Abstract
Aims
This study investigated the impact of canopy cover and seasonality on litter decay in Mediterranean pine forests to enhance climate predictions.
Methods
We conducted litterbag experiments in plots of different tree densities in two Mediterranean pine forests differing in precipitation amounts. In each plot, local litter was placed in forest gaps and under tree canopies for 613 days, starting in the dry season.
Results
Litter mass loss was greater in forest gaps than under tree canopies across forests and tree densities. Similarly, a reduction in tree density tended to increase mass loss. Additionally, while the decay rate slowed down from the first to the second wet season, the decay rate remained constant during the first and the second dry season, and the dry seasons contributed 30% to the overall mass loss.
Conclusions
Reduction in canopy cover enhances litter decay, and the stability and magnitude of the dry season contribution to annual mass loss have the potential to control litter mass loss when accounting also for the dry periods in the wet season. Combined, the ongoing tree mortality and the predicted prolongation of dry periods due to climate change may enhance litter decay, possibly reducing ecosystem carbon stocks in drylands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Semi-arid ecosystems regulate the long-term trend and the interannual variability in the terrestrial carbon (C) cycle (Poulter et al. 2014; Ahlström et al. 2015). The relevance of drylands for the global C cycle arises from their vast spatial extent (~41% of Earth’s land area), the fraction of the global soil organic C stored in drylands (~27%; Safriel et al. 2005), and their large C sequestration potential (Luo et al. 2007; Wohlfahrt et al. 2008; Ahlström et al. 2015). Furthermore, drylands are predicted to increase in range due to desertification processes (D’Odorico et al. 2013), thus their global importance is likely to rise in the future. However, the decomposition of organic plant material in dryland ecosystems is not well understood (Throop and Archer 2009; Poulter et al. 2014; Carvalhais et al. 2014). In particular, the decomposition of above-ground plant litter in biogeochemical models tends to be greatly underestimated (Parton et al. 2007; Adair et al. 2008; Bonan et al. 2013), and thus poses a source for uncertainty in our predictions of the terrestrial C cycle.
It was suggested that this underestimation of litter decay in drylands stems from the fact that models do not include abiotic and biotic “non-rainfall decay mechanisms” operating in dry periods (Barnes et al. 2015; Gliksman et al. 2017). Among the abiotic daytime decay drivers, (i) solar radiation causes photodegradation of litter through mineralization of photo-reactive compounds and photo-oxidation of chemical bonds (Moorhead and Callaghan 1994; King et al. 2012; Barnes et al. 2015), and (ii) high temperatures (>30 °C) lead to thermal degradation of litter (Lee et al. 2012; Van Asperen et al. 2015). These drivers can contribute up to 60% of the annual litter mass loss and the dry-season ecosystem CO2 flux (Austin and Vivanco 2006; Rutledge et al. 2010). Additionally, biotic degradation occurs in rainless periods when high levels of water vapor and dew are absorbed by litter at night, thus facilitating microbial degradation (Dirks et al. 2010; Day et al. 2015; Jacobson et al. 2015), which can control the dry-season mass loss (Gliksman et al. 2017). In addition to their direct contribution to decomposition, these abiotic and biotic decay mechanisms can facilitate each other on a daily scale (Gliksman et al. 2017), and solar radiation can also determine the annual mass loss by facilitating or inhibiting decay (“photopriming”) in subsequent wet periods (Pancotto et al. 2003; Henry et al. 2008; Gallo et al. 2009; Smith et al. 2010; Baker and Allison 2015; Austin et al. 2016). Studies in which photodegradation was simulated showed the great potential of these mechanisms to improve litter decay models in drylands (Foereid et al. 2011; Chen et al. 2016).
The contribution of decay drivers operating in rainless periods can be strongly affected by changes in the tree or shrub canopy cover. Forest ecosystems in drylands are especially vulnerable to changes in climate, land use and fire, which lead to the opening of landscapes due to tree mortality (Allen et al. 2010; Davidson et al. 2012). Reduced canopy cover increases the amount of solar radiation that reaches the litter layer, thus enhancing photochemical and thermal degradation (Mlambo and Mwenje 2010; Araujo and Austin 2015). Similarly, lower canopy cover is likely to increase dew formation through enhanced nighttime heat loss (Baier 1966; Jacobs et al. 1990; Xiao et al. 2009; Pan et al. 2010), thus increasing the microbial degradation of litter (Gliksman et al. 2017). For this reason, there is a need to assess how litter decay will be influenced not only by changes in climate, but also by changes in the vegetation structure.
Beyond the effects of canopy cover on decay, we also have little knowledge concerning the dynamics and magnitude of litter decay in forests when differentiating between dry and wet periods. Dynamics of litter decay are commonly described as a decay function with a fast initial decay which decreases over time (Swift et al. 1979; Cornwell and Weedon 2014). However, such functions may not be accurate if we examine the decay occurring in dry and wet seasons separately. In hot and dry periods without precipitation, abiotic and biotic degradation can alternate on a daily basis in their consumption of ‘recalcitrant’ and ‘labile’ compounds, thus potentially reducing bottle necks for each degradation type (Gliksman et al. 2017), and allowing the decay rate to remain constant over time. Such decay dynamics coupled with long rainless periods can lead to a substantial contribution of the non-rainfall decay mechanisms to the annual mass loss.
Here, we investigated the effects of variations in canopy cover on litter decay in Mediterranean pine forests by using experimental plots of different tree densities and by selecting exposed and shaded microsites within plots. We also compared the impact of the extreme Mediterranean seasonality by following litter decay over two consecutive dry season-wet season cycles. We hypothesized that (1) litter decay in Mediterranean forests increases with the reduction in canopy cover due to a higher contribution of non-rainfall decay mechanisms; (2) the contribution of consecutive dry and wet seasons to litter decomposition decreases as a consequence of increasingly decomposed litter; and (3) the dry periods have a significant contribution to the annual decay due to the long rainless periods in the studied forests.
