Abstract
Background and aims
The aim of this study was to test the effect of Azospirillum brasilense on the superoxide anion production (O2 •−) and enzymes related with redox metabolism in roots of wheat (Triticum aestivum).
Methods
A. brasilense Sp245 and T. aestivum seeds cv Nana F2007 were used in this study. Wheat roots were stained with nitro blue tetrazolium (NBT) to visualize and localize O2 •− production. Superoxide dismutase (SOD) and peroxidase (POX) activities were assayed in native PAGE.
Results
We found that A. brasilense application resulted in a decrease in meristem length and cell size, and in a reduction in the O2 •− level in roots. The bacteria stimulated SOD and soluble POX isoenzymes, particularly in the zone of the root tip. Qualitative O2 •− production in roots treated with LaCl3, a Ca2+ channel blocker, in combination with A. brasilense was comparable to inoculated roots. Similar results were observed with the Ca2+ ionophore A23187.
Conclusions
Our results suggest that O2 •− metabolism is important during the interaction of wheat and A. brasilense, and that the antioxidative enzymes such as SOD and POX are involved in its regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant development and growth are strongly influenced by biotic and abiotic factors encountered by roots within soils. Certain soil microbial populations can be beneficial for plants by improving growth, development, and health. Representative beneficial microbes include plant growth-promoting rhizobacteria (PGPR), which establish associative symbiotic interactions with their host plant (Raaijmakers et al. 2009; Richardson et al. 2009). PGPR are able to colonize plant root systems and enhance plant growth through a variety of mechanisms, including direct effects on nutrient uptake as well as root growth through the production of phytohormones such as auxins, gibberellins, and cytokinins (Richardson et al. 2009; Yang et al. 2009). They can also protect plants against root and/or foliar pathogens (Raaijmakers et al. 2009) through antibiotic production, competition for ecological niches, and induction of systemic resistance (Lugtenberg and Kamilova 2009; Rezzonico et al. 2005).
Among PGPR, Azospirillum is considered as one of the most important rhizobacterial genus and it is used worldwide for improving plant growth and crop yield (Bashan et al. 2004; Jacoud et al. 1998; Okon and Labandera-Gonzalez 1994). The sites of primary root colonization include points of lateral root emergence and the root hair zones (Vande Broek et al. 1993). Plant-beneficial effects of Azospirillum strains result mostly in morphological and physiological changes of the root system (Khalid et al. 2004; Richardson et al. 2009), which increases root proliferation and elongation and leads to an enhanced capacity to access essential nutrients and water (Richardson et al. 2009). These effects are due to the production of phytohormones by the bacteria, especially indole-3-acetic acid, and by deaminating 1-aminocyclopropane-1-carboxylate (ACC), which is a precursor of ethylene that inhibits root growth (Prigent-Combaret et al. 2008; Steenhoudt and Vanderleyden 2000).
In plants, normal root growth is controlled by the activity of the subapical meristem and the elongation of newly formed cells. Several recent studies indicate that reactive oxygen species (ROS) are required for cell expansion during the morphogenesis of organs, such as roots and leaves (Takeda et al. 2008). ROS can function as potent signaling molecules during the growth and development and coordinate responses to biotic and abiotic stresses in plants (Apel and Hirt 2004). Evidence suggests that ROS function through an elaborate network of crosstalk with hormonal networks, thereby allowing plants to regulate developmental processes as well as abiotic and biotic stress tolerance responses (Tognetti et al. 2012).
ROS, such as O2 •−, H2O2, singlet oxygen, and hydroxyl radicals, are produced continuously as a result of cell metabolism, but this low level of ROS homeostasis can be significantly perturbed when plants are exposed to environmental stress conditions (Mittler et al. 2004; Van Breusegem and Dat 2006). Since excessive ROS concentrations result in cellular oxidative damage or the induction of cell death, basal levels need to be maintained by a redox signaling network consisting of several enzymes, such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX). Thus, plants have developed a high degree of control over ROS accumulation through this network and are able to use ROS as signaling molecules to regulate normal and stress-tolerant physiological processes (Foyer and Noctor 2009; Potters et al. 2010). Among ROS, H2O2 plays a central role in a broad range of physiological processes, including senescence (Corpas et al. 2001), photorespiration and photosynthesis (Noctor and Foyer 1998), stomatal movement (Bright et al. 2006), cell cycle (Mittler et al. 2004), growth and development (Foreman et al. 2003), and plant adaptation to the changing environment (Neill et al. 2002; Tognetti et al. 2012). In A. thaliana roots, ROS homeostasis regulates the transition from proliferation to differentiation (Tsukagoshi et al. 2010).
