Abstract
Background and Aims
Verticillium wilt, caused by the soil-borne pathogenic fungus Verticillium dahliae (Kleb), is one of the most severe diseases of olive trees in Mediterranean agriculture. At present, the use of organic amendments is considered an effective means to combat certain soil-borne plant diseases while in turn supplying plants with nutrients. The aim of this study was to determine the early stage effects of Verticillium wilt of olive (VWO) on macronutrient uptake and in planta mobility by analysing nutrients in young olive trees.
Methods
Young olive trees were grown in unamended as well organically amended soils (employing two distinct olive mill waste composts, C1 and C2) in the presence or absence of mineral fertilisation. Half of the soils were inoculated with Verticillium dahliae (Vd) defoliating (D) pathotype, leaving the other half non-inoculated. At 22 and 100 days after inoculation, nutrient content (N, P, K, S, Ca, and Mg) was analysed in mature and young leaves.
Results
Olive mill compost enhanced N, P, K and S availability but did not prevent Verticillium infection in young olive trees. Pathogen inoculation affected nutrient content in mature and young leaves and reduced leaf nutrient input during the early stages of host colonization. Stronger effects were observed in pathogen inoculated plants grown in soil amended with C2, coinciding with high N and P and transiently low S availability. Leaf K input showed the highest sensitivity to V. dahliae inoculation.
Conclusions
In high N conditions, deleterious effects caused by Vd on young olive trees may be overcome by promoting the growth of new shoots. We present evidence that Vd requires S during the initial stages of host colonization, in which plant-pathogen competition for this element is key, and that low levels of S in foliage during such early stages may enhance Verticillium wilt development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Verticillium wilt of olive (VWO), caused by Verticillium dahliae (Vd), a pathogenic fungus in Division Ascomycota, Family Plectosphaerellaceae, (Inderbitzin and Subbarao 2014) is considered to be one of the most important and devastating biotic threats affecting olive tree groves in most Mediterranean countries. This disease was reported for the first time in Italy (Ruggieri 1946) and described for the first time in Spain in 1975 (Jiménez-Díaz et al. 2012).
V. dahliae is a soil-borne fungus that reproduces asexually. Vd produces resistance structures (microsclerotia) that remain viable in soil under unfavourable conditions (Mercado-Blanco and López-Escudero 2011). Wet soils and favourable temperatures (21-27 °C) as well as root exudates stimulate microsclerotia germination, starting an infection cycle when hyphae penetrate roots, colonizing epidermal cells and the cortex (Jiménez-Díaz et al. 2012). Once the root system has been colonized, most hyphae remain in cortex cells forming colonies (Prieto et al. 2009) until hyphae invade xylem vessels where they form conidia. Vascular colonization occurs when conidia are transported upwards by xylem sap. Under these conditions, lytic enzymes produced by the fungus, as well as defence products such as tyloses and polysaccharide gels produced by the plant may partially block xylem vessels (Báidez et al. 2007). This may impede water and nutrient transport to upper parts of the plant. Vascular obstruction may cause chlorosis and symptoms of wilt in leaves and shoots. The mode of infection and symptom expression caused by Vd in olive trees depend on the pathotype; the most virulent one being known as the defoliating pathotype (D), characteristically causing plant wilt, chlorosis, widespread defoliation, a severe reduction of plant growth, and finally plant death (Martos-Moreno et al. 2006; López-Escudero and Mercado-Blanco 2010).
Vd plant vascular invasion can affect nutrient translocation, water transport and photosynthesis (Resende et al. 1996; Walters and Bingham 2007). In the case of olive trees, significant reductions of water consumption and chlorophyll production in leaves of Vd inoculated plants of the cv. Picual have been observed (Birem et al. 2009). These reductions have been characterized to be a consequence of vascular obstruction caused by Vd. Although such obstruction may affect transport of all nutrients, it may have an especially notable effect on nutrients in limiting concentrations, as they may greatly alter plant metabolic responses.
Changes in nutrient availability may induce changes in plant metabolism and growth that in turn may affect plant pathogen resistance (Marschner 2012). The influence of fertilisation on vascular diseases has been studied in a wide range of crops (Pegg and Brady 2002). In the case of olive trees it is assumed that high N fertilisation in irrigated systems may increase the incidence and severity of Vd wilt (López-Escudero and Mercado-Blanco 2010). Similar results have been found in the case of cotton (El-Zik 1985). However, some studies have shown that the use of ammonia may reduce the number of microsclerotia in soils and increase the biological activity of pathogen antagonists (Pegg and Brady 2002). In the case of Pistacia vera L. increasing K and Mg availabilities by means of organic amendments or reducing Ca levels in soil may diminish disease severity (Pegg and Brady 2002). On the other hand, soil microbial populations and activity can directly interact with Vd causing additional effects besides changes in nutrient availability (Gamliel et al. 2000; Goicoechea 2009).
Organic amendments formulated with olive mill waste (Yangui et al. 2008; Yildiz and Benlioglu 2010; Alfano et al. 2011; Papasotiriou et al. 2013; Vitullo et al. 2013; Avilés and Borrero 2017) or other organic sources as well as its extracts (Arriagada et al. 2012) have been found to have the capacity to suppress Verticillium wilt through different biological control mechanisms. In areas with widespread olive oil production, olive mill waste is widely produced as a by-product and is readily available (Morillo et al. 2009); thus, in case of being effective, its use may be viewed as a strategy to derive value from recycling these residues. The use of compost in biocontrol can cause changes in substrate microorganisms, changes in nutrient availability, and can contribute to inducing plant resistance (Segarra et al. 2013; Fernàndez et al. 2014). The application of olive mill compost over the short term generally increases soil microbe populations and their enzymatic activity, but does not generally increase short-term N availability (García-Ruiz and Ochoa 2012). In fact, we hypothesized that the application of olive mill composts to soils would transiently limit N availability. We further hypothesized that the application of mineral fertilisers may reduce this suppressive effect. Thus, our aims were to (i) determine early effects of the pathogen in young olive tree growth in fertilised and unfertilised soils amended with olive mill compost, (ii) to determine the effects of Vd on plant nutrition, and (iii) to identify which nutrients are specifically affected by Vd infection in early stages.
