Abstract
Aims
To evaluate the effects of changes in water stress and variable evaporative demand on water potential (Ψ), relative water content (RWC) and gas exchange during two consecutive years (2005 and 2006) providing insight into the mechanisms of stomatal control in the little-studied cultivar ‘Cobrançosa’.
Methods
Measurements of water potential, gas exchange in olive trees subjected to three irrigation treatments: rainfed (T0), continuous deficit irrigation (T1) and well irrigated (T2) that received 0, 30 and 100 % of estimated crop evapotranspiration.
Results
Both the differences in irrigation amounts and the prolonged summer drought conditions characteristic of the study area (Northeast Portugal) resulted in considerable divergences among treatments, with T2 showing the highest values of both Ψ, RWC and gas exchange rates and T0 the lowest. Stomatal conductance exhibited a strong down-regulation under increasing vapor pressure deficit (VPD), with water stress leading to parallel reductions in stomatal conductance values at any given VPD and sensitivity to changes in VPD. These effects might be associated to the deduced drought-induced declines in leaf specific hydraulic conductance (K L).
Conclusions
The results indicated that Cobrançosa olive trees have a near-isohydric behavior with a tendency to maintain a constant root-to-leaf water gradient (∆Ψ).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Mediterranean ecosystems the summer months are characterized by scarce rainfall, high temperatures and intense solar radiation, conditions that lead to the development of a high vapor pressure deficit. The olive (Olea europaea L.) is an evergreen tree traditionally cultivated in the Mediterranean basin for oil and table olives. This species is well known for its ability to withstand severe drought periods in which leaves show substantial reductions in relative water content (RWC) and water potential (Ψ) (Nogués and Baker 2000). For instance, Rhizopoulou et al. (1991) reported midsummer values of Ψ and RWC as low as −10 MPa and 50 %, respectively, with unimpaired capacity to rehydration. In general, these changes in water status are associated with large decreases on photosynthetic activity. Under conditions of mild to moderate water deficit, decreases in photosynthetic rate has been attributed primarily to stomatal closure (Loreto and Sharkey 1990), whereas in conditions of severe water stress non-stomatal limitations are invoked to explain restrictions in carbon uptake, such as the inhibition of primary photochemical reactions (Angelopoulos et al. 1996). Recently, Flexas et al. (2013) reported that the mesophyll conductance to CO2 assimilation (g m) and the leaf hydraulic conductance (K leaf) are two variables that importantly influence transport of materials within the leaf and they have been increasingly recognized as playing central roles in determining gas exchange rates and plant performance. As a consequence, plants experience low photosynthesis rates under periods of low soil water availability, limiting vegetative growth and final production (Iniesta et al. 2009; Fernandes-Silva et al. 2010; Machado et al. 2013). Irrigation can improve olive tree yields (Goldhamer et al. 1994; Fernandes-Silva et al. 2013) by minimizing the impact or avoiding the effect of water stress on crop’s performance. However, olive trees response’s to irrigation depends on the behavior of each cultivar to management system, soil and environment in field growth conditions demonstrated by some studies (Bosabalidis and Kofidis 2002; Ennajeh et al. 2009, 2010; Torres-Ruiz et al. 2013a).
Changes in stomatal conductance (g s) in response to environmental and physiological signals represent the primary way that plants regulate gas exchange and water flow through the soil-plant-atmosphere continuum over the short-term. As vapor pressure deficit (VPD) between leaf and air increases, stomata generally respond through partial closure. In most cases, stomatal conductance decreases exponentially with increasing VPD (Oren et al. 1999). There are contradictory observations on the response of olive trees to VPD under field conditions. For instance, Giorio et al. (1999) did not observe a correlation between g s and VPD on young olive trees in field conditions, whereas the field observations of Fernández et al. (1997) on well-watered olive trees or under moderate water stress suggested an upper-bound relationship between g s and VPD. Also Moriana et al. (2002) under field conditions, observed, that g s were associated with VPD but the relationship did vary with the level of water stress. Indeed, other studies suggest that soil water content plays an important role in controlling g s (Bongi and Palliotti 1994; Giorio et al. 1999), since g s depend on the transport of soil water transport to the leaves and from these to the atmosphere.
Many aspects of plant water use, particularly in response to drought, may have as their primary basis the alteration of hydraulic conductivity in the soil-root-branch-leaves continuum, and any changes in this parameter may affect gas exchange (Hubbard et al. 1999; Sperry et al. 2002; Torres-Ruiz et al. 2013b; 2014). The relationships between stomatal conductance (g s), leaf water potential (Ψleaf), K (hydraulic conductance) and environmental variables are complex. Feedback mechanisms between these variables (Chaves et al. 2003; Lovisolo et al. 2010) and differences between cultivars (Winkel and Rambal 1990; Fernández et al. 2008) have been reported. As the interaction among the stomata and the environment varies among species (Tardieu and Simonneau 1998, Pou et al. 2012), the influence of hydraulic and chemical signals on stomatal behavior may also vary under increasing water stress and water recovery conditions. Stomatal behavior in relation to transpiration-induced leaf water deficits has led to the distinction between isohydric and anysohydric species (Tardieu and Simonneau 1998). In practice, the distinctions between isohydric and anysohydric strategies are often not clear (Franks et al. 2007). For example, cultivars of poplar (Hinckley et al. 1994) and grapevine (Schultz 2003; Lovisolo et al. 2010) have been shown to exhibit both contrasting hydraulic behaviors. A third mode of behavior was also suggested by Franks et al. (2007), in which the difference between soil and midday water potential (Ψsoil–Ψleaf) keeps relatively constant internal water potential gradients allowing Ψleaf to fluctuate dramatically on a seasonal basis in synchrony with soil water potential.
The isohydric behavior is characterized by a tight stomatal regulation that allows the plant to maintain a constant minimum Ψ preventing excessive xylem cavitation, whereas anisohydric species exhibit less stomatal sensitivity to evaporative demand and soil water deficits, resulting in large fluctuations of Ψ that allows gas exchange to continue over greater increases in soil water deficit or vapor pressure deficit (VPD). The olive has been traditionally included in the latter group (Tognetti et al. 2009; Díaz-Espejo et al. 2012). However, in an experiment conducted with well and deficit-irrigated trees, Cuevas et al. (2010) observed a near-isohydric behavior as both treatments differed in g s but maintained similar midday Ψ throughout the season. Similar discrepancies have been found for other species, being sometimes attributed to contrasting stomatal behavior among cultivars of a same species (Hinckley et al. 1994; Schultz 2003). Furthermore, it has been recently noticed that some grape cultivars can switch from an isohydric-to an anisohydric-like behavior under determined conditions of soil water content and evaporative demand (Domec and Johnson 2012; Rogiers et al. 2012, Zhang et al. 2012). In this paper we will focus attention on the role played by VPD, soil and leaf water status on the mechanism of stomatal control.
