Abstract
Aims
The effects of drying and wetting on soil carbon processes are regulated by the responses of plants, plant-associated microbes, and free-living microbes. Whether these groups respond similarly to drying and wetting is not clear, however, making it difficult to predict the net effect of drought on soil carbon processes.
Methods
We imposed a drought-rewetting event on mesocosms planted with maple (Acer saccharum Marshall; arbuscular mycorrhizal fungi host) or oak (Quercus alba L.; ectomycorrhizal fungi host) saplings. In each mesocosm, we used mesh to create chambers separating roots, mycorrhizal fungi hyphae, and free-living microbes. We compared respiration and carbon degrading enzyme activity across chambers throughout the dry-rewetting cycle.
Results
Respiration from all chambers declined during drought, and chambers differed in their drought-sensitivity. In maple mesocosms, respiration from the Hyphae+Microbes chamber was the most drought-resistant; in oak mesocosms respiration from the Microbes chamber was the most drought-sensitive. Respiration did not recover after rewatering, indicating a persistent drought legacy. In contrast, enzyme activity returned to control functioning after 2 weeks of well-watered conditions.
Conclusions
Our results suggest that belowground biota differ in their sensitivity to and recovery from drought, which affects the carbon processes differently. An improved ability to partition carbon fluxes into biotic sources can help to constrain predicted carbon fluxes under future climate scenarios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Droughts are predicted to become more frequent and more extreme under future climate scenarios (IPCC 2012, 2014; Seidl et al. 2017). Since terrestrial carbon (C) stocks are highly sensitive to precipitation anomalies (Reichstein et al. 2013; Wolf et al. 2016; Zscheischler et al. 2014), future droughts are expected to have strong impacts on terrestrial C cycling. However, accurate predictions of C cycling under future environmental conditions are limited by uncertainty about the relative effects of drought on plants, plant-associated microbes (e.g. mycorrhizal fungi), and free-living microbes, as well as the contribution of these groups to soil carbon efflux. These biota mediate the effects of drought on C processes through their metabolic use of carbon and water (Manzoni et al. 2012 and citations therein), but differences among them in their drought sensitivity can have important repercussions for soil carbon gains and losses. Incorporating and improving the mechanistic representation of rhizosphere processes into earth system models is largely expected to improve their accuracy in predicting global C biogeochemistry under future climates scenarios (Bradford et al. 2016; Schlesinger et al. 2016; Warren et al. 2015). Indeed, models which do not explicitly include root processes perform poorly in predicting ecosystem-specific C fluxes during drought (Hanson et al. 2004), emphasizing the importance of studying belowground processes to understand ecosystem responses to environmental stressors.
Plant roots, mycorrhizal fungi, and free-living soil microbes interact via the exchange of C – the C economy. Photosynthetically fixed C is allocated by plants belowground to grow and maintain plant roots and mycorrhizal symbionts. Fixed C is also exuded from roots into the rhizosphere, where it stimulates microbial activity and decomposition (Kaiser et al. 2014). As roots, fungi, and microbes die, C stored in their tissues is incorporated into soils as necromass (Crowther et al. 2015; Liang et al. 2017; Schweigert et al. 2015). Soil organisms mechanically and enzymatically decompose labile necromass to gain access to C and other nutrients, releasing small monomers and mineral nutrients in the process (Schimel and Bennett 2004; Talbot et al. 2008). The growth and activity of all three biotic groups – plant roots, mycorrhizal fungi, and free-living soil microbes – releases CO2 to the atmosphere through respiration.
During drought, the C economy slows as plants reduce photosynthesis (Ruehr et al. 2009). Bulk soil respiration is fundamentally limited by photosynthetically-derived C (Högberg et al. 2001; van der Molen et al. 2011), so drought-driven reductions in photosynthesis can cause cascading impacts on C transfer among rhizosphere biota (Clarholm 1981). Drought has been shown to reduce the transfer of C from meadow plants to soil bacteria, but not soil fungi (Fuchslueger et al. 2014). Different plant strategies to balance water loss and C gain during drought (i.e. isohydry vs anisohydry; McDowell et al. 2008) likely play an imporant role in the extent to which rhizodeposition is maintained during drought (Preece and Peñuelas 2016) and the net effect of drought on the C economy. Respiration from plant roots ranges from ~10–80% of total respiration across global forests, and accounts for a relatively greater proportion as total respiration increases (Bond-Lamberty et al. 2004; Subke et al. 2006). Since plant root respiration is allometrically related to photosynthesis (Heilmeier et al. 1997; Litton et al. 2007), reductions in C uptake during drought would also reduce root respiration. Mycorrhizal respiration is often included in estimates of root respiration due to sampling limitations, but disentangling the responses of mycorrhizal fungi from those of plant roots is necessary to mechanistically understand ecosystem C flux dynamics. Plants can allocate a substantial fraction (Franklin et al. 2014; Hobbie 2006), and up to 85%, of net photosynthate to mycorrhizal fungi (Treseder and Allen 2000), so reductions in C flux belowground are also likely to reduce mycorrhizal respiration (Talbot et al. 2008). However, differences in the C demand of mycorrhizal types likely affects their response to, and effect on, the altered C economy during drought (Read 1991). Finally, microbial biomass is positively correlated with net primary production, suggesting that microbial respiration may be also limited by photosynthate-C (Zak et al. 1994). Microbial respiration may decline during drought if root turnover and exudate production or microbial and mycorrhizal exoenzyme production is reduced (Kuzyakov and Domanski 2000; Schimel and Weintraub 2003; Wieder et al. 2013).
In addition to uncertainty surrounding the individual responses of roots, mycorrhizal fungi, and microbes to drought, their capacity to recover remains unclear. Rewetting following drought produces a pulse of high CO2 efflux (Hagedorn et al. 2016), which, after a single extreme rain event, can account for up to 10% of annual net ecosystem CO2 production (Lee et al. 2004). The source of this pulse is debated, but is hypothesized to be driven by increases in microbial respiration following an increase in labile C substrate availability (Schimel et al. 2010). These C substrates are derived either from soil organic matter that has been released from physicochemical protection during soil rewetting, or from lysed microbial cells (Fierer and Schimel 2002; Fierer and Schimel 2003). These different mechanisms of C loss after rewetting imply different repercussions for long-term terrestrial C storage, and the overall net effect of drought on the C economy (Kuzyakov 2006). Furthermore, the degree to which plant roots, mycorrhizal fungi, or soil microbes contribute to the observed CO2 pulses following dry-rewetting cycles is not known. In the absence of plants (and presumably mycorrhizal fungi), CO2 production was strongly correlated with new microbial DNA production 3 hours after rewetting in a laboratory incubation (Blazewicz et al. 2014). These findings suggest that part of the CO2 flush observed after rewetting is correlated with the proliferation of microbial biomass. In addition, a 14C tracer study in root-free soil also found that the majority of C respired after rewetting came from microbial biomass (Fierer and Schimel 2003). However, in a field study with roots, mycorrhizal fungi, and free-living microbes, ectomycorrhizal respiration increased during rewetting relative to root and microbial respiration (Heinemeyer et al. 2007). This implies that mycorrhizal respiration may contribute non-negligibly to CO2 pulses after rewetting events, and also underscores the need to disentangle the responses of different biota in order to gain mechanistic insight into an overall ecosystem-level response to drought and rewetting.