Materials and methods
Plant material, substrates and growing conditions
A total of 360 two-year-old Olea europaea cv. Picual cuttings (highly susceptible cultivar) obtained via clonal propagation were transplanted individually in 10 l pots in October 2016, and grew in a greenhouse under partially-controlled conditions at the University of Barcelona (UB) Torribera Food Science Campus in Santa Coloma de Gramenet, Spain. All plants were pruned down to 7–8 leaves per plant on 21/12/2016 in order to obtain an increased homogeneity in size. Three different soils were employed: field soil on its own and in combination with one of two olive mill composts (Soil, Soil-C1, Soil-C2). Soil was a sandy loam (14.5% clay) collected from a commercial olive tree grove in the municipality of Abrera, Spain, in which no symptoms of Verticillium wilt had been observed. No microsclerotia were found in this soil when plated in modified soil extract agar (MSEA) media according to the method of Harris et al. (1993). In order to favour drainage and aeration, the soil was mixed with perlite (Perlite, 2–6 mm, Premium Gramoflor, Germany) at a ratio v/v of 2 soil: 1 perlite. The two olive mill commercial composts were separately mixed with soil (4: soil-perlite mix, 1: compost, v/v). These were selected from a study carried out by Avilés and Borrero (2017) to test their natural VWO suppressiveness. Compost 1 (C1) was found to be the most suppressive of microsclerotia while compost 2 (C2) was less suppressive. C1 was made in Jaen, (Spain) and is composed of wet olive husks, olive waste and sheep manure. C2 was made in Málaga (Spain) and is composed of wet olives husk, olive leaf waste, goat manure and straw. In Table 1 we show the main chemical characteristics of the soil, composts, and soil-compost mixes employed in this study. Soil and soil-compost mixes will be referred to as soils in general terms henceforth.
Experimental design
To evaluate the effects of Vd in young trees we set up an experimental layout with 240 pots (6 inoculated treatments × 30 repetitions and 6 non-inoculated treatments × 10 repetitions) corresponding to to three factors: organic amendment (0, C1 and C2), mineral fertilisation (0, NPK), and Vd inoculation (0, Vd), yielding a total of 12 treatments. For nutrient and plant growth analysis we randomly selected 5 pots per treatment (60 pots). At the end of the sampling period, a lower branch segment from the base (between 0 and 6 cm) was cut in order to detect the putative presence of Vd in randomly selected 20 infected and 10 non-infected plants per treatment. All branch segments were sterilized with 10% bleach for 10 min and plated in Czapek-Dox agar media. All plates were later observed at the microscope.
For mineral fertilisation we used a NPK fertiliser (ENTEC Nitrofoska 14–7-17, EuroChem Agro, Barcelona, Spain) that contained 8% ammonia-N and 6% nitrate-N, 7% P2O5, 17% K2O, 22.5% SO3, 2% MgO, 0.02% B, 0.01% Zn, and 0.8% 3,4-dimethylpyrazole phosphate (DMPP), and was applied at a dose of 142.5 Kg N ha−1, 31.1 Kg P ha−1 and 150.45 kg K ha−1. No calcium was added in mineral fertilisation. A conidial suspension was prepared from a fermenter culture composed of Czapek-Dox Broth and V. dahliae (D pathotype) obtained from 6 Petri dishes in which the fungus had grown previously in Czapek-Dox Agar media for 4 weeks in the dark at 28 °C. This culture was kept during 5 days at an aeration rate of 6 l min−1 (pO2 was adjusted at 100 ± 5%), 1000 rpm, 25 °C, and at non-buffered pH. Plants were inoculated with this conidial suspension at 106 CFU/ml soil of Vd three months after potting. Watering was carried out regularly to near field capacity and weeds were controlled manually as needed. Conditions within the greenhouse varied in accord with the weather, although the greenhouse roof automatically opened and closed adjusting inner temperature to values not much higher than outside values on hot days. Temperatures during the spring growing period ranged from 3.9 to 30.7 °C and relative humidity from 11.3 to 58.7%.
Green area and growth measurements
The green area index (GA) was used as non-destructive measure of plant biomass (Gracia-Romero et al. 2017). GA was derived from HIS (hue, intensity, saturation) colour space and defined as proportion of pixels with 60o < Hue<180 o. This vegetation index was obtained from pixel analysis of RGB photographs taken using a Sony ILCE-QX1 digital camera with an E PZ 16–50 mm F3.5–5.6 OSS lens and a Cat 560 Smartphone as a remote shutter release button controller. A tripod was used to keep the position of the camera steady and four photographs were taken of each plant; two from a zenithal plane and two from a lateral plane, rotating plants slightly for maximum stereoscopy. Images were saved in a 3632 × 5456 pixel JPEG format. Different properties of colour enabled the determination of green area obtained from the images using the source Breedpix 0.2 software adapted to JAVA8 and integrated as a plugin within FIJI (https://github.com/George-haddad/CIMMYT), the final result per replicate being the mean values derived from the 4 pictures taken. Plant growth measurements were taken from the differences in basal diameter between transplanting (day zero) and the end of the experiment. Basal diameter was measured with a digital calliper.
Foliar sampling and analyses
Twenty two days after Vd inoculation 5 plants per treatment were randomly selected for sampling. For each selected plant we sampled two mature leaves and two young leaves that were formed during the 11 weeks of growth after transplanting. Sampled shoots were labelled with a string. One hundred days after inoculation leaves opposite to the leaf sampled as a young leaf were established as mature leaves and sampled, while new grown leaves at the tip of each selected shoot were selected as young leaves. All leaves were rinsed with deionized water to eliminate any nutrients held on the leaf surface. Leaves were oven-dried at 70 °C for 48 h and weighed.
Nutrient content in leaves, except for N, was determined after wet digestion with HNO3 and HClO4 (Zasoski and Burau 1977). All leaves were finely ground in an agate mortar at room temperature. Ten-40 mg of ground plant tissue was pre-treated with nitric acid (HNO3 69.5%) in borosilicate tubes and left overnight. On the following day, all tubes were heated at 80 °C for an hour, let cool, and then 0.5 ml of perchloric acid (HClO4 70%) was added before heating the samples to 180 °C for three hours. All digested samples were filtered and made up to 10 ml volume with deionised water. Element content (P, K, S, Ca, Mg) in the extracts was determined by induced coupled plasma (simultaneous ICP-OES, Perkin Elmer Optima 8300). N content was determined by elemental analyser (Thermo EA 108 CHNS-O, Carlo Erba Instruments) using 2.4–2.8 mg of plant material per sample.
Differences in weight, nutrient content (μg leaf−1) and concentration (μg g−1) between young leaves at time 1 (day 22; T1) and mature leaves (opposite for each leaf) at time 2 (day 100; T2) were used to elucidate nutrient movement within the plant (Weetman and Wells 1990). Leaf nutrient input was calculated as the difference between leaf nutrient content at T2 minus T1. Differences in leaf growth between inoculated and control plants were used as measures of disease and named henceforth as decreased leaf growth.
