Abstract
Background and aims
Herbaspirillum seropedicae (Hs) Z67 a diazotrophic endophyte was genetically engineered for secretion of 2-keto-D-gluconic acid by heterologous expression of genes for pqq synthesis and gluconate dehydrogenase to study its beneficial effect on plants.
Methods
Two plasmids, pJNK5, containing a 5.1 Kb pqq gene cluster of Acinetobacter calcoaceticus and pJNK6, carrying in addition the Pseudomonas putida KT2440 gluconate dehydrogenase (gad) operon were constructed in pUCPM18Gmr under Plac promoter. H. seropedicae Z67 transformants were monitored for P and K solubilization, cadmium (Cd) tolerance and rice growth promotion.
Results
Hs (pJNK5) secreted 23.5 mM gluconic acid and Hs (pJNK6) secreted 3.79 mM gluconic acid and 15.8 mM 2-ketogluconic acid respectively. Under aerobic conditions, Hs (pJNK5) and Hs (pJNK6) solubilized 239.7 μM and 457.7 μM P on HEPES rock phosphate and, 76.7 μM and 222.7 μM K on HRPF (feldspar), respectively, in minimal medium containing 50 mM glucose. Under N free minimal medium, similar effects of P and K solubilization were obtained. Hs (pJNK5) and Hs (pJNK6) inoculation increased the biomass, N, P, K content of rice plants (Gujarat – 17). These plants also accumulated 0.73 ng/g PQQ, and had improved growth and tolerance to CdCl2.
Conclusions
Incorporation of pqq and gad gene clusters in H. seropedicae Z67 imparted additional plant growth promoting traits of P and K solubilization and ability to alleviate Cd toxicity to the host plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Herbaspirillum seropedicae Z67 is a nitrogen-fixing bacterium which colonizes as an endophyte economically important crops such as rice, sugarcane, and wheat (da Silva et al. 2003). Inoculation of this bacterium has been shown to increase biomass and N content of rice plants equivalent to the treatment with 40 kg of N/ha (Pereira and Baldani 1995). It is well known that N fixation by endophytic or symbiotic bacteria is limited by the availability of P and that co-inoculation of N fixing bacteria with phosphate solubilizing microorganisms (PSMs) results in improved N status of plants (Elkoca et al. 2007; Valverde et al. 2007).
PSMs increase the availability of P by secreting low molecular weight organic acids such as citric, oxalic, gluconic, 2-ketogluconic, lactic, malic, etc. which account for their mineral phosphate solubilizing (MPS) ability (Gyaneshwar et al. 2002). Solubilization of mineral P from soils depends on the nature and amount of the organic acid, which varies from 10 to 100 mM from strong to weak organic acids (Archana et al.2012). One of the most well-studied mechanisms of organic acid mediated P solubilization is the secretion of high levels of gluconic acid synthesized by the direct glucose oxidation pathway involving pyrroloquinoline quinone (PQQ)-dependent periplasmic glucose dehydrogenase (GDH) (Goldstein 1995). Many bacteria possess the apo-GDH but lack the ability to synthesize its cofactor PQQ. Heterologous expression of PQQ biosynthesis gene clusters from Pseudomonas., Burkholderia, Azospirilluim brasilense, Deinococcus radiodurans and Serratia marcescens in Escherichia coli have been reported to confer upon E. coli the ability to secrete gluconic acid (Krishnaraj and Dahale 2014). Coexpression of genes for the cofactor (PQQ) and the apoenzyme (GDH) from Serratia marcescens has been reported to confer high MPS ability to E. coli (Farhat et al. 2013). The GDH enzyme and the PQQ biosynthesis pathway have been targeted for genetic manipulation to improve MPS ability in several Gram-negative bacteria (Sashidhar and Podile 2010). We have recently reported the heterologous expression of PQQ biosynthesis operon in the H. seropedicae Z67 (Wagh et al. 2014a). Interestingly, pqqE alone is not sufficient for gluconic acid secretion in H. seropedicae Z67 (as against in E. coli), but the cluster consisting of pqqABCDE resulted in high PQQ levels and the appearance of GDH activity and gluconic acid secretion in this bacterium conferring upon it the MPS ability (Wagh et al. 2014a).
2-Keto-D-gluconic acid (2KDG) is a stronger acid than gluconic acid and can solubilize mineral phosphates more efficiently by chelation of calcium ions from Ca-phosphates in soils (Moghimi et al. 1978). Certain plant growth promoting bacteria are known to secrete 2KDG (Gulati et al. 2010; Park et al. 2010). The production of 2KDG is attributed to the gluconate dehydrogenase enzyme which is part of the direct glucose oxidation pathway. Yum et al. 1997 reported overexpression of Erwinia cypripedii ATCC 29267 gad operon encoding three subunits of membrane-bound GADH in E. coli. The holoenzyme glucose dehydrogenase of E. coli was reconstituted by addition of pyrroloquinoline quinone to the culture medium, and this led to the conversion of D-glucose to 2KDG by recombinant E. coli harboring the cloned GADH gene. Overexpression of Pseudomonas putida KT 2440 gad operon in Enterobacter asburiae PSI3, a P-solubilizing rhizobacterium proficient at gluconic acid secretion, resulted in the extracellular conversion of gluconic acid to 2KDG with enhanced MPS ability (Kumar et al. 2013).
The role of organic acids in solubilizing insoluble K containing minerals in addition to mineral P solubilization is becoming significant. Several bacterial strains solubilize minerals, such as feldspar, biotite, illite etc. by excreting organic acids that either directly dissolve rock K or chelate silicon ions to bring the K in to solution (Sheng and He 2006) and can supply K to plants (Sheng 2005; Basak and Biswas 2009). Organic acid mediated changes in metal mobility are important for alleviation of metal phytotoxicity (Archana et al. 2012). For instance, citric, malic and oxalic acids are implicated in the sequestration and detoxification of Al3+ (Singh and Chauhan 2011). Enterobacter asburiae PSI3, a gluconic acid secreting rhizobacterium could enhance growth of mung bean seedlings in the presence of phytotoxic levels of Cd2+ (Kavita et al. 2008), while multimetal-resistant, gluconic acid secreting Enterobacter sp. C1D promoted plant growth in the presence of toxic levels of Cr3+ . Since 2KDG is a stronger acid (pKa 2.66) than gluconic acid (pKa 3.8), it is of interest to develop strategies to incorporate 2KDG production in plant-beneficial bacteria and study the plant growth promotion ability of the engineered strain.