In this study, we examined belowground C processes (respiration and potential enzyme activity; EA) throughout an extreme drought (in terms of its severity and duration) and extreme rewetting event. In order to understand how plant roots, mycorrhizal hyphae, and free-living microbes independently and collectively mediate soil respiration in dry-rewet conditions, we used mesocosms with mesh barriers with different pore sizes to exclude the growth of different biotic groups (Fig. S1). To quantify soil C losses and transformations, we compared respiration and EA across the chambers in mesocosms planted with sugar maple saplings (Acer saccharum Marshall; which form associations with arbuscular mycorrhizal fungi), and in mesocosms with white oak saplings (Quercus alba L.; which form associations with ectomycorrhizal fungi). We measured photosynthesis throughout the dry and rewetting event to estimate photosynthetic C inputs. We asked: (1) How do belowground biotic groups (i.e. roots, mycorrhizal fungi, and microbes) respond to an extreme dry and rewetting event? (2) Do these groups differ in their sensitivity to and recovery from drought, as evidenced by changes in respiration rate and EA? We hypothesized that both respiration rates and EA would decline across all chambers in response to drought.
Prior to drought, we expected that microbial respiration would be the largest source of CO2, followed by mycorrhizal and plant root respiration in accordance with studies of forest soils (Fenn et al. 2010; Heinemeyer et al. 2007). In maple mesocosms, we expected that mycorrhizal fungi and microbes would be more sensitive to drought than plant roots, since their activity would be limited both by reduced photosynthate-C supply, and by the diffusion of C derived from organic matter degradation. In oak mesocosms, we expected that root respiration would be relatively less sensitive to drought since oaks maintain photosynthetic activity during drought (McDowell et al. 2008; Yi et al. 2017). We also expected that oak mycorrhizal respiration would be less sensitive to drought than microbial respiration, since ectomycorrhizal fungi have an especially high demand for C from the host plant (Read 1991).
Materials and methods
Experimental design
In May 2016, we collected A. saccharum and Q. alba seedlings from a mixed hardwood stand in Oak Ridge, TN, USA. To minimize potential genetic variability, seedlings were collected from a small area (~5 m radius) where both species co-occurred. Initial heights were approximately 15 cm for both species and seedlings were all estimated to be less than 1 year old. To preserve the native microbial community, seedlings were not washed prior to being transplanted into mesocosms.
We constructed 60 mesocosms (38 × 23.5 × 18 cm LxWxH or 15.14 L capacity) with mesh partitions that divided each mesocosm into three chambers (Fig. S1). Small holes (each ~0.75 cm diameter) at the bottom of each mesocosm prevented water logging. One tree seedling was planted into the center chamber of each mesocosm such that 40 mesocosms were planted with A. saccharum and 20 were planted with Q. alba. Seedlings were allowed to acclimate in potting mix (see below) for 2 months prior to being planted in mesocosms. The center chamber included plant roots, mycorrhizal hyphae, and free-living microbes, and will hereafter be referred to as the Roots+Hyphae+Microbes chamber. One mesh partition (35 μm) excluded root growth, allowing only mycorrhizal hyphae and free-living microbes to colonize this chamber, which we hereafter refer to as the Hyphae+Microbes chamber. The second mesh partition (1 μm) excluded roots and mycorrhizal hyphae, allowing only free-living microbes to colonize this chamber which we hereafter refer to as the M chamber. We compare respiration across these chambers, but do not partition respiration into the individual biotic sources since competition among the biota would result in non-additive effects.
Each chamber had a schedule 40 PVC collar (10 cm height, 5 cm diameter) installed for gas sampling (see below). Twelve windows (each 3.7 cm diameter) were drilled into each PVC collar to allow biotic and hydrologic connectivity between soils in adjacent chambers and collars (Fig. S1). Mesocosms were filled with ~2.5 kg Fafard® 52 mixed with slow-release Osmocote Plus Fertilizer (15–9-12, NPK). Fafard® 52 is a commercially available potting soil mix of peat moss, bark, and vermiculate that is formulated for fast drainage of large interior pots. We used a common soil across chambers to eliminate potential differences in biogeochemistry associated with arbuscular mycorrhizal versus ectomycorrhizal fungi colonization (Phillips et al. 2013).
Seedlings in mesocosms grew and acclimated for 4 months prior to the start of the experiment. During this time, mesocosms were watered as needed. Mesocosms were divided evenly between treatment (drought) and control (well-watered) conditions (N = 20 A. saccharum; N = 10 Q. alba per treatment) and randomized on a bench in the greenhouse. The greenhouse was maintained between 18 and 21 °C throughout the duration of the experiment; 1000 watt HPS lights supplemented ambient sunlight to maintain the light intensity in the greenhouse above 250 μmol m−2 s−1 during the day (06:00–22:00).
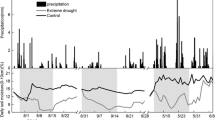
The experiment ran for 11 weeks from September – November 2016 (Chart S1). The pre-drought period (week 1) was followed by 7 weeks of drought (i.e. 49 days; weeks 2–8), and a 3-week recovery period (weeks 9–11). Oak Ridge, TN had not experienced a drought of at least this duration in 69 years; the longest drought (53 days with ≤1 mm precipitation) since 1948 occurred in fall 2016, while this experiment was ongoing (Menne et al. 2012a, b). During the pre-drought period, all mesocosms were maintained under well-watered conditions. During the drought period, control mesocosms were watered generally twice per week to maintain target soil water status while treatment mesocosms were left un-watered and soils progressively dried down. Control mesocosms were initially maintained between −0.90 and − 0.75 MPa (65–75% of field capacity, which was −0.45 MPa) during the drought period. To increase the difference in water availability between drought and control mesocosms, however, we increased water availability of control mesocosms and maintained them at −0.50 MPa (~80% of field capacity) for the remaining 5 weeks of the drought period. During the recovery period, we watered control and treatment mesocosms to saturation, and then immediately allowed them to drain to field capacity. Because the drought increased the hydrophobicity of treatment soils, treatment mesocosms retained less water than control mesocosms after the first watering during the recovery period. Consequently, we re-watered all mesocosms to saturation for two consecutive days. For the first re-watering, we watered mesocosms in the evening and allowed them to drain overnight for 12 h prior to measuring soil respiration (see below) the following morning.
Soil water content
To maintain mesocosms at the target water contents, we weighed mesocosms, calculated the volumetric water content of each mesocosm (based on known mesocosm dry soil mass), and added water by hand at least weekly to bring water volume up to the target level. Dry soil mass was measured by destructively harvesting additional mesocosms. We constructed water release curves using soil subsamples that we progressively dried and re-measured in a WPC4 Water Potential Meter (Decagon Devices; Pullman, USA). We used these curves to estimate the water potential of each mesocosm based on its volumetric water content and known bulk density. Although water release curves constructed by drying wet soils may differ from those constructed by wetting dry soils, our rewetting event during the recovery period aimed to bring soils to full saturation rather than slowly increase soil water availability. For this reason, we did not construct separate water release curves to estimate soil water content during the recovery period. The presence of root and fungal biomass, as well as likely differences in soil structure (e.g. bulk density, water content) among the three chambers of each mesocosm may have increased uncertainty of the actual water potentials in each chamber, although soil moisture measured gravimetrically did not differ among chambers of 12 supplementary mesocosms (ANOVA p = 0.785, data not shown). However, the integrated whole mesocosm estimate still provided a good guide to the relative water stress between mesocosms and should be considered in that context. Finally, the mesh partitions between chambers would continue to allow unsaturated and vapor flow of water from wetter chambers to drier chambers, and would contribute to the equilibration of water availability across chambers even if the root chamber dried at a faster rate. We report water availability as soil water potential (Ψsoil), as calculated from abovementioned water release curves.
