Abstract
Background and aims
Rhizosphere processes are integral to carbon sequestration by terrestrial ecosystems in response to rising concentrations of atmospheric CO2. Yet, the nature and magnitude of rhizosphere responses to elevated CO2, particularly in nutrient and water-limited forest ecosystems, remain poorly understood.
Methods
We investigated rhizosphere responses (enzyme activities and nutrient availability) to atmospheric CO2 enrichment (ambient +150 μmol CO2 mol−1) in a phosphorus-limited mature eucalypt woodland in south-eastern Australia (the EucFACE experiment).
Results
Following 17 months of treatment, the activity of rhizosphere soil exoenzymes related to starch and cellulose degradation decreased between 0 and 10 cm and increased from 10 to 30 cm depth under elevated CO2. This response was concurrent with increases in nitrogen and phosphorus availability and smaller C:P nutrient ratios in rhizosphere soil under elevated CO2.
Conclusions
This nutrient-poor eucalypt woodland exhibited rhizosphere responses to atmospheric CO2 enrichment that increased nutrient availability in rhizosphere soil and suggest accelerated rates of soil organic matter decomposition, both of which may, in turn, promote plant growth under elevated CO2 concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric carbon dioxide (CO2) concentrations have been steadily increasing since pre-industrial times and are predicted to reach 650 μmol mol−1 within the next 30–50 years (Friend et al. 2014). This increase is due to human activities such as fossil fuel combustion and land clearing, which release large amounts of CO2 to the atmosphere, altering the global carbon (C) cycle and changing the climate across the globe (Day et al. 2013; Friend et al. 2014). A large proportion of anthropogenic CO2 emissions is expected to be assimilated and stored by plants via photosynthesis (Liberloo et al. 2007). However, an increase in net primary productivity will only result in significant C accumulation if a substantial proportion of the fixed C is able to enter pools that turn over slowly, for example, soil organic matter, wood and coarse roots (Allen et al. 2000; Trumbore 2000; Stover et al. 2007).
Soil fertility and water availability frequently modulate ecosystem responses to elevated CO2 (eCO2), particularly in forests (de Graaff et al. 2006; McCarthy et al. 2010; Norby et al. 2015). Furthermore, in the absence of external nutrient inputs (e.g., via fertilization or high levels of atmospheric nitrogen (N) deposition), plant growth enhancement under eCO2 may not be maintained in the long-term due to progressive nutrient limitation (de Graaff et al. 2006). This can be especially important for mature ecosystems, which are frequently limited by nutrients such as N and phosphorus (P) (Vitousek et al. 2010). Under such circumstances, enhanced rhizosphere activity may help sustain higher rates of plant growth in response to eCO2 due to a rhizosphere-induced increase in soil nutrient availability (Phillips et al. 2011; Phillips et al. 2012; Dijkstra et al. 2013; Drake et al. 2013). In this context, plants can exude a considerable proportion (estimated at about 17% on average) of their assimilated C belowground as low molecular weight C compounds that can be directly metabolised by microorganisms (Phillips and Fahey 2005; Nguyen 2009; Jones et al. 2009; Finzi et al. 2015). Alternatively, plants can influence nutrient availability via the production of extracellular enzymes and release of carboxylic acids. A recent global meta-analysis demonstrated that eCO2 stimulates rhizodeposition of C and both fine and coarse root production (Nie et al. 2013). This was concomitant with a decrease in the proportion of roots in the topsoil and the expansion of rooting systems deeper into the soil profile, particularly in forests (Pritchard et al. 2008; Iversen 2010; Nie et al. 2013). This suggests that, under eCO2, strategies associated with increased acquisition of soil resources may be especially relevant. However, little is known as to whether this mechanism applies to ancient, highly P-depleted soils such as those in the southern hemisphere and throughout the tropics (Wang et al. 2010; Norby et al. 2015).
Given the paramount importance of forest ecosystems for terrestrial C sequestration and the extent of P limitation across the globe (Wang et al. 2010), two key questions are whether P-limited forest ecosystems respond to increasing atmospheric CO2 concentrations and how important is the rhizosphere in mediating eCO2 effects on nutrient availability (Jin et al. 2015). In a recent study, Hasegawa et al. (2016) found higher P and N availability and mineralization rates under eCO2 in a low-nutrient, mature eucalypt woodland but this increase was only evident during the warmer, summer months (November–February). These responses were attributed to an enhancement of microbial turnover of organic matter and mobilisation of chemically-bound nutrients. However, the study did not provide evidence of the specific mechanisms driving this response, including rhizosphere mechanisms, which remains an under-explored field of research (Zak et al. 2000; de Graaff et al. 2006). In this study, we examine the role of rhizosphere mechanisms in underpinning these observed increases in P and N availability.
Herein, we aimed to quantify nutrient availability and activity of extracellular enzymes (exoenzymes) related to the main nutrient cycles (C, N and P) in the bulk and rhizosphere soil, and their early responses (17 months) to eCO2. We also sought to explore the influence of fine roots on nutrient availability and activity of exoenzymes (i.e., the rhizosphere effect). We predict that eCO2 will increase plant C investment in strategies that facilitate the acquisition of soil resources (particularly P) such as exudation of C-rich compounds and increasing plant- and microbial-derived exoenzymes (Nie et al. 2013). This would, in turn, reduce resource constraints on the CO2 fertilization response.
