Abstract
Aims
The primary aim of this review is to determine if methods based on 15N enrichment (E) and 15N natural abundance (NA) give consistent estimates of the proportional dependence of N2-fixing species on biological N2 fixation (P atm), and secondly to attempt to explain any inconsistencies that may be found.
Methods
Published estimates of the symbiotic dependence of N2-fixing plants based on E and NA techniques applied in the same experiment were compared across scales from glasshouse pots to field plots to landscapes in agricultural and forest ecosystems, which included grain legumes, pasture and forage legumes, and woody perennials. A meta-analysis of the published data was based on correlation coefficients, box-plots and confidence intervals of means.
Results
In some studies, estimates were reference plant dependent for both E and NA techniques, indicating temporal and/or spatial variations in the natural and artificial distribution of 15N, which can sometimes result in erroneous negative estimates of symbiotic dependence. While significant correlations were obtained between E and NA estimates of P atm for each of the three groups of N2-fixing species, the probability that the methods provided estimates of P atm within −5 to +5 % of each other was 0.29 or was 0.54 within −10 to +10 % of each other.
Conclusions
We have identified a number of interacting factors that may contribute to the inconsistent agreement between estimates of P atm by E and NA techniques, which underlines the need for a re-examination of the fundamental assumptions on which each method is based, and whether those assumptions are valid in any given situation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 15N isotope dilution estimation of the proportional dependence of legumes on biological N2 fixation (BNF) was first reported by McAuliffe et al. (1958). The method is based on 15N enrichment of the soil with a labelled fertilizer and the use of paired plots, one containing the legume and the other a non-N2-fixing reference plant. The 15N isotope dilution technique has been used extensively to estimate BNF in cropping, pastoral, forestry and silvo-pastoral systems, including legumes, actinorhizal plants and tropical C4 grasses, and several critical reviews have been written about the technique (e.g., Chalk 1985; Chalk and Ladha 1999).
The use of 15N natural abundance (NA) to estimate legume BNF is a more recent development compared with 15N enrichment (E), with the first soil-based experiments reported by Amarger et al. (1979) and Kohl et al. (1980). The method also requires the use of a non-N2-fixing reference plant, and in addition, it requires the determination of the isotopic fractionation which occurs during BNF (the B-value). The NA method has also been widely applied in systems that include grain and forage legumes, and woody perennials that include legumes and actinorhizal plants, and several critical reviews have been published (e.g., Shearer and Kohl 1986; Boddey et al. 2000).
Both E and NA techniques depend on the use of a non-N2-fixing reference plant to estimate de facto the ratio of labelled (isotope-derived) to unlabelled (soil-derived) N assimilated by the legume. The major assumption in both techniques is that the reference plant accesses the same available soil N pool as the legume. However, as discussed in many previous publications, the application of isotope to confined micro-plots in the field perturbates the system under study and results in a non-uniform temporal and spatial (vertical) distribution of 15N in the soil. Estimates of symbiotic dependence are therefore reference plant-dependent (Chalk 1985; Chalk and Ladha 1999) because of differences in relative rates of N uptake and soil volumes explored by roots of the two species.
On the other hand, there is no disturbance when the NA method is used, and it has been claimed that natural variations in 15N abundance are relatively uniform over time and with soil depth (e.g., Ledgard and Steele 1992). Therefore if this assertion, which was originally based on limited published data, is generally valid, estimates of symbiotic dependence, unlike those obtained with the E method, should be less sensitive to the choice of the reference plant. To our knowledge, this hypothesis has not been tested by comparison of the many published estimates obtained by E and NA methods, particularly those where several reference plants were used. The objective of this review is therefore firstly to determine if the two methods give consistent estimates of symbiotic dependence over a range of scales and N2-fixing systems, and secondly to attempt to explain any observed inconsistencies by considering, where possible, variations in the temporal and spatial distribution of 15N. Attention will also be focused on the NA technique with respect to the reference plant δ-value and the B-value and the method used to determine the B-value.
Methodology
15N enrichment method (E)
The proportion of legume N derived from the atmosphere (P atm) is estimated by Eq. 1, which gives a yield-independent estimate of P atm.
where E is 15N enrichment expressed as excess atom fraction 15N.
In a single case of comparing E and NA estimates of P atm (Stevenson et al. 1995), the A-value modification of the E technique was used, whereby a higher rate of 15N-enriched fertilizer was applied to the reference plant compared with the legume (see review of Chalk 1996).
