Abstract
Background and Aims
Atmospheric nitrogen (N) deposition has elevated rapidly in tropical regions where N2-fixing tree species are widespread. However, the effect of N deposition on litter decomposition in forests with N2-fixing tree species remains unclear. We examined the effect of N addition on litter decomposition and nutrient release in two tropical plantations with Acacia auriculiformis (AA, N2-fixing) and Eucalyptus urophylla (EU, non-N2-fixing) in South China.
Methods
Three levels of N additions were conducted: control, medium-N (50 kg N ha−1 yr.−1) and high-N (100 kg N ha−1 yr.−1) in each plantation.
Results
Initial decomposition rate (k a ) for the control plots was faster in the AA plantation than in the EU plantation, but later in decomposition, larger fraction of slowly decomposing litter (A) remained in the former. N addition increased the slow fraction (A), decreasing soil microbial biomass and reducing acid-unhydrolyzable residue (AUR) degradation in the AA plantation. In the EU plantation, however, N additions significantly increased initial decomposition rate (k a ) and soil N availability. Furthermore, N addition decreased litter carbon and N release (in the AA plantation), while litter phosphorus release also decreased in both plantations.
Conclusions
With ongoing N deposition in future, tropical plantations with N2-fixing tree species would potentially increase carbon accumulation and nutrient retention in forest floor by slowing litter decomposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litter decomposition is a fundamental ecosystem process that regulates nutrient cycling, humus formation and ecosystem carbon (C) storage (Wardle 2002; Osono and Takeda 2004; Berg and McClaugherty 2008). Several factors such as climate, soil properties, litter chemistry, and soil decomposer activity control litter decomposition rate and nutrient release (Trofymow and Moore 2002; Berg et al. 2010). Nitrogen (N), including endogenous (litter) and exogenous (soil) N availability, plays an important role in litter decomposition (Knorr et al. 2005). The initial C:N ratio is higher in the litter (71:1 on average) than that in the microbes (7:1 on average) (Yuan and Chen 2009; Xu et al. 2013); therefore, microbes need to immobilize N from litter and soil to meet the requirements of growth and maintenance in the early stage of litter decomposition (Moore et al. 2006; Parton et al. 2007). In this stage, N may have a positive effect on litter decomposition (Hobbie 2005; Cornwell et al. 2008; Norris et al. 2013). However, during the later stages, when the rate of litter decomposition is dominated by the degradation of lignin and modified lignin-like humification products, N may have a negative influence on lignin degradation, leading to the production of substantial amounts of residue (Berg and McClaugherty 2008).
Global N deposition is expected to reach 200 Tg N yr.−1 in 2050, with the greatest N deposition occurring in tropical regions (Galloway et al. 2004, 2008). In China, the rate of N deposition has reached 30–73 kg N ha−1 yr.−1 in some tropical and subtropical forests (Fang et al. 2011). As a major source for exogenous N for forest ecosystems, N deposition was recently recognized as an important factor that controls litter decomposition. For example, N deposition can affect litter decomposition directly by raising soil N availability and changing the quantity and quality of litter inputs, or indirectly by altering plant or microbial community composition (Manning et al. 2008). The effects of N deposition on litter decomposition and nutrient release in forests depend on the forest nutrient status (N-rich vs. N-poor) and the stages of decomposition (early stage vs. later stages). Previous studies have indicated that N deposition accelerates the early stage of litter decomposition in the N-poor forests by increasing soil N availability or by decreasing litter C/N ratio (Hobbie 2000; Hobbie 2005; Norris et al. 2013). However, N deposition often suppresses litter decomposition in N-rich forests. In these forests, exogenous N may (1) inhibit the synthesis of oxidative enzymes involved in the degradation of acid-unhydrolyzable residue (AUR; formerly referred to as lignin) in the later stages of decomposition (Carreiro et al. 2000); (2) react with polyphenol compounds or products of microbial degradation to form more recalcitrant materials (Hobbie 2008); and (3) alter the composition and activity of decomposers to influence their degradability (e.g., shifting from a fungi-dominated to a bacteria-dominated community) (Frey et al. 2004; Cusack et al. 2011). In addition, some other studies have shown that N additions have no significant effect on litter decomposition rate in the phosphorus (P) limited forests, but significantly change nutrient dynamics by stimulating abiotic and/or microbial immobilization (Hobbie and Vitousek 2000; Kaspari et al. 2008; Mo et al. 2008).
Tropical regions have experienced a dramatic conversion from primary forests to secondary forests and plantations (FAOUN 2010). In China, the total area of forests is 208 million ha, approximately 59 % of which are natural forests and 33 % are plantations (available data from the eighth national forest resources inventory survey of China: http://english.forestry.gov.cn/index.php/information-services/forest-resources/2- forest-resources-in-China). The percentage of forest cover in the Pearl River Delta, Guangdong Province increased from 26 % in 1979 to 56 % in 2005, due to the ecological restoration of degraded land (Peng et al. 2009). Owing to symbiotic N2-fixation, N2-fixing tree species generally produce litter with high N concentration, and improve soil C storage and N availability (Forrester et al. 2005; Gei and Powers 2013). Therefore, N2-fixing tree species have been widely used as pioneer plants to facilitate ecological restoration in eroded and degraded ecosystems (Ren et al. 2008). Based on previous studies (Mo et al. 2006; Hobbie 2008; Hobbie and Vitousek 2000), we concluded that N deposition might have a negative or no effect on litter decomposition in N-rich sites. However, these previous studies did not reveal any direct evidence in terms of the effects of N deposition on N2-fixing species that play an important role in ecological restoration worldwide. Assessing the potential effect of elevated N deposition on litter decomposition and nutrient release is critical for predicating C accumulation and nutrient retention in tropical plantations, especially those with N2-fixing tree species.