Material and methods
Study site
The study was conducted at the Eucalyptus free-air CO2 enrichment (EucFACE) experiment in a P-limited (Crous et al. 2015) remnant Cumberland Plain woodland in the Sydney basin, near Richmond, New South Wales, Australia (33° 24’S, 150° 59’E). The dominant tree species is Eucalyptus tereticornis and the dominant understorey grass is Microlaena stipoides. Other frequent understorey species are the forbs Pratia purpurascens and Commelina cyanea. Mean tree basal area at the study site is 27.6 ± 2.7 m2 ha−1. The soil is a loamy sand of the Clarendon Formation (>75% sand in the surface; Gimeno et al., 2015). Total soil C and N (0–10 cm) at the site range between 1 and 2% and 0.08–0.16%, respectively, whereas total P (extracted with Aqua Regia and, thus, representing the P contained in the soil organic matter pool) ranges between 51.3–102.4 mg P kg soil−1 (Drake et al. 2016). The pH is acidic (pH 5.5; this study). Variations in soil moisture at the site are conditioned by the presence of a hard soil layer that runs in an oblique angle across the whole experimental area (depth ranges between 34 and 67 cm), as well as by microtopography and associated variation in soil texture. Annual rainfall and mean minimum/maximum temperatures at the site in the two years immediately prior to sampling were 824.2 mm and 10.5 °C/24 °C in 2012 and 661 mm, 11.1 °C/25.2 °C in 2013 (Station 067105, Australian Government, Bureau of Meteorology).
Experimental design
EucFACE was designed as a completely randomized experiment and consists of six experimental rings, each 25 m in diameter. Three of these rings are controls, receiving ambient air only, and three are continuously fumigated during daylight hours to maintain a CO2 concentration of 150 μmol CO2 mol−1 above ambient levels. The experiment commenced in September 2012. CO2 concentrations in the fumigated rings were gradually increased by 30 μmol CO2 mol−1 every 4–5 weeks until the final target concentrations (+150 μmol CO2 mol−1) were reached in February 2013 (Gimeno et al. 2015). From February 2013 to February 2014, 5-min average CO2 concentrations measured in the canopy were within 25% of the target 87.5% of the time (Suppl. Fig. 1).
Soil sampling and root standing crop biomass determinations
In February 2014, eight soil samples were collected at depths of 0–10 and 10–30 cm within four pre-established 1 × 1 m plots in each of the 6 rings (96 samples in total), using a slide hammer (4.5 cm diameter). This corresponded to a period when soil P availability, as measured using ion exchange membranes with subsequent Bray 1-P extraction using 0.03 M NH4F (Rayment and Lyons 2011), was observed to be higher under eCO2 (Hasegawa et al. 2016). Samples were transported to the laboratory, weighed and then stored at 4 °C and processed within 15 days. To separate fine roots, soil was sieved and then sorted by hand, collecting any roots <2 mm in diameter. Rhizosphere soil was obtained by shaking fine roots vigorously after hand collection and also by collecting any soil particles attached to the roots. The remaining soil was considered as bulk soil. The roots were then washed, placed in a drying oven at 60 °C for 2 days, after which they were weighed. Bulk and rhizosphere soil samples were refrigerated (4 °C) and processed within 15 days.
Soil measurements
Soil pH was measured using a 1:2.5 ratio of fresh bulk soil to deionised water. We determined the maximum potential activity of seven enzymes related to the main nutrient cycles (C, N and P) both in bulk and rhizosphere soil. Enzymes assayed were: α-1,4-glucosidase (AG; starch degradation), β-1,4-glucosidase (BG; starch degradation), β-xylosidase (XYL; hemicellulose degradation) and β-D-cellobiohydrolase (CBH; cellulose degradation) for the C cycle; β-1,4-N-acetylglucosaminidase (NAG; chitin degradation) and L-leucine aminopeptidase (LAP; protein degradation) for the N cycle; and acid phosphatase (PHOS; phosphorus mineralization) for the P cycle. Soil enzyme activities were assessed fluorometrically following the methods described in Bell et al. (2013). Briefly, assays were conducted by homogenizing 0.3 g of soil in 30 ml of 50 mM sodium acetate buffer (pH 5.5) for 1 min. The homogenised solutions were then added to a 96-deep-well (2 ml) microplate. Control replicates of soil slurry and 4-methylumbellfferone (MUB) or 7-amino-4-methylcoumarin (MUC) standard curves of 0–100 μM were included in each sample. Soil slurries with fluorometric substrates (Sigma-Aldrich: M9766 for AG, M3633 for BG, M7008 for XYL, M6018 for CBH, M2133 for NAG, L2145 for LAP, and M8883 for PHOS) were then incubated for 1.5 h at 35 °C. Following incubation, the supernatant solution was transferred into corresponding wells in a black, flat-bottomed 96-well plate. The plates were then scanned on a microplate fluorometer (2300, EnSpire® Multilabel Reader, PerkinElmer, Boston, MA, USA) using an excitation wavelength of 365 nm and an emission wavelength of 450 nm. Root phosphatase activity was evaluated on ~2-cm fine root segments following the same procedure.