15N natural abundance method (NA)
The proportion of legume N derived from the atmosphere (P atm) is estimated by Eq. 2, which also gives a yield-independent estimate of P atm.
where δ 15N is the \( \frac{15_{\mathrm{N}}}{14_{\mathrm{N}}} \) ratio of the sample relative to the \( \frac{15_{\mathrm{N}}}{14_{\mathrm{N}}} \) ratio of the international standard, atmospheric N2 (Eq. 3).
where by definition, δ 15Nstandard is zero excess atom fraction 15N.
Several methods have been proposed for determining the ‘B-value’ in Eq. 2 which represents the isotopic fractionation which may occur during the N2 fixation process and subsequently during the translocation of biologically-fixed N from the nodulated roots to shoots (Unkovich et al. 2008). The direct and most commonly-used technique (Amarger et al. 1979; Bergersen and Turner 1983; Method 1), particularly with grain legumes, involves growing the legume in pots in a glasshouse in either sterilized sand culture inoculated with the appropriate rhizobial strain or in solution culture, so that BNF is the sole source of N. A second method (Doughton et al. 1992; Method 2) involves paired treatments where symbiotic dependence is estimated by E (P atm(E)) and NA (P atm(NA)) methods in the glasshouse. The B-value is then estimated by Eq. 4 in the form proposed by Okito et al. (2004) by assuming that P atm(E) = P atm(NA). The derived value is then applied in field studies.
A third method involves the determination of an ‘apparent B-value’ equal to the lowest δ value of the legume measured across the experimental area (Eriksen and Høgh-Jensen 1998; Riffkin et al. 1999; Method 3). Another approach is to use a value or the mean of the range of values from the published literature for a given N2-fixing species (Unkovich et al. 2008; Method 4) or to assume B is equal to zero or another value (e.g., Høgh-Jensen and Schjoerring 1994; Jacot et al. 2000; Issah et al. 2014; Method 5).
In some publications (e.g., Bergersen and Turner 1983; Ledgard et al. 1985a; b), NA values for the reference plant were expressed as atom % 15N, the absolute 15N abundance, x(15N). These data were converted to relative δ (‰) values using the expression (Eq. 5) given by Chalk et al. (2015), after 15N natural abundance (0.3663 atom %) was subtracted from the sample 15N abundance to give 15N enrichment, x E(15N), as atom % excess 15N.
A meta-analysis of the published data was based on Pearson correlation coefficients for P atm(E) vs. P atm(NA), box-whisker plots for B-values and reference plants δ-values, and confidence intervals of means of differences in P atm(E) – P atm(NA) . Minitab 17 ® software was used to construct the Figs. and all statistical analysis.
Comparison of estimates of P atm using E and NA techniques
Published estimates of P atm using the two techniques for grain legumes, pasture and forage legumes and woody perennials are summarized in Tables 1, 2 and 3, respectively. The scale of studies ranged from glasshouse pot experiments, to field microplots (unconfined or confined by barriers, usually in the m2 scale) to landscape investigations (Tables 1, 2 and 3).
Negative estimates of symbiotic dependence
As discussed in the Introduction, fixing and reference plants superimposed on the non-uniform temporal and/or spatial distribution of 15N at artificial or natural abundance levels can have a profound effect on estimates of P atm, resulting in negative estimates or a marked reference plant dependency. Thus, while Doughton at al. (1995) found close agreement between NA and E estimates of symbiotic dependence in chickpea at P atm > 30 %, the E method gave impossible negative values when P atm was in the lower range, whereas the NA method provided realistic estimates over the whole range. Chalk (1985) similarly reviewed several studies where negative estimates of P atm were obtained with the E method.
The NA method was reported to yield negative estimates in white clover – perennial ryegrass swards under grazing (Hansen and Vinther 2001), where the variation of δ 15N in the grass varied from −7.0 to +5.7 ‰, most likely due to the random distribution of 15N-depleted urinary N voided on the pasture. Therefore, this result could be considered as atypical of estimates normally obtained by the NA technique in the absence of the confounding effect of grazing. However, negative estimates in some treatments were previously reported for the NA method (Amarger et al. 1979).