This present study reports the overexpression of Acinetobacter calcoaceticus pqq gene cluster and Pseudomonas putida KT2440 gad operon in H. seropedicae Z67 resulting in higher 2KDG secretion imparting upon the engineered strain superior MPS and K solubilizing ability, tolerance to Cd toxicity there by promoting growth of rice plants.
Materials and methods
Bacterial strains, plasmids, growth media, and culture conditions
Bacterial strains and plasmids used for this work are listed in Table 1. DNA manipulation and plasmid constructions were performed using E. coli DH10B as the host strain using standard protocols (Sambrook and Russell 2001). Plasmid bearing derivatives of E. coli DH10B were routinely grown at 37 °C on Luria Bertani broth (LB) and maintained on Luria Bertani Agar (LA) plates with 40 μg ml−1 streptomycin. H. seropedicae Z67 was routinely grown at 30 °C on LB and maintained on semisolid JNFb medium (pH 5.8) plates containing 20 μg ml−1nalidixic acid at 30 °C (Baldani et al. 1986). For growth of H. seropedicae Z67 and its plasmid bearing derivatives under nitrogen fixing conditions, JNFb medium was prepared devoid of a nitrogen source.
Cloning of A. calcoaceticus pqq gene cluster in the broad host range vector pUCPM18Gm under lac promoter
Plasmid pSS2 containing pqq gene cluster of A. calcoaceticus was obtained as a generous gift from Dr. N. Goosen, Department of Molecular Genetics, University of Leiden, Netherlands. pSS2 was digested with EcoR1 and BamHI and the 5.1 Kb insert was purified by using the Invitrogen gel purification Kit (Carlsbad, CA, USA) and ligated with the broad host range vector pUCPM18Gm digested with same restriction endonucleases (REs), which resulted in pJNK5 plasmid. The ligation mixture was transformed in to E. coli DH10B Strr; the screening was done on the basis of antibiotic resistance. The plasmid was isolated and confirmed by RE digestions.
Cloning of gad operon in pJNK5 under lac promoter
Plasmid pCNK12 containing gad operon of P. putida KT 2440 (Kumar et al. 2013) was digested by BamHI and XbaI. The 3.8 Kb fragment containing the gad operon was purified using the Invitrogen gel purification Kit. Plasmid pJNK5 containing the pqq gene cluster of A. calcoaceticus was digested with BamHI and XbaI and the 3.8 Kb fragment was ligated with pJNK5 which resulted in pJNK6 plasmid. The ligation mixture was transformed in to E. coli DH10B Strr; screening was done on the basis of antibiotic resistance. Plasmid was isolated and confirmed by RE digestion. Further, plasmids pUCPM18Gm as vector control, pJNK5, pJNK6 were transformed by electroporation in H. seropedicae Z67 as described by (Unge et al. 1996).
P-solubilizing assay
Fresh cultures of H. seropedicae Z67 and its transformants, obtained by inoculating a single colony in 3 ml LB and growing under shaking conditions at 30 °C, were dispensed in 1.5 ml sterile tubes, centrifuged at 9200 x g and washed thrice with sterile normal saline (0.85 % NaCl). P-solubilizing ability was monitored as di-calcium phosphate (DCP) solubilization on Pikovaskya’s (PVK) agar (Pikovskaya 1948) and acid secretion recorded on rock phosphate containing buffered minimal agar (HRP medium) plates with methyl red as a pH indicator. HRP medium is similar to TRP medium (Buch et al. 2008) with 100 mM HEPES buffer of pH 8.0 instead of 100 mM Tris-Cl pH 8.0 (Wagh et al. 2014a). The culture suspension was aseptically spot inoculated on the HRP agar plates and allowed to dry completely followed by incubation at 30 °C for 2–4 days. P solubilization was determined by monitoring the zone of clearance on PVK agar and red zone on the HRP Methyl red agar plates.
Potassium solublization assay
Washed and resuspended culture suspensions as mentioned above were used to characterize K-solubilizing ability using Feldspar as the mineral K source (Aleksandrov agar plate) (Hu et al. 2006). Culture suspensions were aseptically spot inoculated on the plates and left to dry completely followed by incubation at 30 °C for 2–4 days. K solubilization was determined by monitoring the zone of clearance.
GDH and GADH assays
H. seropedicae Z67 wild type as well as transformants were grown on M9 and HRP minimal medium with 50 mM glucose as the C source and 50 mM HEPES buffer pH 8.0. Cells were harvested (5000 xg for 10 min) after the pH of the culture supernatant was reduced to below 5.0, washed with sterile saline, and resuspended in 50 mM Tris-HCl, pH 8.75, and the whole-cell suspension was employed as the source of the enzyme. Spectrophotometric method was followed for estimation of GDH activity, according to (Matsushita and Ameyama 1982). GADH enzyme activity was measured using D – gluconate as substrate in the assay mixture (Matsushita et al. 1982). GDH and GADH units were defined as nanomoles of 2, 6-dichlorophenol indophenol (DCIP) reduced per minute using glucose and gluconate as substrates, respectively.
PQQ determination
PQQ levels of H. seropedicae Z67 transformants was estimated as described earlier (Wagh et al. 2014a).
Growth characteristics
Growth profile and pH reduction of the spent medium were monitored for wild type as well as H. seropedicae Z67 (Hs) transformants using HRP medium and P released in the supernatant was determined as a measure of rock phosphate solubilization ability (Gyaneshwar et al. 1998). HRP medium was supplemented with 1 mg/ml Feldspar (HRPF) for monitoring K solubilization. Growth curves and pH profiling were performed in 150 ml conical flasks containing 30 ml of relevant medium (Wagh et al. 2014a).