In this study, we conducted a simulated N deposition experiment to explore the effects of N additions on litter decomposition rate, nutrient release, and soil microbial biomass in two tropical plantations with Acacia auriculiformis (AA, N2-fixing tree species) and Eucalyptus urophylla (EU, non-N2-fixing tree species) in South China, where recent estimates of atmospheric N deposition range from 16.2 to 38.2 kg N ha−1 yr.−1 (Fang et al. 2011). We hypothesized that: (1) in the initial stage of decomposition, AA litter would decompose faster but leave a larger slowly decomposing fraction in the later stages than EU litter due to high litter N concentration and soil N availability in the AA plantation; (2) external N addition would decrease initial decomposition rate for AA litter because of the negative N effect on soil microbial biomass. However, N addition would increase initial decomposition rate for EU litter due to the improvement of N availability. In the later stages of decomposition, N addition would have negative effects on decomposition for both litter types, because of the slowdown in AUR degradation; (3) N addition would delay litter N and P release because of chemical immobilization and microbial assimilation in both plantations.
Materials and methods
Site description
The study was conducted at the Heshan National Field Research Station of Forest Ecosystems (112°50ʹE, 22°34ʹN), which is located in the middle of Guangdong Province in South China. The region has a tropical monsoon climate with a distinct wet and dry season. Mean annual precipitation is 1543 mm, with the wet season extending from April to September. Mean annual temperature is 22.5 °C, with an average temperature in the coldest (January) and hottest (July) months of 10.9 and 28.0 °C, respectively (Wu et al. 2011). Ambient atmospheric N deposition in precipitation was about 43.1 ± 3.9 kg N ha−1 yr.−1 in 2011 with 1:1 ratio for NH4 + to NO3 −, which is almost five-fold higher than that in 1995 (8.31 kg N ha−1 yr.−1; Huang et al. 2014). The mean annual increase in the N deposition rate is nearly 2.17 kg N ha−1 yr.−1. We established two research sites, a N2-fixing plantation and a non-N2-fixing plantations, which were located 500 m apart. These plantations were both established on a degraded grassland site in 1984, and each had an area of approximately 5–8 ha. A survey conducted in July 2010 (before the first N addition) showed that the dominant tree species in the N2-fixing plantation was Acacia auriculiformis (AA), and Eucalyptus urophylla (EU) dominated the non-N2-fixing plantation. The soils in both plantations are classified as acrisols (FAOUN 2006). The soil in this area has eroded seriously due to long-term disturbances. The mean annual litter production was 694 ± 25 g air-dried mass m−2 yr.−1 and 590 ± 14 g air-dried mass m−2 yr.−1 in the AA plantation and the EU plantation, respectively (Zhang et al. 2012). Leaf litter production accounted for 75–90 % of the total litter production in the two plantations (measured in 2011, Unpublished data).
Experimental treatments
Nitrogen addition treatments were initiated within these two plantations in July 2010. Three treatments (three replicate plots per each treatment) were established: control (no fertilizer), medium N (MN: 50 kg N ha−1 yr.−1), high N (HN: 100 kg N ha−1 yr.−1). The MN treatment and HN treatment received a total of 100 kg N ha−1yr−1 and 150 kg N ha−1 yr.−1, respectively, via background N deposition plus N addition. The levels of N addition were reasonable to simulate the average projected increase in N deposition rate in the studied region for the years 2030 and 2060. The levels of N addition were comparable to other studies in the tropical zone (Mo et al. 2006; Hall et al. 2003). Eighteen plots with dimensions (10 m × 10 m) were established in these two plantations (nine plots per plantation). All plots and treatments were laid out randomly. Each plot was surrounded by a 10 m wide buffer strip. Ammonium nitrate (NH4NO3) solution has been sprayed every 30 days onto the forest floor as 12 equal doses over the whole year since August 2010. In each N addition event, fertilizers were weighed, dissolved in 10 L water for each plot, and sprayed evenly using a backhand sprayer. The control plots received 10 L water without N added.
Litter decomposition and soil sampling
We collected leaf litter in litter traps located within the control plots during August 2010. All the leaves were air-dried and stored at room temperature. The leaves of an individual species were mixed to obtain a uniform mixture before being placed in the mesh bags. Six subsamples of each species (about 10 g per subsample) were dried at 105 °C to obtain the initial oven-dry weight (conversion factor from air-dry to 105 °C; Mo et al. 2008). Other subsamples for each tree species were dried at 65 °C to a constant weight (Hobbie 2005), and then divided into two parts: one part was dried at 105 °C to obtain the conversion factor (conversion rate from 65 to 105 °C); the other part was analyzed for initial nutrient concentrations and AUR concentration (all results are reported on a 105 °C oven-dried weight basis). A total of 324 litterbags (162 bags for AA leaves, 162 bags for EU leaves) were prepared at the beginning of the study in November 2011. Approximately 10 g air-dried leaf litter was placed in a polyvinyl screen mesh bag (20 cm × 20 cm, mesh size was 0.5 mm at the bottom and 2 mm at the top). Only one litter type was filled in each bag. At the end of November 2011, these litterbags were evenly distributed across each plot (18 bags for each plot) in the appropriate plantation. Three litterbags were retrieved from each plot (nine bags for each treatment) after 3, 6, 9, 12, 15 and 18 months. After collection, we returned the bags to the laboratory for separation and analysis.
Mineral soils (0–10 cm depth) were collected from all plots in December 2011 and July 2012. In each plot, three soil cores (3.5 cm inner diameter) were collected randomly and composited into one sample (a total of 18 samples). The soil samples were delivered to the laboratory as soon as possible for the determination of soil chemistry and microbial biomass carbon, nitrogen, and phosphorus.
Laboratory procedures
In the laboratory, litter was cleaned from any ingrown roots and other material, and then oven-dried in paper bags at 65 °C to a constant weight (Hobbie 2005). Each bag was individually weighed. Litters from the same plot in each plantation were mixed thoroughly and combined into one sample. Subsamples of the 65 °C-dried litter were grounded to pass through a 0.15-mm mesh sieve, and then analyzed for litter nutrient concentrations. The other subsamples for AUR concentration analysis were ground and passed through a 0.5-mm mesh sieve.
