Abstract
Aims
Ecological forest succession can be influenced by plant-plant interactions that exert contrasting effects on early- and late-successional species. In this study, we explored the role of indirect plant-plant interactions and the underlying microbial mechanisms in forest succession.
Methods
In a mesocosm experiment, we used Schima superba, a widespread mid-successional species in subtropical China, as a model species to explore how inoculating the rhizosphere soil of Schima affected the performances of two early-successional species (Pinus massoniana and Rhodomyrtus tomentosa) and two late-successional species (Cryptocarya chinensis and Machilus chinensis). All direct and indirect correlations between plant performance and soil microbial composition were examined using partial least square path models.
Results
Schima inoculum inhibited the growth of the early-successional species but had little effect on the growth of the late-successional species. Inoculation reduced non-arbuscular mycorrhizal fungi (non-AMF) colonization in both species groups but increased arbuscular mycorrhizal fungi (AMF) colonization in the late-successional species. The percentage of root lesions in the early-successional species increased with inoculation, while that in the late-successional species decreased. Plant nutrient acquisition was not responsive to inoculation. According to the path models, soil microbes explained 51% of the growth variances in the early-successional species but barely explained any growth variances in the late species.
Conclusions
Schima may increase the competitive advantage of the late-successional species over early-successional species by inhibiting the mutualistic association between non-AMF and the latter, which in turn may facilitate forest succession.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant community succession represents a predictable, directional and continuous process of vegetation replacement, and it has been the focus of many ecological studies. It is believed that plant succession is the result of differential responses of plant species to environmental changes along a successional chronosequence (Tilman 1985; Grau et al. 1997; Li and Waller 2016). Early-successional plants usually possess traits such as fast growth rate, high fecundity, and high resource consumption efficiency that allow them to rapidly colonize a resource-abundant habitat, while late-successional plants are usually slow growers with relatively low fecundity and resource consumption efficiency (Connell and Slatyer 1977; Rees et al. 2001). Early-successional species will eventually be replaced by late-successional species in the absence of disturbances because abiotic and biotic conditions will eventually favor the latter (Peng and Wang 1993; Cornelissen et al. 1994). For the forests in subtropical China, the effect of the interplay between abiotic conditions and plant characteristics on succession has also been well studied (Mo et al. 2006; Huang et al. 2013). Among all factors, ambient light conditions and plant preference for light have been identified as the most important ones (Cornelissen et al. 1994). However, it is reported that early-successional species might fail to recruit in open gaps in late-successional forest, suggesting the important roles of other factors, such as soil nutrient availability, in limiting the recruitment of early species (Yan et al. 2015).
Because the nature of plant community succession is the replacement of early-successional species by later-successional species (Rees et al. 2001; Peng et al. 2012), any ecological processes that might alter plant competition could alter the course of succession (Connell and Slatyer 1977; Maggi et al. 2011). It is well known that the competitive outcome between two plant species can be altered by a third species that exerts contrasting effects on these two species (Wootton 1994; Metlen et al. 2013). According to the Connell-Slatyer model, the colonization by some key species may affect subsequent species recruitment and plant succession (Connell and Slatyer 1977). Therefore, a plant species has the potential to alter the course of succession by exerting contrasting effects on the performance of early- and late-successional species.
Plant-soil feedbacks play an important role in regulating vegetation dynamics (Klironomos 2002; Bever 2003). Despite a large body of literature indicating the importance of negative plant-soil feedbacks in the facilitation of species coexistence (Klironomos 2002; Mangan et al. 2010; Liu et al. 2012b; Bennett et al. 2017), plant-soil feedbacks have received much less attention in the context of plant succession, especially the succession of forest ecosystems (but see O’Hanlon-Manners and Kotanen 2006; McCarthy-Neumann and Kobe 2008; Krüger et al. 2017). Kardol et al. (2006) reported negative plant-soil feedbacks on early-successional species and positive plant-soil feedbacks on late-successional species, which profoundly contributed to grassland succession. In a follow-up study, Kardol et al. (2007) found that the negative plant-soil feedbacks for early-successional species were driven by soil pathogens, which may facilitate succession by breaking the dominance of early species. In addition to negative plant-pathogen feedbacks, positive plant-mycorrhiza feedback has been reported to facilitate community succession in a volcanic desert (Nara and Hogetsu 2004; Nara 2006). These pieces of evidence all support the idea that plant-soil feedback may play an important role in plant succession.
Forest succession in subtropical China can be roughly divided into three stages. The early stage is characterized by the dominance of fast-growing, heliophytic (shade-intolerant) conifers and shrubs; the middle stage is characterized by a mixture of conifers and heliophytic broadleaf trees; and the late stage is characterized by the dominance of slow-growing, mesophytic (moderately shade-tolerant) broadleaf trees (Peng and Wang 1993; Peng et al. 2012). In subtropical forests, there is a special group of broadleaf trees, with a moderate light preference and growth rate, that occur during the transition from early to middle stage and are not replaced during the middle and late stages. Schima superba (a member of Theaceae family) is a typical representative of this group, and it is one of the most widely distributed species in the forests in subtropical China (Cao 2013). However, it is not clear whether Schima plays any roles in regulating the succession process, despite its long-term persistency across successional stages (Peng and Wang 1993). It is possible that Schima can modify the biotic and abiotic conditions in a way that induces contrasting responses on early- and late-successional species, which in turn alter the course of forest succession. If the modification by Schima benefits early-successional species, then the successional process will be slowed down; if it benefits the late-successional species, then the successional process will be accelerated.