Minimum inhibitory concentration (MIC) and maximum tolerance concentration (MTC) for cadmium of Hs transformants
The Minimal inhibitory concentration (MIC) and maximum tolerance concentration (MTC) of Hs transformants for Cd was determined by broth dilution method of Calomiris et al. 1984. Freshly grown cultures of Hs transformants were washed thrice with saline (0.85 %), resuspended in 1 ml saline and used for inoculation. Cultures were inoculated aseptically in 150 ml conical flask containing different concentrations of CdCl2 (70 μM, 80 μM, 90 μM and 100 μM) and growth was monitored after incubation on a shaker at 30 °C.
Hydroponics experiment for studying the effect of Cd on rice plants
The hydroponic experiment was designed in three replicates according to Crestani et al. (2009). Rice (Oryza sativa, Gujarat-17) seeds were surface sterilized in 70 % ethanol for 1 min and in 1 % sodium hypochlorite (NaClO) for 20 min, rinsed 5–6 times with sterile distilled water and then allowed to germinate for 72 h on sterile moist filter paper in petri plates kept in the overnight at 30 °C. Uniformly grown seedlings with 5 mm radicles were transferred to nylon nets placed on top of beakers containing 500 ml hydroponics nutrient solution for rice suggested by Miyamoto et al. 2001. CdCl2 was added wherever required as independently sterilized solution to nutrient medium. PQQ (100 nM) was also added exogenously to a set of plants (Choi et al. 2008). Rice seedlings were raised for 10 days with CdCl2 (1–10 μM in the ½ strength M.S. medium) and 100 nM PQQ with CdCl2 (1–10 μM in the ½ strength M.S. medium). After 10 d of growth, plants were removed, rinsed with tap water and used for catalase (Cat) and superoxide dismutase (SOD) activity assay. Root length and shoot length were measured to ascertain the result of stress on rice plants.
Superoxide dismutase (SOD) assay
Extracts were prepared by using polyvinylpyrolidone (PVP) according to Costa et al. 2002. Total SOD (EC 1.15.1.1) activity was measured spectrophotometrically based on inhibition in the photochemical reduction of nitroblue tetrazolium (NBT). The 3 ml reaction mixture contained 50 mM sodium phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 2 μM riboflavin, 0.1 mM EDTA and 0.1 ml enzyme extract; riboflavin was added last (Van Rossum et al. 1997). One unit of SOD was defined as the quantity of enzyme required to inhibit the reduction of NBT by 50 % in a reaction mixture. An enzyme unit of SOD was calculated according to the formula given by Constantine and Stanley 1977.
Catalase (CAT) assay
Fresh samples were prepared for catalase assay using polyvinyl pyrolidone (PVP) and a pinch of activated charcoal according to Mahatma et al. (2011). Total catalase (EC 1.11.1.6) activity was determined in the supernatants by measuring the decrease in absorption at 240 nm as H2O2 was consumed according to the method of Aebi 1984 and enzyme activity expressed as mmol H2O2 oxidized min−1 g−1 protein. The 3 ml assay mixture contained 50 mM sodium phosphate buffer (pH 7.0), 30 mM H2O2 and 50 μl enzyme extract.
Plant inoculation experiments to study the effect of Hs transformants
Rice (Oryza sativa, Gujarat-17) seeds were surface sterilized in 70 % ethanol for 1 min and in 1 % sodium hypochlorite (NaClO) for 20 min, and then rinsed 5–6 times with sterile distilled water and then allowed to germinate for 24 h on moist filter paper in petri plates kept in the dark at 30 °C. Pure bacterial cultures were grown in nutrient broth at 30 °C, centrifuged, and diluted to a final concentration of 108 CFU/ml in sterile distilled water. The plants were grown at 30 °C under natural daylight, while grown seeds were dipped with bacterial cultures in petri plates for 2 h and then seeds were rinsed with sterile water, treated seeds were irrigated with 50 mL of sterile distilled water every day. The experiment was conducted on six replicates (three bags per replicate, two plants per bag) for each treatment and was completely randomized. Plant growth parameters were followed as per the method described in Wagh et al. (2014b).
Analytical methods
Modified Lowry’s method (Peterson 1979) was applied for total protein estimation. Cell growth of H. seropedicae Z67 wild type as well as transformants was determined as optical density at 600 nm (OD600). Soluble K content in the supernatant of HRPF medium was determined by flame photometry (Sugumaran and Janarthanam 2007). Different concentrations of KCl were used to prepare a standard curve. HPLC analysis was used to detect organic acid levels and retention time was determined under above mentioned conditions for identification of the organic acid. Pure organic acids were used for quantification according to Wagh et al. 2014b. Physiological parameters like growth rate, biomass yield on glucose and specific glucose reduction rate were calculated as described by Buch et al. 2008, 2009. Graph Pad Prism (version 3.0) and Microsoft Excel were used for statistical analysis of the parameters. Each parameter has been represented as mean ± SD or mean ± SEM as specified in the figure captions.
Results
Growth and MPS ability of Hs transformants with pJNK5 and pJNK6 plasmids
The transformant containing the pqq cluster alone [Hs (pJNK5)] as well as the transformant carrying the pqq cluster conjunction with gad operon [Hs (pJNK6)] showed comparable P solubilization on Pikovaskya’s agar and very good acidification zone on 100 mM HEPES and 50 mM glucose containing HRP medium (Fig. S1). K solubilization was also apparent for both the transformants on Aleksandrov agar plate. The wild type strain and the transformant with the empty plasmid vector failed to display any proficiency at solubilization of mineral phosphates as well as mineral K (Fig. S1).
