling
This study was conducted at two locations in the Colombian Amazon region. The first location was 27 km north of Leticia in the El Zafire Biological Station (ZBS) area of the National University Sede Amazonas (Fig. 1, Table 1). This area has different types of soil with four main forests types: upland terra-firme forests, temporarily flooded forests, white sand forests, and transitional forests within the upland and flooded forests (Peñuela-Mora 2014). Soils are sandy and composed mainly of quartz with the parental material belonging to Tertiary sedimentary units that probably originated from the Guiana Shield (Jiménez et al. 2009). The other location was located in the middle Caquetá region ca. 420 km from El Zafire area (Fig. 1). This middle Caquetá region presents upland terra-firme forests, temporarily flooded forests, and white sand forests (Parrado-Rosseli 2005). In this second location, two sites with similar climatic conditions and approximately 34 km apart were sampled (Fig. 1, Table 1). One was close to Peña Roja village, and the other close to the village of Puerto Santander (Table 1). In these areas, the communities of P. tropenbosii (named PtF) occur in upland terra-firme forests which make part of the Tertiary Sedimentary Plain unit and have a flat to undulating topography with valleys and hills of 20 to 40 m height. Soils in these areas are well drained with a low mineral nutrient content and consist of sands to clays of the Amazonian upper and lower tertiary superior unit (Parrado-Rosseli 2005).
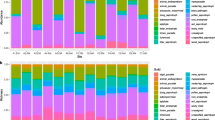
Taxonomic composition of fungal communities from soils of AMF, PtF, and WSF from the Amazon region in Colombia. Lineages with less than five occurrences across all samples (Atractiellomycetes, Basidiobolomycetes, Chrytridiomycetes, Dacrymycetes, Entomophthoromycetes, Incertae sedis, Kickxellomycetes, Pucciniomycetes, Taphrinomycetes) were grouped with the unknown and others for clarity. In total, 2,507 fungal OTUs from 64 orders, 147 families, and 292 genera were detected. Despite differences in community structure, phylogenetic representation at class level appears largely similar between the forest types
In each area, three types of forest were sampled, namely two in terra-firme forests and one in white sand forests (WSF). The selected forests harbor different species and densities of EcM and AM plants (Table 1). The terra-firme forests occur in areas with well-drained soils, which are not flooded by rivers. Floristically, these forests are more diverse than flooded forests and contain plants mainly associated with AM fungi and few individuals of EcM host plants of the genera Coccoloba (Polygonaceae) and Guapira or Neea (Nyctaginaceae) (Importance value index IVI, Polygonaceae = IVI 0.47%, Nyctaginaceae =0.8%) (Parrado-Rosseli 2005). In some areas of the terra-firme forests in Colombia, there are some small patches of P. tropenbosii trees mixed with the typical vegetation of the terra-firme forests. In these areas, P. tropenbosii is one of the most important canopy species (IVI = 16–18%) (Duivenvoorden and Lips 1993; Parrado-Rosseli 2005). Studies showed that this endemic tree is an EcM hosts similar to other species in the family Dipterocarpaceae (Vasco-Palacios et al. 2016). The third type of forest that we studied was a WSF that occurs in areas where the soils have a higher quartz content. The WSF in western Amazonia, generally occurs as small island of habitat surrounded by terra-firme forests. White sand forests have a unique plant composition and the tree diversity is low when compared to terra-firme, but they show a high specialization and high level of plant endemism (Jiménez et al. 2009; Peñuela-Mora 2014; Roy et al. 2016). The genera Dicymbe and Aldina of the family Fabaceae were present in Colombian WSF with an abundance of 25% of all canopy trees (IVI 25%) (Peñuela-Mora 2014). Soil samples were collected during the rainy season in both regions in April 2012.
Sampling design
The sampling design followed protocols of Tedersoo et al. (2014). Circular plots with of 20 m diameter were established. Nine plots were located in the middle Caquetá region (6 PtF and 3 AMF) and nine in the Zafire area (3 WSF, 3 PtF, 3 AMF) (Table 1). The selected WSF patch in the Zafire was less than 5 ha, and surrounded by terra-firme mixed forest. The data about fungal communities were part of a previous large-scale analysis (Tedersoo et al. 2014). In the middle Caquetá area, we did not collect soil samples at WSFs, because the site was disturbed by a herd of wild pigs that disrupted the soil and the litter layer shortly prior to our fieldwork.
In each plot of WSF and PtF 20 trees of the dominant EcM hosts were selected. Two lateral soil cores were collected at 2 m from the trunk of each selected tree. After removal of coarse roots and stones, the 40 core soils were pooled per plot. In the terra-firme plots with dominance of AM fungi (named AMF), 40 soil cores were taken randomly inside the circular plot and pooled. A subset of each pooled soil sample was air-dried, preserved in silica gel, and transported to the Laboratory of Taxonomy and Ecology of Fungi of the Antioquia University, Medellín, for further analysis. About 500 g of the A horizon (i.e. a depth of 5–10 cm after removal of organic litter horizon) were collected from each soil for physicochemical analyses. Soil samples were air-dried and transported to the laboratory for further analysis. Edaphic analyses were conducted at the National Laboratory of the Soils, Instituto Geográfico Agustín Codazzi (IGAC), Bogotá, Colombia and at Tartu University, Estonia (Table 2). pH was measured with a KCL method, total organic carbon content of the soil used the method of Walkley Black; total phosphorous was analyzed by the Bray II method, nitrogen by the Kjeldahl method, levels of Ca2+ were measured on ammonium acetate (pH 7) soil extracts, and Mg2+ Na and K on ammonium lactate (pH 7) soil extracts using atomic absorption spectrophotometry (MAT 253; Thermo Electron, Bremen, Germany) (Table 2).
Molecular analysis
DNA was extracted from 2.0 g of soil using the PowerSoil DNA Isolation Kit (MoBio, Carlsbad, CA). The internal transcribed spacer (ITS) region 2 of the ribosomal DNA (rDNA) was amplified using a mixture of six forward primers analogous to ITS 3 and one degenerate reverse primer analogous to ITS4 following the procedures described by Tedersoo et al. (2014). Negative and positive controls were included during the PCR amplification. Normalized amplicons were subjected to 454-adaptor ligation, emulsion PCR, and 454 pyrosequencing using the Roche GS FLX+ platform and titanium chemistry (Beckman Coulter Genomics, Danvers, MA).
Bioinformatics and sampling analyses
Pyrosequences were curated based on quality information provided by the sequencing company. Adapters were removed using ACACIA 1.52 (Bragg et al. 2012). To exclude short and low quality sequences, these were trimmed in Mothur 1.32.2 (Schloss et al. 2009) using the following parameters: minlength = 200; maxambig = 0; maxhomoP = 10; qwindo-waverage = 35; qwindowsize = 50; and bdiffs = 1. Sequences were demultiplexed based on the MID tags and primers. Putative chimeras were identified and removed with UCHIME (Edgar 2010; Edgar et al. 2011). After these filtering steps, the remaining sequences were pooled and clustered into operational taxonomic units (OTUs) at 97% identity threshold using the UCLUSTER algorithm (Edgar 2010). The longest sequences obtained were selected to assign taxonomic affiliation of each OTU. These representative sequences were subjected to BLASTn against the quality-checked database of ITS, UNITE - INSD (UNITE Community 2017), and an ITS sequencing data gathered from a large scale DNA-barcoding sequencing project of the Westerdijk Fungal Biodiversity Institute database fungal strain collection (Vu et al. 2016, 2019) using the usearch_global tool from USEARCH 6. For each query, the ten best BLASTn hits were annotated as detailed as possible. Species limits were determined based on ≥97% identity for operational taxonomic units (OTUs) (Hughes et al. 2009; Lindahl et al. 2013). We used 90, 85, 80, and 75% sequence identity for assigning names to a genus, family, order, and class respectively (Tedersoo et al. 2014). Fungal OTUs identified to lower taxonomic levels were based on Index Fungorum as featured in UNITE. The designation of the ecological guilds for each genus and family was based on literature when possible (Cannon and Kirk 2007; Tedersoo and Smith 2013; Tedersoo et al. 2010a, 2014). EcM taxa were considered if they matched best (based on ≥97% identity) with any sequence considered to represent EcM lineages (UNITE-database; Tedersoo et al. 2010a; Tedersoo and Smith 2013). Lineage nomenclature followed Tedersoo et al. (2010a) and Tedersoo and Smith (2013). All OTUs represented by one sequence, i.e. singletons, were removed from the analysis to reduce the effect of rare OTUs in the analysis (Bálint et al. 2016; Tedersoo et al. 2010b).
