Abstract
Background and aims
Reliance on nitrogen (N) fertilizers to maintain crop productivity requires a thorough understanding of the transformation of this nutrient within the soil-plant system. Organic matter input from a mixture of crop residues, such intercrop systems, influence N transformations differently compared to sole crops. We tested the hypothesis that N mineralization and immobilization differ between cereal-legume intercrops and sole crops.
Methods
A short-term experiment using 15N isotopic pool dilution was conducted in 2007 and 2012 in maize (Zea mays L.) and soybean (Glycine max L. Merr.) sole crops and 1:2 (1 row maize:2 rows soybean) and 2:3 (two rows maize:3 rows soybean) intercrops. Soil characteristics, gross mineralization and immobilization, and net immobilization to a 10 cm depth were quantified.
Results
Soil characteristics (pH, bulk density, soil organic carbon (C), total N, and C:N) were not significantly different (P < 0.05) among treatments, but differed significantly (P < 0.05) between years (2007 vs. 2012). Soil NH4 +-N was significantly lower (P < 0.05) in the maize sole crop. Gross N mineralization, immobilization and net immobilization, were significantly different (P < 0.05) among treatments and between years. Relative NH4 +-N immobilization was significantly different (P < 0.05) among treatments and between years, showing the lowest values in the intercrops. The amount of NH4 +-N mineralized per day was significantly greater (P < 0.05) in the 2:3 intercrop and was significantly different (P < 0.05) between years in the 2:3 intercrop. Residence time of NH4 +-N was significantly longer (P < 0.05) in the soybean sole crop and 1:2 intercrop followed by the 2:3 intercrop and the maize sole crop.
Conclusions
Intercropping contributed to the long-term immobilization of N and therefore was a more sustainable land-management practice than sole cropping. The adoption of cereal-legume intercrops will curb our currently growing reliance on N fertilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The addition of nitrogen (N) fertilizers to maintain soil fertility and crop productivity has amplified the amount of reactive N in the biosphere and contributed to nitrous oxide (N2O) gas emissions (Jamont et al. 2013). The increase of reactive N in the biosphere also results in eutrophication of ecosystems and a loss of biodiversity (Galloway et al. 2003). As agricultural production continues to intensify, a growing reliance on N fertilizers to meet current and future global demands for food, fiber and fuel, is expected (Baggs et al. 2006). Therefore, understanding N transformations in various agricultural management practices, at the soil and crop level, is essential (Arihara and Srinivasan 2001).
Reducing external N inputs can be achieved through the diversification of cereal crops by integrating legumes either in rotation or as an intercrop. Intercropping, where crop intensification occurs in both time and space is defined as the simultaneous growth of more than one species in the same field (Vandermeer 1992). The concept of cereal-legume, and legume-based intercropping is not new in tropical agroecoystems (Sharma and Behera 2009), and it is gaining recognition in temperate regions (Hauggaard-Nielsen and Jensen 2001; Oelbermann and Echarte 2011). This is because intercropping systems have a smaller environmental foot print (Li et al. 2001) and they are more resilient to local climate change due to their greater structural complexity (Dyer et al. 2012). In low input intercrops, the efficient use of soil resources, via enhanced microbial activity, tighter nutrient cycling, and complementary use of N, increases plant productivity, especially in the non-N fixing crop, compared to sole crops (Bedoussac et al. 2014). However, in high-input systems, the quantity of mineral N fertilizer required in intercrops can be reduced due to dynamic soil-plant interactions (Pelzer et al. 2012) since crops do not compete for the same resource niche, but use N in a complementary way (Hauggaard-Nielsen and Jensen 2001). For example, intercropping rapeseed (Brassica napus L.) with faba bean (Vicia faba L.) accumulated 20 % more N per crop species compared to a rapeseed sole crop (Jamont et al. 2013).
After harvest, the interactions between mixed crop residues affect decomposition differently in intercrops compared to sole crops (Redin et al. 2014). Mixing residues with high and low C/N ratios increases faunal activity and nutrient translocation (Chapman et al. 1988), and improves the synchrony between N supply and crop N demand (Vachon and Oelbermann 2011; Redin et al. 2014). This creates competition for NH4 + by immobilizers and nitrifiers, reducing N losses from the soil-plant system (Liang et al. 2013), causing immediate net N immobilization and delaying the onset of net mineralization (Frimpong et al. 2012). For example, intercropping spring barley (Hordeum vulgare L.) and pea (Pisum sativum) in Scotland reduced NO3 − leaching and N2O emissions compared to sole cropped barley (Pappa et al. 2011).