Data analysis
Nutrient content in leaves and shoot growth was analysed by factorial ANOVA with organic fertilisation, mineral fertilisation and Vd inoculation as fixed factors (SPSS, 24.0 version). The normality of residues was tested by the non-parametric Kolmogorov-Smirnov test. Variables were transformed by the arcsine square root when needed to achieve normality and homogeneity of variance before statistical analyses. Duncan multiple range test (p < 0.05) was used for identifying significant differences between organic amendments. Vd abundance was tested by using a binomial analysis in SPSS Generalized Linear Models (GLM) binomial with a logarithm as link function.
Results
Effects on leaf growth, plant growth and green area
In all cases inoculated plants showed lower values of leaf growth between T1 and T2 when compared to non-inoculated plants, although values were only significant in the soil-C2 treatment (Fig. 1). The highest values of leaf growth were observed in soil-C2 regardless of mineral fertilisation and Vd inoculation. Lower values were obtained in soil-C1 followed by unamended soil. Green area at the end of the experiment was taken as an indication of biomass. A greater biomass was observed in C2 amended plants. Green area was higher in fertilised plots, especially in unamended soils. Green area did not show any effect of inoculation. Basal diameter growth was highest in compost amended soils and showed a positive effect of fertilisation. Vd inoculation diminished diameter growth in comparison to control only in C1 amended soils. In fertilised C2 amended soils increased diameter growth was observed in Vd inoculated plants.
Leaf growth between T1 (22 days after inoculation) and T2 (100 days after inoculation), green area and basal diameter growth at T2 of young control and Verticillium dahliae inoculated olive trees growing in soils amended with compost and mineral fertilisation. V+ refers to inoculated plants and V- to non-inoculated plants. ANOVA significant factors and interactions overall (upper case letters) and within (lower case letters) each substrate are shown. ‘I’ refers to Vd inoculation, S to substrate and F to mineral fertilisation. Capital letter show significant differences between substrates
Measurements of disease
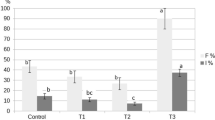
Isolation of Vd was highest in amended soils and lowest in fertilised unamended soils. Unfertilised C2 amended soils showed the highest values (Fig. 2).
Percentage of isolation of V. dahliae from basal branches in inoculated treatments. F refers to fertilised treatments, SC1 to soil + compost 1 and SC2 to soil + compost 2. Binomial test significance between fertilised and non fertilised treatments is shown by an asterisk. Different letters show differences among substrates
Effects on leaf nutrient content
Leaf N levels in unamended soils were directly related to mineral fertilisation, since values obtained were higher than those obtained in non-fertilised treatments, except for young leaves at T1, with values that were not significant when compared to unfertilised plants (Fig. 3). A similar trend could be observed in C1 amended soil, since values for leaf N were significantly higher in fertilised treatments, the only exception being mature leaves at T1, showing a significant interaction between fertilisation and inoculation. In this case, inoculation decreased N concentration only in fertilised plants. In C2 amended soil, N values were higher in fertilised plants at T1 (significant in mature leaves), while at T2 no differences were observable in mature leaves. However, young leaves showed an interaction between inoculation and fertilisation, since values obtained were higher in inoculated treatments when compared to non-inoculated treatments in fertilised plants while showing an opposite trend in unfertilised plants. The effect of inoculation was not significant except in the interactions with fertilisation that in this case increased N concentration in fertilised plants. The lowest values obtained corresponded to unfertilised treatments in unamended soils and C1 amended soils, and presented lower values at T2 when compared to T1.
Mean and standard error (±SE) of leaf N, P and K concentrations in young (Y) and mature (M) leaves sampled 22 days (T1) and 100 (T2) days after inoculation. Young T1 (YT1) and mature T2 (MT2) leaves were sampled from the same verticil. V+ refers to inoculated plants and V- to non-inoculated plants. ANOVA significant factors and interactions within each substrate and leaf type are shown. ‘I’ refers to Verticillium dahliae inoculation and F to mineral fertilisation. Long dashed lines refer to limit of sufficiency for young olive trees (Fernández-Escobar et al. 2016; Jiménez-Moreno and Fernández-Escobar 2017) and dotted line for adult olive trees (Villar and Villar 2016)
Leaf phosphorous levels in compost amended soils increased towards the end of the sampling in mature leaves, while this effect was not observed in unamended soil (Fig. 3). In unamended soils and C1 amended soils leaf phosphorous levels were significantly higher at T2 in fertilised plants. In C2 amended soils an opposite trend was observed, with lower values in fertilised treatments in mature leaves at T2. At T2 in unamended soil, Vd inoculation caused significantly higher leaf phosphorous levels in mature leaves. In C1 amended soil, a similar trend could be observed in inoculated treatments, showing higher levels in mature leaves at T1, while at T2 results showed an opposite effect with significantly lower values for inoculated treatments in both mature and young leaves. Results obtained for young leaves at T1 and mature leaves at T2 in C1 amended plants showed an interaction between fertilisation and inoculation, in which values were significantly lower in inoculated treatments, but only in fertilised plants.
In both C1 and C2 amended soils leaf potassium levels were generally higher when compared to unamended soils (Fig. 3). In all cases young leaves showed a notable increase in potassium levels between T1 and T2, while values obtained in mature leaves decreased, most notably in C2 amended soils. The effect of fertilisation was significant in mature leaves at T2 in unamended soil as well as young leaves at T1 in C2 amended soil. An interaction between fertilisation and inoculation was observable in unamended soils in mature leaves at T1, in which values were lower in inoculated treatments in unfertilised plants and higher in fertilised and inoculated plants. In contrast, the interaction observed in young leaves at T2 showed lower values in inoculated and fertilised plants and no differences in unfertilised plants. In young leaves at T2 in unfertilised inoculated C2 amended soils, values obtained were significantly higher when compared to other treatments.
In unamended soils inoculated plants showed lower levels of S both in mature leaves at T1 and young leaves at T2 (Fig. 4). A similar trend was observed in C1 amended plants. In C2 amended soils mature leaves at T1 showed very low levels of S especially in unfertilised plants. In unamended soils the effect of fertiliser decreased Ca levels in young leaves at T2. A similar trend could be observed in C1 amended plants. In C2 amended soils inoculated plants showed lower levels of Ca in mature leaves at T2. This effect was also observed in young T2 leaves only in fertilised plants. Organic amendments generally reduced foliar Ca levels. In unamended soils inoculation decreased Mg levels at T1 in mature leaves. This effect was also observed in C1 amended soils at T2 in young leaves and in C2 amended soils at T2 in mature leaves. In all cases leaf Mg levels decreased with time with the exception of unfertilised and non-inoculated C2 amended plants.
