Abstract
Aims
Shrub encroachment has profound influences on regional carbon cycling. However, few studies have examined the changes in soil organic carbon (SOC) components at the molecular level along a climate gradient. In this study, we aimed to investigate the effects of biotic and abiotic factors on the patterns of SOC components in the shrub patches and the grassy matrix.
Methods
We analyzed the distribution and controlling factors of SOC components (including free lipids, bound lipids, and lignin-derived phenols) in the topsoil of shrub-encroached grasslands along natural climate gradients in Inner Mongolia, China.
Results
We found that the concentrations of bound lipids and lignin-derived phenols were significantly higher and the vanillic acid to vanillin ratio ((Ad/Al)v) was significantly lower in the shrub patches than in the grassy matrix (p < 0.05). After excluding variables exhibiting collinearity, redundancy analysis showed that shrub patch cover and soil pH were the most important variables that influenced SOC composition in the shrub patches, while herb characteristics and shrub density were the most important in the grassy matrix. Structural equation modeling showed that shrub characteristics at the plot scale greatly contributed to the variance in all components in the grassy matrix, whereas soil properties were more important in the shrub patches.
Conclusions
Our results highlight that although the topsoil carbon content did not change, shrub encroachment altered the SOC components and their drivers in the Inner Mongolian grasslands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shrub encroachment, which is the shift in domination from grasses to shrubs, has become one of the most important issues in grasslands throughout the world (Reynolds et al. 2007). Shrub encroachment typically changes soil biogeochemical cycles (Zhou et al. 2018), and leads to the redistribution of soil nutrients (Eldridge et al. 2011). Many previous studies have demonstrated that soil organic carbon (SOC) changes after shrub encroachment vary with climate (Jackson et al. 2002; Knapp et al. 2008), soil properties (Eldridge et al. 2011) and vegetation (Maestre et al. 2009), however, very few studies have evaluated the response of the molecular components of SOC to shrub encroachment (Zhou et al. 2017).
Understanding the molecular components of SOC can provide an insight into soil carbon dynamics (Kögel-Knabner 2002; Pisani et al. 2016; Kögel-Knabner and Rumpel 2018). Soil organic matter is a heterogeneous mixture derived from plants, microbes and animal residues (Kögel-Knabner 2002). Previous studies have revealed that the composition of SOC is controlled by the combination of input characteristics and decomposition processes (Tamura and Tharayil 2014; Li et al. 2017). The precise identification and quantification of the origin and decomposition of SOC are necessary to improve the understanding of C cycling and the response of SOC to changing environmental conditions (Armas-Herrera et al. 2016). The solvent-extractable (“free”, i.e., n-alkanols, n-alkanes and n-alkanoic acids) and hydrolyzable (“bound”, i.e., cutin and suberin) lipids as well as lignin-derived phenols (i.e., vanillyls, syringyls and cinnamyls) play essential roles in the incorporation of residues into the mineral soil. Therefore, these compounds are regarded as important molecular proxies for tracing the origin and degradation of SOC (Dai et al. 2018; Kögel-Knabner and Rumpel 2018; Zhou et al. 2018). Qualitative and quantitative analyses of these molecular components of SOC have been successfully used to examine the response of SOC to climate change (Feng et al. 2010), vegetation shifts (Filley et al. 2008) and land use change (Zhao et al. 2014; Pisani et al. 2016).
Shrub encroachment has widely occurred in the Inner Mongolian grasslands in response to climate change and increased human disturbance, i.e., long-term overgrazing, (Peng et al. 2013; Chen et al. 2015; Fang et al. 2015) and it has strong potential to influence the structure and biogeochemical cycles of this grassland ecosystem (Peng et al. 2013; Zhou et al. 2017; Zhang et al. 2018). Previous studies showed that shrub encroachment did not significantly change the topsoil organic carbon (OC) content in Inner Mongolia at the regional scale, which was partly due to the different responses in the typical grasslands and desert grasslands (Li et al. 2019). This result indicated that the consequences of shrub encroachment were dependent on the climate, vegetation and soil properties (Li et al. 2019). A recent study revealed that shrub encroachment changed the SOC composition by altering the inputs and soil microbial community in the shrub patches and grassy matrix in a Xilinhot grassland (Zhou et al. 2017). However, how shrub encroachment affects the molecular SOC components at the regional scale in Inner Mongolian grasslands remains unclear (Zhou et al. 2017). The SOC composition in different ecosystems was shown to be driven by climate and soil properties (Feng et al. 2010; Pisani et al. 2016; Cai et al. 2017). For example, Feng et al. (2008) found that cutin-derived components increased while other major compounds remained unchanged (or even decreased) after soil warming in a Canadian forest, and Angst et al. (2018) found that free lipids and bound lipids in a forest subsoil accumulated in the sand- and fine-sized aggregates, respectively. However, very few studies have investigated the vegetation, soil, and climate together to assess their effects on SOC composition (Pisani et al. 2016).
This study aimed to quantify SOC components, including free lipids, bound lipids and lignin-derived phenols, in soils of five shrub-encroached grasslands along a climate gradient in Inner Mongolia. The objectives of this study are to (1) explore the effects of shrub encroachment on the chosen components of topsoil OC and (2) investigate the effects of biotic and abiotic factors on the patterns of these components in the shrub patches and the grassy matrix.