In the same set of bulk and rhizosphere soil samples (February 2014), extractable soil N (NO3 − and NH4 +) and hydrolysable C sources (phenols and hexoses) were evaluated from 0.5 M K2SO4 extracts following the procedures described in Chantigny et al. (2006). Extractable P (PO4 3−, used as a proxy of instantaneous P availability) was also measured, using 2.5% acetic acid (Olsen P) as described in Covelo et al. (2008). In all cases, we used one bulked soil sample per ring and per depth (composite of eight samples) given the scarcity of material, particularly for rhizosphere soil in the deep layer. For this reason, and in order to be able to extract both C and N fractions from the same soil sample, K2SO4 was chosen over other extractants (e.g., KCl) to measure N availability.
Statistical analysis
Three-way nested analysis of variance (ANOVA) was used to evaluate the overall effects of eCO2, sampling depth and soil type (bulk vs. rhizosphere soil) on nutrient availability and enzymatic activities, and their respective stoichiometric ratios. Stoichiometric ratios (C to N, C to P and N to P) were calculated based on the sum of all nutrient concentrations or log-transformed enzyme activities related to the same nutrient cycle. For example, the enzyme C:N ratio was calculated as the logarithm of the sum of AG, BG, CBH and XYL divided by the logarithm of the sum of NAP and LAP (Sinsabaugh et al. 2009). In our model, soil type was nested within depth and depth within ring.
The relative interaction intensity (RII) index, originally developed to measure competition/facilitation in plants (Armas et al. 2004), but recently used in other ecological studies (e.g., soil microbial responses to N addition; Sinsabaugh et al. 2015), was also used to evaluate the relative effect of soil type on these biogeochemical parameters. This index ranges from −1 to 1 and is defined as:
where X is the variable of interest and the subscripts r and b refer to the rhizosphere and the bulk soil, respectively. Relative interaction index values were calculated at the sample level. In our case, RII ± 95% confidence interval values <0 indicate lower enzyme activity/nutrient availability in the rhizosphere soil in relation to the bulk soil (significant negative rhizosphere effect), whereas values >0 represent situations in which the activity/availability is higher in the rhizosphere soil (significant positive rhizosphere effect). Two-way nested ANOVAs were also used to evaluate the effects of eCO2 on RII values and root phosphatase activity. In these analyses, depth was nested within ring.
We also carried out Pearson correlation analyses with soil water content (SWC), soil pH and tree basal area (data obtained from Drake et al. 2016) to investigate the potential mediating role of pre-treatment spatial heterogeneity in resource availability, soil properties and tree basal area in the belowground responses. Similarly, we carried out Pearson correlation analyses between root biomass and, by extension total rhizosphere soil extracted, and all the biogeochemical parameters evaluated both in the bulk and rhizosphere soils in order to investigate the extent of the control exerted by the roots in the soil function. In the case of SWC, we used average values of continuously monitored volumetric soil water content data (from 1/11/2012 to 31/1/2014) from each ring (eight probes per ring, 0–15 cm; CS650-L; Campbell Scientific, Logan, UT, USA).
Statistical analyses were carried out using the ‘lme’ function of the nlme package in R version 3.3.2 (R Core Team 2016). Given the low number of experimental replicates (n = 3), P-values for these analyses were considered significantly different at α = 0.10 (Phillips et al. 2011). Mean ± standard error values are consistently reported throughout. When needed, data were log-transformed prior to analyses to meet normality assumptions.
Results
Enzyme activities
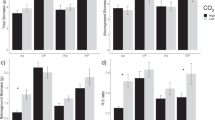
Cellobiohydrolase activity was significantly affected by the interaction between CO2 treatment and depth (P = 0.059; Fig. 1; Table S1), with a three-fold decrease and a four-fold increase in activity between 0 and 10 cm and 10–30 cm, respectively, under eCO2 compared to ambient conditions. Similarly, BG decreased at 0–10 cm and increased at 10–30 cm in the rhizosphere soil under eCO2 (CO2 x depth interaction in the rhizosphere soil; P = 0.06; Fig. 1; Table S1). Phosphorus (PHOS) and N-degrading enzymes (LAP and NAG) were, in contrast, not affected by eCO2 (Fig. 1; Table S1). The enzyme related to the P cycle (i.e., PHOS) was dominant in soil in relation to those enzymes related to C (~3 times higher) and N (~6 times higher) cycles (Fig. 1) and the activity of all C-degrading enzymes, except XYL, was consistently higher in the rhizosphere soil compared to the bulk soil (Fig. 1; Table S1). Similar to PHOS, root phosphatase activity (228.2 ± 28.7 nmol g dry root−1 h−1) was not affected by eCO2 (P = 0.96) nor by depth (P = 0.46), despite a general trend towards higher activity at 10–30 cm (Suppl. Fig. 2). The interaction between eCO2 and depth was also non-significant (P = 0.93).
