Abstract
Aims
The objectives of this study were to determine the partitioning pattern of recently fixed carbon in a plant-soil system and the difference in patterns of carbon flux between fenced and clipped grasslands in the Chinese Loess Plateau (CLP).
Methods
We used an in situ 13C pulse labeling method and determined the plant biomass, carbon content and δ13C value in shoot, root and soil, in order to calculate the 13C amount in the plant-soil system.
Results
Thirty days after labeling, the 13C incorporated into the shoots did not differ significantly between the fenced (30.6 % of recovered 13C) and clipped (27.0 %) grasslands. However, the amount of 13C remaining in the roots and soil in fenced grassland (roots, 9.2 %; soil, 14.7 %) was significantly higher than that in clipped grassland (roots, 2.0 %; soil, 2.5 %). By contrast, the total loss of assimilated 13C was significantly lower in fenced grassland (45.5 %) than that in clipped grassland (68.5 %).
Conclusions
We demonstrate that clipping management results in a higher 13CO2 efflux and a lower 13C allocated belowground, which has a negative effect on carbon sequestration in typical grasslands in the semiarid CLP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The importance of biogeochemical carbon cycles in terrestrial ecosystems is well established. In particular, grassland ecosystems, which cover approximately 40 % of China’s land area, represent approximately 2 % of the global carbon pool (Hill et al. 2007; Fang et al. 2010; Wu et al. 2010; Hafner et al. 2012). Patterns of carbon flux, originating from the fixation of atmospheric CO2 by leaves that is then transferred and accumulated into the roots and soil (Woodin et al. 2009), have a significant influence on the function of carbon inputs and outputs. However, quantitative research on carbon allocation from shoots to roots and soil is rarely conducted in the field at a large spatial scales (Wu et al. 2010), especially in semiarid, fragile ecosystems. A large body of literature on carbon translocation is based on indirect estimates from inventories of carbon stocks in different carbon pools, such as shoots, roots and soil (Guo and Gifford 2002; Qiu et al. 2012). However, little is known about in situ ecosystem carbon acquisition and translocation (Heimann and Reichstein 2008). Thus, there is an urgent need for further investigation of in situ carbon fluxes in field ecosystems.
The 13CO2 pulse labeling method has been increasingly used as a suitable and important approach for understanding and quantifying carbon flows in terrestrial ecosystems. This technology makes it possible to trace carbon flux from shoot to root, to soil and to soil respiration. It can address a wide range of biological and ecological questions, such as in plant metabolism and biochemistry (Barbour et al. 2007; Norby 2009), partitioning patterns of carbon fluxes in plant-soil systems (Ostle et al. 2000; Leake et al. 2006; Woodin et al. 2009; Wu et al. 2010; Reinsch and Ambus 2013) and the influence of different management systems on carbon allocation (Rangel-Castro et al. 2004; De Deyn et al. 2011; Hafner et al. 2012; Gong et al. 2014). Previous research has demonstrated that carbon allocation can vary significantly among plant species and ecosystems. For example, vascular plants in the high Arctic transfer 61 % of what they assimilate to their belowground parts (Woodin et al. 2009); in pasture plants, this value is approximately 30–50 %, and in cereals, it is 20–30 % (Kuzyakov and Domanski 2000). Among all the selected species (grass, forb, legume and moss), the uptake of 13C was greatest and 13C concentrations declined the fastest in Ranunculus repens, while the assimilation was the lowest and the 13C signature remained longer in mosses (De Deyn et al. 2011). In the Inner Mongolia temperate steppe, the dynamics and allocation of recently photo-assimilated carbon varied by site, growth stage and management state (Wang et al. 2007). These differences in plants and regions undoubtedly result in a large difference in carbon partitioning both in plants and within the ecosystem. Therefore, determining the carbon dynamics of specific ecosystems covering large land areas, such as the Stipa grandis community in the Chinese Loess Plateau (CLP), is crucial to understanding regional carbon budgets.
Changes in land use and management are important for the control of carbon fluxes in plant-soil ecosystems (Sanderson 2008). Clipping in the manner effectively mimics hay mowing, a widely practiced land use in large regions of the CLP due to the strict prohibition of grazing by the government. Previous studies have demonstrated that clipping significantly affects the microclimate (Dahlgren and Driscoll 1994; Wan et al. 2002), vegetation characteristics (Bahn et al. 2006; Cheng et al. 2012), soil CO2 efflux (Wan and Luo 2003; Zhou et al. 2007) and soil carbon pool (Belay-Tedla et al. 2009). For instance, a study on the Great Plain Apiaries in the United States found that clipping management tended to decrease both recalcitrant and total carbon pools (Bahn et al. 2006). In the CLP, Cheng et al. (2012) found that plant biomass decreased significantly when clipped once or twice a year, compared to getting clipped once every two years. Soil carbon storage decreased significantly with clipping management. Clearly, the current knowledge of the effects of clipping on carbon change is mostly based on separate estimates of different carbon pools. A quantitative determination of carbon translocation in plant-soil systems is relatively rare, which greatly limits our understanding of the processes of carbon flow.
The ecosystems in the CLP are thought to be fragile and sensitive to climate change and management due to their high altitude and special geographic environment. We suggest that carbon translocation in grasslands in the CLP may have specific characteristics due to its distinct environment relative to other regions, and the management of grassland would affect carbon allocation in the ecosystem. Therefore, we conducted an in situ 13CO2 pulse labeling experiment in a grassland typical of the semiarid CLP. The purpose of this study was to (1) determine the partitioning pattern of recently fixed carbon in shoots, roots and soil in the fenced and clipped grasslands; (2) evaluate differences in the partitioning patterns of recent assimilates between the fenced and clipped grasslands in semiarid CLP under field conditions.