Statistical analysis
Rarefaction curves of OTUs were calculated using the function specaccum with 1,000 permutations in the Vegan package of R (Oksanen et al. 2013; R Core Team 2014). Because samples differed in the number of sequences reads, each sample was standardized to the minimum number of sequences (to 913 sequences) using the function raremax in Vegan (Oksanen et al. 2013). We generated a randomly rarefied community data matrix using the funcion rrarefy in Vegan (Oksanen et al. 2013). All data analyses were performed with this rarefied data. Abundances were scaled between 0 and 1. Differences in fungal communities between types of forest were tested with ANOVA using function aov in R and Tukey-Kramer HSD pairwise mean comparison tests with a significance level of 0.05. In addition, differences in the value of each edaphic parameter between the type of forests were determined with ANOVA. The correlation between localities, types of forest, and edaphic parameters and the complete fungal community data were addressed using a partial Mantel test as implemented in the Ecodist package of R, after 9999 permutations (Goslee and Urban 2007). The test used Bray-Curtis dissimilarity matrix of the OTUs occurrence data, also a Euclidean distance matrix was calculated from types of forest and from edaphic parameters. A similar assessment was accomplished to study the effect of the type of forest and the edaphic parameters on the EcM fungal community. To compare the soil fungal community from the same locality or type of forests (three dissimilarity matrices), we performed a partial test Mantel using the Pearson method as implemented in the Ecodist package (Goslee and Urban 2007). The effect of localities, types of forest, and edaphic parameters was also calculated using Bray-Curtis distance measure implemented in Adonis routine of the Vegan package of R (Oksanen et al. 2013) with 9999 permutations. Significance level of 0.05 was used throughout the study.
Additionally, we conducted a non-metric multidimensional scaling (NMDS) analysis to examine the dissimilarity relationships among the fungal communities across localities and types of forest, and among significant edaphic parameters such as pH and carbon content. The NMDS was run on a rarefied, presence/absence matrix using Jaccard dissimilarity. The ordination was implemented in the Ecodist package of R. In addition, a cluster analysis was performed to analyze the similarity in species composition of plots. Furthest-neighbor cluster analysis was run from a distance matrix calculated with a Bray index of similarity in hclust in Vegan (Oksanen et al. 2013).
Results
Fungal diversity
Pyrosequencing of ITS2 rDNA from 18 pooled soil samples of tropical lowland forests from the Colombian Amazonia recovered 60,247 reads. Curation resulted in 53,971 high quality sequences. After removal of chimeras, 42,136 reads were obtained that clustered in 3,576 OTUs, of which 3,354 (39,621 sequences) were classified as Fungi. The fungal sequences were assembled into 2,507 non-singleton OTUs (39,525 sequences) and 847 singletons OTUs (13%). Out of the total of 39,621 fungal sequences, 4,219 sequences (21%) corresponding to 572 OTUs (11.1%) could not be classified to order level, 11,996 (34.5%) sequences corresponding to 1,303 OTUs (50%) not to family level, and 1,657 OTUs (63%) not to genus level. The orders with the most unidentified OTUs were Auriculariales (88%), Sebacinales (87%), Helotiales (66%), Sordariales (62%), and Xylariales (44%).
The soil sampling revealed representatives of all major phyla and classes of fungi (Fig. 2). The phylum Ascomycota (63% of OTUs, 47% of sequences) was the most diverse, followed by Basidiomycota (32% of OTUs, 36% of sequences), Zygomycota-Mucoromycotina (1.5% of OTUs, 14% of sequences), and Rozellomycota and Chytridiomycota (1% of OTUs, 0.1–0.3% of sequences) (Fig. 2). Archaeorhizomycetes, a recently described phylum from temperate regions (Rosling et al. 2011), was relatively diverse and comprised 6% of the fungal OTUs (4% of sequences). Fungal OTUs were assigned to 64 orders, 147 families, and 292 genera. In Ascomycota, the order Hypocreales (188 OTUs) was the most diverse, followed by Helotiales (183 OTUs) and Chaetothyriales (156 OTUs). Within Basidiomycota, Agaricales (158 OTUs) was the most diverse, followed by Trechisporales (106 OTUs). Overall patterns of taxonomic representation were fairly consistent across the different forest types, but the proportions varied (Fig. 2). In general, WSF harbored a unique composition when compared to the other two forest types. WSF presented less diversity of Agaricomycetes and had a high number of OTUs belonging to Eurotiomycetes (e.g. Penicillium) and Leotiomycetes that comprise many putative plant pathogens (Fig. 2). The obtained species accumulation curve showed that the fungal community was not completely represented (Fig. 3a, b). Overall, soils from terra-firme forests (AMF and PtF) presented a higher fungal diversity than WSFs, because samples differed in the number of OTUs and sequences (182–562; 913–4840 respectively) (Suppl. Fig. 1a). The following genera were found to be most common (>15 OTUs) Umbelopsis (36 OTUs), Penicillium (33 OTUs), Cladophialophora (31 OTUs), Chaetosphaeria (21 OTUs), Cryptococcus (21 OTUs), Meliniomyces (20 OTUs), Bionectria (20 OTUs), Mycena (19 OTUs), Ochroconis (16 OTUs), and Fusarium (15 OTUs).
The main phylogenetic and functional ecological guilds of fungi were present (i.e. saprotrophs, plant-pathogens, EcM, and AM) in all types of forest, but their relative richness varied across plots (Figs. 2, 4). The saprotroph guild was the most diverse group of soil fungi in this study (59% of all OTUs), followed by plant-pathogens (9%), and EcM fungi (6%). Other trophic categories, such as lichenized fungi, mycoparasites, and AM symbionts represented less than 5% of all fungal OTUs (Fig. 4). Altogether, 22% of OTUs grouped in the category “unknown”, because they could not be assigned to any trophic group due to lack of taxonomic resolution. The richness of plant pathogens positively correlated with the fungal community structure by forest type (Mantel r = 0.2489; P < 0.05). The AM fungi were not well represented in any of the ecosystems studied (<1%). EcM fungi comprised 144 OTUs belonging to 21 fungal families, where Telephoraceae and Russulaceae were the most abundant taxa. 129 OTUS were detected from PtF samples, 72 from AMF, and 34 from WSF. The EcM lineages /tomentella-thelephora (36 OTUs, 25%), /russula-lactarius-lactifluus (23 OTUs, 16%), /clavulina (13 OTUs, 9%), /cortinarius (8 OTUs, 5.6%), /boletus (10 OTUs, 6.9%), /pisolithus-scleroderma, /inocybe and /sordariales1 (7 OTUs, 4.9%), and /cantharellus (5 OTUs, 3.5%) were the most diverse in this study (Table 3).