Spatial variability in 15N abundance
Spatial (horizontal and vertical) variability in 15N abundance should be negligible in pots of well-mixed soil, so any variation in estimates of P atm should be due to temporal non-uniformity in the distribution of 15N. In a pot experiment reported by Ledgard et al. (1985 b) estimates of P atm for sub clover using two reference plants, annual ryegrass and Phalaris, which were sampled at 16 and 32 days after sowing (DAS) were strongly reference plant dependent for both E and NA methods at each sampling time (Table 2). Therefore in the presumed absence of spatial variability, these pot experiment results suggest that both E and NA methods were affected by temporal 15N variability. Indeed, Feigin et al. (1974) demonstrated that the δ 15N values of NO3 − released from four Illinois soils increased during the first 35 days of incubation before becoming constant with time.
In experiments conducted in field plots, estimates of P atm for peanuts (Cadisch et al. 2000), sub clover and lucerne (Ledgard et al. 1985 a) were similarly reference plant dependent for both E and NA methods (Tables 1 and 2, respectively). Cadisch et al. (2000) found significant variation in the distribution of δ 15N of total N in E and NA plots between 0–10, 10–20 and 20–30 cm depth, with decreasing values for E and increasing values for NA (+5.7, +7.0 and +9.2 ‰, respectively). However, there was no significant depth difference in the δ 15N signature of mineral N released during incubation of NA soil samples for 21 days. Huss-Danell and Chaia (2005) similarly reported increasing δ 15N values for total N between 0–20 and 20–30 cm from +4 to +7 ‰.
In several studies, samples were taken during crop development and at maturity, or several cuts were taken from pastures during the growing season (Tables 1 and 2). The δ 15N value of both grain and pasture legumes and reference species exhibit a marked seasonal variation (Pate et al. 1994), but in this study the legumes always had lower δ 15N values than the reference plants. However, seasonal trends were not consistent among reference plants. Agreement between E and NA estimates tended to improve with plant age, in line with a concomitant increase in symbiotic dependence (e.g., Bergersen and Turner 1983; Evans et al. 1987; Peoples et al. 1996). When experiments were conducted in successive years (e.g., Cadisch et al. 2000; Carranca et al. 1999) agreement between E and NA techniques were inconsistent.
Lateral variability in 15N abundance
Lateral variability in δ 15N signatures has been studied at different scales ranging from the experimental site (Oberson et al. 2007) to landscapes (Bremer and van Kessel 1990; Androsoff et al. 1995; Stevenson et al. 1995) to regions (Pate et al. 1994). Oberson et al. (2007) found large variations in the δ 15N values of 16 reference weed species across the experimental treatments in their legume-based sward, which ranged from +2.6 ± 0.2 ‰ to +8.1 ± 3.3 ‰. On the other hand, Pate et al. (1994) found less variation at the regional scale encompassing a range of agricultural ecosystems across south-west Australia, with the δ 15Nvalue of a more restricted number of weed species varying from +2 to +5 ‰.
At the landscape scale, Bremer and van Kessel (1990) found that variability in δ 15N of reference plants was site and season dependent. Seasonal patterns among six reference plants were inconsistent, in agreement with the finding of Pate et al. (1994). The variability of δ 15N among reference plants differed between three sites with the greatest range at one site being from +2.8 to +9.3 ‰ over a distance of 67 m. At one site, the δ values of pea and flax were well separated across horizontal distance with the value for pea always less then flax, but at two other sites the δ values of pea and flax or lentil and wheat were not well separated and some crossover occurred, which would give erroneous negative estimates of P atm at some individual sampling points. However, Bremer and van Kessel (1990) found that mean E and NA estimates of P atm for field pea and lentil were not significantly different in 18 out of 21 comparisons despite the site and seasonal dependency of reference plant δ 15N.
Additional studies with field pea were conducted in the same rolling (undulating) landscape by Androsoff et al. (1995) and Stevenson et al. (1995). On a 90 × 100 m sampling grid with 10 m spacing, Stevenson et al. (1995) found poor agreement between E and NA estimates of P atm at flowering at both landform footslopes and shoulder positions, while at maturity agreement between the two methods was only close in the shoulder position (Table 1). Both Stevenson et al. (1995) and Androsoff et al. (1995) found no correlation between individual E and NA estimates of P atm across the landscape, and concluded that while symbiotic dependence of field pea was partly controlled by topography due to the divergent availability of water and mineral N, other unspecified factors operating at the plot scale (i.e., within 3 m) exerted a stronger influence. In an earlier microcosm experiment in the glasshouse, Brendel et al. (1997) also found that there was no correlation between individual estimates of P atm of red clover when E treatments were imposed at two levels of 15N abundance (atom fraction 15N of 0.005 and 0.05) and one 15N depleted level (−16.5 ‰).
