Abstract
Aims
Plant growth forms can influence carbon cycling, particularly in carbon-rich ecosystems like northern peatlands; however, mechanistic evidence of this relationship is limited. Our aim was to determine if northern peatland plant growth forms alter belowground dissolved carbon chemistry and enhance carbon release through stimulated microbial metabolism.
Methods
We used replicated, peat monoliths populated exclusively by Sphagnum mosses, graminoids, or bare peat and quantified changes in belowground dissolved organic carbon chemistry, microbial metabolism, as well as respired CO2.
Results
The graminoid growth form was significantly distinct in belowground dissolved organic carbon chemistry with carbon compound lability 20 % and 11 % greater than bare peat and Sphagnum moss respectively. The labile dissolved organic carbon stimulated the microbial community, as indicated by greater microbial metabolic activity and richness values in conjunction with 50 % higher respired CO2 fluxes under the graminoid treatment.
Conclusions
Our results provide mechanistic evidence that peatland plant growth forms can drive carbon cycling processes by altering dissolved organic carbon chemistry to prompt cascading effects on the microbial community and carbon release — trends suggestive of microbial priming effects. Should climate change increase graminoid prevalence at the expense of Sphagnum moss northern peatland carbon store stability may be threatened by this mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant communities have a central role in ecosystem carbon dynamics, driving carbon uptake via photosynthesis and indirectly regulating carbon release through decomposition (Dorrepaal 2007; Del Giudice and Lindo 2017). Different plant species vary greatly in their capacity to take up or release carbon, with implications for ecosystem level carbon cycling. To address this variation researchers have organized species into plant functional groups, grouping together species with similar effects on ecosystem processes (Lavorel and Garnier 2002). Building evidence has demonstrated the success of this approach, with the plant growth form grouping acting as a particularly effective predictor of carbon dynamics — especially for decomposition processes (Armstrong et al. 2012; Robroek et al. 2016; Walker et al. 2016). In fact, plant growth form is such a strong determinant of carbon dynamics that it can have a greater influence on decomposition rates than even warming conditions (Ward et al. 2015), a well-established control on decomposition (Davidson and Janssens 2006). Yet, despite the clear predictive capacity of plant growth form on carbon release, we have a very limited mechanistic understanding of how different plant growth forms influence belowground carbon processes (Dunn et al. 2015; Ward et al. 2015; Robroek et al. 2016).
It is understood that plant growth form determines the quantity and quality of primary, carbon-rich material introduced into the belowground environment (Dorrepaal et al. 2005; Del Giudice and Lindo 2017) ― factors that in turn drive microbial decomposition rates (Blagodatsky et al. 2010). The quantity and quality of plant contributions to the dissolved organic carbon (DOC) pool are especially salient to decomposition because DOC comprises a key energy source for microbial communities (Allison et al. 2010; Blagodatsky et al. 2010). Simple, high-quality root inputs can be particularly important because labile DOC compounds can significantly enhance decomposition of the soil organic matter by stimulating or ‘priming’ the microbial community (Bengtson et al. 2012). However, the specific effect of different plant growth forms on overall belowground DOC quantity and quality remains unclear, even though this information is key to developing a mechanistic understanding of how plant growth forms influence belowground carbon dynamics.
Plant and soil linkages that dictate ecosystem-level carbon dynamics are particularly pertinent for peatland ecosystems, as peatlands store approximately 30 % of the Earth’s terrestrial carbon (Gorham 1991; Yu 2012). These vast stores of carbon in northern peatlands have accumulated due to the remarkably slow decomposition rates that characterise this ecosystem (Rydin and Jeglum 2013). Plant growth forms, such as Sphagnum moss, can strongly contribute to these slow decomposition rates by producing highly recalcitrant litter (Straková et al. 2010; Pinsonneault et al. 2016). Conversely, other peatland plant growth forms, such as graminoids, may actually stimulate decomposition rates by producing comparatively readily-degradable litter (Bombonato et al. 2010; Straková et al. 2010). Graminoids may also stimulate decomposition rates by introducing simple carbon compounds into the belowground system as root exudates, providing energy that stimulates microbial activity, priming microbial communities for enhanced decomposition (Kuzyakov et al. 2000); however, very few studies have considered such priming effects in peatland systems (but see Fan et al. 2013).
Peatland plant growth forms have the potential to strongly alter peatland belowground carbon dynamics via differing carbon inputs (Dunn et al. 2015; Walker et al. 2016). Among the few studies that have considered peatland plant growth form and belowground DOC relationships, the findings have been largely incongruent. For example, even broad DOC concentration trends are wide ranging in the literature, where both non-significant and significant effects of plant form are both reported (Armstrong et al. 2012; Dunn et al. 2015; Robroek et al. 2016). These difficulties appear to reflect the various limitations of the experimental approaches employed, such as single time point measurements, the absence of living plants, and natural variability in local environmental conditions. Our experiment builds on this knowledge base by studying belowground carbon dynamics in replicated, intact mesocosms populated exclusively by Sphagnum mosses, graminoids, or bare peat maintained under standardized environmental conditions for nine months. In addition, our study presents one of the first applications of Biolog Ecoplates® as a measure of microbial activity in peatland environments. In doing so, our main objective was to determine if different plant growth forms influence belowground DOC quantity and quality to stimulate microbial activity and carbon release. As graminoids produce root exudates and relatively high quality litter, we anticipated this growth form would increase both belowground DOC concentration and biodegradability, resulting in increased microbial activity, decomposition rates, and ultimately carbon release.