Mean and standard error (±SE) of leaf S, Ca and Mg in young (Y) and mature (M) leaves sampled 22 days (T1) and 100 (T2) days after inoculation. Young T1 (YT1) and mature T2 (MT2) leaves were sampled from the same verticil. V+ refers to inoculated plants and V- to non-inoculated plants. ANOVA significant factors and interactions within each substrate and leaf type are shown. ‘I’ refers to Verticillium dahliae inoculation and F to mineral fertilisation. Long dashed lines refer to limit of sufficiency for young olive trees (Fernández-Escobar et al. 2016; Jiménez-Moreno and Fernández-Escobar 2017) and dotted line for adult olive trees (Villar and Villar 2016)
Leaf nutrient input and concentration change
Vd inoculation showed a general trend to reduce N, P, K and S input as compared to control plants (Figs. 5 and 6). For N, K and S these reductions observed in inoculated plants were especially relevant in C2 soils while in the case of P they were especially relevant for C1 soils and were not observed in unamended soils.
Mean and standard error (±SE) of the leaf N, P and K input and concentration change between day 22 and day 100 in each treatment. V+ refers to inoculated plants and V- to non-inoculated plants. ANOVA significant factors and interactions overall (upper case letters) and within (lower case letters) each substrate are shown. ‘I’ refers to Vd inoculation, S to substrate and F to mineral fertilisation. Capital letters show significant differences between substrates
Mean and standard error (±SE) of leaf S, Ca and Mg input and concentration change between day 22 and day 100 for each treatment. V+ refers to inoculated plants and V- to non-inoculated plants. ANOVA significant factors and interactions overall (upper case letters) and within (lower case letters) each substrate are shown. ‘I’ refers to Vd inoculation, S to substrate and F to mineral fertilisation. Capital letters show significant differences between substrates
N concentration changes were not significantly different from zero in any case although they tended to increase in both compost amended soils (Fig. 5). In amended and inoculated plants, N concentration decreased over time in contrast to non-inoculated plants. P concentration changes were clearly positive for organically amended plants and negative for the control treatment in unamended soils. In the case of C1, fertilisation increased P concentration change while it decreased it in C2. In all cases inoculated plants showed lower increases of P concentration. In the case of K, in Vd inoculated C2 treatments there was a large decrease in concentration change. Similar to P, K concentration change in fertilised plants was much lower than in unfertilised plants.
S concentration changes were higher in C1 and C2 amended soils (Fig. 6). In C2 amended plants non-inoculated plants showed the largest increases in concentration changes especially when no fertilised. In such plants, Vd inoculation largely reduced S concentration change. C1 amended plants did not show any effect of inoculation. Similar results were observed for Mg. S in mature leaves at T1 negatively correlated to isolation of the pathogen from basal branches and to leaf growth decrease (Fig. 7). Leaf growth decrease in inoculated plants correlated positively to leaf N, P and Mg input and negatively to K, S and Ca leaf input (Table 2).
Ca concentration changes were always positive and the lowest concentration changes were observed in C1 amended plants (Fig. 6). In C2 amended plants, fertilisation decreased Ca concentration changes. This reduction due to fertilisation also occurred for other nutrients such as Mg, K, P and N. Inoculation reduced the concentration change for all nutrients in C2 amended plants, while in C1 amended plants this was only the case for P (and marginally for N).
Discussion
Foliar diagnosis of olive plants
The limits of sufficiency of leaf nutrients were established by critical levels reported in the literature in young olive trees (Fernández-Escobar et al. 2016; Jiménez-Moreno) and also for adult trees (Villar and Villar 2016). We did not observe any deficiency symptoms in our experiment despite the fact that N levels in mature leaves at T2 were somewhat low in soil and C1 amended unfertilised treatments (Fig. 3). By growing young plants under severe deficiencies Fernández-Escobar et al. (2016) observed deficiency symptoms under 1.28% of N. Our soil unfertilised plants showed even lower values at the end of the experiment without any visual symptoms. Indeed, foliar N was always higher than the local limit of sufficiency.
Foliar levels of P and K were well above all limits of sufficiency throughout the experiment. In contrast, Ca levels were in all cases under the general limits of sufficiency and in many cases they were lower than the limit for visual deficiency symptoms of 6.8 mg/g (Fernández-Escobar et al. 2016). However, no apparent visual symptoms were observed in our plants. Finally, Mg levels in all soils were below the limits of sufficiency at the end of the experimentation period. The lowest levels of Ca and Mg were found in C1 amended soils.
Plant growth and nutrient availability in non-inoculated plants
C2 amended soils showed the highest impact on plant growth as indicated by leaf growth, green area and basal diameter growth. In contrast, N poor C1 compost only improved basal diameter growth compared to unamended soils. Positive effects of mineral fertilisation on plant growth were only observed in unamended soils. However, the impact of the C2 was greater than that of adding mineral nutrients suggesting that this compost had the greatest fertilising capacity. Distinct fertilising capacities of different composts have been reported in other studies (López-López et al. 2016). The low C/N ratio (14.5) of C2 may have contributed to increasing N supply to a greater extent than in C1. Both C1 and C2 present an adequate C/N ratio for composted material (Mathur et al. 1993; Huerta et al. 2010). Despite the relatively high C/N ratio in the C1 substrate, plants grown in this treatment showed sufficient N. This may be due to the fact that the olive mill compost we used was providing sufficient N for plant growth, since olive mill composted materials have been characterized to release N as they mature (Muktadirul Bari Chowdhury et al. 2013). As the lowest levels of foliar N were found in unamended soils and were close to the limits of sufficiency, we suggest that both compost amendments may have contributed to N supply and plant growth from the onset of the experiment. Unlike N, other nutrients such as P, K or S showed high levels towards the end of the experiment suggesting no limitation of such nutrients to plant growth. The extremely low Ca levels observed in compost amended soils are suggestive of Ca limitations to plant growth. This appears to be the case despite the fact that Ca plays a key role in plant growth and development (Hepler 2005).
Plant growth and nutrient availability in inoculated plants
A reduction on leaf growth was the most prominent effect of Vd inoculation. However, as this reduction was not observed for the green area index, it appears that it was compensated by general plant growth. Indeed, inoculation reduced basal diameter growth only in C1 amended soils and somewhat surprisingly increased it in C2 fertilised soils.