Under normal conditions, O2 •− and H2O2 are differentially distributed within a plant root (Dunand et al. 2007), with superoxide mainly accumulating in dividing and expanding cells of the meristem and H2O2 accumulating in the elongation zone. An overlap of both ROS types has been observed within the so-called “transition zone” (Tsukagoshi et al. 2010). In Salix seedlings, the production of ROS in specific regions of roots seems to be essential for the normal growth of this organ being either partially or completely inhibited in the presence of H2O2 or O2 •− scavengers, respectively. O2 •− production has been found to be elevated in the root apex, particularly in the subapical meristem and protodermal zones (Causin et al. 2012). Moreover, apical O2 •− generation activity correlates with a high level of either Cu/Zn superoxide dismutase protein as well as carbonylated proteins. O2 •− production is also high in root hairs during budding, but markedly decreases when the hair begins active elongation. Root hair formation is also increased in the presence of H2O2 scavengers, but suppressed when H2O2 or peroxidase inhibitors are present (Causin et al. 2012).
Calcium is another molecule implicated in plant morphogenesis as well as the elongation of roots and root hairs. Ca2+ influx from the extracellular store is required for cell elongation in roots, and NADPH oxidases appear to control development by generating ROS that promotes plant cell expansion through the activation of Ca2+ channels (Foreman et al. 2003). Taken together, these results suggest that ROS homeostasis may play an important role during root growth regulation by PGPR, which is one aspect that remains poorly studied. Therefore, the aim of the present study was to analyze the effect of the inoculation of wheat roots with A. brasilense Sp245 on O2 •− production and antioxidative enzymes as well as its correlation with changes in root morphology.
Material and methods
Materials and growth conditions
A. brasilense Sp245 (Baldani et al. 1986) was used in this study. Bacteria were grown on LB medium (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl, 0.186 g/L MgSO4, 0.277 g/L CaCl2, 15 g/L Agar) for routine use and maintained in nutrient broth with 15 % glycerol at −80 °C for long-term storage. Cultures were grown 20 h (exponential phase) at 27 °C with rotation at 100 rpm. The cultures were then washed twice in 0.9 % NaCl by centrifugation (4300×g, 10 min, 4 °C), resuspended in sterile water, and adjusted to a final concentration of 1 × 106 colony-forming units (CFU)/mL for use as an inoculum.
T. aestivum seeds, cv Nana F2007, were kindly provided by Dr. Mario González-Chavira (INIFAP- Celaya, Gto, México). Seeds were washed by shaking in 1 % SDS for 3 min, surface sterilized for 5 min in a 1 % sodium hypochlorite solution, washed four times with sterile distilled water, and germinated on filter paper sterilized and wetted with sterile distilled water on Petri dishes for 3 days in the dark at 28 °C. Seedlings were then aseptically transferred to assay tubes (15 cm length and 2.5 cm width) and immersed (only roots) in 5 mL of liquid Murashige and Skoog (MS) medium (pH 5.7). Inoculation with A. brasilense was performed by adding 100 μl of a 1 × 106 cells/mL suspension on MS medium. Root length was measured with a ruler using one primary root. Unless otherwise indicated, chemicals were obtained from Sigma Sigma-Aldrich (St. Louis, MO, USA).
4′,6-diamidino-2-phenylindole (DAPI) and NBT staining
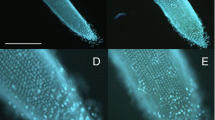
DAPI staining of root tips was performed as follows: root seedlings were fixed in a solution of 4 % paraformaldehyde for 24 h at 4 °C, then washed 3 times in water and mounted on cover slips. The roots were stained with 1 μg/ml DAPI for 30 min and analyzed with an Olympus BX60 fluorescence microscope (excitation 365 nm and emission 420–540 nm; Zeiss, Jena, Germany) (Tsukagoshi 2012). To visualize the localization and/or rate of O2 •− production, seedlings were stained for 15 min with a solution of 0.1 % nitro blue tetrazolium NBT in 50 mM sodium phosphate buffer, pH 7.5 (Causin et al. 2012).