Materials and methods
Study sites
The study was performed in two Aleppo pine (Pinus halepensis) forests in Israel that are part of the long-term ecological research network of the national forest service (JNF-KKL). The first study site was in the Kdoshim forest, located in the dry sub-humid Mediterranean climatic zone, on the lower western slopes of the Jerusalem mountains (400–500 a.s.l.), with topographic aspect ranging from south- to north-western and average slope of 25% (31°78′N, 35°02′E). The long-term (40 yr) mean annual precipitation (MAP) is 550 mm (during the last 10 years, MAP was 15% lower), all of which falls between October and April; minimum and maximum mean monthly air temperatures are 16 and 25°C in the daytime, and 16 and 19°C in the nighttime. The forest canopy is composed of Aleppo pine trees planted in 1968, the understory by broad-leaf trees and shrubs dominated by Quercus calliprinos Webb (tree), Pistacia lentiscus L. (shrub) and Rhamnus lycioides L. (shrub) and a variety of ephemeral herbaceous species. Average pine tree height, stem diameter at breast height (DBH) and leaf area are 12.6 ± 0.5 m, 18.9 ± 0.6 cm and 71.6 ± 9.2 m2, respectively. During the years of this study (2011–12), the forest has suffered 15% tree mortality as a result of consecutive drought years. The soil is a shallow Terra rossa, bedrock is mostly dolomite and lime. More detailed information about this site is provided in Calev et al. (2016).
The second site was in Yatir forest, located in the Mediterranean semi-arid zone on a plateau of the northern Negev (650 a.s.l.; 31°20′N, 35°20′E). The climate is drier, with 280 mm MAP, most of which falls between November and April. Minimum and maximum mean monthly air temperatures are 11 and 28°C in the daytime, and 8 and 21°C in the nighttime. The canopy is composed of Aleppo pine trees planted mostly during 1964–1967, the understory is sparse and dominated by dwarf shrubs, mainly Sarcopoterium spinosum L. and Phagnalon rupestre (L.) DC. and a variety of ephemeral herbaceous species. Before this study began, the forest suffered 5–10% tree mortality (2010–2011) as a result of consecutive drought years (Klein et al. 2014). The substrate is light colored Rendzina above chalk and limestone. Trees are ~12 m high, stand density of ~300 trees ha−1 and DBH of ~21 cm.
Plots of different tree densities were created by thinning in both forests in 2009, as follows: 0, 100 and 300 trees ha−1 in the dry sub-humid forest, 100, 200 and 300 trees ha−1 in the semi-arid forest. Subsequently, monitoring plots were set up, with each plot extending over 3000 m2 (of which the center 1500 m2 were used for measurements) in the dry sub-humid forest and 800 m2 in the semi-arid forest.
Litter collection and litterbag preparation
During June 2011, nets were placed on the ground in both forests to collect freshly shed pine needles (termed as “pine litter”) at the annual peak of needle fall. Additionally, fresh standing-dead material of the dominant annual grass Avena sterilis L. (dry sub-humid forest) and a mixture of Poaceae (semi-arid forest) was harvested and chopped to 4-cm segments (termed “grass litter”, see Table 1 for initial litter quality). Litter was dried at 55 °C for two days, after which 2 g of litter were placed in 10 cm × 10 cm litterbags made of grey fiberglass (facing the ground, 0.5 mm mesh) and transparent polyethylene [facing up, 1 mm mesh, 90% transmittance over the photochemically active wavelengths of 280–550 nm (Austin and Ballaré 2010)].
Local litter experiment: Tree density and canopy cover effect on litter decomposition
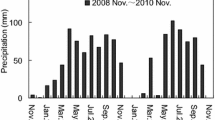
Four plots of each of the following tree densities were selected for the experiment (24 plots in total in both forests): 0, 100 and 300 trees ha−1 (leaf area index, LAI = 0, 1.0 and 1.7, respectively, based on Calev et al. 2016; see Fig. S1 for the radiation distribution under the different tree densities) in the dry sub-humid forest and 100, 200 and 300 trees ha−1 in the semi-arid forest (with LAI in 2011 of about 0.8, 1.3 and 1.8, respectively, unpublished data, for methods see Supporting Information; see Fig. S2 for radiation distribution). In early July 2011, at solar noon time, we selected two microsites in each plot for placing the litterbags: one in the center of the largest gap in the forest canopy (“forest gap”) and the other one in the middle of the largest shade patch under the canopy of pine trees (“canopy cover”). The diameter of the gaps ranged between 5 and 10 m in the high tree-density plots to 10–30 m in the low density plots. In the clear-cut plots (0 trees ha−1) of the dry sub-humid forest, the microsites for litterbags placement were either close to or far from pine stumps. A total of 384 litterbags were placed on the ground in both forests in mid-July 2011, with 4 litterbags (one litterbag for each of four retrieval dates) for each combination of litter type (2), microsite (2), plot (12), and forest (2). The litter in all bags was local, i.e. collected and incubated in the same forest. The deployment of litterbags took place as early as possible following the shedding of needles in late-June. The experiment lasted 613 days, and the four litterbag retrievals were performed at the end of the dry season of 2011 and of 2012 (both retrievals at the end of September) and at the end of the wet season of 2012 and 2013 (both in mid-March). Here, we define the dry season as the rainless period from mid-March till end of September. The dry seasons were without rain except for a single rainy day at the beginning of the second dry season in the dry sub-humid forest (Fig. 1; 9 mm rain, late-March 2012).
