Abstract
Aims
The influence of changing carbon (C) assimilation and relative C partitioning during plant development on the transfer of absolute C amounts (mg C per plant) into the subsurface should be analyzed and estimated. Moreover, an approach to determine the precision of the modeled C transfer values should be developed.
Methods
Several relative 14C partitioning coefficients were derived from pulse labeling experiments at different developmental stages using spring rye as a model plant. Subsequently, relative 14C allocation was dynamically linked with daily shoot C mass increments to obtain precise information about C partitioning at different plant developmental stages and during the entire growing season.
Results
The transfer of assimilated C into the subsurface during the plant development is a highly dynamic process, in which the maximum root growth preceded maximum shoot growth by approximately 17 days. The model simulations indicate that 18 ± 2 % of all assimilated C that was not respired by shoots was transferred into the subsurface during the entire growing season. This flow splits into 9 ± 0.8 % C for root growth, 6 ± 0.7 % C for belowground respiration, and 3 ± 0.3 % C for detectable rhizodeposition. Nearly half of the downward-transferred C flowed into the subsurface between elongation growth and the last boot stage. Moreover, highly correlated relationships between root growth, rhizodeposition, and belowground respiration were found.
Conclusions
Our study clearly shows that the dynamic linking of relative C partitioning with absolute C assimilation can provide detailed and precise information about the downward-transferred C during different developmental stages and the entire growing season.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Precise quantification of plant-derived carbon (C) input into soils has become increasingly important from the perspective of soil fertility and the reduction of anthropogenic impacts on the global C cycle (Bolinder et al. 2012; Janzen 2006; Moureaux et al. 2008; Stockmann et al. 2013; Virto et al. 2012). Determining the magnitude of this C input is critical for understanding the terrestrial C cycle (Chapin et al. 2009) and for improving belowground process models (Litton and Giardina 2008; Wiesmeier et al. 2014). Unfortunately, estimates of the soil C input from plant roots are highly uncertain (Bolinder et al. 2007; Nguyen 2003; Toal et al. 2000; Wiesmeier et al. 2014). The reasons for this uncertainty are numerous and include both methodical and physiological aspects.
Carbon transferred into roots is used for root growth, rhizodeposition, and root respiration. Root respiration provides energy and C skeletons for plant physiological processes, such as maintenance, root growth, and active uptake, as well as the assimilation of nutrients (Bloom et al. 1992; Lambers et al. 1996; Plaxton and Podesta 2006). Rhizodeposition is a collective term that includes the production of exudates (free sugars, amino acids and organic acids), the production of mucilage, and the sloughing-off of root cap cells (Jones et al. 2009). In addition to their importance for supporting and protecting root tips in the process of soil penetration and for improving nutrient uptake, rhizodeposits drive microbial life in the rhizosphere and influence the soil structure (Jones et al. 2009; Rasse et al. 2005). In contrast to some labile substances of rhizodeposition, which can be metabolized within a few hours (Rasse et al. 2005), the microbial turnover of senescent or dead roots requires substantially more time (Pritchard and Rogers 2000; Milchunas 2009; Steingrobe et al. 2001). The respiration of roots, the microbial turnover of rhizodeposits and senescent or dead roots, and the mineralization of older soil C cause continuous mixing of C from different sources, making it difficult to distinguish between plant-derived C and older soil C.
Consequently, an investigation of root growth using root ingrowth and rhizotron techniques or the determination of the shoot/root ratio can provide only sub-flows of soil C input. Moreover, the turnover of senescent or dead roots during plant development must be taken into account, which can distort results (Milchunas 2009). To estimate the total C input from plant roots into the subsurface (the entirety of roots, soil, and soil gas), it is therefore useful to determine relative C partitioning coefficients, which indicate the allocation of assimilated C into shoots and roots, belowground respiration, and rhizodeposition as percentages of fixed C (Nguyen 2003). The precise determination of these coefficients is possible by using pulse labeling of plant shoots, as demonstrated by Remus et al. (2016).
The assimilation of labeled CO 2 allows the tracing and quantification of the flow and turnover of freshly assimilated C within a plant-soil-soil gas system and the separation of recently assimilated C from old soil C (Babst et al. 2005; Dilkes et al. 2004; Keith et al. 1986; Keutgen et al. 1995; Kuzyakov et al. 2001; Meng et al. 2013; Staddon et al. 2003; Swinnen et al. 1994b, a; Swinnen 1994; Wang et al. 1989; Warren et al. 2012). The relative C partitioning coefficients derived from a pulse labeling experiment provide only the distribution of the C assimilated during pulse labeling. Therefore, the averages and/or medians of the relative C partitioning coefficients from different labeling experiments and the aboveground biomass or the combined usage of shoot/root ratios, the average relative C partitioning coefficient of rhizodeposition, and the aboveground biomass have been used to upscale the soil C input during the growing season (Bolinder et al. 2007; Bolinder et al. 2012; Kuzyakov and Domanski 2000; Kuzyakov and Schneckenberger 2004; Nguyen 2003; Wiesmeier et al. 2014).
Unfortunately, we do not know which measure of central tendency, i.e., the average or the median, is more suitable, especially if there are substantial differences between both estimators. For example, a large difference between the two measures has been observed for the total belowground C transfer under barley, where the median was 17 %, and the average was 28 % of the assimilated C (Kuzyakov and Domanski 2000).
Moreover, it must be considered that the relative amount of assimilated C transferred into the subsurface by younger plants is significantly greater than that transferred by older plants, and the relative amount of downward-transferred C continuously but non-linearly decreases during plant development (Swinnen et al. 1994b). For example, a 14C transfer into the subsurface of 33 % to 55 % has been observed under wheat and barley 24 h after pulse labeling at tillering, whereas this transfer was less than 10 % after labeling at grain filling (Gregory and Atwell 1991). Additionally, other authors found that the C transfer into the subsurface depends on the age of the investigated plants (Carbone and Trumbore 2007; Keith et al. 1986; Sauerbeck et al. 1976; Sey et al. 2010; Xu and Juma 1993). Therefore, unbalanced sampling during the growing period can cause a distortion of the results (Jones et al. 2009).
Furthermore, the rate of the daily absolute C assimilation or biomass production of crops (expressed as mg C per plant per day) and the relative C transfer into the subsurface (expressed as a percentage of fixed C) exhibit completely different opposing trends over time. For example, the relative amount of fixed C that a wheat plant uses for root growth decreases exponentially from elongation until the ripening stage (Swinnen et al. 1994b). However, the rate of daily absolute C net assimilation of young plant shoots is low and increases continuously during plant growth until it reaches a maximum, then, subsequently, declines until the end of the growing season. This trend can be fit with the time derivative of a sigmoid growth function, which is typical of the cumulative growth of crops (Causton and Venus 1981; Yin et al. 2009; Zeide 1993).
Based on these considerations, it seems necessary to link dynamic absolute C assimilation of the shoot and relative C partitioning coefficients for the whole vegetation period by a modeling approach to obtain age-balanced C transfer values, which facilitate a description of the process of C allocation within the plant-soil system during plant development with high precision. To the best of our knowledge, the only approach that partially meets these requirements is that presented by Keith et al. (1986), which was further developed by Swinnen et al. (1994b). This approach (hereinafter referred to the Keith-Swinnen method) is based on repeated single-pulse labeling experiments at different plant developmental stages and modeling of the distribution of a C tracer within plant-soil-soil gas systems during plant development. According to the Keith-Swinnen method, the 14C amounts of roots, rhizodeposition, and belowground respiration are dynamically linked with the ratio of 14C incorporation in the shoots and the daily shoot growth rate to calculate the absolute C transfer into the subsurface at each moment during an investigation period. A precondition of this approach is that the partitioning of the C tracer within the plant-soil-soil gas system represents the use of C (assimilated at the time of pulse labeling) for root growth, rhizodeposition as well as root respiration and microbial turnover of rhizodeposits. As demonstrated by Remus et al. (2016), this precondition was fulfilled when sampling occurred between 15 and 21 days after labeling. The details and the underlying idea of the Keith-Swinnen method are described in the Section “Modeling the relative and absolute C fluxes”.