When inoculated in HRP minimal medium with or without feldspar, growth of the transformants and wild type strain was not significantly different. However, a drop in pH was observed in case of Hs (pJNK5) and Hs (pJNK6) bringing pH to 5.0 and 4.6, respectively, within 40 h indicating the secretion of organic acids (Figs. 1a and 1b). Growth under microaerobic conditions was slow for all the strains, however similar observations regarding lowering of pH could be made in case of the transformants carrying the pqq [Hs (pJNK5)] and pqq, gad [Hs (pJNK6)] clusters (Figs. 1b and 1c).It is apparent that the pH of the spent medium is lowered to a greater extent by Hs (pJNK6) than Hs (pJNK5) under aerobic as well as microaerobic conditions. A higher pH drop with pJNK6 than pJNK5 is also well substantiated in Fig. S1 with different media.
Growth and pH profile of H. seropedicae Z67 and its transformants. (a, c- HRP medium and b, d- HRP medium supplemented with 1 mg/ml Feldspar). (a, b – Aerobic, N source supplemented condition and c, d- Microaerobic, N source-free condition) Growth in terms of OD600 is depicted as filled symbols and pH of the extracellular supernatant as open symbols,  ,
, , Hs (wild type);
, Hs (wild type);  ,
,  , Hs (pUCPM18Gm);
, Hs (pUCPM18Gm);  ,
, , Hs (pJNK5);
, Hs (pJNK5);  ,
,  , Hs (pJNK6), Values at each time point are represented as the mean ± SD of six independent observations
, Hs (pJNK6), Values at each time point are represented as the mean ± SD of six independent observations
Quantitative growth parameters for the transformants and the wild type strain for aerobic growth on HRP medium are given in Table 2. Growth rates of H. seropedicae Z67 and the transformants Hs (pUCPM18), Hs (pJNK5) and Hs (pJNK6) were similar on HRP medium. Total glucose depletion of Hs (pJNK5) and Hs (pJNK6) was 2.8 fold higher as against wild type strain and Hs (pUCPM18), respectively, on HRP medium. On the other hand, the specific glucose utilization rate of Hs (pJNK5) and Hs (pJNK6) was 1.1 and 2 fold higher than wild type and Hs (pUCPM18), respectively, on HRP medium. However, glucose uptake was apparently similar in all H. seropedicae Z67 transformants when compared with wild type. In contrast, biomass yield of Hs (pJNK6) was decreased by 2 fold on HRP medium when compared with wild type. Hs (pJNK5) showed a similar biomass yield as the wild type strain. Hs (pJNK5) and Hs (pJNK6) released P up to 239.7 μM and 457.7 μM, respectively, using RP as the P source (Table 4).
Under N free HRP medium, growth rates of Hs (pUCPM18), Hs (pJNK5) and Hs (pJNK6) were similar as compared to the wild type strain (Table 3 ). On the other hand, the specific glucose utilization rate of Hs (pJNK6) was 2 fold higher than wild type, Hs (pUCPM18), and Hs (pJNK5), respectively, on N free HRP medium. In contrast, biomass yield of Hs (pJNK6) was decreased by 2 fold on N free HRP medium when compared with wild type, Hs (pUCPM18) and Hs (pJNK4) showed similar pattern of biomass yield as compared with wild type strain. Wild type strain and Hs (pUCPM18) neither showed acidification nor solubilized RP whereas Hs (pJNK5) and Hs (pJNK6) released P up to 195.6 μM and 440.8 μM, respectively, on 50 mM glucose (Table 3). The extent of P release in microaerobic conditions is similar to that under aerobic conditions.
K solubilizing ability of H. seropedicaeZ67 transformants
Hs (pJNK5) Hs (pJNK6) showed K solubilization, on Aleksandrov agar plates (Fig. S1). H. seropedicae Z6 and its transformants when inoculated in HRP medium supplemented with feldspar (HRPF) medium. No pH change in the case of Hs wild type and Hs (pUCPM18) was observed but Hs (pJNK5) and Hs (pJNK6) showed good growth and a drop in pH up to 5.0 and 4.6, respectively, within 40 h in the presence of glucose (Fig. 1). Hs (pJNK5) and Hs (pJNK6) also released K up to 76.7 μM and 222.7 μM, respectively, on 50 mM glucose (Table 4).
The wild type strain and Hs (pUCPM18) neither showed acidification nor solubilized feldspar while Hs (pJNK5) and Hs (pJNK6) released K up to 63.7 μM and 198.2 μM, respectively, on 50 mM glucose under microaerobic conditions in N free HRPF medium (Table 3). The extent of K release in microaerobic condition is similar to that under aerobic conditions.
PQQ levels, GDH and GADH activities and organic acid secretion in H. seropedicae Z67 transformants
Hs wild type and Hs (pUCPM18) did not secrete PQQ while Hs (pJNK5) and Hs (pJNK6) secreted 1.15 μM and 1.17 μM PQQ, respectively, in M9 minimal medium. Hs wild type and Hs (pUCPM18) did not show any GDH activity in the HRP medium while Hs (pJNK5) and Hs (pJNK6) showed 221.5 U and 234 U of GDH activity, respectively (Table 4). On the other hand, GADH activity was undetectable in Hs wild type, Hs (pUCPM18) and Hs (pJNK5) whereas 414 U of GADH activity was found in Hs (pJNK6) when compared with Hs wild type strain. Organic acid secreted by Hs (pJNK5) was 23.47 mM and Hs (pJNK6) 3.79 mM, while Hs (pJNK5) was unable to produce 2KDG, Hs (pJNK6) secreted 15.83 mM 2KDG acid which is sufficient for RP solubilisation (Table 4) and (Fig 2 ).