Respiration measurements
To assess soil respiration, we measured CO2 efflux at least weekly by sampling the capped PVC collar headspaces (196 cm3) installed in each chamber. PVC collars were capped with PVC caps fitted with butane septa. Caps were installed with needle vents to avoid headspace pressure changes during capping, and the headspace was mixed by pumping 3 × 35 mL with a syringe. The needle vent was removed from the septum and a 1-mL sample was drawn from the headspace and immediately injected into a Li-Cor 6252 infrared gas analyzer (Li-COR Inc., Lincoln, Nebraska, USA), modified to detect small volume CO2 sample injections carried with an N2 carrier gas. PVC collars were left capped for ~1.5 h, and resampled after this incubation. To minimize diel variation in respiration rates, we sampled headspaces only in the morning (08:00–11:00), and sampled mesocosms and chambers in the same order every sampling date. At each sampling time, we used standard CO2 gases to create a standard curve with which we calculated the CO2 concentration in each sample’s headspace. During the drought period, watering events of control mesocosms occurred no closer than 2 days prior to respiration measures to minimize any short term increases in respiration due to the watering event.
Enzyme activity assays
To assess differences in EA between the Roots+Hyphae+Microbes, Hyphae+Microbes, and Microbes chambers, we measured soil EA in all chambers from a subset of mesocosms (N = 5 from each treatment) at the end of the pre-drought, drought, and recovery periods. Soil samples were stored at 4 °C and analyzed within 1 week of collection for EA according to Bell et al. (2013). We targeted four enzymes involved in C acquisition from organic matter degradation: α-glucosidase, β-glucosidase, cellobiohydrolase, and xylosidase. Briefly, we homogenized 1 g field moist soil in 70 mL of 50 mM sodium acetate buffer (pH 4) with an immersion blender. We incubated 800 μL soil slurry with 200 μL fluorescent substrate in 96-well deep-well plates for 2 hours at 25 °C. We prepared separate standard curves for each soil sample by incubating 800 μL soil slurry with 200 μL standard (4-Methylumbelliferone, MUB) in deep-well plates with sample plates. After incubating, we inverted and centrifuged deep-well plates, and transferred 250 μL to black-bottom 96-well plates and read them on a fluorescence microplate reader (365 nm excitation, 450 nm emission). We report EA as the sum of all C degrading enzymes to reduce the number of statistical tests needed and to ease interpretation. See Table S1 for mean activity of individual enzymes.
Photosynthesis and leaf water potential
To assess belowground C dynamics in context of potential availability to roots or mycorrhiza, we measured photosynthesis on a subset of plants (N = 5 of each species in each treatment) with a Li-COR 6400 (Li-COR Inc., Lincoln, Nebraska, USA) at six time points throughout the experiment. One leaf was chosen per plant and measured at each time point. On one plant, the sample leaf abscised during the drought treatment; for all other samples, we measured the same leaf regardless of its wilting status.
To quantify the degree of plant water stress during the drought, we measured pre-dawn leaf water potential from all plants on the last day of the drought period. One leaf was collected from each plant before dawn, placed into a plastic bag with a small piece of slightly moistened paper towel and stored at 4 °C until analysis could be completed over the next several hours using a PMS Pressure Chamber Model 610 (PMS Instrument Co., USA).
Biomass
At the conclusion of the experiment, we harvested above- and belowground plant biomass from each mesocosm. Root growth had filled full chambers, including inside the PVC collars. We excavated the remaining root biomass from the bulk chamber soil and rinsed it in water to remove excess soil. Aboveground and belowground biomass was dried at 70 °C until a constant mass, then weighed. Root:shoot ratios (R:S) were calculated as the root biomass divided by the shoot biomass.
Statistical analyses
Estimates of photosynthesis, soil water potential (Ψsoil), leaf water potential (Ψleaf) and biomass were collected at the mesocosm-level (not at the chamber level). Biomass was non-normally distributed, so we compared biomass estimates (aboveground, belowground, and R:S) across treatments for each species using non-parametric Wilcoxon tests. Photosynthesis, Ψsoil, and Ψleaf were measured multiple times on each mesocosm (or plant), so we used linear mixed effects models to compare differences across treatments (drought and control). We used the lme() function (nlme package version 3.1) in R (3.3.2; Team 2011) with mesocosm as a random effect and treatment (control or drought) and the predictor. We analyzed each species (maple or oak) separately. We report ANOVA results of the linear mixed effects models.
Potential C degrading EA was measured at the chamber-level. To examine how potential C degrading EA was affected by drought and rewetting we built linear mixed effects models. We analyzed each species (maple or oak) separately and tested for the effects of treatment (control or drought), chamber (Roots+Hyphae+Microbes, Hyphae+Microbes, or Microbes), and their interaction on EA at each time point using ANOVA tests. We report Tukey post hoc results.
Respiration was also measured at the chamber-level. To assess how respiration was impacted by drought and rewetting, we examined respiration in two ways. First, we compared the drought-sensitivity of each chamber within each tree species using the CO2 fluxes from the pre-drought and drought time points. We built mixed effect models to explain Respiration during pre-drought and drought time points with mesocosm as a random effect and the following predictors: Ψsoil, chamber type (Roots+Hyphae+Microbes, Hyphae+Microbes, or Microbes), and their interaction. We define drought-sensitivity as the estimated relationship between Ψsoil and CO2 flux (slope coefficient); significantly different regression slopes among chambers (i.e. a significant interaction between Ψsoil and chamber) indicates different drought-sensitivity between chambers. To account for potential differences in respiration due to biomass, we tested whether respiration drought-sensitivity was correlated with final biomass (aboveground, belowground, or R:S). Second, to examine the potential for respiration to recovery after rewetting, we compared the mean respiration from drought versus control chambers after re-watering. We compared respiration at each time point during the recovery period using ANOVAs and Tukey post-hoc tests. Finally, to examine whether drought had affected the underlying relationship between water availability and respiration, we used linear regressions to compare the relationship between CO2 flux and Ψsoil in control versus drought mesocosms (combining all chambers of both species). We examined these relationships both in the pre-drought and recovery periods.
Results
Plant biomass, photosynthesis, and water availability
Drought affected the aboveground and belowground biomass allocation of maples, but not of oaks (Fig. 1). Maples exposed to drought had significantly less aboveground biomass (Wilcoxon Rank Sum test p = 0.020) relative to control maples; this resulted in significantly higher R:S in drought maples than in control maples (Wilcoxon Rank Sum test p = 0.018). Oaks had higher R:S than maples (R:SOak = 2.05 ± 0.04; R:SMaple = 0.86 ± 0.02; F = 35.0, Wilcoxon Rank Sum test p < 0.001).