NBT quantification
Quantification of superoxide was assayed using a method described by Arthikala et al. 2014. Briefly, NBT-stained tissue was ground in liquid nitrogen and the formazan content of the resulting powder was dissolved in 2 M KOH-DMSO (1:1.16 v/v) and then centrifuged for 10 min at 12,000 g. The quantity of NBT was determined by measuring the optical density at 630 nm and comparing with a standard curve using known concentrations of NBT.
SOD and soluble POX enzyme activity
The plant tissues (100 mg) were powdered in liquid nitrogen and homogenized in 100 mM phosphate buffer, pH 7.8. The homogenate was centrifuged at 4 °C for 10 min at 10,000 g. The supernatant containing extracted soluble enzymes was used to measure SOD and POX enzyme activities that might be both of bacterial and plant origins. Wheat root was divided in two root zones of 5 mm each, and named Apical zone and Distal zone to analyze the enzyme activities.
SOD activity was assayed using the method described by Beauchamp and Fridovich (1971). Briefly, samples of supernatant prepared as described above (100 μg per lane) were separated by polyacrylamide gel electrophoresis (PAGE) under non-denaturing conditions. Following electrophoresis in a 12.5 % (w/v) native polyacrylamide gel at 100 V and 4 °C, the gel was immersed in 2.45 mM NBT for 20 min, followed by a 15-min soak in a solution containing 28 mM tetramethylethylenediamine (TEMED), 28 μM riboflavin, and 36 mM potassium phosphate, pH 7.8. SOD activity was detected by illuminating the gel with bright light, which caused the gel to turn uniformly blue except at positions exhibiting SOD activity.
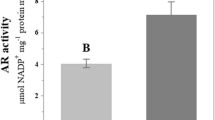
The SOD activity was also measured spectrophotometrically as described by Beyer and Fridovich (1989). In this assay, 1 unit of SOD is defined as the amount required to inhibit the photoreduction of NBT by 50 %. The specific activity of SOD was expressed as units mg−1 protein.
For POX activity, protein samples (30 μg) were dissolved in loading buffer without SDS and thiol-reducing agents and analyzed on 0.5-mm-thick SDS-polyacrylamide gels (10 %) without prior boiling based on the method of Laemmli (1970). Proteins were separated at 10 V cm−1. After separation, the gels were equilibrated for 30 min in 50 mm sodium citrate, pH 5.5, prior to incubation with 1 mm 3,3′-diaminobenzidine DAB and 0.03 % (w/v) H2O2 in fresh sodium citrate buffer. Peroxidase isoforms stained brown color. The protein content of the extracts was determined according to Bradford (1976) using the Bio-Rad dye reagent (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as the standard.
LaCl3, A23187, and indole-3-acetic acid (IAA) treatments
Wheat seeds were germinated for 3 days and transferred to tubes containing 5 ml of liquid MS medium with 1 mM LaCl3, a calcium channel blocker (Lanteri et al. 2006), 1 × 10−4 M A23187, a calcium ionophore (Bibikova et al. 1997), or 1 μM IAA . They were subsequently incubated for 7 days after germination under a cycle consisting of 16 h of light and 8 h of darkness at 22 ± 1 °C.
Statistics
Statistical differences between mean values were determined with the Student’s t-test. Differences at the level of P < 0.05 were considered to be statistically significant. Each experiment was repeated at least three times and results are presented as mean ± standard deviation (SD).
Results
Effect of inoculation on morphology, cell size, and cell number on wheat root
Inoculation of wheat seedlings with A. brasilense Sp245 (1 × 106 CFU ml−1) resulted in a strong decrease in root length 4 days after incubation (Fig. 1a). In addition, root hair proliferation was more pronounced for inoculated plants than for non-inoculated plants (Fig. 1b). To determine if the short-root phenotype in inoculated plants may be due to effects on the organization of the root meristem as a result of changes in cell size or cell number, the root was divided in two main regions: differentiation zone and meristem zone (Fig. 2a). Parameters including cell size, meristem length, and cell number were then measured. The cell size in both the differentiation zone and meristem was smallest in inoculated roots compared to control roots. Meristem length was reduced in inoculated roots, but the number of cells in this zone was notably higher than in the non-inoculated roots (Fig. 2b). Taken together, these data suggest that the reduced root elongation in response to inoculation with A. brasilense could be due to a decrease in cell size.