Extracellular organic acid secretion by H. seropedicae Z67 wild type and its transformants on HRP medium. a: Organic acid levels (mM); b- Organic acid yields expressed as g/g/g indicating g of organic acid produced/g of glucose utilized/g dry cell weight. -  Gluconic acid;
Gluconic acid;  2 ketogluconic acid and
2 ketogluconic acid and  Acetic acid. Values are for cultures grown for 36 h. Results are expressed as Mean ± SEM of 3 independent experiments. Wild type and vector control do not produce detectable levels of gluconic aid nor 2 keto gluconic acid. Values for acetic acid were compared with wild type strain and for gluconic acid in Hs (pJNK6) were compared with Hs (pJNK5). *** P<0.001, * P<0.05, ns=non-significant
Acetic acid. Values are for cultures grown for 36 h. Results are expressed as Mean ± SEM of 3 independent experiments. Wild type and vector control do not produce detectable levels of gluconic aid nor 2 keto gluconic acid. Values for acetic acid were compared with wild type strain and for gluconic acid in Hs (pJNK6) were compared with Hs (pJNK5). *** P<0.001, * P<0.05, ns=non-significant
Effect of Hs transformants Hs (pJNK5) and Hs (pJNK6) on Cd tolerance
Since PQQ is known as antioxidant molecule, Hs transformants were monitored for minimum inhibitory and tolerance levels. Hs wild type and Hs (pUCPM18) were able to tolerate 40 μM of CdCl2 and minimum inhibitory concentration (MIC) was 60 μM. However, Hs (pJNK5) and Hs (pJNK6) were able to grow and tolerate up to 100 μM CdCl2 .
Effect of PQQ on the growth and antioxidant enzyme status of rice seedling against Cd stress
Under hydroponic conditions, rice seedlings treated with 10 μM Cd showed growth inhibition of root and shoot length by 70 % and 60 %, respectively, when compared with control seedlings while PQQ treated seedlings with 10 μM Cd had root and shoot length of rice similar to that of untreated seedlings (Fig. 3 ).
Relative net elongation (RNE) (%) of root and shoot of rice seedling. (a, b- CdCl2 treated, a-% RNE of root; b-% RNE of shoot). (a, b)  - CdCl2;
- CdCl2;  - Exogenous PQQ;
- Exogenous PQQ;  - Hs (wild type);
- Hs (wild type);  - Hs (pUCPM18Gmr);
- Hs (pUCPM18Gmr);  - Hs (pJNK5);
- Hs (pJNK5);  - Hs (pJNK6). The results are expressed as Mean±SEM of readings from six plants of treatments *** P<0.001, ns=non-significant (as compared with wild type culture controls)
- Hs (pJNK6). The results are expressed as Mean±SEM of readings from six plants of treatments *** P<0.001, ns=non-significant (as compared with wild type culture controls)
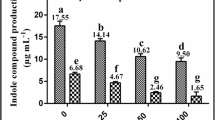
Cd treated rice seedlings exhibited a significant change in the activities of CAT and SOD. CAT activity of control seedlings was 18 U while seedling treated with 10 μM Cd showed a 3.4 fold increase in activity when compared with control plants without Cd. PQQ treated plants showed a significant decrease in CAT activity by 2 fold in presence of 10 μM Cd. SOD activity of 10 μM Cd treated seedlings was increased by 3.8 fold when compared with control plants without Cd. When PQQ was supplemented to plants treated with 10 μM Cd, SOD activity was significantly reduced by 2.3 fold as compared to 10 μM Cd treated seedlings (Fig. 4).
Effect H. seropedicae Z67 transformants (pJNK5) and (pJNK6) on the antioxidant enzyme activity of rice seedlings exposed to cadmium after 10 DAI. 1- CAT and 2 – SOD.  -Control;
-Control;  -Cd 10 μM;
-Cd 10 μM;  - Hs Cd 10 μM;
- Hs Cd 10 μM;  - Hs (pUCPM18 Gm Cd 10 μM);
- Hs (pUCPM18 Gm Cd 10 μM);  - Hs (pJNK5) Cd 10 μM;
- Hs (pJNK5) Cd 10 μM;  - Hs (pJNK6) Cd 10 μM;
- Hs (pJNK6) Cd 10 μM;  - PQQ Cd 10 μM. The results are expressed as Mean ± SEM of readings from six plants of treatments *** P<0.001, ns=non-significant (as compared with wild type culture controls)
- PQQ Cd 10 μM. The results are expressed as Mean ± SEM of readings from six plants of treatments *** P<0.001, ns=non-significant (as compared with wild type culture controls)
Effect of Hs (pJNK5) and Hs (pJNK6) on the nutrient status of rice plants
Shoot length, stem length, fresh weight, dry weight, N, P and K content were monitored in rice plants 30 days after inoculation (DAI) (Table 5 and S1). Hs wild type strain and Hs (pUCPM18) inoculated rice plants showed 1.3 fold increase in shoot length as compared to control uninoculated plants, while Hs (pJNK5) and Hs (pJNK6) inoculations showed 1.7 and 2 fold increase as compared to the plants inoculated with the wild type strain. Root length was increased in both Hs and Hs (pUPM18) treatments by 2 fold when compared with uninoculated plants, while Hs (pJNK5) and Hs (pJNK6) showed 1.7 and 2 fold increase in root length as compared with Hs inoculated plants. Fresh weight of plants inoculated with Hs and Hs (pUCPM18) increased by 2 fold when compared with control set, while Hs (pJNK5) and Hs (pJNK6) showed a significant increase in fresh weight by 1.6 and 2 fold when compared with Hs treated plants. Hs and Hs (pUCPM18) treated plants showed 1.5 fold increased total chlorophyll content when compared with control plants, while both Hs (pJNK5) and Hs (pJNK6) increased chlorophyll content by 1.4 and 1.6 fold respectively when compared with wild type strain colonized plants.