Materials and methods
Site description
The experimental site (36°13′-36°19’N, 106°23′-106°28’E) is located on the National Grassland Reserve of Yunwu Mountain in the central semiarid CLP (Fig. S1); it covers a total area of 6000 ha−1, with an elevation of approximately 2100 m asl. The area is influenced by the East Asian monsoon; it has a sub-arid climate characterized by distinct wet and dry seasons. The average annual air temperature is 5 °C with an extreme maximum of 25 °C in summer and an extreme minimum of −14 °C in winter. The frost-free season averages 137 days. Annual mean precipitation is 424 mm (mean of data from 1980 to 2009), approximately 60 % of which falls from July to September. The landform is mainly a loess hilly landscape. Loessial soil and mountain gray cinnamon soil (Chinese Soil Taxonomic Classification) are the main soil types in the study area (Wei et al. 2012; Liu et al. 2014). Aside from the dominant species (Stipa grandis), the main companion plants are Artemisia vestita, Galium verum L., Thymus mongolicus Ronn, Potentilla acaulis L. and Saussurea alata DC., which are broadly distributed in other regions of the semiarid CLP.
In the study area, a national prohibition against grazing of grasslands applies and fencing has been in place for more than 30 years. On the fenced grassland, a clipping plot (once a year) covering an area of 30 × 30 m was selected in a low-relief area and created over one month prior to labeling in cooperation with the Administrative Office of Yunwu Mountain (Wang et al. 2007). The clipping management was the same as the grassland clipped by local citizens, who clip grassland approximately once a year, with the remaining stubble of clipped grassland being 5 cm high. The plant composition and biomasses (shoot and root) were determined. This site provides the opportunity to study differences in carbon partitioning between fenced and clipped grasslands, as the basic conditions of both sites (such as land use and plant and soil characteristics) were similar prior to clipping.
Experimental set up and 13CO2 pulse labeling
Three replicate plots (50 × 50 cm) were randomly established in fenced and clipped grasslands, respectively. The distance between fenced and clipped grasslands was less than 20 m. The closest distance between two neighboring plots within the same treatment was approximately 3 m. There was an automated weather station 2 km away from the study site, which was used for measuring solar radiation, precipitation and air temperature. An automatic temperature recorder (iButton DS1923, Maxim company, San Jose, CA, U.S.), buried in the soil at a depth of 5 cm, was used for measuring soil temperature. Solar radiation, precipitation, air and soil temperatures are shown in Fig. S2. At least 24 h before 13CO2 pulse labeling, the plots were trenched (once a week after this time) to sever underground connections. Stainless steel frames, with channels on the top to provide an airtight water seal for the chambers, were inserted to a depth of 5 cm (Grau 1995; Wang et al. 2007; Woodin et al. 2009). Soil was packed firmly around the stainless steel frames to reduce gas leakage.
The 13CO2 pulse labeling was conducted at noon on August 2, 2013, which was a clear day. Each plot was labeled using chambers (50 cm × 50 cm × 40 cm) made from transparent plexiglass with a more than 90 % transmittance of photosynthetically active radiation. The inner surface of the chamber was smeared with an anti-fog agent to reduce water vapor condensation during labeling, which helps to avoid a decrease in light intensity (Wu et al. 2010). To avoid gas loss, the chamber was set on a water-tight groove of the stainless steel frame, which was then sealed by injecting water. Following previous protocols, the 13CO2 pulse was produced by injecting 10 ml of 4 mol L−1 sulphuric acid (H2SO4), using springs, into a plastic vial inside the chamber, which contained a solution of distilled water containing 1.0 g sodium carbonate (Na2 13CO3) (Cambridge Isotope Laboratories, Inc., America) enriched with 13C to 99 atom% (Wang et al. 2007; Wu et al. 2010; Hafner et al. 2012). An electric fan (12 V, 0.3 A) was installed in the middle of the top wall inside the chamber to uniformly distribute 13CO2. We used an infrared CO2 analyzer (GXH-3010E, Huayun Instrument Company, Beijing, China) to monitor changes in CO2 concentration inside the chamber. When the CO2 concentration in the chamber reached the initial value before labeling, we considered the label to be complete (Hafner et al. 2012). The 13CO2 pulse lasted for 4–5 h in this study.
Ecosystem compartment sampling
Carbon allocation was traced by 13C over the course of a 30-day chase period in shoots, roots, soil and soil CO2 efflux. In addition to the labeled plots, another three unlabeled plots in fenced and clipped grassland were sampled simultaneously as the background. Air samples were collected 0, 12, 18, and 24 h and 6, 12, 20, and 30 d after labeling using two different sizes of opaque chambers made of PVC (bigger chamber: 20 cm diameter, 25 cm height; smaller chamber: 5 cm diameter, 25 cm height). We defined the CO2 emission generated from shoots as shoot respiration, from roots and soil as soil respiration. The sum of shoot and soil respiration was defined as ecosystem respiration. The bigger chambers were used for the collection of CO2 generated from ecosystem respiration, while the smaller chambers were used for the collection of CO2 generated from soil respiration after clipping the shoots (Wu et al. 2010). The static alkali absorption method was used to trap CO2 generated from ecosystem and soil respiration (Hafner et al. 2012; Singh and Gupta 1977). A wide mouth Teflon bottle containing 20 ml of 1 M NaOH was inserted into the closed opaque chamber to capture CO2 respired from the ecosystem and soil. To determine the isotope composition of respired CO2, 2 M SrCl2 was added to NaOH to produce SrCO3 precipitation, which was then centrifuged and oven dried in the base at the field station as quickly as possible (Hafner et al. 2012).