Common garden experiment
To separate the effect of litter quality from that of the environment, we conducted a common-garden litter decomposition experiment under two contrasting conditions. We chose two microsites in the dry sub-humid forest, one with full exposure to solar radiation (in a clear-cut plot, termed as forest gap) and a second one under dense canopy cover in a 600 trees ha−1 plot. Twenty replicated bags of each of the two types of litter collected from each forest (four litter types overall) were placed for decomposition at each microsite (160 litterbags in total). On each of the four retrieval dates, five litterbags were collected from each litter type and microsite. The placement and retrieval of litterbags was performed simultaneously with the ones in the local litter experiment.
Chemical analyses of litter
Initial litter and litter retrieved from the field were analyzed in the lab for dry weight (55 °C, 48 h) and ash content (550 °C, 4 h) to assess ash-free litter dry mass for calculating the litter mass loss. The C and nitrogen (N) concentration of the initial litter were measured on finely ground powder (Pulverisette 7 Planetary Micro Mill; Fritsch, Idar-Oberstein, Germany) in an elemental analyzer (FlashEA 1112; Thermo, Waltham MA, USA), and was expressed on ash-free base.
Climate monitoring
We placed small data loggers (DS1922L-F5#, iButtons, Embedded Data Systems, Lawrenceburg, KY, USA) between the litterbags and the soil in both forests to monitor litter temperature during the experimental period. In the dry sub-humid forest, the loggers were placed in the two microsites of the common garden experiment (a clear-cut plot and under a dense canopy cover), both under pine and grass litter collected from the dry sub-humid forest (one logger per litter type and microsite, four loggers overall). In the semi-arid forest, loggers were placed under pine and grass litterbags in a forest gap of a 100 trees ha−1 plot and under litterbags below canopy cover in a 300 trees ha−1 plot (overall four loggers). The locations of logger placement in both forests represent the extremes within the range of the canopy cover. Rainfall was collected in meteorological stations positioned in the both forests (for the semi-arid site see Tatarinov et al. 2016). The same station in the dry sub-humid forest measured also relative humidity (RH) at 2.5 m above-ground.
Statistical analysis
In both forests and experiments, we analyzed the decay rates after the first dry season and at the end of the experiments. In the local litter experiment, mass loss in the two microsites of the clear-cut plots in the dry sub-humid forest did not differ over all samplings and litter types (P > 0.31; next to tree stumps or between stumps). Therefore, we combined both microsites to be a “forest gap” microsite, and divided the analysis of litter decay for each litter type in the dry sub-humid forest into two parts. For each litter type, we performed a full factorial ANOVA using the variables microsite (forest gap/canopy cover) and tree density, with two tree densities of 100 and 300 trees ha−1 (excluding the 0 trees ha−1). Then, we used a one-way ANOVA for comparing the three densities in the non-shaded microsite (forest gaps in plots of 0, 100 and 300 trees ha−1). In the semi-arid forest, we used a full factorial ANOVA for each litter type, using the variables microsite and tree density (all levels). We analyzed the common garden experiment in the dry sub-humid forest using a one-way ANOVA for each litter type to find differences between the microsites. Interactions are mentioned when significant. The forests were analyzed separately because of differences in experimental design (densities) and the difference in litter quality of the grass litter collected from each forest.
We calculated the mass loss per month for each of the four seasons to allow comparisons of decay rates between different seasons, while eliminating differences in the length of the season. In the local litter experiment, the rate of decay per month for each litter type is the average of means of mass loss per month from the six combinations of microsite and tree density (five in the dry sub-humid forest). This averaging represents the decay rate for a litter type under the different conditions within a forest with the purpose of receiving uniform results across all litter types to transcend the differences between forests which originate from the different tree densities in each forest. For each litter type we compared the means of two dry seasons or two wet seasons using Student’s t-test. In addition, we performed two full-factorial analyses to examine if the change in monthly decay rates between two consecutive seasons (either dry or wet seasons) was affected by the microsites and litter types, across all densities. In the common garden experiment the rate of decay per month is the average of the mean mass loss per month of the eight combinations of canopy cover and litter type. The Tukey-Kramer HSD test was used for post hoc comparisons of multiple means. When several specific contrasts between means were tested at α = 0.05, Holm’s correction was applied (Rice 1989). When heterogeneity of variance occurred, data were log-transformed prior to statistical analyses. Data were analyzed using JMP 11 software (SAS Institute, Cary, NC, USA).
Results
Over both dry seasons, forests and litter types, the mean daytime maximum temperatures measured under the litterbags (i.e., protected from direct solar radiation) were about 19 °C higher in forest gaps than under canopy cover (Table 2, P < 0.001; t test). Nighttime minimum temperature in the dry seasons was lower by 2 °C in forest gaps than under canopy cover in the dry sub-humid forest, while in the semi-arid forest the trend reversed and the minimum temperature was higher by 1.7 °C in forest gaps than under canopy cover (P < 0.001; t test for both forests). During the wet seasons, no clear trend was obtained for daytime maximum temperature, but minimum temperature at night was lower by 1.7 °C in forest gaps than under canopy cover (P < 0.001; t test across litter types, forests and both wet seasons). The wet seasons differed in rainfall amounts (Fig. 1; 574 vs. 450 mm and 350 vs. 249 mm in the first vs. the second wet season in the dry sub-humid and semi-arid forests, respectively). Nights were with high RH (77.1 ± 0.6, n = 639 days) in the dry sub-humid forest, with small fluctuations between the different seasons (Fig. S3).