A better understanding of how soil water regimes and plant water status influence stomata regulation and hydraulics of olive cultivars is important for an efficient management of water used in irrigated orchards. In this context, this study aims to give insight into the regulation of stomatal aperture in mature olive trees (Cv ‘Cobrançosa’) in relation to both variations in water stress and evaporative demand in natural conditions. ‘Cobrançosa’cultivar is widely grown in Portugal, being one of the prevailing olive cultivars in the region of Trás-os-Montes, the second region of importance to olive growing and oil production. The response of g s to increasing VPD is investigated and the participation of K L on the g s vs VPD response in mature Olea europaea L. (cv. “Cobrançosa”) is assessed under field conditions at different volumetric soil water content imposed by differential irrigation treatments, during two growing seasons. The findings expected from this investigation will contribute for a better understanding of how soil water regimes and plant water status influence stomata regulation and hydraulics of Cv. Cobrançosa cultivar an important knowledge for an efficient management of water used in irrigated orchards in dry hot environments.
Materials and methods
Site description and experimental design
Experiments were performed during two consecutive years (2005 and 2006) in a 10-year-old commercial olive orchard (Cv. ‘Cobrançosa’) located at Vilariça Valley (Vilarelhos: 41.33° N, 7.04° W; 240 m altitude) a typical olive growing area of Northeast Portugal. The climate is typically Mediterranean with an average annual rainfall of 520 mm concentrated mainly from autumn to spring (IPMA 2015b). During the experimental period the daily mean temperature varied between 5 and 9 °C in winter (December/January) and 25–27 °C in summer (July/August). The year 2005 was classified as a year extremely dry, having been recorded the lowest total precipitation since 1931 whereas the agricultural year 2005/2006 was classified as a dry year. The preceding winter 2004/05 was the driest of the last 75 years in relation to climatology normal (1971–2000). Notably, the spring was the 4th driest since 1931 and the summer was the 3rd in the same period (IPMA 2015a). Therefore, the study was carried out in an environment with a fully established drought conditions.
Reference evapotranspiration (ETo) was estimated with the FAO-Penman-Monteith equation (Allen et al. 1998) using daily data from an automatic weather station placed close (less than 100 m) to the experimental orchard. The mean annual ETo over the experimental period was 1133 mm (Fernandes-Silva et al. 2010).
The soil is classified as Eutric Leptosols developed on metamorphic rocks (schists), of sandy loam texture, characterized by poor organic matter content (<10 g kg−1), and a mean apparent bulk soil density of 1.23 g cm−3 over the 0–100 cm soil depth. The upper and lower limits of the soil water content over the soil profile were 0.19 and 0.04 m3 m−3, respectively (Fernandes-Silva et al. 2010). Trees of the orchard had a spacing of 6 m x 6 m and the experimental layout consisted of three adjacent blocks each of these made of four rows with twenty olives trees where only the six central trees were used for sampling. Since planting in 1994 till spring 2004 all blocks were irrigated equally to guarantee a uniform tree development in the entire orchard.
Three different treatments were imposed in the orchard including one rainfed taken as the control (T0) and two differential irrigated treatments receiving a seasonal water amount equivalent to 30 % (T1) and 100 % (T2) of crop evapotranspiration (ETc.), which was calculated using the FAO method (Doorenbos and Pruitt 1977). Detailed information of experimental conditions and quantified ETc. may be found in a previous paper (Fernandes-Silva et al. 2010).
Tree water status measurements
Measurements of Ψ were conducted three times daily (local standard time) –at predawn (ΨP, 4:30–6:30 h), midday (Ψmd, 13:30–14:30) and late afternoon (17:30–18:30). In addition, once a month, measurements were performed on one current-year shoot with fully expanded leaves and on six plants per irrigation treatment. The young leafy shoots were collected from sunny positions at the canopy. After cutting, the shoots were immediately enclosed in plastic bags to prevent water loss and immediately placed into a pressure chamber (model PMS 1000, Oregon, Corvallis, USA). Such process was always completed in the shade and within one to three minutes after shoot removal from the tree. Moreover, additional Ψmd values were determined every 15 days from May to September.
RWC was measured on three leaves per plant and on six plants per treatment. Leaves were detached from similar positions to the shoots used for Ψ measurements. After excision, the petiole was immersed in demineralized water in a glass tube, which was sealed, placed in a cold container and transported to the laboratory, where the increased weight of the tubes was used to determine leaf fresh mass (FM). After 48 h in the dark and 4 °C ambient temperature, the leaves were weighed to obtain FM at full turgor (TM). Dry mass (DM) was measured after oven-drying at 80 °C to a constant weight. The RWC (%) was then calculated as:
Soil water content measurements
Soil water content was measured every 15 days throughout the irrigation season (and monthly for the rest of the year) using a neutron probe (model I.H. III, Didcot Instruments Ltd, UK) previously calibrated for the experimental soil. In each of the plots a single tree was monitored with 16 access tubes, placed in a quarter of unit tree surface area. The set-up of access tubes was designed to sample the soil water content in the row and inter-row according to Fernandes-Silva et al. (2010). The measurements were taken at 0.20 m increments, from 0.20 m to a depth of 1.0 m. At the surface (0–0.10 m) the soil water content was measured by the gravimetric method. Available water content (AWC) was calculated as the difference between the upper and lower limits of soil water content measured in situ with a neutron probe.
Gas exchange measurements
Net photosynthesis (A) and stomatal conductance (g s) were measured in the field using a portable IRGA (ADC-LCA-3, Analytical Development, Hoddesdon, U.K.), operating in the open mode. Readings were taken until steady-state conditions were achieved (around 1 min). Net CO2 assimilation rate (A), stomatal conductance (g s) and transpiration rate per unit leaf area (E) were estimated from measurements of gas exchange of CO2 and H2O, using the equations developed by Von Caemmer and Farquhar (1981) considering that olive is a hypostomatous species.
Sunlit fully expanded leaves were selected as samples (two leaves per tree and four trees per treatment). Measurements were conducted every 15 days at midday (13:30 to 14:30) and once a month early in the morning (09:00–10:00) and at late afternoon (17:30 to 18:30). The photosynthetic photon flux density incident on the leaves was always greater than the saturation light point in these plants (1000–1200 mmol m−2 s−1 according to Natali et al. 1991) and thus light was not a factor limiting the overall rate of photosynthesis. Diurnal patterns of gas exchange rates were very similar throughout the season, therefore only the data obtained on August 30 (2005) and September 19 (2006) (representative of severe drought periods) are presented.
Leaf specific hydraulic conductance
Leaf specific hydraulic conductance of the flow path from soil to leaf (K L) may be described by Darcy’s law:
Where Q L is flux of liquid water per unit leaf area in the xylem tissue (mmol m−2 s−1), and Ψsoil and Ψ (MPa) are soil and leaf water potentials, respectively. In steady state conditions, it is assumed that flow of liquid water in the xylem is close to transpiration rate, so midday measurements of gas exchange of H2O (E) were used to estimate an apparent K L (mmol m−2 s−1 MPa−1). Predawn leaf water potential (ΨP) was used as a surrogate of Ψsoil considering that leaves are in equilibrium with the soil in contact with the root system by that time of day (Hubbard et al. 1999).