Methods and materials
Study site
Intact, vegetated monoliths were collected from a 10.2 ha fen located near the city of White River, Ontario, Canada (48°21’N, 85°21’W). The research area is part of the White River Experimental Watersheds Study, a long-term program that has previously established the plant community structure, hydrology, and carbon dynamics (McLaughlin and Webster 2010; Webster and McLaughlin 2010), which is maintained by the Ontario Ministry of Natural Resources and Forestry - Ontario Forest Research Institute. The 31-year annual average precipitation and temperature are 970 mm and 2.2 °C respectively, with a growing season ranging from 70 to 100 days (McLaughlin and Webster 2010; Webster et al. 2013). In January and July the mean temperature values are −14.2 °C and 14.7 °C respectively; temperatures are rarely above 0 °C during the winter or 30 °C in summer (McLaughlin and Webster 2010). The precipitation at this site dominantly falls as rain, although 40 % occurs as snowfall (McLaughlin and Webster 2010). Previous vegetation surveys of the site have documented that approximately 80 % of the fen is vegetated by graminoid species, primarily Carex spp. The shrub and bryophyte species include sweet gale (Myrica gale L.), leatherleaf (Chamaedaphne calyculata (L.) Moench.), and Labrador tea (Rhododendron groenlandicum (Oeder) Kron and Judd), with Scorpidium spp., Rhizomnium spp., and Sphagnum spp. as dominant bryophytes.
Experimental design
In August 2014, 18 intact peat monoliths (10 cm diameter × 20 cm deep) were collected from the study site, with six monoliths each of exclusively bare soil, Sphagnum moss cover on bare peat, or graminoid cover on bare peat (3 treatments × 6 replicates =18 mesocosms). The dimensions of our mesocosms were modeled from a successful series of publications considering carbon dynamics associated with both Sphagnum mosses and graminoids (see Fenner et al. 2007a, b). The monoliths were extracted from regions of relatively homogenous cover, reflecting treatment conditions. On average the Sphagnum moss monoliths initially contained 39 ± 4 individuals, while the graminoid monoliths initially contained 12 ± 2 individuals. Monoliths were collected by cutting a 10 cm diameter ring using a 40 cm narrow blade saw and then carefully inserting a PVC pipe into the ground (10 cm diameter × 25 cm deep) to extract the intact peat core within the PVC pipe. The collected monolith was then capped at the base and transported to the University of Western Ontario’s Biotron Institute for Experimental Climate Change. Once at the institute a tapered hole was drilled 1 cm from the base of each mesocosm and fitted with ½ inch threaded ABS barbed fitting to form a drain. A 30 cm length of clear vinyl tube was attached to the fitting for water sample collection as discussed below. All mesocosms were then placed in a greenhouse under the ambient growing conditions (described below) for 20 weeks to stabilize after the initial collection disturbance.
The greenhouse used throughout this experiment was an environmentally controlled system, regulating humidity, atmospheric CO2, and temperature conditions through fully automated processes (ARGUS Control Systems Ltd., White Rock, BC, Canada). Specifically, the relative humidity conditions were not less than 60 %, while the atmospheric CO2 concentrations matched outdoor ambient conditions, averaging 426 ppm. The temperature conditions purposefully followed two regimes, reflecting pre-growing and growing seasons. During the pre-growing season (November 1 – April 30) temperature conditions were held constant at 11.5 °C, with a naturally reduced average photoperiod of approximately 12 h per day. These conditions allowed us to quantify and control for differences in belowground chemistry when plant inputs were minimal. Throughout the growing season (May 1 – October 31) temperatures were programmed to the five year running average local daily maximum and minimum with a maximum annual summer temperature of 35 °C on 8 Jul 2016. The photoperiod was also naturally longer throughout the growing season with an average of approximately 14 h of sunlight daily. This climate program allowed us to discern how belowground chemistry changed when plant inputs were at their highest during the growing season. Unlike the other environmental variables, water table height was maintained manually, held at 2 cm below the peat surface. The water table was maintained via biweekly top and bottom water additions of dilute Rudolph’s solution, used to mimic rainwater and subsurface lateral transport nutrient delivery (diluted by a factor of 4 and adjusted to pH 5.8; see Faubert and Rochefort 2002). Dilute Rudolph’s solution was also added below the soil promptly following sampling events to further maintain the appropriate water table height. Finally the mesocosms were randomly relocated within the greenhouse monthly to address any microclimate effects.