Effects of Azospirillum inoculation on the growth of control non-inoculated (−Ab) and Azospirillum-inoculated (+Ab) wheat roots of seedlings a. Wheat root length b. Seedlings were disinfected and germinated on wet filter paper in sealed trays for 3 days and then grown for 4 days in liquid MS medium inoculated with A. brasilense (1 × 106 cells mL−1). n = 20. The asterisk indicates statistically significant differences from control with P < 0.05
Superoxide anion production in roots inoculated with A. brasilense
We next analyzed the production of superoxide anion in inoculated roots since it is known that this ROS is involved in developmental processes and stress responses. O2 •- is often the first reduced form of oxygen to be generated in plant tissues, which subsequently leads to the formation of H2O2 and OH•. Therefore, since O2 •- seems to be important for root growth, we analyzed its production and spatial localization in A. brasilense-treated roots using NBT as a histochemical probe. NBT staining revealed active production of O2 •- in the root apex and beyond the apical region in control non-inoculated roots (Fig. 3a). However, when roots were inoculated with A. brasilense, O2 •− production decreased and was confined to the root tips. Inoculation of roots with an increasing amount of bacteria led to a clear decrease in O2 •− production in the root, including the root apex (Fig. 3b).
Roots of wheat seedlings were stained with NBT to detect O2 •− production a. Seedlings were pretreated with different concentrations of A. brasilense, incubated for 24 h, and then stained with NBT for 15 min b. Representative photographs are shown. Barr = 2 mm. The experiments were repeated at least three times with similar results
An inoculum with a bacteria concentration of 1 × 106 CFU ml−1 was used to analyze the O2 •− production at different times after inoculation. The production of O2 •− decreased with time after inoculation, whereby a sharp decrease in O2 •− production was observed 96 h post treatment (Fig. 4a). We used formazan as an indirect way to quantitate O2 •− production. This analysis supported the decrease in O2 •− production in inoculated roots (Fig. 4c). The changes in O2 •− production in inoculated roots also correlated with an increase in the size and number of lateral roots, with the greatest increase observed closer to the root tip (Fig. 4b).
Production of O2 •− in wheat roots at different times a, lateral root production b, O2 •− accumulation c, and distance from the root tip to the first lateral root d, in non-inoculated and inoculated wheat seedlings. n = 20. Barr = 2 mm. The asterisk indicates statistically significant differences from control with P < 0.05
Superoxide dismutase and soluble peroxidase in A. brasilense-treated roots
We next assessed SOD and POX enzymes activity, using native gels, since these enzymes play central roles in the metabolism of ROS. Enzyme activities were first assayed in whole roots. Three isoenzymes of SOD were clearly detected in total protein extracts and exhibited differential activity in both inoculated and non-inoculated roots. A clear increase in activity was observed for all three isoenzymes in inoculated roots. The SOD enzymes were also assayed separately in protein extracts obtained from segments isolated from apical or distal sections of the roots. We found that the activities of all SOD enzymes were stimulated in the apical zone of roots inoculated with A. brasilense compared to non-inoculated roots. Greater SOD activity was observed in the distal zone of the roots, but this activity was not significantly different between the inoculated and non-inoculated roots. Spectrophotometrical analysis of total SOD activity further indicated that SOD activity was two to three-fold greater in inoculated roots compared to non-inoculated roots (Fig. 5a).
Native gel electrophoresis of POX identified at least seven isoenzymes with all exhibiting a high activity in non-inoculated roots. The same isoforms were detected in inoculated roots; however, the observed activity of all existing POX isoenzymes was increased, and one of these (POX 3) was remarkably enhanced. In the apical zone, POX activity increased after the incubation with A. brasilense. In contrast, the POX activity in the distal zone of the roots was high but equivalent in both groups, inoculated and non-inoculated (Fig. 5b).