Determination of the B-value
In all but one of the studies with grain legumes the B-value was determined by growing the legume in an N-free medium (Table 1). In contrast, in several of the studies with pasture and forage legumes (Table 2) and with woody perennials (Table 3), the B-value was not determined in the same way, but was either an apparent value, a value taken from the published literature or an assumed value. There is therefore a degree of uncertainty with regard to the efficacy of the B-values in such studies, in contrast to the B-values determined directly for the grain legumes.
Several studies have shown that B-values are dependent on the rhizobial strain used as the inoculum in the N-free medium. e.g., groundnut (Cadisch et al. 2000) and soybean (Pauferro et al. 2010). Since this finding is generally applicable to other legumes, it could pose problems, particularly for pasture legumes, where multiple strains could infect the host plant. This is perhaps a further reason for inconsistent agreement between E and NA techniques for pasture and forage legumes. In an attempt to circumvent this problem, Unkovich et al. (2008) recommend that a mixed or soil inocula should be used to determine the B-value if the infecting strains are unknown.
The B-value depends on the particular part of the plant that is sampled (Cadisch et al. 2000; Huss-Danell et al. 2007). B-values are usually determined on the shoot material which may or may not be grown to maturity. Therefore the estimated isotopic fractionation may not necessarily correspond to the actual fractionation that occurs if the legume is grown for a different period of time or if a different plant sample is collected. As can be seen in Tables 1, 2 and 3, there is considerable variation in these parameters among the published data.
The reference plant δ-value
According to Unkovich et al. (2008) the reference plant exerts a strong influence on estimates of P atm when δ 15N is <4 ‰, but does not have a large effect if δ 15N is >6 ‰. Since the relationship between δ 15Nlegume and P atm is linear for a given δ 15Nreference plant, the sensitivity of the final estimate will also be proportional to the δ 15Nreference plant (Unkovich and Pate 2000). The higher the δ 15N value of the reference plant, the more precise the estimate of P atm will be. Unkovich et al. (1994) suggested that, given the analytical precision of ± 0.2 ‰, a reference δ 15N of at least 2 ‰ (about 10 times the precision of measurement) would be required to detect a theoretical change in P atm of around 10 %. Therefore we can assume that estimates of P atm for grain legumes were more precise overall than those of the other categories, as no values of δ 15Nreference plant were < 2 ‰, whereas several δ 15Nreference plant values for the other groups were below this value, and in several cases the reference plant δ values were not given (Tables 2 and 3).
One problem when comparing E and NA techniques in the same experiment is the possibility of cross contamination if labelled treatments are randomized with unlabelled treatments. Brendel et al. (1997) recognized this possibility and separated the individual 15N treatments within different compartments of the same glasshouse. However, it appears that cross contamination may have been a factor in some experiments, as Ledgard et al. (1985 b) reported reference plant δ values of +22.5 to +23.8 in the NA treatment in a pot experiment involving subclover-annual ryegrass and subclover-Phalaris associations, values well outside the normal range expected in plants grown in unlabelled soil. Somado and Kuehne (2006) also found that δ 15N values of the tops and roots of the reference plant (rice) in the NA treatment pots randomized with the E treatment pots fell within the atypical range of +12.7 to +26.4 ‰ when estimating P atm for a green manure legume (Aeschynomene afraspera). Similarly, the reference plant δ values reported by Bergersen and Turner (1983) in the NA treatment also fell outside the expected range in a field study of a subclover-ryegrass sward on a lower footslope position that included confined E treatment microplots (Table 2). These results suggest that estimates of P atm using the NA technique should be treated with caution when reference plant δ values are atypical.
Statistical analysis
Correlations between estimates of P atm using E and NA techniques
Significant correlations were found when estimates of P atm were compared between E and NA methods for each category of N2-fixing species (grain and pasture/forage legumes) and woody perennials, and for all categories combined. The data used for these comparisons were taken from individual treatments within each publication rather than means or selected treatments as shown in Tables 1, 2 and 3. The correlations were higher for grain legumes (r = 0.72, p < 0.001, n = 54) and woody perennials (r = 0.76, p < 0.001, n = 18) than for pasture and forage legumes (r = 0.45, p < 0.004, n = 40). These significant correlations obtained from published data contrast with data obtained in some studies where the individual estimates of P atm obtained by E and NA methods were not correlated (e.g., Stevenson et al. 1995; Androsoff et al. 1995; Brendel et al. 1997).