Hs (pJNK5) and Hs (pJNK6) inoculated rice plants showed presence of PQQ in rice plants while control plants did not contain any PQQ. Plants inoculated with Hs (pJNK5) and Hs (pJNK6) showed 0.726 ng and 0.728 ng of PQQ, respectively (Table 5 ). Nitrogen content in plant leaves was significantly increased with Hs and Hs (pUCPM18) by 1.3 fold as compared with control plants while N levels in Hs (pJNK5) and Hs (pJNK6) treated plants showed 1.5 and 2 fold increase, respectively, when compared Hs plants. N levels in roots in Hs and Hs (pUCPM18) treated plants showed 2 fold increase against uninoculated control, while Hs (pJNK5) and Hs (pJNK6) treatment showed 1.7 and 2.2 fold increase when compared with Hs treatment. Phosphorus content in leaves and roots of both Hs (pJNK5) and Hs (pJNK6) treated plants increased by 2.2 and 3 fold respectively, when compared with Hs inoculated plants. K content of leaves and roots was increased in case of Hs (pJNK5) and Hs (pJNK6) inoculated plants by almost 1.3 and 1.6 fold respectively, when compared against Hs treated plants (Table S1 ).
Discussion
Phosphate solubilizing rhizobacteria primarily secrete gluconic acid by the direct glucose oxidation pathway mediated by periplasmic GDH which requires PQQ as a redox cofactor (Krishnaraj and Dahale 2014). Earlier studies from our laboratory showed that incorporation of 5.1 Kb Acinetobacter calcoaceticus and 13.4 Kb Pseudomonas fluorescens B16 pqq gene clusters independently in H. seropedicae Z67 resulted in high secretion of gluconic acid, leading to mineral phosphate solubilization under aerobic and nitrogen fixing microaerophilic conditions (Wagh et al. 2014a). The present study shows that cloning of the smaller pqq gene cluster of A. calcoaceticus along with the gad operon of P. putida in H. seropedicae Z67 resulted in secretion of gluconic acid as well as 2-keto D-gluconic acid. This work demonstrates the functional establishment of the direct glucose oxidative pathway by the heterologous expression of PQQ biosynthesis genes and GAD encoding operon which act in conjunction with the resident apo-GDH encoded by H. seropedicae Z67 genome. The increased secretion of gluconic acid and 2KDG improved P solubilization and plant growth enhancement ability as compared to the native strain as well as only gluconic acid secreting transformant.
Hs (pJNK6) containing P. putida KT2440 gad operon secreted 15.83 mM 2KDG along with 3.79 mM gluconic acid. As compared to the present work, earlier reports from our lab showed that overexpression of P. putida KT2440 gad operon in E. asburiae PSI3 (which naturally has gluconic acid secreting ability) resulted in the secretiom of 11.63 mM 2KDG along with 21.65 mM gluconic acid (Kumar et al. 2013). GADH activity of Hs (pJNK6) was found to be 414 U in the HRP minimal medium and was similar to E. asburiae PSI3 gad transformant (Kumar et al. 2013). Over expression of gad in E. coli enhanced GADH activity by 2 fold (Yum et al. 1997). Hs (pJNK6) transformant released 2 fold higher P than Hs (pJNK5) indicating 2KDG secretion resulted in more efficient at P solubilization than GA secretion alone.
Interestingly, Hs transformants (pJNK5) and (pJNK6) were able to solubilize potassium minerals. The role of organic acid secretion like oxalic, citric, malic, succinic tartaric and acetic in P solubilizaion is well documented (Archana et al. 2012). A Bacillus sp. that solubilized phosphate also solubilized potassium minerals, the biochemical basis for latter is not fully understood (Hu et al. 2006). Three fold higher potassium solubilization in Hs (pJNK6) than Hs (pJNK5) strongly suggests a significant role of 2KDG in K solubilization which has been well substantiated by earlier observation of Duff et al. (1963) who showed 2KDG secretion by a Pseudomonas strain did result in K solubilization. Higher K solubilization by Hs (pJNK6) compared to the earlier report by Duff et al. (1963) may be due to the strain’s ability to secrete gluconic acid in addition to 2-keto gluconic acid.
Hs (pJNK5) and Hs (pJNK6) produced 1.15 and 1.17 μM of PQQ in the medium after over expression but lesser than that of the E. coli transformant containing Gluconobacter oxydans pqq cluster which produced 6 μM PQQ (Yang et al. 2010), while overexpression of pqq under different promoters in E. coli and Klebsiella pneumonia showed two fold increased PQQ production (Sun et al. 2014). However, Hs transformants showed PQQ secretion in P deficient medium while E. coli transformants in Luria broth. Additionally, both H. seropedicae Z67 pqq and gad transformants showed MPS ability under microaerobic nitrogen fixing conditions showed two fold increased (Wagh et al. 2014b). Incorporation of pqq and gad genes has maintained the biomass, specific glucose utilization and mineral K solubilization in H. seropedicae Z67 under both aerobic and microaerobic conditions in HRP medium.
Phosphate solubilizing bacteria (PSB) are known to enhance yield in rice and maize. A similar increase in growth, yield, N, and P levels were found in wheat plants upon co-inoculation (mixture) of Rhizobium with PSB (Bhardwaj et al. 2014). Plant treatment with transformants Hs (pJNK5) and Hs (pJNK6) resulted in a significant increase in fresh weight dry weight, shoot length, root length and in chlorophyll in comparison with treatment with the control and wild type Hs strain. Increased plant growth promotion was observed by Bhardwaj et al. (2014) in plants that were co-inoculated with phosphate solubilizing bacteria along with nitrogen fixing bacteria. Similarly, overexpression of the citrate operon in H. seropedicae Z67 solubilised mineral phosphate and enhanced plant growth of rice (Wagh et al. 2014b). Rice plants inoculated in autoclaved soil with Hs (pJNK5) and Hs (pJNK6) accumulated PQQ, increased the biomass, N. P and K content. The similar effect of increase in plant growth was found upon inoculation with PQQ secreting P. fluorescens B16 but not with its pqq mutant (Choi et al. 2008). Significant increase in uptake of N, P and K status in plants by the transformants could be attributed to the cumulative effect of the organic acid secretion, nitrogen fixing and PQQ producing ability of bacteria.