Sap flux measurements
Sap flux density was monitored with heat dissipation probes (Granier 1985) consisting of a heated and a reference sensor of 33 mm in length spaced 15 cm in the flow direction. The thermocouple of the probes was located 20 mm from the tip of the needle. Two trees per treatment (two of the individuals in which Ψ, gas exchange and RWC measurements were conducted) were equiped with one thermal dissipation probe 50 cm aboveground in the trunk. Probes were connected to a datalogger (CR10X, Campbell Scientific Inc., Logan, UT, USA) and the temperature gradients between heated and reference probes were recorded every 20 min. Sap flux density was determined in periods of three consecutive days around the latest four dates of 2005 when coinciding measurements of Ψ and E were performed. For the rest of the season, the probes were unheated, allowing the correction for the naturally ocurring temperature gradients. To do so, the equations shown in Do and Rocheteau (2002) were used.
Once sap flux density values were computed, sap flow (F l, mmol m−2 s−1) was obtained multiplying by the trunk xylem section and expressed on a leaf area basis. Required values of leaf area were estimated from the product of canopy volume (measurements available in Fernandes-Silva et al. 2010) and a typical value for leaf area density for olive orchards taken from the literature (Mariscal et al. 2000).
Canopy conductance and stomatal sensitivity to vapor pressure deficit
Leaf-level sensitivity of the stomatal response to VPD for each irrigation treatment was determined by fitting g s and VPD data to the functional form proposed by Oren et al. (1999):
where the parameter -m (−dg s/dlnVPD) quantifies the sensitivity of g s to VPD and b represents reference conductance at VPD = 1 kPa. In addition, the ratio of –m/b and the theoretical VPD for stomatal closure were deduced.
The same analysis was conducted at the canopy-level using the F l estimates to determine a bulk stomatal conductance (G s) by inversion of the imposed evaporation equation:
Where P is atmospheric pressure (kPa). This approach assumes that F l is a surrogate of transpiration rate, neglecting possible divergences associated to hydraulic capacitance between the point of sap flow measurements and the leaves. To minimize both errors arising from that assumption and the impact of low irradiance on G s, only data recorded between 10:00 and 18:00 were considered.
Statistics
The statistical treatment of the data was performed with the Statistix program (Statistix 9 for Windows, Analytical Software, Tallahasse, FL, USA). The significance of differences in Ψ, RWC and gas exchange between irrigation treatments was explored through conventional analyses of variance (ANOVA) using the Tukey HSD test at P < 0.05. For those cases in which assumptions of ANOVA could not be fulfilled, the non-parametric Kruskal-Wallis test was used instead.
Results
Climate conditions
The year 2005, as the previous, was characterized by a far less quantity of rainfall values relatively to the average (1971–2000), being classified as an extremely dry year (Table 1), having been recorded the lowest total precipitation since 1971 (IPMA 2015a). The 2005/06 winter (December, January and February) was classified as very dry. Although the month of March has been very rainy, the spring (March, April and May) was still classified as dry as a result the situation of dryness, which began in late 2004.
Crop responses
Seasonal variations of ΨP and RWC are presented in Fig. 1. Large differences among treatments were found for both years and variables, in particular between T0 and irrigated treatments (T1 and T2). Thus, whereas values obtained under drought conditions reached below −6 MPa (ΨP) and 60 % (RWC), both T1 and T2 trees have always maintained ΨP and RWC values above −2 MPa and 80 %, respectively. Notably, T0 trees exhibited an unimpaired capacity to rehydrate with the early Autumn rains (19 mm in Sep. 9th 2005 and 23 mm in Aug. 18th 2006, respectively DOY 252 and 230) as confirmed by both ΨP and RWC reaching values around −1.5 MPa and 85 %. By contrast, ΨP and RWC trends were more uniform throughout the seasons in the irrigated treatments. In general, ΨP values in T1 were slightly lower than those of T2 while both treatments showed less marked differences in terms of RWC.
Seasonal time course of predawn shoot water potential (ΨP, top panels) and relative water content (RWC, bottom panels) in 2005 (left panels) and 2006 (right panels) at the study site. Vertical bars represent standard deviation (n = 6 and 5 for ΨP and RWC). Different letters below the series denote statistically significant (P < 0.05) differences between irrigation treatments. T0 - Rainfed, T1 and T2 = irrigated with 30 % and 100 % ETc., respectively. DOY day of the year. Dotted lines indicate rainfall events: DOY 252 (19 mm) and DOY 230 (23 mm) in 2005 and 2006, respectively
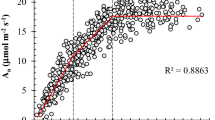
To investigate further, the relations between both ΨP and RWC and the soil AWC were studied and the results are illustrated in Fig. 2. In both cases, the sensitivity of both ΨP and RWC to changes in AWC was low for AWC values above 30 %. On the contrary, below this threshold, both ΨP and RWC declined abruptly reaching minimum values of −6.1 MPa and 59 %, respectively, for AWC ≈ 0. The seasonal course of midday A, g s and E in 2005 and 2006 is shown in Fig. 3. Independently of the year in consideration or variable, all treatments showed fairly similar values during the spring and marked differences throughout the summer. In the most irrigated plants (T2), all the variables A, g s and E showed an increasing trend from spring to autumn, when highest values were attained. By contrast, in T0 a progressive decline was observed in the three variables during the summer, leading to near-zero values, reaching even negative values for A in 2005. In the less irrigated treatment (T1), plants always showed intermediate values. In addition, the patterns of A and g s bore a close resemblance which was corroborated by linear regression analysis for each treatment (P < 0.001 and r 2 > 0.94 in all cases).
Relationship between soil available water content (AWC) and either predawn shoot water potential (ΨP, top panel) or leaf relative water content (RWC, bottom panel). Data included measurements of all irrigation treatments (T0 - Rainfed, T1 and T2 = irrigated with 30 and 100 % ETc., respectively) from 2004 to 2006. Each point is the average of either 6 or 5 measurements of ΨP and RWC, respectively. The fit relationships were: ΨP = −6.86 + 6.59 (1–0.93AWC) [r 2 = 0.95, P < 0.001] and RWC = 50.7 + 41.4 (1–0.93AWC) [r 2 = 0.80, P < 0.001]
Seasonal time course of midday net photosynthesis (A), stomatal conductance (g s) and transpiration rate (E) for the three irrigation treatments (T0 - Rainfed, T1 and T2 = irrigated with 30 % and 100 % ETc., respectively) in 2005 and 2006 (left and right panels, respectively). Vertical bars represents the standard deviation (n = 8) and different letters above the series denote statistically significant (P < 0.05) differences between treatments. Dotted lines indicate rainfall events: DOY 252 (19 mm) and DOY 230 (23 mm) in 2005 and 2006, respectively
The daily course of Ψ for two representative summer days with marked differences among treatments is illustrated in Fig. 4. The highest values were always measured at predawn and the minimum ones were recorded at midday, and there was no sign of recovery in the late afternoon. As expected, T2 showed the highest values of Ψ and T0 the lowest, regardless of the time of day. For instance, Ψmd was −2.5, −3.8 and −6.7 MPa for T2, T1 and T0, respectively. On the other hand, Fig. 5 presents the diurnal course of A, g s and E for the same days. Again, differences among treatments were evident, with the highest values of all variables attained by T2 and lowest values by the T0 water treatment. The patterns of A bore a close resemblance to those of g s, with the highest values being observed in the morning then followed by a decline until midday. No sign of recovery was observed from midday to late afternoon. The slightly higher values of A and g s observed on 19/09/2006 (DOY - day of year 230) were associated with lower evaporative demand (mean VPD for that day was 1.7 kPa against 3.1 kPa on 30/08/2005; DOY 211). Unlike to what was observed on 19/09/2006 there was a clear decline of E, values of T0 and T1 treatments from early morning till midday on 30/08/2005. In the case of T2, there were no marked variations in E values from early morning till the afternoon in any of the dates.