In the local litter experiment, mass loss in the dry sub-humid forest at the end of the first dry season was greater in forest gaps than under canopy cover by a factor of 1.5 for pine and 1.6 for grass litter (Table 3a, Fig. 2, P = 0.002 and 0.018, respectively; full factorial models without clear-cut plots). Moreover, pine litter decayed more in the 100 trees ha−1 plots than in the 300 trees ha−1 plots by a factor of 1.7 (P = 0.037). When including the clear-cut plots and comparing litter mass loss only in forest gaps, tree density did not have a significant effect on litter decay for pine and grass litter (P = 0.207 and 0.8, one-way ANOVA). By the end of the incubation period (after the second wet season), microsite and tree density (without the clear-cut plots) did not significantly affect decomposition of pine (P = 0.429 and 0.366) and grass litter (P = 0.475 and 0.545). When including the clear-cut plots and analyzing forest gaps only, pine litter decomposed more by a factor of 1.2 and 1.3 (P = 0.001, one-way ANOVA) in the clear-cut plots than in the forest gaps at 100 and 300 trees ha−1, respectively, while tree density did not have a significant effect on grass litter mass loss (P = 0.95).
Dynamics of pine and grass litter decay in the two forests and under three tree densities in the local litter experiment. “D1” and “D2“ stand for the two dry seasons that lasted from July 2011 till September 2011 and from March 2012 till September 2012, and “W1” and “W2“stand for the two wet seasons that lasted from September 2011 till March 2012 and from September 2012 till March 2013. The solid symbols (and full lines) represent different tree densities in the forest gap microsites of the dry sub-humid forest (♦, ■ and ▲ for 0, 100 and 300 trees ha−1) and the semi-arid forest (■, ● and ▲ for 100, 200 and 300 trees ha−1). The open symbols (and the dashed lines) represent the above densities for the canopy cover microsites. Mean ± SE; n = 2–8 litterbags per category
In the semi-arid forest, pine litter decay was greater by a factor of 3.1in forest gaps than under canopy cover by the end of the first dry season (Table 3b, Fig. 2, P = 0.001; marginally significant effect for density P = 0.079 with the highest mass loss at the lowest tree density). Neither microsite nor tree density significantly affected grass litter decomposition in this forest (P = 0.362 and 0.887). By the end of the incubation period, mass loss for both pine and grass litter was 1.6 times greater in forest gaps than under canopy cover (P ≤ 0.001 and P = 0.002, for pine and grass litter types, respectively). There was a marginally significant effect of tree density on pine litter decay (P = 0.057; with highest mass loss at the lowest tree density) and no significant effect on grass litter decay (P = 0.854).
In the common garden experiment, decomposition of pine and grass litter from the dry sub-humid forest and grass from the semi-arid forest was greater by a factor of 1.6, 2.7 and 1.5 in forest gaps than under canopy cover by the end of the first dry season (Table 4, Fig. 3, P = 0.043, 0.001 and 0.021), while pine litter from the semi-arid forest decayed similarly in both microsites (P = 0.812). By the end of the incubation period, the decay was greater in forest gaps than under canopy cover for all litter types by a factor of 1.1–1.3 (P = 0.03 and 0.029 for pine and grass litter from the dry sub-humid forest; P = 0.008 and 0.006 for pine and grass litter from the semi-arid forest).
Dynamics of pine and grass litter decay originating from the dry sub-humid and semi-arid forests in the common garden experiment conducted in the dry sub-humid forest, in two different microsites over the entire experimental period. “D1” and “D2“ stand for the two dry seasons that lasted from July 2011 till September 2011 and from March 2012 till September 2012, and “W1” and “W2“stand for the two wet seasons that lasted from September 2011 till March 2012 and from September 2012 till March 2013. Mean ± SE; n = 4–5 litterbags per combination of litter type, sampling and microsite
We compared the monthly rate of litter decay separately between the two dry seasons and the two wet seasons in the local litter experiment. While there was a trend for higher decay rates in the first compared with the second dry season (by a factor of 1.13 on average), this trend was not statistically significant across all litter types and forests (P = 0.20–0.81, t-test on means of monthly rates of mass loss; Fig. 4). In contrast, the rate of mass loss per month in the first wet season was significantly greater by a factor of 1.79 on average across all litter types and forests (P < 0.05, except for pine from the dry sub-humid forest, P = 0.053) compared to the second wet season. Thus, the monthly mass loss rate of the dry season in relation to that of the subsequent wet season increased from the first year to the second year from 45% to 56% in the dry sub-humid forest and from 50% to 112% in the semi-arid forest (averages of both litter types in each forest). Additionally, we did not detect any effect of the microsite on the difference in monthly decay between the two dry seasons or the two wet seasons (Table S1). In the common garden experiment, there was no difference in rate of mass loss per month between the dry seasons (P = 0.27, pooling of litter types; Fig. 4), while the rate was significantly higher in the first wet season than in the second wet season by a factor of 2.5 (P = 0.004). Thus, the monthly mass loss rate of the dry season in relation to that of the subsequent wet season increased from the first year to the second year from 46% to 90% (over all litter types).