Fungal community composition between type of forests
The number of OTUs ranged between 182 (WSF- Zafire plot) and 562 OTUs (PtF-Peña Roja plot). WSF showed a remarkably lower diversity within all samples (Suppl. Fig. 1a). The soil fungal community from PtF presented the highest richness of all studied plots (1,828 OTUs, 72%). Peña Roja (562 OTUs, 22%) and the Zafire area (523 OTUs, 21%) also showed a high richness of OTUs. Despite differences in the number of detected OTUs, the composition of the communities at the taxonomic and the ecological level was similar within types of forest (P > 0.05) independent of the forest type (Figs. 2, 4).
Our data showed that the fungal communities are mainly shaped by type of forest and sites (F2,17 = 1.3, P = 0.001; F2,17 = 1.53, P = 0.004). Overall, soil fungal community composition was more similar in samples from plots located in terra-firme (AMF and PtF) than those from WSF (Suppl. Fig. 2). The non-metric multidimensional scale (NMDS) ordinations showed community similarities of soil fungi between samples from terra-firme plots (Fig. 5 a). However, in the Zafire area, the fungal community from AMF plots presented significant differences with the PtF plots (Suppl. Fig. 2c). Samples from forests with the ectomycorrhizal tree P. tropenbosii exhibit the most homogeneous fungal community despite the geographical distance, even among plots that are separated more than 400 km (Fig. 5a–c, Suppl. Fig. 2c; Mantel P < 0.5; Suppl. Table 1). A high number of common OTUs (321 OTUs) were detected across the PtF (Mantel r = 0.0071; P < 0.05). The sampled WSF presented the most unique fungal composition compared with the other two forest types (Mantel r = 0.0071; P < 0.05). The Tukey HSD test showed that the main differences between WSF and the other type of forests were due to differences in richness (Suppl. Fig. 1b). A similar trend was observed for the EcM fungal community (Fig. 5b, Suppl. Fig. 2b). The Tukey HSD test showed differences between EcM fungal community from PtF and the other two forests (Suppl. Fig. 1b). WSF in the Zafire area presented 14 OTUs of EcM fungi, while the most diverse was PtF-Peña Roja with 46 OTUs (Table 2; Suppl. Fig. 1b).
a Nonmetric multidimensional scaling (NMDS) ordination plot representing similarity of the fungal soil community in different forest types in Colombia Amazonia. Stress value = 0.1074; R2 = 0.982. For all plots colors represent different sites: Peña Roja in red, Puerto Santander in blue, and El Zafire in green. Figures represent different type of forests: circle indicated terra-firme plots with dominance of AM fungi, triangle indicated terra-firme plots with P. tropenbosii and cross indicated white sand forests . b NMDS ordination plot representing similarity of the EcM fungal soil community in different forest types in Colombia Amazonia. c NMDS ordination plot representing similarity of the fungal community and carbon content per plot. d NMDS ordination plot representing similarity of the fungal soil community and pH in different forest types in Colombia Amazonia
Edaphic factors that shape the fungal communities
Carbon content and soil pH are the edaphic factors that showed a significant relationship with fungal composition. In general, carbon content and pH were not significantly different in both forests and sites (ANOVA P < 0.5). However, differences in soil carbon were detected among terra-firme plots (PtF and AMF) (ANOVA P < 0.5), with soil carbon levels ranging from 40 g/Kg at the middle Caquetá region to 13 g/Kg at El Zafire area (Fig. 5c), and this differences explained fungal variation (Fig. 5c, F2,18 = 0.8, P = 0.007). Soil pH also do not present significan differences with respect to forests and plots, and neither soil pH nor wood pH showed a clear pattern in the NMDS (Fig. 5d). pH values were low, but it is important to take in consideration that the pH values (KCL extraction) are generally one pH unit lower than pH (H2O) values. Fungal functional guilds were differentially affected by edaphic parameters such as carbon content and soil pH. These two factors correlated with the presence of EcM, saprotrophic, and plant pathogenic fungi (Suppl. Tables 1, 2), but a pattern was also not found in the NMDS (5c-d). Other nutrients, such as N, phosphorous, potassium, calcium, and magnesium did not correlate with the fungal composition (Suppl. Table 1).
Discussion
Fungal diversity of Colombian Amazon forests
In this study, a high diversity of soil fungi was observed in three types of forests from the Amazon region in Colombia. We identified 2,507 OTUs belonging to 64 orders, 147 families, and 292 genera. South America is a hotspot for fungi, and Tedersoo et al. (2014) found that the diversity of soil fungi peaked in tropical forests with the highest diversity observed in the Colombian Amazonia, including the 18 plots here described. A previous metabarcoding study from similar ecosystems in Western Amazonia revealed 1,776 fungal OTUs (Peay et al. 2013). Differences in the diversity between Peay et al. data (2013) and our study may be due to sampling intensity (10 samples versus 18 pooled samples in this study), and sequencing depth. The fungal richness observed in our studies corroborated those made in the Brazilian Amazonia where analyses of land use types revealed 4,810 OTUs of fungi (Mueller et al. 2016). The main phylogenetic and functional groups of fungi were present in all three studied forests and the taxonomic profile was comparable with previous findings from Amazonia (Lucheta et al. 2016; Mueller et al. 2014, 2016; Peay et al. 2013; Tedersoo et al. 2014; Vasco-Palacios et al. 2018). The lack of a full understanding of the fungal diversity in the tropics was reflected in the large number of OTUs that remained unidentified at lower taxonomic levels, i.e. family or genus level with 50% and 63% unidentified OTUs respectively. Although high-throughput sequencing methods provide deeper insight into fungal diversity compared with traditional methods, they still do not reflect the complete species composition present (Bálint et al. 2016; Tedersoo et al. 2010b). The efficiency of both high-throughput sequencing techniques and statistical methods to analyze data have improved rapidly (Bálint et al. 2016). In addition, the Illumina platform offers an increase in the depth of coverage and the ability to detect rare OTUs (Bálint et al. 2016; Estrada-Peña et al. 2018). However, the lack of representative DNA sequences in databases from tropical and subtropical species, has limited ecological and large-scale biogeographical studies (Looney et al. 2016; Matheny et al. 2009). Thus, ecological studies using molecular approaches reported a high level of unidentified taxa from tropical areas, with up to 80% of all soil-fungal taxa remaining unidentified at the species level (Tedersoo et al. 2017).
The AM fungi were not well represented in any of the ecosystems studied (<1%), which was similar to results found in soils from northern South America and Panama (Lucheta et al. 2016; Peay et al. 2013; Tedersoo et al. 2014). The low diversity of AM fungi could be due to the barcode gene used in this study, as ITS2 is less efficient for detecting Glomales compared to nuclear SSU rRNA (Lee et al. 2008; Simon et al. 1992). In order to include a better overview of soil fungal community it will be important to include specific primers for Glomales during future molecular analysis. A comparison of the soil fungal composition with other ecosystems is difficult, because there are still relatively few studies using high throughput sequencing data from Neotropical ecosystems (Camenzind et al. 2014; Lucheta et al. 2016, 2017; Mueller et al. 2014, 2016; McGuire et al. 2010; Peay et al. 2013; Tedersoo et al. 2014). In the Colombian Amazonia region, around 60 species of AM fungi have been reported to be associated to cultivated manioc roots; are not available data from natural ecosystems (Peña-Venegas and Vasco-Palacios 2019). The interaction between AM fungi and other soil biota can accelerate decomposition of organic materials (Bunn et al. 2019). AM fungi may release labile carbon into the soil and rhizosphere, stimulating activity of microbial decomposers and decreasing degradation rates of soil organic matter (Bunn et al. 2019). This feedback between soil microbes could be an important dynamic to be considered in future studies.