Effect of internal Ca2+ concentrations during A. brasilense-colonization of wheat roots
As an initial step for investigating the involvement of Ca2+ during root colonization by A. brasilense, seedlings were grown for 4 days after germination in liquid MS medium containing either a Ca2+ channel blocker (LaCl3) or Ca2+ ionophore (A23187) with the rhizobacteria. No effect on root length was detected after 24 h, regardless of treatment. However, a decrease in root length was observed in inoculated roots after 96 h of incubation. While treatment with LaCl3 alone had no effect on root length, combining LaCl3 with inoculation with A. brasilense caused a decrease in root length most similar to that observed with inoculation with A. brasilense alone. This result suggests that LaCl3 did not interfere with the effect of A. brasilense on root length. In contrast, the production of lateral roots was inversely affected by treatment with LaCl3 alone or A. brasilense alone: treatment with LaCl3 resulted in a decrease in the number of lateral roots and inoculation with A. brasilense resulted in an increase in the number of lateral roots. Combining inoculation with A. brasilense and treatment with LaCl3 had an additive effect and no significant change in the number of lateral roots could be detected (Fig. 6a). This latter result suggests that the effect of A. brasilense on lateral roots number is distinct from that caused by LaCl3 treatment.
Superoxide anion production did not show changes when LaCl3 was combined with A. brasilense or when applied alone (Fig. 6b).
Root length was slightly reduced in plants treated with A23187 alone, after 96 h of incubation relative to the non-inoculated control. Root length was also significantly reduced in plants inoculated with A. brasilense alone and this effect was similar to that observed in plants treated with A23187 in combination with inoculation with A. brasilense. This suggests that the effect of A23187 and A. brasilense on root length is not additive and could possibly be mediated by a common mechanism (Fig. 7a).
Treatment with A23187 alone led to a slight decrease in superoxide production compared with control, which was additionally reduced in the presence of A. brasilense, and was similar to that observed in inoculated roots (Fig. 7b).
Taken together, the results are consistent with Ca2+ homeostasis being critical for normal root growth and development, and indicate that Ca2+ signaling is unlikely to mediate the plant roots response to inoculation with A. brasilense.
Our results indicated that inoculation of the roots with A. brasilense leads to a reduction in root length, increase in the number of lateral roots, and superoxide production and these effects could be mimicked by exogenous addition of IAA (Fig. 7b), suggesting that IAA alone, likely produced by the bacteria, can mediate these root phenotypes.
Discussion
Increasing evidence suggests that ROS plays a role in root growth, but its role in the interaction between plants and rhizobacteria remains elusive. Given the role of O2 •− as an important mediator of cellular effects on roots, and in controlling various ROS-mediated plant growth processes, we explored the putative role of this molecule during the interaction of A. brasilense with wheat roots. We observed that wheat roots inoculated with A. brasilense exhibited a change in meristem morphology and an increase in cell number that correlated with changes in O2 •− production detected in root tissues. The reduced levels of O2 •− observed in wheat roots inoculated with Azospirillum are likely due to an increase in SOD activity, resulting in reduced elongation of the roots. The enzymatic modulation of O2 •− production indicated that the production of this ROS is regulated during the interaction between Azospirillum and wheat. Interestingly, this regulation was specific to the meristem zone where cell growth occurs. These results are consistent with those obtained in a recent study with Arabidopsis where similar changes in root morphology were detected in response to inoculation by rhizobacteria (Zamioudis et al. 2013).
ROS production has been linked in the normal growth of plants and probably results from an active cellular metabolism in growing plant organs. For example, the production of ROS in specific regions of Salix seedlings was shown to be essential for normal growth (Causin et al. 2012). In these studies, O2 •− was predominantly located in the apoplast of the cells in the elongation zone, whereas H2O2 accumulated in the differentiation zone and in growing root hairs. Treatments that decrease O2 •− concentration reduced root elongation and root hair formation, while scavenging H2O2 promoted root elongation and suppressed root hair formation (Dunand et al. 2007). Active production of O2 •− in the root apex has also been reported for some plant model systems, including maize (Liszkay et al. 2004), cucumber (Renew et al. 2005), and A. thaliana (Dunand et al. 2007). Although it is clear that O2 •− is essential for the control of root growth, the mechanism involved remains controversial.