Response to the oxidative stress generated by heavy metals leads to increase in the activity of oxidative enzymes, which play a major role in antioxidative defense system (Choi et al. 2008). Cd stress tolerance in rice seedlings is associated with a significant increase in the activities of these enzymes (Bhardwaj et al. 2014). Cd stress in this study showed increased SOD and CAT activities, but rice plants inoculated with Hs (pJNK5) and Hs (pJNK6) showed normal levels of CAT and SOD signifying that PQQ secreted by these transformants appear to be sufficient to counter the toxic effects of Cd. This is supported by the fact that PQQ as a potent water soluble antioxidant neutralizes reactive species by scavenging reactive oxygen species (ROS) and plays a key role in the DNA repair and reduces oxidative stress (Misra et al. 2012). Additionally, PQQ protects cells against oxidative stress caused by γ radiation.
In conclusion, H. seropedicae Z67 possessing pqq and gad gene clusters secretes high amount of PQQ, gluconic and 2-ketogluconic acids which confer MPS and potassium solubilization abilities. Inoculation of these transformants on rice plants promoted overall growth, increased the N and P status, and provided tolerance to Cd. Thus, the present study demonstrates that incorporation of 2KDG secretion in nitrogen fixing bacterial isolates could be a good strategy for enhancing their potential as biofertlizers.
References
Adhikary H, Sanghavi PB, Macwan SR, Archana G, Naresh Kumar G (2014) Artificial citrate operon confers mineral phosphate solubilization ability to diverse fluorescent pseudomonads. PLoS One 9(9):e107554. doi:10.1371/journal.pone.0107554
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Archana G, Buch A, Naresh Kumar G (2012) Pivotal role of organic acid secretion by rhizobacteria in plant growth promotion. Satayanarayan T, Johri BN, Prakash A, editors. Microorganisms in Sustainable Agriculture and Biotechnology Springer, In, pp. 35–53
Baldani JI, Baldani VLD, Seldin L, Dobereiner J (1986) Characterization of Herbaspirillum seropedicae gen. Nov., sp. nov., a root-associated nitrogen-fixing bacterium. Int J Syst Bacteriol 30:86–93
Basak BB, Biswas DR (2009) Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by Sudan grass (Sorghum vulgare Pers.) grown under two Alfisols. Plant Soil 317:235–255. doi:10.1007/s11104-008-9805-z
Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N (2014) Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Factories 13:66
Buch AB, Archana G, Naresh Kumar G (2008) Metabolic channeling of glucose towards gluconate in phosphate-solubilizing Pseudomonas aeruginosa P4 under phosphorus deficiency. Res Microbiol 159:635–642
Buch AD, Archana G, Naresh Kumar G (2009) Enhanced citric acid biosynthesis in Pseudomonas fluorescens ATCC 13525 by overexpression of the Escherichia coli citrate synthase gene. Microbiology 155:2620–2629
Calomiris JJ, Armstrong JL, Seidler RJ (1984) Association of metal tolerance with multiple antibiotic resistance of bacteria isolated from drinking water. Appl Environ Microbil 47:238–1242
Choi O, Kim J, Kim JG, Jeong Y, Moon JS, Park CS, Hwang I (2008) Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol 146:657–668
Constantine N, Stanley KR (1977) Superoxide dismutases. Occurrence in higher plants. Plant Physiol 59:309–314
Costa H, Gallego SM, Tomaro ML (2002) Effects of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci 162:939–945
Crestani M, da Silva JAG, de Souza VQ, Hartwig I, Luche HS, de Sousa RO, de Carvalho FIF, de Oliveira AC (2009) Irrigated rice genotypes performance under excess iron stress in hydroponic culture. Crop Breed Appl Biotechnol 9:87–95
da Silva LG, Miguens FC, Olivares FL (2003) Herbaspirillum seropedicae and sugarcane endophytic interaction investigated by using high pressure freezing electron microscopy. Braz J Microbiol 34:69–71
Duff RB, Webley DM, Scott RP (1963) Solubilization of min-erals and related materials by 2-ketogluconic acid producing bacteria. Soil Sci 95:105–114
Elkoca E, Kantar F, Sahin F (2007) Influence of nitrogen fixing and phosphorus solubilizing bacteria on the nodulation, plant growth, and yield of chickpea. J Plant Nutr 31:157–171
Farhat MB, Fourati A, Chouayekh H (2013) Coexpression of the pyrroloquinoline quinone and glucose dehydrogenase genes from Serratia marcescens CTM 50650 conferred high mineral phosphate-solubilizing ability to Escherichia coli. Appl Biochem Biotechnol 170:1738–1750. doi:10.1007/s12010-013-0305-0
Goldstein AH (1995) Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram-negative bacteria. Biol Agric Hort 12:185–193
Goosen N, Horsman HP, Huinen RG, van de Putte P (1989) Acinetobacter calcoaceticus genes involved in biosynthesis of the coenzyme pyrrolo-quinoline-quinone nucleotide sequence and expression in Escherichia coli K-12. J Bacteriol 171:447–455
Gulati A, Sharma N, Vyas P, Sood S, Rahi P, Pathania V, Prasad R (2010) Organic acid production and plant growth promotion as a function of phosphate solubilization by Acinetobacter rhizosphaerae strain BIHB 723 isolated from the cold deserts of the trans-Himalayas. Arch Microbiol 192:975–983
Gyaneshwar P, Naresh Kumar G, Parekh LJ (1998) Effect of buffering on the phosphate-solubilizing ability of microorganisms. World J Microbiol Biotechnol 14:669–673
Gyaneshwar P, Naresh Kumar G, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245:83–93
Hester KL, Lehman J, Najar F, Song L, Roe BA, MacGregor CH, Hager PW, Phibbs PV, Sokatch JR (2000) Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J Bacteriol 182:1144–1149