The Keith-Swinnen method has two major flaws. First, Swinnen and colleagues only investigated the timespan between elongation growth and ripening. During this time period, one linear and three simple exponential functions were sufficient to model relative C partitioning within the plant-soil-soil gas system (Swinnen et al. 1994b). Therefore, it is unknown whether it is possible to model relative C partitioning with sufficient precision on each day and over the whole growing season when more complex functions are needed. Second, the Keith-Swinnen method cannot provide information about the precision of the calculated absolute C fluxes. Hence, the aim of our study was to address the identified shortcomings of the Keith-Swinnen method and to provide detailed and precise information about the process of C allocation during plant development.
Our research had the following objectives:
-
Modeling relative 14C partitioning within the plant-soil-soil gas system and shoot growth from emergence to the ripening stage with sufficient precision.
-
Modeling and quantifying absolute C transfer into the subsurface during the growing season.
-
Investigating the influence of different observation periods on the estimation of soil C input.
-
Further developing the Keith-Swinnen method by designing an approach to estimate modeling precision based on model simulations.
-
Analyzing the relationships among root growth, rhizodeposition, and belowground respiration.
To obtain data on shoot growth and relative C partitioning between plant emergence and ripening, an outdoor and a field experiment were simultaneously conducted using spring rye as the model plant.
Materials and methods
Cultivation of plants, labeling dates, and sampling
To determine the relative 14C partitioning within the plant-soil-soil gas system at different plant developmental stages and the shoot growth of spring rye, an outdoor experiment and a field experiment were simultaneously performed.
The field experiment was conducted on loamy sand (albic luvisol) at the Leibniz Center for Agricultural Landscape Research (ZALF), Müncheberg, Germany (N 52 ∘ 31’, E 14 ∘ 07’). Approximately two weeks before rye seeds were sown in the field, 19 plastic pots (length: 110 cm, outer diameter: 16 cm, inner diameter: 15 cm) made from commercially available KG sewer pipes and accessories (KG DN 160 from Marley, Germany) were placed in the field using a specially manufactured driller. To accommodate these pots, holes of 110 cm depth were drilled in the field. The obtained soil was placed stepwise in portions of approximately 10 cm soil depths on plastic wrap. Afterward, the pots were embedded in the field and filled with the obtained soil portions to create a 100 cm long soil column with a similar soil profile as in the field. The dry mass of the soil (including stones) in the pots was approximately 28 kg. The field (including open pots) was fertilized with 94 kg of Ca ha −1, 114 kg of K ha −1, 12 kg of Mg ha −1, 20 kg of P ha −1, and 16 kg of S ha −1 using a commercial mineral fertilizer (Thomaskali, K+S KALI GmbH, Germany) on the day before sowing. Subsequently, the field was fertilized with nitrogen by applying liquid fertilizer (60 kg N ha −1 per application; NTS urea-ammonium nitrate solution, Nutri-Tech Solutions, Australia) 4 and 40 days after sowing. Consequently, each pot received 189 mg Ca, 201 mg K, 24 mg Mg, 35 mg P, and 28 mg S through fertilization with Thomaskali and 106 mg N on each of the two nitrogen-application dates.
The seeds were sown in the field in order to have the pots in the plant stand. A single plant was grown in each pot. Between sowing and harvesting, the average daytime temperature was 11 ∘C. Total precipitation in the whole year was 549 L m −2 and did not differ from the average precipitation at this site. At the time of labeling, three to four pots were dug out and transferred to an open space that was equipped for labeling and CO 2 collection from the head space of the pots (see Section “ 14C pulse labeling”). Between labeling and harvest (21 days), the pots were irrigated with deionized water using an amount that was equivalent to the local precipitation.
In the field experiment, ca. 120 (non-labeled) plant shoots (per sampling) were harvested at 11 different sampling dates between emergence and ripening to model the shoot growth (see Section “Modeling the relative and absolute C fluxes”).
Tracer application, preparation, and analysis
14C pulse labeling
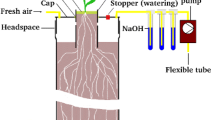
In the outdoor experiment, each plant was separately labeled with 14 CO 2 at six different developmental stages between emergence and the late milk-ripening stage. The top of each pot was closed by a perforated cap with two tubing connections (inlet and outlet) 24 h before labeling (a figure of the pot design is presented by Remus et al. (2016). Subsequently, the holes in the cap around the reduction tubes and the holes in the bottom of the pot were sealed with semi-solid acid-free silicon rubber. The soil surface within the reduction tube was sealed with a liquid silicon rubber (TACOSIL 170 with 3 % cross-linker no. 28 from Thauer & Co. KG, Germany) in order to separate the soil gas in the pots from the atmosphere. Before labeling, the tubing connections in the caps were closed, and the plants were covered with transparent plastic bags. Then, each plant was labeled with up to 10 MBq 14C using a locking opening of the bag. After removing the plastic bags, the tubing connections were opened and connected to flexible tubes to supply fresh air and to suck gas out of the soil headspace. The CO 2 of the soil gas was continuously trapped in an NaOH solution to determine the 14C activity of the belowground respiration. The NaOH solution (3 x 12 ml 1 M NaOH) was renewed daily until harvest (21 days after labeling).
Sample preparation
Rye shoots were cut at their base at harvest (21 days after labeling) and dried in an oven at 104 ∘C. Subsequently, the pots (sewer pipes) were dissected into five pats, each 20 to 30 cm long. The soil (including roots) of each pat were immediately frozen at -26 ∘C and later defrosted separately at 4 ∘C overnight to separate the roots from the soil. After hand-picking of roots from the soil portions of approximately 5 to 6 kg, the roots were washed with 250 ml of deionized water in an Erlenmeyer flask on a horizontal shaker for 1 h to remove the adhesive soil. Next, the roots were dried at 104 ∘C. The soil that was separated from the roots by washing was operationally defined as rhizosphere soil. The washing water and the rhizosphere soil were transferred to the top of a sieve with a 300- μm mesh size. A wet-sieving procedure was started to separate small root fragments and rhizodeposits. Sieving was considered to be completed when only white sand was visible on the top of the sieve. Then, the sand (including root residues) from the top of the sieve was dried at 104 ∘C. The soil that passed through the sieve were dried at 104 ∘C after complete evaporation of water (approximately 1 to 2 L) at 80 ∘C to obtain all 14C labeled material from the rhizosphere soil.
After hand-picking of the roots, each soil portion (approximately 5 to 6 kg) was homogenized by hand. The dry soil mass of each bulk soil portion was determined in 50 g of fresh weight (two replications). In addition, two bulk soil aliquots (50 g each) were taken from each soil portion. Consequently, ten aliquots were taken from bulk soil of each pot. Each bulk soil aliquot was dissolved in 250 ml of deionized water and shaken in an Erlenmeyer flask on a horizontal shaker for 1 h. Then, the water and the dissolved bulk soil were transferred to the top of a sieve with a 300- μm mesh size. Next, a wet-sieving procedure was started to separate rhizodeposits and small root fragments, which could not be selected by hand-picking from bulk soil. Sieving was considered complete when only white sand was visible on the top of the sieve. The sand (including root fragments) from the top of the sieve, the washing water (approximately 1 - 2 L), and the soil fraction (passing the sieve) were treated similarly to the rhizosphere soil in order to obtain root fragments (≥ 300 μm) that could not be obtained by hand-picking and detectable rhizodeposits (< 300 μm) from bulk soil aliquots.
Fragmentation into roots, rhizosphere soil, and bulk soil, as well as soil portions of different soil depths, was performed to obtain representative samples because 14C activities in roots, rhizosphere, and bulk soil, as well as at different soil depths, considerably differ. More information about this procedure is presented by Remus et al. (2016).
C analysis
The C content and 14C activity of shoot, root, soil samples (≥ 300 μm and < 300 μm) from the rhizosphere and bulk soil were determined by combustion at 1200 ∘C in a continuous O 2 flow. The CO 2 from the elemental analyzer (CS 500, ELTRA GmbH, Germany) was absorbed in a CO 2 trap Qureshi et al. (1985), which was filled with 7 ml of Carbo-Sorb E (PerkinElmer, Rodgau, Germany). Then, 3 ml aliquots of CarboSorb E were mixed with 12 ml of Permafluor-Scintillator (PerkinElmer) to measure the 14C activity in a liquid scintillation counter (LS 6000SC, Beckman, Germany). The 14C activities, released by belowground respiration, were determined using 3 ml aliquots of the NaOH solution from the CO 2 traps of the labeling experiments after the aliquots were mixed with 12 ml of UltimaGold-Scintillator (PerkinElmer). The liquid scintillation counter was calibrated using 14C, 3H, and background standards from Beckman according to the manual for the device.