Litter carbon concentration was measured by potassium dichromate oxidation titration with Fe2+ solution (Liu et al. 1996). Litter N and P concentration was determined using Kjeldahl digestion followed by the indophenol blue method and the Mo-Sb colorimetric method, respectively, on a UV-8000 spectrophotometer (Metash Instruments Corp., Shanghai, China) (Liu et al. 1996; Hobbie and Vitousek 2000). For the initial potassium (K), calcium (Ca), magnesium (Mg) and manganese (Mn) concentration, subsamples were determined by atomic absorption spectroscopy (AAS, contrAA 700, Analytik Jena, German), after digestion with a sulfuric acid-perchloric acid (H2SO4–HClO4) solution (Liu et al. 1996). The AUR concentration in the litter samples was estimated by gravimetric analysis after using a hot sulfuric acid digestion (King and Heath 1967) and following the methods used by Hagiwara et al. (2012). AUR integrates the least soluble and most hydrolysis-resistant organic structures, including true lignin, cutin, waxes, and condensed tannins in varying proportions (Preston et al. 2009).
Soil from each treatment was sieved (2 mm) and mixed thoroughly by hand. Soil pH was determined in a 1:2.5 ratio of soil to water suspension using a pH meter (Meltler-Toledo Instruments Co., Ltd., Shanghai, China). One 20 g subsample from each composite sample was shaken for 1 h in 100 ml 2 M KCl solution, filtered through Whatman no.1 filters, and then frozen immediately for later analysis. Soil NH4 + concentration was determined by the indophenol blue colorimetric analysis, and NO3 − concentration was determined after cadmium reduction to NO2 −, followed by sulfanilamide-NAD reaction (Liu et al. 1996). Soil available N was the sum of NH4 + and NO3 −. Soil organic carbon (SOC), soil total nitrogen (TN) and soil total phosphorus (TP) was determined by the same methods used for the litter C, N and P concentration. Soil available P was determined by colorimetric analysis after extraction with 50 mL Bray-1 (0.03 M NH4F-0.025 M HCl) extractants (Anderson and Ingram 1989). Microbial biomass C, N and P were estimated after chloroform fumigation. Briefly, fresh samples of fumigated and no-fumigated soil were shaken with 0.5 M K2SO4 for 1 h (soil to solution ratio was 1:5; Brookes et al. 1985; Vance et al. 1987). Organic C and TN were determined simultaneously on a TOC-VCHN analyzer (Shimadzu Corp., Japan) after a ten-fold dilution of the extracts. Microbial biomass P was extracted in a 1:4 soil to solution ratio with Bray-1 extractants based on a method described by Oberson et al. (1997) as modified in Wu et al. (2000). TP was determined spectrophotometrically after digestion with 0.2 mL H2O2 (30 %, v/v) and 0.5 mL HClO4 (70 %, v/v). Microbial biomass C, N and P were calculated from the difference between the fumigated and non-fumigated samples using the conversion factors k C = k N = 0.45 and kP = 0.40 (Jenkinson et al. 2004). The C:N, N:P and C:P ratios in soil microbial biomass were expressed on a molar basis. Subsamples of litter and soils were dried at 105 °C to a constant weight (at least 24 h), and all results are reported on a 105 °C dry weight basis (Mo et al. 2006).
Data calculations and analyses
We fitted the fraction of initial litter mass remaining (and litter carbon remaining) against time to two exponential decay models (Olson 1963; Weider and Lang 1982): a single-exponential decay model, \( X={{\mathrm{e}}^{-}}^{k_st} \), and an asymptote function, \( X=A+\left(1-A\right){{\mathrm{e}}^{-}}^{k_at} \), where X is the fraction of mass remaining at time t (year), “e” is the base of natural logarithm. In the single-exponential model, k s is the decomposition constant (year −1) over the whole decomposition period. In the asymptotic model, A is the fraction of the initial mass with a decomposition rate of zero (i.e., the asymptote), and k a is the initial decomposition rate (year−1). Berg and McClaugherty (2008) and Hobbie et al. (2012) suggested that the decomposition rate would never actually equal zero, so A can be regarded to the “slow fraction”, (1 - A) is equal to the “limit value” and represents the maximum accumulated mass loss before decomposition gradually slows to zero. These two decomposition models were fitted to the data using least squares regression of the natural logarithm of fractional mass remaining or carbon remaining (Jensen and Nybroe 1999). Litter C, N and P contents were determined by multiplying the nutrient concentration by the mass and were expressed as the fraction of the original nutrient content remaining (Hobbie and Vitousek 2000).
One-way analysis of variance (ANOVA) with Tukey’s HSD test was used to test the difference in soil chemical characteristics, microbial biomass and its nutrient ratios, decomposition parameters (k s , k a , and A), litter mass and nutrient (C, N and P) remaining, and AUR remaining among N treatments. Repeated measure ANOVA with the MIXED model was performed to examine the effect of N additions on litter mass and nutrient remaining over time in both plantations. The t-test was performed to test the difference in soil chemistries, initial litter quality and decomposition parameters between the two plantations. Linear regressions were performed using the GLM model in SAS. All data were tested to fulfill the assumptions of normality and homogeneity of variance, and transformations were carried out when necessary. All analyses were conducted using SAS 8.0 for Windows (SAS Institute Inc., Cary NC, USA). Graphic illustrations were generated using Origin 8.0 software (Origin Lab Corporation, Northampton, MA, USA). Statistically significant differences were identified when P-values <0.05, unless otherwise stated. Mean values ±1 standard error are reported in the text.
Results
Soil characteristics and initial litter quality
The control plots in the AA plantation had higher soil available N, SOC and TN, but a lower pH value than in the EU plantation in mineral soil layer (0–10 cm) (all P < 0.05; Table 1). In the AA plantation, HN addition significantly increased soil TN concentration (P = 0.001) and soil available N concentration (P = 0.003), but decreased soil C:N ratio (P = 0.0001). However, there was no significant difference between the control and MN addition plots. There was also no significant difference in the concentrations of SOC, soil available P, and soil pH among treatments in the AA plantation (all P > 0.05; Table 1). In the EU plantation, soil available N concentration was significantly increased by N additions (P = 0.0016; Table 1). However, N addition had no significant effect on SOC, TN, the C:N ratio and pH value in the 0–10 cm mineral soil layer in the EU plantation (all P > 0.05; Table 1).
