Abstract
Aims
The purpose of this research was to compare root exudation of major organic components, effects of PGPR on root exudates and the response of different soybean varieties to inoculation with PGPR to understand variety-dependent relationships between these traits.
Methods
Growth and root exudation of soybean varieties Nice-Mecha, Bara and Svapa in the absence and presence of PGPR Pseudomonas oryzihabitans Ep4 was studied using gnotobiotic hydroponic system and UPLC techniques. Rhizobacterial effects on growth, seed yield quality, nodulation by Bradyrhizobium japonicum 634b and uptake of nutrients was investigated under field conditions.
Results
Genotypic differences between soybean varieties in root exudation, rhizobacterial effects on exudation and interactions with Ps. oryzihabitans Ep4 were revealed. Variety Bara had greatest root biomass with least root exudation and least Ps. oryzihabitans Ep4 colonisation. In both hydroponic experiments and field trials, the varieties Nice-Mecha and particularly Svapa responded more actively to Ps. oryzihabitans Ep4 than the variety Bara. Several mechanisms related to root exudation rate, exudate composition, bacterial production of siderophores and auxins are proposed to explain variety dependent interactions of plants with PGPR.
Conclusions
The variety specific plant response to PGPR is mediated by genotypic differences in root exudation and the ability of PGPR to metabolize and/or transform the exuded organic acids, sugars and amino acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant growth-promoting rhizobacteria (PGPR) influence plant growth, nutrition, and adaptation to unfavorable climate and soil conditions via multiple mechanisms including nitrogen fixation, production or destruction of biologically active substances, mobilization of nutrient elements in the rhizosphere, induction of systemic resistance and biocontrol of phytopathogens (Lugtenberg et al. 2001; Vessey 2003; Bashan et al. 2004; Glick et al. 2007; Dodd et al. 2010; Maheshwari 2010; Vacheron et al. 2013; Duca et al. 2014; Nascimento et al. 2014). Although responses of various agricultural crops and other plant species to inoculation with different bacterial strains was intensively studied, intra-species variation in responses to a single PGPR strain has received little attention (Smith and Goodman 1999). In particular, only one of two potato varieties responded positively to inoculation with Pseudomonas oryzihabitans Ep4 increasing root biomass of germinated tubers by 51% (Belimov et al. 2015a). Safronova et al. (2006) described different effects of Ps. brassicacearum Am3 and Ps. marginalis Dp1 on uptake of nutrients (N, P, K, Cf, S and Fe) by four pea varieties from metal contaminated soil. Significant difference in growth response to inoculation with Azospirillum brasilense was also reported for sorghum (Das et al. 1997), wheat (Saubidet and Barneix 1998) and common bean (Remans et al. 2008) varieties, as well as to inoculation with Azospirillum lipoferum of maize varieties (Walker et al. 2011). Variety specific effects of three PGPR strains, namely Az. lipoferum 137, Agrobacterium radiobacter 10 or Arthrobacter mysorens 7, and yield and nitrogen uptake by barley (Belimov et al. 1995a, 1995b) and yield, titratable acidity and sugar content in tomato fruits (Belimov et al. 1995b) were described. In a pot experiment with 11 varieties of Indian mustard, the plant growth-promoting effect of Variovorax paradoxus negatively correlated with Cd tolerance and shoot Cd concentration of the plants grown in Cd-supplemented soil (Belimov et al. 2015b). Inoculation with Az. lipoferum revealed differences in two rice varieties in expression of genes related to auxin and ethylene signaling (Drogue et al. 2014). Several reports showed significant (3–50-fold) variation in root colonizing ability of PGPR depending on plant variety (Belimov et al. 1995b; Saubidet and Barneix 1998; Okubara et al. 2004). However the reasons for intraspecies variation in such plant-bacteria interactions are scarcely understood.
PGPR stimulate the formation and development of nitrogen-fixing symbiosis between legume plants and nodule bacteria (reviewed by Belimov and Kozhemiakov 1992; Vessey 2003; Safronova et al. 2011; Pérez-Montaño et al., 2014). In soybeans, combined inoculations with the nodule-forming Bradyrhizobium japonicum and the PGPR Pseudomonas fluorescens (Polonenko et al. 1987; Zhang et al. 1996; Chebotar et al. 2001), Az. brasilense (Hungria et al. 2013) or Bacillus subtilis (Bai et al. 2002; Atieno et al. 2012; Masciarelli et al. 2014) increased nodulation and biomass production. The rhizobacterial enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase plays an important role in this symbiotic interaction by decreasing root ethylene biosynthesis and alleviating its negative effect on nodulation, particularly under environmental stress conditions (Belimov et al. 2009, 2012; Belimov and Safronova 2011; Ahmad et al. 2011; Kong et al. 2016; Gamalero and Glick 2015; Nascimento et al. 2016). Furthermore, auxin-producing PGPR also stimulate nodule formation of various legume crops (Remans et al. 2008; Masciarelli et al. 2014; Pérez-Montaño et al., 2014). Cultivar variation in beneficial effects of PGPR on legume-rhizobia symbiosis were described in soybean (Dashti et al. 1998; Atieno et al. 2012), pea (Belimov et al. 2012) and chickpea (Imran et al. 2015), however mechanisms explaining the observed variations remain unknown.
Plant roots exude various organic compounds such as organic acids, sugars and amino acids to the rhizosphere. These substances serve as an important nutrient source for microorganisms and thereby modulate composition, activity and functions of rhizosphere microbial communities (reviewed by Bais et al. 2006; Badri et al. 2009; Dennis et al. 2010; Bakker et al. 2012). Significant intraspecies variation in exudation of organic acids was found in sorghum (Krotzky et al., 1988), barley (Gahoonia et al. 2000), maize (Gaume et al. 2001), rice (Yang et al. 2007) and pea (Kuzmicheva et al. 2014). Differences in exudation of sugars and amino acids were also reported for maize and mung bean (Singh et al. 2009), peanut (Li et al. 2013), pea (Kuzmicheva et al. 2014) and wheat (Shaposhnikov et al. 2016). Nevertheless, relationships between intraspecies variability in root exudation and response of varieties to inoculation with PGPR are scarcely described. High root exudation of malate and succinate, which favour proliferation of the associative nitrogen fixers Azospirillum (Dobereiner 1980), by Sorghum nutans cv. CSV was associated with high nitrogen fixation activity in the rhizosphere (Krotzky et al., 1988). High symbiotic potential of the new pea variety Triumph was associated with active exudation of succinate and pyruvate, traits inherited from its parental primitive variety k_8274 which had prominent efficiency of symbiosis with nodule bacteria and arbuscular mycorrhizal fungi (Kuzmicheva et al. 2014). The peanut variety Ganhua-5, which was susceptible to the soil-borne pathogens Fusarium oxysporum and F. solani, exuded more sugars and amino acids but less phenolic acids than the resistant variety Quanhua-7 (Li et al. 2013). Moreover, Arabidopsis thaliana mutant abcg30 (Atpdr2) with decreased exudation of sugars but increased exudation of lactate and indole-3-acetic acid had relatively greater abundance of potential PGPR in the rhizosphere (Badri et al. 2009). These few reports indciate that varietal differences in root exudation make a significant contribution to interactions with PGPR and deserve more detailed study.