Dissolved organic carbon sampling and analysis
Porewater samples were collected monthly from the vinyl tubes at the base of the mesocosms from February 2015 – October 2015. The porewater (200 mL) was collected in clean, pre-rinsed 500 mL Nalgene® bottles and filtered using ashed, Macherey-Nagel GF-2 glass filters (pore size 0.5 μm). The filtrate was used to determine the dissolved organic carbon content measured as DOC in mg L−1, and carbon quality measured as the specific ultraviolet absorbance at a wavelength of 254 nm (SUVA254) in L mg C−1 m−1. SUVA254 quantifies the aromaticity of DOC (Weishaar et al. 2003), where more aromatic carbon compounds indicate less biodegradable carbon (Kalbitz et al. 2003). Carbon quality was also determined using excitation-emission matrix (EEMs) fluorescence spectroscopy analysis and summarized using three common indices, the fluorescence index (FI), the humification index (HIXEM), and the freshness index (BIX). The FI, HIXEM, and BIX indices indicate the carbon source, the degree of humification, and carbon freshness respectively. Microbially sourced, less humified, fresh carbon tends to be more biodegradable (Fellman et al. 2010). Filtrate for DOC and SUVA254 analysis was stored in 30 mL HDPE bottles, while filtrate for EEMs was stored in 20 mL acid-washed, glass bottles. All samples were stored in the dark at 4 °C until analyzed.
The DOC content of the porewater samples was determined using the persulfate wet oxidation method using an iTOC Aurora 1030 (OI Analytical, College Station, TX, USA). The SUVA254 character was quantified using methods outlined by Weishaar et al. (2003). Specifically, the ultraviolet absorbance at λ = 254 for each sample was determined using a quartz cuvette with a 1 cm path length in conjunction with a Spectramax® M2 spectrophotometer (Molecular Devices Corp., Sunnyvale, CA, USA). Absorbance values were then converted to m−1 and divided by the DOC concentration of the sample to determine the SUVA254 value. The fluorescence excitation and emission was determined using a Horiba Aqualog® spectrofluorometer with a xenon lamp in the Watershed Hydrology Group Laboratory at McMaster University, Ontario, Canada. The excitation wavelengths ranged from 240 to 600 nm with 10 nm increments and the emission wavelengths ranged from 210 to 620 nm with 3.27 nm increments, with an integration time of 0.5 s. Samples were diluted 1:1 with ultra pure deionised water to diminish any attenuation of the light by the sample itself (inner filtering effects). All samples were normalized using the area under the water Raman peak at an excitation of 350 nm to address natural changes in lamp intensity due to decay (Lawaetz and Stedmon 2009). The produced excitation and emission matrices were then used to calculate the aforementioned carbon quality indices using R software (R Core Team 2012) in combination with the most recent index equations described by Fellman et al. (2010).
Respired carbon dioxide
Respired carbon dioxide from combined autotrophic and heterotrophic sources was determined monthly from February 2015 – October 2015 following the methodology established by Carroll and Crill (1997). This approach resulted in nine separate measures of respired carbon dioxide from all mesocosms, corresponding with repeated porewater sample collection. The dark static chamber measurements were completed using a Licor-8100A infrared gas analyzer (LI-COR, Lincoln, NE, USA) in combination with a custom chamber head. The chamber head was 2.4 L in volume (10 cm diameter, 30 cm height), with a foam seal, and was constructed of non-translucent PVC to stop photosynthesis during readings. No internal fans were placed in the chamber head as initial trials demonstrated the sampling unit could adequately mix the gas within the relatively small chamber head. The LI-8100A unit was programmed to record the air carbon dioxide concentration every 0.5 s, averaging these values every 4 s over a 2.5 min collection period. The final carbon dioxide flux values were corrected for the chamber volume, soil temperature (top 5 cm), air moisture content, initial carbon dioxide concentration, and initial pressure as measured by the LI-8100A unit. The final carbon dioxide flux values are presented as μmol of CO2 per m2 per second (μmol CO2 m−2 s−1).
Vegetative biomass and peat collection
All mesocosms were destructively sampled after nine months to determine vegetative biomass and collect peat substrate for microbial community level physiological profiling. Aboveground vegetation was clipped at the surface before the monoliths were carefully extracted from their containers and the coarse live root biomass (diameter > 0.2 cm) was removed by hand. The collected vegetative biomass was dried at 60 °C to a constant weight to give aboveground and root biomass in g as summarized in Supplementary Table S1. All of the peat substrate for each mesocosm was then fully homogenized by hand, placed in an individual plastic bag, and stored in the dark at 4 °C until further processing for microbial community profiling.