In this context, we explored the role of Schima superba in forest succession from the perspective of plant-soil feedbacks. In an outdoor common garden, we assessed the effect of the rhizosphere soil of Schima superba on the growth performance, nutrient acquisition status, root health status and root mycorrhiza colonization of the early- and late-successional trees via soil inoculation. Furthermore, we used phospholipid fatty acid analysis (PLFA) to determine the composition of the rhizosphere microbial community of the study species under different inoculum treatments. Specifically, we asked the following two questions: i) Does the rhizosphere soil of Schima superba exert contrasting effects on the performance of the early- vs. late- successional species? ii) If so, to which extent can the observed changes in plant performance be attributed to Schima-induced changes in soil microbial community? Addressing the above questions enables us to have a better understanding of the importance of indirect plant-plant interactions in regulating forest succession, shedding light on the microbial mechanism that drives the observed indirect plant-plant interactions.
Materials and methods
Schima rhizosphere soil collection
The rhizosphere soil of Schima was collected from two different forest types in Dinghushan Biosphere Reserve in Guangdong Province, China (112°10′E, 23°10′N) in May 2016: a mixed pine and broadleaf forest developed from a 1930 plantation effort, and an old-growth broadleaf forest protected from anthropological disturbances for more than 400 years (Mo et al. 2006). The relative frequencies of Schima in these two types of forest were 15.7 and 4.4%, respectively (Peng et al. 2012). Six Schima individuals 10–15 m in height and 30–50 m apart from each other were chosen in each forest type. Two forest types were chosen to account for the effects of different plant communities on the rhizosphere microbial community of Schima, because Barberan et al. (2015) suggested that the composition of the rhizosphere microbial community of a species is dictated by both the species itself and its neighbors. For each individual, five soil cores were sampled randomly within a 0.5 m radius from tree stem. Litter was removed before soil cores of ~900 cm3 (20 cm depth × 7.5 cm diameter) were collected. The soil cores from the same individual were thoroughly mixed and sieved over a 2 mm sieve to remove large rocks and root tissues. Because refrigeration can only preserve the soil samples for a few days to avoid fungi desiccation (Brundrett et al. 1996), the sieved soil samples were then stored at −20 °C for two weeks before we started our common garden experiment. Previous studies from polar and temperate regions have shown that freezing does not significantly reduce the abundance of mycorrhiza and soil bacteria (Männistö et al. 2009; Kilpeläinen et al. 2016). However, it is not clear whether freezing will alter the composition of the soil microbial community in subtropical regions.
Study species
To explore the effect of the rhizosphere soil of Schima on early- vs. late-successional species, we selected two fast-growing early-successional species (Pinus massoniana [Pinaceae] and Rhodomyrtus tomentosa [Myrtaceae]) and two slow-growing late-successional species (Cryptocarya chinensis [Lauraceae] and Machilus chinensis [Lauraceae]). Both early-successional species are fast-growing heliophytes (Wang and Peng 1987; Cao 2013), which are frequently found to associate with ectomycorrhizal fungi (ECM) (Howard et al. 2000; Huang et al. 2014). Both late-successional species are slow-growing mesophytes (Wang and Peng 1987; Cao 2013), which are frequently found to associate with arbuscular mycorrhizal fungi (AMF) (Chen et al. 2017). Two Lauraceae species were chosen to represent late-successional mesophytes because Lauraceae species are the only typical late-successional species that dominate late-successional forest stands but are not observed in early- and mid-successional stands at Dinghushan Biosphere Reserve (Mo et al. 2006; Cao 2013).
Experimental set-up
A soil inoculation experiment was conducted in an outdoor common garden near Huolushan Forest Park, Guangdong Province, China (113°24′E, 23°11′N) from July 2016 to April 2017. We prepared the growth medium by placing 0.4 L soil inocula on the surface of 8 L ‘background’ soil in 10 L pots (20 cm depth×25 cm diameter). Except for the portion of background soil that was used as inoculum, the rest of background soil was sterilized by autoclaving once per day for three consecutive days. The background soil was collected from an abandoned plantation near the outdoor common garden. It was a typical red soil with low pH and low nutrient content (supplementary materials Table S1), which is similar to the initial condition for secondary succession in subtropical China (Liu et al. 2012a). Three types of inocula were used: i) background soil (labeled as CK), ii) Schima inoculum from mixed pine and broadleaf forest (labeled as MF) and iii) Schima inoculum from broadleaf forest (labeled as BF).
In July 2016, two- or three-year-old seedlings of Pinus, Rhodomyrtus, Cryptocarya, and Machilus were purchased from local plantations and got transplanted into the pots. Because in a preliminary experiment we found that thorough removal of rhizosphere soil profoundly reduced seedling survival rates of the late-successional species, Cryptocarya, and Machilus (i.e. the survival rate of the seedlings without any original rhizosphere soil was lower than 10% of that of the seedlings which were transplanted without removing any original rhizosphere soil), we had to keep a minimum amount of rhizosphere soil that came with the purchased seedling to ensure seedling survival. The initial height, basal diameter and biomass of every individual were recorded.
For each species, each inoculum treatment was replicated 6 times, with CK including replicated samples of the background soil and MF/BF including the independently sampled rhizosphere soil of the six Schima individuals from mixed/broadleaf forest stands. As a result, a total of 72 seedlings were grown (4 species × 3 inoculum treatments × 6 replicates). All plants were watered once a day during the whole experiment except rainy days.