Major fungal functional guilds, such as saprotrophs, plant-pathogens, EcM, and AM, were present in all types of forest, but their relative proportion varied across samples. However, the functional structure of the fungal community was similar between all plots. Saprotroph fungi were the most abundant and evenly distributed in the three forest types, although the species composition varied between plots and forests. The analysis showed that saprotrophic fungal species compositions were significantly related to the type of forests and pH and carbon content, and the community from WSF was the most particular one. These results suggest that different saprotrophic species inhabit different habitats, and differences in the composition of the litter layer and the low water retention capacity could be shaping the saprotrophic community in the WSF. Also, a competition for nutrients between mycorrhizal fungi and saprotrophs have been proposed, but the mechanisms and effects are still unknown (Verbruggen et al. 2017). The EcM and plant pathogenic fungi were evenly distributed in the tropical lowland forests studied and occurred in similar proportions as previously reported in other tropical lowland forests (Peay et al. 2013; Tedersoo et al. 2014). The abundance and diversity of plant pathogenic fungi was significantly different across forests types, likely because plant pathogens often exhibit host specificity (Gilbert and Webb 2007). Peay et al. (2013) found that western Amazonian forests maintain different lineages of putative pathogenic fungi in white-sand, flooded, and terra-firme forests, with very low overlap in OTUs across habitats. Furthermore, the number of species of plant pathogenic fungi increases in the tropics and plays an important role to maintain tree species diversity in forests (Augspurger 1983; Tedersoo et al. 2014). But we still have a very limited understanding of the actual roles played by fungal pathogens in natural ecosystems.
In this study, 144 fungal EcM OTUs (5.7% OTUs), belonging to 21 families were detected. The 89 species of EcM fungi reported here is similar to the one previously found from WSFs and PtFs in Colombia based on an analysis of fruiting bodies and root tips (Vasco-Palacios et al. 2018) (Table 3). The number of detected OTUs is also close to 174 species of EcM fungi reported from forests dominated by Fabaceae and the EcM tree Pakaraimaea dipterocarpacea (Cistaceae) in Guyana based an analysis of fruiting bodies and root tips (Henkel et al. 2002, 2012; Smith et al. 2013) (Table 3). The EcM lineages /tomentella-thelephora and /russula-lactarius were the most abundant in the Colombian lowland forests (Table 3). These lineages are known to be associated with all major plant host taxa in a number of globally occurring ecosystems (Brearley 2012; Tedersoo et al. 2010a, 2010b, 2010c; Vasco-Palacios et al. 2018), even though knowledge about /tomentella-thelephora and /sebacina is poorly known in many tropical countries. In Colombia, species of Tomentella, Thelephrora, and EcM-Sebacina were found to be dominant in root-tips on Dicymbe and Pseudomonotes species (Vasco-Palacios et al. 2018). Lineages that have been reported rare or absent from tropical ecosystems, such as /hygrophorus, /descolea or the dessert truffle /terfezia (truffle-like)-like, were detected in our dataset. It is well known that our knowledge about the diversity and distribution of fungi in tropical regions is still incomplete, for example Terfezia is an EcM fungi belonging to the Pezizales, that are typical from North Africa and Asia, but related taxa could be present in the tropics (falta cita). Hypogeous fungi are one of the fungal groups poorly known in tropics (Sulzbacher et al. 2017), mainly due to their habit that make them difficult to find. However, a high diversity that include new taxa, have been discovered in forests in Guyana (Sulzbacher et al. 2017). The distribution patterns of fungal species have changed as we do more studies on fungal diversity. Recently, five species of Sarcodon from PtF and WSF in Colombia, and Pakaraima mountains in Guyana were described as new species, Sarcodon is a genus previously considered restricted to boreal areas (Grupe et al. 2016).
PtF showed the highest number of EcM OTUs (129 OTUs), followed by AMF and the lowest diversity was observed in WSF (61 and 34 OTUs, respectively). However, the low fungal diversity observed in WSF may be due to the fact that only one site was sampled, because soil in the WSF in the middle Caquetá region was strongly disturbed by a herd of wild pigs just prior to our field work. Recent studies in white sand areas from Brazil, Guyana and Colombia showed a high diversity and heterogeneity of EcM fungal communities that seems to be irrespective of the soil type (Roy et al. 2016; Vasco-Palacios et al. 2018). The terra-firme forests with dominance of AM-plants revealed an unexpected high diversity of EcM fungi, similar to that occurring in terra-firme forests where P. tropenbosii was the most important EcM tree. The occurrence of EcM fungi in AM-dominant forests could be explained by the presence of EcM plant hosts, such as Coccoloba, Guapira, and Neea, that are geographically scattered and occur in low abundance (<1% IVI). In western Amazonia in Peru, 38 species of EcM fungi from roots associated to Coccoloba, Guapira, or Neea were reported (Tedersoo et al. 2010)). Importantly, our results showed that some OTUs were shared between the two types of terra-firme forests, AMF and PtF, and even with WSF (57 OTUs in common with PtF and 15 with WSF). This contrasts with the assumed host specificity previously reported for Coccoloba, Guapira, and Neea (Tedersoo et al. 2010c). Unfortunately, little is known about the EcM fungal community associated with these EcM plants in terra-firme forests, and the role of those host in the fungal distribution of the EcM fungal species throughout the Amazon region. It is important to highlight that in this region, the EcM-forests (WSF and PtF) are patchily distributed within a matrix of AM-dominated forests and a strong exchange of fungal species may be happening (Vasco-Palacios et al. 2018).
Determinants of fungal communities
Our results show that fungal communities have a strong correlation with forest types. Plant composition may affect fungal richness and species composition selectively via host-specific symbiosis, root structures, the production of root exudates, and the presence of recalcitrant litter (Burns et al. 2015; Geml et al. 2014; Hanson et al. 2012; McGuire et al. 2010; Peay et al. 2013). The composition of OTUs was relatively similar in all forests with P. tropenbosii despite the geographical distance. Populations of P. tropenbosii are only known from Colombia, forming small patches inside the terra-firme forests (Parrado-Rosseli 2005). The specific conditions of the environment that allowed P. tropenbosii to be established in particular areas of the terra-firme are still unclear, and the soil chemistry did not show significant differences between type of forests that explained this patchy distribution. The fungal communities in PtF were also similar with those from the surrounded AM-dominant forests, except in the Zafire area where more variation on community structure were detected in samples of AMF. It is important to note that both, AMF and PtF correspond to the terra-firme type of forests, and they have a similar vegetation structure and soil chemistry.
The soil fungal community present in the WSF was unique and differed significantly from the other two types of forest. However, it should be noted that despite the differences in species richness, there was no difference in the functional structure of fungal communities between forests (Fig. 4). The WSFs are a highly specialized habitats with high levels of plant endemism, and the EcM genera Dicymbe and Aldina (Fabaceae) are common in these forests (Fine and Baraloto 2016; Fine et al. 2010; Peñuela-Mora 2014). There is evidence than EcM fungi can influence degradation of organic matter and subsequently acquire and transfer a portion of released nutrients to their associated host plants, facilitating the establishment of plants in poor soils (Corrales et al. 2018; Fukami et al. 2017). Plant and fungal communities thrive on low nutrient soils composed mainly of quartz sands (Peñuela-Mora 2014). These soils are acidic with a low phosphorus concentration, are leached and have a low water-holding capacity (Peñuela-Mora 2014).