Soybean is an important legume crop and significantly depends on symbiosis with microorganisms such as nodule bacteria and PGPR. The expansion of the area of soybean cultivation envisages the creation of varieties resistant to low temperatures and relatively nutrient-poor soils. In Russia, the most northern area of soybean production is the Orel region for which the early-ripening varieties of the northern ecotypes Nice-Mecha, Bara and Svapa have recently been selected. However, little is known about their symbiotic peculiarities and potential, although it is expected that PGPR containing ACC deaminase and producing auxins should promote better adaptation of plants to such soil and climatic conditions. This report aimed to compare these soybean varieties with respect to root exudation and response to inoculation with PGPR to better understand relationships between variety-dependent rhizobacterial effects on root exudation and plant growth promotion.
Materials and methods
Plants and bacteria
Super elite seeds of early-ripening soybean (Glycine max (L.) Merr.) varieties Nice-Mecha and Svapa were obtained from The All-Russia Research Institute of Legumes and Groat Crops (Orel, Russian Federation, http://www.vniizbk.ru/en.html) and variety Bara was obtained from Ltd. “Soy Complex” (Krasnodar, Russian Federation, http://www.co-ko.ru/). Strain of ACC-utilizing and auxin-producing bacteria Pseudomonas oryzihabitans Ep4 (Belimov et al. 2001) and its derivative strain Ep4gfp marked with the gene encoding green fluorescent protein (Suarez et al. 1997) and described previously (Belimov et al. 2015a) were obtained from the Russian Collection of Agricultural Microorganisms (St.-Petersburg, Russian Federation, http://www.arriam.ru/kollekciya-kul-tur1/). Bacteria were maintained on agar Bacto-Pseudomonas F (BPF) medium (Glick et al. 1995) supplemented with antibiotics (20 μg ml−1 rifampicin, 30 μg ml−1 kanamycin and 15 μg ml−1 gentamycin) to maintain strain Ep4gfp. To prepare inoculum of Ps. oryzihabitans Ep4 for the field experiment, the bacteria were cultivated in liquid BPF medium for 4 days and the obtained suspension was diluted with sterile tap water to a final concentration 107 cells ml−1. A commercial bio-preparation Rhizotorfin produced by the company ECOS (St-Petersburg, Russia, http://ekosspb.ru/o_predpriyatii/) and consisting of sterile peat as a carrier and the nodule bacterium Bradyrhizobium japonicum strain 634b (109 cells g−1 peat) was also used in the field experiment. Siderophore production by Ps. oryzihabitans Ep4 and Ep4gfp was determined using a chrome azurol S (CAS) shuttle solution as described by Schwyn and Neilands (1987) and the assay was calibrated using deferoxamine mesylate (DFM). The protein concentration of cell suspensions was determined by the method of Bradford (1976).
Utilization of components of root exudates by bacteria in vitro
Strain Ps. oryzihabitans Ep4 and its derivative Ep4gfp were cultivated at 28 °C in test tubes containing 5 ml liquid MSMN medium (Belimov et al. 2005) supplemented with 5 g l−1 of organic acids (acetic, citric, fumaric, lactic, malic, propionic, pyroglutamic, pyruvic, succinic) or sugars (fructose, glucose, ribose, xylose) as a sole carbon source. Utilization of amino acids by Ps. oryzihabitans Ep4 as a sole source of carbon or nitrogen in batch culture was studied previously (Belimov et al. 2015a). Here we performed similar experiments with derivative strain Ep4gfp on utilization of all proteinogenic amino acids and several non-proteinogenic amino acids (ACC, β-alanine, α-aminobutyric acid, β-aminobutyric acid, γ-aminobutyric acid, N-butyryl-DL-homoserine lactone, L-canavanine, L-citrulline, dopamine, DL-homoserine, D-glucosamine, L-mimosine, L-ornithine, serotonin) which have relation to plant-microbe interactions (Vranova et al. 2011). The ability of bacteria to utilize these substances and to grow was monitored visually at the 5th day, by comparing turbidity with the inoculated medium containing no carbon source.
Hydroponic experiments
Seeds were surface sterilized and scarified by treatment with 0.1% HgCl2 for 10 min and then 5% NaOCl for 8 min, rinsed thoroughly with sterile tap water and germinated on sterile filter paper in Petri dishes for two days at 25 °C in the dark. The uniformly germinated seedlings were transferred to polypropylene pots OS140BOX (Duchefa, Netherlands). Four pots with 5 seedlings each were prepared per variety and treatment and filled with 200 ml nutrient solution (μM): KH2PO4, 400; KNO3, 1200; Ca(NO3)2, 60; MgSO4, 250; KCl, 250; CaCl2, 60; Fe-tartrate, 12; H3BO3, 2; MnSO4, 1; ZnSO4, 3; NaCl, 6; Na2MoO4, 0.06; CoCl2, 0,06; CuCl2, 0.06; NiCl2, 0,06. Overnight culture of Ps. oryzihabitans Ep4gfp was prepared, washed with sterile water by centrifugation at 9000 g for 5 min and the nutrient solution was supplemented with bacteria in a final concentration 5 × 105 cells ml−1. Unsupplemented solution was used as a control treatment. Plants were cultivated for 10 days in a growth chamber ADAPTIS-A1000 (Conviron, UK) with 200 μmol quanta m−2 s−1, 12 h photoperiod and minima/maxima temperatures of 18 °C/23 °C respectively. Then plants were removed from pots and nutrient solution collected to determine root exudates. The presence of GFP-tagged bacteria on roots was visualized using a fluorescent microscope Axio Imager A2 (Carl Zeiss, Germany). To determine root colonization by P. oryzihabitans Ep4gfp, root samples (3 replicates per treatment) were homogenized in sterile tap water with sterile mortar and pestle. Homogenates were serially diluted in 10-fold steps and 50 μl aliquots were plated in two replicates on BPF agar medium with and without antibiotics. Aliquots of diluted nutrient solution were also plated in similar way. Characteristic colonies of the introduced strain, which had green fluorescence under UV light, were counted after incubation at 30 °C for 4 days. The presence of colonies without fluorescence (contamination) was monitored in all inoculated and control samples. The data were expressed as the number of colony forming units (CFU) per mg of root dry weight (DW). Then roots and shoots were dried at 50 °C and DW determined. Experiments for each soybean variety were repeated three times.
Determination of root exudates
On harvest day, the nutrient solution (containing root exudates) was centrifuged (Model 5804R, Eppendorf, USA) for 10 min at 4500 g, vacuum filtered through 0.45 μm filters (Corning, Germany) and concentrated at 45 °C using a rotary vacuum evaporator BUCHI R-200 (BUCHI, Switzerland). The concentrates were passed through a column of ion exchange resin DOWEX 50Wx8 resulting in 2 fractions: (1) sugars and organic acids; (2) amino acids. The obtained fractions were vacuum evaporated to dryness and dissolved in 0.5 mL of deionized water for subsequent chromatographic analysis using the UPLC system Waters ACQUITY H-Class (Waters, USA). Sugars were separated on column SUPELCOSIL LC-NH2 (Supelco Gland, Switzerland) and determined using refractometric detector Waters-2414. Amino acids were analysed by the Waters AccQ-Tag method as described by the manufacturer. Organic acids were separated on column ACQUITY CSH C18 (Waters, USA) and determined using UV detector at 210 nm. The content of L-tryptophan was analyzed in the same samples, but without treatment with ACQ reagent, using column Waters UPLC RP-18 Shield and a fluorescent detector. Standards comprised sugars, organic acids, tryptophan and non-proteinogenic amino acids from analytical grade reagents of Sigma-Aldrich (USA), as well as proteinogenic amino acids from Amino Acid Hydrolysate Standard H (L enantiomers, Thermo Fisher Scientific Inc., USA).