Apart from the dependency of P atm on the reference plant per se for both methods due to the non-uniform temporal and spatial distribution of 15N as discussed previously, there are other possible reasons for inconsistencies between the two methodologies, which relate to the determination of the B-value and the reference plant δ-value for the NA technique.
Variability of B-values and reference plant δ-values
Box-plots show that the variability of reference plant δ-values was much larger compared to the variability of B-values in the published data (Fig. 1). For example, Ofori et al. (1987) found that the B-values of cowpea varied from +0.2 to +0.3 ‰, while the maize reference plant δ-values ranged from +5.0 to +7.5 ‰ in a field study and from +2.4 to +4.1 ‰ in a glasshouse experiment. Similarly, Oberson et al. (2007) showed the δ-values of weeds used as reference plants ranged from +3.2 ± 0.4 to +6.5 ± 1.5 ‰ in different treatments (cropping system and growth stage), while B-values of soybean varied from only −1.2 to −0.9 ‰ at flowering and maturity, respectively.
Data for pasture and forage legumes show higher variability of B-values and reference plant δ-values than grain legumes and woody perennials, including the outliers (Fig. 1). This high variability for δ-values may partly explain the weaker correlation of E and NA estimations for pasture/forage legumes than for grain legumes and woody perennials. Therefore, we believe that the impact of the reference plant δ-value on estimates of P atm by the NA technique may be greater than expected due to the large variability found for this parameter in the published data, although in theory the impact of the B-value should be higher than that of the reference plant δ-value when P atm is >60 % (Unkovich et al. 2008).
Consistency in the estimates of P atm
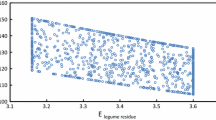
A scatter plot of P atm(NA) vs. P atm(E) is shown in Fig. 2. It appears that there is a tendency for P atm(NA) to give lower values than P atm(E) at high values of symbiotic dependence (i.e., when P atm(E) > 60 %), and higher values than P atm(E) when P atm(E) < 60 %), except for woody perennials that show P atm(E) > P atm(NA) in 15 out of 18 comparisons (Fig. 2).
Scatter plot of P atm (E) vs. P atm (NA). The dashed line represents the hypothetical boundary along which estimates are equal (i.e., where P atm (E) = P atm (NA)). The dispersion of points around the line shows the magnitude of the discrepancy between the estimates. GL grain legumes; PA/FL pasture and forage legumes; WP woody perennials
When the outliers were removed, the differences in P atm(E) – P atm(NA) were found to be within the range from −30.0 to +34.0 %, with 50 % of data from −7.0 to +10.3 %, and the median equal to 2.0 % (normal distribution, Anderson-Darling test, p = 0.134). Means and 95 % confidence intervals of the differences in P atm(E) − P atm(NA) for each group of species are shown in Fig. 3. The mean difference for the grain legumes was significantly less (t-test, p < 0.05) than for the other groups, but there was no significant difference between woody perennials and pasture/forage legumes. Therefore estimates of P atm(E) and P atm(NA) for grain legumes were more consistent than estimates obtained for the other groups (Fig. 3). For the standardized normal distribution of data for all species combined the probability that the methods gave similar estimates within an arbitrarily selected range of P atm(E) − P atm(NA) of −5 to +5 % was 0.29, while the corresponding probability within the range of −10 to +10 % was 0.54 (Table 4).
Conclusions
On the basis of our examination of the published literature we believe that the methods do not provide consistent estimates of the proportional dependence of N2-fixing species on biological N2 fixation over a range of scales and settings. The reasons for the generally poor agreement overall are complex and may include one or more of the following factors: (i) non-uniform temporal and spatial distribution of the 14N and 15N isotopes (ii) asynchrony of mineral N uptake by legume and reference plants (iii) error in the estimation of the B-value (iv) insufficient difference in δ values between the atmosphere and soil available N (v) cross contamination between E and NA treatments. The limited observations neither contradict nor support the often-stated hypothesis that NA should be influenced less by the non-uniform distribution of 15N compared with E. While it is possible to identify potential reasons for discrepancies in individual studies, in many cases it is not possible because essential data on B-values, reference plant δ values and the spatial/temporal distribution of the N isotopes were not provided.