Besides the type of forests, soil pH and carbon content correlated with fungal community structure. A significant impact of soil pH on the fungal communities has been reported before at local and global scales (Burns et al. 2015; Geml et al. 2014; Högberg et al. 2007; Lucheta et al. 2016; Tedersoo et al. 2014). In this study, soil pH had a strong influence on fungal communities among forests, but no detectable pattern was apparent. For example, in the Zafire area pH ranged between 2.64 and 2.87, while in the middle Caqueta region pH ranged between 2.51 and 2.89. In particular, the role of pH is important because it affects the availability of nutrients and alters competitive interaction between fungi and other soil microorganisms (Finlay 1995; Rousk et al. 2010). We suggest that mycorrhizal fungi may play an important role enhancing nutrition uptake by the host plants in the studied forests. Nevertheless, the extension of this effect to plant communities in the studied forests is still unclear. More plots and the study of other factors such as the sand and clay proportion, aluminum content and variations in carbon contents during the rainy season and dry season must be consider in future. Soil pH may affect directly fungal composition by physiologically restricting fungal growth and survival. However, the major fungal functional guilds present in the studied samples did not show significant different responses to soil pH levels (Suppl. Table 1).
Soils in the Zafire area (13.67–42.68 g/kg) have a higher carbon content than those in the middle Caquetá region (11.4–40.09 g/kg). Previous studies in the Zafire area found a higher carbon content in soils and fine roots of WSF than in terra-firme forests (Jiménez et al. 2009). In addition, the production of fine roots in WSF was twice than in terra-firme (Jiménez et al. 2009). The high carbon allocation was explained due to limitations in soil resources in WSF (Jiménez et al. 2009). We found that the carbon content also correlated with EcM fungal communities (Mantel test >0.0.1). EcM fungi cause variations in root production in response to limitations of soil resources when nutrient absorption surface may be needed. As our study focuses on more general aspects, disentangling such causal relationships is beyond the scope of our work. In agreement with previous results, other fungal groups, such as saprotrophs, are shown to be influenced by carbon content (Mantel test >0.0.1). For instance, carbon content is expected to strongly affect the dominance of fungal decomposer communities in natural ecosystems and forest plantations (Högberg et al. 2007; Strickland and Rousk 2010). However, in this study this tendency was not observed.
Other nutrients, such as N, phosphorous, and calcium that were previously found as predictors of fungal richness in Amazonian ecosystems (López-Quintero et al. 2012; Lucheta et al. 2016; Tedersoo et al. 2014) were not found to be significant in this study. This is the case of N, were differences were not significantly within type of forests or sites. Major fungal functional guilds also did not show a significantly different response to soil N levels. The low N concentrations (0.6–1.5 g/Kg) was detected in WSF and AMF from the Zafire area. These values may correspond to N limitation, which has been reported for tropical forests occurring on podzolized soils in Amazonia and WSF (Lips and Duivenvoorden 1996; Quesada et al. 2011). Nonetheless, EcM fungi have the enzymatic ability to access organic N directly from soil organic matter, including the litter layer (Hobbie and Högberg 2012). In ecosystems in which N availability limits growth of plants and microbes, a strong competition exists between EcM and other microorganisms and fungi may regulate rates of soil N (Averill et al. 2014; Orwin et al. 2011). Studies have shown than N cycling and soil N availability shift at regional scales depending on soil heterogeneity and fungal and forests composition (Waring et al. 2016). Fertility of poor soils in Amazonia is extremely depending on biological cycles that maintain nutrients held in biomass and drive the recycling process. Our study showed that there is a link between the distribution of tropical fungal diversity with respect to local soil environments and forest types. It is important to consider that there was a non-independent process that generated the pattern that we observed. In one hand, plants and fungi could be responding independently to soil, and the EcM fungi could drive host distributions improving the adaptation of plants to white sand soils. On the other hand, EcM plant host could drive species distribution in the Amazonian region.
In summary, this study described in detail fungal communities from three forest types in the Amazonia region of Colombia. We observed that fungal soil communities have a spatial variation related to forest type (i.e. terra-firme and WSF), and carbon content and soil pH. The AMF and PtF were similar, not only in terms of fungal community composition and functional structure, but also in terms of edaphic parameters. Fungal communities in PtF are the most similar despite the geographical distances and significant differences in carbon content and soil pH. WSFs harbored a distinct fungal composition reflecting the special type of soil and plant composition of these forests. Despite these results and due to the strong correlation between vegetation and soil fertility in Amazonia, it is difficult to understand the effect of those factors on the fungal communities. EcM fungi occurred in the three types of forests, including forests dominated by AM fungi and a high number of species were shared among forests. Future work in Amazonian forests to elucidate the factors that structure fungi in tropical soils should be more integrative, combining plant and fungal community data with environmental data. Such combined approaches can provide important insights to understand the role of fungi in the nutrient cycling and how these keystone organisms affect the overall community structure of tropical lowland forests. However, this type of approach is not possible without an enrichment of fungal sequence reference databases using vouchered specimens of tropical fungal species, that help improving the identification of the 63% of OTUS that could not be identified at the genus level. Long-term studies using permanent plots are also needed to better understand the dynamics of fungal communities and abiotic factors during dry and rainy periods in the light of climate change.