14C calculations for all components
The amounts of 14C resulting from belowground respiration were calculated using the specific 14C activity of the NaOH solution in the CO 2 traps (expressed as Bq per ml NaOH) multiplied by the total volume of NaOH in the trap. Finally, the respired 14C activities for each day were added together to obtain the total 14C activity of the belowground respiration.
The total amount of 14C for shoots and hand-picked roots was calculated by multiplying the specific 14C activity (Bq per mg shoot or root material) by the total weight of shoots and roots, respectively. The total 14C amount of the root fragments that could not be recovered by hand was calculated using the specific 14C activity (Bq per mg) of the ≥ 300 μm samples of rhizosphere and bulk soil and the weight of the soil fractions ≥ 300 μm in the rhizosphere and bulk soil from the entire pot. Next, the amounts of 14C in root fragments and roots were summed to obtain the total amount of 14C in the entire root system. The amount of 14C from rhizodeposition was determined by multiplying the specific 14C activity (Bq per mg) of the soil fractions < 300 μm, which was obtained by wet-sieving rhizosphere and bulk soil, by the total weight of these fractions in the whole root system and pot, respectively. These two amounts of 14C were summed to obtain the entire detectable rhizodeposition. The 14C-labeled substances of this fraction were operationally defined as detectable rhizodeposition because a portion of the labeled rhizodeposits was surely released as labeled CO 2 by microbial turnover between labeling and plant harvest.
Modeling and quantification of the C fluxes during the growing season
The different 14C fluxes
The sum of the 14C recovered in the shoots, roots, and rhizodeposits on the day of harvest and the 14C from the belowground respiration collected after labeling until harvest (21 days after labeling) is defined as the 14C amount of the plant-soil-soil gas system. The 14C activity of the shoot included the 14C of the stem, leaves, and ears (if present). The 14C activity of the root includes the 14C activities of the handpicked roots and the root fragments from the sand of the rhizosphere and bulk soil fractions that could not be selected by hand-picking. The 14C-labeled detectable rhizodeposition was determined as the sum of the 14C found in the soil fractions < 300 m of the rhizosphere and bulk soil (see Section “Sample preparation”). In this study, we were unable to distinguish between the respiration activities of roots and microbes. Therefore, the 14C-labeled belowground respiration includes the 14C-labeled CO 2 released by root respiration as well as the microbial turnover of 14C-labeled rhizodeposits.
To obtain the relative C partitioning coefficients from each investigated system, the sum of all 14C amounts recovered in the plant-soil-soil gas system was adjusted to 100 %. Afterward, the relative C partitioning coefficients for each component were calculated and expressed as percentages.
Modeling the relative and absolute C fluxes
The modeling process following the Keith-Swinnen method is presented in Fig. 1. As shown in this flow chart, the process starts with modeling shoot growth during the growing season after sampling non-labeled plant shoots in the field experiment (top of Fig. 1). In this step, a non-linear regression based on the amount of C (mg C per plant shoot) in the rye shoots is performed. Next, the first derivative of the best-fitting shoot-growth function is generated to obtain shoot growth rates, expressed as mg C per plant per day (upper-right corner of Fig. 1).
Figure 1 also indicates that after pulse labeling (top left), the amount of 14C in each component is determined, and the relative C partitioning coefficients are calculated (middle). Based on these data, suitable models for the relative distribution of 14C into shoots, roots, detectable rhizodeposition, and belowground respiration during the plant development are sought. Therefore, 10,200 combinations of four possible partitioning functions (4-tuple) with an acceptable goodness-of-fit of each curve are compared. The 4-tuple of the 14C partitioning functions whose sum exhibits the smallest deviation from the 100 % line (see Fig. 2) is used to model the 14C partitioning within the plant-soil-soil gas system, expressed as a percentage of the total 14C amount recovered in the investigated system 21 days after labeling (middle-right of Fig. 1). The idea underlying the Keith-Swinnen method is that the ratio of 14C incorporation into the structural C pool of the shoot and the shoot growth rate must equal the ratio of 14C incorporation into the structural C pool from the roots and the root growth rate on the labeling day, provided that the distribution of the assimilated 14C and its respiratory turnover in the plant is nearly finished at sampling. Based on this idea, a proportionality equation (Eq. 1) is used. In this equation, the time-dependent functions of the 14C partitioning coefficients of the shoot (14 C % s h o o t(t)) and root (14 C % r o o t(t)), respectively, are in the numerator. These two functions provide the relative fractions of net fixed 14C incorporated into shoots and roots, respectively.
Partitioning of 14C into shoots, roots, detectable rhizodeposition (rhizodep.), and belowground respiration (belowgr. resp.) during the growing season. The amount of 14C is expressed as a percentage of the 14C amount recovered in the plant-soil-soil gas system 21 days after labeling. The dashed black line represents 100 % of this 14C amount, whereas the dotted gray line indicates the actual sum of all four functions at each point in time. The modeling of 14C partitioning is based on the means of 3 pots per date. Error bars represent the standard deviation of these values. The 14C partitioning in the gray areas is extrapolated because no measurements are available for these periods
In the denominator on the left side, the first derivative of the shoot growth function is represented as the growth rate of the shoot (dC shoot/dt), calculated as mg C per plant per day. The denominator on the right side represents the time-dependent root growth rate (dC root/dt), which cannot be measured in an experiment by direct weighing because growth and decomposition of roots can occur simultaneously. However, after rearrangement of Eq. 1, the root growth rate (dC root/dt, Eq. 2) of each day can be modeled in terms of C increment per day (mg C per root per day) by dynamically linking the daily incorporation of 14 C into the shoots and roots with the daily increment of the shoot growth, as shown in the bottom of Fig. 1.
The substitution of 14C incorporation into the roots using the other 14C partitioning functions (Fig. 1; dotted lines) allows for modeling of the absolute C flux rates of the detectable rhizodeposition (dC rhizodep./dt, Eq. 3) and the belowground respiration (dC belowgr. resp./dt, Eq. 4).
Furthermore, modeling the total C transfer rate into the subsurface (dC total transfer/dt, Eq. 5) is made possible by summing the individual C flux rates. Consequently, integrating the C flux rates over a time interval or the whole growing season provides the total absolute amount of C that flowed into the different components over the investigation period.
Modeling and analysis of age-balanced relative 14C partitioning coefficients
As described in the Introduction, the C transfer into the subsurface depends on the age distribution of the investigated plants. Therefore, age-balanced datasets of relative 14C partitioning coefficients were created by calculating these coefficients for each day of the sampling period (from day 16 until 84 after emergence) and over the whole growing season (120 days) using modeled time-dependent partitioning of 14C into roots, detectable rhizodeposition, and belowground respiration (see Fig. 2). Subsequently, the mean, median, standard deviation, and range of each dataset, with 69 and 120 coefficients, respectively, were determined.
Determination of the reliability and precision of the modeled C input
Development of an approach to quantify modeling precision
The modeling error of the Keith-Swinnen method cannot be estimated using Gaussian error propagation because C partitioning and shoot growth are time-dependent non-linear processes. Therefore, the modeling error was estimated using 125,000 model simulations; this method is similar to the method for error calculation described by Hoffmann et al. (2015). For these simulations, 50 prediction intervals of the best-fitting functions of shoot growth and the relative 14C partitioning into shoots, roots, rhizodeposition, and belowground respiration were generated.
In contrast to the study of Hoffmann et al. (2015) in which equidistant prediction intervals were used, in our study, the probability levels of the prediction intervals were determined using a vector of 50 normally distributed numbers to avoid assigning equal weight to less and more probable prediction functions. The vector was generated from a normal distribution with an expected value (μ) of 0 and a standard deviation (σ) of 0.33 because for a normal distribution, more than 99 % of all possible values are located within the range of μ±3∗σ, i.e., in our study in the interval between -0.99 to +0.99.