Leaf growth reduction in inoculated plants was more noticeable in high N and P environments such as C2 amended soils. As a result, in C2 soils Vd reduced leaf nutrient input of all studied nutrients, while in C1 soils such reductions only affected N, P, K and S leaf input, with no significant effects observed in plants grown in unamended soils. These findings suggest reduced leaf nutrient uptake in inoculated treatments was greatest in C2. In our experiment, the effects of Vd inoculation in young olive trees were observed in a period spanning 3 to 14 weeks during which plants were visually asymptomatic. Other studies carried out with young olive trees growing in sterilized soils coincide in showing effects of Vd in similar time frames (Calderón et al. 2014). However, in our case, no visual symptoms of Verticillium wilt were observed during this time period. This lack of visual symptoms may be due to the fact that during early stages of infection (biotrophic phase) Vd does not cause severe reductions in plant performance other than competing for nutrients. Indeed Vd has been described as a hemibiotrophic fungi (Scholz et al. 2018). It has been shown that certain substrates (including soil and compost) may be suppressive to Verticillium wilt expression by reducing pathogen soil proliferation (Malandraki et al. 2008) or microsclerotia concentration (Goicoechea 2009; Castaño and Avilés 2013; Avilés and Borrero 2017). Specifically, Avilés and Borrero (2017), working with different batches of the same compost sources as in our study found that C1 was able to quantitatively reduce Vd microsclerotia to a greater extent than C2. We present evidence of the presence of Vd in all inoculated substrates, with the strongest effects of Verticillium wilt being found in C2 plants. C2 infected plants, growing in conditions of high nutrient availability, may try to overcome infection by developing new vessels, thus increasing basal diameter growth.
Nutrient availability and Verticillium wilt
Nutrient availability can either increase or decrease incidence and severity of fungal diseases. Disease management is complex and multifactorial and includes the direct effects of mineral nutrient levels on the pathogen, on plant growth and on mechanisms of plant resistance (Walters and Bingham 2007).
Nitrogen
An excess of N can generally favour the presence of fungal diseases in plants (Marschner 2012) and this phenomenon has been specifically described in Verticillium wilt (Pegg and Brady 2002). In our experiment, the effects of Verticllium wilt, although plants have remained visually asymptomatic, have been more intense in soils with high N availability, as indicated by the positive correlation between leaf growth decrease and leaf N input. This supports the idea that high N availability may favour Vd disease. It is known that Vd as a vascular pathogen can block N transport within plants and decrease the availability of N and plant growth (Wheeler and Johnson 2016). However, in our experiment growth of C2 fertilised plants increased after inoculation. As these plants showed high N levels in T2 young leaves we suggest a strong N mobilization to the shoot tips may have also involve to a lesser extent other nutrients, as was the case of S and P. N and S are both components of proteins. Increased production of proteins involved in cell-wall remodelling in Vd infected plants, growing below established levels for N sufficiency, has been reported in the literature (Chu et al. 2015). Cell-wall remodelling in our fertilised C2 plants may have been enhanced N levels in plant as well as mobility of other nutrients. Such increased nutrient mobilization in our infected plants may have contributed in overcoming the effects of Verticillium wilt on plant growth. Other studies have also found reductions of Verticillium wilt incidence in N and P fertilised plants (Davis et al. 1994).
Phosphorus
High P in plants may counteract the effects of fungal diseases by inducing systemic resistance (Orober et al. 2002; Walters and Bingham 2007). Indeed, it has been shown that adequate P levels can suppress Verticillium wilt in potato plants. In our experiment, the P levels were in most cases well above the limit of sufficiency. Moreover, both tested composts increased leaf P at the end of the experiment and Verticillium wilt effects were highest in unfertilised C2 treatments, showing extremely high levels of P in infected mature leaves. Consequently, it appears that in our experimental conditions of P sufficiency, increasing P availability did not reduce the incidence of Verticillium wilt. Similar to N, Vd inoculation hindered P supply to leaves throughout all studied treatments. However, high P and N availabilities increased the effects of inoculation on leaf growth.
As expected, mineral fertilisation increased foliar P in plants grown in unamended soils and in soil amended with nutrient poor C1. Interestingly, plants growing in soils amended with nutrient rich C2 showed decreased P availability after adding mineral nutrients. This may be due to an increased microbial demand for P triggered by adding mineral nutrients to soil and may indicate a nutrient limitation to soil microbiota in C2 amended soils.
Potassium
It has been shown that both an excess of K or a deficit of K can decrease plant resistance to Verticillium wilt (Burge and Simmons 1982). On the other hand, Verticillium wilt may reduce K supply to plants, since certain studies have found K deficiencies in cotton plants infected with Vd (Devay et al. 1974). In our experiment, foliar K levels were well above the limit of sufficiency (4 mg g−1 according to Fernández-Escobar et al. 2016) in all cases, and in contrast to N and P, leaf K input was negatively correlated with decreased leaf growth as a result of Vd inoculation. This indicates that leaf K input was highly reduced in plants showing greater incidence of Verticillium wilt. However, in spite of such reductions, K levels in young leaves at the end of the experiment were very high, suggesting no K shortages even in inoculated plants. Inoculated unfertilised C2 amended plants showed the largest post-inoculation decreases in leaf growth and clearly showed increased levels of K at T2 in young leaves as compared to other treatments. This may suggest a high capacity of Vd inoculated plants to mobilize K up to the shoot tips.
Olive mill composts are generally a source of K for olive trees (Alburquerque et al. 2004). In our experiment, high levels of foliar K occurred in compost amended plants, with C1 amended plants showing the highest levels of K.