To address the increasing reliance on N fertilizers at a global scale, multiple studies have focused on plant N availability and grain yield in a variety of cropping systems, including intercrops (Pappa et al. 2011; Hauggaard-Nielsen et al. 2012; Jamont et al. 2013; Chapagain and Riseman 2014). However, knowledge on the underlying process of mineralization leading to N availability, when different intercrop configurations are used over the short-term, remains scarce. Therefore, understanding the process of mineralization provides further insight into the amount of N available for plant uptake or nutrient input requirements under different agroecosystem management practices (Davidson et al. 1991). Although Vachon and Oelbermann (2011) evaluated the rate of mineralization of crop residues at the same site as the current study, using a 9-month litterbag decomposition experiment, their results only yielded values on net N mineralization. Using 15N stable isotope techniques however, advances our understanding of gross N mineralization and immobilization in various agroecosystem management practices (Powlson and Barraclough 1993). To date, most N mineralization studies focused on laboratory incubations whose results are extrapolated to understand this process at the field-scale (Mary et al. 1996). But laboratory-scale studies underestimate mineralization rates compared to those at the field-scale (Sanchez et al. 2002). The primary goal of this study was to evaluate the dynamics of gross mineralization and immobilization, and net immobilization in two differently configured cereal-legume intercrops and in cereal and legume sole crops. This was achieved by conducting a short-term field study in 2007 and in 2012 using the 15N isotopic pool dilution technique. It was hypothesized that N mineralization rates will be greater in the intercrops because of the different quantity and mixing of residues compared to the sole crops. Our study provided further understanding on the response of residue input from single or mixed crops and its effect on soil N transformations.
Materials and methods
Experimental site
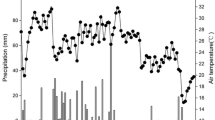
The research site was located in the southern Argentine Pampa, outside the city of Balcarce (37°45′S, 58°18′W). Previous studies conducted at this intercrop site included residue decomposition, greenhouse gas emissions, land equivalent ratio, and changes in soil characteristics as a result of intercropping (Oelbermann and Echarte 2011; Vachon and Oelbermann 2011; Dyer et al. 2012; Echarte et al. 2011; Oelbermann et al. 2015). The climate in this area was classified as mesothermal subhumid–humid (Thornthwaite classification) or as temperate humid without dry season (Köpen classification). The mean annual rainfall, potential evapotranspiration, and the annual mean air temperature (1980–2012) were 860 mm yr−1, 856 mm yr−1, and 14.3 °C (maximum 24.2 °C and minimum 7.6 °C), respectively (Unidad Integrada Balcarce Weather Station, 37°45′ S, 58°18′ W, 130 m.a.s.l.) (Fig. 1). The soil was classified as a Typic Agridudoll (US Soil Taxonomy) or Luvic Phaeozem (FAO Soil Classification) and was part of the Mar del Plata series, with a soil texture of 41.1 % sand, 35.8 % silt and 23.1 % clay (INTA 1979). The Balcarce soils were formed from an Aeolian accretion of calcareous loess during the Holocene epoch (Blanco and Stoops 2007), and were composed of illite (80 %) and montmorillionite (20 %) clay minerals. Ammonium fixation was negligible; only 0.01 % of the NH4 +-N added to the Phaeozems of Balcarce was fixed to clays. The soil (0–20 cm) was moderately acid with a pH of 5.77, had low available phosphorus (P) of 7.83 mg kg−1 (Bray-extractable P), and a high soil organic C (SOC) content of 30.6 g kg−1. The slope was 2 %, indicating little to no erosion.
Experimental design and management
The experimental intercrop and sole crop plots were established in 2007. This was a continual experiment, and for this study two intercrop years (2007 and 2012) were examined. The experimental plots were established on land previously under experimental use of alternating crop and pasture. The previous crop was sunflower (Helianthus annuus L.) cultivated for 2 years using a disk harrow followed by a spike harrow. The study was a randomized complete block design (RCBD) with four treatments: maize sole crop, soybean sole crop, 1:2 intercrop (one row of maize and two rows of soybeans) and 2:3 intercrop (two rows of maize and three rows of soybeans). Each treatment was replicated three times, and each treatment plot was 8.8 × 12 m. The maize and soybean sole crops were rotated annually, but the intercrops were not. In this study, treatments plots referred to as maize sole crop were under maize in 2008–09, 2010–11 and 2012–13, and under soybean in 2007–08 and 2009–10. Treatments plots referred to as soybean sole were under soybean in 2008–09, 2010–11 and 2012–13, and under maize in 2007–08 and 2009–10.
Plant density (plants m−2) was 4.3 (1:2 intercrop), 5.3 (2:3 intercrop), 8.0 (maize sole crop) and 29 (soybean sole crop), with a 0.52 m distance between crop rows in all treatments. The site was disk harrowed three times and spike harrowed before planting. Weeds were controlled by N-phosphonomethyl glycine (Glyphosate). All crops received P fertilizer (35 kg P ha−1). Maize in the sole crop and in the intercrops received N fertilizer (150 kg N ha−1) in the form of urea. Fertilizer was applied by hand at the bottom of the maize stems at the 6th leaf stage in the intercrops. Soybeans were inoculated with Bradyrhizobium japonicum. Maize was seeded in late October or early November and harvested in April; soybeans were seeded in November and harvested in May. Crop residues were returned to all treatments after each harvest from 2007 to 2012. Mean values, in all treatments, of C and N concentration of crop residue biomass was 42.2 % (C) and 0.66 % (N) for maize, and 44.8 % (C) and 1.4 % (N) for soybeans (Vachon and Oelbermann 2011).