References
Augspurger CK (1983) Seed dispersal of the tropical tree, Platypodium elegans, and the escape of its seedlings from fungal pathogens. J Ecol 71:759–771. https://doi.org/10.2307/2259591
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545. https://doi.org/10.1038/nature12901
Baas-Becking LG (1934) Geobiologie; of inleiding tot de milieukunde. WP Van Stockum Zoon NV, The Hague
Bahram M, Peay KG, Tedersoo L (2015) Local-scale biogeography and spatiotemporal variability in communities of mycorrhizal fungi. New Phytol 205:1454–1463. https://doi.org/10.1111/nph.13206
Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, Bengtsson-Palme J, Anslan S, Coelho LP, Harend H, Huerta-Cepas J (2018) Structure and function of the global topsoil microbiome. Nature 560:233–237. https://doi.org/10.1038/s41586-018-0386-6
Bálint M, Bahram M, Eren AM, Faust K, Fuhrman JA, Lindahl B, O'hara RB, Öpik M, Sogin ML, Unterseher M, Tedersoo L (2016) Millions of reads, thousands of taxa: microbial community structure and associations analyzed via marker genes. FEMS Microbiol Rev 29:686–700. https://doi.org/10.1093/femsre/fuw017
Bragg L, Stone G, Imelfort M, Hugenholtz P, Tyson G.W (2012) Fast, accurate error-correction of amplicon pyrosequences using Acacia. Nat Methods 9: 425–426. doi: https://doi.org/10.1038/nmeth.1990
Brearley FQ (2012) Ectomycorrhizal associations of the Dipterocarpaceae. Biotropica 44:637–648. https://doi.org/10.1111/j.1744-7429.2012.00862.x
Bunn RA, Simpson DT, Bullington LS, Lekberg Y, Janos DP (2019) Revisiting the ‘direct mineral cycling’hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? The ISME journal 1
Burns JH, Anacker BL, Strauss SY, Burke DJ (2015) Soil microbial community variation correlates most strongly with plant species identity, followed by soil chemistry, spatial location and plant genus. AoB Plants 7. https://doi.org/10.1093/aobpla/plv030
Camenzind T, Hempel S, Homeier J, Horn S, Velescu A, Wilcke W, Rillig MC (2014) Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Glob Chang Biol 20:3646–3659. https://doi.org/10.1111/gcb.12618
Cannon PF, Kirk PM (2007) Fungal families of the world. CABI, England
Comita LS, Muller-Landau HC, Aguilar S, Hubbell SP (2010) Asymmetric density dependence shapes species abundances in a tropical tree community. Science 329:330–332
Corrales A, Turner BL, Tedersoo L, Anslan S, Dalling JW (2017) Nitrogen addition alters ectomycorrhizal fungal communities and soil enzyme activities in a tropical montane forest. Fun Ecol 27:14–23. https://doi.org/10.1016/j.funeco.2017.02.004
Corrales A, Mangan SA, Turner BL, Dalling JW (2016) An ectomycorrhizal nitrogen economy facilitates monodominance in a neotropical forest. Ecol Lett 19:383–392. https://doi.org/10.1111/ele.12570
Corrales A, Henkel TW, Smith ME (2018) Ectomycorrhizal associations in the tropics–biogeography, diversity patterns and ecosystem roles. New Phytol 220:1076–1109. https://doi.org/10.1111/nph.15151
Duivenvoorden JF, Duque A (2010) Composition and diversity of northwestern Amazonian rainforests in a geoecological context. In: Hoorn C, Wesselingh FP (eds) Amazonia, landscape and species evolution: a look into the past. Blackwell Publishing, West Sussex, pp 360–372
Duivenvoorden JF, Lips JM (1993) Landscape ecology of the middle Caquetá basin; explanatory notes to the maps. Tropenbos, Colombia
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Estrada-Peña A, Cabezas-Cruz A, Pollet T, Vayssier-Taussat M, Cosson JF (2018) High throughput sequencing and network analysis disentangle the microbial communities of ticks and hosts within and between ecosystems. Front Cell Infect Mi 8:1–12. https://doi.org/10.3389/fcimb.2018.00236
Eva HD, Glinni A, Janvier P, Blair-Myers C (1999) Vegetation map of tropical South America. TREES Publications Series D, Luxembourg
Fearnside PM (2005) Deforestation in Brazilian Amazonia: history, rates, and consequences. Conserv Biol 19:680–688. https://doi.org/10.1111/j.1523-1739.2005.00697.x
Fine PV, Baraloto C (2016) Habitat endemism in white-sand forests: insights into the mechanisms of lineage diversification and Community assembly of the Neotropical Flora. Biotropica 48:24–33. https://doi.org/10.1111/btp.12301
Fine PV, Garcia–Villacorta R, NCA P, Mesones I, Kembel SW (2010) A floristic study of the white sand forest of Peru. Ann Missouri Bot Gard 97:283–305. https://doi.org/10.3417/2008068
Finlay RD (1995) Interactions between soil acidification, plant growth and nutrient uptake in ectomycorrhizal associations of forest trees. Ecol Bull 1:197–214
Frąc M, Hannula SE, Bełka M, Jędryczka M (2018) Fungal biodiversity and their role in soil health. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.00707
Fukami T, Nakajima M, Fortunel C, Fine PV, Baraloto C, Russo SE, Peay KG (2017) Geographical variation in community divergence: insights from tropical forest monodominance by ectomycorrhizal trees. Am Nat 190:S105–S122. https://doi.org/10.1086/692439
Geml J, Pastor N, Fernandez L, Pacheco S, Semenova TA, Becerra AG, Nouhra ER (2014) Large-scale fungal diversity assessment in the Andean Yungas forests reveals strong community turnover among forest types along an altitudinal gradient. Mol Ecol 23:2452–2472. https://doi.org/10.1111/mec.12765
Gentry AH (1988) Tree species richness of upper Amazonian forests. Proc Natl Acad Sci 85:156–159. https://doi.org/10.1073/pnas.85.1.156
Gilbert GS, Webb CO (2007) Phylogenetic signal in plant pathogen-host range. Proc Natl Acad Sci 104:4979–4983. https://doi.org/10.1073/pnas.0607968104
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19. https://doi.org/10.18637/jss.v022.i07
Grupe AC, Vasco-Palacios AM, Smith ME, Boekhout T, Henkel TW (2016) Sarcodon in the Neotropics II: four new species from Colombia and a key to the regional species. Mycologia 108:791–805. https://doi.org/10.3852/15-254
Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JB (2012) Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol 10:497–506. https://doi.org/10.1038/nrmicro2795
Henkel TW, Terborgh J, Vilgalys RJ (2002) Ectomycorrhizal fungi and their leguminous hosts in the Pakaraima Mountains of Guyana. Mycol Res 106:515–531. https://doi.org/10.1017/s0953756202005919
Henkel TW, Aime MC, Chin MML, Miller SL, Vilgalys R, Smith ME (2012) Ectomycorrhizal fungal sporocarp diversity and discovery of new taxa in Dicymbe monodominant forests of the Guiana shield. Biodivers Conserv 21:2195–2220. https://doi.org/10.1007/s10531-011-0166-1
Hobbie EA, Högberg P (2012) Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196:367–382. https://doi.org/10.1111/j.1469-8137.2012.04300.x
Högberg MN, Högberg P, Myrold DD (2007) Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150:590–601. https://doi.org/10.1007/s00442-006-0562-5