The AA leaf litter had significantly higher litter C, N, and AUR concentrations, as well as higher C:P, N:P (all P < 0.001), and AUR:N (P = 0.014) ratios, but lower P, K, Ca, Mg, and Mn concentration (all P < 0.05), and C:N ratios (P = 0.0030) than the EU leaf litter (Table 2).
Decomposition rates and patterns
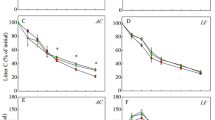
All data of litter mass remaining gave reasonably good fits to both two functions (the adjusted-R2 values ranged from 0.81 to 0.95 for the single-exponential model, and from 0.80 to 0.97 for the asymptotic model). The patterns of litter mass loss among treatments were characterized by a fast initial decomposition rate (k a ; ranged from 1.50 to 2.21 y−1), followed by a subsequent slow rate because a large “slow fraction” (A; ranged from 0.3 to 0.59) remained in the later stages of decomposition for both litter types (Fig. 1).
Mean decomposition parameters obtained from fitting the single-exponential and asymptotic models to data of litter mass remaining (a, b, and c) and carbon remaining (d, e, and f) to show differences between two plantations, and among treatments in the AA plantation and EU plantation. Values are means ±1SE in parentheses. Decomposition parameters are indicated as: k s , single-exponential model decomposition rate; k a , asymptotic model decomposition rate; A, asymptotes (the fraction of initial litter with the decomposition rate of zero). Different capital letter indicated significant difference in control plots between two plantations (P < 0.05). Different low letter indicated significant difference among treatments in each plantation (P < 0.05). CK: control (without N addition), MN: medium-N addition (50 kg N ha yr.−1), HN: high-N addition (100 kg N ha yr.−1)
Patterns of leaf litter decomposition in the control plots reflected the natural decomposition process without N addition. Compared to the EU litter, the AA litter had higher initial decomposition rate (k a: 1.94 ± 0.04 y−1 and 1.50 ± 0.22 y−1 for the AA and EU plantation, respectively; P = 0.02), and a larger “slow fraction” (A: 0.50 ± 0.02 and 0.30 ± 0.06 for the AA and EU plantations, respectively; P = 0.039; Fig. 1a). The pattern of k s (which is a constant rate over the whole decomposition period) did not reflect the pattern of k a , because a large slow fraction remained in both litter types.
In the AA plantation, N addition had no significant effect on the initial decomposition rate (k a , y−1), while HN addition significantly increased the value of A (the asymptote, the fraction of litter whose decomposition rate approached zero) from 0.50 ± 0.02 in the control plots to 0.59 ± 0.02 in the HN-addition plots (P = 0.019; Fig. 1b). Therefore, it is reasonable that the result showed that the values of k s decreased following increasing levels of N addition, and the difference between the HN-addition plots and the control plots was significant (P = 0.004; Fig. 1b). Repeated measures ANOVA with Tukey’s HSD test also indicated that litter mass loss was significantly slower in the HN plots than in the control plots on the third, fourth, and fifth sampling dates (P = 0.0001; Fig. 2a).
Changes of litter mass loss and nutrient release (represented as fraction of initial remaining) in decomposing litter for two dominant tree species in various N treatments in the AA plantation (a, c, e, and g) and the EU plantation (b, d, e, and f). Values are means; Bars are ±1SE. Fraction of original nutrient remaining >1 means net nutrient accumulation; fraction of original nutrient remaining <1 means net nutrient release. Asterisk (*) indicates significant difference at least between two treatments at P < 0.05 (n = 3)
In the EU plantation, N addition had a positive effect on the initial decomposition rate (k a , y−1; P = 0.0034), which increased from 2.24 ± 0.11 (the control plots) to 3.7 ± 0.17 (the MN-addition plots) and 2.9 ± 0.09 (the HN-addition plots), while the slow fraction (A) showed a decreasing trend with increasing levels of N addition (P = 0.035; Fig. 1c). The pattern of k s was inconsistent with those for k a, showing a reduction following the increasing of A (Fig. 1c). Furthermore, repeated measures ANOVA with Tukey’s HSD test showed that the effect of N addition on litter mass loss was not significant over the entire experimental period (P = 0.083; Fig. 2b).
Litter C, N and P content in decomposing litter
All data of litter C remaining gave reasonably good fits to both two functions (the adjusted-R2 values ranged from 0.78 to 0.99 for the single-exponential model, and from 0.88 to 0.99 for the asymptotic model). Patterns of k s , k a , and A (here A means the fraction of initial carbon that decomposed very slowly) for carbon decomposition for the control plots in these two plantations were similar to those for litter mass decomposition, but the differences were not significant (Fig. 1d). The effect of N addition on litter C decomposition was consistent with that for litter mass decomposition in both plantations. In the AA plantation, HN-addition significantly increased A (P = 0.005), and decreased k s (P = 0.021; Fig. 1e). Repeated measures ANOVA showed that the effect of N addition on litter C remaining was significant (P = 0.023). After 18-months of decomposition, the fraction of initial C remaining in MN and HN addition plots was 17 and 22 % higher than that in the control plots, respectively (Fig. 2c). In the EU plantation, N addition (both MN and HN addition) significantly increased k a (P = 0.004), but had no significant effect on A (Fig. 1f).
N additions had a positive effect on litter N content in the AA plantation over the whole study period (P < 0.001; Fig. 2e), but there was no significant effect in the EU plantation (Fig. 2f). For the AA plantation, net N accumulation occurred in the MN-addition and HN-addition plots on the first sampling date, following a net N release over the next nine months. After 12-months of decomposition, the fraction of initial N remaining in the MN and HN plots were 25 and 23 % higher than that in the control plots, respectively.
The suppressing effect of N additions on litter P release was more pronounced in the AA plantation than in the EU plantation (P = 0.0001 for the AA plantation and P = 0.003 for the EU plantation; Fig. 2g, h). In the AA plantation, the fractions of initial P remaining in the MN and HN plots were 32 and 39 % higher than in the control plots, respectively, after 12-months of decomposition. However, in the EU plantation, the fractions of initial P content in the MN and HN plots were 19 and 22 % higher than in the control plots, respectively, on the fourth sampling date.