Field experiment
This was performed at the experimental field of the Orel State Agrarian University named after N.V. Parahin (village Lavrovo, Orel region, 2013) in a dark gray forest soil having the following characteristics determined by standard methods as described by Arinushkina (1970): total carbon, 3.8%; available N, 42 mg kg−1; available P, 129 mg kg−1; available K, 159 mg kg−1; pHKCl = 5.0. Mean air temperature (°C) and total rainfall (mm) were respectively: May, 18.0 and 21.4; June, 19.8 and 22.8; July, 18.8 and 16.5; August, 19.0 and 11.1; September, 10.6 and 36.2. Farming system was of the seven-grain crop rotation type with fallow as the precursor. On May 15, seeds were treated with fungicide fludioxonil, to which the studied bacterial strains showed resistance at applied concentrations, inoculated with Rhizotorfin (strain B. japonicum 634b) in the amount of 2 g kg−1 following manufacturer’s instructions and sown using a seed drill (Plotseed XL, Wintersteiger, Austria). Strain Ps. oryzihabitans Ep4 was introduced manually as a diluted bacterial suspension (107 cells ml−1) supplied at 200 ml m2 to the germinated seedlings (May 26) and at the 2–3 true leaf stage (June 12) stages just at the root-shoot junction. Four replicates (plots of 10 m2 in size with 70 seeds m2) were prepared for each treatment (uninoculated control, B. japonicum 634b, Ps. oryzihabitans Ep4, mixture of B. japonicum 634b and Ps. oryzihabitans Ep4) in a completely randomized design. Mineral fertilizers N10P26K26 were added 7 days before sowing. No irrigation was applied during crop growth.
At the flowering stage (July 10), chlorophyll a fluorescence emission by intact leaves was measured to determine quantum yield efficiency of photosystem II (ϕPSII) using a photosynthesis yield analyzer (Mini-PAM II, Walz, Germany) with at least 10 determinations for each variety and treatment. To determine nitrogen-fixing activity on roots, the acetylene reduction assay (Hardy et al. 1973) was applied. For this purpose the roots of 5 plants were collected from each plot and washed with tap water. After nodule counting, the roots were placed in 250 ml sealed bottles and incubated for 1 h in the presence of 10% C2H2 at 25 °C. The reaction was stopped by addition of 2.5% formalin solution and ethylene content was determined using FGH-1 gas chromatograph (Ltd ECAN, Russian Federation). Leaves from 15 plants from each plot were collected, dried and used for elemental analysis.
Two weeks before harvest the plants were desiccated by treatment with Reglon Super BP (Syngenta, Switzerland) and then (on September 25) seeds were harvested by combiner TERRION-SAMPO SR2010 (Agrotechmash, Russian Federation). The contents of protein and oil in seeds were determined using infrared grain analyzer Infratec™ 1241 (FOSS, Denmark) following manufacturer’s instructions.
Elemental analysis
The dry leaves were ground and digested in a mixture of concentrated HNO3 and 38% H2O2 at 70 °C using digestion system DigiBlock (LabTech, Italy). Contents of nutrient elements (B, Ca, Co, Cu, Fe, K, Mg, Mn, Mo, Ni, P, S and Zn) in digested plant samples were determined using an inductively coupled plasma emission spectrometer ICPE-9000 (Shimadzu, Japan) following manufacturer instructions. Total N in leaf samples was determined using a Kjeltec 2300 Auto distillation unit (FOSS Analytical, Denmark).
Statistical analysis
Statistical analysis of the data was performed and figures were designed using the software STATISTICA version 10 (StatSoft Inc., USA). Student’s t test and Fisher’s least significant difference (LSD) test (ANOVA) were applied to analyze differences between means.
Results
Strains Ps. oryzihabitans Ep4 and Ep4gfp grew actively in the presence of all organic acids and sugars tested (data not shown), with the only exception being poor growth in the presence of xylose. Abundant growth of strain Ps. oryzihabitans Ep4gfp was observed: (i) in the presence of alanine, β-alanine, γ-aminobutyric acid, asparagine, aspartic acid, L-citrulline, dopamine, glutamic acid, glutamine, glycine, histidine, leucine, lysine, L-ornithine, proline, threonine, tyrosine and valine both as C and N source; (ii) in the presence of isoleucine as a sole C source; (iii) in the presence of ACC, γ-aminobutyric acid, arginine, L-canavanine, cysteine, methionine and tryptophan as a sole N source (data not shown). Both strains Ps. oryzihabitans Ep4 and Ep4gfp produced siderophores in the amount of 75 ± 3 and 70 ± 4 μM DFM mg−1, respectively.
Ps. oryzihabitans Ep4gfp inoculation of varieties Nice-Mecha and Svapa grown in hydroponics stimulated root growth (Fig. 1a). At the end of the experiments, the number of introduced bacteria in nutrient solution and attached to the roots of these varieties was similar, but was about 5 and 25 times higher than the variety Bara (Fig. 1b). Microscopy observations showed that single cells and micro colonies (Fig. 2) of bacteria were present on intact roots. The pattern of root colonization by bacteria was similar for all varieties. No contamination with other microorganisms was observed in solution and on plant roots either by microscopy or on agar plates with BPF medium.
Root biomass of soybean varieties Nice-Mecha, Bara and Svapa (a) and the number of Ps. oryzihabitans Ep4gfp (Ep4) in the root zone (b) of inoculated plants cultivated in hydroponics. Data are means of 3 experiments with 1 replica each. Vertical bars show standard errors. Different letters show significant differences between treatments (least significant difference test, P < 0.05). DW stands for dry weight and CFU stands for colony forming unit
The qualitative composition of root exudates, such as organic acid anions (Fig. 3), sugars (Fig. 4) or proteinogenic amino acids (Fig. 5) detected in the control treatments (without bacteria) was similar for all varieties. The only exception was that succinate was not detected in exudates of variety Bara (Fig. 3). At the same time the amount of several substances exuded by the control plants significantly varied depending on variety. Among non-proteinogenic amino acids, only L-ornithine was detected (Fig. 5).