Diurnal time course of shoot water potential (Ψ, MPa) in the three irrigation regimes (T0 - Rainfed, T1 and T2 = irrigated with 30 and 100 % ETc., respectively) for two representative late summer days, when differences among treatments are the largest. Columns are means (n = 6) and vertical bars represent the standard deviation. In all cases, differences between treatments were statistically significant (P < 0.05). DOY day of year
Diurnal time course of net photosynthesis (A), stomatal conductance (g s), transpiration rate (E) for the three irrigation treatments (T0 - Rainfed, T1 and T2 = irrigated with 30 and 100 % ETc., respectively) for two representative late summer days, when differences among treatments are the largest. Columns are means and vertical bars represents the standard deviation (n = 8). In all cases, differences between treatments were statistically significant (P < 0.05). DOY day of year
The seasonal courses of Ψmd (previously published in Fernandes-Silva et al. 2010) and ΨP (Fig. 1) followed rather similar patterns in all treatments and throughout the two seasons. Figure 6a confirms this fact showing a good correlation between them (r 2 = 0.966, P < 0.001) across the range of ΨP measured (open symbols). On the contrary, poor fits were found between Ψmd and g s (Fig. 6b, open symbols). Apparently, large fluctuations of g s occurred with Ψmd remaining slightly more to −3 MPa and low values of Ψmd (< −4.5 MPa) always were associated with low g s (<50 mmol m−2 s−1). On the other hand, the apparent water potential gradient from roots to shoots (∆Ψ) in T1 and T2 plants, calculated as the difference between ΨP and Ψmd, remained within a relatively stable range (1.90 ± 0.05 SE MPa) despite the significant changes in ΨP and g s (Fig. 6a&b, closed symbols). In T0 plants, however, ∆Ψ presented a tendency to decrease (in absolute terms) under conditions of very low ΨP and g s.
Midday shoot water potential (Ψmd, open symbols) and midday water potential gradient from shoots to roots (−∆Ψ, closed symbols) versus predawn water potential (ΨP) (a) and stomatal conductance (g s) (b). Different symbol types (circles, squares and triangles) correspond to the three treatments (T0 - Rainfed, T1 and T2 = irrigated with 30 and 100 % ETc., respectively)
Figure 7 depicts the time course of K L for the two years of study. Once again, large differences among treatments were observed. For instance, T2 plants exhibited values ranging from 2.3 to 4.1 mmol m−2 s−1 MPa−1, while K L was always between 0.6 and 2.7 mmol m−2 s−1 MPa−1 in T1. The rainfed treatment showed the lowest K L, with values from 1.5 mmol m−2 s−1 MPa−1 on 180 DOY in 2006 to almost zero in 2005 and 2006 summers (minimum of 0.06 mmol m−2 s−1 MPa−1). In line with the differences among treatments, K L was well correlated with AWC (r 2 = 0.71, P < 0.001, data not shown). A good linear fit was also found between K L and E (r 2 = 0.94, P < 0.001, Fig. 8a). In addition, further evidence of the latter relationship was found when plotting the values of K L against those of E deduced from sap flow data (Fig. 8a). Besides that, the plot of K L versus g s was better predicted by a curvilinear relationship (r 2 = 0.87, P < 0.001, Fig. 8).
Time course of estimated midday leaf specific hydraulic conductivity (K L) for each irrigation treatment (T0 - Rainfed, T1 and T2 = irrigated with 30 and 100 % ETc., respectively) in the two years of study. DOY – day of year.). Vertical bars represents the standard deviation (n = 6) and different letters above the series denote statistically significant (P < 0.05) differences between treatments
Relationships between estimated midday leaf specific hydraulic conductance (K L) and midday transpiration (E) (a) and stomatal conductance (g s) (b) and between midday water potential gradient from roots to shoots (∆Ψ) and midday transpiration (c). Each symbol type corresponds to a different treatment (T0 - Rainfed, T1 and T2 = irrigated with 30 and 100 % ETc., respectively). Grey symbols in panel A correspond to the plot of K L versus E values deduced from the available sap flow records in 2005. The fit relationships were: K L = 0.4683 E + 0.2684 [r 2 = 0.96, P < 0.001], K L = 0.1607 g s 0.5833 + 0.0108 [r 2 = 0.82, P < 0.001] and ∆Ψ = 0.2995 ln(E) + 1.4825 [r 2 = 0.57, P < 0.001]
The responses of g s and G s to VPD as a function of the irrigation treatment are illustrated in Fig. 9. Strong relationships were always found regardless of the level (i.e. leaf or canopy) or treatment considered (Table 2). In general, both the sensitivity of stomatal response (−m) and reference conductance at VPD = 1 kPa (b) differed greatly among treatments, with T2 and T0 showing the highest and the lowest values, respectively. Such values were several times greater at the leaf-level, but the ratio of stomatal sensitivity to reference conductance (i.e. –m/b) was almost the same (within the range 0.45–0.50), again independently of the level and treatment considered. Finally, the extrapolation of the regression lines yielded the average value of VPD 8.6 kPa for theoretical stomatal closure (0.63 mmol m−2 s−1).