Materials and methods
Site description and field sampling
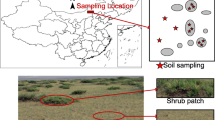
Shrub encroachment forms a mosaic landscape that shrub patches in the grassy matrix, which is significantly different from pure grasslands (Maestre et al. 2009; Chen et al. 2015; Fig. S1). We conducted a field survey along a precipitation gradient from mid-July to September 2013 (Chen et al. 2015). Five study sites distributed in the typical shrub-encroached grassland area in Inner Mongolia were selected. At each study site, we established three 20 × 20 m plots, each containing four 10 × 10 m subplots. The number, size and height of the shrub patches in two randomly selected subplots were measured to assess the shrub characteristics at the plot scale. In each 20 × 20 m plot, we established a pair of 1-m2 quadrats, with one quadrat within the shrub patch and one in the neighboring grassy matrix. We recorded the size, cover and height of chosen shrub patch, and then recorded the herb richness in the paired 1-m2 quadrats. Then, each 1-m2 quadrat was divided into four 0.25 m2 subquadrats. We recorded the number and height of each herbaceous species and clipped all aboveground biomass (AGB), including that of shrubs and herbs, at the soil surface in a randomly selected 0.25 m2 subquadrat. Herb abundance was calculated as the sum of individual herbs in the subquadrat. Topsoil (0–10 cm) samples and herb belowground biomass (BGB) were also collected from the subquadrats using a stainless-steel corer with a diameter of 5 cm. Each soil core was divided into an air-dried sample for the analysis of nutrient contents, pH and texture, and a frozen sample (−20 °C) for biomarker analysis. The vegetation and soil characteristics were listed in Table 1.
Laboratory analyses
All air-dried soil samples were sieved through a 2-mm sieve, and the plant roots were carefully removed. The soil total carbon (STC) and soil total nitrogen (STN) were measured using an elemental analyzer (Vario El III, Elementar, Germany). The SOC content was calculated as the difference between the STC and the soil inorganic carbon, which was measured using a carbonate content analyzer (Eijkelkamp 08.53, Netherlands). The soil pH was measured from a soil and water suspension (1:2.5 w/v) using a pH meter. After treatment with H2O2 and HCl, the soil texture was measured using a particle size analyzer (Mastersizer 2000, Malvern, UK).
Biomarker extraction
Solvent extraction, base hydrolysis and copper(II) oxide (CuO) oxidation were conducted to separate free lipids, bound lipids and lignin-derived phenols (Feng et al. 2010; Zhou et al. 2017, 2018). Briefly, approximately 6 g of soil samples were extracted using ultrasonication for 15 min and then centrifuged at 3000 rpm for 10 min using 15 mL of chromatography-grade dichloromethane: methanol (1:1; v: v). The combined solvent extracts (free lipids) were concentrated using rotary evaporation.
After solvent extraction, approximately 3 g of soil residue was sealed with 15 mL of 1 M methanolic KOH in Teflon-lined bombs and heated at 100 °C for 3 h. Then, the suspension was acidified to pH 1 with 6 M HCl. The base hydrolysis extracts were recovered using liquid-liquid extraction with 20 mL ethyl acetate. Then, the concentrated products were methylated with 1 mL of 14% BF3: MeOH (90 min at 70 °C). The bound lipids were obtained using liquid-liquid extraction with 2 mL hexane.
The remaining residues after solvent extraction were sealed with 1 g of CuO, 100 mg of ammonium iron (II) sulfate hexahydrate and 15 mL of 2 M NaOH and heated at 170 °C for 2.5 h after oxygen exhaustion. The mixture was centrifuged at 3000 rpm for 10 min, and the supernatant was then acidified to pH 1 with 6 M HCl. The extracts were kept in the dark for 1 h. After centrifugation (3000 rpm, 10 min), the lignin-derived phenols were recovered using 20 mL ethyl acetate.
All products were converted to trimethylsilyl derivatives using 100 μL N, O-bis-(trimethylsilyl) trifluoroacetamide and 100 μL pyridine at 60 °C for 2 h. The biomarkers were identified and quantified using an Agilent 7890A gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) coupled to an Agilent 5973 N quadruple mass selective detector and a flame ionization detector, respectively. The free lipids and bound lipids were quantified by comparison with the internal standards (squalane and 5α-cholestane, respectively), whereas the lignin-derived phenols were quantified using the calibration curves of eight lignin-phenol standards (Zhao et al. 2014; Zhou et al. 2017, 2018). All the biomarkers were normalized to the SOC contents.
Statistical analysis
Climate data from 1982 to 2011 were obtained from the China Meteorological Data Sharing Service System (http://cdc.nmic.cn). Differences in variables between the shrub patches and grassy matrix were examined using paired t tests. Climate, shrub, herb and soil characteristics were expected to explain the variance in SOC components. Details of the explanatory variables were provided in Table 2. The relationships between each biomarker and the explanatory variables were examined using Pearson correlations. Then, the explanatory variables were subjected to redundancy analysis (RDA) to determine the important drivers. To decrease the collinearity, the explanatory variables with the highest variance inflation factor (vif) values were removed one by one until the vifs of all variables in the RDA were lower than 10. Finally, structural equation modeling (SEM) was further conducted to evaluate the relative importance of climate, shrub, herb and soil characteristics in controlling the SOC components. The data of all the environmental variables were Z-score normalized and included in the principal component analysis (PCA). The first component (PC1) of each explanatory group was used as input in the subsequent SEM analysis. The fit of our model was evaluated using a chi-square test and the root mean square error of approximation (Grace 2006). The statistical analysis was conducted using the R 3.3.1 platform (R Foundation for Statistical Computing, Vienna, AT), and the SEM analysis was conducted in AMOS 17.0 (Amos Development Corporation, Chicago, IL, USA).