The choice of which method to use will ultimately depend on practical considerations such as the cost of 15N-enriched fertilizer, the scale of the experiment, the analytical and instrumental facilities available, and the work required to determine the B-value. Pauferro et al. (2010) and Oberson et al. (2007) considered that NA is the most easily applied ‘on farm’ technique. Oberson et al. (2007) also commented that NA was better than E for the determination of the amount of N2 fixed due to restriction of root growth by the laterally-confined microplots often used for the E technique. A practical guide for the application of both techniques can be found in Unkovich et al. (2008).
The overall problem of the dependency of estimates of P atm on the reference plant for both E and NA methods can only be overcome by seeking a way to discard the reference plant altogether. Two approaches have been proposed to make the reference plant redundant. For the E method the temporal decline in the 15N enrichment of available N in the topsoil can be accommodated by fitting an exponential equation to the experimental data, which provides an integrated estimate of the 15N enrichment of the available N pool over the measurement period (Chalk et al. 1996). For the NA method Wanek and Arndt (2002) demonstrated experimentally that the difference (Δ15N) between the δ 15N values of the shoot and nodulated roots (i.e., Δ15N = δ 15Nshoot – δ 15Nnodulated root) was linearly and highly correlated with reference plant estimates of P atm of soybean in solution and soil (pot) culture. Furthermore, they showed similar significant relationships for published data for soybean at different growth stages under glasshouse or field conditions, for different cowpea cultivars in the field and for tagasaste in hydroponic culture. The authors claimed that this approach overcomes the problem with the NA method when the relative 15N abundance of soil mineral N is close to zero. Both of these reference-plant-free approaches represent conceptual advances in response to the reference plant dilemma, aptly described by Chalk and Ladha (1999) as the ‘Achilles heel’ of the 15N methodology. However, more field testing is required before the potential of these alternative methodologies can be confidently assessed.
References
Amarger N, Mariotti A, Mariotti F, Durr JC, Bourguignon C, Lagacherie B (1979) Estimate of symbiotically fixed nitrogen in field grown soybeans using variations in 15N natural abundance. Plant Soil 52:269–280
Androsoff GL, van Kessel C, Pennock DJ (1995) Landscape-scale estimates of dinitrogen fixation by Pisum sativum by nitrogen-15 natural abundance and enriched isotope dilution. Biol Fertil Soils 20:33–40
Bergersen FJ, Turner GL (1983) An evaluation of 15N methods for estimating nitrogen fixation in a subterranean clover-perennial ryegrass sward. Crop Past Sci 34:391–401
Boddey RM, Peoples MB, Palmer B, Dart PJ (2000) Use of the 15N natural abundance technique to quantify biological nitrogen fixation by woody perennials. Nutr Cycl Agroecosyst 57:235–270
Bouillet JP, Laclau JP, Gonçalves JLM, Moreira MZ, Trivelin PCO, Jourdan C, Silva EV, Piccolo MC, Tsai SM, Galiana A (2008) Mixed-species plantations of Acacia mangium and Eucalyptus grandis in Brazil. 2: nitrogen accumulation in the stands and biological N2 fixation. For Ecol Manag 255:3918–3930
Bremer E, van Kessel C (1990) Appraisal of the nitrogen-15 natural abundance method for quantifying dinitrogen fixation. Soil Sci Soc Am J 54:404–411
Brendel O, Wheeler C, Handley L (1997) A statistical comparison of the two-source δ15N and 15N isotope dilution methods for estimating plant N2-fixation using Trifolium pratense and Lolium perenne. Funct Plant Biol 24:631–636
Burchill W, James EK, Li D, Lanigan GJ, Williams M, Iannetta PPM, Humphreys J (2014) Comparisons of biological nitrogen fixation in association with white clover (Trifolium repens L.) under four fertiliser nitrogen inputs as measured using two 15N techniques. Plant Soil 385:287–302
Cadisch G, Hairiah K, Giller KE (2000) Applicability of the natural 15N abundance technique to measure N2 fixation in Arachis hypogaea grown on an Ultisol. NJAS - Wageningen J Life Sci 48:31–45