Relationship between either leaf- (g s, panel a) or canopy-level (G s, panel b) stomatal conductance and the natural logarithm of vapor pressure deficit (VPD). In the first case, measurements were performed along three periods of the day (8:00–9:00; 13:30–14:30; 17:30–18:30) for several dates in 2005 and 2006, respectively, each point representing the average of 8 measurements. In the later, each data point is obtained from the average of 20-min interval sap flux density measurements in two trees. Only measurements between 10:00 and 18:00 on 12 days of 2005 (in groups of three around the same dates as in a) are considered. Separate plots are shown for each treatment (T0 - Rainfed, T1 and T2 = irrigated with 30 and 100 % ETc., respectively). The fit relationships were: g s = 490.4–218.4 ln(VPD) [r 2 = 0.82, P < 0.001, panel A, T2], g s = 248.7–122.7 ln(VPD) [r 2 = 0.76, P < 0.001, panel A, T1], g s = 75.2–34.7 ln(VPD) [r 2 = 0.47, P < 0.001, panel A, T0], G s = 39.6–18.3 ln(VPD) [r 2 = 0.80, P < 0.001, panel B, T2], G s = 25.9–11.6 ln(VPD) [r 2 = 0.82, P < 0.001, panel B, T1] and G s = 11.9–5.9 ln(VPD) [r 2 = 0.64, P < 0.001, panel B, T0]
Discussion
Meteorological conditions during the experiments were typically of a Mediterranean environment and representative of the study area. These conditions (no summer rainfalls, high temperatures and low atmospheric moisture) resulted in a high evaporative demand (Table 1) that led to a rapid depletion of the soil water reserves and, hence, to large differences in plant water status and gas exchange among irrigation treatments. Thus, T0 plants reached values of ΨP and Ψmd as low as −6 and −7 MPa. Such values were similar to those reported by Moriana et al. (2003) who worked with rainfed ‘Picual’; but much lower than those observed for other cultivars, at the same time (Table 3). The origin of these discrepancies may be partly ascribed to the particular environmental conditions of each experiment. For instance, the higher values of Ψ observed by Tognetti et al. (2004, 2006 and 2009) could be ascribed to the scattered summer rainfalls which frequently occurred in their study site. On the contrary, significantly higher values have been reported for ‘Manzanilla de Sevilla’ (Table 3) despite both evaporative demand and precipitation amounts and patterns being relatively similar to those in the present study. With regard to T2, summer Ψmd values typically ranged between −2.5 and −3 MPa, which resulted relatively low as compared to those found in other well-irrigated olive cultivars (Table 3). By contrast, other cultivars such as ‘Frantoio’ or ‘Leccino’ presented lower values in terms of ΨP. In the light of all the above, it is concluded that the use of Ψ as a water stress indicator is compromised by the influence of environmental conditions and cultivar. Future research efforts should address more thoroughly this issue.
Differences in AWC and Ψ among treatments were translated into different levels of dehydration as evidenced by the measurements of RWC. Thus, during the periods of maximum water stress RWC declined to values around 60 % in T0 plants (Fig. 1). Even lower values (45 %) were described by Rhizopoulou et al. (1991) in wild olives, but the levels of water stress experienced by their trees might have been more severe (they measured Ψmd of up to −10 MPa). On the other hand, higher values of RWC than those found by Rhizopoulou et al. (1991) for both rainfed and irrigated olive trees can be found in the literature (Lo Gullo and Salleo 1988; Giorio et al. 1999; Wahbi et al. 2005). However, the data of this study revealed higher values of RWC (>85 %) than those observed by the aforementioned authors (RWC = 75–80 %) under similar conditions of Ψmd (around −3 MPa). The high levels of RWC observed in this study may be an intrinsic characteristic of CV Cobrançosa which may suggest that, in relation to other olive cultivars, this cultivar has a more efficient control mechanism in preventing desiccation, allowing the maintenance of higher photosynthesis rates. Indeed, Santos et al. (2013) reported that Cobrançosa leaves exhibit a thick, waxy cuticle with abundant trichome layers, mainly in the abbatial surface and a high density of foliar tissue. These morfo-anatomical traits should protect Cobrançosa leaves from excessive water loss in dry environments (Fernández 2014).
The water deficits experienced by T1 and T0 plants resulted in reduced values of A and g s as compared to the well-irrigated ones, which is also in agreement with previous observations (Giorio et al. 1999; Moriana et al. 2003; Sofo et al. 2008). The negative values of midday A found in late summer for one of the study years (Fig. 5) indicate that respiratory processes were greater in magnitude than gross photosynthetic rates. Despite negative values of A during the day are not very common, similar observations have been reported in other species under water stress conditions (Syros et al. 2004). The RWC in T0 plants was permanently below the turgor loss point for olive, considered to be 77 % (Hinckley et al. 1980), producing therefore osmotic stress and dehydration at the cellular level. Thus, the very low values of RWC most likely have inhibited photosynthesis during the summer. In this regard, Giorio et al. (1999) observed that a reduction in RWC from 84 to 74 % induced decreases of 67 % in photosynthesis capacity and 48 % in carboxylation efficiency. In our study, from mid-May to early June, we observed that a reduction of 25 % in of AWC, from 47 to 22 % in T0 olive plants, induced a 50 % drop in ΨP (Fig. 2), from −0.5 to −1.0 MPa. Simultaneously, morning E was reduced by about 57 % while morning A decreased 42 % in relation to well irrigated plants. In the same period, T1 plants experienced a comparable reduction in AWC, although its absolute values were slightly higher (from 55 to 35 %). In this case, smaller decreases were recorded both for ΨP (from −0.45 to −0.51 MPa), and in morning values of E (30 %) and A (16 %). It is worth highlighting the little impact that the moderate water stress had on in carbohydrate synthesis in this treatment.
The combination of Ψ and gas exchange measurements (E) allowed us to obtain estimates of the apparent K L for the experimental trees. Since its calculation was conditioned by several more or less realistic simplifications (mainly ΨP representing Ψsoil and a lack of scaling from ‘leaf-level transpiration rate’ measurements to ‘canopy-level transpiration rate’), our estimates may differ from the actual values of K L. However, such estimates are meaningful for analyzing relative variations over time and differences among treatments. With regard to the later, it was evident that K L was affected by water stress as significantly lower values were observed for T0 and T1 in relation to T2 (Fig. 7). Similarly, but working with a long leaf pine undergoing periods of contrasting soil water status, Addington et al. (2004) noticed that K L fluctuated in association to AWC, with the lowest values coinciding with drought periods. According to these authors, we attribute the drought-induced decline in K L to decreases in the hydraulic conductance of either the vascular (e.g. induced by xylem cavitation) or non-vascular (e.g. by root suberization or inhibition of aquaporines) pathways. Regarding to this issue, Torres-Ruiz et al. (2014a) evaluated on 1-year-old Arbequina potted olive plants, the dynamics of chemical and hydraulic signals at leaf, stem and root level, and their effect on the regulation of stomatal conductance (g s) during water stress and recovery. They found a strong hydraulic segmentation in olive plants, with higher hydraulic functioning losses in roots and leaves than in stems. Furthermore, the dynamics of hydraulic conductance of roots and leaves observed as water stress developed could explain both a protection of the hydraulic functionality of larger organs of the plant (i.e., branches, etc.) and a role in the down-regulation of g s. On the other hand, they reported that abscisic acid (ABA) content also increased, displaying an analogous behavior to g s dynamics; however its effect on g s in response to water stress cannot be ruled out.