Results
Plant community and soil properties
At the plot scale, the density, cover, mean area and height of the shrub patches ranged from 13 to 215 individuals/200 m2, 2.8 to 23.1%, 0.2 to 2.9 m2 and 18.3 to 41.8 cm, respectively (Table 2). In addition, the patch area, cover, height and AGB of the chosen shrub individuals ranged from 0.7 to 9.2 m2, 55.0 to 81.7%, 30.1 to 47.5 cm and 36.9 to 84.7 g/0.25 m2, respectively (Table 2).
The herb richness in the grassy matrix was significantly higher than that in the shrub patches (7–13 vs. 4–12/m2, respectively; p = 0.03). However, significant differences in herb abundance (34–164 individuals/m2), height (7.6–19.0 cm), AGB (8.8–42.9 g/0.25 m2) and BGB (129.2–525.5 g/0.05 m3) were not observed (p > 0.05). Similarly, pH (7.4–9.5), STN (0.02–0.12%) and SOC (0.16–0.99%) also did not differ between the shrub patches and the grassy matrix (p > 0.05). The soil clay content was significantly higher in the grassy matrix than in the shrub patches (11.3–27.6% vs. 4.7–19.5%, respectively; p < 0.01; Table 2).
SOC components
The dominant free lipids from the solvent extraction were C27-C31n-alkanes, C18-C30n-alkanols and C16-C30n-alkanoic acids, with normalized concentrations of 0.44–2.37 mg/g OC, 0.90–14.89 mg/g OC and 0.82–5.02 mg/g OC, respectively (Table 3). The bound lipids from base hydrolysis mainly included C14-C16 alkanoic acids (1.81–3.81 mg/g OC), C16-C26α, ω-alkanedioic acids (DAs, 0.25–0.89 mg/g OC) and C16-C24ω-hydroxyalkanoic acids (OH FAs; 0.02–0.83 mg/g OC). Regarding the molecular characteristics of the local plants (Zhou et al. 2017, 2018), we defined the C16 and C18ω-OH FAs with mid-chain hydroxy or epoxy groups as cutin components, and their concentrations were very low in this study (Table 3). The C16 and C18ω-OH FAs and DAs were components in both cutin and suberin (∑CS, 0.13–0.73 mg/g OC), and the long-chain α, ω-DAs and ω-OH FAs were defined as suberin components (0.09–0.80 mg/g OC) in this study. Eight lignin-derived phenols (vanillyls: vanillin, acetovanillone, vanillic acid; syringyls: syringaldehyde, acetosyringone, syringic acid; cinnamoyls: p-coumaric acid and ferulic acid) were identified in the CuO products; among them, the vanillyls were the most abundant class of components, with concentrations of 5.67–13.55 mg/g OC (Table 3).
Factors affecting the SOC components
Vegetation, climate and soil all affect the SOC components in shrub-encroached grasslands. First, we used Pearson correlation to analyze how these factors affect the SOC components. Our data indicated that the n-alkanols in the grassy matrix were significantly and negatively correlated with herb richness and AGB (p < 0.05; Fig. 1a). The n-alkanes in both the shrub patches and grassy matrix were also significantly and negatively correlated with the soil carbon and nitrogen (p < 0.05; Fig. 1).
Pearson correlations between different soil organic carbon biomarkers and explanatory variables in the grassy matrix (a) and shrub patches (b). Different colors represent different coefficients. Significance is indicated by asterisks: *, p < 0.05; **, p < 0.01. FA, alkanoic acid; ∑CS, components in both cutin and suberin; C/V, cinnamyls/vanillyls; S/V, syringyls/vanillyls; (Ad/Al)s, syringic acid/syringaldehyde; (Ad/Al)v, vanillic acid/vanillin; PE, potential evaporation; AI, aridity index; MAT, mean annual temperature; SR, shrub richness; SDp, SCp, SAp and SHp represent shrub density, cover, area and height at the plot scale, respectively; SAi, SCi, SHi and SBi represent the size, cover, height and biomass of the individual shrub patches, respectively; HR, herb richness; HA, herb abundance; HH, herb height; AGB, aboveground biomass; BGB, belowground biomass; clay, silt and sand represent the particles of soil; TN, TC and SOC represent soil total nitrogen, total carbon and organic carbon contents, respectively
Suberin showed significant positive correlations with the cover of individual shrub patches and soil clay, carbon and nitrogen and was significantly and negatively correlated with the mean annual temperature (MAT) and potential evaporation (PE) in both the shrub patches and grassy matrix (p < 0.05; Fig. 1). Cutin and ∑CS were mainly positively correlated with the soil clay but negatively correlated with the pH in the grassy matrix (p < 0.05; Fig. 1a). However, these two components were mainly negatively correlated with the MAT in the shrub patches (p < 0.05; Fig. 1b). The lignin-derived phenols and the indicators of the botanical origin of the lignin (cinnamyls/vanillyls (C/V) and syringyls/vanillyls (S/V)) were negatively correlated with the mean annual precipitation (MAP), AGB, and soil carbon and nitrogen and positively correlated with the sand content of the soil in both the shrub patches and grassy matrix (Fig. 1). In addition, the lignin-derived phenols were significantly and positively correlated with the herb BGB in the grassy matrix (p < 0.05; Fig. 1a), whereas the indicator of the stage of lignin degradation, vanillic acid to vanillin ratio ((Ad/Al)v), showed the opposite correlation (p < 0.05; Fig. 1).We then conducted RDA to explore the effects of biotic and abiotic variables on the SOC components. Our RDA explained 77.3% of the variance in the SOC composition in the grassy matrix and 67.2% of that in the shrub patches (Fig. 2, S2). Herb AGB, richness, shrub density and herb height were the most important variables explaining the SOC composition in the grassy matrix, with explanatory powers of 29.2%, 14.3%, 12.4% and 12.2%, respectively (Fig. 2a). In addition, the cover of individual shrub patches and pH were the most important variables and explained 21.5% and 14.3% of the variance in the shrub patches, respectively (Fig. 2b).