Hu XF, Chen J, Guo JF (2006) Two phosphate and potassium solubilizing bacteriaisolated from Tiannu mountain, Zhejiang, China. World J Micro Biotech 22:983–990
Kavita B, Shukla S, Naresh Kumar G, Archana G (2008) Amelioration of phytotoxic effects of Cd on mung bean seedlings by gluconic acid secreting rhizobacterium Enterobacter asburiae PSI3 and implication of role of organic acid. World J Microbiol Biotechnol 24:2965–2972
Krishnaraj PU, Dahale S (2014) Mineral phosphate solubilization: concepts and prospects in sustainable agriculture. Proc Indian Natn Sci Acad 80(2):389–405
Kumar C, Yadav K, Archana G, Naresh Kumar G (2013) 2-ketogluconic acid secretion by incorporation of Pseudomonas putida KT 2440 gluconate dehydrogenase (gad) operon in Enterobacter asburiae PSI3 improves mineral phosphate solubilization. Curr Microbiol 67:388–394
Mahatma MK, Bhatnagar R, Mittal GK, Mahatma L (2011) Antioxident metabolism in pearl millet genotype during compatible and incompatible interaction with downy mildew pathogen. Arch Phytopathol. Plant Protect 44(9):911–924
Matsushita K, Ameyama M (1982) D-glucose-dehydrogenase from Pseudomonas fluorescens, membrane bound. Methods Enzymol 89:149–154
Matsushita K, Shinagawa E, Ameyama M (1982) D-gluconate dehydrogenase from bacteria, 2-keto-D-gluconate yielding, membrane-bound. Methods Enzymol 89:187–193
Misra HS, Rajpurohi YS, Khairna NP (2012) Pyrroloquinoline-quinone and its versatile roles in biological processes. J Biosci 37:313–325
Miyamoto N, Steudle E, Hirasawa T, Lafitte R (2001) Hydraulic conductivity of rice roots. J Exp Bot 52:1835–1846
Moghimi A, Tate ME, Oades JM (1978) Characterization of rhizosphere products especially 2-ketogluconic acid. Soil Biol Biochem 10:283–287
Park KH, Lee OM, Jung HI, Jeong JH, Jeon YD, Hwang DY, Lee CY, Son HJ (2010) Rapid solubilization of insoluble phosphate by a novel environmental stress-tolerant Burkholderia vietnamiensis M6 isolated from ginseng rhizospheric soil. Appl Microbiol Biotechnol 86:947–955
Pereira JAR, Baldani JI (1995) Selection of Azospirillum spp. and Herbaspirillum seropedicae strains to inoculate rice and maize plants. In: International symposium on sustainable agriculture for the tropics: the role biological nitrogen fixation. Angra dos REIS, RJ, Brazil, pp. 220–221
Peterson GL (1979) Review of the Folin phenol quantitation method of Lowry, Rosenberg, Farr and Randall. Anal Biochem 100:201–220
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiol 17:362–370
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Sashidhar B, Podile AR (2010) Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol 109:1–12
Sheng XF (2005) Growth promotion and increased potassium uptake of cotton and rape by a potassium releasing strain of Bacillus edaphicus. Soil Biol Biochem 37:1918–1922
Sheng XF, He LY (2006) Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can J Microbiol 52:66–72
Singh D, Chauhan SK (2011) Organic acids of crop plants in aluminium detoxification. Curr Sci 100:1509–1515
Sugumaran P, Janarthanam B (2007) Solubilization of potassium containing mineralsby bacteria and their effect on plant growth. World J Agric Sci 3:350–355
Sun J, Han Z, Ge X, Tian P (2014) Distinct promoters affect pyrroloquinoline Quinone production in recombinant Escherichia coli and Klebsiella pneumoniae. Curr Microbiol 69(4):451–456. doi:10.1007/s00284-014-0607-7
Unge A, Tombolini R, Davey ME, de Bruijn FJ, Jansson JK (1996) GFP as marker gene. Section 6.1.13. In: Akkermans ADL, Van Elsas JD, de Bruijn FJ (eds) Molecular microbial ecology manual. Academic Publishers, Dordrecht, The Netherlands, pp. 1–16
Valverde A, Burgos A, Fiscella T, Rivas R, Velazquez E, Rodrıguez-Barrueco C, Cervantes E, Chamber M, Igual JM (2007) Differential effects of coinoculations with Pseudomonas jessenii PS06 (a phosphate-solubilizing bacterium) and Mesorhizobium ciceri C-2/2 strains on the growth and seed yield of chickpea under greenhouse and field conditions. Plant Soil 287:43–50
Van Rossum MWPC, Alberda M, Van der Plas LHW (1997) Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci 130:207–216
Wagh J, Shah S, Bhandari P, Archana G, Naresh Kumar G (2014a) Heterologous expression of pyrroloquinoline quinone (pqq) gene cluster confers mineral phosphate solubilization ability to Herbaspirillum seropedicae Z67. Appl Microbiol Biotechnol 98:5117–5129
Wagh J, Bhandari P, Shah S, Archana G, Naresh Kumar G (2014b) Overexpression of citrate operon in Herbaspirillum seropedicae Z67 enhances organic acid secretion, mineral phosphate solubilization and growth promotion of Oryza sativa. Plant Soil 383:73–86
Yang XP, Zhong GF, Lin JP, Mao DB, Wei DZ (2010) Pyrroloquinoline quinone biosynthesis in Escherichia coli through expression of the Gluconobacter oxydanspqqABCDE gene cluster. J Ind Microbiol Biotechnol 37:575–580
Yum DY, Lee YP, Pan JG (1997) Cloning and expression of a gene cluster encoding three subunits of membrane-bound gluconate dehydrogenase from Erwinia cypripedii ATCC 29267 in Escherichia coli. J Bacteriol 179:6566–6572
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stéphane Compant .
Rights and permissions
About this article
Cite this article
Wagh, J., Chanchal, K., Sonal, S. et al. Inoculation of genetically modified endophytic Herbaspirillum seropedicae Z67 endowed with gluconic and 2-ketogluconic acid secretion, confers beneficial effects on rice (Oriza sativa) plants. Plant Soil 409, 51–64 (2016). https://doi.org/10.1007/s11104-016-2937-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2937-7