After early autumn rains, leaf water status recovered from drought earlier than photosynthetic variables, alike the T2 plant values. As previous studies have shown (Moriana et al. 2007; Torres-Ruiz et al. 2013b ; Perez-Martin et al. 2014), olive has a high capacity for recovery from water stress after rewatering. A similar pattern has been reported in tobacco (Galle et al. 2009). The value of g s showed the slowest recovery of all the variables studied. The reasons for this commonly slow recovery of g s after restoration of leaf water status is not yet clear, although it has been related to both hydraulic limitations (Brodribb and Cochard 2009) and chemical limitations (Lovisolo et al. 2008). In this study, the very low values of Ψ reached at the end of the drought period (below −7 MPa) may suggest that a large loss of stem hydraulic conductivity could be attributed to a chemical and physical hydraulic factor conditioning. This supposition can be supported by the findings of Perez-Martin et al. (2014) on 5-year-old O. europaea L. var. Manzanilla potted plants. Concerning to chemicals limitations, several authors have pointed out the accumulation of abscisic acid (ABA) during stress accumulation as a circumstance that might prevents g s from fully recovering once soil water is available again and plant water status has been restored (Davies and Zhang 1991; Lovisolo et al. 2008). This role played by ABA in the recovery stage as a way of controlling transpiration rate during the embolism-repairing time. However, the conclusions of that work, carried out in grapevine, are not necessarily applicable to olive, especially as the former has been reported to have a great refilling capacity of embolized vessels (Brodersen et al. 2010). Moreover, even in grapevine, contradictory results have been obtained, with no recovery of g s once ABA accumulation was fully reversed after a few days (Pou et al. 2008). Finally, several authors have reported that recovery from water stress depends on the level and velocity of the stress imposition (Galle et al. 2011). In olive plants Torres-Ruiz et al. (2014) indicated that neither hydraulic nor non-hydraulic factors were able to explain the delay in the full recovery of g s after soil water availability was restored. Thus, this subject needs to be better explored on future investigations targeted to gene expression with all the potential photosynthesis-limiting factors (gs, gm and biochemistry) during water stress and recovery under field conditions.
Both measurements of g s and estimates of G s revealed a strong stomatal down-regulation under increasing evaporative demand and the functional form proposed by Oren et al. (1999) was satisfactorily fitted in all cases (Table 2, Fig. 9). The higher values found at the leaf-level were already expected, as g s was measured from sun-exposed leaves while G s integrates the whole canopy, including shaded leaves. Apart from that, we should note that the calculation of G s from sap flux density measurements was subjected to several more or less realistic assumptions (e.g. the whole trunk section was considered conductive sapwood and only one probe per tree was installed so that both azimuthal and radial variations in sap flux density were neglected) and, hence, absolute values should be taken with care. Regardless of the level considered, both Fig. 9 and Table 2 puts in evidence that water stress had a profound impact on the stomatal response to VPD. Thus, T2 plants exhibited higher sensitivity to VPD and higher values of g s and G s at any given value of VPD than those of T1 and T0. These results are in line with those of Addington et al. (2004), who highlighted a possible link between K L dynamics and changes in the stomatal behavior in response to VPD.
Olive has been described both as an anisohydric (Tognetti et al. 2009; Díaz-Espejo et al. 2012) and a near-isohydric (Cuevas et al. 2010; Torres-Ruiz et al. 2013a; Perez-Martin et al. 2014) species. Isohydric behavior is generally attributed to the strong stomatal control of transpiration rate, which results in the observed similarity in midday leaf water potential in drought and well-watered plants (Tardieu and Simonneau 1998). Anisohydric plants typically exhibit less stomatal control over evaporative demand and soil moisture, allowing large fluctuations in leaf water potential (Franks et al. 2007). The experiment reported here clearly shows that both T2 and T1 plants were able to regulate their g s in order to maintain the midday water potential above around −3.0 MPa (Fig. 6b). The g s values recorded in T2 and T1 plants usually varied from >250 to 100 mmol m−2 s−1 whereas slight variations were observed for Ψmd. However, when plants were under more severe water stress conditions (T0), i.e. for low AWC value (Fig. 2), it was observed a large decline in Ψ and RWC. Thus, it seems that T0 plants lost their capacity for regulating the stomatal behavior to maintain a relatively constant Ψmd, leading to drastic reductions in water status in relation to well irrigated trees. Under these circumstances, stomatal closure was almost reached to prevent excessive dehydration, which might have threatened the survival of the tree. Besides that, an interesting finding illustrated in Fig. 6 is that the root-to-shoot ∆Ψ was maintained at similar levels (1.90 ± 0.05 SE MPa) over low and high values of ΨP and g s in all treatments except in some situations of very severe water stress (i.e. when predawn equilibrium between roots and shoots Ψ was probably not reached). This pattern of hydraulic behavior, referred to as ‘isohydrodynamic’, had been previously observed in Eucalyptus gomphocephala (Franks et al. 2007), some grape cultivars (Zhang et al. 2012; Shellie and Bowen 2014) and in both annuals crops (Helianthus annuus L.) and three angiosperm and gymnosperm tree species, growing in a common garden (Simonin et al. 2015) but never in olive. According to Franks et al. (2007), for ∆Ψ to be maintained, a direct relationship between K L and E is required, a condition that was satisfied in our experiments (Fig. 8a). As stated by Simonin et al. (2015) leaves operating with this mechanism show a nonlinear relationship between ΔΨ and E, with ΔΨ increasing as E increases. This condition was observed in leaves of cv. Cobrançosa (Fig. 8c). It seems, that the variation in K L observed in our study may have occurred to maximize leaf gas exchange while minimizing variation in ΔΨ, that increase the maximum potential of g s and A for a given VPD. As a final remark, the discrepancies found in olive, both between the data reported elsewhere and between our findings and the literature published, might be linked to different hydraulic regulation strategies among cultivars—as in the case of grape (Schultz 2003)—or to the environmental conditions in which trees grow.
Conclusions
Our results showed that stomatal conductance exhibited a strong down-regulation under increasing vapor pressure deficit (VPD), with water stress leading to parallel reductions in stomatal conductance values at any given VPD and sensitivity to changes in VPD. These effects might be associated to the deduced drought-induced declines in leaf specific hydraulic conductance (K L). Cobrançosa olive trees presented an isohydric or near-isohydric behavior with a tendency to maintain a constant root-to-leaf water gradient (∆Ψ), which is the first observation of an isohydrodynamic behavior in olive trees.
The findings of the present work could be of importance for olive genetic improvement for low g s mechanism as an important approach for drought tolerance and water economy in agriculture of hot and dry Mediterranean environments.