Shoots, roots and soil samples were collected at 1, 6, 12, 20, and 30 d when taking CO2 samples. All shoot samples 5 cm in diameter were collected, only the green parts of which were used to determine the isotope composition later. According to previous research, soil organic matter and plant roots mainly accumulate in the top 30 cm of the soil profile (Wei et al. 2011; Wei et al. 2012). Thus, soil cores 5 cm in diameter were taken from three layers: 0–10, 10–20 and 20–30 cm. In the laboratory, all visible roots were picked out from the soil by hand for carbon content and isotope analysis. The samples of shoots and roots were washed with deionized water to remove attached soil, oven-dried at 40 °C, ground in an agate mortar, sieved through a 100 mesh screen and homogenized. Approximately 5 g sieved soil was steeped in 2 M HCl for 24 h to remove inorganic carbon. The samples were then washed with deionized water until the pH > 5 and oven-dried again.
Carbon content and isotopic composition
The carbon content and isotope composition (δ13C) were measured with an elemental analyzer (Vario EL III, Hanau, Germany) and a MAT-252 gas source mass spectrometer (Thermo Finnigan, Bremen, Germany) (Wei et al. 2012; Liu et al. 2014). The samples of shoots, roots (approximately 1 mg) and soil (approximately 0.2–0.5 g) were combusted for 4 h at 850 °C in an evacuated sealed quartz tube in the presence of silver foil, cupric oxide and copper. CO2 gas was extracted and purified cryogenically, and then the isotope composition of extracted CO2 was analyzed using a MAT-252 mass spectrometer with a dual inlet system. The SrCO3 precipitation sample was performed online, using a MAT-252 mass spectrometer with an automated carbonate preparation device (Kiel II) (Liu et al. 2014). The carbon isotope results are expressed in delta (δ) notation relative to the V-PDB standard:
where R is the 13C/12C ratio. The typical standard deviation for repeated analyses of laboratory standards is ±0.1 ‰.
Calculations.
To facilitate comparisons with other studies, we have expressed the 13C enrichment values as 13C atom% excess. According to Eq. 1, we can calculate the Rsample from the data obtained (δ13C) using the MAT-252 mass spectrometer as follows:
where Rsample is the 13C/12C ratio of the sample, and RPDB is the 13C/12C ratio of the standard, with a value of 0.011237.
To determine the 13C atom% of the sample:
The 13C atom% excess of labeled samples was calculated by subtracting the amount of 13C of background from the amount of 13C in the labeled samples:
The amount of 13C incorporated into the ecosystem compartments (shoot, root and soil) during the chase period was calculated as follows:
where Cc is the carbon content in the shoots, roots and soil during the chase period.
The carbon storage (Cs) in the soil layers 0–10, 10–20 and 20–30 cm were calculated as follows:
where i is the soil layer, Di is the soil depth (cm), Bi is the soil bulk density (g cm−3), and Oi is the average SOC content (g kg−1) at a depth of i.
The weighted 13C (13C recovered, % in total recovered 13C ), recovered in a carbon pool at time t (13C t pool, % of total added 13C) after labeling was related to the recovery time immediately after labeling (13C t0 pool, % in total added 13C) in the corresponding plot.
Losses of assimilated 13C by shoot respiration were not measured. Instead, shoot respiration (13C in shoot respiration, % in total recovered 13C) was calculated as follows:
13C in shoot respiration =100 – (13C in shoot +13C in belowground) (8).
The sum of recovered 13C in shoots (% in total recovered 13C) and belowground carbon pools (% in total recovered 13C; including 13CO2 efflux from soil) was subtracted from 100.
Statistical analysis
Statistical analysis was performed in SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Mean values (n = 3) of the parameters representing biomass, carbon content, 13C atom% excess and 13C amount with standard errors (SE) both in fenced and clipped grasslands were presented in figures and tables. One-way ANOVA was used to evaluate significant differences between treatments with respect to each parameter at each measurement period. Where homogeneity of variances was confirmed, a Tukey’s HSD test was affiliated. If the variance cannot meet the needs of homogeneity, the non-parametric Mann-Whitney U-test was employed to evaluate statistical differences in the 13C (% in total recovered 13C) between fenced and clipped grasslands. The figures were handled with Origin 8.0.
Results
Plant biomass and 13C amount
The composition and abundance of plant species showed no significant change in either fenced or clipped grassland during the chase period (Fig. S3). The shoot biomass differed significantly between fenced and clipped grassland at the end of the chase period (P < 0.05), which averaged 236.2 ± 47.2 g m−2 and 111.5 ± 13.9 g m−2, respectively (Fig. 1). The root biomass was 963.5 ± 221.0 g m−2 in fenced grassland, which was also higher (but not significantly; P > 0.05) than that in clipped grassland, which had a mean of 633.1 ± 101.0 g m−2. The root biomass mainly accumulated in the top 10 cm in both the fenced and clipped grasslands, averaging 871.3 ± 220.0 g m−2 and 497.2 ± 58.2 g m−2, respectively. The carbon content of shoots, roots and soil was 42.1 %, 41.2 %, and 2.35 % and 41.1 %, 40.6 %, and 2.29 % in fenced and clipped grasslands, respectively, during the chase period. These values were used to calculate the carbon flow in the plant-soil system. Approximately 486 mg 13C m−2 was applied to each chamber during the chase period, of which approximately 28.4 % (137.9 mg m−2) and 28.1 % (136.7 mg m−2) was recovered in the ecosystem in fenced and clipped grasslands, respectively, immediately after the labeling.