Microbial community level physiological profiling
Differences in the microbial community metabolic profiles of peat substrate were determined using Biolog Ecoplates® (Biolog Inc., Hayward, CA, USA) for nine of the eighteen mesocosms (3/6 replicates). Ecoplates are 96 well microplates that contain a triplicate set of 31 different carbon substrates as well as three water control wells. The carbon substrates vary in quality including ten carbohydrates, nine carboxylic/acetic acids, four polymers, six amino acids, and two amine/amide compounds (see Table 3 in Frąc et al. 2012). The metabolism of a particular carbon substrate by a microbial community is indicated by a colour change caused by the redox-indicator tetrazolium. To determine the microbial metabolic profile, peat samples were processed using the methods established by Garland (1996). Specifically, 10 g fresh weight, fully homogenized peat was suspended in 100 mL of sterile ultra pure water using sterile techniques, and shaken for 10 min on a wrist shaker at maximum speed. The sample then settled for 10 min before being serially diluted to 10−3. A plate was then inoculated with the resultant suspension by adding 150 μl of it to each well. Three replicate sets of carbon substrates within a plate were averaged for each mesocosm examined. The well absorbance at 590 nm was determined using a Spectramax® M2 spectrophotometer (Molecular Devices Corp., Sunnyvale, CA, USA) at the time of inoculation, and every 24 h for 7 days while being incubated at 22 °C.
The raw absorbance data were corrected by subtracting both the control values and the absorbance values recorded at the time of inoculation. All resultant negative values were converted to zero as suggested by Garland (1997). For each time point the carbon substrate utilization richness of the microbial community was determined as an indicator of metabolic richness by deriving the number of wells with metabolic activity (absorbance at 590 > 0.25). The average well colour development (AWCD) was also determined as an indicator of overall microbial metabolic activity using following equation:
where C is the raw absorbance value at 590 nm for each well containing carbon substrate, and R is the raw absorbance value of the control well (Garland and Mills 1991).
Statistics
A one-way repeated measures Anova (RM-Anova) with a Tukey post-hoc test was used to consider the effects of plant growth form on DOC, SUVA254, the fluorescence index, the humification index, the freshness index, respired carbon dioxide, carbon substrate utilization richness, and AWCD over time (Statistica 7, StatSoft. Inc. 2004). The same test was also used to determine significant differences in root biomass and aboveground biomass among treatments. A priori contrasts were used to separate the sampling time points into pre-growing and growing season for the respired carbon dioxide flux data. However, as the transition between pre-growing and growing seasons (May) elected high variability in respiration, a subsequent RM-Anova was conducted without May for the respired carbon dioxide flux data, considering the effects of plant growth form.
Results
Dissolved organic carbon quantity and quality
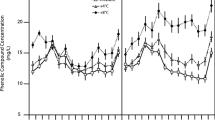
The concentration of DOC varied seasonally throughout the experiment, with the lowest values reported for all mesocosms during the pre-growing season and at the end of the growing season (F 8,120 = 37.31, P < 0.001) (Fig. 1a). Throughout the pre-growing season the different treatment types had relatively similar DOC concentrations, ranging from 17 to 40 mg L−1 across all treatments. During the growing season, however, DOC concentrations significantly increased and were significantly different among treatments (Time × Treatment: F 16,120 = 1.88, P = 0.029) (Fig. 1a). Specifically, Sphagnum moss and bare soil produced significantly more DOC in porewater than the graminoid treatment during the growing season. In July differences between treatments were maximized with Sphagnum moss and bare soil generating 20 % and 15 % more DOC than the graminoid treatment. The concentrations of DOC under the Sphagnum moss and bare peat treatments were relatively similar throughout the entire experiment. At the end of the growing season all treatments began to converge, tending towards pre-growing season DOC concentrations.
The influence of plant growth form over time on a dissolved organic carbon concentration and b carbon quality measured as specific ultraviolet absorbance at the wavelength 254 nm (SUVA254) in peatland mesocosm porewater (mean ± SE). The vertical dashed line denotes the two temperature regimes implemented with the pre-growing season occurring from February 2015 to April 2015 (left of the dashed line), and the growing season occurring May 2015 to October 2015 (right of the dashed line)
The SUVA254 measurements, like DOC, differed significantly among treatments over time (F 16,120 = 4.02, P < 0.001), with the highest values for all treatments occurring during the pre-growing season, indicating higher molecular aromaticity (Fig. 1b). All SUVA254 values decreased briefly during the early growing season, but diverged and increased after July 2015 in the following order: graminoid < Sphagnum moss < bare peat. Differences between treatments were maximized at the experiment’s end with graminoid SUVA254 values 11–20 % lower than the bare peat and Sphagnum moss treatments. The EEMs fluorescence measurements, conversely, did not show any clear seasonal trends, but instead were relatively consistent throughout the experiment (Supplementary Fig. S1). Unlike the humification and freshness indices, the fluorescence index did indicate that Sphagnum moss communities produce significantly and consistently higher FI values (more microbial-like carbon) throughout the experiment (F 2,14 = 17.66, P < 0.001).