Quantification of soil chemical and physical characteristics
Prior to applying 15N, three soil samples were extracted randomly from each treatment replicate to a 10 cm depth using a 7 cm inner diameter soil corer. A 20 g subsample of field moist soil was oven-dried (105 °C, 48 h) to determine bulk density. Bulk density was calculated using the inner diameter of the core sampler and the oven dry weight of the soil. Bulk density was not adjusted for rock volume (mineral particles ≥ 2 mm) because these soils had minimal rock content. A 5 g subsample of field moist soil was extracted in 50 mL of 2 M KCl, and analyzed for NH4 +-N and NO3 −-N by micro-distillation (Bremner 1965). Data for NH4 +-N and NO3 −-N were available only for 2012.
The remaining soil was air-dried and sieved to 2 mm. Soil pH was quantified using a 20 g subsample in a 1:1 soil:water suspension (BioKit AB 15B, Houston, TX, USA). Prior to the analysis of SOC and N, soil carbonates were removed by adding 150 mL of 0.5 M HCl to 2 g of sieved soil. The mixture was stirred three times over 24 h, and washed by pipetting the HCl from the settled soil by adding ultrapure water to the soil. This washing procedure was repeated daily for 4 days after which the soil was dried in an oven at 40 °C for 2 days (Midwood and Boutton 1998). The acid treated soil was ground in a ball mill (Retsch® ZM1, Haan, Germany) and analyzed for SOC and N (Costech 4010, Cernusco, Italy). Soil organic C and total N stocks were determined by multiplying SOC and N concentration (%) by the amount of soil per square meter, using soil bulk density, to a 10 cm depth.
Nitrogen immobilization and mineralization using isotope pool dilution
The 15N isotope pool dilution technique was used to quantify gross N mineralization and immobilization (Davidson et al. 1991). The labelled ammonium sulfate (15NH4)2SO4 (~350 μg N mL−1, 10 % 15N) fertilizer was added in 2007, at the start of the experiment, to the sole crops and the 2:3 intercrop. After 5 years, in 2012, the labelled solution was added to all sole crop and intercrop treatments. Gross mineralization rates were measured just prior to crop sowing, to minimize the impact on N mineralization and immobilization by the growing crop. In 2007 four PVC cylinders with a 6 cm inner diameter and 13 cm long, and in 2012 six PVC cylinders of the same size, were randomly inserted into the soil to a 12 cm depth within each treatment replicate.
A multiple point injection device, with the same diameter as the PVC cylinders was used to ensure the application of the 15N label to the soil was homogenous. The injection device contained seven hypodermic syringes (Monaghan 1995) and an acrylic disc, to which seven spinal needles were fixed. The device with the syringes was connected to the needles. When the syringes were pulled up, the syringe plungers were pushed down, injecting 15N solution into the soil. Injection of the labelled ammonium sulfate fertilizer occurred at a rate of ~10 μg N per g of dry soil. The volume of the solution injected was dependent on the soil moisture, and was adjusted to increase soil moisture by a maximum of 20–25 % (Monaghan 1995).
After 24 h (t = 0), half of the PVC cylinders from each treatment replicate were removed, ensuring that initial immobilization of the label had ceased (Hood et al. 2003). The remaining PVC cylinders were removed after 96 h (t = 1), allowing sufficient time for N transformations to occur but before re-mineralization began (Videla 2004). Twenty grams of the collected field moist soil from within each cylinder was extracted with 100 mL of 2 M KCl.
Potassium chloride extracts were analyzed for exchangeable NH4 + using steam distillation in the presence of MgO as described by Bremner (1965). We measured 15N using the polytetrafluoroethylene (PTFE) diffusion technique described by Sørensen and Jensen (1991) and Mulvaney et al. (1997). Twenty milliliters of KCl extract solution was weighed into 100 mL jars, a PTFE envelope containing a quartz filter paper disc (Whatman GF/D) of 5 mm diameter acidified with 10 μL of 2.5 M KHSO4 was added to the solution, approximately 0.2 g of MgO was then added to the vessel, which was closed immediately, shaken at 50 rev min−1 for 72 h in the dark at 30 °C (Mulvaney et al. 1997). After shaking, the discs were dried overnight in a desiccator with silica gel and a trap of 50 mL concentrated H2SO4. The dry discs were placed into 5× 9 mm tin capsules (Costech, Cernusco, Italy) and analyzed for 15N:14N ratio with a Delta V mass spectrometer equipped with a Conflo IV Interface (ThermoScientific, Waltham, USA).
Gross N mineralization and immobilization rates were quantified as described by Kirkham and Bartholomew (1954):
where m is the gross N mineralization rate (μg N g−1 soil d−1); i is the gross N immobilization rate (μg N g−1 soil d−1); NH4 + is the total soil ammonium content (μg N g−1 soil); Δt is the related time interval (days); APE is the atom percent 15N excess of NH4 +; 0 (t = 0) and t (t = 1) indicate the two sampling time points.
The net rate of immobilization was calculated by subtracting the gross rate of mineralization from the gross rate of immobilization. Relative NH4 +-N immobilization is the ratio between the gross rates of N immobilization and mineralization. The percentage of NH4 +-N mineralized per day (%) was defined as the gross N mineralization rate divided by soil total N, and multiplied by 100.