Effects of elevated CO2 on enzyme activities related to C, N and P cycles in the bulk and rhizosphere soil at two incremental depths. Standard error bars are shown (n = 3). α-1,4-glucosidase (AG), β-1,4-glucosidase (BG), β-xylosidase (XYL) and β-D-cellobiohydrolase (CB) for the C cycle; β-1,4-N-acetylglucosaminidase (NAG) and L-leucine aminopeptidase (LAP) for the N cycle; phosphatase (PHOS). * and **indicate significant differences in relation to control conditions at P < 0.1 and P < 0.05, respectively for either bulk or rhizosphere soils by depth
Soil chemistry and rhizosphere effect
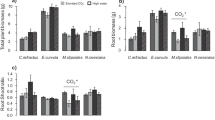
Extractable soil P concentrations were 4.91 ± 1.35 mg kg soil−1 and did not vary in absolute terms with depth (P = 0.78), indicating an overall depletion of this nutrient across the soil profile (Fig. 2; Table S1). Phosphorus concentrations were also unaffected by eCO2 (P = 0.34) in both rhizosphere (P = 0.26) and bulk (P = 0.83) soil. This contrasts with the consistent trend towards higher P levels in eCO2 rings and the significant effects reported by Hasegawa et al. (2016) during the same month. Soil P concentrations were consistently greater in the rhizosphere compared to bulk soil (P = 0.01) and there was also a significant interaction between CO2 treatment and soil type (bulk/rhizosphere, P = 0.06) indicating greater P availability in rhizosphere soil under eCO2 regardless of soil depth. Extractable P concentrations were also greater in rhizosphere soil (compared to bulk) under eCO2 when evaluated with the RII (P = 0.09; Fig. 3; Tables S1 and S2), although the rizhosphere effect was only clearly evident at 0–10 cm depth. Inorganic N and hydrolysable C fractions were not affected by eCO2 (Fig. 2; Tables S1 and S2). Ammonium-N was the main inorganic N fraction in soil (~3 times higher than NO3 −-N). This form was particularly dominant in the rhizosphere (~12 times higher), possibly reflecting high rates of N mineralization in root-associated soil and/or a lower rhizosphere pH (Fig. 2). As was found for P availability, there was a significant interaction between CO2 treatment and bulk/rhizosphere soil (P = 0.07) for total inorganic N availability, with greater concentrations in rhizosphere soil under eCO2. This effect was particularly evident at 0–10 cm depth (P = 0.06), although the rhizosphere effect was significant at both depths (Figs. 2 and 3; Table S2). Hydrolysable C, both in the form of phenols and hexoses, was also much greater in the rhizosphere soil compared to bulk soil (~3 times higher), particularly at 10–30 cm depth (significant rhizosphere effect regardless of eCO2), which suggests an active release of organic C compounds by roots (i.e. rhizodeposition; Figs. 2 and 3; Table S1). Soil pH was not affected by eCO2 (P = 0.20), despite an evident negative trend and previous findings of a significant reduction (P = 0.09) (Hasegawa et al. 2016).
Effects of elevated CO2 and soil depth on a hydrolysable C, b N (DIN), and c P (PO4) availability RII index. Values above the zero line indicate a positive rhizosphere effect, whereas negative values indicate higher P and/or N availability in the bulk soil (negative rhizosphere effect). Means and 95% confidence intervals are shown. * indicates a significant (P < 0.05) rhizosphere effect
With regard to stoichiometric responses, we found a significant interaction between CO2 treatment and bulk/rhizosphere soil in terms of the C:P nutrient ratio. This was largely due to the relative increase in P availability with respect to concentrations of hydrolysable C in the rhizosphere soil under eCO2, particularly at 10–30 cm (Fig. 4a; Table S3). In contrast, the C:P enzyme ratio increased under eCO2 in the rhizosphere soil between 10 and 30 cm (Fig. 4b; Table S4).
Effects of elevated CO2 on extractable nutrient and enzyme stoichiometric ratios in the bulk and rhizosphere soil at two incremental depths. Standard error bars are shown (n = 3). **indicates significant differences in relation to control conditions at P < 0.05 for either bulk or rhizosphere soils by depth
Root biomass
Fine root biomass (0–30 cm) within the rings in February 2014 was estimated to be 238.6 ± 18.6 g m−2, with total root biomass estimated at 1027.7 ± 286.87 g m−2. Coarse root biomass therefore represented 69.8% of total root biomass; however, coarse root biomass estimates, based on small diameter cores, did not include any taproot or large diameter coarse roots and are thus likely to be an underestimate. Root biomass at the study site varied among plots, and was positively related to SWC, P availability, tree basal area and most of the measured soil factors (Table 1).