Tree seedling photosynthetic rates were highly variable throughout the experiment. Across all experimental periods, photosynthetic rates differed significantly between species (t = 5.58, p < 0.001) but there were no significant differences between control and drought plants at any sampling time point. Maples and oaks in the drought treatment and during the drought period had mean photosynthetic rates of 0.43 ± 0.02 and 3.92 ± 0.15 μmol CO2 m−2 s−1, respectively. Maple and oak individuals in the control treatment had photosynthetic rates of 1.20 ± 0.08 and 5.00 ± 0.15 μmol CO2 m−2 s−1 during the same time period. Although some plants abscised the individual leaves we used for photosynthesis measurements, all plants survived the drought.
Pre-drought, Ψsoil was −0.92 ± 0.01 in maple mesocosms and − 0.89 ± <0.01 MPa in oak mesocosms (Fig. S2) and did not differ between control and drought mesocosms. At the end of the drought, Ψsoil differed significantly between control and drought mesocosms for both maples and oaks (p < 0.001 for both). At the end of the drought, Ψsoil in drought mesocosms was −1.74 ± 0.01 MPa (maple) and − 1.73 ± 0.02 MPa (oak), versus −0.64 ± <0.01 MPa (maple) and − 0.60 ± 0.01 MPa (oak) in control mesocosms. These differences in soil water availability corresponded to differences in the pre-dawn leaf water potential (Ψleaf) between control and drought mesocosms at the end of the drought period. For maple, Ψleaf of control and drought plants was −0.42 ± 0.04 MPa and − 1.24 ± 0.24 MPa, respectively (p = 0.003). Oak Ψleaf of control and drought plants was −0.59 ± 0.09 and − 1.34 ± 0.26 MPa (p = 0.021). After 2 weeks of well-watered conditions during the recovery period, differences in Ψsoil between drought and control mesocosms were greatly reduced (Fig. S2) but persisted for maple mesocosms (p = 0.023) and was ameliorated for oaks (p = 0.165). At the end of the recovery period, Ψsoil of control and drought mesocosms was −0.70 ± 0.01 MPa and − 0.66 ± 0.01 MPa in maple mesocosms, and − 0.66 ± 0.01 MPa and 0.69 ± <0.01 MPa for oaks.
Enzyme activity
Potential C degrading EA did not differ across chambers for either tree species, at any time period. Prior to drought, EA also did not differ between control and drought treatments (maple mesocosms: 0.20 ± 0.01 nmol C gds hour−1; oak mesocosms: 0.19 ± 0.01 nmol C gds−1 h−1; Fig. 2). At the end of the drought, potential C degrading EA in maple mesocosms was 50% lower in drought mesocosms (0.20 ± 0.02 01 nmol C gds−1 h−1) compared to control mesocosms (0.37 ± 0.05 01 nmol C gds−1 h−1; Tukey HSD p = 0.007; Fig. 2). For oaks, too, EA was lower in drought mesocosms (0.18 ± 0.03 nmol C gds−1 h−1) than in control mesocosms (0.45 ± 0.05 nmol C gds−1 h−1; Tukey HSD p < 0001; Fig. 2). At the end of the recovery period (i.e. after 2 weeks of well-watered conditions) EA remained ~40% lower in the drought mesocosms relative to the control mesocosms (maple: Tukey HSD p = 0.032, oak: Tukey HSD p = 0.015; Fig. 2).
Potential carbon degrading enzyme activity (EA) over a dry-rewetting cycle. EA did not differ among chambers, but differed between control and drought mesocosms at the end of the drought (weeks 2–8) and recovery periods (weeks 9–11). The grey area denotes the drought period; the recovery period following the drought was initiated by rewatering all mesocosms 12 h prior to the week 9 measurements. See text for statistical details
Respiration sensitivity to drought across biotic groups
Respiration varied over time and declined with the drought (Fig. 3). Pre-drought, respiration from maple mesocosms was higher from Roots+Hyphae+Microbes chambers (0.22 ± 0.02 mg C m−2 min−1) than Microbes chambers (0.18 ± 0.01 mg C m−2 min−1; p < 0.001) and did not differ between Roots+Hyphae+Microbes and Hyphae+Microbes chambers (0.21 ± 0.02 mg C m−2 min−1; p = 0.386). In oak mesocosms, respiration from Roots+Hyphae+Microbes chambers (0.19 ± 0.01 mg C m−2 min−1) was higher than respiration from both Hyphae+Microbes (0.16 ± 0.01 mg C m−2 min−1; p = 0.007) and Microbes chambers (0.15 ± 0.01 mg C m−2 min−1; p = 0.001). Respiration was not correlated with aboveground or belowground plant biomass in any chamber for either species. By the end of the drought, respiration from drought mesocosms was reduced to <10% of that of control mesocosms (p < 0.001 for both species).
Respiration from all chambers declined as soils dried, but chambers differed in their sensitivity to drought (i.e. regression slopes; Fig. 4). This resulted in differences in the relative C flux from each chamber relative to control mesocosms. In control mesocosms, the C flux from each chamber stayed remarkably constant over time at an approximate 1:1:1 ratio. In maple mesocosms, respiration from the Hyphae+Microbes chamber was less sensitive to drought (i.e. shallower slope; slope = 1.76) than the Roots+Hyphae+Microbes chamber (slope = 2.32) and the Microbes chamber (slope = 2.40; both p = 0.001; Fig. 4). By the last week of the drought, respiration from Hyphae+Microbes chambers of maple mesocosms in the drought treatment (0.05 ± 0.01 mg C m−2 min−1) was ~20% higher than the Roots+Hyphae+Microbes chamber, and 40% higher than the Microbes chamber. In oak mesocosms, the Microbes chamber was significantly more sensitive to drought (i.e. steeper slope; slope = 2.97) than the Roots+Hyphae+Microbes (p < 0.001; slope = 1.97) and the Hyphae+Microbes (slope = 2.27; p = 0.006) chambers (Fig. 4). In oak mesocosms in the drought treatment, respiration from the Microbes chamber (0.02 ± 0.01 mg C m−2 min−1) was 40% lower than the Hyphae+Microbes chamber (0.03 ± 0.01 mg C m−2 min−1), and 60% lower than the Roots+Hyphae+Microbes chamber (0.05 ± 0.01 mg C m−2 min−1).
Respiration-sensitivity differs across chambers for each species. Best models included a significant interaction term (p < 0.001 for each species) indicating that the regression line slopes differed across chambers. Solid, dashed, and dotted lines correspond to the Microbes, Hyphae+Microbes, and Roots+Hyphae+Microbes chambers, respectively. See text for additional statistical details
Respiration recovery from drought
To control for the effects of increasing water availability following the drought treatment, we saturated the soils in both control and drought mesocosms at the start of the recovery period. Immediately (12 h) after rewatering, respiration from control mesocosms increased by 20%, and respiration of drought mesocosms increased by nearly 550% (Fig. 5a). Maximum respiration values were observed 36 h after rewatering (i.e. week 9.5), but respiration from previously-droughted mesocosms was still lower than from control mesocosms despite being maintained under the same water availability (Tukey post-hoc test p < 0.001 for both species; Fig. 5b). During this respiration pulse, CO2 flux from maple mesocosms was 0.65 ± 0.03 mg C m−2 min−1 in the control treatment, and 0.36 ± 0.02 mg C m−2 min−1 in the drought treatment. During this pulse, respiration did not differ across chambers for control maple mesocosms, but differed across chambers of drought maple mesocosms (Fig. 5a). Respiration from the Hyphae+Microbes chamber was ~1.3 times higher than the Roots+Hyphae+Microbes and Microbes chambers (p < 0.001 for both). Respiration from oak mesocosms was 0.61 ± 0.04 mg C m−2 min−1 in the control treatment, and 0.31 ± 0.02 mg C m−2 min−1 in the drought treatment. Respiration did not differ across chambers of either control or drought oak mesocosms during the respiration pulse (Fig. 5a).