Effects of Schima inocula on plant growth and nutrient acquisition status
During the nine-month experimental period, we repeatedly measured plant height and basal diameter at three-month intervals. In April 2017, all plants were harvested, dried at 60 °C for 72 h and weighted. Because of the differences in initial size among different individuals, all growth measurements were quantified by relative growth rate (RGR), which was calculated as follows (Hoffmann and Poorter 2002):
where M1 is the value of a growth measurement at time one (t1) and M2 is the value of a growth measurement at time two (t2).
In addition to growth performance, we measured plant nutrient acquisition status by determining leaf nitrogen (N) and phosphorous (P) contents at the end of the first growing season in October 2016. We collected, dried (at 60 °C for 72 h), and ground eight to ten mature leaves per individual. We then determined leaf N and P contents following the protocol by Lu (1999).
Effects of Schima inocula on root lesion and mycorrhiza root colonization
Before the plant harvest in April 2017, two soil cores of ~200 cm3 (10 cm depth×5 cm diameter) were taken from the rhizosphere of each individual. All roots were extracted from the soil samples, washed, cut into 2 cm fragments and stored in 60% ethanol. For the assessment of root lesions, root fragments were cleared with 10% KOH and subjected to microscopic inspection at 100 intersections at 100× magnification for any necrotic lesions on the root surface (McGonigle et al. 1990). The root fragments were then stained with trypan blue (Phillips and Hayman 1970) and inspected at 100 intersections at 250× magnification for the presence of arbuscular/vesicular hyphae (typical AMF structures) and the presence of regularly septate hyphae (a typical non-AMF structure) (McGonigle et al. 1990).
Effects of Schima inocula on rhizosphere soil microbial community
At the beginning of the experiment, differences in the species composition of the soil microbial community were assessed for the three soil inocula (i.e., CK, MF and BF). To assess changes in the rhizosphere microbial community, we sampled and analyzed the rhizosphere soil of every individual plant at the end of the experiment. The species composition of the soil microbial community was determined by phospholipid fatty acid analysis (PLFA) following the protocol of Bossio and Scow (1998). Microbial lipids were first extracted with single-phase mixture of chloroform: methanol: buffer solution (1:2:0.8 v/v/v). The extracted lipids were then separated into neutral lipids, glycolipids and phospholipids on a silicic acid column. The fraction of phospholipids was methylated before being analyzed by chromatography–mass spectrometry (GC-MS). Following Brockett et al. (2012), we assigned the phospholipids into seven major groups (i.e., G+bacteria, G− bacteria, actinomycetes, methanogens, AMF, ECM and saprophytic fungi) and calculated the abundance of each group of soil microbes.
Statistical analysis
In a preliminary analysis, we found that the effect of inoculum treatment was similar for the species belonging to an identical successional stage (supplementary materials Table S2, Fig. S1). Because our goal was to determine whether the Schima inocula (CK, MF and BF) affected the performance of early- and late-successional species differently, we used general linear mixed models (LMMs) with inoculum treatment, group (early species vs. late species), and their interactions as fixed factors, while species nested within group was selected as a random factor. Because the interaction between inoculum treatment and group was significant for most measurements, we further analyzed the effect of inoculum treatment separately for early and late species. In these models, inoculum treatment was selected as a fixed factor, while species was selected as a random factor. For RGR of height and RGR of basal diameter, which were measured three times, we included time (i.e., 1st, 2nd and 3rd measurement) as a random factor to account for the variance among different measurement times. Because using identical CK inocula is likely to result in less variation in the CK treatment comparing to MF and BF treatments, we had to correct for data homogeneity and normality through data transformation (see the normality test for model residuals in supplementary materials Fig. S2). The LMMs were conducted with R package ‘lme4’ (Bates et al. 2015). Although it is recommended that the minimum number of levels for a random factor be eight, LMMs using non-focal factors as random factors are easier to interpret (Gelman and Hill 2006). Moreover, the results of LMMs were similar to those of fixed-effect linear models (LMs; see LM outputs in supplementary materials Table S3). Post hoc analyses were conducted for the multiple comparisons among inocula treatments using the ‘glht’ function (‘multcomp’ package; Hothorn et al. 2008) for all the above models.
To test the effect of Schima inocula on the rhizosphere microbial community for each study species, we conducted a non-metric multidimensional scaling (NMDS) on the abundance of the seven major groups of microbes using the ‘metaMDS’ function (‘vegan’ package; Oksanen et al. 2016). Because the distributions of the soil microbial groups of the four tree species on NMDS axes were not distinctive, we combined the datasets of all four species and conducted an overall NMDS on the combined dataset. Additionally, we conducted a NMDS on the microbial groups of the original soil inocula. Then, the scores of the first two axes from NMDS analysis for each individual and original soil inocula were extracted, which were subjected to post hoc analysis for the inoculum effect on axis scores.
To test whether the rhizosphere soil microbial community and root-associated mycorrhiza account for the variances in plant growth, a partial least squares (PLS) path model was conducted to detect all significant direct and indirect correlations between growth performance and soil microbes for early and late species using the ‘plspm’ function from the ‘plspm’ package (Sanchez 2013). Because there is a suggested sample size for PLS path model (Marcoulides and Saunders 2006), we compiled a dataset of 54 entries for each species. The three repeated measurements of growth, RGR of height and RGR of basal diameter, of each individual were treated as three different entries; thus, for the 18 individuals per species we obtained 54 entries. The one-time measurements of nutrient acquisition status, root health status, mycorrhiza root colonization and rhizosphere microbial community were triplicated for each individual. An a priori model was constructed using six latent variables with corresponding indicators (see model set-up in Fig. 1). As mentioned above, our goal was to see whether the early- and late-successional species responded differently to the inocula treatments. By conducting separate PLS path models for individual species, we confirmed that the species within the same group responded in a similar fashion (see supplementary materials Fig. S3; Table S7, 8). Thus, we controlled for between-species variance following Lankau (2013) and combined the datasets of the two species within the same group from the same successional stage. The predicted model for each group was obtained and confirmed by bootstrapping following Sanchez (2013) (see steps for model construction in supplementary materials Note S1). All statistics were conducted in R version 3.4.1 (R Core Team 2017).