The results of redundancy analysis based on the biomarkers and the explanatory variables in the grassy matrix (a) and shrub patches (b) in Inner Mongolia. S/C, suberin/cutin; ∑CS, components in both cutin and suberin; S/V, syringyls/vanillyls; (Ad/Al)v, vanillic acid/vanillin; 16, ω-C16/∑C16; AGB, aboveground biomass; BGB, belowground biomass; HR, herb richness; HH, herb height; HA, herb abundance; SDp, SCp and SHp represent shrub density, cover and height at the plot scale, respectively. SCi, SAi, SBi and SHi represent the cover, size, biomass and height of the individual shrub patches, respectively. SOC represent the soil organic carbon
Furthermore, we applied SEM analysis to quantify the relative contributions of climate and the shrub, herb and soil characteristics to the variations in the SOC components. The models could explain 15–91% of the variance in the SOC components (Fig. 3). In the grassy matrix, the herb and soil characteristics had direct and negative effects on the lignin-derived phenols (Fig. 3c), whereas climate had significant direct and negative effects on the bound lipids and lignin-derived phenols in the shrub patches (Fig. 3bc).
Structure equation modeling examining the multivariate effects of climate, shrub, herb and soil characteristics on the free lipids (a), bound lipids (b) and lignin-derived phenols (c) of the grassy matrix (left) and shrub patches (right) in Inner Mongolia. Single-headed arrows indicate the hypothesized direction of causation between the related variables. Double-headed arrows indicate covariance between the related variables. The numbers adjacent to the arrows are the standard path coefficients. Herbg, Herbs, Soilg, and Soils represent the herb community and soil characteristics in the grassy matrix and shrub patches, respectively. Shrubp and Shrubin represent the shrub characteristics at the plot scale and the characteristics of the individual shrub patches, respectively. R2, the proportion of variance explained. All models adequately fit the data (p-values of χ2 test≥0.60, RMSEA = 0.00 in all cases)
Discussion
Differences in SOC components between the shrub patches and grassy matrix
Relative to the Inner Mongolian grasslands that have been subject to different land uses, the shrub-encroached grasslands in this region were characterized by low bound lipid concentrations and extremely high lignin-derived phenol concentrations (Zhao et al. 2014), indicating that shrub encroachment has altered the SOC composition in the grasslands. Our results showed that the main components that differed were also the bound lipids and lignin-derived phenols (i.e., bound n-alkanoic acids, ∑CS, syringyls and vanillyls; p < 0.05; Table 3), which were more abundant in the shrub patches than in the grassy matrix. In contrast, the lignin degradation parameter (Ad/Al)v was much lower in the shrub patches than that in the grassy matrix (p < 0.05; Table 3; Fig. S3). These results suggested the occurrence of more inputs and a low degradation state in the shrub patches (Otto and Simpson 2006; Angst et al. 2016). In our study, the concentration of cutin (a tracer of aboveground inputs) significantly differed between the shrub patches and grassy matrix (Table 3; Fig. S3), whereas a difference in suberin (the tracer of belowground inputs) was not observed, indicating that aboveground inputs made greater contributions in the shrub patches than in the grassy matrix (Kögel-Knabner 2002; Feng et al. 2010; Angst et al. 2018). This result agreed with findings regarding the accumulation of cutin under the tree canopy after 5 years of cultivation in a grassland in New Zealand (Huang et al. 2011). These results could reasonably be explained by the following mechanisms: (1) shrubs produce more litter than herbaceous species (Table 2; Knapp et al. 2008; Eldridge et al. 2011); (2) leguminous shrubs increase microbial decomposition in the grassy matrix by providing additional available resources (i.e., a low C: N ratio) (Zhang et al. 2018). A previous study in an Inner Mongolian shrub-encroached grassland showed that the SOC in shrub patches was mainly derived from aboveground inputs, whereas that in the grassy matrix originated from both aboveground and belowground inputs (Zhou et al. 2017). Aboveground inputs are incorporated into the organic layer or mineral soil via decomposition by microbes over time (Six et al. 2004; Wardle et al. 2004; Wilkinson et al. 2009), whereas belowground inputs, such as rhizodeposition, released by living roots, can be rapidly consumed by soil microorganisms in situ (Kögel-Knabner 2002; Angst et al. 2016). In addition, leguminous shrubs can transfer nitrogen to the soils under the grassy matrix via their horizontal root system (Temperton et al. 2007; Maestre et al. 2009; Zhang et al. 2018; Zhou et al. 2018), which can partly explain the increased degradation of the lignin-derived phenols in the grassy matrix. The effects of shrubs on the grassy matrix have been reported in many studies (Maestre et al. 2009; Rascher et al. 2012; Rasmussen et al. 2013). Rascher et al. (2012) found that the area influenced by nitrogen fixers could be at least 3.5-fold greater than the area of their canopy in a sand dune ecosystem. In Mongolian grasslands, the distance over which nitrogen was transferred by shrub species was even found to reach 5 m (Zhang et al. 2016).