Effect of Ps. oryzihabitans Ep4gfp (Ep4) on concentration of organic acid anions exuded by roots of soybean varieties Nice-Mecha, Bara and Svapa cultivated in hydroponics. Data are means of 3 experiments with 1 replica each. Vertical bars show standard errors. Asterisks show significant effect of Ps. oryzihabitans Ep4 for each compound and cultivar (Student’s t test, P < 0.05), respectively. DW stands for dry weight
Effect of Ps. oryzihabitans Ep4gfp (Ep4) on concentration of sugars exuded by roots of soybean varieties Nice-Mecha, Bara and Svapa cultivated in hydroponics. Data are means of 3 experiments with 1 replica each. Vertical bars show standard errors. Asterisks show significant effect of Ps. oryzihabitans Ep4 for each compound and cultivar (Student’s t test, P < 0.05), respectively. DW stands for dry weight
Effect of Ps. oryzihabitans Ep4gfp on concentration of amino acids exuded by roots of soybean varieties Nice-Mecha, Bara and Svapa cultivated in hydroponics. Data are means of 3 experiments with 1 replica each. Vertical bars show standard errors. Asterisks show significant effect of Ps. oryzihabitans Ep4 for each compound and cultivar (Student’s t test, P < 0.05), respectively. DW stands for dry weight
Inoculation of varieties Nice-Mecha and Svapa with Ps. oryzihabitans Ep4gfp decreased content of all organic acid anions (Fig. 3) and sugars (Fig. 4) in the nutrient solution as compared to control treatments. Rhizobacterial inoculation of variety Bara had no significant effect on content of acetate, lactate, pyroglutamate and pyruvate, as well as on glucose and xylose contents. The bacteria decreased contents of many amino acids exuded by variety Nice-Mecha with exception of glycine, L-ornithine, proline, threonine and tyrosine (Fig. 5). Contents of all amino acids exuded by variety Svapa were decreased by inoculation. Contrary to this, only arginine, proline and serine contents decreased when variety Bara was inoculated with Ps. oryzihabitans Ep4gfp. Comparison of total contents of the studied root exudate fractions showed that variety Nice-Mecha exuded more organic acids and sugars, compared to other varieties, whereas Svapa exuded more sugars than Bara (Fig. 6a). This comparison also confirmed that the bacterial effects (expressed as percent changes related to uninoculated controls) were more pronounced for varieties Nice-Mecha and Svapa, as compared to Bara (Fig. 6b).
Total content of exudate fractions (a) and the effect of Ps. oryzihabitans Ep4gfp on concentration of root exudates (b) of soybean varieties Nice-Mecha, Bara and Svapa cultivated in hydroponics. Data are means of 3 experiments with 1 replica each. Vertical bars show standard errors. Different letters show significant differences between varieties for each exudate fraction (least significant difference test, P < 0.05). DW stands for dry weight
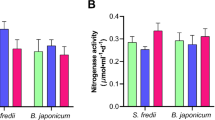
Under field conditions, application of nodule bacterium B. japonicum 634b (commercial bio-preparation Rhizotorfin) alone and in combination with Ps. oryzihabitans Ep4 increased leaf biomass at the flowering stage and seed yield at harvest in all soybean varieties (Table 1). These treatments both increased seed protein and oil contents of variety Nice-Mecha. The combined treatment also increased seed protein and oil contents of Svapa. Inoculation with single Ps. oryzihabitans Ep4 increased leaf biomass and seed protein content of Nice-Mecha, as well as leaf ϕPSII and seed yield of Svapa (Table 1). Observation of roots at flowering stage showed that all treatments with B. japonicum 634b induced formation of symbiotic nodules and co-inoculation with Ps. oryzihabitans Ep4 significantly stimulated nodulation on Svapa roots by 3.7 times (Table 1). Nitrogen fixation (acetylene reduction) activity on Nice-Mecha, Bara and Svapa roots treated with B. japonicum 634b was 2.1 ± 0.3, 0.5 ± 0.2 and 2.0 ± 0.1 μM C2H4 plant−1 h−1, respectively. Inoculation with Ps. oryzihabitans Ep4 had no additional effects on nitrogen fixation activity (data not shown). Since plants from the control and single Ps. oryzihabitans Ep4 treatments lacked nodules, their nitrogen fixation activity was not determined.
Application of B. japonicum 634b universally increased foliar nitrogen concentrations (Table 2). Across all replicate plots of all three varieties (n = 12), the nodule number positively correlated with leaf biomass (r = +0.73, P = 0.007), leaf N content (r = +0.83, P = 0.001), seed yield (r = +0.78, P = 0.003), seed protein content (r = +0.72, P = 0.008) and seed oil content (r = +0.67, P = 0.016). Although co-inoculation with strain Ps. oryzihabitans Ep4 had no effect on foliar N, it increased Fe and Zn content in leaves of Svapa and Zn content in leaves of Nice-Mecha (Table 3). A combined inoculation increased contents of Ca, S, B and Co in leaves of Nice-Mecha and contents of all elements present in Tables 2 and 3, with exception of Fe and Zn, in leaves of Svapa. Inoculations did not affect leaf Mn, Cu and Ni contents (data not shown).
Discussion
Experiments with batch cultures showed that strains Ps. oryzihabitans Ep4 and Ep4gfp utilized all organic acids, sugars and amino acids (the last group as carbon and/or nitrogen source, except phenylalanine) detected in root exudates of the studied soybean varieties. Ps. oryzihabitans Ep4 and its derivative Ep4gfp did not differ in amino acid utilization, as previously reported for strain Ep4 (Belimov et al. 2015a), as well as in utilization of organic acids and sugars (present study), confirming relevancy of comparing two strains in further experiments related to root exudation.
The composition of root exudates was qualitatively similar between different soybean varieties, but significantly differed in the specific amounts of the exuded compounds, with Nice-Mecha exuding about two times more organic acids, variety Svapa exuding more sugars than variety Bara and the latter variety having the lowest exudation rates of all measured fractions (Fig. 6a). Since Ps. oryzihabitans Ep4 can efficiently utilize many of these soybean root exudates (Fig. 3, 4 and 5), these observations positively correlated with greater number of bacteria in the root zone of Nice-Mecha and Svapa, as compared with Bara (Fig. 1a). However the bacteria decreased concentration of root exudates of Nice-Mecha and Svapa to a greater extent than of Bara (Fig.1b). Therefore, better bacterial proliferation in this case could not be explained only by greater nutrient substrate availability in the root zone.
One possibility is that variety Bara exuded larger quantities of compounds that inhibit growth of Ps. oryzihabitans Ep4gfp, compared to other two varieties. Many plant species, including soybean, exude various compounds, mostly phenolic, with antimicrobial activity (Kramer et al. 1984; Siqueira et al. 1991; Bais et al. 2005; Badri et al. 2009). For example, a peanut variety susceptible to Fusarium oxysporum exuded more sugars but less phenolic acids than a resistant variety (Li et al. 2013). Another possibility may be that bacteria alter the efflux of organic compounds from plant roots. Decreased phenylalanine concentrations in the root zone of inoculated Nice-Mecha and Svapa, along with an inability of P. oryzihabitans Ep4gfp to utilize this compound in batch culture, supports this hypothesis. Similarly, P. oryzihabitans Ep4gfp decreased concentrations of glutamic acid and phenylalanine in the potato rhizosphere, but did not utilize these compounds (Belimov et al. 2015a). Moreover, the effect of bacteria on exudation of many amino acids was more pronounced in potato variety Swift than variety Nevsky (Belimov et al. 2015a). Variety-dependent effect of PGPR on root exudation was also observed in rice, where inoculation with Corynebacterium sp. or Rhizobium sp. stimulated efflux of amino acids in two varieties, but inhibited this process in a third (Naher et al. 2008). Also, the cytokinin producing Bacillus subtilis stimulated deposition of amino acids to the rhizosphere by wheat roots (Kudoyarova et al. 2014). Several Pseudomonas spp. stimulated efflux, but inhibited influx, of amino acids by alfalfa and wheat roots due to production of 2,4-diacetylphloroglucinol (Bonsall et al. 1997; Phillips et al., 2004).Thus bacterial regulation of root metabolite efflux is an important determinant of rhizosphere chemistry.