A priori PLS path model. Rectangles denote indicators that were measured in our experiments. Circles denote latent variables that were estimated by corresponding indicator(s). An arrow between two latent variables denotes a direct correlation between the two variables. In total, six latent variables were included: growth, nutrient acquisition status, root health status, mycorrhiza root colonization, structure of the soil microbial community and inoculum treatment. The model was built under the following assumptions: i) nutrient acquisition status directly affects plant growth (Santiago et al. 2012); ii) root lesion directly affects plant growth by inducing tissue repair or compensatory regrowth (Maschinski and Whitham 1989), or indirectly affects plant growth by affecting nutrient acquisition status (Olff and Ritchie 1989); iii) root mycorrhiza colonization directly affects plant growth by enhancing stress tolerance (Carrillo-Garcia et al. 1999) or indirectly affects plant growth through affecting root health status (Schulz and Boyle 2005) and nutrient acquisition status (Smith and Smith 2012); iv) the soil microbial community only indirectly accounts for growth variances by affecting root health status (Li et al. 2012), nutrient acquisition status (Rodríguez and Fraga 1999) and root mycorrhiza colonization (Bardgett and Shine 1999); and v) inoculation only indirectly affects plant growth by affecting the microbial community in the rhizosphere and plant-microbe interactions. Leaf N-P: leaf N-P ratio; BD: basal diameter
Results
Inoculum effect on growth performance and nutrient acquisition status
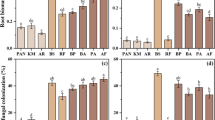
The growth performance of the early species was inhibited by the BF inoculum (Table 1; Fig. 2a–c): RGR of height, basal diameter and biomass decreased by 33 ± 9% (CK vs. BF post hoc z = 4.061, P < 0.001), 24 ± 8% (CK vs. BF post hoc z = 3.238, P = 0.003) and 35 ± 10% (CK vs. BF post-hoc z = 3.310, P = 0.003), respectively. In addition, there was a trend that the MF inoculum inhibited the RGR of height of the early species (Table 1; Fig. 2; CK vs. MF post hoc z = 2.171, P = 0.076). In contrast, the growth performance of the late-successional species was not responsive to the inoculum treatments (Table 1; Fig. 2a–c): the post hoc z values for CK vs. BF were − 0.234 (P = 0.970), 0.812 (P = 0.696) and − 0.206 (P = 0.977) for RGR of height, basal diameter and biomass, respectively. Because the effects of the random factors, species and time, were mostly negligible for the models conducted in this study, we only present the effect sizes of random factors in the supplementary materials (Table S4).
The effect of inoculation on RGR of height (a), RGR of basal diameter (b), RGR of biomass (c), leaf N content (d), leaf P content (e), leaf N-P ratio (f), root non-AMF colonization rate (g), root AMF colonization rate (h) and root lesion percentage (i) of early- and late-successional species. Means with 1 SE are shown. Significant differences between these two groups are denoted by different upper-case letters, while significant differences among different inoculum treatments within each group are denoted by different lower-case letters, based on post hoc tests. Refer to statistics in Table 1. See post hoc z and P values in Results
Leaf nitrogen and phosphorous contents remained unaffected by inoculum treatments, suggesting that the nutrient stoichiometry is rather strong for all species (Table 1; Fig. 2d–f).
Inoculum effect on root mycorrhiza root colonization and root lesions
Non-AMF root colonization in both the early and late species was inhibited by the BF inoculum comparing to the CK inoculum: the reductions in colonization rates were 51 ± 16% and 57 ± 9%, respectively (for the early species: post hoc z = 3.274, P = 0.003; for the late species: post hoc z = 6.366, P < 0.001). The MF inoculum also reduced the non-AMF colonization in the late species by 32 ± 9% (post hoc z = 3.610, P < 0.001).
AMF root colonization in the early species remained unaltered across inoculum treatments (Table 1; Fig. 2h): the post hoc z values for CK vs. MF and CK vs. BF were 0.613 (P = 0.813) and − 0.508 (P = 0.868), respectively. However, AMF root colonization in the late species was increased in response to the BF inocula by 224 ± 47% (post hoc z = −4.806, P < 0.001). There was a trend of increased AMF root colonization in the late species under the MF treatment (post hoc z = 2.216, P = 0.068).
Inoculation significantly affected the root lesion percentage of both the early and late species. For the early species, the MF and BF inocula increased root lesions by 40 ± 16% (post hoc z = −2.438, P = 0.039) and 70 ± 16% (post hoc z = −4.267, P < 0.001). For the late species, the MF and the BF inocula decreased root lesions by 40 ± 16% (post hoc z = 2.472, P = 0.035) and 51 ± 16% (post hoc z = 3.164, P = 0.004), respectively.