Statistical analysis
Prior to any statistical analyses, all data were examined for normality (Shapiro-Wilk test of normality) and homogeneity of variance (Levene test of equality of variances). Data for soil chemical characteristics was tested using the univariate general linear model (ANOVA) in SPSS v20 (SPSS Science Inc. 1989). Treatment-by-year interactions were not significant. Significant differences between means (main effects) were separated by Tukey’s multiple comparison test for factors with three or more levels (e.g., treatment); and significant differences for factors with two levels (e.g., year) were derived from the F-statistic (Steel et al. 1997). The threshold probability level for determining significant differences for all statistical analyses was set at P < 0.05.
Results
Soil chemical and physical characteristics
Soil pH and bulk density were not significantly different among treatments in 2007 and 2012. However, bulk density increased significantly (P = 0.005) from 2007 to 2012 in all treatments (Table 1), with a relative increase ranging from 9 to 17 % (Table 2). Soil organic C concentration and stock increased significantly (P = 0.001) between 2007 and 2012 (Table 1). Among all treatments, SOC concentration and stock increased up to 29 % and up to 45 %, respectively (Table 2). Soil total N concentration increased significantly (P = 0.05) between 2007 and 2012 in the soybean sole crop only (Tables 1 and 2). Between 2007 and 2012, soil total N stock increased significantly (P = 0.001) in all treatments (Table 1) with a relative increase ranging from 21 to 36 % (Table 2). The soil C/N ratio increased significantly (P = 0.001) between 2007 and 2012 (Table 1); with a relative change ranging from 12 to 19 % (Table 2).
In 2012, soil ammonium N (NH4 +-N) was significantly different (P = 0.001) among treatments prior to seeding and at harvest. Prior to crop seeding, NH4 +-N was significantly greater in the 1:2 intercrop than in other modalities (Table 1), however at crop harvest it was significantly greater in the maize sole crop (23.90 ± 10.44 μg g−1), followed by the soybean sole crop (11.63 ± 1.05 μg g−1), 1:2 intercrop (9.53 ± 0.50 μg g−1) and 2:3 intercrop (7.87 ± 2.23 μg g−1). NH4 +-N was significantly greater (P = 0.001) at crop harvest than at crop seeding. Soil nitrate N (NO3 −-N) was not significantly different among treatments prior to crop seeding in 2012 (Table 1) and at crop harvest. However, NO3 −-N was significantly lower (P = 0.001) at crop harvest [maize sole crop (9.90 ± 1.29 μg g−1); soybean sole crop (10.47 ± 0.93 μg g−1); 1:2 intercrop (10.70 ± 1.40 μg g−1); 2:3 intercrop (10.63 ± 1.99 μg g−1)] than prior to crop seeding (Table 1).
Nitrogen mineralization and immobilization
Gross N mineralization was significantly greater (P = 0.001) in the sole crops in 2007, and in the 2:3 intercrop in 2012 (Fig. 2). The gross N mineralization rate rose significantly (P = 0.001) between 2007 and 2012 in the 2:3 intercrop with a relative increase of 274 %. Between years, the maize and soybean sole crops had a relative increase of 37 and 25 % respectively (Table 2). Gross N immobilization was significantly different (P = 0.001) among treatments in 2012, where the greatest rate occurred in the 2:3 intercrop (Fig. 3). Nitrogen immobilization rose significantly (P = 0.001) between 2007 and 2012 in all treatments and had a relative increase ranging from 49 to 125 % (Table 2). Net N immobilization was significantly different (P = 0.001) among treatments in 2007 and 2012 (Fig. 4). In both years, net immobilization was greatest in the soybean sole crop followed by the intercrops and was lowest in the maize sole crop. Net N immobilization increased significantly (P = 0.001) between 2007 and 2012, with a relative increase ranging from 2 to 66 % (Table 2).
Gross nitrogen mineralization (0–10 cm) in maize and soybean sole crops and in 1:2 and 2:3 intercrops in 2007 and 2012, Balcarce, Argentina. Bars with the same upper case letters, comparing differences between years and within treatments, are not significantly different at P < 0.05 using the Student’s t-test. Bars with the same lower case letters, comparing differences among treatments and within years, are not significantly different at P < 0.05 using Tukey’s multiple comparison test
Gross nitrogen immobilization or microbial consumption of NH4 + (0–10 cm) in maize and soybean sole crops and in 1:2 and 2:3 intercrops in 2007 and 2012, Balcarce, Argentina. Bars with the same upper case letters, comparing differences between years and within treatments, are not significantly different at P < 0.05 using the Student’s t-test. Bars with the same lower case letters, comparing differences among treatments and within years, are not significantly different at P < 0.05 using Tukey’s multiple comparison test
Net nitrogen immobilization (0–10 cm) in maize and soybean sole crops and in 1:2 and 2:3 intercrops in 2007 and 2012, Balcarce, Argentina. Bars with the same upper case letters, comparing differences between years and within treatments, are not significantly different at P < 0.05 using the Student’s t-test. Bars with the same lower case letters, comparing differences among treatments and within years, are not significantly different at P < 0.05 using Tukey’s multiple comparison test
Relative NH4 +-N immobilization was significantly greater in the soybean sole crop in 2007 (P = 0.05) and in 2012 (P = 0.05) (Table 3). Relative NH4 +-N immobilization also increased significantly (P = 0.001) between years. The greatest relative increase occurred in the maize sole crop (29 %), but decreased (−16 %) in the soybean sole crop (Table 2). NH4 +-N mineralized per day was significantly greater (P = 0.05) in the maize sole crop in 2007, whereas in 2012, it was significantly greater in the 2:3 intercrop (Table 3). The NH4 +-N mineralized per day was significantly greater (P = 0.05) in 2012 than in 2007, with a relative change ranging from −3 to +139 % (Table 2). NH4 +-N residence time (data available for 2012 only) was significantly different (P = 0.05) among treatments (Table 3). The shortest NH4 +-N residence time occurred in the maize sole crop and the 2:3 intercrop, whereas the longest residence time occurred in the 1:2 intercrop and soybean sole crop.