Dynamics of δ13C values during the chase period
The δ13C value for ecosystem respiration declined rapidly from 68.2 ‰ to −20.3 ‰ and from 120.1 ‰ to −23.3 ‰ in fenced and clipped grassland, respectively, during the 30-day chase period (Fig. 2a). A very high 13C loss rate in ecosystem respiration (the δ13C value declined 87.5 ‰ and 118.1 ‰ in fenced and clipped grassland, respectively) was observed during the first 12 h immediately following labeling for both land use types, illustrating rapid carbon loss by shoot respiration and belowground (the sum of root and soil) allocation and a dilution effect by 12CO2. The range of δ13C values in the dynamics of soil respiration, however, was relatively small; it decreased from −13.5 ‰ to −22.1 ‰ and from −9.4 ‰ to −22.9 ‰ in fenced and clipped grasslands, respectively, during the 30-day chase period (Fig. 2b). The δ13C value in shoots increased to 82.4 ‰ and 214.6 ‰ immediately after labeling in fenced and clipped grasslands; these values then exhibited a significant decrease during the chase period (Fig. 2c). The δ13C values ranged from −23.1 ‰ to −19.5 ‰ and from −21.7 ‰ to −16.8 ‰ in roots and from −25.4 ‰ to −25.1 ‰ and from −25.1 ‰ to −24.5 ‰ in soil in fenced and clipped grasslands, respectively (Fig. 2d and e).
Temporal variation in δ13C values of ecosystem respiration (a), soil respiration (b), shoot (c), root (d) and soil (e) in fenced and clipped grasslands during the 30-day chase period. All the time in x-axle refers to the time after the end of labeling. ER and SR represent ecosystem respiration and soil respiration, respectively. Error bars are standard errors (n = 3)
Patterns of 13C translocation in the plant-soil system
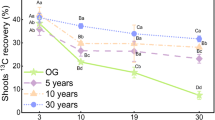
The shoots within the fenced grassland assimilated as much 13C as did the clipped grassland immediately after labeling, averaging 118.5 and 120.9 mg m−2, respectively (Table 1). However, the dynamics of 13C during the chase period differed significantly between the two land use types. During the chase period, 43 % of the 13C in shoots decreased within the first 6 days in the fenced grassland (Fig. 3). Afterwards, the 13C decreased gradually with a proportion of 30.6 % of assimilated 13C remaining in shoots on the 30th day. Relative to the 13C amount immediately after labeling, the total loss of 13C in shoots during the first 6 days only amounted to 18.4 % in clipped grassland; it exhibited a linear decreasing trend during the whole chase period and retained a proportion of 27.0 % of assimilated 13C on the 30th day. As shoot respiration was higher in the clipped grassland during the chase period, 11.7 % and 36.5 % of the total fixed 13C was lost in the fenced and clipped grasslands, respectively. At the end of the chase period, the total loss of 13C as ecosystem respiration amounted to 45.5 % and 68.5 % of recovered 13C in fenced and clipped grasslands, respectively.
Temporal variations of 13C (% of total recovered 13C) in shoots (a) and belowground parts (the sum of root and soil) (b) in fenced and clipped grasslands during the 30-day chase period. All the time in x-axle refers to the time after the end of labeling. Error bars are standard errors (n = 3). *Denotes significant differences at P < 0.05 between the fenced and clipped grasslands
The proportion of 13C allocated to belowground parts was significantly higher in fenced grassland, with a value of 57.7 % during the chase period (Fig. 3). The maximum 13C allocation in belowground parts appeared on the 12th day in fenced grassland, which was later than in clipped grassland, where the maximum 13C allocation occurred on the 6th day. The amount of 13C remaining in belowground parts at the end of the chase period was significantly higher in fenced grassland.
The 13C allocation in roots was slightly higher in clipped grassland, with a value of 6.7 % immediately after labeling (Fig. 4). Afterwards, the amount of 13C increased and reached a maximum value of 22.2 % on the 6th day. Then, the 13C in the roots decreased rapidly in clipped grassland. The 13Cin the roots in fenced grassland exhibited similar trends to that in clipped grassland; it reached a maximum value on the 12th day with a relative proportion of 27.5 %. Compared with the clipped grassland, the amount of 13C was always higher in fenced grassland starting 12 days after labeling. To further understand the carbon allocation in roots, we determined the amount of 13C in different soil layers. With the exception of the first day after labeling, the proportion of 13C at a depth of 0–10 cm accounted for more than 90 % of the total recovered 13C in the roots from any sample during the chase period in fenced grassland. The dynamics of 13C in the clipped grassland exhibited similar trends to those in the fenced grassland. At the end of the chase period, the amount of 13C remaining in the roots in the fenced grassland (9.2 %) was over four times higher than that in the clipped grassland (2 %).
Temporal variation of 13C (% in total recovered 13C) in roots at different depths in fenced (a) and clipped (b) grasslands and the difference of 13C (% in total recovered 13C) between fenced and clipped grasslands (c) during the 30-day chase period. All the time in x-axle refers to the time after the end of labeling. Error bars are standard errors (n = 3). Where homogeneity of variances was confirmed, one-way ANOVA followed by a Tukey’s HSD test was used to evaluate significant differences in the 13C (% in total recovered 13C) for every layer between fenced and clipped grasslands. If the variance cannot meet the needs of homogeneity, the non-parametric Mann-Whitney U-test was affiliated. *Denotes significant differences at P < 0.05 in different depths between the fenced and clipped grasslands
Although there were similar dynamics of 13C between roots and soil, the proportion of 13C allocated to soil was always lower than that in the roots during the chase period in fenced grassland. The maximum amount of 13C in the soil appeared later than it did in the roots, with a maximum value of 26.8 % on the 12th day in the clipped grassland (Fig. 5a). To further understand the carbon allocation in soil, we determined the amount of 13C in different soil layers. The dynamics of 13C exhibited little difference between the different layers, with the maximum amount of 13C occurring on the 12th day at all depths (i.e., 0–10, 10–20 and 20–30 cm) in both fenced and clipped grasslands. The difference in the amount of 13C was relatively small in different layers in the fenced grassland, whereas the amount of 13C was usually higher at a depth of 0–10 cm than in other soil layers in the clipped grassland. The amount of 13C remaining in soil was significantly higher in the fenced grassland than in the clipped grassland at the end of the chase period; it averaged 14.7 % and 3.4 % in fenced and clipped grasslands, respectively.