The influences of vegetation, climate, and soil on SOC components
Plants are believed to be the critical factor that controls the SOC dynamics and composition (Kögel-Knabner 2002; Kögel-Knabner and Rumpel 2018). We found that the free lipids were strongly correlated with herb richness and AGB in the grassy matrix (p < 0.05; Fig. 1a), while the free lipids, cutin and suberin were significantly correlated with the shrub characteristics in the shrub patches (p < 0.05; Fig. 1b), indicating the different contributions of vegetation in this landscape (Zhou et al. 2017). Shrubs can influence the bound lipids in the grassy matrix via root inputs, evidenced by the significant correlations of shrub characteristics (i.e., individual shrub patch cover) with suberin compounds and the suberin/cutin ratio in the grassy matrix (p < 0.05; Fig. 1a; Angst et al. 2016). In the grassy matrix, lignin-derived phenols were positively correlated with herb BGB (p < 0.05; Fig. 1a), but negatively correlated with the shrub characteristics (p < 0.05; Fig. 1a), whereas lignin degradation parameter (Ad/Al)v showed the opposite correlations with these variables (Fig. 1a). These results support our previous findings that the lignin-derived phenols in the grassy matrix are mainly derived from herbaceous roots and shrubs can mediate the process of decomposition in the grassy matrix via their horizontal root system (Zhang et al. 2016; Zhou et al. 2018). In our study area, the dominant shrub Caragana has a complex root system that can extend several meters horizontally (Maestre et al. 2009; Zhang et al. 2016).
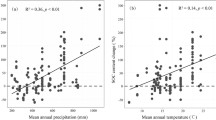
Cutin and suberin were significantly and negatively correlated with the MAT, especially in the shrub patches (Fig. 1). This result contrasted with the increase in cutin components observed in Duke Forest after 14 months of soil warming (Feng et al. 2008), this difference may be attributed to the water limitations in our study area, as rising temperatures can be expected to lead to increased water stress (Bai et al. 2007). In both the shrub patches and grassy matrix, the MAP and aridity index (AI) had strongly negative effects on the lignin-derived phenols but opposite effects on the (Ad/Al)v (Fig. 1). This finding was consistent with the patterns of lignin-derived phenols observed in Inner Mongolian pure grasslands (Ma et al. 2018). Previous studies showed that precipitation influenced the patterns of lignin-derived phenols indirectly by changing the soil microbial activities, soil moisture and vegetation rather than via direct control (Filley et al. 2008; Pisani et al. 2016). Li et al. (2017) found that the biomass of fungi (the major decomposers of lignin-derived phenols) increased with increasing precipitation in Inner Mongolian shrub-encroached grasslands. We inferred that water was the main factor driving degradation and that increased precipitation enhanced lignin-phenol degradation by facilitating microbial activity (Ma et al. 2018).
Our results demonstrated that the bound lipids (i.e., suberin and ∑CS) were positively correlated with the soil clay content (p < 0.05; Fig. 1), suggesting that these compounds were physically protected by the clay fractions. Similarly, Lin and Simpson (2016) found that cutin and suberin were physically protected in grassland, forest and agricultural soils, and Angst et al. (2018) found similar results, showing that bound lipids accumulated in the clay fractions of forest subsoil. These results may have occurred because clay fractions have a greater capacity than coarse particles to adsorb alkyl-C onto their surface (Feng et al. 2005). Consistent with other findings in pure Inner Mongolian grasslands, the lignin-derived phenols were negatively correlated with the soil nutrients and positively correlated with the sand fractions (p < 0.05; Fig. 1); however, the degradation parameter (Ad/Al)v showed the opposite correlations with these soil properties (Ma et al. 2018). These results indicated that lignin-derived phenols decrease with increasing degradation under nutrient-rich conditions (Otto and Simpson 2006; Feng et al. 2010; Ma et al. 2018). These results further suggested that the lignin-phenols were not selectively preserved in the clay fraction, which was consistent with the findings from grasslands in North America (Amelung et al. 1999).
The relative importance of environmental factors in driving SOC components
We conducted RDA to examine the effects of the chosen variables on the SOC composition (Fig. 2). The shrub characteristics at the plot scale, which represented the degree of shrub encroachment, had higher explanatory power than the characteristics of the individual shrub patches in explaining the variation in SOC components in the grassy matrix (Fig. 2, S2; 77.3% vs. 61.5%, respectively). The highest explanatory power of herb AGB suggested herb productivity was an important resource in the grassy matrix (29.2%; Fig. 2a). The critical role of herb richness was ascribed to increasing herb productivity via complementary resource use (Chen et al. 2018). Moreover, the shrub density, which is an important indicator of shrub encroachment, also explained 12.4% of the variance in the grassy matrix, which highlights the effects of shrub encroachment (Eldridge et al. 2011). By compassion, individual shrub patch cover and soil pH were the most important variables explaining 21.5% and 14.3% of the variance in SOC components in the shrub patches, respectively (Fig. 2b). High shrub patch cover facilitates the addition of shrub litter inputs into the soil (Table 1; Knapp et al. 2008), and high shrub patch cover is usually associated with complex root systems, which indicates a heterogeneous microenvironment under shrub canopies (Maestre et al. 2009; Soliveres and Eldridge 2014). Positive or negative effects of soil pH on free lipids (i.e., n-alkanoic acids), hydrolysable lipids and lignin-derived phenols have been reported in grasslands, shrublands and forests; and can likely be ascribed to mediation of the soil fauna (Bull et al. 2000; Nierop et al. 2003; Cai et al. 2017; Ma et al. 2018).