Discussion
Soil chemical characteristics
Soil physical and chemical characteristics were similar to those reported Studdert and Echeverría (2000), Aparicio and Costa (2007), and Domínguez et al. (2009) from the same region of Argentina as our study. In 2009 and 2010, Dyer et al. (2012) conducted a study at the same site and found a lower bulk density (0–10 cm) in all treatments compared to our results. Over time, conventional tillage practices caused soil compaction, increasing soil bulk density, which was also observed by Tolon-Becerra et al. (2011) in the Argentine Pampa.
A greater crop residue input from maize and soybeans in all treatments compared to sunflower, produced at this site prior to 2007, increased SOC and soil total N by 2012. Andrade (1995) reported aboveground residue input from sunflower in Balcarce, Argentina was 958 g m−2. Comparatively, the mean residue input over six growing seasons from maize was 2237 g m−2, 824 g m−2 from soybeans, 1688 g m−2 from the 1:2 intercrop, and 1775 g m−2 from the 2:3 intercrop (Regehr 2013). However, maize and soybean sole crops were rotated annually, and the treatment referred to as maize sole crop had a 6 year mean crop residue input of 1622 g m−2 and that referred to as soybean sole crop had a 6 year mean crop residue input of 1432 g m−2. This greater input of organic matter from crop residues in all treatments led to an accumulation of SOC and N between 2007 and 2012.
The short-term nature of this study failed to detect measurable changes in SOC and N among treatments in 2007 and in 2012. Quantifyable differences in soil characteristics are expected to occur 10+ years after initiating intercropping (Oelbermann and Echarte 2011). For example, Alvarez et al. (1998) detected a measureable increase in SOC after more than 5 years of initiating land-management changes in the Argentine Pampa, whereas Studdert and Echeverria (2000) found measureable changes after 11 years. Increases in SOC and N over the short-term in soil with an inherent high SOC content, such as that of the Canadian Prairies, the Corn Belt in the Midwest USA, or the Argentine Pampa, cannot be detected as statistically significant within less than a decade (Malhi et al. 2008; Kutsch et al. 2012).
The low NH4 +-N concentration indicated that N was mineralized rapidly and converted to NO3 −-N since no plants were available to take up N prior to crop seeding. Variation in NH4 +-N prior to crop seeding was due to differences in the amount of residual N available from the previous cropping season. Differences in NH4 +-N between sole crops and intercrops at harvest were also observed by Chapagain and Riseman (2014) in a pea-barley intercrop and pea sole crop in western Canada. This was due to the distribution of maize and soybean roots in the intercrops, and their interaction, affecting NH4 +-N at harvest. The dynamic interaction between crop roots and changes in the structure of rhizospheric microbial communities as a result of biological N fixation and associated chemical changes, allowed maize plants to use NH4 +-N present in the soybean rows, leading to a lower residual of this nutrient throughout the growing season.
Soybean nodules from roots and decomposed residues from previous cropping seasons did not affect NO3 −-N in the intercrops (Reents and Möller 2000; Matusso et al. 2014). A lower soil NO3 −-N at harvest, also reported by Li et al. (2005), was due to differences in plant N uptake. For example, the maize sole crop had a greater demand for N, and therefore a greater plant N uptake compared to soybean, which supplied its N through N2-fixation (Zhang and Li 2003).