Some of the observed differences in soybean root exudation of the measured compounds may be involved in variety-dependent interactions with PGPR. Under axenic conditions, Ps. oryzihabitans Ep4gfp actively proliferated in the root zone and stimulated root growth of varieties Nice-Mecha and Svapa, but not of variety Bara. Along with this, Bara exuded much less malate and did not exude succinate, thus less malate was utilized by bacteria in the root zone of Bara. These organic acids play important role in interactions with PGPR by acting as preferred nutrient compounds and improving root colonization by the beneficial bacteria (Kravchenko et al. 1993b; Lugtenberg et al., 2001; de Weert et al. 2002; Kamilova et al. 2006; Rudrappa et al. 2008). In addition, sorghum variety with intensive exudation of malate and succinate was characterized by high nitrogen fixing activity (Krotzky et al., 1988). High symbiotic potential of pea cultivar Triumph was associated with increased succinate exudation (Kuzmicheva et al. 2015). These observations suggest that a high exudation of organic acids, particularly malate and succinate, by varieties Nice-Mecha and Svapa may be involved in more efficient integration between components of the studied plant-bacteria associations.
Many PGPR convert the amino acid tryptophan into the phytohormone indole-3-acetic acid (IAA), thereby stimulating plant growth (e.g. see reviews Bashan et al. 2004; Patten and Glick 1996; Spaepen et al. 2007; Dodd et al. 2010; Duca et al. 2014). Intensive production of IAA from tryptophan by Ps. oryzihabitans Ep4 was described previously (Belimov et al. 2015a). Tryptophan was detected in root exudates of various plant species such as wheat, barley, cucumber (Kravchenko and Leonova, 1993a), radish and tomato (Kamilova et al. 2006). Although tryptophan was detected in root exudates of three soybean varieties (Fig. 5), inoculation with Ps. oryzihabitans Ep4gfp reduced tryptophan concentration in root zone of Nice-Mecha and Svapa only. Part of this tryptophan was likely a precursor for bacterial biosynthesis of IAA resulting in stimulation of root growth. In line with this proposal, the greater growth-promoting effect of Ps. fluorescens WCS365 on radish, as compared to tomato, was related to more active exudation of tryptophan by the former species (Kravchenko et al. 2004; Kamilova et al. 2006).
While bacterial IAA production may be important in stimulating growth, Ps. oryzihabitans Ep4 also has a high activity of ACC deaminase and this trait was probably involved in plant growth promotion (Belimov et al. 2001). Although Ps. oryzihabitans Ep4gfp actively utilized ACC in the potato rhizosphere and stimulated root growth (Belimov et al. 2015a), ACC was not detected in root exudates of the studied soybean varieties. It has been proposed that bacterial ACC deaminase outcompetes plant ACC oxidase, the plant enzyme hydrolyzing this compound inside plant tissues, thus root cells actively “pump” ACC into bacterial cells even without root exudation of ACC (Glick et al. 1998). Therefore the possibility that ACC deaminase of Ps. oryzihabitans Ep4gfp promoted soybean root growth and stimulated nodulation cannot be ruled out, however more detailed experiments (e.g. using ACC deaminase knock out mutants) are required to prove this hypothesis.
The PGPR strain Ps. fluorescens B16 produces pyrroloquinoline quinone (PQQ), which promotes plant growth by acting as an antioxidant, whereas its mutants that did not produce PQQ did not stimulate plant growth (Choi et al. 2008). The amino acids glutamine and tyrosine are precursors in PQQ biosynthesis (Houck et al. 1991) and inoculation with Ps. oryzihabitans Ep4gfp decreased concentrations of these amino acids in root zone of varieties Nice-Mecha and Svapa (Fig. 5). If allowed to assume that Ps. oryzihabitans Ep4gfp produces PQQ from glutamine and/or tyrosine, this trait could also be involved in variety-dependent response on soybean to the bacteria.
Positive effects of mono-inoculation with Ps. oryzihabitans Ep4 on growth and nutrient uptake by soybean varieties Nice-Mecha and/or Svapa (such as leaf biomass, leaf Fe and Zn content, seed yield and seed protein content) were observed in the field experiment. Furthermore, co-inoculation of B. japonicum 634b with Ps. oryzihabitans Ep4 increased uptake of many nutrients (N, Ca, Mg, S, B, Co, Fe, Cu and/or Mo) in these soybean varieties, particularly Svapa, suggesting positive additive effects on plant mineral nutrition. Moreover, only a combined inoculation increased seed protein and oil contents of Svapa. In variety Bara, such growth-promoting and nutritional effects were significant for leaf N and B content only. Many rhizosphere-inhabiting presudomonads produce siderophores which chelate Fe and other metal ions, thereby making these elements more available for plants (Braud et al. 2009; Cornelis, 2010; Rajkumar et al. 2010; Johnstone and Nolan 2015). Ps. oryzihabitans Ep4 also produces siderophores (this report) in amounts comparable to other PGPR (Belimov et al. 2005). Bacterial siderophore complexes contain peptide chains consisting mostly of amino acids alanine, glycine, lysine, ornithine and threonine (Meyer et al. 2002; Mashiach and Meijler 2013). The concentrations of these amino acids were substantially decreased by Ps. oryzihabitans Ep4gfp in root exudates of Svapa (Fig. 5), suggesting efficient utilization (or transformation) of the compounds in association with this variety. Under field conditions, we propose that part of the exuded amino acids are involved in bacterial biosynthesis of siderophores, particularly in the rhizosphere of the inoculated variety Svapa, thus improving nutrient uptake. This hypothesis connects root exudation, siderophore production and nutritional effects of PGPR on plants.
Co-inoculation of B. japonicum 634b with Ps. oryzihabitans Ep4 increased nodulation of variety Svapa by 3.7 times, as in previous reports where PGPR (Ps. fluorescens or Ps. putida) benefited the legume-rhizobia symbiosis via increasing soybean nodulation by 3.8 times (Chebotar et al. 2001), 2.6 times (Polonenko et al. 1987) or 38% (Zhang et al. 1996). Positive effects of Ps. oryzihabitans Ep4 on nodulation, nitrogen-fixing activity and growth were previously described in our experiments with pea (Malkov et al. 2012). Co-inoculation of soybean with B. japonicum and PGPR Serracia spp. enhanced nodulation efficiency, the amount of fixed nitrogen and yield only of the variety AC Bravor (of the 2 varieties inoculated), which was characterized by big biomass but few and small nodules after rhizobial-only inoculation (Dashti et al. 1998). As with the relationship between the effect of bacteria on nodulation and root exudation, Ps. oryzihabitans Ep4 utilized sugars and amino acids (including tryptophan, a precursor in auxin biosynthesis) exuded by Svapa most actively, suggesting better integration of the introduced bacteria with this variety. However the role of plant genotype in intraspecies variability of PGPR effects needs more detailed study. The described mechanisms proposed to explain stimulation of nodulation by PGPR include decreased plant ethylene biosynthesis by ACC deaminase activity and production of auxins by rhizobacteria (Belimov et al., 2012; Pérez-Montaño et al., 2014, Nascimento et al. 2016). Although little is known about the role of rhizobacterial ACC deaminase and IAA in variety-dependent responses of legume-rhizobia symbiosis to inoculation with PGPR, such effects could be related to differences in response of common bean varieties to exogenous auxins produced by Az. brasilense: since variety BAT477 increased root biomass while variety DOR364 increased nodulation frequency (Remans et al. 2008). The ACC deaminase activity and auxin production of Ps. oryzihabitans Ep4 suggests it can influence nodule formation, but the specific roles of each bacterial trait in determining variety-specific differences in soybean nodulation (Table 1) requires further work.