Respired carbon dioxide
Respired carbon dioxide levels responded strongly to the transition from the pre-growing to the growing temperature schemes, resulting in a spike in respired carbon dioxide flux values in May 2015. During this month some individual measures were 139 % greater than average carbon flux values for the experiment. This disturbance masked much of the treatment response over time, as substantiated by the a priori contrast test, which demonstrated that pre-growing season respiration (Feb 2015 – Apr 2015) was significantly lower than the growing season (Jun 2015 – Oct 2015) when May was excluded from the contrasts (F 3,43 = 4.53, P = 0.008). As such, the May respiration values were dropped from the subsequent RM-Anova analysis. Prior to the change to variable temperature regimes, respired carbon dioxide rates were highly uniform across treatments, but from July until the end of the growing season, the graminoid community produced significantly higher values than either the Sphagnum moss or bare peat treatments (F 14,105 = 2.74, P = 0.002) (Fig. 2). Specifically, during the month of July the graminoid treatment released approximately 50 % more respired carbon dioxide then either of the other treatments.
Microbial community level physiological profiling
The AWCD and microbial metabolic richness both indicated that the microbial community characteristics significantly differed among treatments (Fig. 3). The AWCD demonstrated that after 72 h of incubation the graminoid and Sphagnum moss communities had significantly higher microbial activity compared to the bare peat treatment (Incubation Time × Treatment: F 12,36 = 3.24, P = 0.003), with the numerically greatest microbial activity occurring under the graminoid treatment. The microbial metabolic richness measures indicated that after 72 h of incubation the graminoid treatment had a microbial community that utilized a greater number of carbon substrates than either the Sphagnum moss or bare peat treatments (Incubation Time × Treatment: F 12,36 = 2.07, P = 0.045). These differences were most apparent after 168 h of incubation with graminoid microbial metabolic richness values approximately 20 % higher than either the Sphagnum moss or bare peat.
Measures of microbial activity and microbial metabolic richness as indicated by a average well colour development and b carbon substrate utilization richness (mean ± SE). Both average well colour development and carbon substrate utilization richness were determined from peat samples collected at the experiment’s end and incubated in Biolog Ecoplates® for seven days
Discussion
Our experiment demonstrates that plant growth form, specifically graminoids, can influence ecosystem carbon dynamics directly through increased labile carbon contributions with cascading effects through stimulation of the microbial community. These results are important for northern peatlands as they store globally significant quantities of terrestrial carbon (Gorham 1991; Yu 2012), which if released into the atmosphere could intensify climate change conditions. Recent studies have shown that northern peatlands, when exposed to climate change scenarios, will undergo vegetation restructuring events, increasing the prevalence of vascular growth forms like graminoids at the expense of Sphagnum moss (Weltzin et al. 2000; Wiedermann et al. 2007; Dieleman et al. 2015). Alongside these vegetative restructuring events we predict there will be an increase in the prevalence of simple, non-aromatic DOC compounds belowground via senescing fine root biomass and root exudate inputs (Crow and Wieder 2005; Robroek et al. 2016) in the short-term, and through changes in leaf litter quality in the long-term (Pinsonneault et al. 2016). These predicted vegetative restructuring events are also anticipated to be self-reinforcing, altering the local environment to increase resource cycle rates, and thus resource availability (Eppinga et al. 2009). Our data adds support to this prediction, indicating graminoid inputs can be completely mineralized throughout the growing season when plant inputs are maximized, keeping DOC concentrations low and carbon dioxide release high.
Belowground, labile DOC compounds stemming from graminoids can function as a readily available microbial energy source (Fenchel et al. 1998; Philippot et al. 2013), as shown through increasing microbial activity and metabolic richness in our study. These trends fit well with work by Robroek et al. (2016) who demonstrated the removal of peatland vascular vegetation (graminoids, shrubs, graminoids/shrubs) consistently lowers overall potential microbial activity, most likely due to the loss of simple root exudate inputs (Kuzyakov 2010). Increased DOC lability, metabolic activity and richness, alongside increased respired carbon dioxide are suggestive of microbial priming effects. Microbial priming effects refers to the addition of organic or mineral substances that stimulate soil organic material (SOM) turnover rates, and are indicated by increased respired carbon dioxide release produced via elevated microbial metabolism, microbial biomass production, and microbial exoenzyme release (Blagodatskaya and Kuzyakov 2008). Microbial priming effects have been well established in mineral soils, however relatively few studies in organic soils have considered the phenomenon, particularly in a peatland context (Fan et al. 2013). We provide three points of support on the phenomenon of microbial priming effects in this study: 1) associative trends with root exudates and the presence of labile carbon belowground, 2) increased microbial metabolic richness and activity, and 3) enhanced respiration.
Root exudates are composed of predominantly simple, labile carbon compounds, and are hypothesized to be produced by plants to induce microbial priming effects as a competitive strategy to gain nutrients in the rooting zone (Gunina and Kuzyakov 2015). The significantly lower SUVA254 values found under the graminoid treatment in our study indicates this plant growth form was introducing simple carbon compounds belowground during the growing season, presumably as root exudates (Crow and Wieder 2005). That said the clear effect of different plant growth forms on SUVA254 values did not become apparent until July 2015, after which the bare peat, Sphagnum moss, and graminoid treatments diverged. These trends fit well with established soil microbial biomass seasonal trends, where the microbial community rapidly builds biomass using readily available carbon resources from April until June, only to have notable decline or stabilization in biomass for the remainder of the growing season (see Buckeridge et al. 2013). These seasonal trends in SUVA254 values may also have been due to natural variation in root exudate quality, as a number of environmental variables that vary with season influence root exudate composition (Badri and Vivanco 2009). In addition to SUVA254 we used EEMs to monitor changes in DOC quality. However, EEMS measures proved to be a less sensitive indicator of plant induced changes in DOC lability, with only the Fluorescence Index (FI) demonstrating a difference in DOC quality with treatment. Still, it is clear graminoids are providing a source of labile carbon compounds for the belowground environment, a key component of microbial priming effects (Kuzyakov et al. 2000; Zhu et al. 2014).