Soil microbial biomass and nutrient ratios
Microbial biomass C in the control plots was no significantly different between the two plantations. N addition significantly decreased microbial biomass C in the AA plantation (P = 0.031; Fig. 3a). The mean microbial biomass C concentration was 334 ± 16 mg C kg−1 in the control plots, 301 ± 18 mg C kg−1 in the MN plots, and 253 ± 13 mg C kg−1 in the HN plots, which represented a decrease of 10 and 24 %, respectively (Fig. 3a). In the EU plantation, microbial biomass C also decreased with N addition, but the differences were not statistically significant (Fig. 3a).
Soil microbial biomass carbon, nitrogen, phosphorus, and their stoichiometric ratios in CK and N fertilized plots in the AA and EU plantations. Soil samples (0-10 cm) were collected in July 2012. Different letters indicated a significant difference (P < 0.05) between treatments in each plantation (n = 3)
Microbial biomass N was not affected by N addition in either plantation (Fig. 3b), while N addition significantly increased microbial biomass P in the AA plantation (P = 0.0001; Fig. 3c). The mean microbial biomass P concentration was 14.5 ± 0.6 mg P kg−1 in the control plots, 20.0 ± 0.3 mg P kg−1 in the MN plots and 21.6 ± 0.6 mg P kg−1 in the HN plots, which represented an increase of 38 and 49 %, respectively. However, no significant differences were found in the EU plantation.
Nitrogen addition significantly decreased the microbial biomass C:N ratio in the AA plantation (P = 0.0097; Fig. 3d). The mean microbial biomass C:N ratio was 6.1 ± 0.2 in the control plots, 5.0 ± 0.2 in the MN-addition plots, and 4.8 ± 0.1 in the HN-addition plots. The microbial biomass C:P ratio significantly decreased with N addition in the AA plantation from 63 ± 4 in the control plots to 31 ± 1 in the HN-addition plots (P = 0.0003; Fig. 3f). The microbial biomass N:P ratio in the AA plantation also significantly decreased with N addition from 10.4 ± 0.5 to 6.4 ± 0.6 (P = 0.004; Fig. 3f). However, the microbial biomass C:N, C:P, and N:P ratios were not affected by N addition in the EU plantation (Fig. 3d, e, f).
Discussion
By fitting the asymptotic model to our data (litter mass and C remaining), we found significant residue (C, the asymptotic residue ranging from 0.3 to 0.59 for litter mass, and from 0.39 to 0.48 for litter carbon) in all plots. Furthermore, the asymptotic model enables us to estimate the effects of N addition on initial rates of decomposition and the value of the asymptotes (the fraction of initial mass decomposing very slowly), as mentioned by Berg and Matzner (1997) and Hobbie et al. (2012). Therefore, we mainly discuss the decomposition patterns between the control plots of two plantations, and the patterns among treatments in each plantation using the decomposition parameters calculated from the asymptotic model unless otherwise stated.
The litter decomposition patterns in the control plots
The litter decomposition rates (k s , yr.−1) observed in the control plots of the AA and EU plantations (0.514–0.768 yr.−1) were similar to previous results for temperate sites (from 0.3 to 0.7 yr.−1; Hobbie 2008), whereas they were higher than those in some coniferous forests/plantations (from 0.17 to 0.31 yr.−1; Mo et al. 2006; Perakis et al. 2012; Hobbie 2008). Higher rates of litter decomposition than our result were also reported from other tropical forests (ranging from 1.12 to 4.41 yr.−1), probably due to the higher microbial biomass and activity in those forests (Mo et al. 2006; Cleveland et al. 2006).
The initial decomposition rate (k a ) for AA litter was significantly higher than that for EU litter, which was consistent with our hypothesis and supported previous reports which showed that the litter mass loss of N2-fixing species (e.g. grey alder (Alnus incana), red alder (Alnus rubra)) was faster than that of non-N2-fixing species (e.g. white birch (Betula pubescens), Scots pone (Pinus sylvestris), lodgepole pine (Pinus contorta) and Douglas-fir (Pseudotsuga menziesii)) in early stage of decomposition (Berg and Ekbohm 1991; Perakis et al. 2012). As mentioned in the results, AA litter had significantly higher initial N concentration than the EU litter. Moreover, the AA plantation had higher soil N availability than the EU plantation due to symbiotic N2-fixation. Under these conditions, decomposers (who often have higher C:N ratio than litter) can acquire sufficient N resource from litter and soil, and secrete more enzymes to degrade carbon (Allison and Vitousek 2005; Hobbie et al. 2012).
The AA litter had a larger slow fraction (the asymptote, A) than the EU litter in the later stages of decomposition, as expected, and consistent with some previous studies which showed that the higher N concentration in litter, the larger the slow fraction remained (or the lower level of “limit value”) (Berg and Ekbohm 1991, Berg and McClaugherty 2008 and Hobbie et al. 2012). Previous studies have shown that the slowly decomposing fraction (A) was positively correlated with initial litter N concentration, but negatively correlated with litter Mn and Ca concentrations (Berg 2000; Berg et al. 2010; Davey et al. 2007). In the present study, initial AUR concentration was significantly higher in the AA litter (429 ± 14 mg g−1) than in the EU litter (303 ± 9 mg g−1). High litter N and soil inorganic N concentrations can suppress the colonization by ligninolytic fungi and the secretion of ligninolytic enzymes by white-rot fungi, and thus slow the degradation of AUR (Keyser et al. 1978; Berg and Matzner 1997; Berg and McClaugherty 2008; Hagiwara et al. 2012). Soil inorganic N might bond with AUR to form more recalcitrant substances, leading to a hampering effect on decomposition in the later stages of decomposition (Fog 1988; Davidson et al. 2003). In the present study, Mn concentration, which plays an important role in the synthesis of Mn-peroxidase by white rot fungi (Berg and McClaugherty 2008; Hatakka and Hammel 2010), was significantly higher in the EU litter than that in the AA litter (P < 0.0001; Table 2). Litter Ca and P concentrations, which likely are positively related to limit values (Berg and Ekbohm 1991; Berg and Matzner 1997; Hobbie et al. 2012), were higher in the EU litter than in the AA litter. We suggest that the difference in the decomposition rates for the whole decomposition period between the two plantations depends on the combined effect of factors controlling litter decomposition in the initial period (N and P) and in the later period (N, AUR, Ca, Mn).