Conclusion
Within the studied soybean varieties, there were substantial genotypic differences in a number of traits such as root exudation of major organic components (organic acids, sugars and amino acids), root colonization by Ps. oryzihabitans Ep4 and the effects of bacteria on growth, photosynthesis, nodulation, yield quality and uptake of nutrients. In both hydroponic experiments and the field trial, the varieties Nice-Mecha and particularly Svapa responded more positively to Ps. oryzihabitans Ep4 than the variety Bara. We propose that variety specific plant response to PGPR is mediated by differences in root exudation and the ability of rhizobacteria to metabolize and/or transform such compounds.
Abbreviations
- ACC:
-
1-aminocyclopropane-1-carboxylate
- BPF:
-
Bacto-Pseudomonas F
- CAS:
-
chrome azurol S
- IAA:
-
indole-3-acetic acid
- PGPR:
-
Plant growth-promoting rhizobacteria
- PQQ:
-
pyrroloquinoline quinone
References
Ahmad M, Zahir ZA, Asghar HN, Asghar M (2011) Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 57(7):578–589. doi:10.1139/W11-044
Arinushkina EV (1970) Guidelines for the chemical analysis of soils. Moscow State University Press, Moscow
Atieno M, Herrmann L, Okalebo R, Lesueur D (2012) Efficiency of different formulations of Bradyrhizobium japonicum and effect of co-inoculation of Bacillus subtilis with two different strains of Bradyrhizobium japonicum. World J Microbiol Biotechnol 28(7):2541–2550. doi:10.1007/s11274-012-1062-x
Badri DV, Quintana N, El-Kassis EG, Kim HK, Choi YH, Sugiyama A, Verpoorte R, Martinoia E, Manter DK, Vivanco JM (2009) An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol 151:2006–2017. doi:10.1104/pp.109.147462
Bai Y, D’Aoust F, Smith DL, Driscoll BT (2002) Isolation of plant-growth-promoting Bacillus strains from soybean root nodules. Can J Microbiol 48:230–238. doi:10.1139/w02-014
Bais HP, Prithiviraj B, Jha AK, Ausubel FM, Vivanco JM (2005) Mediation of pathogen resistance by exudation of antimicrobials from roots. Nature 434:217–221. doi:10.1038/nature03356
Bais HP, Weir T, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. doi:10.1146/annurev.arplant.57.032905.105159
Bakker MG, Manter DK, Sheflin AM, Weir TL, Vivanco JM (2012) Harnessing the rhizosphere microbiome through plant breeding and agricultural management. Plant Soil 360:1–13. doi:10.1007/s11104-012-1361-x
Bashan Y, Holguin G, de Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50:521–577. doi:10.1139/w04-035
Belimov AA, Kozhemiakov AP (1992) Use of mixed cultures of nitrogen fixing bacteria in agriculture. Agricul Biol 5:77–87 (In Russian)
Belimov AA, Safronova VI (2011) ACC deaminase and plant-microbe interactions (review). Agricul Biol 3:23–28
Belimov AA, Kozhemyakov AP, Chuvarliyeva GV (1995a) Interaction between barley and mixed cultures of nitrogen fixing and phosphate solubilizing bacteria. Plant Soil 173:29–37. doi:10.1007/BF00155515
Belimov AA, Kunakova AM, Gruzdeva EV, Vasilyeva ND, Vorobyev NI, Kojemyakov AP, Khamova OF, Postavskaya SM, Sokova SM (1995b) Relationship between survival rates of associative nitrogen fixers on roots and yield response of plants to inoculation. FEMS Microbiol Ecol 17:187–196. doi:10.1016/0168-6496(95)00023-4
Belimov AA, Safronova VI, Sergeyeva TA, Egorova TN, Matveyeva VA, Tsyganov VE, Borisov AY, Tikhonovich IA, Kluge C, Preisfeld A, Dietz KJ, Stepanok VV (2001) Characterisation of plant growth-promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 47:642–652. doi:10.1139/cjm-47-7-642
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol Biochem 37:241–250. doi:10.1016/j.soilbio.2004.07.033
Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ (2009) Rhizosphere bacteria containing ACC deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol 181:413–423. doi:10.1111/j.1469-8137.2008.02657.x
Belimov AA, Demchinskaya SV, Safronova VI (2012) Reaction of pea plants on inoculation by rhizosphere 1-aminocyclopropane-1-carboxylate (ACC) utilizing bacteria in the presence of endomycorrhizal fungus Glomus intraradices. Agricul Biol 3:90–97. doi:10.15389/agrobiology.2012.3.90eng
Belimov AA, Dodd IC, Safronova VI, Shaposhnikov AI, Azarova TS, Makarova NM, Davies WJ, Tikhonovich IA (2015a) Rhizobacteria that produce auxins and contain ACC deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Ann Appl Biol 167:11–25. doi:10.1111/aab.12203
Belimov AA, Puhalsky IV, Safronova VI, Shaposhnikov AI, Vishnyakova MA, Semenova EV, Zinovkina NY, Makarova NM, Wenzel W, Tikhonovich IA (2015b) Role of plant genotype and soil conditions in symbiotic plant-microbe interactions for adaptation of plants to cadmium polluted soils. Water Air Soil Pollut 226(8):1–15. doi:10.1007/s11270-015-2537-9
Bonsall RF, Weller DM, Thomashow LS (1997) Quantification of 2,4-diacetylphloroglucinol produced by fluorescent Pseudomonas spp. in vitro and in the rhizosphere of wheat. Appl Environ Microbiol 63:951–955
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–258. doi:10.1016/0003-2697(76)90527-3
Braud A, Hannauer M, Mislin GLA, Schalk IJ (2009) The Pseudomonas aeruginosa pyochelin-iron uptake pathway and its metal specificity. J Bacteriol 191(11):3517–3525. doi:10.1128/JB.00010-09
Chebotar VK, Asis CA, Akao S (2001) Production of growth-promoting substances and high colonization ability of rhizobacteria enhance the nitrogen fixation of soybean when coinoculated with Bradyrhizobium japonicum. Biol Fertil Soils 34(6):427–432. doi:10.1007/s00374-001-0426-4
Choi O, Kim J, Kim JG, Jeong Y, Moon JS, Park CS, Hwang I (2008) Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol 146:657–668. doi:10.1104/pp.107.112748
Cornelis P (2010) Iron uptake and metabolism in pseudomonads. Appl Microbiol Biotechnol 86:1637–1645. doi:10.1007/s00253-010-2550-2
Das SK, Sharma KL, Neelam S, Srinivas K (1997) Effect of cultivars, nitrogen sources and soil types on response of sorghum (Sorghum bicolor L.) to Azospirillum inoculation. Ann Agric Sci 18:313–317
Dashti N, Zhang F, Hynes R, Smith DL (1998) Plant growth promoting rhizobacteria accelerate nodulation and increase nitrogen fixation activity by field grown soybean [Glycine max (L.) Merr.] under short season conditions. Plant Soil 200:205–213. doi:10.1023/A:1004358100856