Legacy of drought after re-watering. a Control (solid lines) and drought mesocosms (dashed lines) of both species experienced a spike in respiration rates 36 h after re-watering, but respiration remained significantly reduced in drought mesocosms relative to control (p < 0.001 for each time point). b The relationship between respiration and Ψsoil did not differ between control and drought mesocosms prior to drought (solid lines), but after re-watering, respiration from drought mesocosms remained lower than control mesocosms over the same Ψsoil range (dashed lines). See text for statistical details. In panel (a), the black line indicates the time of re-watering, 12 h prior to the Week 9 measurement
Similar patterns persisted across the full recovery period. By the last week of the recovery period, respiration from control maple mesocosms was generally highest in the Roots+Hyphae+Microbes chamber (Roots+Hyphae+Microbes vs Hyphae+Microbes chamber p = 0.074; Roots+Hyphae+Microbes vs Microbes chamber p = 0.013; Fig. 5a). Respiration from previously-droughted maple mesocosms (i.e. drought treatment) however remained highest in the Hyphae+Microbes chamber (Hyphae+Microbes vs Roots+Hyphae+Microbes p = 0.003; Hyphae+Microbes vs Microbes p = 0.008; Fig. 5a). Respiration did not differ across chambers of either control or drought oak mesocosms.
We compared the relationship of respiration and Ψsoil between treatments in the pre-drought and recovery periods. The slope (Treatment*Ψsoil) of the correlation between respiration and Ψsoil did not differ between treatment groups (control vs drought) either in the pre-drought (p = 0.704) or recovery periods (p = 0.656) indicating no legacy effects on the overall effect of water availability on respiration (Fig. 5b). However, the intercept of the regression line differed significantly between treatment groups only in the recovery period (Fig. 5b; p < 0.001), suggesting that the drought resulted in a persistently reduced C flux 2 week even after ample water availability.
Discussion
Respiration from forests dominated by arbuscular mycorrhizal or ectomycorrhizal trees are controlled by different environmental factors (Vargas et al. 2010), indicating that plants with different mycorrhizal associations vary in their sensitivity to different stressors. In this study, we examined the responses of belowground respiration and potential carbon (C) degrading enzyme activity (EA) throughout a cycle of extreme drought followed by saturated rewetting; we contrasted the responses among belowground biotic groups (i.e. roots, mycorrhizal fungi, and free-living microbes) in maple and oak mesocosms. We found that drought strongly reduced respiration and EA across all groups relative to control. While EA recovered somewhat after 2 weeks of well-watered conditions, respiration did not. We also observed different responses of respiration across biotic groups, particularly after the rewatering event. These asymmetrical responses of C processes over time and across biota suggest that belowground biota may differ in their physiological responses to drought (Lennon et al. 2012).
Enzyme activity is resistant to drought
Despite the severity (minimum mean Ψsoil of drought treatment was −1.73 ± 0.02 MPa) and duration of our extreme drought, all plants survived the experiment. Both respiration and C degrading potential decreased during the drought, and increased during rewatering, but neither processes completely shut down. Furthermore, over the drought period, there appeared to be stronger increases in these processes in the control mesocosms compared to decreases in these processes in the drought mesocosms. Particularly for C degrading potential, differences in EA observed between control and drought mesocosms during the drought belied important temporal dynamics. That is, the differences between control and drought mesocosms during the drought and recovery periods are likely attributable to increases in EA in the control mesocosms (Henry 2012), more so than decreases in EA in drought mesocosms. Thus, we speculate that, while additional enzymes were not produced during the drought, enzymes were also not degraded throughout the drought in the drought mesocosms. We note, however, that other enzymes involved in C degradation, such as oxidases, were not measured and could respond differently to drought. If potential EA responds minimally to drought (Steinweg et al. 2013), enzymes may remain active in soil aggregates under dry conditions and continue degrading C substrates even as soils become hydrologically disconnected. Then, when soils rewet, the release of monomers from soil aggregates may be an important C source for microbial activity (Kieft et al. 1987), and has been hypothesized to contribute to the observed CO2 pulse upon rewetting (Fierer and Schimel 2003).
Mycorrhizal fungi buffer soil respiration responses to drought
Belowground biota differed in their respiration-sensitivity to drought, and the relative performance of plant roots, mycorrhizal fungi, and free-living microbes differed in maple and oak mesocosms. We discuss these differences in the context of each mesocosm separately. We caution against drawing comparisons between the responses of maples (arbuscular mycorrhizal fungi hosts) and oaks (ectomycorrhizal fungi hosts), since we only examined two tree species which also differ in drought response strategy (i.e. isohydry vs anisohydry). In both maple and oak mesocosms, microbial respiration was the most sensitive to drying soils. This supports our original hypothesis that (free-living) microbial activity would be the most strongly C-limited during drought, since C supplies from root exudates and microbial access to soil organic matter are both reduced under drought. In addition, soil bacteria are thought to be less drought-tolerant than fungi (Yuste et al. 2011), since they have a more limited capacity to accumulate osmolytes and scavenge for water than fungi (Schimel et al. 2007). However, because we did not assess biomass or composition of the microbial community, we cannot assess the extent to which drought caused a shift in fungal or bacterial abundances or composition.
Although we expected C limitation to also reduce the performance of arbuscular mycorrhizal fungi, in maple mesocosms respiration from the Hyphae+Microbes chamber was the least sensitive to drought. Arbuscular mycorrhizal fungi produce a protein, glomalin, which increases soil aggregation and is thought to enhance water holding capacity (Rillig 2004). The production of intracellular osmolytes is a C-expensive process (Schimel et al. 2007), so if microbial sensitivity to drought is buffered in the presence of arbuscular mycorrhizal fungi this may alter ecosystem C cycling dynamics in rhizosphere versus bulk soil. Although the production of glomalin provides a plausible mechanism to explain the sustained respiration of arbuscular mycorrhizal fungi relative to soil microbes, it fails to explain why the full chamber did not also exhibit sustained respiration. We speculate that, in the full chamber of maple mesocosms, increased competition by roots for water (Augé 2001) may have counteracted the positive effects of glomalin on soil water availability. Because we measured Ψsoil at the mesocosm level, we cannot discount the possibility that chambers differed in water availability within a mesocosm.