Temporal variation of 13C (% in total recovered 13C) in soil at different depths in fenced (a) and clipped (b) grasslands and the difference of 13C (% in total recovered 13C) between fenced and clipped grasslands (c) during the 30-day labeling period. All the time in x-axle refers to the time after the end of labeling. Error bars are standard errors (n = 3). One-way ANOVA followed by a Tukey’s HSD test was used to evaluate significant differences in the 13C (% in total recovered 13C) for every layer between fenced and clipped grasslands. *Denotes significant differences at P < 0.05 in different depths between the fenced and clipped grasslands
Discussion
Dynamics of δ13C value during the chase period
For both land use types, the δ13C value in ecosystem respiration showed a peak value immediately after labeling with a subsequent sharp decline within the next 6 days. The sharp decline tendency was in agreement with the results reported by Wu et al. (2010), who found a loss rate that diminished exponentially during the 84 h after labeling. It was roughly matched with the temporal variations of 13C amount in shoots, similar with the result of Ostle et al. (2000), which could be explained by 13C loss resulting from shoot respiration and partitioning of 13C from shoots to roots and soil (Johnson et al. 2002; Leake et al. 2006; Wang et al. 2007; Wu et al. 2010; De Deyn et al. 2011). The shoot δ13C value in clipped grassland, however, was significantly higher (P < 0.05) than that in fenced grassland 6–20 days after labeling, likely for two reasons. First, the plant biomass is higher in fenced grassland, which will result in a higher dilution effect (Wang et al. 2007). Second, plants under clipping management exhibit over-compensatory growth with a higher relative growth rate and a stimulation of compensatory photosynthesis to the remnant leaves compared with those of unclipped plants, resulting in a higher uptake rate of 13CO2 in clipped grassland (Detling et al. 1979; Zhao et al. 2008). In grassland ecosystems, the roots always accumulate more carbon than shoots (Fig. 1), resulting in the 13C assimilation from shoots to roots being inevitably diluted by 12C. This phenomenon led to a significantly lower δ13C value in roots than in shoots in both land use types to some extent (Fig. 2).
Patterns of 13C allocation in the plant-soil system
The total amount of 13C remaining in the plant-soil system decreased significantly throughout the chase period (Table 1). This result was similar with previous research in fenced Leymus chinensis grassland, in which the amount of 13C also gradually decreased for approximately 20 % of total assimilated 13C (Wang et al. 2007). In a Kobresia humilis meadow, however, the amount of 13C was relatively stable during the chase period (Wu et al. 2010). The main reason for the discrepancy was probably due to the variance in biomass turnover of species (i.e., root), and the proportions of fixed 13C partitioned to respiration loss (i.e., approximately 60 % and 29.6 % in Leymus chinensis grassland and Kobresia humilis meadow, respectively) between studies.
In this study, clipping management had little effect on shoot fixation of 13C, with a similar amount in fenced (118.5 mg m−2) and clipped (120.9 mg m−2) grasslands. Combined with the significantly lower plant biomass in the clipped grassland, we can conclude that the photosynthesis rate of clipped grassland might be higher than that of fenced grassland due to the contribution of new leaves. The destinations of the 13C fixed by shoots may be released to the atmosphere as 13CO2, used in the components of new shoot growth or relocated to the belowground parts (i.e., roots and soil) (Staddon et al. 2003; Leake et al. 2006; Kaštovská and Šantrůčková 2007; De Deyn et al. 2011). In the fenced grassland, the rate of carbon loss or the export of newly fixed 13C with respect to the total assimilated 13C was 43 %, significantly higher than that in clipped grassland, within the first 6 d after labeling. This rate was slightly higher than that found in a previous study (Wu et al. 2010) but lower than that in two other studies, which identified a range of 32–51 % loss within the first 24 h (Johnson et al. 2002) and a 77.4 % loss within 48 h in an upland grassland in the UK (Ostle et al. 2000). Moreover, the percentage of 13C remaining in the shoots was stable at approximately 30 % between 12 and 30 days of the chasing period in the fenced grassland, which was consistent with a previous study of the Qinghai-Tibetan Plateau, which found a value of approximately 29 % (Wu et al. 2010). However, the shoot 13C declined gradually during the whole chase period in clipped grassland. Therefore, comparing the allocation pattern for both land use types, the main differences at the current development stage of the grasses occur in the relocation of newly fixed 13C. At the end of the chase period, 55.4 % and 61.4 % of total assimilated 13C were exported from shoots in the fenced and clipped grassland, respectively, which were similar to the results of Wu et al. (2010) (55.5 %), and lower than the results of Hill et al. (2007) (67–75 %) and Kaštovská and Šantrůčková (2007) (76 %).
The carbon fluxes relocated belowground were lowered with clipping management: a larger proportion of newly assimilated 13C was found in belowground carbon pools (57.7 %) in fenced grassland compared to clipped grassland (36.5 %) (Table 1). For fenced grassland, a similar percentage of 13C relocated belowground was found in typical Kobresia grassland at Haibei Research Station (58.7 %) (Wu et al. 2010), which was significantly higher than that in Kobresia grassland at Xinghai (40 %) in the Qinghai-Tibetan Plateau (Hafner 2010; Hafner et al. 2012) and Leymus chinensis grassland (22 %) in the Inner Mongolia Plateau (Wang et al. 2007). Although the 13C relocated belowground in the fenced grassland in this study, and the typical Kobresia grassland at Haibei Research Station showed a similar pattern, the 13C remaining belowground at the end of the chase period was significantly higher in the typical Kobresia grassland, probably due to the higher root/shoot ratio in alpine regions (IPCC 2007).