De Weert S, Vermeiren H, Mulders IH, Kuiper I, Hendrickx N, Bloemberg GV, Vanderleyden J, de Mot R, Lugtenberg BJ (2002) Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol Plant Microb In 15:1173–1180. doi:10.1094/MPMI.2002.15.11.1173
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72:313–332. doi:10.1111/j.1574-6941.2010.00860.x
Dobereiner J (1980) Forage grasses and grain crops. In: Bergersen FS (ed) Methods for evaluating biological nitrogen fixation. John Wiley & Sons Ltd, New York, pp 535–555
Dodd IC, Zinovkina NY, Safronova VI, Belimov AA (2010) Rhizobacterial mediation of plant hormone status. Ann Appl Biol 157:361–379. doi:10.1111/j.1744-7348.2010.00439.x
Drogue B, Sanguin H, Chamam A, Mozar M, Llauro C, Panaud O, Prigent-Combaret C, Picault N, Wisniewski-Dyé F (2014) Plant root transcriptome profiling reveals a strain-dependent response during Azospirillum-rice cooperation. Front Plant Sci 5(607):1–14. doi:10.3389/fpls.2014.00607
Duca D, Lory J, Patten CL, Rose D, Glick BR (2014) Indole-3-acetic acid in plant–microbe interactions. A Van Leeuw J Microb 106:85–125. doi:10.1007/s10482-013-0095-y
Gahoonia TS, Asmar F, Giese H, Gissel-Nielsen G, Nielsen NE (2000) Root-released organic acids and phosphorus uptake of two barley cultivars in laboratory and field experiments. Eur J Agron 12:281–289. doi:10.1016/S1161-0301(00)00052-6
Gamalero E, Glick BR (2015) Bacterial modulation of plant ethylene levels. Plant Physiol 169:13–22. doi:10.1104/pp.15.00284
Gaume A, Mächler F, Frossard E (2001) Aluminum resistance in two cultivars of Zea mays L.: root exudation of organic acids and influence of phosphorus nutrition. Plant Soil 234:73–81. doi:10.1023/A:1010535132296
Glick BR, Karaturovic DM, Newell PC (1995) A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can J Microbiol 41:533–536. doi:10.1139/m95-070
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68. doi:10.1006/jtbi.1997.0532
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339. doi:10.1007/s10658-007-9162-4
Hardy RWF, Bums RC, Holsten RD (1973) Application of the C2H2-C2H4 assay for measurement of nitrogen fixation. Soil Biol Biochem 5:47–82. doi:10.1016/0038-0717(73)90093-X
Houck DR, Hanners JL, Unkefer CJ (1991) Biosynthesis of pyrroloquinoline quinone. 2. Biosynthetic assembly from glutamate and tyrosine. J Am Chem Soc 113:3162–3166. doi:10.1021/ja00008a053
Hungria M, Nogueira M, Araujo R (2013) Co-inoculation of soybeans and common beans with rhizobia and azospirilla: strategies to improve sustainability. Biol Fertil Soils 49:791–801. doi:10.1007/s00374-012-0771-5
Imran A, Mirza MS, Shah TM, Malik KA, Hafeez FY (2015) Differential response of kabuli and desi chickpea genotypes toward inoculation with PGPR in different soils. Front Microbiol 6:859. doi:10.3389/fmicb.2015.00859
Johnstone TC, Nolan EM (2015) Beyond iron: non-classical biological functions of bacterial siderophores. Dalton T 44:6320–6339. doi:10.1039/c4dt03559c
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova TS, Makarova NM, Lugtenberg B (2006) Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microb In 19(3):250–256. doi:10.1094/MPMI-19-0250
Kong Z, Deng Z, Glick BR, Wei G, Chou M (2016) A nodule endophytic plant growth-promoting Pseudomonas and its effects on growth, nodulation and metal uptake in Medicago lupulina under copper stress. Ann Microbiol 67:49–58. doi:10.1007/s13213-016-1235-1
Kramer RP, Hindorf H, Jha HC, Kallage J, Zilliken F (1984) Antifungal activity of soybean and chickpea isoflavones and their reduced derivatives. Phytochemistry 23:2203–2205. doi:10.1016/S0031-9422(00)80520-8
Kravchenko LV, Leonova EI (1993a) Utilization of root metabolite tryptophan in biosynthesis of indolyle-3-acetic acid by associative bacteria. Microbiol-USSR 62(3):282–286
Kravchenko LV, Azarova TS, Dostanko OY (1993b) Effect of root exometabolites of wheat with different genome ploidy on growth of Azospirillum brasilense. Microbiol-USSR 62(5):517–520
Kravchenko LV, Azarova TS, Makarova NM, Tikhonovich IA (2004) The effect of tryptophan present in plant root exudates on the phytostimulating activity of rhizobacteria. Microbiol-USSR 73(2):156–158. doi:10.1023/B:MICI.0000023982.76684.9d
Krotzky A, Bergold R, Werner D (1988) Plant characteristics limiting associative N2 fixation with two cultivars of sorghum mutants. Soil Biol Biochem 20:157–162
Kudoyarova GR, Melentiev AI, Martynenko EV, Timergalina LN, Arkhipova TN, Shendel GV, Kuz’mina LY, Dodd IC, Veselov SY (2014) Cytokinin producing bacteria stimulate amino acid deposition from wheat roots. Plant Physiol Biochem 83:285–291. doi:10.1016/j.plaphy.2014.08.015
Kuzmicheva YV, Shaposhnikov AI, Azarova NS, Petrova SN, Naumkina TS, Borisov AY, Belimov AA, Kravchenko LV, Parakhin NV, Tikhonovich IA (2014) Composition of root exometabolites of the symbiotically effective pea cultivar triumph and its parental forms. Russ J Plant Physiol 61(1):112–118. doi:10.1134/S1021443714010087
Kuzmicheva YV, Tychinskaya IL, Petrova SN, Parakhin NV (2015) Efficiency of introduction of ACCutilizing rhizobacteria into soybean agrocenosis in Orel region. Agricultural Biology 50:377–383
Li XG, Zhang TL, Wang XX, Hua K, Zhao L, Han ZM (2013) The composition of root exudates from two different resistant peanut cultivars and their effects on the growth of soil-borne pathogen. Int J Biol Sci 9:164–173. doi:10.7150/ijbs.5579
Lugtenberg B, Dekkers L, Bloemberg GV (2001) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39:461–490. doi:10.1146/annurev.phyto.39.1.461
Maheshwari DK (ed) (2010) Plant growth and health promoting bacteria. Springer, Heidelberg Dordrecht London New York. doi:10.1007/978-3-642-13612-2
Malkov NV, Zinovkina NY, Safronova VI, Belimov AA (2012) Increase in resistance of legume-rhizobial complex to cadmium using rhizosphere bacteria containing ACC deaminase. Achieve Sci Eng AIC 9:53–57 (in Russian)
Masciarelli O, Llanes A, Luna V (2014) A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol Res 169:609–615. doi:10.1016/j.micres.2013.10.001
Mashiach R, Meijler MM (2013) Total synthesis of pyoverdin D. Org Lett 15:1702–1705. doi:10.1021/ol400490s