Increasing evidence indicates that the regulation of the redox state play a major role in mediating the interaction of plants with beneficial microbes. Gluconacetobacter diazotrophicus, an aerobic diazotrophic plant-growth-promoting bacterium, upregulated the transcript levels of SOD and glutathione reductase (GR) genes at early stages of colonization showing the importance of ROS during root colonization (Alquéres et al. 2013). Similarly, inoculation of A. brasilense to wheat (Camilios-Neto et al. 2014) or A. thaliana (Spaepen et al. 2014) modulated plant and bacteria ROS metabolism, suggesting that ROS are produced during the initiation of Azospirillum –plant roots associations.
The production of phytohormones is a key factor for the observed effects of Azospirillum inoculation on plant growth (Vande Broek et al. 2000). In addition to auxins, such as indole-3-acetic acid (IAA) (Tsavkelova et al. 2007; Martinez-Morales et al. 2003), Azospirillum spp. produce cytokinins (Tsavkelova et al. 2006), and gibberellins (Bottini et al. 2004; Perrig et al. 2007). Auxin production has been implicated in regulating ROS homeostasis in plant roots (Ivanchenko et al. 2013), via modulation of H2O2 production (Zelinová et al. 2011) and regulation of the activities of ROS scavenging enzymes, such as SOD, catalase and peroxidase (Tyburski et al. 2009). Although auxin production by A. brasilense Sp245 was not quantified in our assays, its production has been previously reported (Crozier et al. 1988). The direct role of IAA produced by A. brasilense Sp245 in regulating plant growth is suggested by the observation that a mutant lacking ipdC (coding for the indole-3-pyruvic acid decarboxylase) had a 90 % reduction in IAA biosynthesis and inoculation of wheat seeds with this mutant failed to yield a significant root growth promotion, in contrast to the effect of the parental Sp245 strain (Dobbelaere et al. 1999). Further, comparison of changes in gene expression in A. thaliana inoculated with A. brasilense Sp245 or a derivative overproducing IAA confirmed the role of bacterial auxin production in modulating changes in root growth (Spaepen et al. 2014). The molecular mechanisms involving redox regulation during the interaction of PGPR with root plants are poorly understood, but several genetic and biochemical studies suggest that auxin produced by these bacteria is responsible for the control of root growth (Spaepen et al. 2014). The effects of A. brasilense Sp245 inoculation on morphology and superoxide anion production in wheat roots described here could be mimicked with the exogenous addition of auxins, suggesting that this ROS is also under auxin regulation, and reveal a connection between superoxide anion and auxins to regulate root growth by rhizobacteria. Whether this effect of auxin is direct remains to be determined. Further insight into the mechanisms by which beneficial rhizobacteria such as Azospirillum spp. modulates plant ROS production is needed to establish how auxin production is linked to ROS generation in plant tissues. However, in Arabidopsis, the auxin-induced ROS production appears to be mediated by the NADPH oxidase RbohD (respiratory burst oxidase homologue D) (Peer et al. 2013). Although all of the molecular links between stimulus and ROS production have not been identified, it is known that the binding of phosphatidyl 3-phosphate (PI3P), produced by the activity of phosphatidyl inositol 3-kinase, to one of the components of NADPH oxidase can activate the enzyme complex (Ellson et al. 2001). Interestingly, it has been reported that the activation of phosphatidylinositol 3-kinase is needed to auxin-induced reactive oxygen species production in A. thaliana (Joo et al. 2005).
Here, we found that chemically manipulating Ca2+ homeostasis had no effect on O2 •− production in wheat roots inoculated with A. brasilense, indicating that Ca+2-mediated signaling is not critical for the ability of these beneficial rhizobacteria to promote plant growth, under the conditions of the experiments. This is in contrast to the role of Ca2+ in the establishment of symbiosis between the roots of legumes and their rhizobial partners (Ehrhardt et al. 1996). Changes in Ca2+ concentration elicit physiological and developmental responses in plants, including gravitropism (Toyata et al. 2008), closure of stomata (Geiger et al. 2011), and root growth (Zhao et al. 2010). Our results suggest that signaling events underlying the establishment of beneficial rhizobacteria on plant roots and their promoting growth effects are not Ca2+-mediated and are distinct from the signaling cascade implicated in legume-rhizobia symbiosis.