Inocula effect on rhizosphere microbial community
The soil microbial component of the CK inoculum significantly differed from that of the MF and BF inocula, with a significantly higher abundance of ECM and a significantly lower abundance of all other microbial groups (e.g., G+ bacteria, G− bacteria, AMF, etc.) in the CK inoculum (Fig. 3a; see scores for inoculum treatments and microbial groups in supplementary materials Table S5, 6). All study plant species had altered rhizosphere microbial communities during the experiment. The soil microbial components of the different inoculum treatments were not distinctive for Rhodomyrtus, Cryptocarya and Machilus (Fig. 3c–e). However, the abundance of saprophytic fungi and ECM were lower and the abundance of actinomycetes and G+ bacteria were higher under the CK treatment than under the BF treatment for Pinus (Fig. 3b; see scores for inoculum treatments and microbial groups in supplementary materials Table S5, 6).
Biplots for the first two axes of the PCA for soil inocula (a), Pinus massoniana (b), Rhodomyrtus tomentosa (c), Cryptocarya chinensis (d) and Machilus chinensis (e). Open circles denote the CK treatment, gray triangles denote the MF treatment, and black squares denote the BF treatment. Major microbial groups are mapped based on their scores on the first two axes
Direct and indirect correlations between growth and soil microbes
Fifty-one percent of the variation in growth performance in the early species could be explained by the direct and indirect effects of soil microbes (Fig. 4a). The growth of the early species was directly correlated with root mycorrhiza colonization, root health status and leaf nutrient acquisition status (Fig. 4a): a higher percentage of non-AMF colonization, stronger N vs. P acquisition and fewer root lesions corresponded to faster growth. The standard beta coefficients quantifying the strengths and directions of the direct correlations between growth and mycorrhiza root colonization, nutrient acquisition status and root health status were 0.48, 0.29, and − 0.10, respectively. In addition, root non-AMF colonization indirectly improved the growth performance of the early species through two synergistic paths (Fig. 4a; standard beta coefficient = 0.10): i) reducing root lesion (standard beta coefficient = −0.14); and ii) enhancing the relative strength of N vs. P acquisition (standard beta coefficient = 0.29). Inoculation with Schima rhizosphere soil significantly increased the percentage of root lesions in the early species (standard beta coefficient = 0.60), and it increased the abundance of saprophytic fungi in the rhizosphere of the early species (standard beta coefficient = −0.53). Because saprophytic fungi were strongly negatively loaded on soil microbial NMDS2 (see supplementary materials Table S6), saprophytic fungi appeared to indirectly inhibit the growth of the early species through three synergistic paths (Fig. 4a; standard beta coefficient = 0.44): i) increasing root lesion (standard beta coefficient = −0.11); ii) decreasing the relative strength of N vs. P acquisition (standard beta coefficient = 0.29); and iii) decreasing root non-AMF colonization (standard beta coefficient = 0.34). See bootstrapped indicator loadings and path coefficients in supplementary materials Table S7, 8.
Predicted PLS path model for the direct and indirect correlations between the growth performance and soil microbes for the early-successional species (a) and late-successional species (b). Circles represent latent variables. Indicators for corresponding latent variables are shown beside/below the circles. To make all indicators positively loaded on corresponding latent variable, the following indicators were transformed before being used: i) for the early species, the values of leaf P content were multiplied by −1; ii) for the late species, the values of AMF colonization rate and leaf P content were multiplied by −1. All statistics shown are the result of 500-iteration bootstrapping. R2 values with 1 SE are shown for all endogenous latent variables (i.e., the ones that are pointed to at least one arrow). Significant R2 values are marked by asterisks (*P < 0.05, **P < 0.01, *** P < 0.001). Dark gray solid lines and arrows denote facilitation. Light gray lines denote inhibition. Thicker lines indicate stronger correlations. See indicator loadings and path coefficients in supplementary materials Table S7 and S8, respectively
Only 4 % of the variation in the growth performance of the late species could be explained by the direct and indirect effects of soil microbes (Fig. 4b). The growth of the late species was directly correlated only with leaf nutrient acquisition status (Fig. 4b; standard beta coefficient = −0.15): a stronger P vs. N acquisition corresponded to faster growth. Because root non-AMF and AMF functioned antagonistically (see model construction in supplementary materials Note S1), root mycorrhiza colonization exerted a very weak indirect effect on growth performance (Fig. 4b; standard beta coefficient = −0.03) by altering nutrient acquisition status (standard beta coefficient = −0.26). Inoculation with Schima rhizosphere soil significantly reduced the percentage of root lesions in the late species (standard beta coefficient = −0.36), while it increased the abundance of root-associated AMF but reduced the abundance of root-associated non-AMF (standard beta coefficient = −0.76). Because soil saprophytic fungi functioned antagonistically against soil AMF and methanogens (see supplementary materials Table S7), rhizosphere microbes also had little indirect effect on plant growth by affecting leaf nutrient acquisition status (Fig. 4b; standard beta coefficient = 0.02): saprophytic fungi increased, while AMF and methanogens decreased the relative strength of N vs. P acquisition (standard beta coefficient = −0.13). See bootstrapped indicator loadings and path coefficients in supplementary materials Table S7, 8.
Discussion
Although indirect plant-plant interactions through plant-soil feedbacks have been identified as important drivers of plant community dynamics, only a few studies have explored their roles in forest succession. Our study is among the first to demonstrate the importance of plant-soil interactions in subtropical forest succession by showing that the interplay between plants and soil microbes may enhance the competitive advantage of late- over early-successional species. Specifically, we examined the effects of Schima-induced changes in its rhizosphere microbial community on the growth, leaf nutrient content, root lesions and root-mycorrhiza association of the species from different successional stages. Our experimental design allowed us to test the relative explanatory power of all possible direct and indirect correlations between soil microbes and plant growth.