Our study presents one of the first applications of Biolog Ecoplates® as a measure of microbial activity and metabolic richness in peatland environments, providing data that support the presence of microbial priming effects. Microbial activity and metabolic richness were greatest under the vegetated treatments, with the highest values observed under the graminoid treatments. This result suggests that the microbial community was more active and was able to decompose a wider range of carbon compounds. These findings fit well with work by Fontaine et al. (2007), who demonstrated microbial priming effects in organic soils require a constant labile carbon source in order to decompose recalcitrant carbon stores. Such a microbial community would be highly active with a structurally complex metabolism, able to breakdown both simple and complex compounds — as we observed under the graminoid treatment.
Lastly, the release of ‘extra’ respired carbon dioxide from amended soil in comparison to unamended soils is a hallmark of microbial priming effects (Kuzyakov 2010). That said, in order to incorporate the contributions of living plant material to belowground carbon cycling, we utilized fully vegetated, intact peat monolith in our experiment, and in doing so included live plant respiration in our total respired carbon dioxide values. In many mineral soil ecosystems these autotrophic respiration contributions can be substantial, comprising 61–74 % of respired carbon dioxide (Griffis et al. 2004; Gomez-Casanovas et al. 2012). Peatlands, however, are a unique ecosystem where microbial heterotrophic respiration alone can constitute 70 % of the respired carbon dioxide (Dorrepaal et al. 2009) — rendering microbial respiration the probable driver of our respired carbon dioxide trends. For added confidence we regressed the total destructively-sampled plant biomass against average respired carbon dioxide values from each mesocosm in our study and found no significant relationship (data not shown). These results do not negate the contribution of live plants to respired mesocosm-level carbon dioxide, but suggest that respired carbon dioxide trends were not driven by autotrophic respiration.
To better simulate the carbon dynamics of Sphagnum moss and graminoid communities we used intact peat monoliths. This allowed the supporting peat material to vary naturally amongst the treatments, potentially driving carbon dynamics. To isolate and quantify the effect of the supporting peats on carbon dynamics we instated a pre-growing season, where cooler temperatures and shorter photoperiod conditions combined to minimize plant productivity. Accordingly, any detectable and stable differences amongst the treatments during the pre-growing season would be driven by the supporting peats. Instead, differences amongst treatments tended to be minimized during the pre-growing period for most variables, especially DOC concentration and respired CO2. Even in the case of SUVA254 clear, consistent differences between treatments did not arise until well into the growing season when plant inputs were maximized. Thus, even though supporting peats undoubtedly influence carbon dynamics, labile root exudates generated rapidly by plants during the growing season are most likely driving our trends.
While our study presents evidence for microbial priming-like effects in peatland ecosystems, it is not possible to confidently discern if these priming-like effects are what the literature terms ‘real’ or ‘apparent’ (Blagodatskaya and Kuzyakov 2008). Real priming effects occur when the microbial community is stimulated to breakdown SOM, releasing the soil carbon as carbon dioxide. In the context of peatland carbon storage real priming effects would result in the breakdown and release of ‘old’ carbon stored in the peat matrix. Apparent priming effects also increase carbon dioxide release, but are due to intensified microbial metabolism and biomass turnover, leaving SOM decomposition rates unaltered (Blagodatsky et al. 2010). That said, apparent priming effects are generally considered highly transient, lasting less than 14 days (Blagodatsky et al. 2010; Blagodatskaya et al. 2011). Real priming effects, conversely, can be sustained for several months (Blagodatsky et al. 2010). Thus, the increased carbon dioxide response under the graminoid treatment that was maintained for the duration of the growing season in our study is supportive of real priming effects. Real priming effects in organic soils are also associated with microbial communities capable of metabolizing an increased range of carbon compounds (Fontaine et al. 2007), a characteristic we observed under the graminoid treatment. Additionally, real priming effects corroborate emerging research on peatland plant growth forms that demonstrates graminoids enhance ancient SOM release (Walker et al. 2016). Our research suggests labile carbon from the graminoids primes microbes to decompose ancient SOM — in turn providing a mechanistic explanation for the findings of Walker et al. (2016).