References
Addington RN, Mitchell RJ, Oren R, Donovan LA (2004) Stomatal sensitivity to vapor pressure deficit and its relationship to hydraulic conductance in Pinus palustris. Tree Physiol 24:561–569
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration.Guidelines for computing crop water requirements. FAO Irrigation and Drainage Paper 56. FAO, Rome
Angelopoulos K, Dichio B, Xiloyannis C (1996) Inhibition of photosynthesis in olive trees (Olea europaea L.) during water stress and rewatering. J Exp Bot 47:1093–1100
Bongi G, Palliotti A (1994) Olive. In: handbook of environmental physiology of fruit crops: temperate crops, vol. I, CRC Press, Boca Raton, Florida, pp. 165–187
Bosabalidis AM, Kofidis G (2002) Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci 163:375–379
Brodersen CR, McElrone AJ, Choat B, Matthews MA, Shackel KA (2010) The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography. Plant Physiol 154:1088–1095
Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149:575–584
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
Cuevas MV, Torrez-Ruiz JM, Alvarez R, Jiménez MD, Cueva J, Fernandez JE (2010) Assessment of trunk diameter variation derived indices as water stress indicators in mature olive trees. Agric Water Manag 97:1293–1302
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Díaz-Espejo A, Buckley TN, Sperry JS, Cuevas MV, de Cires A, Elsayed-Faraga S, Martin-Palomo MJ, Muriel JL, Perez-Martina A, Rodriguez-Dominguez CM, Rubio-Casal AE, Torres-Ruiz JM, Fernández JE (2012) Steps toward an improvement in process-based models of water use by fruit trees: a case study in olive. Agric Water Manag 114:37–49
Do F, Rocheteau A (2002) Influence of natural temperature gradients on measurements of xylem sap flow with thermal dissipation probes. 1. Field observations and possible remedies. Tree Physiol 22:641–648
Domec JC, Johnson DM (2012) Does homeostasis or disturbance of homeostasis in minimum leaf water potential explain the isohydric versus anisohydric behavior of Vitis vinifera L. cultivars? Tree Physiol 32:245–248
Doorenbos J, Pruitt WO (1977) Crop water requirements. FAO irrigation and drainage paper 24. FAO, Rome
Ennajeh M, Vadel AM, Khermira H (2009) Osmoregulation and osmoprotection in the leaf cells of two olive cultivars subjected to severe water deficit. Acta Physiol Plantarum 31:711–721
Ennajeh M, Vadel AM, Cochard H, Khermira H (2010) Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J Hort Sci Biot 85:289–294
Fernandes-Silva AA, Ferreira TC, Correia CM, Malheiro AC, Villalobos FJ (2010) Influence of different irrigation regimes on crop yield and water use efficiency of olive. Plant Soil 333:35–47
Fernandes-Silva AA, Gouveia JB, Vasconcelos P, Ferreira TC, Villalobos FJ (2013) Effect of different irrigation regimes on the quality attributes of monovarietal virgin olive oil from cv. “Cobrançosa”. Grasas Aceites 64:41–49
Fernández JE (2014) Understanding olive adaptation to abiotic stresses as a tool to increase crop performance. Plant Cell Environ 103:158–179
Fernández JE, Moreno F, Girón IF, Blázquez OM (1997) Stomatal control of water use in olive tree leaves. Plant Soil 190:179–192
Fernández JE, Díaz-Espejo A, Infante JM, Durán P, Palomo MJ, Chamorro V, Girón JF, Villagarcía L (2006) Water relations and gas Exchange in olive trees under regulated deficit irrigation and partial rootzone drying. Plant Soil 284:273–291
Fernández JE, Diaz-Espejo A, D’Andria R, Sebastiani L, Tognetti R (2008) Potential and limitations of improving olive orchard design and management through modelling. Plant Biosyst 142:130–137
Flexas J, Scoffoni C, Gago J, Sack L (2013) Leaf mesophyll conductance and leaf hydraulic conductance: an introduction to their measurement and coordination. J Exp Bot 64:3965–3981
Franks PJ, Drake PL, Froend RH (2007) Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ 30:19–30
Galle A, Florez-Sarasa ID, Tomas M, Pou A, Medrano H, Ribas-Carbo M, Flexas J (2009) The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): Acclimation or limitation? J Exp Bot 60:2379–2390
Galle A, Florez-Sarasa I, El Aououad H, Flexas J (2011) The Mediterranean evergreen Quercus ilex and the semi-deciduous Cistus albidus differ in their leaf gas exchange regulation and acclimation to repeated drought and re-watering cycles. J Exp Bot 62:5207–5216
Gimenez C, Fereres E, Ruz C, Orgaz F (1996) Water relations and gas exchange of olive trees: diurnal and seasonal patterns of leaf water potential, photosynthesis and stomatal conductance. Acta Hortic 449:411–415
Giorio P, Sorrentino G, d’Andria R (1999) Stomatal behaviour, leaf water status and photosynthetic response in field-grown olive trees under water deficit. Environ Exp Bot 42:95–104
Goldhamer DA, Dunai J, Ferguson LF (1994) Irrigation requirements of trees and responses to sustained deficit irrigation. Acta Hortic 356:172–175
Granier A (1985) Une nouvelle method pour la mesure des flux de séve dans le tronc des arbres. Ann Sci For 42:193–200
Hinckley TM, Duhme F, Hinckley AR, Kichter H (1980) Water relations of drought hardy shrubs: osmotic potential and stomatal reactivity. Plant Cell Environ 3:131–140
Hinckley TM, Brooks JR, Cermák J, Ceulemans R, Kucera J, Meinzer FC, Roberts DA (1994) Water flux in a hybrid poplar stand. Tree Physiol 14:1005–1018
Hubbard RM, Bond BJ, Ryan MG (1999) Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa tress. Tree Physiol 19:165–167
Iniesta F, Testi L, Orgaz F, Villalobos FJ (2009) The effects of regulated and continuous deficit irrigation on the water use, growth and yield of olive trees. Eur J Agron 30:258–265
IPMA (2015) Climate normals, Köppen classification. Instituto Português do Mar e da Atmosfera. Acedido em 08/06/2015. Url: http://www.ipma.pt/pt/oclima/normais.clima/
IPMA (2015a) Climate normals. Instituto Português do Mar e da Atmosfera. Acedido em 8/06/2015. Url: http://www.ipma.pt/en/oclima/normais.clima/1971-2000/002/
Lo Gullo MA, Salleo S (1988) Different strategies of drought resistance in three Mediterranean sclerophyllous trees growing in the same environmental conditions. New Phytol 108:267–276
Loreto F, Sharkey TD (1990) Low humidity can cause uneven photosynthesis in olive (Olea europaea L.) leaves. Tree Physiol 6:409–415
Lovisolo C, Perrone I, Hartung W, Schubert A (2008) An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytol 180:642–651
Lovisolo C, Perrone I, Carra A, Ferrandino A, Flexas J, Medrano H, Schubert A (2010) Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update. Funct Plant Biol 37:98–116
Machado M, Felizardo C, Fernandes-Silva AA, Nunes FM, Barros A (2013) Polyphenolic compounds, antioxidant activity and L-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different water irrigation. Food Res Int 51:412–421
Mariscal MJ, Orgaz F, Villalobos FJ (2000) Modelling and measurement of radiation interception by olive canopies. Agric For Meteorol 100:183–197
Moreno F, Fernández JE, Clothier BE, Green SR (1996) Transpiration and root water uptake by olive trees. Plant Soil 184:85–96
Moriana A, Fereres E (2002) Plant indicators for scheduling irrigation of young olive trees. Irrig Sci 21:83–90