Contrasting effects of Schima superba on early- and late-successional species
The rhizospheric soil of Schima superba exerted contrasting effects on the early vs. late-successional species used in this study: Schima inoculum inhibited the performance of the early-successional species but had little effects the performance of the late-successional species. Although we only chose two species to represent each group, with the early species both being heliophytes and the late species both being mesophytes, it is important to note that heliophytes are nearly as common as mesophytes in mid- and late-successional forests in subtropical China (Liu et al. 2012a; Cao 2013). In a parallel experiment on the effect of Schima inocula on Syzygium rehderianum, a common heliophyte in mid- and late-successional stands (Mo et al. 2006), we found that the performance of Syzygium was inhibited by inoculation, as was observed for early-successional heliophytes in the current study (unpublished data; Liao et al.). We thus demonstrate negative soil feedbacks on heliophytes and slightly positive feedbacks on mesophytes (Fig. 2). These results together suggest that Schima superba has the potential to accelerate forest succession by inhibiting heliophytes, the major component of early- and mid-successional forests. As a matter of fact, the “tolerance” model proposed by Connell and Slatyer (1977) also describes a similar scenario to what we observed in the current study: the colonization of particular species restricted the recruitment of early-successional species by modifying environmental conditions, while they had little effects on the colonization of late-successional species. Though the Connell-Slatyer model is a good theoretical tenet, there is not much empirical evidence in support of it. Our study showed that Schima may function as an early occupant in Connell-Slatyer model and has the potential to facilitate succession.
Explanatory hypothesis for the contrasting effects of Schima on early- and late-successional species
Differences in the strength and direction of soil feedbacks for different groups were apparent in our results. Because nutrient acquisition status had similar explanatory power for the growth of early and late species (Fig. 4), the much greater percentage of growth variance explained for early than for late species was due to root mycorrhiza colonization and root lesions instead of nutrient acquisition status (Fig. 4). In the current study, the negative soil feedback for the early-successional species seemed to result from the lack of association with non-AMF (Fig. 2g and Fig. 4a): a lower percentage of non-AMF colonization corresponded to more root lesions and slower growth. The early-successional species, Pinus massoniana, is a known ECM-associated plant (Huang et al. 2014). Myrtaceae (the family of the other early species, Rhodomyrtus tomentosa) has been reported to mainly but not exclusively associate with ECM (Howard et al. 2000). In contrast, Lauraceae (the family of the late species, Cryptocarya chinensis and Machilus chinensis) was found to associate with AMF (Chen et al. 2017). A recent study showed that while ECM-associated plants seldom form AMF associations, AMF-associated plants are often found to be infected by ECM to some extent (Toju et al. 2014). Thus, it is reasonable that the early species, Pinus and Rhodomyrtus, suffered more from the decreased non-AMF colonization than the late species, Cryptocarya and Machilus.
Strong explanatory power of soil microbes on the growth responses to inocula treatment
In the current study, we found that soil microbes had both directly and indirectly affected plant growth, especially for early species (Fig. 4).
Root mycorrhiza has been reported to directly facilitate plant growth by enhancing plant tolerance to environmental stress, such as drought (Carrillo-Garcia et al. 1999) and acidity (Gupta and Krishnamurthy 1996). Consistent with previous studies, we did find a significant direct effect of root mycorrhiza on the growth performance of early species (Fig. 4a). Because seasonal drought and strong soil acidity have been proposed as key limiting factors for plant colonization and establishment in subtropical China (Peng and Wang 1993), ECM may greatly contribute to plant stress tolerance and thus allow initial establishment of pioneer and early-successional species.
In addition, soil microbes indirectly affected plant growth through affecting nutrient acquisition status. On one hand, soil microbes have been proposed to facilitate plant nutrient consumption (Rodríguez and Fraga 1999; Nakano-Hylander and Olsson 2007; Smith and Smith 2012). On the other hand, soil microbes have been reported to compete for soil nitrogen with host plant (Bardgett et al. 2003). Our results showed that for the early-successional species, rhizosphere saprophytic fungi negatively correlated with the relative strength of N vs. P acquisition (Fig. 4a). For the late-successional species, root-associated non-AMF increased, and root-associated AMF reduced the relative strength of N vs. P acquisition (Fig. 4b), which confirmed the well-known roles of non-AMF, such as ECM, in N uptake (Landeweert et al. 2001) and AMF in P uptake (Smith and Smith 2012). Since both of the study groups suffered slightly from N limitation (i.e., leaf N-P ratio < 10) instead of P limitation (i.e., leaf N-P ratio > 20) (Fig. 2f), the more prominent benefit from non-AMF on N uptake is reasonable.
Moreover, soil microbes played an important role in regulating resistance to belowground pathogenic/herbivory attacks. Strong association with non-AMF had alleviated the root lesion in early-successional species (Fig. 4a), while strong association with AMF had alleviated the root lesions in the late-successional species (Fig. 4b). This may have resulted from the protection on host plants by mycorrhiza (Moosavi and Zare 2012). However, we also found that the root lesion percentage in the late-successional species increased as non-AMF colonization rate increased (Fig. 4b), where an increased amount of saprophytic fungi tended to increase the possibility of pathogenic/herbivory attack on the roots (Fig. 4a,b). The positive correlation between the abundance of saprophytic fungi and root lesion might be due to direct parasitism (Liang et al. 2015) or competition between saprophytes and beneficial microbial groups, such as mycorrhiza (Allen et al. 1995).