Using this distribution, the upper and lower mean prediction bands of the best-fitting curves were generated. Positive random numbers were used to determine the probability levels of the upper bands, whereas absolute values of negative random numbers were used for the probability levels of the lower mean prediction bands. In this manner, for each curve, a function family of 50 curves, which were normally distributed around the best-fitting curve, was generated. The values of the 50 random numbers used had an average of -0.00017 and a standard deviation of 0.30719, and they ranged from -0.71596 to 0.51516.
In the next step, the time derivatives of the 50 mean prediction functions of the shoot growth curve were calculated and combined with the 50 14C partitioning functions (mean prediction bands) of the shoots and roots. Hence, 125,000 different combinations of functions were created and used for modeling root growth. Afterward, from all combinations, the arithmetical mean, median, standard deviation, spread, and coefficients of variation of the amount of C used for root growth during the growing season were calculated using Eq. 2.
In the same manner, statistics regarding the C flux into rhizodeposition and belowground respiration, as well as the total subsurface C input, were calculated using Eqs. 3, 4, and 5 with 125,000 simulation runs. In addition to the statistics of the entire growing season, statistics were also estimated for the sampling period.
Influence of the observation period on C input estimates
In our study, the youngest plants were labeled 16 days after emergence, and the oldest plants were labeled at the late milk-ripening stage (84 days after emergence). Therefore, it was necessary to extrapolate the C fluxes for the periods between days 0 and 15 and between days 85 and 120 to estimate the C transfer into the subsurface for the entire growing season. In contrast to our study, Swinnen and colleagues investigated spring wheat between elongation growth and the dough-ripe stage (Swinnen et al. 1994b), which corresponds to day 34 and 98 after emergence in our experiments. The observation period in our study is thus 4 days longer than that of the study of Swinnen et al. (1994b). In addition, the length and position of the extrapolated periods differ in both studies. To investigate the influence of such differences on the estimates of the downward-transferred C, the extrapolated downward-transferred C values from day 0 until day 15, from day 0 until day 33, from day 85 until day 120, and from day 99 until day 120 after emergence were calculated using our models and the parameters from the Keith-Swinnen method.
Relationships among the recently assimilated C in roots, rhizodeposition, and belowground respiration
To investigate the relationships among the amounts of recently assimilated C in roots, rhizodeposition, and belowground respiration over all of the observed developmental stages, the relative amounts of 14C (expressed as a percentage of the total recovered 14C of the plant-soil-soil gas system) of each component were contrasted with each other. Afterward, correlation and regression analyses for each set of paired data were performed. In addition to the measured 14C, the modeled transfers of recently assimilated C into these components were contrasted.
Statistical analyses
Non-linear regressions were used to determine the best-fitting models to describe relative 14C partitioning and shoot growth during the growing season. The coefficients of determination (R 2) for all non-linear regression analyses were calculated using 1-(SS error / SS corrected total), based on Kvålseth (1985). Furthermore, Spearman rank correlation analyses and non-linear regression analyses were used to analyze the relationships among root growth, detectable rhizodeposition, and belowground respiration (see the Section “Relationships among C fluxes of recently assimilated C in the subsurface”). All statistical calculations were performed using the Mathematica 9 software package (Wolfram Research).
Results
Modeling, quantification, and comparison of C fluxes
Modeling of shoot growth and 14C partitioning
The relative distribution of 14C in the plant-soil-soil gas system rapidly changed during the investigated period and depended on plant age (see Fig. 2). In the plant-soil-soil gas system, 32 % of the assimilated 14C that was recovered 21 days after labeling remained in the shoots, which were labeled after emergence, whereas 68 % was transferred into the subsurface (root growth: 28 %, rhizodeposition: 12 %, respiration of root and microbes: 28 %). In contrast, when labeled at the late milk-ripening stage, 93 % of the assimilated 14C remained in the shoots, and only 7 % was transferred into the subsurface (root growth: 3 %, rhizodeposition: 2 %, respiration of roots and microbes: 2 %).
All the tested models featured high goodness-of-fit, and in contrast to the rhizodeposition curve, all parameters of the other curves were statistically significant (p < 0.01; Table 1). Figure 2 also shows the sum of the four models used to describe the relative partitioning of 14C within the plant-soil-soil gas system. This sum exceeds the 100 % line by up to 0.7 % on day 10 and falls below the 100 % line by up to 1.2 % on day 31 after emergence. The cumulative sum up to t = 120 (days after emergence) was 12,000.4, compared to a desired value of 12,000. Therefore, the modeled 14C partitioning had an inaccuracy of approx. +0.003 % (overestimation) over the entire growing season.
As shown in Fig. 3, the Gompertz model was suitable (R 2 = 0.966) for describing the shoot growth of field-grown rye plants. All three parameters (a, b, and c) of the Gompertz model were statistically significant with p-values < 0.001. Figure 3 also shows the curve of the growth rate, created by differentiating the Gompertz equation of shoot growth with respect to time (t). The maximum rate of shoot growth was reached on day 56 after emergence.
Growth of spring rye shoots (field experiment), shown as the amount of C per plant shoot throughout the growing season (each point represents a pooled sample of 120 plant shoots, \(C_{shoot}=a*e^{-e^{b - c*t}}\), R 2 = 0.966, black line, left axis), and growth rate of the shoot, shown as the increment of C per plant shoot per day (gray line, right axis)
Modeling absolute C fluxes
All modeled C fluxes of the plant-soil-soil gas system are presented in Fig. 4. In contrast to the shoot growth maximum on day 56, the C transfer into the subsurface reached a maximum on day 38 after emergence. It should be noted that the curve of the C transfer into the subsurface corresponds to the sum of C incorporated into roots and the flow into rhizodeposition and belowground respiration. The C flux curves of belowground respiration, rhizodeposition, and root growth reached their maxima on day 36, 38, and 39 after emergence, respectively. This pattern indicates that root growth preceded shoot growth because their maxima were temporally separated by approximately 17 days.
Modeled C flux rates of spring rye during the growing season: sum of C that was used for shoot growth and was transferred into the subsurface (total C), shoot growth (shoot), transfer into the subsurface (transfer), root growth (root), belowground respiration (belowgr.), and detectable rhizodeposition (rhizo.)
Total C fluxes and their distribution in the plant-soil-soil gas system were calculated by integrating the different C flux rate curves (shown in Fig. 4) and are summarized in Table 2. The largest amount of C was transferred into the subsurface during stem elongation up to the late boot stage. Considering the absolute C values used for root growth, belowground respiration, and rhizodeposition over the whole growing period (211.1, 155.9, and 77.1 mg C/plant), which are presented in Table 2, and those values for the timespan between stem elongation and the late boot stage (97.8, 67.9, and 37.0 mg C/plant), the C amount for root growth, belowground respiration, and detectable rhizodeposition during stem elongation until the late boot stage amounted to 46 %,44 % and 48 %, respectively. Table 2 also shows that 18.3 % of the assimilated C (of the plant-soil-soil gas system) was transferred into the subsurface during the whole growing period and split into 8.7 % for root growth and 9.6 % for detectable rhizodeposition and belowground respiration.
Influence of initial labeling time on estimates of C input
In this study, the youngest and oldest plants were labeled 16 and 84 days after emergence, respectively. Therefore, it was necessary to extrapolate the C fluxes between days 0 and 15 and between days 85 and 120 to estimate the downward-transfer of C over the entire growing season (Table 3). Table 3 also presents the C fluxes that would be extrapolated if the first and last labelings were performed on day 34 and 98 after emergence, respectively, i.e., at elongation growth and the dough-ripe stage, as described by Swinnen et al. (1994b).
The sum of the extrapolated absolute C transfer into the subsurface between days 0 and 15 and between days 85 and 120 only amounted to approximately 5 % of the modeled C flux over the whole growing season (Table 3). Moreover, the low shoot growth rates during the first period and the low relative C transfer into the subsurface during the last period (see the Section “Modeling of shoot growth and 14C partitioning”) indicate that a deviation from the assumed curve progression will only have a small influence on the total calculated C amount. In contrast, the extrapolated C amount for sampling between day 34 and 98 after emergence amounts to approximately 30 % of the modeled C flux over the whole growing season.