The SEM in this study was well established, as indicated by the nonsignificant chi-square test result (p > 0.05) and low RMSEA (<0.05) (Fig. 3; Table S1; Delgado-Baquerizo et al. 2017). The standard effects analyses based on SEM showed that all group variables collectively influenced the SOC composition of the grassy matrix (Fig. 4). Among these variables, free lipids were mostly controlled by herb characteristics (−0.60; Fig. 4a), bound lipids were mainly controlled by the soil characteristics (0.52; Fig. 4b), and lignin-derived phenols were mainly controlled by the climate (−0.79) and shrub characteristics (0.65; Fig. 4c). By comparison, soil properties had the greatest standard effects on the free lipids and bound lipids in the shrub patches (−0.74 and 0.85; Fig. 4ab), while the climate was the most important factor driving the lignin-derived phenols (−0.76; Fig. 4c). Conversely, herb characteristics had little effect on the SOC components in the shrub patches. These results further confirm that shrub encroachment exerts important influences on the SOC composition in the grasslands, including the grassy matrix (Fig. 4; Eldridge et al. 2011; Zhou et al. 2018). Previous studies have demonstrated that SOC composition is mainly controlled by vegetation (Kohl et al. 2017). However, our results indicate that the soil properties were also important drivers (Fig. 4). The unexplained variance in SOC composition might be ascribed to variables that were not included in our models, such as topography (Wu and Archer 2005) and the microbial community (Li et al. 2017).
Structural equation modeling showing the standard total effects (black), direct effects (red) and indirect effects (blue) of climate, shrub, herb and soil characteristics on the free lipids (a), bound lipids (b) and lignin-derived phenols (c) in grassy matrix (left) and shrub patches (right) in Inner Mongolia
Conclusions
Our field investigation along a climate gradient represents the first assessment of the relative contributions of the biotic and abiotic factors that drive the SOC components in Inner Mongolian shrub-encroached grasslands (Fig. 5). Our study highlights that although shrub encroachment did not significantly change the topsoil OC contents, it could still increase the soil heterogeneity by changing the SOC components, especially the bound lipids and lignin-derived phenols. In addition, leguminous shrubs could influence the degradation of lignin-derived phenols by transferring nitrogen to the grassy matrix via their horizontal root system. The Pearson correlations showed that the bound lipids and lignin-derived phenols were strongly influenced by temperature and precipitation, respectively. The herb BGB made a great contribution to the SOC of the grassy matrix. In addition, the bound lipids were protected by the clay fractions, while the physical protection of lignin-derived phenols was not observed in our study area. The herb characteristics and shrub density were the most important variables that controlled the SOC components in the grassy matrix, while the individual patch cover and soil pH were the most important variables in the shrub patches. Furthermore, our SEM analysis revealed that the climate, vegetation and soil characteristics collectively influenced the SOC compounds in the grassy matrix, while the soil properties had this effect in the shrub patches. Our findings provide new insights into the response and relevant mechanisms of soil carbon to vegetation shifts. Our results will also be helpful in the development of carbon models and guiding grassland management.
References
Amelung W, Flach KW, Zech W (1999) Lignin in particle-size fractions of native grassland soils as influenced by climate. Soil Sci Soc Am J 63:1222–1228. https://doi.org/10.2136/sssaj1999.6351222x
Angst G, John S, Mueller CW, Kögel-Knabner I, Rethemeyer J (2016) Tracing the sources and spatial distribution of organic carbon in subsoils. Sci Rep 6:29478. https://doi.org/10.1038/srep29478
Angst G, Nierop KGJ, Angst Š, Frouz J (2018) Abundance of lipids in differently sized aggregates depends on their chemical composition. Biogeochemistry 140:111–125. https://doi.org/10.1007/s10533-018-0481-7
Armas-Herrera CM, Dignac MF, Rumpel C, Arbelo CD, Chabbi A (2016) Management effects on composition and dynamics of cutin and suberin in topsoil under agricultural use. Eur J Soil Sci 67:360–373. https://doi.org/10.1111/ejss.12328
Bai Y, Wu J, Pan Q et al (2007) Positive linear relationship between productivity and diversity: evidence from the Eurasian steppe. J Appl Ecol 44:1023–1034. https://doi.org/10.1111/j.1365-2664.2007.01351.x
Bull ID, van Bergen PF NCJ, Poulton PR, Evershed RP (2000) Organic geochemical studies of soils from the Rothamsted classical experiments-VI. The occurrence of organic acids in an experimental grassland soil. Soil Biol Biochem 32:1367–1376. https://doi.org/10.1016/S0038-0717(00)00054-7