Sulphur
It has been determined that S is an essential element for soil fungi and that such fungi have developed enzymatic systems to obtain and assimilate S (Marzluf 1997). S interchanges between soil bacteria involved in sulfonate desulphurization and soil fungi have been described (Gahan and Schmalenberger 2015). The use of S rich specific proteins is required by Vd to grow within the xylem vessels (Hoppenau et al. 2014) in which the access of S and other nutrients is limited (Singh et al. 2010). On the other hand, it is known that elemental S can induce plant resistance of Verticillium wilt (Resende et al. 1996) or other fungal diseases (Williams and Cooper 2003). Thus, it appears that plant-pathogen competition for S may be crucial in early phases of Vd infection. We observed a consistent reduction of leaf S concentration in mature leaves soon after Vd inoculation except in unfertilised C2 plants, in which S levels were very low even in controls. As during the first weeks after inoculation foliar S differences between infected and uninfected plants were large and consistent, it appears Vd utilized part of the S supply at the expense of the plant. This suggests a competition between plant and pathogen occurring during the first three weeks after inoculation. Low levels of S in mature leaves in C2 soils at the beginning of the experiment could be observed in both inoculated and non-inoculated soils, suggesting a high demand of S by amended soil microbiota. High microbial demand is also supported by the lack of response to the addition of mineral S in C2 amended plants. Somewhat surprisingly, C2 was much richer in S than C1. High levels in young leaves of S in C2 plants at T1 suggests that plants mobilized S from mature to young leaves in response to the high demand of S by Vd and other soil microbiota. Plant-pathogen competition for S was still observed at the end of the experiment only in non-amended and C1 soils. C2 plants showed high S levels irrespective of our treatment, suggesting microbiota in C2 amended soil released S during the course of the sampling period. Therefore, the effect of these two olive mill composts in enhancing microbial competition may delay the use of S by plants while not producing long lasting shortages of S.
The effect of Vd inoculation in young olive trees increased in plants showing low S levels in the early stages of Vd growth. Both, abundance of Vd in basal branches and decreases in leaf growth due to Verticillium inoculation were higher in plants that showed the lowest levels of S in mature leaves at the onset of the experiment. This may indicate a strong competition for S in early phases of Vd growth within the plant and a greater incidence of disease under conditions of low S availability. Low foliar S levels, soon after Vd inoculation, may have contributed to spread Vd in such plants as high S levels have been reported to impair the spread of Vd in infected plants (Bollig et al. 2013). Increased levels of foliar S towards the end of the experiment may have helped plants to counteract the effects of Vd. Indeed, several works have pointed out the beneficial effects of sulphur as a component of defence against fungal diseases. Sulphur supply has been shown to enhance Vd resistance by the synthesis of Sulphur containing defence compounds (Williams and Cooper 2003; Fu et al. 2016).
Calcium
Calcium was the only element for which an effect of inoculation was not observable on leaf input during leaf growth. A reduction of Ca in inoculated plants was only observable in the compost 2 treatment. Despite of showing very large contents on Ca, ranging from 7 to 9%, both composts reduced the availability of Ca as indicated by the low levels of Ca in mature leaves and by the reduced leaf Ca input in organically amended plants. The Ca reduction in plants amended with compost was highest in compost 1 and lasted for the whole experiment. Compost 2 was richer in Ca and plants growing in C2 amended soils recovered their Ca levels towards the end of the experiment. It is known that composts and other organic amendments rich in P are typically high in Ca (Vandecasteele et al. 2017), as is the case of composts used in this experiment. Decreased Ca availability on applying compost may be related to reduced solubility of Ca at alkaline pH (Monem Balba 1995) as well as microbial processes. Indeed, in calcareous areas with high abundance of basic soils it has been proposed that the addition of phosphogypsum to olive mill compost may improve the supply of both Ca and P to plants and thus enhance crop growth (Kammoun et al. 2017).
Magnesium
Magnesium levels tended to decrease towards the end of the experimental period and in many cases were well below the limit of sufficiency (in control and compost 1 plants), suggesting that plants grew under conditions of Mg limitation. This is further supported by the fact that addition of mineral Mg did not improve foliar Mg in most cases. Moreover, in compost amended plants Vd inoculation reduced foliar Mg levels at the end of the experiment and reduced leaf Mg inputs and concentration changes mainly in C2 plants. Despite the fact that foliar Mg in inoculated plants was generally low and tended to decrease during the experimental period, leaf Mg input increased with leaf growth decrease. This may suggest that Mg was not likely to be the main driver of Vd effects on leaf growth. Although other authors have found decreases in leaf Mg due to Vd (Goicoechea et al. 2004), we have not found any information in the literature linking Verticillium wilt with Mg metabolism.
Conclusions
Vd activity was detected in all inoculated treatments three months after inoculation as indicated by reductions in leaf growth and N, K and S leaf inputs. However, plant growth parameters were only negatively affected by Vd in low/medium fertility treatments but not in the most fertile treatment in which high N and the mobility of other nutrients to shoot tips may help the plant to overcome the effects of infection. Reduced S availability may favour disease development. Vd inoculated plants showed large reductions in leaf K input over the three months post inoculation.
Olive mill compost amendments may decrease N and S availability over the first three months after compost application. However, this effect was transient as they favoured N, P, K and S availabilities during the following months. In contrast, they decreased the availability of Ca throughout the experiment, likely due to increased pH.
References
Alburquerque JA, Gonzálvez J, García D, Cegarra J (2004) Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour Technol 91:195–200