Carranca C, de Varennes A, Rolston DE (1999) Biological nitrogen fixation estimated by 15N dilution, natural 15N abundance, and N difference techniques in a subterranean clover-grass sward under Mediterranean conditions. Eur J Agron 10:81–89
Chalk PM (1985) Estimation of N2 fixation by isotope dilution: an appraisal of techniques involving 15N enrichment and their application. Soil Biol Biochem 17:389–410
Chalk PM (1996) Estimation of N2 fixation by 15N isotope dilution – The A-value approach. Soil Biol Biochem 28:1123–1130
Chalk PM, Ladha JK (1999) Estimation of legume symbiotic dependence: an evaluation of techniques based on 15N dilution. Soil Biol Biochem 31:1901–1917
Chalk PM, Smith CJ, Hopmans P, Hamilton SD (1996) A yield-independent, 15N-isotope dilution method to estimate legume symbiotic dependence without a non-fixing reference plant. Biol Fertil Soils 23:196–199
Chalk PM, Inácio CT, Craswell ET, Chen D (2015) On the usage of absolute (x) and relative (δ) values of 15N abundance. Soil Biol Biochem 85:51–53
Domenach AM, Kurdali F, Bardin R (1989) Estimation of symbiotic dinitrogen fixation in alder forest by the method based on natural 15N abundance. Plant Soil 118:51–59
Doughton JA, Vallis I, Saffigna PG (1992) An indirect method for estimating 15N isotope fractionation during nitrogen fixation by a legume under field conditions. Plant Soil 144:23–29
Doughton JA, Saffigna PG, Vallis I, Mayer RJ (1995) Nitrogen fixation in chickpea. II. Comparison of 15N enrichment and 15N natural abundance methods for estimating nitrogen fixation. Crop Past Sci 46:225–236
Eriksen J, Høgh-Jensen H (1998) Variations in the natural abundance of 15N in ryegrass/white clover shoot material as influenced by cattle grazing. Plant Soil 205:67–76
Evans J, O’Connor GE, Turner GL, Bergersen FJ (1987) Influence of mineral nitrogen on nitrogen fixation by lupin (Lupinus angustifolius) as assessed by 15N isotope dilution methods. Field Crop Res 17:109–120
Feigin A, Kohl DH, Shearer G, Commoner B (1974) Variation in the natural nitrogen-15 abundance in nitrate mineralized during incubation of several Illinois soils. Soil Sci Soc Am J 38:90–95
Hairiah K, Van Noordwijk M, Cadisch G (2000) Quantification of biological N2 fixation by hedgerow trees in Northern Lampung. NJAS – Wageningen J Life Sci 48:47–59
Hansen JP, Vinther FP (2001) Spatial variability of symbiotic N2 fixation in grass-white clover pastures estimated by the 15N isotope dilution method and the natural 15N abundance method. Plant Soil 230:257–266
Høgh-Jensen H, Schjoerring JK (1994) Measurement of biological dinitrogen fixation in grassland: comparison of the enriched 15N dilution and the natural 15N abundance methods at different nitrogen application rates and defoliation frequencies. Plant Soil 166:153–163
Hossain SA, Waring SA, Strong WM, Dalal RC, Weston EJ (1995) Estimates of nitrogen fixation by legumes in alternate cropping systems at Warra, Queensland, using enriched-15N dilution and natural 15N abundance techniques. Crop Past Sci 46:493–505
Huss-Danell K, Chaia E (2005) Use of different plant parts to study N2 fixation with 15N techniques in field-grown red clover (Trifolium pratense). Physiol Plant 125:21–31
Huss-Danell K, Chaia E, Carlsson G (2007) N2 fixation and nitrogen allocation to above and below ground plant parts in red clover-grasslands. Plant Soil 299:215–226
Issah G, Kimaro AA, Kort J, Knight JD (2014) Quantifying biological nitrogen of agroforestry shrub species using 15N dilution techniques under greenhouse conditions. Agrofor Syst 88:607–617
Jacot KA, Lüscher A, Nösberger J, Hartwig UA (2000) Symbiotic N2 fixation of various legume species along an altitudinal gradient in the Swiss Alps. Soil Biol Biochem 32:1043–1052
Kohl DH, Shearer G, Harper JE (1980) Estimates of N2-fixation based on differences in the natural abundance of I5N in nodulating and non-nodulating isolines of soybeans. Plant Physiol 66:61–65
Kurdali F, Domenach AM, Bardin R (1990) Alder-poplar associations: determination of plant nitrogen sources by isotope techniques. Biol Fertil Soils 9:321–329
Ledgard SF, Steele KW (1992) Biological nitrogen fixation in mixed legume/grass pastures. Plant Soil 141:137–153