Cai Y, Tang Z, Xiong G, Xie Z, Liu Z, Feng X (2017) Different composition and distribution patterns of mineral-protected versus hydrolyzable lipids in shrubland soils. J Geophys Res-Biogeo 122:2206–2218. https://doi.org/10.1002/2017JG003759
Chen L, Li H, Zhang P, Zhao X, Zhou L, Liu T, Hu H, Bai Y, Shen H, Fang J (2015) Climate and native grassland vegetation as drivers of the community structures of shrub-encroached grasslands in Inner Mongolia, China. Landsc Ecol 30:1627–1641. https://doi.org/10.1007/s10980-014-0044-9
Chen S, Wang W, Xu W, Wang Y, Wan H, Chen D, Tang Z, Tang X, Zhou G, Xie Z, Zhou D, Shangguan Z, Huang J, He JS, Wang Y, Sheng J, Tang L, Li X, Dong M, Wu Y, Wang Q, Wang Z, Wu J, Chapin FS III, Bai Y (2018) Plant diversity enhances productivity and soil carbon storage. P Natl Acad Sci USA 115:4027–4032. https://doi.org/10.1073/pnas.1700298114
Dai G, Ma T, Zhu S, Liu Z, Chen D, Bai Y, Chen L, He JS, Zhu J, Zhang Y, Lü X, Wang X, Han X, Feng X (2018) Large-scale distribution of molecular components in Chinese grassland soils: the influence of input and decomposition processes. J Geophys Res-Biogeo 123:239–255. https://doi.org/10.1002/2017jg004233
Delgado-Baquerizo M, Eldridge DJ, Ochoa V, Gozalo B, Singh BK, Maestre FT (2017) Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol Lett 20:1295–1305. https://doi.org/10.1111/ele.12826
Eldridge DJ, Bowker MA, Maestre FT, Roger E, Reynolds JF, Whitford WG (2011) Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol Lett 14:709–722. https://doi.org/10.1111/j.1461-0248.2011.01630.x
Fang J, Bai Y, Wu J (2015) Towards a better understanding of landscape patterns and ecosystem processes of the Mongolian plateau. Landsc Ecol 30:1573–1578. https://doi.org/10.1007/s10980-015-0277-2
Feng X, Simpson MJ (2008) Temperature responses of individual soil organic matter components. J Geophys Res 113:G03036. https://doi.org/10.1029/2008jg000743
Feng X, Simpson AJ, Simpson MJ (2005) Chemical and mineralogical controls on humic acid sorption to clay mineral surfaces. Org Geochem 36:1553–1566. https://doi.org/10.1016/j.orggeochem.2005.06.008
Feng X, Simpson AJ, Schlesinger WH, Simpson MJ (2010) Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the duke forest. Glob Chang Biol 16:2104–2116. https://doi.org/10.1111/j.1365-2486.2009.02080.x
Filley TR, Boutton TW, Liao JD, Jastrow JD, Gamblin DE (2008) Chemical changes to nonaggregated particulate soil organic matter following grassland-to-woodland transition in a subtropical savanna. J Geophys Res 113. https://doi.org/10.1029/2007jg000564
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Huang Z, Davis MR, Condron LM, Clinton PW (2011) Soil carbon pools, plant biomarkers and mean carbon residence time after afforestation of grassland with three tree species. Soil Biol Biochem 43:1341–1349. https://doi.org/10.1016/j.soilbio.2011.03.008
Jackson RB, Banner JL, Jobbágy EG, Pockman WT, Wall DH (2002) Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418:623–626. https://doi.org/10.1038/nature00910
Knapp AK, Briggs JM, Collins SL et al (2008) Shrub encroachment in north American grasslands: shifts in growth form dominance rapidly alters control of ecosystem carbon inputs. Glob Chang Biol 14:615–623. https://doi.org/10.1111/j.1365-2486.2007.01512.x
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162. https://doi.org/10.1016/S0038-0717(01)00158-4
Kögel-Knabner I, Rumpel C (2018) Advances in molecular approaches for understanding soil organic matter composition, origin, and turnover: a historical overview. Advances Agron 149:1–48. https://doi.org/10.1016/bs.agron.2018.01.003
Kohl L, Philben M, Edwards KA, Podrebarac FA, Warren J, Ziegler SE (2017) The origin of soil organic matter controls its composition and bioreactivity across a Mesic boreal forest latitudinal gradient. Glob Chang Biol 242:458–473. https://doi.org/10.1111/gcb.13887
Li H, Zhang J, Hu H, Chen L, Zhu Y, Shen H, Fang J (2017) Shift in soil microbial communities with shrub encroachment in Inner Mongolia grasslands, China. Eur J Soil Sci 79:40–47. https://doi.org/10.1016/j.ejsobi.2017.02.004
Li H, Shen H, Zhou L, Zhu Y, Chen L, Hu H, Zhang P, Fang J (2019) Shrub encroachment increases soil carbon and nitrogen stocks in temperate grasslands in China. Land Degrad Dev 30:756–767. https://doi.org/10.1002/ldr.3259
Lin LH, Simpson MJ (2016) Enhanced extractability of cutin- and suberin-derived organic matter with demineralization implies physical protection over chemical recalcitrance in soil. Org Geochem 97:111–121. https://doi.org/10.1016/j.orggeochem.2016.04.012
Ma T, Zhu S, Wang Z, Chen D, Dai G, Feng B, Su X, Hu H, Li K, Han W, Liang C, Bai Y, Feng X (2018) Divergent accumulation of microbial necromass and plant lignin components in grassland soils. Nat Commun 9:3480. https://doi.org/10.1038/s41467-018-05891-1