Relationships between fine root biomass and soil biogeochemistry
Fine root biomass and, by implication, the total amount of rhizosphere soil in the extracted set of soil cores (r = 0.683; P = 0.014), were positively related to the concentrations of most of the bulk soil nutrients and enzymes relating to C, N and P cycles (Table 1), suggesting a tight association between the amount of fine roots and soil microbial activity and nutrient availability. Phosphorus availability in the bulk soil was, however, more strongly (positively) related to coarse root biomass and total root biomass than to fine root biomass alone (Table 1). In contrast, associations between fine root biomass and associated rhizosphere soil amount with soil nutrients and enzymes measured in the rhizosphere were less clear, except in the case of hexoses (i.e. carbohydrates) that, in contrast to the bulk soil, showed a strong, negative association with fine root biomass and associated total rhizosphere soil. Total and coarse root biomass were highly positively related to tree basal area (27.2 ± 2.4 m2 ha−1) and SWC (10.9 ± 0.1%), and negatively related to soil pH (5.30 ± 0.06; Table 1).
Root phosphatase activity was not related to any of the soil variables measured, including rhizosphere/bulk soil phosphatase activity or P availability (data not shown). However, root phosphatase activity was positively associated with the rhizosphere effect (RII values) of NAG, N:P enzyme ratio and, to a lesser extent, PHOS (Suppl. Fig. 3). There was a significant positive log-linear relationship between hydrolysable C concentrations (i.e. sum of dissolved hexoses and phenols) and P availability, a response mainly driven by a significant relationship in the rhizosphere soil but not in the bulk soil (Fig. 5a). We did not find the same clear association between N availability and hydrolysable C concentrations in the rhizosphere soil, although there was an overall positive relationship between these two variables (Fig. 5b).
Discussion
Our study provides novel evidence that indicates that plant and microbial communities from P-limited eucalypt woodlands are able to respond rapidly to eCO2 by altering the production of starch and cellulose degrading enzymes, which indirectly may have resulted in a relative increase of inorganic P and N in the rhizosphere compared to bulk soil. Plant responses at the rhizosphere level appear to be driven by an induced nutritional imbalance (increased C availability, i.e. eCO2, in relation to mineral P), as suggested by stoichiometric changes in rhizosphere chemistry (smaller C:P nutrient ratios under eCO2). Similar shifts in nutrient and exo-enzyme stoichiometry under eCO2 have also been reported for a grassland ecosystem (Dijkstra et al. 2013). The observed increase in C-degrading enzyme activities in the deeper soil layers may be related to a rhizosphere priming effect and, therefore, to an increase in microbial nutrient mining. In contrast, the smaller activity of C-degrading enzymes in the shallower layers, where P turnover rates are typically much faster, could possibly reflect a functional response of mycorrhizal plants to reduce microbial competition for limiting nutrients such as P (Fontaine et al. 2003). Although eucalypt roots can typically explore the soil down to several meters, the presence of a hard layer at depths ranging between 34 and 67 cm may limit the exploration of roots for extra P much deeper, making our 10–30 cm layer a good functional representation of deep exploration as compared to the shallow, 0–10 cm layer. Taken together, these two apparently contrasting, depth-dependent strategies may allow plants to take advantage of the extra CO2 present in the atmosphere, at least in the short term, by increasing soil nutrient availability and uptake (Dijkstra et al. 2013).
Soil nutrients and enzymes
Our findings of increased enzyme activities related to starch and cellulose degradation between 10 and 30 cm under eCO2 are similar to those of Finzi et al. (2006) in a temperate forest ecosystem. Interestingly, and in contrast to the latter study, N-degrading soil enzymes and, more importantly, potential soil and root phosphatase activities were not affected by eCO2 (Dijkstra et al. 2013; Carrillo et al. 2014). Despite the lack of significant CO2 effects, phosphatase activity was greater than that of any other C- or N-degrading enzyme, which is also in agreement with a global synthesis study (Sinsabaugh et al. 2009). In contrast to individual nutrient concentrations and enzyme activities, stoichiometric ratios were relatively consistent between rhizosphere and bulk soil and depths, suggesting a certain degree of homeostasis in the system (Sinsabaugh et al. 2009). Potential soil and root phosphatase activities were also decoupled from one another, as evidenced by the lack of correlation between these variables.
Strikingly, only those enzyme activities responsible for the degradation of starch (BG) and cellulose (CBH) were affected by eCO2, while NAG (chitin degradation) and XYL (hemicellulose degradation), responsible for the degradation of recalcitrant compounds, were not affected. These results suggest that plant roots may be releasing labile C compounds to the rhizosphere resulting in a depth-dependent increase in microbial activity as a consequence of priming (Carrillo et al. 2014). The up-regulation of enzymes responsible for the degradation of recalcitrant compounds may only happen after longer exposure times once labile P and more accessible bound P fractions have been depleted. Microbial priming has been reported to release nutrients locked in SOM, particularly N and P, and may be the underlying mechanism explaining the larger relative and absolute P availability in response to eCO2 observed here and by Hasegawa et al. (2016). This evidence is further supported by an increase in soil respiration rates under eCO2 early in EucFACE (Drake et al. 2016) and the lack of up-regulation of phosphatase activity, both in rhizosphere and bulk soil and in the roots. In contrast to Finzi et al. (2006), C-degrading enzymes in our study, particularly BG and CBH, markedly decreased in the rhizosphere soil between 0 and 10 cm under eCO2, which is consistent with the typically greater availability of soil nutrients in the shallower soil layers and presumably reduced benefits to plant nutrient acquisition from microbial priming (Dijkstra et al. 2013). In fact, slower decomposition has been previously described in other experiments in response to eCO2 under controlled, greenhouse conditions (Carrillo et al. 2014). This depth-dependent up- and down-regulation of microbial activity in the rhizosphere soil under eCO2 may be responsible for the relative increase in availability of inorganic N and P in the rhizosphere soil and also in the ratio of inorganic P to hydrolysable C across the whole soil profile. These results highlight the important role played by the rhizosphere in the previously described increase in N and P availability under eCO2 in the EucFACE experiment (Hasegawa et al. 2016). Our results also suggest the importance of carrying out long-term mechanistic studies in order to fully understand ecosystem responses to eCO2 that can then be appropriately integrated into global biogeochemical models (Norby et al. 2015). Furthermore, the significant positive relationship found between hydrolysable C concentrations and P availability in the rhizosphere soil, together with the lack of effect of eCO2 on phosphatase activity, suggests an additional potential role of root C exudates (e.g., chelators such as carboxylic acids, phenolics, etc.) in the desorption/solubilisation of P from mineral surfaces through ligand exchange and dissolution (Badri and Vivanco 2009; Dijkstra et al. 2013; Lambers et al. 2015).