Considering that the period between day 16 and 84 is only 4 days longer than the period between day 34 and 98, our findings indicate great uncertainty in the C calculation if the investigation does not start several days after plant emergence. However, the expansion of the investigated period from the late milk-ripening stage to the dough-ripening stage provides less improvement in the C calculation, as indicated by the results presented in Table 3.
Precision of modeled C transfer into the subsurface
As described in the Section “Development of an approach to quantify modeling precision”, for each component of the subsurface (root growth, belowground respiration, and detectable rhizodeposition), 125,000 model simulations of the Keith-Swinnen approach were performed to obtain C fluxes with different probabilities. Subsequently, the C-transfer values of each component were added to obtain a set of 125,000 values of the total C transfer into the subsurface. Means, medians, standard deviations (Sd), ranges, and coefficients of variation (CV) generated by model simulations and integration of the C flux rates over the sampling period (days 16-84) and the whole growing season (days 0-120) for each component and the whole subsurface are presented in Table 4.
The simulation runs of the Keith-Swinnen approach provided similar means and medians for each component and period. The greatest difference was 8 mg C, which was observed for total C transfer into the subsurface. This difference was only 1.8 % of the C (445 mg C) transferred into the subsurface during the whole growing season. As expected, means and medians were always greater for the entire growing period than for the shorter period. The differences between the means and medians of the C transfer into the subsurface indicate that over the longer period, 24 mg (median) to 27 mg (mean) more C was downward-transferred than in the shorter period; this difference is plausible. Moreover, all the other statistics show plausible values. Table 4 also shows that the variability of the 125,000 C transfer values of each component and entire subsurface are acceptable because the lowest and highest coefficients of variation (CV) of sampling period and whole growing season ranged between 7 % and 8 % and between 9 % and 11 %, respectively. In addition, the averaged C transfer values of each component and the entire subsurface of the whole growing season (days 0-120) are very close to the corresponding values (right column) of Table 2, which were modeled with the best-fitted shoot growth (Fig. 3) and C partitioning curves (Fig. 2).
As shown in Table 2, in total, 444.1 mg C per plant was transferred into the subsurface over the entire growing season. This value represents 18.3 % of the total C that was incorporated into shoot (shoot C mass) and transferred into the subsurface during plant development. Consequently, the value of C transfer into the subsurface (445 mg C) generated by model simulations (see Table 4) represents 18.34 % of the total C transferred into the subsurface and used for shoot growth. Using the corresponding standard deviation (43 mg C) indicates that 445 ± 43 mg C were transferred into the subsurface over the entire growing season. This range represents 18.34 ± 1.77 % of the total assimilated C that was not respired by the shoot. Using the C values for root growth, belowground respiration, and detectable rhizodeposition and their standard deviations from the whole growing season, presented in Table 4, in the same way, the calculation shows that 8.7 ± 0.8 %, 6.4 ± 0.7 %, and 3.2 ± 0.3 % of the C was used for root growth and flowed into belowground respiration as well as detectable rhizodeposition, respectively.
Relationships among C fluxes of recently assimilated C in the subsurface
A Spearman rank correlation analysis of the 14C in the roots, rhizodeposition, and belowground respiration provided correlation coefficients of 0.912, 0.923, and 0.953 for pairwise comparisons of the 14C amount of rhizodeposition, roots, and belowground respiration. Each correlation coefficient was statistically verified with a p-value < 0.001.
The correlation and regression analyses indicate that the flows of 14C into the roots, belowground respiration, and detectable rhizodeposition were strictly positively correlated with each other (Fig. 5a). A high flow of 14C into the roots is associated with a high flow of 14C into belowground respiration and detectable rhizodeposition. All three relationships could be described with the same two-parametric model (a∗x/(1 + b∗x 2)), with a coefficient of determination ranging between 0.748 and 0.851.
a) Comparisons of 14C (expressed as the percentage of 14C recovered in the plant-soil-soil gas system 21 days after labeling) determined during plant development in the roots, rhizodeposition (rhizodep.), and belowground respiration (below. resp.) with each other, and b) comparisons of the modeled absolute C flux rates (expressed in mg C per plant per day) of root growth, rhizodeposition (Rhizodep.), and belowground respiration (Below. resp.) with one another. It should be noted that the data on the horizontal axes were sorted according to their magnitude and not by time (as in the other figures)
The comparison of the modeled absolute rates of freshly assimilated C that flowed into the roots, rhizodeposition, and belowground respiration (Fig. 5b) indicates positively correlated relationships between the flow of C in each component of the subsurface. Moreover, the hysteresis curves (Fig. 5b) imply that root growth is related to higher belowground respiration and rhizodeposition until the highest C flow into the root was reached; after this point, the root growth is related to lower belowground respiration and rhizodeposition. A similar pattern was also obtained for the relationship between the C flux rates of the detectable rhizodeposition and belowground respiration. Admittedly, values before and after the reversal point of this curve are only slightly different from each other. Thus, the relationship between the C flux rates of the detectable rhizodeposition and belowground respiration seems to be nearly linear.
Discussion
Modeling 14C partitioning and shoot growth
Our results indicate that the relative 14C partitioning of the assimilated C strongly depends on the developmental stage of the plant. In general, the amount of 14C in the shoots increased during plant development. This result is consistent with the varying axial sink during plant development (Lambers et al. 2008; Yasumura 2009). In contrast to the spring wheat study of Swinnen et al. (1994b), who reported an increase only up to ∼ 83 % of all 14C recovered in the plant-soil-soil gas system 19 days after labeling, we observed incorporation into the shoots of up to ∼ 93 % during the ontogeny of spring rye. Furthermore, Sey et al. (2010) determined that more than 90 % of the 13 CO 2 assimilated by corn at the milking stage was retained in the shoot. Gregory and Atwell (1991) also observed that more than 90 % of the 14C assimilated by wheat and barley at grain filling was retained in the shoots.
Corresponding to the increase in the relative C partitioning to the shoots over time, a continuous decrease in the relative C transfer into the subsurface including roots, belowground respiration, and detectable rhizodeposition was observed. In contrast to the results of Swinnen et al. (1994b), in which a nearly constant flow of 5 % of assimilated 14C into the detectable rhizodeposits was observed between elongation growth and ripening, our study found a continuous decrease of this component from approximately 12 % at emergence to approximately 2 % at the milk-ripening stage (see Fig. 2).
Such differences may be caused by differences in plant species or environmental conditions. Therefore, systematic investigations over the entire growing season are required to determine how C partitioning varies depending on the plant species or cultivars and environmental conditions. Such investigations are required before the generalizability of the estimates of root-derived C input into the soil yielded by the Keith-Swinnen method can be evaluated.
However, the first aim of our study was to model the relative 14C partitioning within the plant-soil-soil gas system with sufficient precision from emergence to the ripening stage. This is a challenge for the Keith-Swinnen method because a set of functions that fits the partitioning data well and sums up to 100 % at each point in time during the investigated period (see Fig. 2) is required for precise C modeling. For Swinnen et al. (1994b), it was possible to describe the relative C partitioning in the plant-soil-soil gas system between elongation growth and ripening using a set of four simple functions (three exponential and one linear function) that amounted to exactly 100 % at each moment during the growing season. In this study, the 14C partitioning at emergence and tillering was also analyzed. The regression analyses of the 14C partitioning data indicated that it was not possible to use the 14C partitioning functions presented by Swinnen et al. (1994b) because these functions did not fit our data.
However, a set of functions that had maximum positive and negative deviations from the 100 % line of +0.7 % and -1.2 %, respectively, and a negligible inaccuracy of +0.003 % (overestimation) over the whole growing season was found. This set included the Gompertz function to describe the 14C partitioning of the shoots and three other non-linear functions, as described in the Section “Modeling of shoot growth and 14C partitioning”.
Shoot growth was also well described by the Gompertz model, which is a typical model for shoot growth (Causton and Venus 1981; Yin et al. 2009; Zeide 1993). In contrast to the study of Swinnen et al. (1994b), in which the authors used a numerical method to obtain the daily increments of shoot growth, an algebraic derivative was used in this study.