Meyer J-M, Geoffroy VA, Baysse C, Cornelis P, Barelmann I, Taraz K, Budzikiewicz H (2002) Siderophore-mediated iron uptake in fluorescent Pseudomonas: characterization of the pyoverdine-receptor binding site of three cross-reacting pyoverdines. Arch Biochem Biophys 397:179–183. doi:10.1006/abbi.2001.2667
Naher UA, Radziah O, Halirni MS, Shamsuddin ZH, Razi IM (2008) Effect of inoculation on root exudates carbon sugar and amino acids production of different rice varieties. Res J Microbiol 3:580–587. doi:10.3923/jm.2008.580.587
Nascimento FX, Rossi MJ, Soares CRFS, McConkey BJ, Glick BR (2014) New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS One 9(6):e99168. doi:10.1371/journal.pone.0099168
Nascimento FX, Brígido C, Glick BR, Rossi MJ (2016) The role of rhizobial ACC deaminase in the nodulation process of leguminous plants. Int J Agron 1369472:9. doi:10.1155/2016/1369472
Okubara PA, Kornoely JP, Landa BB (2004) Rhizosphere colonization of hexaploid wheat by Pseudomonas fluorescens strains Q8rl-96 and Q2-87 is cultivar-variable and associated with changes in gross root morphology. Biol Control 30:392–403. doi:10.1016/j.biocontrol.2003.11.003
Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42:207–220. doi:10.1139/m96-032
Pérez-Montaño F, Alías-Villegas C, Bellogín RA, del Cerro P, Espuny MR, Jiménez-Guerrero I, López-Baena FJ, Ollero FJ, Cubo T (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169:325–336. doi:10.1016/j.micres.2013.09.011
Phillips DA, Fox TC, King MD, Bhuvaneswari TV, Teuber LR (2004) Microbial products trigger amino acid exudation from plant roots. Plant Physiol 136:2887–2894. doi:10.1104/pp.104.044222
Polonenko DR, Scher FM, Kloepper JW, Singgleton CA, Laliberte M, Zaleska I (1987) Effects of roots colonizing bacteria on nodulation of soybean roots by Bradyrhizobium japonicum. Can J Microbiol 33:498–503. doi:10.1139/m87-083
Rajkumar M, Ae N, Prasad MN, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149. doi:10.1016/j.tibtech.2009.12.002
Remans R, Beebe S, Blair M, Manrique G, Tovar E, Rao I, Croonenborghs A, Torres-Gutierrez R, El-Howeity M, Michiels J, Vanderleyden J (2008) Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.) Plant Soil 302:149–161. doi:10.1007/s11104-007-9462-7
Rudrappa T, Czymmek KJ, Pare PW, Bais HP (2008) Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol 148:1547–1556. doi:10.1104/pp.108.127613
Safronova VI, Stepanok VV, Engqvist GL, Alekseyev YV, Belimov AA (2006) Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol Fertil Soils 42:267–272. doi:10.1007/s00374-005-0024-y
Safronova VI, Piluzza G, Bullitta S, Belimov AA (2011) Use of legume-microbe symbioses for phytoremediation of heavy metal polluted soils: advantages and potential problems (review). In: Golubev IA (ed) Handbook for phytoremediation. Nova Science Publishers, Inc., New York, pp 443–469
Saubidet MI, Barneix AJ (1998) Growth stimulation and nitrogen supply to wheat plants inoculated with Azospirillum brasilense. J Plant Nutr 21(12):2565–2577. doi:10.1080/01904169809365588
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. doi:10.1016/0003-2697(87)90612-9
Shaposhnikov AI, Morgunov AI, Akin B, Makarova NM, Belimov AA, Tikhonovich IA (2016) Comparative characteristics of root systems and root exudation of synthetic, landrace and modern wheat varieties. Agricultural Biology 51(3):68–78. Doi: 10.15389/agrobiology.2016.1.68eng
Singh G, Singh N, Marwaha TS (2009) Crop genotype and a novel symbiotic fungus influences the root endophytic colonization potential of plant growth promoting rhizobacteria. Physiol Mol Biol Plants 15:87–92. doi:10.1007/s12298-009-0009-7
Siqueira JO, Nair MG, Hammerschmidt R, Safir GR, Putnam AR (1991) Significance of phenolic compounds in plant-soil-microbial systems. Crit Rev Plant Sci 10:63–121. doi:10.1080/07352689109382307
Smith KP, Goodman RM (1999) Host variation for interactions with beneficial plant-associated microbes. Annu Rev Phytopathol 37:473–491. doi:10.1146/annurev.phyto.37.1.473
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448. doi:10.1111/j.1574-6976.2007.00072.x
Suarez A, Guttler A, Stratz M, Staendner LH, Timmis KN, Guzman CA (1997) Green fluorescent protein-based reporter systems for genetic analysis of bacteria including monocopy applications. Gene 196:69–74. doi:10.1016/S0378-1119(97)00197-2
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356. doi:10.3389/fpls.2013.00356
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586. doi:10.1023/A:1026037216893
Vranova V, Rejsek K, Skene KR, Formanek P (2011) Non-protein amino acids: plant, soil and ecosystem interactions. Plant Soil 342:31–48. doi:10.1007/s11104-010-0673-y
Walker V, Bertrand C, Bellvert F, Mënne-Loccoz Y, Bally R, Comte G (2011) Host plant secondary metabolite profiling shows a complex, strain-dependent response of maize to plant growth-promoting rhizobacteria of the genus Azospirillum. New Phytol 189:494–506. doi:10.1111/j.1469-8137.2010.03484.x
Yang JC, Chang EH, Zhans WJ, Wang ZQ, Liu LJ (2007) Relationship between root chemical signals and grain quality of rice. Agric Sci China 6(1):47–57. doi:10.1016/S1671-2927(07)60016-9
Zhang F, Dashti N, Hynes RK, Smith DL (1996) Plant growth gromoting ghizobacteria and goybean [Glycine max (L.) Merr.] nodulation and nitrogen fixation at suboptimal root zone temperatures. Ann Bot 77:453–460. doi:10.1006/anbo.1996.0055
Acknowledgements
We are grateful to Professor Ian Dodd for critically reading the manuscript and help in improving the English language. The work was supported by the Russian Science Foundation (grant 16-16-00080 for hydroponic pot experiments, grant 14-26-00094 for UPLC for root exudates and grant 14-16-00137 for field experiment and elemental analysis of plants).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yoav Bashan.
Rights and permissions
About this article
Cite this article
Kuzmicheva, Y.V., Shaposhnikov, A.I., Petrova, S.N. et al. Variety specific relationships between effects of rhizobacteria on root exudation, growth and nutrient uptake of soybean. Plant Soil 419, 83–96 (2017). https://doi.org/10.1007/s11104-017-3320-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3320-z