Roots as a driver of biogeochemical processes in response to elevated CO2
In February 2014, fine root biomass values of 238.6 g m−2 were slightly smaller, but in the same order of magnitude, than those reported by Macinnis-Ng et al. (2009) (490 g m−2) for remnant Cumberland Plain woodlands within the same region. Our data do, however, represent just a single snapshot of root biomass and so further sampling is needed to determine how variable fine root biomass and productivity are across seasons and years to enable robust tests of eCO2 effects and the role of environmental factors mediating any response.
Elevated CO2 concentrations can stimulate forest NPP through increased photosynthesis, which often results in greater C allocation to root biomass (Palmroth et al. 2006; Carol Adair et al. 2011; Drake et al. 2011). Matamala & Schlesinger (2000) reported a significant increase in fine root biomass production (86%, 0–20 cm) only after two years of fumigation in a comparatively young forest stand, while de Graaff et al. (2006) reported an overall increase (34%) in belowground productivity across multiple eCO2 experiments, but only where extra N had been supplied. Our early data suggest that belowground responses to eCO2 in the mid to long-term could be mediated by spatial and temporal variations in the availability of soil resources (e.g., water and nutrients) and also by pre-treatment variability in tree cover. Future sampling campaigns will, however, determine the long-term ability of this system to increase standing root biomass and productivity in response to eCO2 and the role of environmental factors mediating this response.
In parallel, fine root biomass and, by extension, total rhizosphere soil amount in the extracted soil cores were highly related to the activity of four enzymes in the bulk soil, and to bulk soil nutrient availability. In contrast, root phosphatase activity was upregulated by a positive rhizosphere effect on the chitinase activity and N:P enzyme ratio, suggesting that root-derived enzymes may be also important in maintaining a balanced supply of N and P for plant growth. Overall, our results support the concept of plants controlling nutrient release and uptake either through active root foraging or via enhanced or decreased activity of the microbial communities in the rhizosphere soil, more than directly through the release of plant-derived nutrient-releasing enzymes, although this last mechanism can also play a role (Berendsen et al. 2012; Finzi et al. 2015). In this sense, it has been shown that plants can shape the composition of their rhizosphere microbiome, which is crucial for plant health and the tolerance of biotic and abiotic stresses (Berendsen et al. 2012; Philippot et al. 2013). This is, however, an unexplored field of research, particularly in the context of climate change, that deserves further attention given its implications for C sequestration (Berg and Smalla 2009). Our data also support a broader and more functional definition of rhizosphere as the portion of soil affected by the presence of roots (plant-soil interface sensu lato), and not only restricted to the narrow portion of soil directly attached to them (Hinsinger et al. 2009). In fact, this broader definition coincides better with the original definition of rhizosphere given by Lorenz Hiltner in 1904 (Hartmann et al. 2008) and that implicitly given in the first editorial of the journal Rhizosphere (Adl 2016).
Our study provides novel evidence suggesting that rhizosphere processes in a mature eucalypt forest ecosystem can respond rapidly (<2 years) to atmospheric CO2 enrichment. Here we show that several early belowground responses of a P-limited forested ecosystem to eCO2 are associated with an alteration in the allocation of resources to strategies that enhance the ability of plants to acquire resources (particularly inorganic P and N) necessary to maintain increased plant metabolism and potentially, therefore, growth under eCO2. In particular, we described a synchronized down- (0–10 cm) and up-regulation (10–30 cm) of rhizosphere activity that was associated with an overall increase in inorganic P and N availability in the rhizosphere across the entire soil profile studied. Our findings suggest that rhizosphere-mediated increases in soil organic matter decomposition and enhanced nutrient availability may thus be an important mechanism in promoting plant growth in response to elevated CO2 in this and perhaps other nutrient-poor ecosystems.