References
Alquéres S, Meneses C, Rouws L, Rothballer M, Baldani I, Schmid M, Hartmann A (2013) The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol Plant-Microbe Interact 26:937–945
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arthikala M-K, Sanchez-Lopez R, Nava N, Santana O, Cardenas L, Quinto C (2014) RbohB, a Phaseolus vulgaris NADPH oxidase gene, enhances symbiosome number, bacteroid size, and nitrogen fixation in nodules and impairs mycorrhizal colonization. New Phytol 202:886–900
Baldani VLD, de B. Alvarez MA, Baldani JI, Dobereiner JD (1986) Establishment of inoculated Azospirillum spp in the rhizosphere and in roots of field grown wheat and sorghum. Plant Soil 90:35–46
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997– 2003). Can J Microbiol 50:521–577
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beyer WF, Fridovich I (1989) Characterization of a superoxide dismutase mimic prepared from desferrioxamine and MnO2. Arch Biochem Biophys 271:149–156
Bibikova TN, Zhigilei A, Gilroy S (1997) Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta 203:495–505
Bottini R, Cassán F, Piccoli P (2004) Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl Microbiol Biotechnol 65:497–503
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Camilios-Neto D, Bonato P, Wassem R, Tadra-Sfeir MZ, Brusamarello-Santos LCC, Valdameri G, Donatti L, Faoro H, Weiss VA, Chubatsu LS, Pedrosa FO, Souza EM (2014) Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. BMC Genomics 15:378
Causin HF, Roqueiro G, Petrillo E, Láinez V, Pena LB, Marchetti CF, Gallego SM, Maldonado SI (2012) The control of root growth by reactive oxygen species in Salix nigra Marsh. Seedlings. Plant Sci 183:197–205
Corpas FJ, Barroso JB, del Río LA (2001) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci 6:145–150
Crozier A, Arruda P, Jasmim JM, Monteiro AM, Sandberg G (1988) Analysis of indole-3-acetic acid and related indoles in culture medium from Azospirillum lipoferum and Azospirillum brasilense. Appl Environ Microbiol 54:2833–2837
Dobbelaere S, Croonenborghs A, Thys A, Vande BA, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:155–164
Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
Ehrhardt DW, Wais R, Long SR (1996) Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85:673–681
Ellson CD, Gobert-Gosse S, Anderson KE, Davidson K, Erdjument-Bromage H, Tempst P, Thuring JW, Coope MA, Lim ZY, Holmes AB, Gaffney PR, Coadwell J, Chilvers ER, Hawkins PT, Stephens LR (2001) PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat Cell Biol 3:679–682
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Red Signal 11:861–905
Geiger D, Maierhofer T, Al-Rasheid KA, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, Romeis T, Hedrich R (2011) Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal 4:ra32
Ivanchenko MG, den Os D, Monhausen GB, Dubrovsky JG, Bednarova A, Krishnan N (2013) Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum lycopersicum) root tips while inhibiting root growth. Ann Bot 112:1107–16
Jacoud C, Faure D, Wadoux P, Bally R (1998) Development of a strain-specific probe to follow inoculated Azospirillum lipoferum CRT1 under field conditions and enhancement of maize root development by inoculation. FEMS Microbiol Ecol 27:43–51
Joo JH, Yoo HJ, Hwang I, Lee JS, Nam KH, Bae YS (2005) Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Lett 579:1243–1248
Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473–480
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lanteri ML, Pagnussat GC, Lamattina L (2006) Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin- induced adventitious root formation in cucumber. J Exp Bot 57:1341–1351
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2 •-, H2O2, and •OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Martinez-Morales LJ, Soto-Urzúc L, Baca BE, Sanchez-Ahécdo JA (2003) Indole-3-butyric acid (IBA) production in culture medium by wild strain Azospirillum brasilense. FEMS Microbiol Lett 228:167–173
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) The reactive oxygen gene network in plants. Trends Plant Sci 9:490–498
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signaling molecules in plants. J Exp Bot 53:1237–1247
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Okon Y, Labandera-Gonzalez CA (1994) Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol Biochem 26:1591–1601