In accordance with our expectations in oak mesocosms, respiration from the full and root-exclusion chambers were less sensitive to drought than respiration from the hyphal-exclusion chamber. We hypothesized that, because oaks are anisohydric and therefore maintain photosynthesis and C flux belowground, this would maintain a C supply for root and ectomycorrhizal respiration throughout a drought. Although we found that oaks had higher rates of photosynthesis than maples, unfortunately out ability to draw conclusions based on this variable is limited because droughted leaves senesced and prevented repeated photosynthesis measures during and after drought. However, a reduction in photosynthetic rate during drought is well documented, as is that photosynthesis of anisohydric plants, such as oaks, is relatively unresponsive to drought (McDowell et al. 2008; Yi et al. 2017). Additional research on the drought strategies of mycorrhizal fungi, in particular, can help to resolve their contribution to the relative performance differences observed among belowground biota.
Drought leaves a legacy on soil respiration
By the end of the drought, respiration was <10% that of the control mesocosms and C degrading potential was half that of control mesocosms. Respiration exhibited a strong pulse of high activity upon rewatering, but even when CO2 fluxes were highest, respiration from the droughted mesocosms was still only ~50% that of the control mesocosms. We found a strong drought legacy that continued to suppress respiration even after 2 weeks of high water availability. After 2 weeks of well-watered conditions, respiration from previously-droughted mesocosms was 60% (for maple) and 50% (for oak) that of control mesocosms. This finding contradicts a number of studies that have found a rapid return to background respiration rates correlated with recuperating microbial activity (Barnard et al. 2013; Blazewicz et al. 2014; Placella et al. 2012), and suggests that patterns of respiration during dry-rewetting cycles cannot be attributed solely to free-living microbial activity. It is possible, however, that the extreme fluctuations in water availability between drought and recovery periods may have acted as consecutive stressors on the microbial community (Lennon et al. 2012) as opposed to a drought stress followed by the alleviation of that stress. Nevertheless, our finding may be explained in different ways. Rewatering may have flushed newly labile resources out of our mesocosms (Kieft et al. 1987), leaving behind a depleted resource supply for belowground biota relative to control mesocosms (He and Dijkstra 2014). If rewatering leaches resources from soils, the long-term performance of plants after drought may be influenced by nutrient-limitation. The rapid flushing of nutrients and ions from soils upon rewetting also has important implications for groundwater and aquatic ecosystem functioning (Evans et al. 2006). Alternatively, reduced respiration following drought may occur if the free-living microbial community composition shifted towards one that is more drought-tolerant (Evans and Wallenstein 2014), and therefore exhibited faster reactivation of microbial growth (Meisner et al. 2013, 2015; Ochoa-Hueso et al. 2018). A detailed assessment of microbial composition could help clarify these results.
Differences in the respiration recovery potential of belowground biota, especially in the context of competition, have important implications for predicting C fluxes across ecosystems (Hanson et al. 2004) and under different environmental stresses (Scott-Denton et al. 2006). These differences may be particularly important under extreme droughts, when prolonged C limitation further reduces belowground respiration relative to plant productivity (Shi et al. 2014). Arbuscular mycorrhizal fungi are thought to have shorter turnover times (Soudzilovskaia et al. 2015; Staddon et al. 2003) than ectomycorrhizal fungi (Ekblad et al. 2016), and this may have contributed to the faster respiration recovery of the Hyphae+Microbes chamber of droughted maple mesocosms. These findings suggest that arbuscular mycorrhizal fungi can play an important role in the CO2 pulses that occur following dry-rewetting cycles. We speculate that that recovery of arbuscular mycorrhizal activity could also help to initiate plant recovery following an extreme drought.
Conclusions
An improved understanding of the mechanisms underlying soil respiration is needed to refine predictions of terrestrial CO2 emissions under future climate scenarios (Bradford et al. 2016; Friedlingstein et al. 2014). In particular, as extreme climatic events increase in severity and frequency, it is important to understand whether our current framework for understanding soil respiration can be extended to extreme environmental conditions (Vicca et al. 2014). Our results suggest that ecosystems dominated by different tree species and their associated mycorrhizal fungi may respond to and recover from drought differently in terms of respiration and enzyme activity. In addition to physiological differences between tree hosts, resource use patterns of belowground biota may influence respiration responses from rhizosphere and bulk soil. Incorporating such ecologically-relevant nuances into our understanding of the drought-responses of soil as a whole may improve the spatial accuracy, and thus our ability to scale up, estimates of terrestrial respiration.
Data availability
Data will be archived at https://doi.org/10.3334/CDIAC/ornlsfa.011 following acceptance.
References
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42. https://doi.org/10.1007/s005720100097
Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. The ISME Journal 7:2229–2241. https://doi.org/10.1038/ismej.2013.104
Bell CW, Fricks BE, Rocca JD, Steinweg JM, McMahon SK, Wallenstein MD (2013) High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J Vis Exp (81):e50961. https://doi.org/10.3791/50961
Blazewicz SJ, Schwartz E, Firestone MK (2014) Growth and death of bacteria and fungi underlie rainfall-induced carbon dioxide pulses from seasonally dried soil. Ecol 95:1162–1172. https://doi.org/10.1890/13-1031.1
Bond-Lamberty B, Wang C, Gower ST (2004) A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob Chang Biol 10:1756–1766. https://doi.org/10.1111/j.1365-2486.2004.00816.x
Bradford MA, Wieder WR, Bonan GB, Fierer N, Raymond PA, Crowther TW (2016) Managing uncertainty in soil carbon feedbacks to climate change. Nat Clim Chang 6:751–758. https://doi.org/10.1038/nclimate3071
Clarholm M (1981) Protozoan grazing of Bacteria in soil-impact and importance. Microb Ecol 7:343–350
Crowther TW, Sokol NW, Oldfield EE, Maynard DS, Thomas SM, Bradford MA (2015) Environmental stress response limits microbial necromass contributions to soil organic carbon. Soil Biol Biochem 85:153–161. https://doi.org/10.1016/j.soilbio.2015.03.002
Ekblad A, Mikusinska A, Ågren GI, Menichetti L, Wallander H, Vilgalys R, Bahr A, Eriksson U (2016) Production and turnover of ectomycorrhizal extramatrical mycelial biomass and necromass under elevated CO2 and nitrogen fertilization. New Phytol 211:874–885. https://doi.org/10.1111/nph.13961