Alfano G, Lustrato G, Lima G, Vitullo D, Ranalli G (2011) Characterization of composted olive mill wastes to predict potential plant disease suppressiveness. Biol Control 58:199–207. https://doi.org/10.1016/j.biocontrol.2011.05.001
Arriagada C, Garcia-Sanchez M, Diaz R et al (2012) Suppressive effect of olive residue and saprophytic fungi on the growth of Verticillium dahliae and its effect on the dry weight of tomato (Solanum lycopersicum L.). J Soil Sci Plant Nutr 12:303–313. https://doi.org/10.4067/S0718-95162012000200010
Avilés M, Borrero C (2017) Identifying characteristics of Verticillium wilt suppressiveness in olive mill composts. Plant Dis 101:1568–1577
Báidez AG, Gómez P, Del Río JA, Ortuño A (2007) Dysfunctionality of the xylem in Olea europaea L. plants associated with the infection process by Verticillium dahliae Kleb. Role of phenolic compounds in plant defense mechanism. J Agric Food Chem 55:3373–3377. https://doi.org/10.1021/jf063166d
Birem F, Alcántara E, Blanco-López MA, López-Escudero FJ (2009) Physiologycal differences expressed by susceptible and resistant olive cultivars inoculated with Verticillium dahliae. 10th Int Verticillium Symp B Abstr Corfu Island, Hellas 71:
Bollig K, Specht A, Myint SS, Zahn M, Horst WJ (2013) Sulphur supply impairs spread of Verticillium dahliae in tomato. Eur J Plant Pathol 135:81–96. https://doi.org/10.1007/s10658-012-0067-5
Burge M, Simmons J (1982) The influence of potassium and phosphorus in predisposition of tomato to Verticillium wilt. Chem Ecol 1:83–92
Calderón R, Lucena C, Trapero-Casas JL, Zarco-Tejada PJ, Navas-Cortés JA (2014) Soil temperature determines the reaction of olive cultivars to verticillium dahliae pathotypes. PLoS One 9:e110664. https://doi.org/10.1371/journal.pone.0110664
Castaño R, Avilés M (2013) Factors that affect the capacity of growing media to suppress Verticillium wilt. Acta Hortic (1013):465–471
Chu J, Li W-F, Cheng W, Lu M, Zhou KH, Zhu HQ, Li FG, Zhou CZ (2015) Comparative analyses of secreted proteins from the phytopathogenic fungus Verticillium dahliae in response to nitrogen starvation. Biochim Biophys Acta - Proteins Proteomics 1854:437–448
Davis JR, Stark JC, Sorensen LH, Schneider AT (1994) Interactive effects of nitrogen and phosphorus on verticillium wilt of russet Burbank potato. Am Potato J 71:467–481
Devay J, Forester L, Garber R, Butterfi E (1974) Characteristics and concentration of propagules of Verticillium dahliae in air-dried field soils in relation to prevalence of Verticillium wilt in cotton. Phytopathology 64:22–29. https://doi.org/10.1094/Phyto-64-22
El-Zik KM (1985) Integrated control of Verticillium wilt of cotton. Plant Dis 69:1025–1032
Fernàndez E, Segarra G, Trillas MI (2014) Physiological effects of the induction of resistance by compost or Trichoderma asperellum strain T34 against Botrytis cinerea in tomato. Biol Control 78:77–85. https://doi.org/10.1016/j.biocontrol.2014.06.012
Fernández-Escobar R, Guerreiro M, Benlloch M, Benlloch-González M (2016) Symptoms of nutrient deficiencies in young olive trees and leaf nutrient concentration at which such symptoms appear. Sci Hortic (Amsterdam) 209:279–285. https://doi.org/10.1016/j.scienta.2016.07.002
Fu X, Li C, Zhou X, Liu S, Wu F (2016) Physiological response and sulfur metabolism of the V. dahliae-infected tomato plants in tomato/potato onion companion cropping. Sci Rep 6:1–11. https://doi.org/10.1038/srep36445
Gahan J, Schmalenberger A (2015) Arbuscular mycorrhizal hyphae in grassland select for a diverse and abundant hyphospheric bacterial community involved in sulfonate desulfurization. Appl Soil Ecol 89:113–121. https://doi.org/10.1016/j.apsoil.2014.12.008
Gamliel A, Austerweil M, Kritzman G (2000) Non-chemical approach to soilborne pest management—organic amendments. Crop Prot 19:847–853
García-Ruiz R, Ochoa M (2012) Improved soil quality after 16 years of olive mill pomace application in olive oil groves. Agron Sustain Dev 32:803–810. https://doi.org/10.1007/s13593-011-0080-7
Goicoechea N (2009) To what extent are soil amendments useful to control Verticillium wilt. Pest Manag Sci 65:831–839
Goicoechea N, Aguirreolea J, García-Mina JM (2004) Alleviation of verticillium wilt in pepper (Capsicum annuum L.) by using the organic amendment COA H of natural origin. Sci Hortic (Amsterdam) 101:23–37. https://doi.org/10.1016/j.scienta.2003.09.015
Gracia-Romero A, Kefauver SC, Vergara-Díaz O, Zaman-Allah MA, Prasanna BM, Cairns JE, Araus JL (2017) Comparative performance of ground vs. aerially assessed RGB and multispectral indices for early-growth evaluation of maize performance under phosphorus fertilization. Front Plant Sci 8:1–13. https://doi.org/10.3389/fpls.2017.02004
Harris DC, Yang JR, Ridout MS (1993) The detection and estimation of Verticillium dahliae in naturally infested soil. Plant Pathol 42:238–250
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell Online 17:2142–2155. https://doi.org/10.1105/tpc.105.032508
Hoppenau CE, Tran VT, Kusch H, Aßhauer KP, Landesfeind M, Meinicke P, Popova B, Braus-Stromeyer SA, Braus GH (2014) Verticillium dahliae VdTHI4, involved in thiazole biosynthesis, stress response and DNA repair functions, is required for vascular disease induction in tomato. Environ Exp Bot 108:14–22. https://doi.org/10.1016/j.envexpbot.2013.12.015
Huerta O, López-Martínez M, Soliva M (2010) Procés de compostatge: caracterització de mostres. In: Diputació. Diputació de Barcelona, Barcelona
Inderbitzin P, Subbarao KV (2014) Verticillium systematics and evolution: how confusion impedes Verticillium wilt management and how to resolve it. Phytopathology 104:564–574. https://doi.org/10.1094/PHYTO-11-13-0315-IA
Jiménez-Díaz RM, Cirulli M, Bubici G, del Mar Jiménez-Gasco M, Antoniou PP, Tjamos EC (2012) Verticillium wilt, a major threat to olive production: current status and future prospects for its management. Plant Dis 96:304–329. https://doi.org/10.1094/PDIS-06-11-0496
Jiménez-Moreno MJ, Fernández-Escobar R (2017) Influence of nutritional status of phosphorus on flowering in the olive (Olea europaea L.). Sci Hortic (Amsterdam) 223:1–4. https://doi.org/10.1016/j.scienta.2017.05.028
Kammoun M, Ghorbel I, Charfeddine S, Kamoun L, Gargouri-Bouzid R, Nouri-Ellouz O (2017) The positive effect of phosphogypsum-supplemented composts on potato plant growth in the field and tuber yield. J Environ Manag 200:475–483. https://doi.org/10.1016/j.jenvman.2017.06.016
López-Escudero FJ, Mercado-Blanco J (2010) Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 344:1–50. https://doi.org/10.1007/s11104-010-0629-2