Effects of N additions on litter decomposition rates
Consistent with our hypothesis and some previous studies (Berg and Ekbohm 1991; Berg and Matzner 1997; Hobbie et al. 2012), our results showed that N addition had no effect on the initial decomposition rate (k a ) in the AA plantation, but significantly decreased the level of “limit value” (the accumulated mass loss before the decomposition rate approached zero; which decreased from 50 % in the control plots to 41 % in the HN-addition plots), and increased the fraction of slowly decomposing litter in the later stages of litter decomposition. Therefore, the value of k s for the HN-addition plots was lower than that for the control plots during the overall decomposition period.
The inhibitory effect of N addition on lignin-degrading enzymes can partly explain the negative N effect on litter decomposition. In our study, we found that (1) the negative N effect on litter decomposition was more significant in the AA litter (high AUR:N ratio) than in the EU litter (low AUR:N ratio; Table 2) and (2) a determination of AUR after 12-months of decomposition showed that 78 % of the initial AUR was remained in the HN-addition plots, while 66 % of the initial AUR was remained in the control plots in the AA plantation. The reduction in AUR decay may be due to the suppressive effect of N addition on the formation of ligninases (Edwards et al. 2011, Hobbie et al. 2012). Sinsabaugh et al. (2002) indicated that N addition (20 kg N ha−1 yr.−1 and 80 kg N ha−1 yr.−1) significantly depressed the activity of polyphenol oxidase, leading to a decrease in litter mass loss of red oak in a mixed deciduous woodland in Armonk, NY, USA. Another reason for a slowdown in AUR decay is that inorganic N reacts with AUR chemically, which is the first step of the humification process (Gerber et al. 2010).
Another explanation is that microbial decomposers synthesize phenolic compounds and/or break down litter lignin and other polyphenolic compounds during decomposition. The by-products of these processes then react with inorganic N to form more resistant compounds (Davidson et al. 2003). There was a close relationship among inorganic N addition, formation of resistant compounds, and litter decomposition rate (Berg and Matzner 1997). In our study, we also found that litter decomposed in the HN-addition plots had less accumulated mass loss than that in the control plots in the AA plantation (41 and 50 % in the control and HN-addition plots, respectively; Fig. 1b).
Nitrogen can alter the microbial biomass and community composition, which are closely related to decomposition ability. Excess N input alters the balance between the N and P concentrations and/or decreases the pH value of the soil environment, which affects microbial growth and maintenance (Smolander et al. 1994; Ramirez et al. 2012). A study conducted in four sugar maple (Acer saccharum Marsh)-dominated northern hardwood sites distributed across lower and upper Michigan, USA, showed that atmospheric NO3 − deposition exerted a direct and negative effect on microbial activity, which slowed the decomposition of aboveground litter and led to the accumulation of soil organic matter and N in the forest floor (Zak et al. 2008). Our results show that high N addition significantly decreased soil microbial biomass C and microbial biomass C:N ratios in soil (Fig. 3a, d). This may suggest that there has been a shift in the microbial decomposers community from a highly efficient, fungus-dominated community with high overall C:N ratio to a less efficient, bacteria-dominated community with low C:N ratio (Frey et al. 2004; Högberg et al. 2007). A meta-analysis also show that microbial biomass declined 15 % on average under N addition, and the abundance of microbes and fungi declined significantly when chronic, high amounts of N had been added (Treseder 2008).
The negative effect of N addition on the later stages of decomposition in the AA plantation suggests that ongoing N deposition would increase the fraction of slowly decomposing litter (or reduce the “limit value”), leading to an increase in the accumulation of carbon in the forest floor. This inference is supported by the results of C loss from decomposing litter in the HN-addition plot (a higher fraction of recalcitrant carbon remained in the HN-addition plots), and by some previous reports (Keeler et al. 2009; Gerber et al. 2010; Whittinghill et al. 2012).
In contrast to the AA plantation, N addition had a positive effect on initial litter decomposition (k a ) in the EU plantation. Due to land-use history and high nutrient depletion by the growth of EU species (fast-growing species), the EU plantation had lower soil N availability than the AA plantation (Table 1), which suggests that there may be a strong competition for N uptake between plants and microbes in the EU plantation. N addition significantly increased soil available N concentration (from 18.5 to 21.7 mg kg−1) in this plantation, which supplied sufficient N resource for decomposers in the early stage of decomposition. In this case, decomposers can invest more energy and N to produce polysaccharide hydrolases, causing an acceleration in litter decomposition (Sinsabaugh et al. 2002; Allison and Vitousek 2005). This result is similar to those from a tropical forest in southern China, which showed that N addition had a positive effect on decomposition in N-poor pine forest (Mo et al. 2006) and some temperate forests with low-N status (Berg and Matzner 1997; Hobbie et al. 2012).
Effects of N addition on litter net release of N and P
Our results indicated that N addition significantly inhibited litter N release in the AA plantation, but not in the EU plantation. One possible explanation is that N addition altered the balance of C, N, and P requirement in microbial decomposers by improving soil N availability. Because N addition decreased the microbial biomass C:N ratio (Fig. 3d), the gap between the microbial biomass C:N ratio and the litter C:N ratio was wider in the N-addition plots than in the control plots at the beginning of litter decomposition. This gap gradually narrowed in the decomposing process. When N-limitation of litter decomposition was relieved by N addition, the negative effect of N on litter N release was stronger in the N-rich litter types than the N-poor litter types (Mo et al. 2008). Moore et al. (2006) suggested that litter nutrient loss was slower in nutrient-rich sites than in nutrient-poor sites. N can be retained in litter by microbial uptake (fungal hyphae transfer N from decomposing litter and soil) or chemical immobilization (ammonia-N can react with by-products of microbial breakdown and humus) (Vitousek and Hobbie 2000; Hobbie 2008).