The carbon stores of northern peatlands have been the focus of a remarkable research effort, resulting in the discovery of numerous carbon cycling controls (Limpens et al. 2008). Recent research efforts have demonstrated the importance of plant growth forms in governing carbon processing in peatlands (Ward et al. 2015; Walker et al. 2016), although a mechanistic understanding of these findings has remained limited (Robroek et al. 2016). As climate change conditions are anticipated to restructure peatland plant communities (Wiedermann et al. 2007; Dieleman et al. 2015) this knowledge gap has become important to anticipating the stability of peatland carbon stores in the future (Armstrong et al. 2015). Our work demonstrates that graminoids significantly alter belowground processes by introducing labile DOC, which in turn increases microbial activity and metabolic richness to increase respired carbon dioxide release. These findings are suggestive of microbial priming effects demonstrated in mineral soils. Thus, climate change driven shifts in peatland plant communities, from Sphagnum moss to graminoid dominated systems, may prime belowground environments and potentially enhance the release of carbon stores.
Abbreviations
- AWCD:
-
Average well colour development
- BIX:
-
Freshness index
- CO2 :
-
Carbon dioxide
- DOC:
-
Dissolved organic carbon
- FI:
-
Fluorescence index
- HIXEM :
-
Humification index
- SOM:
-
Soil organic material
- SUVA254 :
-
Specific ultraviolet absorbance at 254 nm
References
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Armstrong A, Holden J, Luxton K, Quinton JN (2012) Multi-scale relationship between peatland vegetation type and dissolved organic carbon concentration. Ecol Eng 47:182–188
Armstrong A, Waldron S, Ostle NJ, Richardson H, Whitaker J (2015) Biotic and abiotic factors interact to regulate northern peatland carbon cycling. Ecosystems 18:1395–1409
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Bengtson P, Barker J, Grayston SJ (2012) Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects. Eco Evol 2:1843–1852
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Blagodatskaya E, Yuyukina T, Blagodatsky S, Kuzyakov Y (2011) Three-source-partitioning of microbial biomass and of CO2 efflux from soil to evaluate mechanisms of priming effects. Soil Biol Biochem 43:778–786
Blagodatsky S, Blagodatskaya E, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42:1275–1283
Bombonato L, Siffi C, Gerdol R (2010) Variations in the foliar nutrient content of mire plants: effects of growth-form based grouping and habitat. Plant Ecol 211:235–251
Buckeridge KM, Banerjee S, Siciliano SD, Grogan P (2013) The seasonal pattern of soil microbial community structure in Mesic low arctic tundra. Soil Biol Biochem 65:338–347
Carroll P, Crill P (1997) Carbon balance of a temperate poor fen. Global Biogeochem Cy 11:349–356
Crow SE, Wieder RK (2005) Sources of CO2 emission from a northern peatland: root respiration, exudation, and decomposition. Ecology 86:1825–1834
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Del Giudice R, Lindo Z (2017) Short-term leaching dynamics of three peatland plant species reveals how shifts in plant communities may affect decomposition processes. Geoderma 285:110–116
Dieleman CM, Branfireun BA, McLaughlin JW, Lindo Z (2015) Climate change drives a shift in peatland ecosystem plant community: implications for ecosystem function and stability. Glob Change Biol 21:388–395
Dorrepaal E (2007) Are plant growth-form-based classifications useful in predicting northern ecosystem carbon cycling feedbacks to climate change? J Ecol 95:1167–1180
Dorrepaal E, Cornelissen JHC, Aerts R, Wallén B, van Logtestijn RSP (2005) Are growth forms consistent predictors of leaf litter quality and decomposability across peatlands along a latitudinal gradient? J Ecol 93:817–828
Dorrepaal E, Toet S, van Logtestijn RSP, Swart E, van de Weg MJ, Callaghan TV, Aerts R (2009) Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460:616–620
Dunn C, Jones TG, Roberts S, Freeman C (2015) Plant species effects on the carbon storage capabilities of a blanket bog complex. Wetlands 36:47–58
Eppinga MB, Rietkerk M, Wassen MJ, De Ruiter PC (2009) Linking habitat modification to catastrophic shifts and vegetation patterns in bogs. Plant Ecol 200:53–68
Fan Z, McGuire AD, Turetsky MR, Harden JW, Waddington JM, Kane ES (2013) The response of soil organic carbon of a rich fen peatland in interior Alaska to projected climate change. Glob Change Biol 19:604–620
Faubert P, Rochefort L (2002) Response of peatland mosses to burial by wind-dispersed peat. Bryologist 105:96–103
Fellman JB, Hood E, Spencer RGM (2010) Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: a review. Limnol Oceanogr 55:2452–2462
Fenchel T, King GM, Blackburn H (1998) Bacterial biogeochemistry: the Ecophysiology of mineral cycling. Academic Press, San Diego
Fenner N, Freeman C, Lock MA, Harmens H, Reynolds B, Sparks T (2007a) Interactions between elevated CO2 and warming could amplify DOC exports from peatland catchments. Environ Sci Technol 41:3146–3152
Fenner N, Ostle NJ, McNamara N, Sparks T, Harmens H, Reynolds B, Freeman C (2007b) Elevated CO2 effects on peatland plant community carbon dynamics and DOC production. Ecosystems 10:635–647