Moriana A, Villalobos FJ, Fereres E (2002) Stomatal and photosynthetic response of (Olea europaea L.) leaves to water deficits. Plant Cell Environ 25:395–405
Moriana A, Orgaz F, Pastor M, Fereres E (2003) Yield responses of a mature olive orchard to water deficits. J Am Soc Hortic Sci 128:425–431
Moriana A, Pérez-López D, Gómez-Rico A, Salvador MD, Olmedilla N, Ribas F, Fregapane G (2007) Irrigation scheduling for traditional, low density olive orchards: water relations and influence on oil characteristics. Agric Water Manag 87:171–179
Natali S, Bignami C, Fusari A (1991) Water consumption, photosynthesis, transpiration and leaf water potential in Olea europaea L. cv. “Frantoio”, at different levels of available water. Agric Med 121:205–212
Nogués S, Baker NR (2000) Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot 51:1309–1317
Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schäfer KVR (1999) Survey and synthesis of intra-and interspecific variation in stomatal sensitivity to vapor pressure deficit. Plant Cell Environ 24:561–569
Pérez-López D, Gijón MC, Moriana A (2008) Influence of irrigation rate on the rehydration of olive tree plants. Agric Water Manag 95:1161–1166
Perez-Martin A, Michelazzo C, Torres-Ruiz JM, Flexas J, Fernández JE, Sebastiani L, Antonio Diaz-Espejo A (2014) Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: correlation with gene expression of carbonic anhydrase and aquaporins. J Exp Bot 65:3143–3156
Pou A, Flexas J, Alsina MM et al (2008) Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). Physiol Plantarum 134:313–323
Pou A., Medrano H, Tomàs M, Martorell S Ribas-Carbó M, Flexas J (2012) Anisohydric behaviour in grapevines results in better performance under moderate water stress and recovery than isohydric behavior. Plant Soil 359:335–349
Rhizopoulou S, Meletiou-Christou MS, Diamantaglou S (1991) Water relations for sun and shade leaves of four Mediterranean evergreen sclerophylls. J Exp Bot 42:627–635
Rogiers SY, Greer DH, Hatfield JM, Hutton RJ, Clark SJ, Hutchinson PA, Somers A (2012) Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abcisic acid. Tree Physiol 32:249–261
Santos SAP, Silva S, Pinto G, Torres LM, Nogueira AJA (2013) The effect of sooty mold on fluorescence and gas exchange properties of olive tree. Turk J Biol 37:620–628
Schultz HR (2003) Differences in hydraulic architecture account for near-isohydric and anisohydric behavior of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ 26:1393–1405
Shellie KC, Bowen P (2014) Isohydrodynamic behavior in deficit-irrigated Cabernet Sauvignon and Malbec and its relationships between yield and berry composition. Irrig Sci 32:87–97
Simonin KA, Burns E, Choat B, Barbour MM, Dawson TD, Franks PJ (2015) Increasing leaf hydraulic conductance with transpiration rate minimizes the water potential drawdown from stem to leaf. J Exp Bot 66:1303–1315
Sofo A, Manfreda S, Fiorentino M, Dichio B, Xiloyannis C (2008) The olive tree: a paradigm for drought tolerance in Mediterranean climates. Hydrol Earth Syst Sci 12:293–301
Sperry JS, Hacke UG, Oren R, Comstock JP (2002) Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ 25:251–263
Syros T, Yupsanis T, Omirou M, Economou A (2004) Photosynthetic response and peroxidases in relation to water and nutrient deficiency in gerbera. Environ Exp Bot 52:23–31
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modeling isohydric and anisohydric behaviors. J Exp Bot 49:419–432
Tognetti R, d’Andria R, Morelli G, Calandrelli D, Fragnito F (2004) Irrigation effects on daily and seasonal variations of trunk sap flow and leaf water relations in olive trees. Plant Soil 263:249–264
Tognetti R, d’Andria R, Lavini A, Morelli G (2006) The effect of deficit irrigation on crop yield and vegetative development of Olea europaea L. (cvs. Frantoio and Leccino). Eur J Agron 25:356–364
Tognetti R, Giovannelli A, Lavini A, Morelli G, Fragnito F, d’Andria R (2009) Assessing environmental controls over conductance through the soil–plant–atmosphere continuum in an experimental olive tree plantation of southern Italy. Agric Forest Meteorol 149:1229–1243
Torres-Ruiz JM, Diaz-Espejo A, Morales-Sillero A, Martín-Palomo MJ, Mayr S, Beikircher B, Fernández JE (2013a) Shoot hydraulic characteristics, plant water status and stomatal response in olive trees under different soil water conditions. Plant Soil 373:77–87
Torres-Ruiz JM, Diaz-Espejo A, Perez-Martin A, Hernandez-Santana V (2013b) Hydraulic vulnerability to cavitation at leaf, stem and root level and its role in the stomatal behaviour during drought in olive trees. Acta Hortic 991:333–339
Torres-Ruiz JM, Diaz-Espejo A, Perez-Martin A, Hernandez-Santana V (2014) Role of hydraulic and chemical signals in leaves, stems and roots in the stomatal behaviour of olive trees under water stress and recovery conditions. Tree Physiol. doi:10.1093/treephys/tpu055
Von Caemmer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Wahbi S, Wakrim R, Aganchich B, Tahi H, Serraj R (2005) Effects of partial rootzone drying (PRD) on adult olive tree (Olea europaea) in field conditions under arid climate: I. physiological and agronomic responses. Agr Ecosyst Environ 106:289–301
Winkel T, Rambal S (1990) Stomatal conductance of some grapevines growing in the field under a Mediterranean environment. Agric For Meteorol 51:107–121
Zhang Y, Oren R. Kang S (2012) Spatiotemporal variation of crown-scale stomatal conductance in an arid Vitis vinifera L. cv. Merlot vineyard: direct effects of hydraulic properties and indirect effects of canopy leaf area. Tree Physiol 32: 262-279.
Acknowledgments
We are very grateful to Eng. Manuel Afonso for providing the experimental orchard and to technical officer Mário Sérgio Graça for helping with the field measurements. We also thank to Professor Jóse Mountinho Pereira and to Assistant Professor Margarida Arrobas, for the loan of the pressure chamber, IRGA and neutron probe. Our thanks also go to Professor Fernando Raimundo for the help given towards the field classification of the soil used in the experimental study. Finally, we are most grateful to all anonymous reviewers for their valuable feedback.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by project AGRO 175 of AGRO-INIA programme of Ministério da Agricultura (Portugal) and Fundação para a Ciência e a Tecnologia do Ministério da Ciência, Tecnologia e do Ensino Superior (Portugal) by the doctoral fellowship (SFRH/BD/18441/2004) awarded to AA Fernandes-Silva. This study has also benefited from additional funding through projects AGL-2010-20766 of Spanish Ministry of Natural, Rural and Marine Environment and by the European Community’s Seven Framework Programme-FP7 (KBBE.2013.1.4-09) under Grant Agreement No. 613817 (MODEXTREME, modextreme. org).
Additional information
Responsible Editor: Rafael S. Oliveira.
Rights and permissions
About this article
Cite this article
Fernandes-Silva, A.A., López-Bernal, Á., Ferreira, T.C. et al. Leaf water relations and gas exchange response to water deficit of olive (cv. Cobrançosa) in field grown conditions in Portugal. Plant Soil 402, 191–209 (2016). https://doi.org/10.1007/s11104-015-2786-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2786-9