Conclusions
In conclusion, Schima superba most probably facilitates forest succession in subtropical China by inhibiting the growth of heliophytes, the major component of early- and mid-successional communities, but having little effect on mesophytes, the major component of late-successional communities. These different effects on trees from different successional stages likely resulted from the changes in the rhizosphere microbial community that weaken the root-non-AMF association of heliophytes but strengthen the root-AMF association of mesophytes. Given that the major group of tree-associated mycorrhiza in tropical or temperate regions is either AMF or ECM, while in subtropical regions AMF- or ECM-associated trees often co-occur at comparable abundances (Toju et al. 2014), the interplay between AMF and ECM in shaping the aboveground vegetation can be exceptionally important in subtropical forests. It is important to note that this study was conducted using a mesocosm experiment; thus, the true effects of Schima superba on forest succession have to be tested further in field experiments.
References
Allen EB, Allen MF, Helm DJ, Trappe JM, Molina R, Rincon E (1995) Patterns and regulation of mycorrhizal plant and fungal diversity. Plant Soil 170:47–62
Barberan A, McGuire KL, Wolf JA, Jones FA, Wright SJ, Turner BL et al (2015) Relating belowground microbial composition to the taxonomic, phylogenetic, and functional trait distributions of trees in a tropical forest. Ecol Lett 18:1397–1405
Bardgett RD, Shine A (1999) Linkages between plant litter diversity, soil microbial biomass and ecosystem function in temperate grasslands. Soil Biol Biochem 31:317–321
Bardgett RD, Streeter TC, Bol R (2003) Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 84:1277–1287
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bennett JA, Maherali HR, Reinhart KO, Lekberg Y, Hart MM, Klironomos J (2017) Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355:181–184
Bever JD (2003) Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol 157:465–473
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Brockett BFT, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol Biochem 44:9–20
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. AClAR Monograph, Australia
Cao H (2013) Dinghushan lower subtropical forest dynamics plot: tree species and their distribution pattern. China Forestry Publishing House, Beijing, China
Carrillo-Garcia A, de la Luz JLL, Bashan Y, Bethlenfalvay GJ (1999) Nurse plants, mycorrhizae, and plant establishment in a disturbed area of the Sonoran Desert. Restor Ecol 7:321–335
Chen L, Zheng Y, Gao C, Mi XC, Ma KP, Wubet T, Guo L (2017) Phylogenetic relatedness explains highly interconnected and nested symbiotic networks of woody plants and arbuscular mycorrhizal fungi in a Chinese subtropical forest. Mol Ecol 26:2563–2575
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144
Cornelissen JHC, Werger MJA, Zhong Z (1994) Effects of canopy gaps on the growth of tree seedlings from subtropical broad-leaved evergreen forests of southern China. Vegetatio 110:43–54
Gelman A, Hill J (2006) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, New York
Grau HR, Arturi MF, Brown AD, Acenolaza PG (1997) Floristic and structural patterns along a chronosequence of secondary forest succession in Argentinean subtropical montane forests. For Ecol Manag 95:161–171
Gupta R, Krishnamurthy KV (1996) Response of mycorrhizal and nonmycorrhizal Arachis hypogaea to NaCl and acid stress. Mycorrhiza 6:145–149
Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Howard K, Dell B, Hardy GE (2000) Phosphite and mycorrhizal formation in seedlings of three Australian Myrtaceae. J Bot 48:725–729
Huang W, Liu J, Wang YP, Zhou G, Han T, Li Y (2013) Increasing phosphorus limitation along three successional forests in southern China. Plant Soil 364:181–191
Huang J, Nara K, Zong K, Wang J, Xue S, Peng K et al (2014) Ectomycorrhizal fungal communities associated with masson pine (Pinus massoniana) and white oak (Quercus fabri) in a manganese mining region in Hunan Province. China. Fungal Ecol 9:1–10
Kardol P, Bezemer TM, van der Putten WH (2006) Temporal variation in plant-soil feedback controls succession. Ecol Lett 9:1080–1088
Kardol P, Cornips NJ, van Kempen MML, Bakx-Schotman JMT, van der Putten WH (2007) Microbe-mediated plant-soil feedback causes historical contingency effects in plant community assembly. Ecol Monogr 77:147–162
Kilpeläinen J, Vestberg M, Repo T, Lehto T (2016) Arbuscular and ectomycorrhizal root colonisation and plant nutrition in soils exposed to freezing temperatures. Soil Biol Biochem 99:85–93
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Krüger C, Kohout P, Janoušková M, Püschel D, Frouz J, Rydlová J (2017) Plant communities rather than soil properties structure arbuscular mycorrhizal fungal communities along primary succession on a mine spoil. Front Microbiol 8:719
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breemen N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–254
Lankau RA (2013) Species invasion alters local adaptation to soil communities in a native plant. Ecology 94:32–40
Li D, Waller D (2016) Long-term shifts in the patterns and underlying processes of plant associations in Wisconsin forests. Glob Ecol Biogeogr 25:516–526
Li X, Wheeler GS, Ding J (2012) A leaf-rolling weevil benefits from general saprophytic fungi in polysaccharide degradation. Arthropod Plant Interact 6:417–424