A large body of literature has reported significant 13C enrichment in the roots of herbaceous vegetation immediately after labeling, peaking within 48 h (Ostle et al. 2000; Johnson et al. 2002; Leake et al. 2006; Wu et al. 2010). In the current study, however, 13C enrichment in the roots was found to reach a peak value on approximately the 12th day after labeling in the fenced grassland. In a Tibetan montane pasture, the maximum 13C enrichment in roots was found on the 18th day after labeling, which was even later than what we found in the fenced grassland. Although we found a peak value of 13C enrichment in the roots on the 6th day after labeling in the clipped grassland, we cannot simply conclude that the rate of 13C enrichment was lower than previous research (peaking within 48 h). It is difficult to confirm the precise time that the peak value appeared under the current resolution of sampling. The lower speed of carbon allocation belowground suggests the lesser importance of newly fixed carbon for rhizodeposition (Wu et al. 2010). Additionally, roots allocated the lowest proportion of newly fixed 13C in the plant-soil system, similar to the amount of 13C remaining in soil for both land use types (Table 1). This is inconsistent with several previous studies, in which roots were the main carbon sink within the belowground pools (Domanski et al. 2001; Wang et al. 2007; Wu et al. 2010). Hafner et al. (2012) considered that these differences were caused by a variance in the plant development stage, which would influence carbon incorporation into the roots. In this study, however, the main reason might be the direct utilization of non-structural carbon for root respiration and for rhizodeposition, which is demonstrated by the higher proportion of respiration loss (Table 1) and the subsequent maximum 13C in soil (Fig. 5). The proportion of 13C relocated to the roots averaged 9.2 % in fenced grassland at the end of the chase period, which was over four times more than that in clipped grassland (2 %), in parallel with the results of Kaštovská and Šantrůčková (2007) (1.3 %) and Wang et al. (2007) (0.7–2.3 %). The higher proportion of 13C in the roots in the fenced grassland than in the clipped grassland might be attributed to two reasons. Firstly, clipping stimulated root respiration and reduced the belowground carbon allocation (Wilsey 1996; Mackie-Dawson 1999). Secondly, a large amount of 13C in roots might be relocated to aboveground parts and accumulated in the stable components of new leaves in clipped grassland (Bardgett et al. 1998; Schmitt et al. 2013).
Previous studies found that clipping has an important influence on carbon relocation to soil. For example, clipping increased root exudation (Bokhari and Singh 1974; Paterson and Sim 1999; Kuzyakov et al. 2002), which increased microbial biomass and activity (Waters and Borowicz 1994; Uhlířová et al. 2005; Blagodatskaya et al. 2009) and further accelerated soil organic matter turnover (Holland et al. 1996) and respiration loss (Table 1). For instance, in a tallgrass prairie in the US Great Plains, both recalcitrant and total carbon pools have been reported to be decreased, while the microbial biomass carbon increased under clipping treatment (Belay-Tedla et al. 2009). In this study, the 13C remaining in soil was over four times higher in fenced grassland (14.7 %) than in clipped grassland (3.4 %) at the end of the chase period, while the initial assimilated 13C was similar. This illustrated that clipping reduced the storage of soil carbon in the study area. This result is supported by another researcher, who determined the species diversity and productivity under a mowing disturbance in a typical steppe in the CLP and found a marked decrease both in species and productivity under a regime of mowing once or twice a year (Cheng et al. 2012).
Many studies have investigated the effect of clipping on soil respiration; however, the results have been contradictory. A slight increase (Thorne and Frank 2009), no change (Bahn et al. 2006; Zhou et al. 2006; Jia et al. 2012; Schmitt et al. 2013), or a decrease (Kuzyakov et al. 2002; Gavrichkova et al. 2010) in the proportion of 13C allocated to soil CO2 have been noted. These differences depended on plant species compositions and the microenvironment. Compared with the fenced grassland in the current study, only a slightly lower amount of 13C in soil respiration was found in clipped grassland (33.8 % vs. 32 %), which was similar to the results of Thorne and Frank (2009).
Effect of clipping on carbon stock
By establishing clipped grassland, we simulated two contrasting grassland regimes. Overall, clipping management in the CLP affects plant biomass production and carbon sequestration. Clipping management (once a year) decreased the biomass of shoots and roots and therefore had a negative effect on carbon stocks in Stipa grandis in the CLP (Figs. 1 and 5; Cheng et al. 2012).
The carbon allocated to roots and soil was considered the major determinant of terrestrial carbon pools in grassland ecosystems (Stewart and Metherell 1999). In this study, the plants allocated less carbon to belowground parts in the top 30 cm. It was confirmed by the partitioning pattern of recent assimilations, which showed that the portion remaining belowground was significantly lower in clipped grassland (root, 2.0 %; soil, 2.5 %) than that in fenced grassland (root, 9.2 %; soil, 14.7 %; Table 1). Previous research indicated that root litter and transformation products are more resistant to degradation and enhance soil organic matter stabilization due to higher lignin/nitrogen ratio, direct input of particulate organic matter and stabilization of rhizodeposits (Rasse et al. 2005). Therefore, the significantly lower root biomass and lower carbon relocation to root in clipped grassland largely decreased the source to soil organic carbon, which reduced the potential for belowground storage of plant-derived carbon. In view of the observed reduction in plant biomass and the relocation of 13C to belowground parts, we conclude that the lower belowground carbon assimilation in clipping management has a significant negative effect on carbon sequestration.
The 13CO2 efflux from soil respiration showed little difference between the land use types. However, the 13CO2 efflux from shoot respiration was significantly higher in clipped grassland than that in fenced grassland. Although the total assimilated 13C of the fenced and clipped grasslands was similar, the share of total 13C loss from ecosystem respiration was higher in clipped grassland. Thus we conclude that the higher 13CO2 efflux in clipped grassland is another important influencing factor on transfer of plant carbon to soil.