Hoorn C, Wesselingh FP (2010) Introduction: Amazonia, landscapes and species evolution. In: Hoorn C, Wesselingh FP (eds) Amazonia, landscape and species evolution: a look into the past. Blackwell Publishing, West Sussex, pp 1–6
Hoorn C, Wesselingh FP, Ter Steege H, Bermudez MA, Mora A, Sevink J, Antonelli A (2010) Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330:927–931. https://doi.org/10.1126/science.1194585
Houbraken J, López-Quintero C, Frisvad JC, Boekhout T, Theelen B, Franco-Molano AE, Samson RA (2011) Five new Penicillium species, P. araracuaraense, P. ellenii, P. penarojaense, P. vanderhammenii and P. wotroi, from Colombian leaf litter. Int J Syst Evol Microbiol 61:1462–1475. https://doi.org/10.1099/ijs.0.025098-0
Hughes KW, Petersen RH, Lickey EB (2009) Using heterozygosity to estimate a percentage DNA sequence similarity for environmental species’ delimitation across basidiomycete fungi. New Phytol 182:795–798. https://doi.org/10.1111/j.1469-8137.2009.02802.x
IGAC (2003) Mapa Digital Integrado fisico politico departamento del Amazonas. Instituto Geografico Agustin Codazzi
Jiménez EM, Moreno FH, Peñuela MC, Patiño S, Lloyd J (2009) Fine root dynamics for forests on contrasting soils in the Colombian Amazon. Biogeosciences 6:2809–2827. https://doi.org/10.5194/bg-6-2809-2009
Jiménez EM, Peñuela-Mora MC, Sierra CA, Lloyd J, Phillips OL, Moreno FH, Navarrete D, Prieto A, Rudas A, Álvarez E, Quesada CA, Grande-Ortíz MA, García-Abril A, Patiño S (2014) Edaphic controls on ecosystem-level carbon allocation in two contrasting Amazon forests. J Geophys Res Biogeosci 119:1820–1830. https://doi.org/10.1002/2014jg002653
Lee J, Lee S, Young JP (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbio Ecol 65:339–349. https://doi.org/10.1111/j.1574-6941.2008.00531.x
Lindahl BD, Tunlid A (2015) Ectomycorrhizal fungi–potential organic matter decomposers, yet not saprotrophs. New Phytol 205:1443–1447. https://doi.org/10.1111/nph.13201
Lindahl BD, Nilsson RH, Tedersoo L et al (2013) Fungal community analysis by high-throughput sequencing of amplified markers – a user’s guide. New Phytol 199:288–299. https://doi.org/10.1111/nph.12243
Lips J, Duivenvoorden J (1996) Fine litter input to terrestrial humus forms in Colombian Amazonia. Oecologia 108:138–150. https://doi.org/10.1007/bf00333225
Looney BP, Ryberg M, Hampe F, Sánchez-García M, Matheny PB (2016) Into and out of the tropics: global diversification patterns in a hyperdiverse clade of ectomycorrhizal fungi. Mol Ecol 25:630–647. https://doi.org/10.1111/mec.13506
López-Quintero CA, Straatsma G, Franco-Molano AE, Boekhout T (2012) Macrofungal diversity in Colombian Amazon forests varies with regions and regimes of disturbance. Biodivers Conserv 21:2221–2243. https://doi.org/10.1007/s10531-012-0280-8
López-Quintero CA, Atanasova L, Franco-Molano AE, Gams W, Komon-Zelazowska M, Theelen B, Müller WH, Boekhout T, Druzhinina I (2013) DNA barcoding survey of Trichoderma diversity in soil and litter of the Colombian Amazonian rainforest reveals Trichoderma strigosellum sp.nov. and other species. Antonie Van Leeuwenhoek 104:657–674. https://doi.org/10.1007/s10482-013-9975-4
Lucheta AR, de Souza C, Roesch LFW, Tsai SM, Kuramae EE (2016) Fungal Community assembly in the Amazonian dark earth. Microb Ecol 71:962–973. https://doi.org/10.1007/s00248-015-0703-7
Lucheta AR, de Souza C, Tsai SM, Kuramae EE (2017) Amazonian dark earth and its black carbon Particles Harbor different fungal abundance and diversity. Pedosphere 27:832–845. https://doi.org/10.1016/s1002-0160(17)60415-6
Matheny PB, Aime MC, Bougher NL, Buyck B, Desjardin DE, Horak E, Kropp BR, Logde DJ, Soytong K, Trappe JM, Hibbett DS (2009) Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. J Biogeogr 36:577–592. https://doi.org/10.1111/j.1365-2699.2008.02055.x
Mayor J, Bahram M, Henkel TW, Buegger F, Pritsch K, Tedersoo L (2015) Ectomycorrhizal impacts on plant nitrogen nutrition: emerging isotopic patterns, latitudinal variation and hidden mechanisms. Ecol Lett 18:96–107. https://doi.org/10.1111/ele.12377
McGuire KL (2007) Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 88:567–574
McGuire KL, Zak DR, Edwards IP, Blackwood CB, Upchurch R (2010) Slowed decomposition is biotically mediated in an ectomycorrhizal, tropical rain forest. Oecologia 164:785–795. https://doi.org/10.1007/s00442-010-1686-1
McGuire KL, Fierer N, Bateman C, Treseder KK, Turner BL (2012) Fungal community composition in Neotropical rain forests: the influence of tree diversity and precipitation. Microb Ecol 63:804–812. https://doi.org/10.1007/s00248-011-9973-x
Moyersoen B (2006) Pakaraimaea dipterocarpacea is ectomycorrhizal, indicating an ancient Gondwanaland origin for the ectomycorrhizal habit in Dipterocarpaceae. New Phytol 172:753–762. https://doi.org/10.1111/j.1469-8137.2006.01860.x
Moyersoen B, Weiß M (2014) New neotropical Sebacinales species from a Pakaraimaea dipterocarpacea forest in the Guayana region, southern Venezuela: structural diversity and phylogeography. PLoS ONE 9: e107078. https://doi.org/10.1371/journal.pone.0103076
Mueller RC, Paula FS, Mirza BS, Rodrigues JL, Nüsslein K, Bohannan BJ (2014) Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J 8:1548–1550. https://doi.org/10.1038/ismej.2013.253
Mueller RC, Rodrigues JL, Nüsslein K, Bohannan BJ (2016) Land use change in the Amazon rain forest favours generalist fungi. Funct Ecol 30:1845–1853. https://doi.org/10.1111/1365-2435.12651
Mueller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley and Sons, New York
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Wagner H (2013) Package ‘vegan’. R Packag ver 254:20–28
Orwin K, Kirschbaum M, St. John M, Dickie I (2011) Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecol Lett 14:493–502. doi:10.1111/j.1461-0248.2011.01611.x
Parrado-Rosseli A (2005) Fruit availability and seed dispersal in terra-firme forest of Colombian Amazonia. Wageningen, Tropenbos-International
Paula FS, Rodrigues JL, Zhou J, Wu L, Mueller RC, Mirza BS, Bohannan BJ, Nüsslein K, Deng Y, Tiedje JM, Pellizari VH (2014) Land use change alters functional gene diversity, composition and abundance in Amazon forest soil microbial communities. Mol Ecol 23:2988–2999. https://doi.org/10.1111/mec.12786
Peay KG, Baraloto C, Fine PV (2013) Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J 7:1852–1861. https://doi.org/10.1038/ismej.2013.66
Peña-Venegas C, Vasco-Palacios AM (2019) Endo- and Ectomycorrhizas in tropical ecosystems of Colombia. In: Pagano M, Lugo M (eds) Mycorrhizal Fungi in South America. Fungal Biology. Springer, Cham, pp 111–146. https://doi.org/10.1007/978-3-030-15228-4_6
Peñuela-Mora MC (2014) Understanding Colombian Amazonian white sand Forest. Dissertation. Utrecht University, Bogota D.C.