Liang M, Liu X, Etienne RS, Huang F, Wang Y, Yu S (2015) Arbuscular mycorrhizal fungi counteract the janzen-connell effect of soil pathogens. Ecology 96:562–574
Liu L, Gundersen P, Zhang T, Mo J (2012a) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38
Liu X, Liang M, Etienne RS, Wang Y, Staehelin C, Yu S (2012b) Experimental evidence for a phylogenetic Janzen-Connell effect in a subtropical forest. Ecol Lett 15:111–118
Lu R (1999) Agricultural chemical analysis of the soil. China Agricultural Science and Technology Press, Beijing
Maggi E, Bertocci I, Vaselli S, Benedetti-Cecchi L (2011) Connell and slatyer's models of succession in the biodiversity era. Ecology 92:1399–1406
Mangan SA, Schnitzer SA, Herre EA, Mack KML, Valencia MC, Sanchez EI et al (2010) Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466:752–U10
Männistö MK, Tiirola M, Häggblom MM (2009) Effect of freeze-thaw cycles on bacterial communities of arctic tundra soil. Microb Ecol 58:621–631
Marcoulides GA, Saunders C (2006) Editor's comments: PLS: a silver bullet? Manag Inf Syst Q 30:iii–iix
Maschinski J, Whitham TG (1989) The continuum of plant responses to herbivory: the influence of plant association, nutrient availability, and timing. Am Nat 134:1–19
Mccarthy-Neumann S, Kobe RK (2008) Tolerance of soil pathogens co-varies with shade tolerance across species of tropical tree seedlings. Ecology 89:1883–1892
Mcgonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Metlen KL, Aschehoug ET, Callaway RM (2013) Competitive outcomes between two exotic invaders are modified by direct and indirect effects of a native conifer. Oikos 122:632–640
Mo J, Brown S, Xue J, Fang Y, Li Z (2006) Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 282:135–151
Moosavi MR, Zare R (2012) Fungi as biological control agents of plant-parasitic nematodes. Springer Netherlands, Dordrecht
Nakano-Hylander A, Olsson PA (2007) Carbon allocation in mycelia of arbuscular mycorrhizal fungi during colonisation of plant seedlings. Soil Biol Biochem 39:1450–1458
Nara K (2006) Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol 171:187–198
Nara K, Hogetsu T (2004) Ectomycorrhizal fungi on established shrubs facilitate subsequent seedling establishment of successional plant species. Ecology 85:1700–1707
O’Hanlon-Manners DL, Kotanen PM (2006) Losses of seeds of temperate trees to soil fungi: effects of habitat and host ecology. Plant Ecol 187:49–58
Oksanen J, Blanchet GF, Friendly M, Kindt R, Legendre P, McGlinn D et al (2016) vegan: community ecology package. https://CRAN.R-project.org/ package=vegan
Olff H, Ritchie ME (1989) Effects of herbivores in grassland plant diversity. Trend Ecol Evol 13:261–265
Peng S, Wang B (1993) Forest succession at dinghushan, Guangdong, China. Journal of Tropical and Subtropical Botany 1:34–42
Peng S, Zhou T, Liang L, Ren W (2012) Landscape pattern dynamics and mechanisms during vegetation restoration: a multiscale, hierarchical patch dynamics approach. Restoration Ecol 20:95–102
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
R Core Team (2017) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. URL: http://www.R-project.org/
Rees M, Condit R, Crawley M, Pacala S, Tilman D (2001) Long-term studies of vegetation dynamics. Science 293:650–655
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Sanchez G (2013) PLS path modeling with R. Trowchez Editions, Berkeley, USA
Santiago LS, Wright SJ, Harms KE, Yavitt JB, Korine C, Garcia MN et al (2012) Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J Ecol 100:309–316
Schulz B, Boyle C (2005) The endophytic continuum. Mycol Res 109:661–686
Smith SE, Smith FA (2012) Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 104:1–13
Tilman D (1985) The resource-ratio hypothesis of plant succession. Am Nat 125:827–852
Toju H, Sato H, Tanabe AS (2014) Diversity and spatial structure of belowground plant-fungal symbiosis in a mixed subtropical forest of ectomycorrhizal and arbuscular mycorrhizal plants. PLoS One 9:e86566
Wang B, Peng S (1987) Quantitative dynamics of the dominant population in the forest communities of dinghushan. Acta Ecol Sin 7:24–31. (in Chinese with English abstract)
Wootton J (1994) The nature and consequences of indirect effcts in ecological communitites. Annu Rev Ecol Syst 25:443–466
Yan J, Li K, Peng X, Huang Z, Liu S, Zhang Q (2015) The mechanism for exclusion of Pinus massoniana during the succession in subtropical forest ecosystems: light competition or stoichiometric homoeostasis? Sci Rep 5:10994
Acknowledgements
We appreciate the help from Yaru Yuan, Meiyu Lu, Peng Zhou, Shuangbo Chen, Xiangping Tan, Chuanyin Xiang and Dingsheng Mo during soil sampling, experimental set-up and PLFA experiment. We thank Wenbo Luo and three anonymous reviewers for their thoughtful comments on the manuscript.
Funding
This project was funded by National Natural Science Foundation of China (NSFC 31700450, NSFC 31670479), Natural Science Foundation of Guangdong Province (China) (2017A03031 0386) and Fundamental Research Funds for the Central Universities (China) (17lgpy104).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Felipe E. Albornoz.
Rights and permissions
About this article
Cite this article
Liao, H., Huang, F., Li, D. et al. Soil microbes regulate forest succession in a subtropical ecosystem in China: evidence from a mesocosm experiment. Plant Soil 430, 277–289 (2018). https://doi.org/10.1007/s11104-018-3733-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3733-3