Although the shoots assimilated similar 13C amount in fenced and clipped grasslands, the lower 13C allocated belowground and higher 13CO2 efflux in clipped grassland suggests that clipping management decreases carbon sequestration in typical grassland in the semiarid CLP.
References
Bahn M, Knapp M, Garajova Z, Pfahringer N, Cernusca A (2006) Root respiration in temperate mountain grasslands differing in land use. Glob Chang Biol 12:995–1006
Barbour MM, McDowell NG, Tcherkez G, Bickford CP, Hanson DT (2007) A new measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant Cell Environ 30:469–482
Bardgett RD, Wardle DA, Yeates GW (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 30:1867–1878
Belay-Tedla A, Zhou XH, Su B, Wan SQ, Luo YQ (2009) Labile, recalcitrant, and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol Biochem 41:110–116
Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuzyakov Y (2009) Contrasting effects of glucose, living roots and maize straw on microbial growth kinetics and substrate availability in soil. Eur J Soil Sci 60:186–197
Bokhari UG, Singh JS (1974) Effects of temperature and clipping on growth, carbohydrate reserves, and root exudation of western wheatgrass in hydroponic culture. Crop Sci 14:790–794
Cheng JM, Cheng J, Shao HB, Yang XM (2012) Effects of mowing disturbance on the community succession of typical steppe in the loess Plateau of China. Afr J Agric Res 7:5224–5232
Dahlgren RA, Driscoll CT (1994) The effects of whole-tree clear-cutting on soil processes at the Hubbard Brook experimental Forest, New Hampshire, USA. Plant Soil 158:239–262
De Deyn G, Quirk H, Oakley S, Ostle N, Bardgett R (2011) Rapid transfer of photosynthetic carbon through the plant-soil system in differently managed species-rich grasslands. Biogeosciences 8:1131–1139
Detling JK, Dyer MI, Winn DT (1979) Net photosynthesis, root respiration, and regrowth of Bouteloua gracilis following simulated grazing. Oecologia 41:127–134
Domanski G, Kuzyakov Y, Siniakina S, Stahr K (2001) Carbon flows in the rhizosphere of ryegrass (Lolium perenne). J Plant Nutr Soil Sc 164:381–387
Fang JY, Yang YH, Ma WH, Anniwaer M, Shen HH (2010) Ecosystem carbon stocks and their changes in China’s grasslands. Sci China Life Sci 53:757–765
Gavrichkova O, Moscatelli MC, Kuzyakov Y, Grego S, Valentini R (2010) Influence of defoliation on CO2 efflux from soil and microbial activity in a Mediterranean grassland. Agric Ecosyst Environ 136:87–96
Gong XY, Berone GD, Agnusdei MG, Rodrίguez Palma RM, Schäufele R, Lattanzi FA (2014) The allocation of assimilated carbon to shoot growth: in situ assessment in natural grasslands reveals nitrogen effects and interspecific differences. Oecologia 174:1085–1095
Grau A (1995) A closed chamber technique for field measurement of gas exchange of forage canopies. New Zeal J Agr Res 38:71–77
Guo LB, Gifford R (2002) Soil carbon stocks and land use change: a meta analysis. Glob Chang Biol 8:345–360
Hafner S (2010) Effect of grazing on C partitioning in Tibetan montane pasture revealed by 13CO2 pulse labeling. PhD Thesis. University of Bayreuth, Germany.
Hafner S, Unteregelsbacher S, Seeber E, Lena B, Xu XL, Li XG, Guggenberger G, Miehe G, Kuzyakov Y (2012) Effect of grazing on carbon stocks and assimilate partitioning in a Tibetan montane pasture revealed by 13CO2 pulse labeling. Glob Chang Biol 18:528–538
Heimann M, Reichstein M (2008) Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451:289–292
Hill PW, Marshall C, Williams GG, Blum H, Harmens H, Jones DL, Farrar JF (2007) The fate of photosynthetically-fixed carbon in Lolium perenne grassland as modified by elevated CO2 and sward management. New Phytol 173:766–777
Holland JN, Cheng WX, Crossley DA Jr (1996) Herbivore-induced changes in plant carbon allocation: assessment of below-ground C fluxes using carbon-14. Oecologia 107:87–94
IPCC (2007) Climate change 2007: the physical science basis. Cambridge University Press, Cambridge, UK and New York, USA
Jia XX, Shao MA, Wei XR (2012) Responses of soil respiration to N addition, burning and clipping in temperate semiarid grassland in Northern China. Agric For Meteorol 166:32–40
Johnson D, Leake JR, Ostle N, Ineson P, Read DJ (2002) In situ 13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol 153:327–334
Kaštovská E, Šantrůčková H (2007) Fate and dynamics of recently fixed C in pasture plant–soil system under field conditions. Plant Soil 300:61–69
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review J Plant Nutr Soil Sc 163:421–431
Kuzyakov Y, Biryukova O, Kuznetzova T, Mölter K, Kandeler E, Stahr K (2002) Carbon partitioning in plant and soil, carbon dioxide fluxes and enzyme activities as affected by cutting ryegrass. Biol Fert Soils 35:348–358
Leake JR, Ostle NJ, Rangel-Castro JI, Johnson D (2006) Carbon fluxes from plants through soil organisms determined by field 13CO2 pulse-labelling in an upland grassland. Appl Soil Ecol 33:152–175
Liu WG, Wei J, Cheng JM, Li WJ (2014) Profile distribution of soil inorganic carbon along a chronosequence of grassland restoration on a 22-year scale in the Chinese loess Plateau. Catena 121:321–329