The monthly decay rate for each of the four seasons in both experiments. “Dry sub-h.” and “Semi-arid” stands for the dry sub-humid and semi-arid forests. The common garden experiment (“Cg”) was conducted in the dry sub-humid forest. In the local litter experiment, n = 5 for litter in the dry sub-humid forest and n = 6 for litter from the semi-arid forest as averages of the combinations of microsite and tree density. In the common garden experiment, n = 8 as averages of litter type and microsite combinations. * indicates significant difference between the microsites at P < 0.05, and (*) represent a marginally significant difference P = 0.053. Means ± SE are shown
Discussion
Our results show the importance of decay in rainless periods in determining the turnover of litter. First, following our hypothesis reductions in canopy cover led to enhanced litter decay. The higher decay rate at the end of the first dry season in forest gaps than under canopy cover (greater by 1.77 times on average over all litter types and experiments) can only be attributed to non-rainfall decay mechanisms. Litter located in forest gaps was exposed to considerably higher daytime temperatures than litter under canopy cover, which likely resulted in higher rates of thermal degradation (Lee et al. 2012). Those higher temperatures in forest gaps were most probably caused by higher irradiance. Thus, we can assume that litter in gaps experienced more photodegradation than litter under canopy cover. Our choice of microsites (forest gaps/canopy cover) were justified by the radiation measurements, which showed that across both forests solar radiation is either mostly blocked or mostly transmitted, with little area exposed to medium levels of radiation (Figs. S1, S2). We expected to find lower minimum temperatures in forest gaps due to greater nighttime heat loss which can lead to higher dew deposition rates and decay (Gliksman et al. 2017). This pattern in temperature was present in the dry sub-humid forest, but the opposite trend was present in the semi-arid forest, which makes the prediction of the extent of nighttime microbial degradation challenging.
At the end of the experiments, the trend of higher decomposition rates in forest gaps than under canopy cover remained (greater by 1.25 times on average over all litter types and experiments after the second wet season), but in this case, multiple processes can lead to higher decay in forest gaps. First, also the wet seasons include long dry periods in which the non-rainfall decay mechanisms can operate and enhance decay in forest gaps. More importantly, enhanced exposure to solar radiation in forest gaps during the dry seasons can lead to photopriming of decay during wet periods by causing changes in the size and composition of microbial populations, and by altering litter chemistry and structure through breakdown of recalcitrant materials, such as lignin (Henry et al. 2008; Smith et al. 2010; Austin and Ballaré 2010; Barnes et al. 2015; Austin et al. 2016). Finally, during rain events the canopy reduces the amount of rain reaching the ground and the litter (Llorens and Domingo 2007), thus potentially reducing decay under canopy cover compared to forest gaps. Our results also show that reduced tree density led to increased decay, and while the overall effect was small, it was in agreement with the findings that reduced canopy cover enhances decay. Furthermore, the limited effect of the tree density on decay in relation to the stronger effect of canopy cover indicates that the local microclimate created even by a single tree (commonly occurring in lower tree densities) is more influential than microclimate dictated by the plot density (Bernaschini et al. 2016). Incorporating such a mosaic of microclimatic patterns into spatially explicit models can enhance our predictions for litter decay.
An important difference in the effect of canopy cover on decay was present when comparing the forests and their MAP. The dry sub-humid forest exhibited much less differentiation in decay between microsites than the semi-arid forest. A similar pattern was observed under relatively low annual precipitation (MAP of 249–600 mm) when reduction in canopy cover tended to enhance litter decay following thinning and clear-cut treatments (Bates et al. 2007; Mlambo and Mwenje 2010; but see Lado-Monserrat et al. 2015). However, with the increase in MAP (750–912 mm) this effect was reversed and higher canopy cover was related to greater decay rates (Cortina and Vallejo 1994; Blanco et al. 2011). In our study, both forests in both seasons were below 600 mm MAP and litter decay increased with the decrease in canopy cover, but to a lower degree in the dry sub-humid forest than the semi-arid forest. It appears that there may be a MAP threshold between 600 and 750 mm beyond which the influence of canopy cover on decay is reversed. The cause for such a threshold may be that with the reduction in precipitation there is an overall reduction in decay but an increase in the relative contribution of the non-rainfall decay mechanisms in areas with lower canopy cover.
Our second key result was that the monthly decay rate occurring in both dry seasons was stable and similar, contrasting with our hypothesis, while rates strongly declined from the first to the second wet season as we predicted. Therefore, the decay during dry seasons did not follow the classic diminishing decay rate over time (Cornwell and Weedon 2014), and its contribution to the annual litter decay increased over the experimental period in relation to the declining contribution of the wet season. Thus, we can support the suggestion that the higher than predicted decay rates in drylands are caused by mechanisms operating during rainless periods (Parton et al. 2007; Adair et al. 2008; Bonan et al. 2013).
The stability of decay during the dry seasons may stem from the co-occurrence of non-rainfall decay drivers. In the dry season, photochemical, thermal and microbial degradation alternate and interact on a daily basis (Gliksman et al. 2017). Photodegradation and thermal degradation operate during daytime and are efficient in degrading litter fractions that are recalcitrant for microorganisms (Austin and Ballaré 2010; Lee et al. 2012). Then, following absorption of water vapor and dew by litter at night, microorganisms can consume the labile fraction (which is likely hydrophilic) with greater ease, exposing recalcitrant tissues for the abiotic decay drivers operating at day. Combined, alternating degradation of different litter constituents can lead to mutual facilitations that maintain the decay during the dry seasons at a similar pace (Gliksman et al. 2017). The decline in decay in the wet seasons on the other hand can be attributed to the observed reduction in precipitation from the first to the second year of the experiment in both forests, and to an increase in the concentration of recalcitrant fractions (Berg and McClaugherty 2008). In both analyses of consecutive dry or wet seasons, we did not find a relation to the microsite, thus it is likely that these dynamics are not influenced by the difference in microclimate between the microsites.