As shown by Remus et al. (2016), sampling 15 days after labeling should provide exact information about the partitioning of the tracer, if plants grow under favorable conditions. Indeed, we decided to harvest all labeled plants after the same time, 21 days after labeling, because we observed a delay of 14C turnover in soil and young leaves of oilseed rape under severe drought stress after labeling, which was stimulated again after re-watering (not yet published). An increased mean residence time of recently assimilated C in leaf biomass under drought stress was also described by Brüggemann et al. (2011). However, no drought-stress symptoms were observed in the outdoor experiment, and the grain yield of the spring rye field experiment was 4.163 ± 0.454 t per ha. The grain yield of winter rye ranged between 6 and 7 t per ha on our experimental area in the last 20 years. Consequently, the grain yield of spring rye was 59 to 69 % of that of winter rye. This pattern also indicates that the spring rye grows under normal conditions.
Modeling all absolute C fluxes
The linkage of the relative 14C partitioning (as a percentage of the amount of 14C recovered in the plant-soil-soil gas system 21 days after labeling) with the shoot growth rate (mg C per plant and day) provided functions of absolute C partitioning that resembled asymmetric bell-shaped curves. Although we used different functions to describe the C partitioning in the plant-soil-soil gas system than Swinnen et al. (1994b), similar trends for the absolute C fluxes were observed.
Our investigation demonstrated that spring rye transfers significantly less (18.3 %) C into the subsurface than spring wheat (approximately 29 % of the assimilated C) over the whole growing season (Swinnen et al. 1994b). However, the use of downward-transferred C for root growth, rhizodeposition, and belowground respiration seems similar between wheat and rye. Wheat and rye use 41 % and 47 %, respectively, of the total transferred C into the subsurface for root growth and 59 % and 53 %, respectively, of the downward-transferred C flows into rhizodeposition and belowground respiration in wheat and rye.
The modeled total C transfer of rye into the subsurface indicates that the maximum flux rate of the C transfer to roots, belowground respiration, and detectable rhizodeposition took place at stem elongation and preceded the maximum C assimilation rate of shoot growth. This result indicates that not only the relative C partitioning but also the plant biomass production combined with the relative C partitioning determines the absolute C fluxes in the plant-soil-soil gas system.
In our investigation, maximum shoot growth occurred 17 days after maximum root growth. Investigations of Swinnen et al. (1994b) also indicate that the maximum root growth rate precedes the maximum shoot growth rate. This temporal offset may explain the temporal offset of the nutrient uptake by wheat plants and plant growth reported by Linser H (1972). This author observed that the point of inflection for the phosphorus, potassium, and nitrogen uptake curves preceded the point of inflection of the dry matter growth curve with a temporal offset of 5 to 10 days.
However, the decrease in the root growth rate after day 39 was possibly caused by a stronger C sink in the shoots after this time, which implies that the C sink in the shoots had already exceeded the C sink in the roots before the plant switched from the vegetative to the generative phase.
Precision of modeled C fluxes
Our model simulations showed that the Keith-Swinnen method is able to provide absolute C fluxes with high precision: the coefficients of variation of the calculated C amounts, which were transferred into subsurface and different components, ranged between 7 % and 8 % and between 9 % and 11 % for sampling period and whole growing season, respectively (see Table 4). Admittedly, a vector of 50 random numbers is only suitably restricted to represent symmetrically distributed data, and any substantial extension of the vector requires an enormous computation time because of the rapid increase in the number of possible combinations of prediction curves for each component. Therefore, a second simulation with 50 unique distributed mean prediction bands and equal distance between all of them was performed similar to Hoffmann et al. (2015), to compare their results (data not shown) with those of our favored simulation approach. Moreover, the probability levels of the upper and lower mean prediction bands in this second simulation were extended to p = 0.9, where computation was still possible or did not stop, to analyze the effect of prediction curves with a wider range. For example, the histograms of the downward-transferred C into the subsurface of the sampling period from the first (favored) and second simulation are shown in Fig. 6.
Frequency analyses of the C input values of the entire subsurface for the sampling period (16 to 84 days after emergence). Histogram of 125,000 simulation runs with 50 random normally distributed mean prediction bands (above) and a histogram of 125,000 simulation runs with 50 equidistant and unique distributed mean prediction bands (below). CV: coefficient of variation
The vector used for the probability levels of the mean prediction bands has an influence on the symmetry and variation of the estimated values of the downward-transferred C. However, Fig. 6 clearly indicates that the two different simulation approaches yielded means that differed by only approximately 0.2% and medians that differed by only approximately 0.5 %; these differences are negligible. Moreover, the extension of the probability level to p = 0.9 and the equal weighting of more and less probable prediction curves around the best-fitting curve provided a coefficient of variation of only ∼ 15 %.
Such a precise calculation of the C transfer into the subsurface is not possible to obtain by a simple estimation method based on using the mean or median of the relative C partitioning coefficients and the shoot biomass obtained at the end of the growing period because the relative partitioning of 14C is extremely different in young and old plants. For example, the modeled relative 14C partitioning coefficients of the C transfer into the subsurface (see Fig. 7) decreased from 68.9 % at emergence (day 16) to 5.3 % at the late milk-ripening stage (day 84). The standard deviation and coefficient of variation of the modeled daily relative 14C partitioning coefficients of the C transfer into the subsurface from this timespan (n = 69) amounted to 18.6 and 72.4 %, respectively. Using the mean (25.7 %) of the modeled daily relative 14C partitioning coefficients of the C transfer into the subsurface from emergence until the late milk-ripening stage and the shoot C increment from day 16 to day 84 (1590.3 mg C per plant) provides an average C transfer into the subsurface of 550 mg C (= 1590.3*25.7/74.3), whilst the median (19.6 %) provides a value of 388 mg C (= 1590.3*19.6/80.4). Consequently, the simple estimation method leads to an overestimation of 32 % using the mean or to an underestimation of 6 % using the median. Considering that the CV of relative 14C partitioning coefficients of the C transfer into the subsurface amounted to 72.4 % and the variation of the shoot C increment was not included by this simple method, the estimation of C values has a high degree of uncertainty.
Relative transfer of 14C (expressed as percentage of 14C recovered in the plant-soil-soil gas system 21 days after labeling; black line) and the rate of total transfer of C (expressed as mg C per plant per day; gray line) into the subsurface during the growing season. The curve segments in the gray regions are extrapolated
Using the mean and median of the relative 14C partitioning coefficients of the C transfer into the subsurface from the entire growing season and shoot C mass at ripeness, the simple method overestimates the C transfer into the subsurface by 56.6 % and underestimates this transfer by 32 % with a CV of 106 % (calculation not shown here). In addition to the high variation of the relative C partitioning coefficients, which lead to a high uncertainty, the fact that this simple method is not able to consider the different trends of relative and absolute C partitioning within the plant-soil-soil gas system (see Fig. 7) leads to an over- or underestimation of the C transfer depending on the measure of central tendency, i.e., average or median. Overall, these results confirm the suspicion that a simple method based on the averages or medians of the relative C partitioning coefficients and aboveground biomass can provide only raw estimations for C allocation within plant-soil-soil gas systems (Kuzyakov and Domanski 2000).
One approach that considers plant growth based on repeated pulse labeling experiments was suggested by Werth and Kuzyakov (2008). However, this approach is only suitable for estimating the soil C input over a short period in which the shoot growth is nearly linear, whereas in general, shoot growth follows a sigmoid curve progression over the whole growing season (Causton and Venus 1981; Yin et al. 2009; Zeide 1993).
Another approach to estimate the soil C input during the growing season was suggested by Bolinder et al. (2007), who used the shoot/root ratio and an average relative C partitioning coefficient for the rhizodeposition. However, apart from the precision of the average relative C partitioning coefficient, it can be difficult to determine the amount of root C using root sampling because sample preparation can cause considerable losses (Böhm 1979; Box 1996; Oliveira et al. 2000; Swinnen et al. 1994b). Furthermore, roots may be contaminated with soil particles even after washing, requiring a correction to obtain the real amount of the root C. For example, in our study, the C concentration of the washed root samples averaged 34 % of the C concentration of the dry mass, whereas the shoot samples exhibited a C concentration of 44 % (data are not shown).