Maestre FT, Bowker MA, Puche MD, Belén Hinojosa M, Martínez I, García-Palacios P, Castillo AP, Soliveres S, Luzuriaga AL, Sánchez AM, Carreira JA, Gallardo A, Escudero A (2009) Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecol Lett 12:930–941. https://doi.org/10.1111/j.1461-0248.2009.01352.x
Nierop KGJ, Naafs DFW, Verstraten JM (2003) Occurrence and distribution of ester-bound lipids in Dutch coastal dune soils along a pH gradient. Org Geochem 34:719–729. https://doi.org/10.1016/s0146-6380(03)00042-1
Otto A, Simpson MJ (2006) Evaluation of CuO oxidation parameters for determining the source and stage of lignin degradation in soil. Biogeochemistry 80:121–142. https://doi.org/10.1007/s10533-006-9014-x
Peng H, Li X, Jiang Z et al (2013) Shrub encroachment with increasing anthropogenic disturbance in the semiarid inner Mongolian grasslands of China. Catena 109:39–48. https://doi.org/10.1016/j.catena.2013.05.008
Pisani O, Haddix ML, Conant RT, Paul EA, Simpson MJ (2016) Molecular composition of soil organic matter with land-use change along a bi-continental mean annual temperature gradient. Sci Total Environ 573:470–480. https://doi.org/10.1016/j.scitotenv.2016.08.154
Rascher KG, Hellmann C, Maguas C, Werner C (2012) Community scale 15N isoscapes: tracing the spatial impact of an exotic N2 -fixing invader. Ecol Lett 15:484–491. https://doi.org/10.1111/j.1461-0248.2012.01761.x
Rasmussen J, Gylfadóttir T, Loges R, Eriksen J, Helgadóttir Á (2013) Spatial and temporal variation in N transfer in grass–white clover mixtures at three northern European field sites. Soil Biol Biochem 57:654–662. https://doi.org/10.1016/j.soilbio.2012.07.004
Reynolds JF, Smith MS, Lambin EF et al (2007) Global desertification: building a science for dryland development. Science 316:847–851. https://doi.org/10.1126/science.1131634
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Till Res 79:7–31. https://doi.org/10.1016/j.still.2004.03.008
Soliveres S, Eldridge DJ (2014) Do changes in grazing pressure and the degree of shrub encroachment alter the effects of individual shrubs on understorey plant communities and soil function? Funct Ecol 28:530–537. https://doi.org/10.1111/1365-2435.12196
Tamura M, Tharayil N (2014) Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol 203:110–124. https://doi.org/10.1111/nph.12795
Temperton VM, Mwangi PN, Scherer-Lorenzen M, Schmid B, Buchmann N (2007) Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia 151:190–205. https://doi.org/10.1007/s00442-006-0576-z
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633. https://doi.org/10.1126/science.1094875
Wilkinson MT, Richards PJ, Humphreys GS (2009) Breaking ground: pedological, geological, and ecological implications of soil bioturbation. Earth-Sci Rev 97:257–272. https://doi.org/10.1016/j.earscirev.2009.09.005
Wu XB, Archer SR (2005) Scale-dependent influence of topography-based hydrologic features on patterns of woody plant encroachment in savanna landscapes. Landsc Ecol 20:733–742. https://doi.org/10.1007/s10980-005-0996-x
Zhang H, Yu Q, Lu X, Trumbore SE, Yang JJ, Han XG (2016) Impacts of leguminous shrub encroachment on neighboring grasses include transfer of fixed nitrogen. Oecologia 180:1213–1222. https://doi.org/10.1007/s00442-015-3538-5
Zhang H, Lü X, Knapp AK et al (2018) Facilitation by leguminous shrubs increases along a precipitation gradient. Funct Ecol 32:203–213. https://doi.org/10.1111/1365-2435.12941
Zhao L, Wu W, Xu X, Xu Y (2014) Soil organic matter dynamics under different land use in grasslands in Inner Mongolia (northern China). Biogeosciences 11:5103–5113. https://doi.org/10.5194/bg-11-5103-2014
Zhou L, Li H, Shen H, Xu Y, Wang Y, Xing A, Fang J (2017) Shrub-encroachment induced alterations in input chemistry and soil microbial community affect topsoil organic carbon in an Inner Mongolian grassland. Biogeochemistry 136:311–324. https://doi.org/10.1007/s10533-017-0396-8
Zhou L, Li H, Shen H, Xu Y, Wang Y, Xing A, Zhu Y, Zhou S, Fang J (2018) Effects of shrub encroachment on vertical changes in soil organic carbon in Mongolian grasslands: using a multi-biomarker approach. Plant Soil 431:217–230. https://doi.org/10.1007/s11104-018-3761-z
Acknowledgements
This study was funded by the National Natural Science Foundation of China (31330012, 31470525), the National Basic Research Program of China on Global Change (2014CB954001), the Key Research Program of Frontier Sciences, CAS, (QYZDY-SSW-SMC011), and the China Postdoctoral Science Foundation (2018M641074).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Ingrid Koegel-Knabner.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 997 kb)
Rights and permissions
About this article
Cite this article
Zhou, L., Shen, H., Xu, Y. et al. Soil organic carbon components in inner Mongolian shrub-encroached grasslands. Plant Soil 442, 199–213 (2019). https://doi.org/10.1007/s11104-019-04166-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04166-y