Climate change projections for the Mediterranean Basin include a reduction in precipitation amounts, and an increase in mean air temperature, the frequency of high temperature extremes and drought events, evapotranspiration rates, and the length of the dry season (Diffenbaugh et al. 2007; Giorgi and Lionello 2008; Giannakopoulos et al. 2009; Fischer and Schär 2010; IPCC 2013). According to these projections, we expect that the dry-season contribution to litter decay will increase in relation to that of the wet season. For example, with longer dry periods and higher temperatures the dry season abiotic degradation will likely increase. In our study, the combined decay during the dry seasons contributed 30% to the overall mass loss, confirming our hypothesis of a significant contribution of the rainless period to the annual mass loss. However, this value is likely below the actual contribution of the non-rainfall decay mechanisms because most days in the wet season are rainless (23% and 12% rainy days during the wet seasons in the dry sub-humid and semi-arid forest, respectively). Since most of the rainy days were clustered, we can assume that during most of the wet season the litter was actually dry. Furthermore, even when we added 3–4 wet days as the expected drying period following each rain event (Raison et al. 1986; Harpole and Haas 1999; applied here for days with >1 mm of rain), there was only a minor change to the percentage of days in which the litter was wet. Finally, while we did not account for possible legacy effects, other studies showed that the dry season can facilitate most of the wet season mass loss through photopriming, as an additional important contribution to the annual mass loss (Henry et al. 2008; Gallo et al. 2009; Baker and Allison 2015). Combined, the dry season is likely to dominate the annual litter mass loss in the Mediterranean climate zone, and possibly in other regions, with low precipitation.
Harsher climate conditions can also affect the canopy cover and litter decomposition through changes in the vegetation composition, and may pose a positive feedback on litter decay during rainless periods. The changing climate is predicted to increase tree mortality in the Mediterranean Basin and in other locations world-wide (Allen et al. 2015), which may enhance litter decay according to our results. However, other factors may attenuate the reduction in canopy cover, such as woody encroachment (Hickler et al. 2012; D’Odorico et al. 2013) and the global increase in CO2 levels, which can enhance tree growth and canopy cover (Donohue et al. 2013; Zhu et al. 2016). Furthermore, litter production may also vary, with a decrease in tree density which can reduce tree litter production (Caldentey et al. 2001), but can increase the herbaceous biomass production (Bates et al. 2000; Malkisnon et al. 2011). Since our results show that grass decomposes faster than pine litter under all conditions (both experiments), there can be a substantial increase in the turnover of litter with the increase of the grass fraction. Furthermore, it should be taken into account that litter quality can also play an important role when the vegetation composition changes. In the common garden experiment, we observed higher mass loss in the forest gap for grass litter from the semi-arid forest than grass litter from the dry sub-humid forest, likely due to lower initial C:N in the grass from the semi-arid forest (Table 1 and Fig. 3).
In conclusion, under climate scenarios that are currently unfolding, the dry season will have a greater role in controlling litter turnover in drylands. It is important to incorporate not only the non-rainfall decay mechanisms into our prediction efforts, but also shifts in vegetation cover, which may enhance the contribution of these decay mechanisms. Integration of these factors into our modelling efforts can reduce substantially the uncertainty in the predictions of carbon cycling in drylands.
Abbreviations
- C:
-
Carbon
- DBH:
-
Stem diameter at breast height
- LAI:
-
Leaf area index
- MAP:
-
Mean annual precipitation
- N:
-
Nitrogen
- RH:
-
Relative humidity
References
Adair EC, Parton WJ, Del Grosso SJ et al (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Chang Biol 14:2636–2660. doi:10.1111/j.1365-2486.2008.01674.x
Ahlström A, Raupach MR, Schurgers G et al (2015) The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 348:895–899. doi:10.1126/science.aaa1668
Allen CD, Breshears DD, McDowell NG (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6:1–55. doi:10.1890/ES15-00203.1
Allen CD, Macalady AK, Chenchouni H et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684. doi:10.1016/j.foreco.2009.09.001
Araujo PI, Austin AT (2015) A shady business: pine afforestation alters the primary controls on litter decomposition along a precipitation gradient in Patagonia, Argentina. J Ecol 103:1408–1420. doi:10.1111/1365-2745.12433
Austin AT, Ballaré CL (2010) Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci U S A 107:4618–4622. doi:10.1073/pnas.0909396107
Austin AT, Méndez MS, Ballaré CL (2016) Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems. Proc Natl Acad Sci 113:4392–4397. doi:10.1073/pnas.1516157113
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558. doi:10.1038/nature05038
Baier W (1966) Studies on dew formation under semi-arid conditions. Agric Meteorol 3:103–112. doi:10.1126/science.146.3651.1601
Baker NR, Allison SD (2015) Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate. Ecology 96:1994–2003. doi:10.1890/14-1482.1