López-López N, Segarra G, Vergara O, López-Fabal A, Trillas MI (2016) Compost from forest cleaning green waste and Trichoderma asperellum strain T34 reduced incidence of fusarium circinatum in Pinus radiata seedlings. Biol Control 95:31–39. https://doi.org/10.1016/j.biocontrol.2015.12.014
Malandraki I, Tjamos SE, Pantelides IS, Paplomatas EJ (2008) Thermal inactivation of compost suppressiveness implicates possible biological factors in disease management. Biol Control 44:180–187
Marschner H (2012) Mineral nutrition of higher plants, third edit. Academic Press, London
Martos-Moreno C, López-Escudero F, Blanco-López MA (2006) Resistance of olive cultivars to defoliating pathotipe of Verticillium dahliae. HortScience 41:1313–1316
Marzluf GA (1997) Molecular genetics of sulfur assimilation in filamentous Fungi and yeast. Annu Rev Microbiol 51:73–96. https://doi.org/10.1146/annurev.micro.51.1.73
Mathur SP, Dinel H, Owen G, Schnitzer M, Dugan J (1993) Determination of compost via maturity II optical density of water extracts of composts as a reflection of their maturity. Biol Agric Hortic 10:87–108
Mercado-Blanco J, López-Escudero FJ (2011) Verticillium wilt of olive and its control: the heat is on. Plant Soil 355:17–21. https://doi.org/10.1007/s11104-011-1091-5
Monem Balba A (1995) Management of problem soils in arid ecosystems. In: CRC press. Florida, Boca Raton
Morillo JA, Antizar-Ladislao B, Monteoliva-Sánchez M, Ramos-Cormenzana A, Russell NJ (2009) Bioremediation and biovalorisation of olive-mill wastes. Appl Microbiol Biotechnol 82:25–39
Muktadirul Bari Chowdhury AKM, Akratos CS, Vayenas DV, Pavlou S (2013) Olive mill waste composting: a review. Int Biodeterior Biodegrad 85:108–119
Orober M, Siegrist J, Buchenauer H (2002) Mechanisms of phosphate-induced disease resistance in cucumber. Eur J Plant Pathol 108:345–353. https://doi.org/10.1023/A:1015696408402
Papasotiriou FG, Varypatakis KG, Christofi N, Tjamos SE, Paplomatas EJ (2013) Olive mill wastes: a source of resistance for plants against Verticillium dahliae and a reservoir of biocontrol agents. Biol Control 67:51–60
Pegg G, Brady B (2002) Verticillium wilts. In: CAB Intern. CAB International, Wallingford
Prieto P, Navarro-Raya C, Valverde-Corredor A, Amyotte SG, Dobinson KF, Mercado-Blanco J (2009) Colonization process of olive tissues by Verticillium dahliae and its in planta interaction with the biocontrol root endophyte Pseudomonas fluorescens PICF7. Microb Biotechnol 2:499–511. https://doi.org/10.1111/j.1751-7915.2009.00105.x
Resende MLV, Flood J, Ramsden D et al (1996) Novel phytoalexins including elemental Sulphur in the resistance of cocoa ( Theobroma cacao L .) to Verticillium wilt ( Verticillium dahliae Kleb .). Physiol Mol Plant Pathol 48:347–359. https://doi.org/10.1006/pmpp.1996.0028
Ruggieri G (1946) Una nuova malatia dell’olivo. L’Italia Agric 83:369–372
Scholz SS, Schmidt-Heck W, Guthke R, Furch ACU, Reichelt M, Gershenzon J, Oelmüller R (2018) Verticillium dahliae-Arabidopsis interaction causes changes in gene expression profiles and jasmonate levels on different time scales. Front Microbiol 9:1–19. https://doi.org/10.3389/fmicb.2018.00217
Segarra G, Santpere E, Elena G, Trillas MI (2013) Enhanced Botrytis cinerea resistance of Arabidopsis plants grown in compost may be explained by increased expression of defense related genes, as revealed by microarray analysis. PLoS One 8:e56075
Singh S, Braus-Stromeyer SA, Timpner C et al (2010) Silencing of Vlaro2 for chorismate synthase revealed that the phytopathogen Verticillium longisporum induces the cross-pathway control in the xylem. Appl Microbiol Biotechnol 85:961–976
Vandecasteele B, Willekens K, Steel H, D’Hose T, van Waes C, Bert W (2017) Feedstock mixture composition as key factor for C/P ratio and phosphorus availability in composts: role of biodegradation potential, biochar amendment and calcium content. Waste and Biomass Valorization 8:2553–2567. https://doi.org/10.1007/s12649-016-9762-3
Villar P, Villar J (2016) Guia De La Fertilitat Dels Sòls I La Nutrició Vegetal En Producció Integrada, Consell Ca. Generalitat de Catalunya, Lleida
Vitullo D, Altieri R, Esposito A, Nigro F, Ferrara M, Alfano G, Ranalli G, de Cicco V, Lima G (2013) Suppressive biomasses and antagonist bacteria for an eco-compatible control of Verticillium dahliae on nursery-grown olive plants. Int J Environ Sci Technol 10:209–220. https://doi.org/10.1007/s13762-012-0145-4
Walters DR, Bingham IJ (2007) Influence of nutrition on disease development caused by fungal pathogens: implications for plant disease control. Ann Appl Biol 151:307–324. https://doi.org/10.1111/j.1744-7348.2007.00176.x
Weetman GF, Wells GG (1990) Plant analyses as an aid in fertilizing forests. In: Westerman RL (ed) Soil Testing and Plant Analyses, 3rd edn. Soil Science Society of America, Madison, Wisconsin USA, pp 659–690
Wheeler DL, Johnson DA (2016) Verticillium dahliae infects, alters plant biomass, and produces inoculum on rotation crops. Phytopathology 106:602–613. https://doi.org/10.1094/PHYTO-07-15-0174-R
Williams JS, Cooper RM (2003) Elemental Sulphur is produced by diverse plant families as a component of defence against fungal and bacterial pathogens. Physiol Mol Plant Pathol 63:3–16. https://doi.org/10.1016/j.pmpp.2003.08.003
Yangui T, Rhouma A, Gargouri K, Triki MA, Bouzid J (2008) Efficacy of olive mill waste water and its derivatives in the suppression of crown gall disease of bitter almond. Eur J Plant Pathol 122:495–504. https://doi.org/10.1007/s10658-008-9317-y
Yildiz A, Benlioglu S (2010) Effects of soil solarization and some amendments to control Verticillium wilt in established olive orchards. African J Biotechnol 9:6660–6665. https://doi.org/10.5897/AJB10.507
Zasoski RJ, Burau RG (1977) Rapid nitric-perchloric acid digestion method for multi-element tissue analysis. Commun Soil Sci Plant Anal 8:425–436
Acknowledgements
This research was supported by the Compovert project (AGL2015-66684-R) of the Spanish Ministry of Science MINECO/FEDER. We also thank the Fermentation Unit of the Faculty of Biology of the UB and the Greenhouse Services of the Torribera Food Sciences Campus of the UB for facilitating the experimentation of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Rights and permissions
About this article
Cite this article
Romanyà, J., Sancho-Adamson, M., Ortega, D. et al. Early stage effects of Verticillium wilt of olive (WVO) on nutrient use in young olive trees grown in soils amended with compost and mineral fertilisation. Plant Soil 436, 193–209 (2019). https://doi.org/10.1007/s11104-018-03923-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-03923-9