Ledgard SF, Simpson JR, Freney JR, Bergersen FJ (1985a) a) Field evaluation of 15N techniques for estimating nitrogen fixation in legume-grass associations. Crop Past Sci 36:247–258
Ledgard SF, Simpson JR, Freney JR, Bergersen FJ (1985b) b) Effect of reference plant on estimation of nitrogen fixation by subterranean clover using 15N methods. Crop Past Sci 36:663–676
McAuliffe C, Chamblee DS, Uribe-Arango H, Woodhouse WW Jr (1958) Influence of inorganic nitrogen on nitrogen fixation by legumes as revealed by N15. Agron J 50:334–337
Oberson A, Nanzer S, Bosshard C, Dubois D, Mäder P, Frossard E (2007) Symbiotic N2 fixation by soybean in organic and conventional cropping systems estimated by 15N dilution and 15N natural abundance. Plant Soil 290:69–83
Ofori F, Pate JS, Stern WR (1987) Evaluation of N2-fixation and nitrogen economy of a maize/cowpea intercrop system using15N dilution methods. Plant Soil 102:149–160
Okito A, Alves BRJ, Urquiaga S, Boddey RM (2004) Isotopic fractionation during N2 fixation by four tropical legumes. Soil Biol Biochem 36:1179–1190
Pate JS, Unkovich MJ, Armstrong EL, Stanford P (1994) Selection of reference plants for 15N natural abundance assessment of N2 fixation by crop and pasture legumes in south-west Australia. Crop Past Sci 45:133–147
Pauferro N, Guimarães AP, Jantalia CP, Urquiaga S, Alves BJR, Boddey RM (2010) 15N natural abundance of biologically fixed N2 in soybean is controlled more by the Bradyrhizobium strain than by the variety of the host plant. Soil Biol Biochem 42:1694–1700
Peoples MB, Palmer B, Lilley DM, Duc LM, Herridge DF (1996) Application of 15N and xylem ureide methods for assessing N2 fixation of three shrub legumes periodically pruned for forage. Plant Soil 182:125–137
Riffkin PA, Quigley PE, Kearney GA, Cameron FJ, Gault RR, Peoples MB, Theis JE (1999) Factors associated with biological nitrogen fixation in dairy pastures in south-western Victoria. Crop Past Sci 50:261–272
Shearer G, Kohl DH (1986) N2 fixation in field settings: estimates based on natural 15N abundance. Funct Plant Biol 13:699–756
Somado EA, Kuehne RF (2006) Appraisal of the 15N-isotope dilution and 15N natural abundance methods for quantifying nitrogen fixation by flood-tolerant green manure legumes. Afr J Biotechnol 5:1210–1214
Stevenson FC, van Kessel C, Knight JD (1995) Dinitrogen fixation in pea: controls at the landscape- and micro-scale. Soil Sci Soc Am J 59:1603–1611
Tobita S, Ito O, Matsunaga R, Rao TP, Rego TJ, Johansen C, Yoneyama T (1994) Field evaluation of nitrogen fixation and use of nitrogen fertilizer by sorghum/pigeonpea intercropping on an Alfisol in the Indian semi-arid tropics. Biol Fertil Soils 17:241–248
Unkovich MJ, Pate JS (2000) An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crop Res 211:211–228
Unkovich MJ, Pate JS, Sanford P, Armstrong EL (1994) Potential precision of the δ15N natural abundance method in field estimates of nitrogen fixation by crop and pasture legumes in S.W. Australia. Crop Past Sci 45:119–132
Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey R., Giller K, Alves B, Chalk, P (2008) Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems. Australian Centre for International Agricultural Research, Canberra. 258 pp. http://aciar.gov.au/files/node/10169/mn136_measuring_plant_associated_nitrogen_fixation_19979.pdf
Wanek W, Arndt SK (2002) Difference in δ15N signatures between nodulated roots and shoots of soybean is indicative of the contribution of symbiotic N2 fixation to plant N. J Exp Bot 371:1109–1118
Acknowledgments
The senior author thanks the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for a visiting scientist fellowship (Pesquisador Visitante No. 101.466/2014) and EMBRAPA-Solos as the host Institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Euan K. James.
Rights and permissions
About this article
Cite this article
Chalk, P.M., Inácio, C.T., Balieiro, F.C. et al. Do techniques based on 15N enrichment and 15N natural abundance give consistent estimates of the symbiotic dependence of N2-fixing plants?. Plant Soil 399, 415–426 (2016). https://doi.org/10.1007/s11104-015-2689-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2689-9