Barnes PW, Throop HL, Archer SR et al (2015) Sunlight and soil–litter mixing: drivers of litter decomposition in drylands. Prog Bot 76:273–302. doi:10.1007/978-3-319-08807-5
Bates JD, Miller RF, Svejcar TJ (2000) Understory dynamics in cut and uncut western juniper woodlands. J Range Manag 53:119–126. doi:10.2307/4003402
Bates JD, Svejcar TS, Miller RF (2007) Litter decomposition in cut and uncut western juniper woodlands. J Arid Environ 70:222–236. doi:10.1016/j.jaridenv.2006.12.015
Berg B, McClaugherty C (2008) Plant litter: decomposition, humus formation, carbon sequestration, 2nd edn. Springer Verlag, Berlin
Bernaschini ML, Moreno ML, Pérez-Harguindeguy N, Valladares G (2016) Is litter decomposition influenced by forest size and invertebrate detritivores during the dry season in semiarid Chaco Serrano? J Arid Environ 127:154–159. doi:10.1016/j.jaridenv.2015.11.009
Blanco JA, Imbert JB, Castillo FJ (2011) Thinning affects Pinus sylvestris needle decomposition rates and chemistry differently depending on site conditions. Biogeochemistry 106:397–414. doi:10.1007/s10533-010-9518-2
Bonan GB, Hartman MD, Parton WJ, Wieder WR (2013) Evaluating litter decomposition in earth system models with long-term litterbag experiments: an example using the community land model version 4 (CLM4). Glob Chang Biol 19:957–974. doi:10.1111/gcb.12031
Caldentey J, Ibarra M, Hernández J (2001) Litter fluxes and decomposition in Nothofagus pumilio stands in the region of Magallanes, Chile. For Ecol Manag 148:145–157. doi:10.1016/S0378-1127(00)00532-6
Calev A, Zoref C, Tzukerman M et al (2016) High-intensity thinning treatments in mature Pinus halepensis plantations experiencing prolonged drought. Eur J For Res 135:551–563. doi:10.1007/s10342-016-0954-y
Carvalhais N, Forkel M, Khomik M et al (2014) Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 514:213–217. doi:10.1038/nature13731
Chen M, Parton WJ, Adair EC et al (2016) Simulation of the effects of photodecay on long-term litter decay using DayCent. Ecosphere 7:e01631. doi:10.1002/ecs2.1631
Cornwell WK, Weedon JT (2014) Decomposition trajectories of diverse litter types: a model selection analysis. Methods Ecol Evol 5:173–182. doi:10.1111/2041-210X.12138
Cortina J, Vallejo VR (1994) Effects of clearfelling on forest floor accumulation and litter decomposition in a radiata pine plantation. For Ecol Manag 70:299–310. doi:10.1016/0378-1127(94)90095-7
D’Odorico P, Bhattachan A, Davis KF et al (2013) Global desertification: drivers and feedbacks. Adv Water Resour 51:326–344. doi:10.1016/j.advwatres.2012.01.013
Davidson EA, de Araújo AC, Artaxo P et al (2012) The Amazon basin in transition. Nature 481:321–328. doi:10.1038/nature10717
Day TA, Guénon R, Ruhland CT (2015) Photodegradation of plant litter in the Sonoran Desert varies by litter type and age. Soil Biol Biochem 89:109–122. doi:10.1016/j.soilbio.2015.06.029
Diffenbaugh NS, Pal JS, Giorgi F, Gao X (2007) Heat stress intensification in the Mediterranean climate change hotspot. Geophys Res Lett 34:L11706. doi:10.1029/2007GL030000
Dirks I, Navon Y, Kanas D et al (2010) Atmospheric water vapor as driver of litter decomposition in Mediterranean shrubland and grassland during rainless seasons. Glob Chang Biol 16:2799–2812. doi:10.1111/j.1365-2486.2010.02172.x
Donohue RJ, Roderick ML, McVicar TR, Farquhar GD (2013) Impact of CO2 fertilization on maximum foliage cover across the globe’s warm, arid environments. Geophys Res Lett 40:3031–3035. doi:10.1002/grl.50563
Fischer EM, Schär C (2010) Consistent geographical patterns of changes in high-impact European heatwaves. Nat Geosci 3:398–403. doi:10.1038/ngeo866
Foereid B, Rivero MJ, Primo O, Ortiz I (2011) Modelling photodegradation in the global carbon cycle. Soil Biol Biochem 43:1383–1386. doi:10.1016/j.soilbio.2011.03.004
Gallo ME, Porras-Alfaro A, Odenbach KJ, Sinsabaugh RL (2009) Photoacceleration of plant litter decomposition in an arid environment. Soil Biol Biochem 41:1433–1441. doi:10.1016/j.soilbio.2009.03.025
Giannakopoulos C, Le Sager P, Bindi M et al (2009) Climatic changes and associated impacts in the Mediterranean resulting from a 2°C global warming. Glob Planet Change 68:209–224. doi:10.1016/j.gloplacha.2009.06.001
Giorgi F, Lionello P (2008) Climate change projections for the Mediterranean region. Glob Planet Change 63:90–104. doi:10.1016/j.gloplacha.2007.09.005
Gliksman D, Rey A, Seligmann R et al (2017) Biotic degradation at night, abiotic degradation at day: positive feedbacks on litter decomposition in drylands. Glob Chang Biol 23:1564–1574. doi:10.1111/gcb.13465
Harpole DN, Haas CA (1999) Effects of seven silvicultural treatments on terrestrial salamanders. For Ecol Manag 114:349–356. doi:10.1016/S0378-1127(98)00365-X
Henry HAL, Brizgys K, Field CB (2008) Litter decomposition in a California annual grassland: interactions between photodegradation and litter layer thickness. Ecosystems 11:545–554. doi:10.1007/s10021-008-9141-4
Hickler T, Vohland K, Feehan J et al (2012) Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Glob Ecol Biogeogr 21:50–63. doi:10.1111/j.1466-8238.2010.00613.x
IPCC (2013) Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Contribution of WGI to the 5th assessment report of the IPCC. Cambridge University Press, Cambridge
Jacobs AFG, Van Pul WAJ, Van Dijken A (1990) Similarity moisture dew profiles within a corn canopy. J Appl Meteorol 29:1300–1306. doi:10.1175/1520-0450(1990)029<1300:SMDPWA>2.0.CO;2
Jacobson K, Van Diepeningen A, Evans S et al (2015) Non-rainfall moisture activates fungal decomposition of surface litter in the Namib Sand Sea. PLoS One 10:e0126977. doi:10.1371/journal.pone.0126977