Nitrogen mineralization and immobilization
Rates of gross N mineralization and immobilization in the sole crops were within the range of those reported by Bengston et al. (2003), Burger and Jackson (2003), Flavel and Murphy (2006), and Griffin (2007). The greater gross N mineralization and immobilization rates in all treatments in 2012 were due to the recent accumulation of soil organic matter (SOM), since a significant proportion of the newly accumulated N became incorporated into the active SOM pool (Nieder and Benbi 2008). The greater rate of gross N mineralization in the intercrops was due to differences in the magnitude of N cycling through this treatment compared to the sole crops (Flavel and Murphy 2006). This occurred because residues from cereal and legume crops are present in different ratios in intercrops compared to the sole crops. The mixing of crop residues causes complex interactions, influencing N mineralization and immobilization differently in intercrops than in the sole crops (Barrios et al. 1996; Redin et al. 2014). Additionally, N mineralization is a biotic process, and soil with a higher microbial activity and soil microbial biomass (SMB) has greater N mineralization rates (Bengston et al. 2003). For example, in 2012, SMB-C (0–10 cm) was greater in the 2:3 intercrop compared to the other treatments (Oelbermann et al. 2015). Significant correlations between the rate of net and gross rate of N mineralization and SMB-C were also observed in a Wyoming grassland (Tracy and Frank 1998) and in white clover (Trifolium repens L.), ryegrass (Lolium perenne L.) and pasture lands of New Zealand (Zaman et al. 1999). However, in 2007, Oelbermann and Echarte (2011) also observed a greater SMB-C in the 2:3 intercrop, but in this year, the rate of gross N mineralization was lower than in 2012. A greater SMB does not always correspond to a higher N mineralization rate, due to differences in the proportion of the active SMB among treatments (Flavel and Murphy 2006). Field studies on gross N mineralization are difficult to conduct due to the heterogeneous distribution of mineral N and interference from other processes such as nitrate movement in soil, root adsorption, volatilization and denitrification, in addition to variations in soil temperature and humidity, causing inconsistent results when comparing within and between cropping systems (Mary et al. 1996).
Rates of gross N immobilization and the release of inorganic N were affected by the differences in residue quality among treatments (Burger and Jackson 2003). Crop residues with high C/N ratios had lower rates of mineralization and higher rates of immobilization. In a previous study, Vachon and Oelbermann (2011) reported C/N ratios of 66 (maize sole crop), 31 (soybean sole crop), 56 (1:2 intercrop) and 62 (2:3 intercrop). Treatments that received crop residues with high C/N ratios had a greater rate of gross N immobilization and a lower rate of net N immobilization. Therefore, availability of easily decomposable substrates in the initial phase of decomposition was lower in the intercrops compared to the soybean sole crop (Luxhoi et al. 2006).
The incorporation of large quantities of maize residue in the sole crop limited N availability. However, mixing maize and soybean residues in the intercrops resulted in a greater availability of N to the growing crops (Sakala et al. 2000). For example, Vachon and Oelbermann (2011) determined that maize residue contributed 70 % C and 55 % N in the 1:2 intercrop, and 78 % C and 63 % N in the 2:3 intercrop. The greater rate of net immobilization indicated a more active microbial population and therefore a reduced potential for inorganic N loss from the intercrops (Accoe et al. 2004). This is because N available from the N-rich soybean residue was immobilized in greater quantities by microbes growing on the N-poor maize residue as a result of an increased availability of N (Redin et al. 2014).
Relative NH4 + immobilization (the ratio between gross N mineralization and immobilization) was influenced by the type of residues added to the soil (Mendham et al. 2004). A lower relative NH4 + immobilization in 2012 in both intercrop configurations minimized the potential of decreasing the NH4 +-N pool, which was due to the mixed crop residue input in these treatments (Vervaet et al. 2004). A relative NH4 +-N immobilization value of ≥1 indicates an N-limited soil, whereas a value of≈0.5 indicates N saturation (Aber 1992). Accordingly, all treatments in 2007 and 2012 were N-limited, and this limitation was greatest in the sole crops and lowest in the 2:3 intercrop.
Although the large amount of NH4 +-N mineralized per day in the maize sole crop showed that available N was highest in this treatment, the potential to minimize a decrease the NH4 +-N pool was greatest in the intercrops (Vervaet et al. 2004). The high NH4 +-N mean residence time in the soybean sole crop caused a slower turnover of the N pool due to a lower C availability to drive the internal N cycle (Hart et al. 1994). A shorter residence time in the 2:3 intercrop showed that the NH4 +-N pool size was relatively small (Flavel and Murphy 2006), and that this intercrop configuration had a lower potential for nitrification and NO3 −-N leaching (Verchot et al. 2001), minimizing the loss of N as N2O (Frimpong et al. 2012).
In conclusion, our data suggest that both intercrop and sole crop agroecosystem management practices had a positive effect on soil characteristics as a result of greater crop residue input compared to land management practices prior to 2007. Due to the dynamics of mixing crop residues, complex interactions in the soil-plant system stimulated a more active microbial community that modified soil N transformations differently in the intercrops than in the sole crops. Both intercrop configurations proved to be a more sustainable land management option compared to sole cropping, since they contributed to the long-term immobilization of N, with the potential to reduce nitrification and NO3 −-N leaching. Reduced soil nitrification rates also moderate N2O losses causing intercrops to abate the overall contribution of this greenhouse gas to global climate change. Our data contributed to a more thorough understanding of N transformations in soil at the crop level, and provided new knowledge on the most effective agroecosystem management practice and intercrop configuration(s) to curb our growing global dependence on N fertilizers with the added potential of reducing water pollution and moderating N2O emissions, and to meet current and future global demands for food, fiber and fuel.