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–281
Frąc M, Oszust K, Lipiec J (2012) Community level physiological profiles (CLPP) characterization and microbial activity of soil amended with dairy sewage sludge. Sensors 12:3253–3268
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28:213–221
Garland JL (1997) Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol Ecol 24:289–300
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57:2351–2359
Gomez-Casanovas N, Matamala R, Cook DR, Gonzalez-Meler MA (2012) Net ecosystem exchange modifies the relationship between the autotrophic and heterotrophic components of soil respiration with abiotic factors in prairie grasslands. Glob Change Biol 18:2532–2545
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Griffis TJ, Black TA, Gaumont-Guay D, Drewitt GB, Nesic Z, Barr AG, Morgenstern K, Kljun K (2004) Seasonal variation and partitioning of ecosystem respiration in a southern boreal aspen forest. Agric For Meteorol 125:207–223
Gunina A, Kuzyakov Y (2015) Sugars in soil and sweets for microorganisms: review of origin, content, composition and fate. Soil Biol Biochem 90:87–100
Kalbitz K, Schmerwitz J, Schwesig D, Matzner E (2003) Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 113:273–291
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1362–1371
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the holy grail. Funct Ecol 16:545–556
Lawaetz AJ, Stedmon CA (2009) Fluorescence intensity calibration using the raman scatter peak of water. Appl Spectrosc 63:936–940
Limpens J, Berendse F, Blodau C, Canadell JG, Freeman C, Holden J, Roulet N, Rydin H, Schaepman-Strub G (2008) Peatlands and the carbon cycle: from local processes to global implications – a synthesis. Biogeosciences 5:1475–1491
McLaughlin JW, Webster KL (2010) Alkalinity and acidity cycling and fluxes in an intermediate fen peatland in northern Ontario. Biogeochemistry 99:143–155
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799
Pinsonneault AJ, Moore TR, Roulet NT, Lapierre J-F (2016) Biodegradability of vegetation-derived dissolved organic carbon in a cool temperate ombrotrophic bog. Ecosystems 19:1023–1036
Robroek BJM, Albrecht RJH, Hamard S, Pulgarin A, Bragazza L, Buttler A, Jassey VEJ (2016) Peatland vascular plant functional types affect dissolved organic matter chemistry. Plant Soil 407:135–143
Rydin H, Jeglum JK (2013) The biology of peatlands 2nd ed. Oxford University Press, Oxford
Straková P, Anttila J, Spetz P, Kitunen V, Tapanila T, Laiho R (2010) Litter quality and its response to water level drawdown in boreal peatlands at plant species and community level. Plant Soil 335:501–520
Walker TN, Garnett MH, Ward SE, Oakley S, Bardgett RD, Ostle NJ (2016) Vascular plants promote ancient peatland carbon loss with climate warming. Glob Change Biol 22:1880–1889
Ward SE, Orwin KH, Ostle NJ, Briones MJI, Thomson BC, Griffiths RI, Oakley S, Quirk H, Bardgett RD (2015) Vegetation exerts a greater control on litter decomposition than climate warming in peatlands. Ecology 96:113–123
Webster KL, McLaughlin JW (2010) Importance of the water table in controlling dissolved carbon along a fen nutrient gradient. Soil Sci Soc Am J 74:2254–2266
Webster KL, McLaughlin JW, Kim Y, Packalen MS, Li CS (2013) Modelling carbon dynamics and response to environmental change along a boreal fen nutrient gradient. Ecol Model 248:148–164
Weishaar JL, Aiken GR, Bergamashi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Weltzin JF, Pastor J, Harth C, Bridgham SD, Updegraff K, Chapin CT (2000) Response of bog and fen plant communities to warming and water-table manipulations. Ecology 81:3464–3478
Wiedermann MM, Nordin A, Gunnarsson U, Nilsson MB, Ericson L (2007) Global change shifts vegetation and plant-parasite interactions in a boreal mire. Ecology 88:454–464
Yu ZC (2012) Northern peatland carbon stocks and dynamics: a review. Biogeosciences 9:4071–4085
Zhu B, Gutknecht JLM, Herman DJ, Keck DC, Firestone MK, Cheng W (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192
Acknowledgments
We are grateful to Dr. C. Dean, Dean of the Western Faculty of Science, for supporting our use of the Biotron, funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant program (ZL, BB), Canada Research Chairs program (BB), NSERC Strategic Network support to the Canada Network for Aquatic Ecosystem Services (BB) and the Ontario Graduate Scholarship program (CD). We thank Dr. J. McLaughlin (Ontario Ministry of Natural Resources and Forestry) for access to the White River, ON field site and his continued support with our research program. We also thank Dr. C. Oswald (Ryerson University) for advice and aid on EEMs techniques and Dr. S. Carey (McMaster University) for access to laboratory instruments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Zucong Cai.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Dieleman, C.M., Branfireun, B.A. & Lindo, Z. Northern peatland carbon dynamics driven by plant growth form — the role of graminoids. Plant Soil 415, 25–35 (2017). https://doi.org/10.1007/s11104-016-3099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3099-3