King JY, Brandt LA, Adair EC (2012) Shedding light on plant litter decomposition: advances, implications and new directions in understanding the role of photodegradation. Biogeochemistry 111:57–81. doi:10.1007/s10533-012-9737-9
Klein T, Rotenberg E, Cohen-Hilaleh E et al (2014) Quantifying transpirable soil water and its relations to tree water use dynamics in a water-limited pine forest. Ecohydrology 7:409–419. doi:10.1002/eco.1360
Lado-Monserrat L, Lidón A, Bautista I (2015) Erratum to: Litterfall, litter decomposition and associated nutrient fluxes in Pinus halepensis: influence of tree removal intensity in a Mediterranean forest. Eur J For Res 134:833–844. doi:10.1007/s10342-015-0923-x
Lee H, Rahn T, Throop H (2012) An accounting of C-based trace gas release during abiotic plant litter degradation. Glob Chang Biol 18:1185–1195. doi:10.1111/j.1365-2486.2011.02579.x
Llorens P, Domingo F (2007) Rainfall partitioning by vegetation under Mediterranean conditions. A review of studies in Europe J Hydrol 335:37–54. doi:10.1016/j.jhydrol.2006.10.032
Luo H, Oechel WC, Hastings SJ et al (2007) Mature semiarid chaparral ecosystems can be a significant sink for atmospheric carbon dioxide. Glob Chang Biol 13:386–396. doi:10.1111/j.1365-2486.2006.01299.x
Malkisnon D, Wittenberg L, Beeri O, Barzilai R (2011) Effects of repeated fires on the structure, composition, and dynamics of Mediterranean maquis: short- and long-term perspectives. Ecosystems 14:478–488. doi:10.1007/s10021-011-9424-z
Mlambo D, Mwenje E (2010) Influence of Colophospermum mopane canopy cover on litter decomposition and nutrient dynamics in a semi-arid African savannah. Afr J Ecol 48:1021–1029. doi:10.1111/j.1365-2028.2010.01208.x
Moorhead DL, Callaghan T (1994) Effects of increasing ultraviolet B radiation on decomposition and soil organic matter dynamics: a synthesis and modelling study. Biol Fertil Soils 18:19–26. doi:10.1007/BF00336439
Pan Y, Wang X, Zhang Y (2010) Dew formation characteristics in a revegetation-stabilized desert ecosystem in Shapotou area, northern China. J Hydrol 387:265–272. doi:10.1016/j.jhydrol.2010.04.016
Pancotto VA, Sala OE, Cabello M et al (2003) Solar UV-B decreases decomposition in herbaceous plant litter in Tierra del Fuego, Argentina: potential role of an altered decomposer community. Glob Chang Biol 9:1465–1474. doi:10.1046/j.1365-2486.2003.00667.x
Parton W, Silver WL, Burke IC et al (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364. doi:10.1126/science.1134853
Poulter B, Frank D, Ciais P et al (2014) Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 509:600–603. doi:10.1038/nature13376
Raison RJ, Woods PV, Khanna PK (1986) Decomposition and accumulation of litter after fire in sub-alpine eucalypt forests. Aust J Ecol 11:9–19. doi:10.1111/j.1442-9993.1986.tb00913.x
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225. doi:10.2307/2409177
Rutledge S, Campbell DI, Baldocchi D, Schipper LA (2010) Photodegradation leads to increased carbon dioxide losses from terrestrial organic matter. Glob Chang Biol 16:3065–3074. doi:10.1111/j.1365-2486.2009.02149.x
Safriel U, Adeel Z, Niemeijer D, Puigdefabres J, White R, Lal R et al (2005) Dryland systems. In: Hassan R, Scholes R, Ash N (eds) Ecosystems and human well-being: current state and trends, Vol. 1. Island Press, Washington, DC, pp 623–662
Smith WK, Gao W, Steltzer H et al (2010) Moisture availability influences the effect of ultraviolet-B radiation on leaf litter decomposition. Glob Chang Biol 16:484–495. doi:10.1111/j.1365-2486.2009.01973.x
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley
Tatarinov F, Rotenberg E, Maseyk K et al (2016) Resilience to seasonal heat wave episodes in a Mediterranean pine forest. New Phytol 210:485–496. doi:10.1111/nph.13791
Throop HL, Archer SR (2009) Resolving the dryland decomposition conundrum: some new perspectives on potential drivers. Prog Bot 70:171–194. doi:10.1007/978-3-540-68421-3_8
Van Asperen H, Warneke T, Sabbatini S et al (2015) The role of photo- and thermal degradation for CO2 and CO fluxes in an arid ecosystem. Biogeosci Discuss 12:2429–2457. doi:10.5194/bgd-12-2429-2015
Wohlfahrt G, Fenstermaker LF, Arnone JA III (2008) Large annual net ecosystem CO2 uptake of a Mojave Desert ecosystem. Glob Chang Biol 14:1475–1487. doi:10.1111/j.1365-2486.2008.01593.x
Xiao H, Meissner R, Seeger J et al (2009) Effect of vegetation type and growth stage on dewfall, determined with high precision weighing lysimeters at a site in northern Germany. J Hydrol 377:43–49. doi:10.1016/j.jhydrol.2009.08.006
Zhu Z, Piao S, Myneni RB et al (2016) Greening of the earth and its drivers. Nat Clim Chang 6:791–795. doi:10.1038/NCLIMATE3004
Acknowledgements
We acknowledge the support of Rita Dumbur, Avner Zinger, Ken Karu, Meron Berniker, Mor Ashkenazi, Ohad Abramovich, Nili Brukental, Nitsan Amiti, Sivan Ben Ari, Yuki Nahmias and Yossi Haddad for field and lab assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research project was financially supported by Keren Kayemet Leyisrael - Jewish National Fund (KKL).
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Responsible Editor: Fernando T. Maestre.
Electronic supplementary material
ESM 1
(DOCX 722 kb)
Rights and permissions
About this article
Cite this article
Gliksman, D., Haenel, S., Osem, Y. et al. Litter decomposition in Mediterranean pine forests is enhanced by reduced canopy cover. Plant Soil 422, 317–329 (2018). https://doi.org/10.1007/s11104-017-3366-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3366-y