The suppressing effect of N addition on litter P release can also be explained by the nutrient-status theory. The initial litter C:P and N:P ratios were significantly higher for the AA litter than for the EU litter (Table 2). However, N addition significantly reduced the soil microbial biomass C:P ratio in the AA plantation (Fig. 3e). For this reason, N addition may further enhance P-limitation during litter decomposition and stimulate microbial P immobilization in the decomposing litter in the AA plantation. Our results indicate that N addition had a stronger suppressing effect on litter P release in the AA plantation than in the EU plantation. Our results were consistent with other studies in tropical forests, which reported that N fertilization can induce litter nutrient retention due to the fairly plastic C:N and C:P ratios of decomposers, even when additional N has no (or a negative) effect on litter decomposition (Hobbie and Vitousek 2000; Mo et al. 2008).
Conclusion
In the present study, litter decomposition and nutrient release following N addition were measured in two tropical plantations with N2-fixing and non-N2-fixing tree species. Since litter N concentration and soil inorganic N was higher in the AA plantation, litter decomposition in this plantation was faster in the early stage of decomposition, but a larger, very slowly decomposing fraction was found in later stages, compared to the EU plantation. N addition did not increase the initial decomposition rate (k a ), but significantly slowed down decomposition in later stages in the AA plantation. In the EU plantation, N addition significantly increased the initial decomposition rate, but had a negative effect in the later stages. For the AA plantation, the negative effect of N addition on litter decomposition was due to a reduction in the decay of AUR and microbial biomass. In addition, N addition also affected the loss of litter C, N (only in the AA plantation) and P (in both plantations) during the decomposition process by stimulating chemical and microbial immobilization. To the best of our knowledge, our study is the first to investigate the effect of N deposition on litter decomposition and nutrient dynamics between plantations with N2-fixing tree species and non-N2-fixing species. Our results suggest that future N deposition would potentially decrease litter decomposition, which would lead to increasing carbon accumulation and nutrients' retention in the forest floor in plantations with N2-fixing tree species. Considering that N deposition and expansion of reforestation worldwide will continue to increase in the future, similar studies should be conducted in other regions to explore the selection of tree species for reforestation and relevant forest management.
References
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient input. Soil Biol Biochem 36:285–296
Anderson JM, Ingram JSI (1989) Tropical soil biology and fertility: a handbook of methods. CAB Int, Wallingford
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133:13--22
Berg B, Ekbohm G (1991) Litter mass-loss rates and decomposition patterms in smoe needle and leaf litter types. Long-term decomposition in a Scots pine forest VII. Can J Bot 69(7):1449--1456
Berg B, Matzner (1997) Effect of N deposition on decomposition of plant litter and soil organic matter in forest ecosystem. Environ Rev 5(1):1–25
Berg B, McClaugherty C (2008) Plant litter decomposition, humus formation, carbon sequestration. Springer, Verlag Berlin Heidelberg
Berg B, Davey M, De MA, Emmett B (2010) Factors influencing limit values for pine needle litter decomposition: a synthesis for boreal and temperate pine forest systems. Biogeochemistry 100(1–3):57–73
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81(9):2359–2365
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87(2):492–503
Cornwell WK et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11(10):1065–1071
Cusack DF, Silver WL, Torn MS, Burton SD, Firestone MK (2011) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Eology 92(3):621–632
Davey M, Berg B, Emmett B, Rowland P (2007) Controls of foliar litter decomposition and implications for C sequestration in oak woodlands. Can J For Bot 85:16–24
Davidson EA, Chorover J, Dail DB (2003) A mechanism of abiotic immobilization of nitrate in forest ecosystems: the ferrous wheel hypothesis. Glob Chang Biol 9:228–236
Edwards IP, Zak DR, Kellner H, Eisenlord SD, Pregitzer KS (2011) Simulated atmospheric N deposition alters fungal community composition and suppresses ligninolytic gene expression in a northern hardwood forest. PLoS ONE 6(6):e20421
Fang YT, Gundersen P, Vogt RD, Koba K, Chen FS, Chen XY, Yoh MK (2011) Atmospheric deposition and leaching of nitrogen in Chinese forest ecosystems. J For Res 16(5):341–350
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic-matter. Biol Rev Camb Philos Soc 63:433e462
Food and Agriculture Organization of the United Nations (FAOUN) (2006) World reference base for soil resources: a framework for international classification, correlation and communication, world soil resources report, vol. 103. Food and Agric. Org. of the U. N, Rome
Food and Agriculture Organization of the United Nations (FAOUN) (2010) Global forest resources assessment 2010: main report, FAO forestry paper, vol. 163. Food and Agric. Org. of the U. N, Rome
Forrester DI, Bauhus J, Cowie AL (2005) Nutrient cycling in a mixed-species plantation of Eucaluptus globulus and Acacia mearnsii. Can J For Res 35(12):2942–2950
Frey SD, Knorr M, Parrent JL, Simpson RT (2004) Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For Ecol Manag 196:159–171
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth SP, Asner CC, Cleveland PP, Green PA, Holland EA, Kari DM, Michaels AF, Porter JH, Townsend AR, Vöosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gei MG, Powers JS (2013) Do legumes and non-legumes tree species affect soil properties in unmanaged forests and plantations in costa Rican dry forests? Soil Biol Biochem 57:264–272
Gerber S, Hedin LO, Oppenheimer M, Pacala SW, Shevliakova E (2010) Nitrogen cycling and feedbacks in a global dynamic land model. Glob Biogeochem Cycles 24: GB1001
Hagiwara Y, Osono T, Ohta S (2012) Colonization and decomposition of leaf litter by ligninolytic fungi in Acacia mangium plantations and adjacent secondary forests. J For Res 17:51–57
Hall S, Matson P (2003) Nutrient status of tropical rain forests influences soil N dynamics after N additions. Ecol Monogr 73(1):107–129