Evans SE, Wallenstein MD (2014) Climate change alters ecological strategies of soil bacteria. Ecol Lett 17:155–164. https://doi.org/10.1111/ele.12206
Evans CD, Chapman PJ, Clark JM, Monteith DT, Cresser MS (2006) Alternative explanations for rising dissolved organic carbon export from organic soils. Glob Chang Biol 12:2044–2053. https://doi.org/10.1111/j.1365-2486.2006.01241.x
Fenn KM, Malhi Y, Morecroft MD (2010) Soil CO2 efflux in a temperate deciduous forest: environmental drivers and component contributions. Soil Biol Biochem 42:1685–1693. https://doi.org/10.1016/j.soilbio.2010.05.028
Fierer N, Schimel JP (2002) Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34:777–787
Fierer N, Schimel JP (2003) A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci Soc Am J 67:798. https://doi.org/10.2136/sssaj2003.0798
Franklin O, Näsholm T, Högberg P, Högberg MN (2014) Forests trapped in nitrogen limitation – an ecological market perspective on ectomycorrhizal symbiosis. New Phytol 203:657–666. https://doi.org/10.1111/nph.12840
Friedlingstein P, Meinshausen M, Arora VK, Jones CD, Anav A, Liddicoat SK, Knutti R (2014) Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J Clim 27:511–526. https://doi.org/10.1175/JCLI-D-12-00579.1
Fuchslueger L, Bahn M, Fritz K, Hasibeder R, Richter A (2014) Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytol 201:916–927. https://doi.org/10.1111/nph.12569
Hagedorn F, Joseph J, Peter M, Luster J, Pritsch K, Geppert U, Kerner R, Molinier V, Egli S, Schaub M, Liu JF, Li M, Sever K, Weiler M, Siegwolf RTW, Gessler A, Arend M (2016) Recovery of trees from drought depends on belowground sink control. Nature Plants 2:16111. https://doi.org/10.1038/nplants.2016.111
Hanson PJ, Amthor JS, Wullschleger SD, Wilson KB, Grant RF, Hartley A, Hui D, Hunt Jr ER, Johnson DW, Kimball JS, King AW, Luo Y, McNulty SG, Sun G, Thornton PE, Wang S, Williams M, Baldocchi DD, Cushman RM (2004) Oak forest carbon and water simulations: model intercomparisons and evaluations against independent data. Ecol Monogr 74:443–489. https://doi.org/10.1890/03-4049
He M, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a meta- analysis. New Phytol 204:924–931. https://doi.org/10.1111/nph.12952
Heilmeier H, Erhard M, E-D S (1997) Biomass allocation and water use under arid conditions. In: Bazzaz F, Grace J (eds) Plant resource allocation. Academic Press, San Diego, pp 93–112
Heinemeyer A, Hartley IP, Evans SP, Carreira De La Fuente JA, Ineson P (2007) Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Glob Chang Biol 13:1786–1797. https://doi.org/10.1111/j.1365-2486.2007.01383.x
Henry HAL (2012) Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47:53–59. https://doi.org/10.1016/j.soilbio.2011.12.026
Hobbie EA (2006) Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecol 87:563–569. https://doi.org/10.1890/05-0755
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792. https://doi.org/10.1038/35081058
IPCC (2012) Special report of the IPCC: managing the risks of extreme events and disasters to advance climate change adaptation. Cambridge University Press, Cambridge
IPCC (2014) Climate change 2014: synthesis report. Contribution of working groups I. II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Geneva
Kaiser C, Franklin O, Dieckmann U, Richter A (2014) Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol Lett 17:680–690. https://doi.org/10.1111/ele.12269
Kieft TL, Soroker E, Firestone MK (1987) Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biol Biochem 19:119–126. https://doi.org/10.1016/0038-0717(87)90070-8
Kuzyakov Y (2006) Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol Biochem 38:425–448. https://doi.org/10.1016/j.soilbio.2005.08.020
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review J Plant Nutr Soil Sci 163:421–431. https://doi.org/10.1002/1522-2624(200008)163:4<421::AID-JPLN421>3.0.CO;2-R
Lee X, Wu HJ, Sigler J, Oishi C, Siccama T (2004) Rapid and transient response of soil respiration to rain. Glob Chang Biol 10:1017–1026. https://doi.org/10.1111/j.1365-2486.2004.00787.x
Lennon JT, Aanderud ZT, Lehmkuhl BK, Schoolmaster DR (2012) Mapping the niche space of soil microorganisms using taxonomy and traits. Ecol 93:1867–1879. https://doi.org/10.1890/11-1745.1
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nature Microbiology 2:17105. https://doi.org/10.1038/nmicrobiol.2017.105
Litton CM, Raich JWJWJW, Ryan MG (2007) Carbon allocation in forest ecosystems. Glob Chang Biol 13:2089–2109. https://doi.org/10.1111/j.1365-2486.2007.01420.x
Manzoni S, Schimel JP, Porporato A (2012) Responses of soil microbial communities to water stress : results from a meta-analysis. Ecol 93:930–938
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739. https://doi.org/10.1111/j.1469-8137.2008.02436.x
Meisner A, Bååth E, Rousk J (2013) Microbial growth responses upon rewetting soil dried for four days or one year. Soil Biol Biochem 66:188–192. https://doi.org/10.1016/j.soilbio.2013.07.014
Meisner A, Rousk J, Bååth E (2015) Prolonged drought changes the bacterial growth response to rewetting. Soil Biol Biochem 88:314–322. https://doi.org/10.1016/j.soilbio.2015.06.002
Menne MJ et al. (2012a) Global historical climatology network - daily (GHCN-daily), version 3.22. NOAA National Climatic Data Center. https://doi.org/10.7289/V5D21VHZ
Menne MJ, Durre I, Vose RS, Gleason BE, Houston TG (2012b) An overview of the global historical climatology network-daily database. J Atmos Ocean Technol 29:897–910. https://doi.org/10.1175/JTECH-D-11-00103.1
Ochoa-Hueso R, Collins SL, Delgado-Baquerizo M et al (2018) Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob Change Biol 24:2818–2827. https://doi.org/10.1111/gcb.14113
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199:41–51. https://doi.org/10.1111/nph.12221
Placella SA, Brodie EL, Firestone MK (2012) Rainfall-induced carbon dioxide pulses result from sequential resuscitation of phylogenetically clustered microbial groups. Proc Natl Acad Sci U S A 109:10931–10936. https://doi.org/10.1073/pnas.1204306109
Preece C, Peñuelas J (2016) Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil 409:1–17. https://doi.org/10.1007/s11104-016-3090-z
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391
Reichstein M, Bahn M, Ciais P, Frank D, Mahecha MD, Seneviratne SI, Zscheischler J, Beer C, Buchmann N, Frank DC, Papale D, Rammig A, Smith P, Thonicke K, van der Velde M, Vicca S, Walz A, Wattenbach M (2013) Climate extremes and the carbon cycle. Nature 500:287–295. https://doi.org/10.1038/nature12350
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754. https://doi.org/10.1111/j.1461-0248.2004.00620.x
Ruehr NK, Offermann CA, Gessler A, Winkler JB, Ferrio JP, Buchmann N, Barnard RL (2009) Drought effects on allocation of recent carbon: from beech leaves to soil CO2 efflux. New Phytol 184:950–961. https://doi.org/10.1111/j.1469-8137.2009.03044.x
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecol 3:591–602. https://doi.org/10.1890/03-8002
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563. https://doi.org/10.1016/S0038-0717(03)00015-4