Moreover, parts of the root system can die and mineralize during plant development (Box 1996; Milchunas 2009; Pritchard and Rogers 2000; Saggar and Hedley 2001; Smithwick et al. 2014; Steingrobe et al. 2001). Therefore, the root biomass at sampling does not necessarily correspond to the root biomass actually formed prior to the sampling; consequently, an approach based on the shoot/root ratio can only be an approximation of the downward-transferred C, as noted by Bolinder et al. (2007).
In contrast to the aforementioned method, the turnover of senescent or dead roots does not matter in the Keith-Swinnen method because the daily formed root mass (expressed as root C) is calculated and integrated over the investigated time period in the latter approach. Furthermore, the use of the C tracer in this approach enables a complete balance of the recently assimilated C within the plant-soil-soil gas system along with estimates of the downward-transferred C with a known and acceptable precision.
Influence of the observation period on C input estimates
The investigation presented in the Section “Influence of initial labeling time on estimates of C input” indicated that shortening the extrapolated timespan at the beginning of the growing season by labeling at emergence instead of during the stage of elongation growth had a greater effect on the uncertainty of the C estimates than decreasing the extrapolated timespan at ripening. Starting the investigation at emergence caused only approximately 5 % of the estimated transfer of C into the subsurface to be located in the extrapolated periods, provided that the C transfer follows the used models. In contrast, starting the investigation at elongation caused approximately 30 % of the estimated transfers to be located in the extrapolated periods.
Moreover, the absolute amount of downward-transferred C strongly varied among different developmental stages. For example, the largest amount of C was transferred into the subsurface from stem elongation until the late boot stage. During this period, which was only 21 days, approximately 46 % of the total downward-transferred C flowed into the subsurface.
This finding supports the importance of a balanced age structure for the investigated plants for the calculation of absolute C fluxes during the growing period (Jones et al. 2009) and stresses the importance of sampling throughout the entire growing season.
Relationships among fluxes of recently assimilated C in the subsurface
The correlation and regression analyses of root growth, belowground respiration, and detectable rhizodeposition clearly indicated dependencies among these components (see Fig. 5). The correlation analysis showed that the 14C flows into the root and the detectable rhizodeposition were significantly positively correlated. This pattern confirms the results of Xu and Juma (1993), who observed a positive correlation between the 14C activities of roots and soil. This pattern also confirms the observation of Dilkes et al. (2004) indicating that the exudation of 14C-labeled soluble material depends more on the rate of C input to the roots than on the rate of photosynthesis of wheat.
Moreover, highly significant positive correlations between the 14C flow to the roots and the belowground respiration as well as between detectable rhizodeposition and belowground respiration were determined. This result indicates that high root growth is connected not only with high rhizodeposition but also with a high respiratory turnover of recently assimilated C in the subsurface. Part of the rhizodeposits will be respired by microbes; therefore, the production of rhizodeposits should be partially linked to belowground respiration.
Based on sample preparation (see the Section “Sample preparation”) that included washing of roots to remove rhizosphere soil and wet sieving of rhizosphere and bulk soil to separate root fragments and rhizodeposits, one can assume that the observed relationships between root growth and rhizodeposition are artificial because roots can lose C due to washing procedures (Swinnen et al. 1994b). However, as shown by Remus et al. (1985), nearly all 14C of the mobile C pool was respired, exuded, or incorporated into the structural C pool of the plant at harvesting (21 days after labeling). Therefore, the amount of 14C that is able to leave the root by diffusion should be negligible. Moreover, the correlations between root growth and belowground respiration as well as between detectable rhizodeposition and belowground respiration, which were obtained in undisturbed root-soil-soil gas systems, support the natural origin of the observed relationships.
In addition, our results show that the relationships between the relative and absolute C fluxes of root growth, belowground respiration, and detectable rhizodeposition are very similar during the growing season, which implies that rhizodeposition and belowground respiration depend on root growth. Comparisons with the relative C partitioning presented by Keith et al. (1986) and Swinnen et al. (1994b) suggest that the same conclusion applies to wheat.
Moreover, a strong, nearly linear relationship was observed between the absolute belowground respiration and detectable rhizodeposition (Fig. 5b). Based on this finding, it seems possible to calculate the actual rhizodeposition for the whole growing season if it is possible to determine the share of rhizodeposition that was respired from labeling until harvest on day 21.
Conclusions
As shown in this study, it is possible to model relative 14C partitioning within the plant-soil-soil gas system from emergence to the ripening stage with sufficient precision, although a set of complex functions is necessary. This study clearly demonstrates that relative C partitioning coefficients alone can provide only limited information about the actual C allocation within plant-soil-soil gas systems, whereas dynamic linking of relative C partitioning coefficients with shoot growth at each moment during the growing season can provide detailed information about the partitioning process and quantity of absolute C fluxes. Such information can be obtained for single developmental stages as well as for the entire growing season. However, this study also showed that it is necessary to investigate the whole vegetation period to obtain reliable data about the transfer of C into the subsurface because the transfer of C into the subsurface during the plant development is a highly dynamic process.
Based on model simulations, an approach could be presented that enables calculation of the precision of the C-transfer values calculated by the Keith-Swinnen method; thereby, this method could be improved. The model simulations indicate that 18.3 ± 1.7 % of all assimilated C that was not respired by shoots was transferred into the subsurface during the entire growing season. This flow splits into 8.7 ± 0.8 % C for root growth, 6.4 ± 0.7 % C for belowground respiration, and 3.2 ± 0.3 % C for detectable rhizodeposition.
Although the Keith-Swinnen method is very labor intensive, this approach has several advantages compared to other methods discussed in this study. Moreover, because the types of the applied models are easy to link with plant growth or yield models, the Keith-Swinnen method has a large potential for the precise estimation of C inputs for a wide range of plants under different environmental conditions and management practices. To meet this objective, phenologically dependent relative C partitioning coefficients of relevant crops should be determined, and possible ranges of variation of relative C partitioning coefficients on different genotypes as well as environmental conditions should be analyzed.