Peer WA, Cheng Y, Murphy AS (2013) Evidence of oxidative attenuation of auxin signalling. J Exp Bot 64:2629–2639
Perrig D, Boiero ML, Masciarelli OA, Penna C, Ruiz OA, Cassán FD, Luna MV (2007) Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculants formulation. Appl Microbiol Biotechnol 75:1143–1150
Potters G, Horemans N, Jansen MAK (2010) The cellular redox state in plant stress biology – a charging concept. Plant Physiol Biochem 48:292–300
Prigent-Combaret C, Blaha D, Pothier JF, Vial L, Poirier M-A, Wisniewski-Dyé F, Moënne-Loccoz Y (2008) Physical organization and phylogenetic analysis of acdR as leucine-responsive regulator of the 1-aminocyclopropane-1- carboxylate (ACC) deaminase gene acdS in phytobeneficial Azospirillum lipoferum 4B and other Proteobacteria. FEMS Microbiol Ecol 65:202–219
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moenne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soil borne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Renew S, Heyno E, Schopfer P, Liszkay A (2005) Sensitive detection and localization of hydroxyl radical production in cucumber roots and Arabidopsis seedlings by spin trapping electron paramagnetic resonance spectroscopy. Plant J 44:342–347
Rezzonico F, Binder C, Défago G, Moënne-Loccoz Y (2005) The type III secretion system of biocontrol Pseudomonas fluorescens KD targets the phytopathogenic chromista Pythium ultimum and promotes cucumber protection. Mol Plant-Microbe Interact 18:991–1001
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Spaepen S, Bossuyt S, Engelen K, Marchal K, Vanderleyden J (2014) Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol 201:850–861
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319:1241–1244
Tognetti VB, Per M, Frank VB (2012) Stress homeostasis – the redox and auxin perspective. Plant Cell Environ 35:321–333
Toyata M, Furuichi T, Tatsumi H, Sokabe M (2008) Critical consideration on the relationship between auxin transport and calcium transients in gravity perception of Arabidopsis seedlings. Plant Signal Behav 3:521–524
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI (2006) Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Microbiol 42:117–126
Tsavkelova EA, Cherdyntseva TA, Botina SG, Netrusov AI (2007) Bacteria associated with orchid roots and microbial production of auxin. Microbiol Res 162:69–76
Tsukagoshi H (2012) Defective root growth triggered by oxidative stress is controlled through the expression of cell cycle-related genes. Plant Sci 197:30–39
Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143:606–616
Tyburski J, Krzeminski L, Tretyn A (2009) Exogenous auxin affects ascorbato metabolism in roots of tomato seedlings. Plant Growth Regul 54:203–215
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
Vande Broek A, Michiels J, Van Gool A, Vanderleyden J (1993) Spatial-temporal colonization patterns of Azospirillum brasilense on the wheat root surface and expression of the bacterial nifH gene during association. Mol Plant-Microbe Interact 6:592–600
Vande Broek A, Dobbelaere S, Vanderleyden J, Van Dommeles A (2000) Azospirillum- plant root interactions: signaling and metabolic interactions. In: Triplett EW (ed) Prokariotic nitrogen fixation: a model system for the analysis of a biological process. Horizon Scientific Press, Wymondham, pp 761–777
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse CMJ (2013) Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol 162:304–318
Zelinová V, Halušková L, Mistrík I, Tamás L (2011) Abiotic stress–induced inhibition of root growth and ascorbic acid oxidase activity in barley root tip is associated with enhanced generation of hydrogen peroxide. Plant Soil 349:281–289
Zhao X, X-w Z, He H, Y-x W, Zhang X (2010) Mechanisms of extracellular NO and Ca2+ regulating the growth of wheat seedling roots. J Plant Biol 53:275–281
Acknowledgments
This study was supported by the Coordinación de la Investigación Científica, Universidad Michoacana de San Nicolás de Hidalgo, México.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Choong-Min Ryu.
Rights and permissions
About this article
Cite this article
Méndez-Gómez, M., Castro-Mercado, E., Alexandre, G. et al. Superoxide anion production in the interaction of wheat roots and rhizobacteria Azospirillum brasilense Sp245. Plant Soil 400, 55–65 (2016). https://doi.org/10.1007/s11104-015-2709-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2709-9