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecol 88:1386–1394. https://doi.org/10.1890/06-0219
Schimel J, Boot C, Holden P, Roux-michollet D, Parker S, Schaeffer S, Treseder K (2010) The biogeochemistry of drought world congress. Soil Sci 2010:55–58
Schlesinger WH, Dietze MC, Jackson RB, Phillips RP, Rhoades CC, Rustad LE, Vose JM (2016) Forest biogeochemistry in response to drought. Glob Chang Biol 22:2318–2328. https://doi.org/10.1111/gcb.13105
Schweigert M, Herrmann S, Miltner A, Fester T, Kästner M (2015) Fate of ectomycorrhizal fungal biomass in a soil bioreactor system and its contribution to soil organic matter formation. Soil Biol Biochem 88:120–127. https://doi.org/10.1016/j.soilbio.2015.05.012
Scott-Denton LE, Rosenstiel TN, Monson RK (2006) Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob Chang Biol 12:205–216. https://doi.org/10.1111/j.1365-2486.2005.01064.x
Seidl R, Thom D, Kautz M, Martin-Benito D, Peltoniemi M, Vacchiano G, Wild J, Ascoli D, Petr M, Honkaniemi J, Lexer MJ, Trotsiuk V, Mairota P, Svoboda M, Fabrika M, Nagel TA, Reyer CPO (2017) Forest disturbances under climate change. Nat Clim Chang 7:395–402. https://doi.org/10.1038/nclimate3303 https://www.nature.com/articles/nclimate3303#supplementary-information
Shi Z, Thomey ML, Mowll W, Litvak M, Brunsell NA, Collins SL, Pockman WT, Smith MD, Knapp AK, Luo Y (2014) Differential effects of extreme drought on production and respiration: synthesis and modeling analysis. Biogeosciences 11:621–633. https://doi.org/10.5194/bg-11-621-2014
Soudzilovskaia NA, van der Heijden MGA, Cornelissen JHC, Makarov MI, Onipchenko VG, Maslov MN, Akhmetzhanova AA, van Bodegom PM (2015) Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling. New Phytol 208:280–293. https://doi.org/10.1111/nph.13447
Staddon PL, Thompson KEN, Jakobsen I, Grime JP, Askew AP, Fitter AH (2003) Mycorrhizal fungal abundance is affected by long-term climatic manipulations in the field. Glob Chang Biol 9:186–194
Steinweg JM, Dukes JS, Paul EA, Wallenstein MD (2013) Microbial responses to multi-factor climate change: effects on soil enzymes. Front Microbiol 4:1–11. https://doi.org/10.3389/fmicb.2013.00146
Subke JA, Inglima I, Cotrufo MF (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Chang Biol 12:921–943. https://doi.org/10.1111/j.1365-2486.2006.01117.x
Talbot JM, Allison SD, Treseder KK (2008) Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol 22:955–963. https://doi.org/10.1111/j.1365-2435.2008.01402.x
Team RDC (2011) R: a language and environment for statistical computing, 3.3.2 edn. R Foundation for statistical computing, Vienna
Treseder KK, Allen MF (2000) Mycorrhizal fungi have a potential role in soil carbon storage under elevated CO2 and nitrogen deposition. New Phytol 147:189–200
van der Molen MK, Dolman AJ, Ciais P, Eglin T, Gobron N, Law BE, Meir P, Peters W, Phillips OL, Reichstein M, Chen T, Dekker SC, Doubková M, Friedl MA, Jung M, van den Hurk BJJM, de Jeu RAM, Kruijt B, Ohta T, Rebel KT, Plummer S, Seneviratne SI, Sitch S, Teuling AJ, van der Werf GR, Wang G (2011) Drought and ecosystem carbon cycling. Agric For Meteorol 151:765–773. https://doi.org/10.1016/j.agrformet.2011.01.018
Vargas R, Baldocchi DD, Querejeta JI, Curtis PS, Hasselquist NJ, Janssens IA, Allen MF, Montagnani L (2010) Ecosystem CO2 fluxes of arbuscular and ectomycorrhizal dominated vegetation types are differentially influenced by precipitation and temperature. New Phytol 185:226–236. https://doi.org/10.1111/j.1469-8137.2009.03040.x
Vicca S, Bahn M, Estiarte M, van Loon EE, Vargas R, Alberti G, Ambus P, Arain MA, Beier C, Bentley LP, Borken W, Buchmann N, Collins SL, de Dato G, Dukes JS, Escolar C, Fay P, Guidolotti G, Hanson PJ, Kahmen A, Kröel-Dulay G, Ladreiter-Knauss T, Larsen KS, Lellei-Kovacs E, Lebrija-Trejos E, Maestre FT, Marhan S, Marshall M, Meir P, Miao Y, Muhr J, Niklaus PA, Ogaya R, Peñuelas J, Poll C, Rustad LE, Savage K, Schindlbacher A, Schmidt IK, Smith AR, Sotta ED, Suseela V, Tietema A, van Gestel N, van Straaten O, Wan S, Weber U, Janssens IA (2014) Can current moisture responses predict soil CO2 efflux under altered precipitation regimes? A synthesis of manipulation experiments. Biogeosciences 11:2991–3013
Warren JM, Hanson PJ, Iversen CM, Kumar J, Walker AP, Wullschleger SD (2015) Root structural and functional dynamics in terrestrial biosphere models - evaluation and recommendations. New Phytol 205:59–78. https://doi.org/10.1111/nph.13034
Wieder WR, Bonan GB, Allison SD (2013) Global soil carbon projections are improved by modelling microbial processes. Nat Clim Chang 3:909–912. https://doi.org/10.1038/nclimate1951
Wolf S et al. (2016) Warm spring reduced carbon cycle impact of the 2012 US summer drought. PNAS:1519620113- https://doi.org/10.1073/pnas.1519620113
Yi K, Dragoni D, Phillips RP, Roman DT, Novick KA (2017) Dynamics of stem water uptake among isohydric and anisohydric species experiencing a severe drought. Tree Physiol 37:1379–1392. https://doi.org/10.1093/treephys/tpw126
Yuste JC et al (2011) Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Glob Chang Biol 17:1475–1486. https://doi.org/10.1111/j.1365-2486.2010.02300.x
Zak DR, Tilman D, Parmenter RR, Rice CW, Fisher FM, Vose J, Milchunas D, Martin CW (1994) Plant production and soil microorganisms in late-successional ecosystems: a continental-scale study. Ecol 75:2333–2347. https://doi.org/10.2307/1940888
Zscheischler J, Michalak AM, Schwalm C, Mahecha MD, Huntzinger DN, Reichstein M, Berthier G, Ciais P, Cook RB, el-Masri B, Huang M, Ito A, Jain A, King A, Lei H, Lu C, Mao J, Peng S, Poulter B, Ricciuto D, Shi X, Tao B, Tian H, Viovy N, Wang W, Wei Y, Yang J, Zeng N (2014) Impact of large-scale climate extremes on biospheric carbon fluxes: an intercomparison based on MsTMIP data global biogeochemical. Cycles 28:585–600. https://doi.org/10.1002/2014GB004826
Acknowledgements
We would like to thank Richard Marinos for help designing the experiment, and Joanne Childs, David McLennan, Sarah Childs, and Anirban Guha for help with data collection. We would also like to thank Emily Bernhardt, Jim Heffernan, Marissa Lee, and two anonymous reviewers for constructive comments on the framing of and details within this manuscript. Research was sponsored by the Biological and Environmental Research Program in the Office of Science, United States Department of Energy, the Graduate Opportunities (GO!) Program, and by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory, managed by UT-Battelle, LLC, for the United States Department of Energy under contract DEAC05-00OR22725.
Author information
Authors and Affiliations
Contributions
Both authors designed the research, collected data, and wrote the manuscript. CDF analyzed data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Thom W. Kuyper.
Electronic supplementary material
ESM 1
(DOCX 14169 kb)
Rights and permissions
About this article
Cite this article
Ficken, C.D., Warren, J.M. The carbon economy of drought: comparing respiration responses of roots, mycorrhizal fungi, and free-living microbes to an extreme dry-rewet cycle. Plant Soil 435, 407–422 (2019). https://doi.org/10.1007/s11104-018-03900-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-03900-2