Quesada CA, Lloyd J, Anderson LO, Fyllas NM, Schwarz M, Czimczik CI (2011) Soils of Amazonia with particular reference to the RAINFOR sites. Biogeosciences 8: 1415–1440. https://doi.org/10.5194/bg-8-1415-2011
Quesada CA, Lloyd J, Schwarz M, Baker TR, Phillips OL, Patiño S, Ramírez H (2009) Regional and large-scale patterns in Amazon forest structure and function are mediated by variations in soil physical and chemical properties. Biogeosci Discuss 6:3993–4057. https://doi.org/10.5194/bgd-6-3993-2009
Quesada CA, Phillips OL, Schwarz M, Czimczik CI, Baker TR, Patiño S, Fyllas NM, Hodnett MG, Herrera R, Almeida S, Dávila EA (2012) Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 9:2203–2246
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org
Rosling A, Cox F, Cruz-Martinez K, Ihrmark K, Grelet GA, Lindahl BD, James TY (2011) Archaeorhizomycetes: unearthing an ancient class of ubiquitous soil fungi. Science 333:876–879. https://doi.org/10.1126/science.1206958
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010)Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME J 4:1340–1351. https://doi.org/10.1038/ismej.2010.58
Roy M, Schimann H, Braga-Neto R, Da Silva RAE, Duque J, Frame D, Wartchow F, Neves MA (2016) Diversity and distribution of ectomycorrhizal fungi from Amazonian lowland white-sand forests in Brazil and French Guiana. Biotropica 48:90–100. https://doi.org/10.1111/btp.12297
Roy M, Vasco-Palacios AM, Geml J, Buyck B, Delgat L, Giachini A, Grebenc T, Harrower E, Kuhar F, Magnano A, Rinaldi AC, Schimann H, Selosse MA, Wartchow F, Neves MA (2017) The (re) discovery of ectomycorrhizal symbioses in Neotropical ecosystems sketched in Florianópolis. New Phytol 214:920–923. https://doi.org/10.1111/nph.14531
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/aem.01541-09
Simon L, Lalonde M, Bruns TD (1992) Specific amplification of 18S fungal ribosomal genes from VA endomycorrhizal fungi colonizing roots. Appl Environ Microbiol 58:291–295
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. 3rd. Academic Press New York, p 815
Smith ME, Henkel TW, Aime MC, Fremier AK, Vilgalys R (2011) Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a Neotropical rainforest. New Phytol 192: 699–712. doi https://doi.org/10.1111/j.1469-8137.2011.03844.x
Smith ME, Henkel TW, Uehling JK, Fremier AK, Clarke HD, Vilgalys R (2013) The ectomycorrhizal fungal community in a Neotropical forest dominated by the endemic dipterocarp Pakaraimaea dipterocarpacea. PLoS One 8:e55160. https://doi.org/10.1371/journal.pone.0055160
Strickland MS, Rousk J (2010) Considering fungal: bacterial dominance in soils–methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395. https://doi.org/10.1016/j.soilbio.2010.05.007
Sulzbacher MA, Grebenc T, Giachini AJ, Baseia IG, Nouhra ER (2017) Hypogeous sequestrate fungi in South America – how well do we know them? Symbiosis 71:9–17
Talbot JM, Bruns TD, Taylor JW, Smith DP, Branco S, Glassman SI, Erlandson S, Vilgalys R, Liao HL, Smith ME, Peay KG (2014) Endemism and functional convergence across the north American soil mycobiome. Proc Natl Acad Sci U S A 111:6341–6346. https://doi.org/10.1073/pnas.1402584111
Tedersoo L, Smith ME (2013) Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Bio Rev 27:83–99. https://doi.org/10.1016/j.fbr.2013.09.001
Tedersoo L, May TW, Smith ME (2010a) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263. https://doi.org/10.1007/s00572-009-0274-x
Tedersoo L, Nilsson RH, Abarenkov K, Jairus T, Sadam A, Saar I, Bahram M, Bechem E, Chuyong G, Koljalg U (2010b) 454-pyrosequencing and sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol 188:291–301. https://doi.org/10.1111/j.1469-8137.2010.03373.x
Tedersoo L, Sadam A, Zambrano M, Valencia R, Bahram M (2010c) Low diversity and high host preference of ectomycorrhizal fungi in Western Amazonia, a Neotropical biodiversity hotspot. ISME J 4:465–471. https://doi.org/10.1038/ismej.2009.131
Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Vasco-Palacios AM, De Kesel A et al (2014) Global diversity and geography of soil fungi. Science 346:4168–4183. https://doi.org/10.1111/mec.12849
Tedersoo L, Bahram M, Puusepp R, Nilsson RH, James TY (2017) Novel soil-inhabiting clades fill gaps in the fungal tree of life. Microbiome 5:42. https://doi.org/10.1186/s40168-017-0259-5
ter Steege H, ATDN, RAINFOR (2010) Contribution of current and historical processes to patterns of tree diversity and composition of the Amazon. In: Hoorn C, Vonhof H, Wesselingh F (eds) Amazonia, landscape and species evolution: a look into the past. Wiley-Blackwell, UK, Oxford, pp 349–359
ter Steege H, Pitman NC, Phillips OL, Chave J, Sabatier D, Duque A, Molino JF, Prévost AMF, Spichiger R, Castellanos H, von Hildebrand P, Vásquez R (2006) Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443:444–447. https://doi.org/10.1038/nature05134
UNITE Community (2017): UNITE mothur release. Version 2016-08-22. https://unite.ut.ee/repository.php. Accessed 10 June 2017
Van der Heijden MG, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423. https://doi.org/10.1111/nph.13288
Vasco-Palacios AM (2016) Ectomycorrhizal fungi in Amazonian tropical forests in Colombia (Doctoral dissertation, Utrecht University
Vasco-Palacios AM, Hernández J, Peñuela-Mora MC, Franco-Molano AE, Boekhout T (2018) Ectomycorrhizal fungi diversity in a white sand forest in western Amazonia. Fungal Ecol 31:9–18. https://doi.org/10.1016/j.funeco.2017.10.003
Verbruggen E, Pena R, Fernandez CW, Soong JL (2017) Mycorrhizal interactions with saprotrophs and impact on soil carbon storage. 441-460. In: Collins N, Gehring C and Jansa J. Mycorrhizal Mediation of Soil. Elsevier. https://doi.org/10.1016/B978-0-12-804312-7.00024-3
Vu D, Groenewald M, Szöke S, Cardinali G, Eberhardt U, Stielow B, de Vries M, Verkleij GJ, Crous PW, Boekhout T, Robert V (2016) DNA barcoding analysis of more than 9 000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud Mycol 1:91–105
Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol 92:135–154. https://doi.org/10.1016/j.simyco.2016.11.007
Waring BG, Adams R, Branco S, Powers JS (2016) Scale-dependent variation in nitrogen cycling and soil fungal communities along gradients of forest composition and age in regenerating tropical dry forests. New Phytol 209:845–854. https://doi.org/10.1111/nph.13654
Yilmaz N, López-Quintero CA, Vasco-Palacios AM, Frisvad J, Theelen B, Boekhout T, Samson R, Houbraken J (2016) Four novel Talaromyces species isolated from leaf litter from Colombian Amazon rain forests. Mycol Prog 10-11:1041–1056. https://doi.org/10.1007/s11557-016-1227-3
Acknowledgements
This research was supported in part by grants to Aida Vasco-Palacios from The Netherlands Fellowship Programmes (NFP) of the Netherlands organization for international cooperation in higher education (NUFFIC), The Faculty for the Future - Schlumberger Foundation (FFTF Grant 2011–2013), and The International Foundation of Science (IFS Grant D/5052–1, 2011-2-13f) and Utrecht University 2014-2015. We would also like to thank Miguel Arcangel and Eduardo Paki and his family for their advice on the forests; Maria Cristina Peñuela for her contributions to the knowledge of white-sand forests in Colombia and for facilitating our work in the biological station El Zafire, and the Laboratorio TEHO of la Universidad de Antioquia. To Wilson López for his support and advice with the statistical analysis in R and to Diego Fernando Ramírez Guerrero for his valuable comments, which helped to improve the manuscript. Research permission for this study was number 07, 01-March-2012 (Autoridad Nacional de Licencias Ambientales, ANLA-Colombia). Access to genetic resources for scientific research contract, with non-commercial interest No 73, 21 May 2013 (Ministerio de Ambiente y Desarrollo Sostenible, MADS-Colombia).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Bernardo M Flores.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. 1
a Differences in species richness between AMF, PtF, and WSF in Colombia. Species richness residuals and standard deviation of species richness recovered from rainforest soils in the Amazon region in Colombia. The internal black bar within the boxes is the standard deviation. The external bars indicate the lowest and highest data of species richness. b Differences in species richness of EcM fungi between AMF, PtF, and WSF in Colombia (PNG 32 kb)
Fig. 2
Cluster analysis showing similarity values of the fungal community compositions between plots based on the Jaccard index. Scale 0–1, with 1 indicating maximal similarity. Color brown represents plots from WSFs, green from PtFs and orange from AMFs. Plots were established in three localities, the biological station El Zafire in red color, and in the Middle Caquetá region, the localities of Peña Roja (PR) in blue color and Puerto Santander (PS) in violet color (PNG 305 kb)
ESM 1
(DOCX 35 kb)
Rights and permissions
About this article
Cite this article
Vasco-Palacios, A.M., Bahram, M., Boekhout, T. et al. Carbon content and pH as important drivers of fungal community structure in three Amazon forests. Plant Soil 450, 111–131 (2020). https://doi.org/10.1007/s11104-019-04218-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04218-3