References
Adl S (2016) Rhizosphere, food security, and climate change: a critical role for plant-soil research. Rhizosphere 1:1–3. doi:10.1016/j.rhisph.2016.08.005
Allen AS, Andrews JA, Finzi AC et al (2000) Effects of free-air CO2 enrichment (FACE) on belowground processes in a Pinus taeda forest. Ecol Appl 10:437–448. doi:10.1890/1051-0761(2000)010[0437:EOFACE]2.0.CO;2
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85(10):2682–2686. doi:10.1890/03-0650
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681. doi:10.1111/j.1365-3040.2009.01926.x
Bell CW, Fricks BE, Rocca JD et al (2013) High-throughput fluorometric measurement of potential soil extracellular enzyme activities J Vis Exp:e50961. doi:10.3791/50961
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486. doi:10.1016/j.tplants.2012.04.001
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Carol Adair E, Reich PB, Trost JJ, Hobbie SE (2011) Elevated CO2 stimulates grassland soil respiration by increasing carbon inputs rather than by enhancing soil moisture. Glob Chang Biol 17:3546–3563
Carrillo Y, Dijkstra FA, Pendall E et al (2014) Plant rhizosphere influence on microbial C metabolism: the role of elevated CO2, N availability and root stoichiometry. Biogeochemistry 117:229–240. doi:10.1007/s10533-014-9954-5
Chantigny MH, Angers DA, Kaiser K, Kalbitz K (2006) Extraction and characterization of dissolved organic matter. In: Carter MR, Gregorich E (eds) Soil sampling and methods of analysis., Second ed. CRC Press, Boca Raton, p 617–635
Covelo F, Rodríguez A, Gallardo A (2008) Spatial pattern and scale of leaf N and P resorption efficiency and proficiency in a Quercus robur population. Plant Soil 311:109–119. doi:10.1007/s11104-008-9662-9
Crous KY, Ósvaldsson A, Ellsworth DS (2015) Is phosphorus limiting in a mature Eucalyptus woodland? Phosphorus fertilisation stimulates stem growth. Plant Soil 391:293–305. doi:10.1007/s11104-015-2426-4
Day FP, Schroeder RE, Stover DB et al (2013) The effects of 11 yr of CO2 enrichment on roots in a Florida Scrub-oak ecosystem. New Phytol 200:778–787. doi:10.1111/nph.12246
de Graaff M-A, van Groenigen K-J, Six J et al (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Chang Biol 12:2077–2091. doi:10.1111/j.1365-2486.2006.01240.x
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:1–8. doi:10.3389/fmicb.2013.00216
Drake JE, Darby BA, Giasson M-A et al (2013) Stoichiometry constrains microbial response to root exudation- insights from a model and a field experiment in a temperate forest. Biogeosciences 10:821–838. doi:10.5194/bg-10-821-2013
Drake JE, Gallet-Budynek A, Hofmockel KS et al (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14:349–357. doi:10.1111/j.1461-0248.2011.01593.x
Drake JE, Macdonald C, Tjoelker MG et al (2016) Short-term carbon cycling responses of a mature eucalypt woodland to gradual stepwise enrichment of atmospheric CO2 concentration. Glob Chang Biol 22:380–390. doi:10.1111/gcb.13109
Finzi AC, Abramoff RZ, Spiller KS et al (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Chang Biol 21:2082–2094. doi:10.1111/gcb.12816
Finzi AC, Sinsabaugh RL, Long TM, Osgood MP (2006) Microbial community responses to atmospheric carbon dioxide enrichment in a warm-temperate forest. Ecosystems 9:215–226. doi:10.1007/s10021-005-0078-6
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843. doi:10.1016/S0038-0717(03)00123-8
Friend AD, Lucht W, Rademacher TT et al (2014) Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc Natl Acad Sci U S A 111:3280–3285. doi:10.1073/pnas.1222477110
Gimeno TE, Crous KY, Cooke J et al (2015) Conserved stomatal behaviour under elevated CO2 and varying water availability in a mature woodland. Funct Ecol 30:700–709. doi:10.1111/1365-2435.12532
Hartmann A, Rothballer M, Schmid M (2008) Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 312:7–14. doi:10.1007/s11104-007-9514-z
Hasegawa S, Macdonald CA, Power SA (2016) Elevated carbon dioxide increases soil nitrogen and phosphorus availability in a phosphorus-limited eucalyptus woodland. Glob Chang Biol 22:1628–1643. doi:10.1111/gcb.13147
Hinsinger P, Bengough a. G, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152. doi: 10.1007/s11104-008-9885-9
Iversen CM (2010) Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol 186:346–357. doi:10.1111/j.1469-8137.2009.03122.x
Jin J, Tang C, Sale P (2015) The impact of elevated carbon dioxide on the phosphorus nutrition of plants: a review. Ann Bot doi: 10.1093/aob/mcv088
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33. doi:10.1007/s11104-009-9925-0
Lambers H, Martinoia E, Renton M (2015) Plant adaptations to severely phosphorus-impoverished soils. Curr Opin Plant Biol 25:23–31. doi:10.1016/j.pbi.2015.04.002