Hatakka A, Hammel KE (2010) Fungal biodegradation of lignocelluloses. In: Hofrichter M (ed) Industrial applications, 2nd edn. The Mycota X. Springer-Verlag Berlin, Heidelberg
Hobbie SE (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3(5):484–494
Hobbie SE (2005) Contrasting effect of substrate and fertilizer nitrogen on the early stages of decomposition. Ecosyst 8(6):644–656
Hobbie SE (2008) Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89(9):2633–2644
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81(7):1867–1877
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82(3):389–405
Högberg MN, Högberg P, Myrold DD (2007) Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all Three? Oecologia 150:590–601
Huang J, Zhang W, Zhu XM, Gilliam FS, Chen H, Lu XK, Mo JM (2014) Urbanization in China changes the composition and main sources of wet inorganic nitrogen deposition. Environ Sci Pollut Res. doi:10.1007/s11356-014-3786-7
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36(1):5–7
Jensen LE, Nybroe O (1999) Nitrogen availability to Pseudomonas fluorescens DF57 is limited during decomposition of barley straw in bulk soil and in the barley rhizosphere. Appl Environ Microbiol 65(10):4320–4328
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11:35–43
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12(1):1–15
Keyser P, Kirk TK, Zeikus JG (1978) Ligninolytic enzyme system of phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol 35(3):790–797
King HGC, Heath GW (1967) The chemical analysis of small samples of leaf material and the relationship between the disappearance and composition of leaves. Pedobiologia 7:192–197
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86(12):3252–3257
Liu GS, Jiang NH, Zhang LD, Liu ZL (1996) Soil physical and chemical analysis and description of soil profiles. Standards Press of China, Beijing
Manning P, Saunders M, Bardgett RD, Bonkowski M, Bradford MA, Ellis RJ, Kandeler E, Marhan S, Tscherko D (2008) Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol Biochem 40(3):688–698
Mo JM, Brown S, Xue JH, Fang YT, Li ZA (2006) Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 282(1–2):135–151
Mo JM, Fang H, Zhu WX, Zhou GY (2008) Decomposition response of pine (Pinus massoniana) needles with two different nutrient-status to N deposition in a tropical pine plantation in southern China. Ann For Sci 65:405
Moore TR, Trofymow JA, Prescott CE, Fyles J, Titus BD (2006) Pattern of carbon, nitrogen and phosphorous dynamics in decomposing foliar litter in Canadian forests. Ecosystems 9(1):46–62
Norris M, Avis P, Reich P, Hobbie S (2013) Positive feedbacks between decomposition and soil nitrogen availability along fertility gradients. Plant Soil 367(1–2):347–361
Oberson A, Friesen DK, Morel C, Tiessen H (1997) Determination of phosphorus released by chloroform fumigation from microbial biomass in high P sorbing tropical soils. Soil Biol Biochem 29:1579–1583
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44(2):322–331
Osono T, Takeda H (2004) Accumulation and release of nitrogen and phosphorous in relation to lignin decomposition in leaf litter of 14 tree species. Ecol Res 19(6):593–602
Parton WA, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315(5810):361–364
Peng SL, Hou YP, Chen BM (2009) Vegetation restoration and its effects on carbon balance in Guangdong province, China. Restor Ecol 17(4):487–494
Perakis SS, Matkins JJ, Hibbs DE (2012) N2-fixing red alder indirectly accelerates ecosystem nitrogen cycling. Ecosystems 15(7):1182–1193
Preston CM, Nault JR, Trofymow JA (2009) Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 2. 13C abundance, solid-state 13C NMR spectroscopy and the meaning of “lignin”. Ecosystems 12(7):1078–1102
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18(6):1918–1927
Ren H, Yang L, Liu N (2008) Nurse plant theory and its application in ecological restoration in lower subtropics of China. Prog Nat Sci 18(2):137–142
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Smolander A. Kurka A, Kitunen V, Mälkönen E (1994) Microbial biomass C and N, and respiratory activity in soil of repeatedly limed and N-and P-fertilized Norway spruce stands. Soil Biol Biochem 26(8): 957–962.
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11(10):1111–1120
Trofymow J, Moore T (2002) Rates of litter decomposition over 6 years in Canadian forests: influence of litter quality and climate. Can J For Res 32(5):789–804
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707
Wardle DA (2002) Communities and ecosystem: linking the aboveground and belowground components. (Vol. 34). Princeton University Press, New Jersey
Weider RK, Lang GE (1982) A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–1642
Whittinghill K, Currie W, Zak D (2012) Anthropogenic N deposition increases soil C storage by decreasing the extent of litter decay: analysis of field observations with an ecosystem model. Ecosystems 15(3):450–461
Wu J, He ZL, Wei WX, O’Donnell (2000) Quantifying microbial biomass phosphorus in acid soil. Biol Fertil Soils 32:500–507
Wu JP, Liu ZF, Wang XL, Sun YX, Zhou LX, Lin YB, Fu SL (2011) Effects of understory removal and tree girdling on soil microbial community composition and litter decomposition in two eucalyptus plantations in south China. Funct Ecol 25(4):921–931
Xu XF, Thornton PE, Post WM (2013) A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob Ecol Biogeogr 22(6):737–749
Yuan ZY, Chen HYH (2009) Global trends in senesced-leaf nitrogen and phosphorus. Glob Ecol Biogeogr 18(5):532–542
Zak DR, Holmes WE, Burton AJ, Pregitzer KS, Talhelm AF (2008) Simulated atmospheric NO3 − deposition increases soil organic matter by slowing decomposition. Ecol Appl 18(8):2016–2027
Zhang W, Zhu XM, Liu L, Fu SL, Chen H, Huang J, Lu XK, Liu ZF, Mo JM (2012) Large difference of inhibitive effect of nitrogen deposition on soil methane oxidation between plantations with N-fixing species and non-N-fixing tree species. J Geophys Res 117:G00N16
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (NO: 41273143 and 41473112) and the National Key Basic Research 973 Program (2011CB403204). The authors wish to acknowledge Shengxing Fu, Ruifang Hu and Mozheng Li for their fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Harry Olde Venterink.
Rights and permissions
About this article
Cite this article
Zhu, X., Chen, H., Zhang, W. et al. Effects of nitrogen addition on litter decomposition and nutrient release in two tropical plantations with N2-fixing vs. non-N2-fixing tree species. Plant Soil 399, 61–74 (2016). https://doi.org/10.1007/s11104-015-2676-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2676-1