References
Aber JD (1992) Nitrogen cycling and nitrogen saturation in temperate forest ecosystems. Trends Ecol Evol 7:220–223
Accoe F, Boeckx P, Busschaert J, Hofman G, Van Cleemput O (2004) Gross N transformation rates and net N mineralization rates related to the C and N contents of soil organic matter fractions in grassland soils of different age. Soil Biol Biochem 36:2075–2087
Alvarez CR, Alvarez R, Grigera MS, Lavado RS (1998) Associations between organic matter fractions and the active soil microbial biomass. Soil Biol Biochem 30:767–773
Andrade FH (1995) Analysis of growth and yield of maize, sunflower and soybean growth at Balcarce, Argentina. Field Crop Res 42:1–12
Aparicio V, Costa JL (2007) Soil quality indicators under continuous cropping systems in the Argentinean Pampa. Soil Tillage Res 96:155–165
Arihara J, Srinivasan A (2001) Significance of nutrient uptake mechanisms in cropping systems. In: Noriharu AE, Arihara J, Okada K, Srinivasan A (eds) Plant nutrient acquisition-new perspectives. Springer, Japan, pp 487–503
Baggs EM, Chebii J, Ndufa JK (2006) A short-term investigation of trace gas emissions following tillage and no-tillage of agroforestry residues in western Kenya. Soil Tillage Res 90:69–76
Barrios E, Buresh RJ, Sprent JI (1996) Nitrogen mineralization in density fractions of soil organic matter from maize and legume cropping systems. Soil Biol Biochem 28:1459–1465
Bedoussac L, Justes E, Journet ÉP, Hauggaard-Nielsen H, Naudin C, Corre-Hellou G, Prieur L, Jensen (2014) Intercropping – applying ecological principles for improved nitrogen use efficiency in organic farming systems. In: Bellon S, Penvern S (eds) Organic farming, Prototype for sustainable agricultures. Springer Science, Dordrecht, pp 47–64
Bengston G, Bengston P, Mansson KF (2003) Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol Biochem 35:143–154
del Blanco CM, Stoops G (2007) Genesis of pedons with discontinuous argillic horizons in the Holcene loess mantle of the southern Pampean landscape, Argentina. J S Am Earth Sci 23:30–45
Bremner JM (1965) Inorganic forms of nitrogen. In: Black CA (ed) Methods of soil analysis part II: chemical and microbiological properties. American Society of Agronomy, Madison, pp 1149–1176
Burger M, Jackson LE (2003) Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol Biochem 25:29–36
Chapagain T, Riseman A (2014) Barley-pea intercropping: effects on land productivity, carbon and nitrogen transformations. Field Crop Res 166:18–25
Chapman K, Whittaker JB, Heal OW (1988) Metabolic and faunal activity in litter of tree mixtures compared with pure stands. Agric Ecosyst Environ 24:33–40
Davidson EA, Hart SC, Shanks CA, Firestone MK (1991) Measuring gross nitrogen mineralization, immobilization, and nitrification by 15N isotopic pool dilution in intact soil cores. J Soil Sci 42:349–355
Domínguez G, Diovisalvi N, Studdert G, Monterubbianesi M (2009) Soil organic C and N fractions under continuous cropping with contrasting tillage systems on Mollisols of the southeastern Pampas. Soil Tillage Res 102:93–100
Dyer L, Oelbermann M, Echarte L (2012) Soil carbon dioxide and nitrous oxide emissions during the growing season from temperate maize-soybean intercrops. J Plant Nutr Soil Sci 175:394–400
Echarte L, Della Maggiora A, Cerrudo D, Gonzalez VH, Abbate P, Cerrudo A, Sadra VO, Calviño P (2011) Yield response to plant density of maize and sunflower intercropped with soybean. Field Crop Res 121:423–429
Flavel TC, Murphy DV (2006) Carbon and nitrogen mineralization rates after application of organic amendments to soil. J Environ Qual 35:183–193
Frimpong KA, Yawson DO, Agyarko K, Baggs EM (2012) N2O emission and mineral N release in a tropical Acrisol incorporated with mixed cowpea and maize residues. Agronomy 2:167–186
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356
Griffin TS (2007) Estimates of gross transformation rates of dairy manure N using 15N pool dilution. Commun Soil Sci Plant Anal 38:1451–1465
Hart SC, Nason GE, Myrold DD, Perry DA (1994) Dynamics of gross nitrogen transformations in an old-growth forest: the carbon connection. Ecology 75:880–891
Hauggaard-Nielsen H, Jensen ES (2001) Evaluating pea and barley cultivars for complementarity in intercropping at different levels of soil N availability. Field Crop Res 72:185–196
Hauggaard-Nielsen H, Mundus S, Jensen ES (2012) Grass-clover undersowing affects nitrogen dynamics in a grain legume-cereal arable cropping system. Field Crop Res 136:23–31
Hood R, Bautista E, Heiling M (2003) Gross mineralization and plant N uptake from animal manures under non-N limiting conditions, measured using the 15N isotope dilution technique. Phytochem Rev 2:113–119