Liberloo M, Tulva I, Raïm O et al (2007) Photosynthetic stimulation under long-term CO2 enrichment and fertilization is sustained across a closed Populus canopy profile (EUROFACE). New Phytol 173:537–549. doi:10.1111/j.1469-8137.2006.01926.x
Macinnis-Ng CMO, Fuentes S, O’Grady AP et al (2009) Root biomass distribution and soil properties of an open woodland on a duplex soil. Plant Soil 327:377–388. doi:10.1007/s11104-009-0061-7
Matamala R, Schlesinger WH (2000) Effects of elevated atmospheric CO2 on fine root production and activity in an intact temperate forest ecosystem. Glob Chang Biol 6:967–979. doi:10.1046/j.1365-2486.2000.00374.x
McCarthy HR, Oren R, Johnsen KH et al (2010) Re-assessment of plant carbon dynamics at the Duke free-air CO2 enrichment site: interactions of atmospheric [CO2] with nitrogen and water availability over stand development. New Phytol 185:514–528. doi:10.1111/j.1469-8137.2009.03078.x
Nguyen C (2009) Rhizodeposition of organic C by plants: mechanisms and controls. In: Navarrete M, Debaeke P et al (eds) Eric Lichtfouse. Sustainable Agriculture, Springer Netherlands, pp 97–123
Nie M, Lu M, Bell J et al (2013) Altered root traits due to elevated CO2: a meta-analysis. Glob Ecol Biogeogr 22:1095–1105. doi:10.1111/geb.12062
Norby RJ, De Kauwe MG, Domingues TF et al (2015) Model-data synthesis for the next generation of forest free-air CO2 enrichment (FACE) experiments. New Phytol 209:17–28. doi:10.1111/nph.13593
Palmroth S, Oren R, McCarthy HR et al (2006) Aboveground sink strength in forests controls the allocation of carbon below ground and its [CO2]-induced enhancement. Proc Natl Acad Sci U S A 103:19362–19367. doi:10.1073/pnas.0609492103
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799
Phillips RP, Fahey TJ (2005) Patterns of rhizosphere carbon flux in sugar maple (Acer saccharum) and yellow birch (Betula allegheniensis) saplings. Glob Chang Biol 11:983–995. doi:10.1111/j.1365-2486.2005.00959.x
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194. doi:10.1111/j.1461-0248.2010.01570.x
Phillips RP, Meier IC, Bernhardt ES et al (2012) Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol Lett 15:1042–1049. doi:10.1111/j.1461-0248.2012.01827.x
Pritchard SG, Strand AE, McCormack ML et al (2008) Fine root dynamics in a loblolly pine forest are influenced by free-air-CO2 -enrichment: a six-year-minirhizotron study. Glob Chang Biol 14:588–602. doi:10.1111/j.1365-2486.2007.01523.x
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rayment G, Lyons D (2011) Soil chemical methods - Australasia. CSIRO Publishing, Collingwood, Victoria
Sinsabaugh RL, Belnap J, Rudgers J et al (2015) Soil microbial responses to nitrogen addition in arid ecosystems. Front Microbiol 6:819. doi:10.3389/fmicb.2015.00819
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798. doi:10.1038/nature08632
Stover DB, Day FP, Butnor JR, Drake BG (2007) Effect of elevated CO2 on coarse-root biomass in Florida Scrub detected by ground-penetrating radar. Ecology 88:1328–1334. doi:10.1890/06-0989
Trumbore S (2000) Age of soil organic matter and soil respiration: radiocarbon constraints on belowground C dynamics. Ecol Appl 10:399–411. doi:10.1890/1051-0761(2000)010[0399:AOSOMA]2.0.CO;2
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Wang YP, Law RM, Pak B (2010) A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences 7:2261–2282. doi:10.5194/bg-7-2261-2010
Zak DR, Pregitzer KS, Curtis PS, Holmes WE (2000) Atmospheric CO2 and the composition and function of soil microbial communities. Ecol Appl 10:47–59. doi:10.1890/1051-0761(2000)010[0047:ACATCA]2.0.CO;2
Acknowledgements
We are grateful to Prof. David Ellsworth, Burhan Amiji, Dr. Craig Barton, Dr. Vinod Kumar and Steven Wohl for managing the EucFACE facility. EucFACE is an initiative supported by the Australian Government through the Education Investment Fund, the Department of Industry and Science, and the Australian Research Council in partnership with the Western Sydney University. Facilities at EucFACE were built as an initiative of the Australian Government as part of the Nation-building Economic Stimulus Package. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philip John White.
Electronic supplementary material
Table S1
(DOCX 36 kb)
Table S2
(DOCX 24 kb)
Table S3
(DOCX 28 kb)
Table S4
(DOCX 24 kb)
Suppl. Fig. 1
(DOCX 2492 kb)
Suppl. Fig. 2
(DOCX 25 kb)
Suppl. Fig. 3
(DOCX 49 kb)
Rights and permissions
About this article
Cite this article
Ochoa-Hueso, R., Hughes, J., Delgado-Baquerizo, M. et al. Rhizosphere-driven increase in nitrogen and phosphorus availability under elevated atmospheric CO2 in a mature Eucalyptus woodland. Plant Soil 416, 283–295 (2017). https://doi.org/10.1007/s11104-017-3212-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3212-2