Mackie-Dawson LA (1999) Nitrogen uptake and root morphological responses of defoliated Lolium perenne (L.) to a heterogeneous nitrogen supply. Plant Soil 209:111–118
Norby RJ (2009) Introduction to a virtual special issue: probing the carbon cycle with 13C. New Phytol 184:1–3
Ostle N, Ineson P, Benham D, Sleep D (2000) Carbon assimilation and turnover in grassland vegetation using an in situ 13CO2 pulse labelling system. Rapid Commun Mass Sp 14:1345–1350
Paterson E, Sim A (1999) Rhizodeposition and C-partitioning of Lolium perenne in axenic culture affected by nitrogen supply and defoliation. Plant Soil 216:155–164
Qiu LP, Wei XR, Zhang XC, Cheng JM, Gale W, Guo C, Long T (2012) Soil organic carbon losses due to land use change in a semiarid grassland. Plant Soil 355:299–309
Rangel-Castro JI, Prosser JI, Scrimgeour CM, Smith P, Ostle N, Ineson P, Meharg A, Killham K (2004) Carbon flow in an upland grassland: effect of liming on the flux of recently photosynthesized carbon to rhizosphere soil. Glob Chang Biol 10:2100–2108
Rasse DP, Rumpel C, Dignac M (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 169:341–356
Reinsch S, Ambus P (2013) In situ 13CO2 pulse-labeling in a temperate heathland–development of a mobile multi-plot field setup. Rapid Commu Mass Sp 27:1417–1428
Sanderson MA (2008) Upland switchgrass yield, nutritive value, and soil carbon changes under grazing and clipping. Agron J 100:510–516
Schmitt A, Pausch J, Kuzyakov Y (2013) Effect of clipping and shading on C allocation and fluxes in soil under ryegrass and alfalfa estimated by 14C labelling. Appl Soil Ecology 64:228–236
Singh JS, Gupta SR (1977) Plant decomposition and soil respiration in terrestrial ecosystems. Bot Rev 43:449–528
Staddon PL, Ostle N, Dawson LA, Fitter AH (2003) The speed of soil carbon throughput in an upland grassland is increased by liming. J Exp Bot 54:1461–1469
Stewart DPC, Metherell AK (1999) Carbon (13C) uptake and allocation in pasture plants following field pulse-labelling. Plant Soil 210:61–73
Thorne MA, Frank DA (2009) The effects of clipping and soil moisture on leaf and root morphology and root respiration in two temperate and two tropical grasses. Plant Ecol 200:205–215
Uhlířová E, Šimek M, Šantrůčková H (2005) Microbial transformation of organic matter in soils of montane grasslands under different management. Appl Soil Ecol 28:225–235
Wan SQ, Luo YQ (2003) Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment. Glob Biogeochem Cycles 17. doi:10.1029/2002GB001971
Wan SQ, Luo YQ, Wallace LL (2002) Changes in microclimate induced by experimental warming and clipping in tallgrass prairie. Glob Chang Biol 8:754–768
Wang ZP, Li LH, Han XG, Li ZQ, Chen QS (2007) Dynamics and allocation of recently photo-assimilated carbon in an inner Mongolia temperate steppe. Environ Exp Bot 59:1–10
Waters JR, Borowicz VA (1994) Effect of clipping, benomyl, and genet on 14C transfer between mycorrhizal plants. Oikos 71:246–252
Wei J, Liu WG, Cheng JM, Li WJ (2011) Dynamics of soil organic carbon storage following restoration of grassland on Yunwu Mountain. Acta Ecol Sinica 31:271–275
Wei J, Cheng JM, Li WJ, Liu WG (2012) Comparing the effect of naturally restored forest and grassland on carbon sequestration and its vertical distribution in the Chinese loess Plateau. PLoS One 7:e40123
Wilsey BJ (1996) Urea additions and defoliation affect plant responses to elevated CO2 in a C3 grass from Yellowstone national Park. Oecologia 108:321–327
Woodin SJ, Van der Wal R, Sommerkorn M, Gornall JL (2009) Differential allocation of carbon in mosses and grasses governs ecosystem sequestration: a 13C tracer study in the high Arctic. New Phytol 184:944–949
Wu YB, Tan HC, Deng YC, Wu J, Xu XL, Wang YF, Tang YH, Higashi T, Cui XY (2010) Partitioning pattern of carbon flux in a Kobresia grassland on the Qinghai-Tibetan Plateau revealed by field 13C pulse-labeling. Glob Chang Biol 16:2322–2333
Zhao W, Chen SP, Lin GH (2008) Compensatory growth responses to clipping defoliation in Leymus chinensis (Poaceae) under nutrient addition and water deficiency conditions. Plant Ecol 196:85–99
Zhou XH, Sherry RA, An Y, Wallace LL, Luo Y (2006) Main and interactive effects of warming, clipping, and doubled precipitation on soil CO2 efflux in a grassland ecosystem. Glob Biogeochem Cycles 20:GB1003. doi:10.1029/2005GB002526
Zhou XH, Wan SQ, Luo YQ (2007) Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob Chang Biol 13:761–775
Acknowledgments
This research was financially supported by National Natural Science Foundation of China (41403015, 41373022), the Key Deployment Project from the Chinese Academy of Sciences (No. KZZD-EW-04-06). We thank the anonymous reviewers for their insightful comments, which significantly improved this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson.
Electronic supplementary material
ESM 1
(DOCX 320 kb)
Rights and permissions
About this article
Cite this article
Wei, J., Liu, W., Wan, H. et al. Differential allocation of carbon in fenced and clipped grasslands: a 13C tracer study in the semiarid Chinese Loess Plateau. Plant Soil 406, 251–263 (2016). https://doi.org/10.1007/s11104-016-2879-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2879-0