References
Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, Thorpe MR, Orians CM (2005) Jasmonic acid induces rapid changes in carbon transport and partitioning in populus. New Phytol 167:63–72
Bloom AJ, Sukrapanna SS, Warner RL (1992) Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol 99:1294–1301
Böhm W (1979) Methods of studying root systems. Ecological Studies. Springer
Bolinder MA, Janzen HH, Gregorich EG, Angers DA, VandenBygaart AJ (2007) An approach for estimating net primary productivity and annual carbon inputs to soil for common agricultural crops in Canada. Agric Ecosyst Environ 118:29–42
Bolinder M, Katterer T, Andren O, Parent L (2012) Estimating carbon inputs to soil in forage-based crop rotations and modeling the effects on soil carbon dynamics in a swedish long-term field experiment. Can J Soil Sci 92:821–833
Box J (1996) Modern methods for root investigations. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. 2nd edn. Marcel Dekker, Inc., New York, pp 193–237
Brüggemann N, Gessler A, Kayler Z, Keel S, Badeck F, Barthel M, Boeckx P, Buchmann N, Brugnoli E, Esperschütz J, Gavrichkova O, Ghashghaie J, Gomez-Casanovas N, Keitel C, Knohl A, Kuptz D, Palacio S, Salmon Y, Uchida Y, Bahn M (2011) Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences 8:3457–3489
Carbone MS, Trumbore SE (2007) Contribution of new photosynthetic assimilates to respiration by perennial grasses and shrubs: residence times and allocation patterns. New Phytol 176:124–135
Causton DR, Venus JC (1981) The biometry of plant growth. Edward Arnold Publishers, London
Chapin III FS , McFarland J, McGuire AD, Euskirchen ES, Ruess RW, Kielland K (2009) The changing global carbon cycle: linking plant-soil carbon dynamics to global consequences. J of Ecol 97:840–850
Dilkes NB, Jones DL, Farrar J (2004) Temporal dynamics of carbon partitioning and rhizodeposition in wheat. Plant Physiol 134:706–715
Gregory PJ, Atwell BJ (1991) The fate of carbon in pulse-labeled crops of barley and wheat. Plant Soil 136:205–213
Hoffmann M, Jurisch N, Albiac Borraz E, Hagemann U, Drösler M, Sommer M, Augustin J (2015) Automated modeling of ecosystem CO2 fluxes based on periodic closed chamber measurements: A standardized conceptual and practical approach. Agric For Meteorol 200:30–45
Janzen HH (2006) The soil carbon dilemma: Shall we hoard it or use it? Soil Biol Biochem 38:419–424
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321:5–33
Keith H, Oades JM, Martin JK (1986) Input of carbon to soil from wheat plants. Soil Biol Biochem 18:445–449
Keutgen N, Roeb GW, Minchin PEH, Fuhr F (1995) Use of transfer-function and compartmental analysis to quantify C-11 labeled photoassimilate export from wheat leaves. J Exp Bot 46:489–496
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Rev J Plant Nutr Soil Sci 163:421–431
Kuzyakov Y, Schneckenberger K (2004) Review of estimation of plant rhizodeposition and their contribution to soil organic matter formation. Arch Agron Soil Sci 50:115–132
Kuzyakov Y, Ehrensberger H, Stahr K (2001) Carbon partitioning and below-ground translocation by lolium perenne. Soil Biol Biochem 33:61–74
Kvålseth TO (1985) Cautionary note about R 2. Am Stat 39:279–285
Lambers H, Scheurwater I, Atkin OK (1996) Respiratory patterns in roots in relation to their functioning. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. 2nd edn. Marcel Dekker, Inc., New York, pp 323–362
Lambers H, Chapin III FS, Pons T L (2008) Plant Physiological Ecology, 2nd. Springer
Linser H (1972) Wachstum und Ertragsbildung. In: Handbuch der Pflanzenernährung und Düngung. 2nd edn. Springer, pp 595–662
Litton CM, Giardina CP (2008) Below-ground carbon flux and partitioning: global patterns and response to temperature. Funct Ecol 22:941–954
Meng F, Dungait JAJ, Zhang X, He M, Guo Y, Wu W (2013) Investigation of photosynthate-C allocation 27 days after 13C-pulse labeling of Zea mays L. at different growth stages. Plant Soil 373:755–764
Milchunas D (2009) Estimating root production: Comparison of 11 methods in shortgrass steppe and review of biases. Ecosystems 12:1381–1402
Moureaux C, Debacq A, Hoyaux J, Suleau M, Tourneur D, Vancutsem F, Bodson B, Aubinet M (2008) Carbon balance assessment of a belgian winter wheat crop (Triticum aestivum L.) Glob Change Biol 14:1353–1366
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23:375–396
Oliveira M d R G, Van Noordwijk M, Gaze SR, Brouwer G, Bona S, Mosca G, Hairiah K (2000) Auger sampling, ingrowth cores and pinboard methods. In: Smit AL, Bengough AG, Engels C, Van Noordwijk M, Pellerin S, Van de Geijn SC (eds) Root methods: a handbook. Springer, pp 175– 210
Plaxton WC, Podesta FE (2006) The functional organization and control of plant respiration. Crit Rev Plant Sci 25:159–198
Pritchard SG, Rogers HH (2000) Spatial and temporal deployment of crop roots in CO2-enriched environments. New Phytol 147:55–71
Qureshi RM, Fritz P, Drimmie RJ (1985) The use of CO2 absorbers for the determination of specific 14C activities. Int J Appl Radiat Isot 36:165–170
Rasse D, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant and Soil 269:341–356
Remus R, Hüve K, Pörschmann J, Augustin J (2016) Determining the timepoint when 14 C tracer accurately reflect photosynthate use in the plant-soil system. Plant Soil. doi:10.1007/s11104-016-3002-2
Saggar S, Hedley CB (2001) Estimating seasonal and annual carbon inputs, and root decomposition rates in a temperate pasture following field C-14 pulse-labelling. Plant and Soil 236:91–103
Sauerbeck D, Johnen B, Six R (1976) Respiration, decomposition and excretion of wheat roots during their development. Landwirtsch Forsch Sonderheft 32:49–58
Sey BK, Manceur AM, Whalen JK, Gregorich EG, Rochette P (2010) Root-derived respiration and nitrous oxide production as affected by crop phenology and nitrogen fertilization. Plant Soil 326:369–379
Smithwick EAH, Lucash MS, McCormack ML, Sivandran G (2014) Improving the representation of roots in terrestrial models. Ecol Model 291:193–204
Staddon PL, Ostle N, Dawson LA, Fitter AH (2003) The speed of soil carbon throughput in an upland grassland is increased by liming. J Exp Bot 54:1461–1469
Steingrobe B, Schmid H, Claassen N (2001) Root production and root mortality of winter barley and its implication with regard to phosphate acquisition. Plant Soil 237:239–248
Stockmann U, Adams MA, Crawford JW, Field DJ, Henakaarchchi N, Jenkins M, Minasny B, McBratney AB, De Courcelles VdR, Singh K, Wheeler I, Abbott L, Angers DA, Baldock J, Bird M, Brookes PC, Chenu C, Jastrowh JD, Lal R, Lehmann J, O’Donnell AG, Parton WJ, Whitehead D, Zimmermann M (2013) The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric Ecosyst Environ 164:80–99
Swinnen J (1994) Rhizodeposition and turnover of root-derived organic material in barley and wheat under conventional and integrated management. Agric Ecosyst Environ 51:115–128
Swinnen J, Van Veen JA, Merckx R (1994a) C-14 pulse-labeling of field-grown spring wheat - an evaluation of its use in rhizosphere carbon budget estimations. Soil Biol Biochem 26:161–170
Swinnen J, Van Veen JA, Merckx R (1994b) Rhizosphere carbon fluxes in field-grown spring wheat - model calculation based on C-14 partitioning after pulse-labeling. Soil Biol Biochem 26:171–182
Toal ME, Yeomans C, Killham K, Meharg AA (2000) A review of rhizosphere carbon flow modelling. Plant Soil 222:263–281
Virto I, Barre P, Burlot A, Chenu C (2012) Carbon input differences as the main factor explaining the variability in soil organic C storage in no-tilled compared to inversion tilled agrosystems. Biogeochemistry 108:17–26
Wang GM, Coleman DC, Freckman DW, Dyer MI, McNaughton SJ, Acra MA, Goeschl JD (1989) Carbon partitioning patterns of mycorrhizal versus non-mycorrhizal plants - real-time dynamic measurements using CO2-C-11. New Phytol 112:489–493
Warren JM, Iversen CM, Garten JCT, Norby RJ, Childs J, Brice D, Evans RM, Gu L, Thornton P, Weston DJ (2012) Timing and magnitude of C partitioning through a young loblolly pine (Pinus taeda L.) stand using C-13 labeling and shade treatments. Tree Physiol 32:799–813
Werth M, Kuzyakov Y (2008) Root-derived carbon in soil respiration and microbial biomass determined by C-14 and C-13. Soil Biol Biochem 40:625–637
Wiesmeier M, Hübner R, Dechow R, Maier H, Spörlein P, Geuß U, Hangen E, Reischl A, Schilling B, Von Lützow M, Kögel-Knabner I (2014) Estimation of past and recent carbon input by crops into agricultural soils of southeast germany. Eur J Agron 61:10–23
Xu JG, Juma NG (1993) Aboveground and belowground transformation of photosynthetically fixed carbon by 2 barley (Hordeum vulgare L.) cultivars in a typic cryoboroll. Soil Biol Biochem 25:1263–1272
Yasumura Y (2009) The effect of altered sink-source relations on photosynthetic traits and matter transport during the phase of reproductive growth in the annual herb chenopodium album. Photosynthetica 47:263–270
Yin X, Guo W, Spiertz JH (2009) A quantitative approach to characterize sink-source relationships during grain filling in contrasting wheat genotypes. Field Crop Res 114:119–126
Zeide B (1993) Analysis of growth equations. Forest Sci 39:594–616
Acknowledgments
We are grateful to Christine Ewald, Krystyna Herrendorf, and Susanne Remus from the ZALF in Müncheberg, Germany, for preparing the soil, root and shoot samples. In addition, we thank the DFG for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Remus, R., Augustin, J. Dynamic linking of 14C partitioning with shoot growth allows a precise determination of plant-derived C input to soil. Plant Soil 408, 493–513 (2016). https://doi.org/10.1007/s11104-016-3006-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3006-y