INTA (Instituto Nacional de Tecnología Agropecuaria) (1979) Carta de Suelos de la República Argentina. Hoja: 3757–31, Balcarce., Instituto Nacional de Tecnología Agropecuaria, Secretaría de Agricultura, Ganadería y Pesca, Buenos Aires, Argentina
Jamont M, Piva G, Fustec J (2013) Sharing N resources in the early growth of rapeseed intercropped with faba been: does N transfer matter? Plant Soil 371:641–653
Kirkham D, Bartholomew WV (1954) Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci Soc Am J 18:33–34
Kutsch WL, Bahn M, Heinemeyer A (2012) Soil carbon relations: an overview. In: Kutsch WL, Bahn M, Heinemeyer A (eds) Soil carbon dynamics-an integrated methodology. Cambridge University Press, Cambridge, pp 1–15
Liang B, Zhao W, Yang X, Zhou J (2013) Fate of nitrogen-15 as influenced by soil and nutrient management history in a 19-year wheat-maize experiment. Field Crop Res 144:124–134
Li W, Li L, Sun J, Guo T, Zhang F, Bao X, An P, Tang C (2005) Effects of intercropping and nitrogen application on nitrate present in the profile of an Orthic Anthrosol in northwest China. Agric Ecosyst Environ 105:483–491
Li L, Sun J, Zhang F, Li X, Yang S, Rengel Z (2001) Wheat-maize or wheat-soybean strip intercropping I. Yield advantage and interspecific interactions on nutrients. Field Crop Res 71:123–137
Luxhoi J, Bruun S, Stenberg B, Breland TA, Jensen LS (2006) Prediction of gross and net nitrogen mineralization-immobilization turnover from respiration. Soil Sci Soc Am J 70:1121–1128
Malhi SS, Moulin AP, Johnston AM, Kutcher HR (2008) Short-term and long-term effects of tillage and crop rotation on soil physical properties, organic C and N in a black Chernozem in northeastern Saskatchewan. Can J Soil Sci 88:273–282
Mary B, Recous S, Darwis D, Robin D (1996) Interactions between decomposition of plant residues and nitrogen cycling in soil. Plant Soil 181(7):71–82
Matusso JMM, Mugwe JN, Mucheru-Muna M (2014) Effects of different maize (Zea mays L.) – soybean (Glycine max (L.) Merrill) intercropping patterns on soil mineral-N, N-uptake and soil properties. Afr J Agric Res 9:42–55
Mendham DS, Kumaraswamy S, Balasundran M, Sandaran KV, Corbeels M, Grove TS, O’Connel AM, Rance SJ (2004) Legume cover cropping effects on early growth and soil nitrogen supply in eucalypt plantations in south-western India. Biol Fertil Soils 39:375–382
Midwood AJ, Boutton TW (1998) Soil carbonate decomposition by acid has little effect on δ13C of organic matter. Soil Biol Biochem 30:1301–1307
Monaghan R (1995) Errors in estimates of gross rates of nitrogen mineralization due to non- uniform distributions of 15N label. Soil Biol Biochem 27:855–859
Mulvaney RL, Khan SA, Stevens WB, Mulvaney CS (1997) Improved diffusion methods for determination of inorganic nitrogen in soil extracts and water. Biol Fertil Soils 24:413–420
Nieder R, Benbi DK (2008) Anthropogenic activities and soil carbon and nitrogen. In: Nieder R, Benbi DK (eds) Carbon and nitrogen in the terrestrial environment. Springer, Germany, pp 161–218
Oelbermann M, Regehr A, Echarte L (2015) Changes in soil characteristics after six seasons of cereal-legume intercropping in the southern Pampa. Geoderma Reg 4:100–107
Oelbermann M, Echarte L (2011) Evaluating soil carbon and nitrogen dynamics in recently established maize-soyabean inter-cropping systems. Eur J Soil Sci 62:35–41
Pappa VA, Rees RM, Walker RL, Baddeley JA, Watson CA (2011) Nitrous oxide emissions and nitrate leaching in an arable rotation resulting from the presence of an intercrop. Agric Ecosyst Environ 141:153–165
Pelzer E, Bazot M, Makowski D, Corre-Hellou G, Naudin C, Al Rifai M, Baranger E, Bedoussac L, Biarnes V, Boucheny P, Carrouee B, Dorvillez D, Foissy D, Gaillard B, Guichard L, Mansard MC, Omon B, Prieur L, Yverginaux M, Justes E, Jeuffroy MH (2012) Pea-wheat intercrops in low-input conditions combine high economic performances and low environmental impact. Eur J Agron 40:39–52
Powlson DA, Barraclough D (1993) Mineralization and assimilation in soil-plant systems. In: Knowles R, Blackburn TH (eds) Nitrogen isotope techniques. Academic Press, Inc., San Diego, pp 209–242
Redin M, Recous S, Aita C, Dietrich G, Caitan Skilaude A, Hytalo Ludgke W, Schmatz R, Giancomini SJ (2014) How the chemical composition and heterogeneity of crop residue mixtures decomposing at the soil surface affects C and N mineralization. Soil Biol Biochem 78:65–75
Reents HJ, Möller K (2000) Effects of different green manure catch crops grown after peas on nitrate dynamics in soils and on yield and quality of subsequent potatoes and wheat. Proceedings of the 13th International IFOAM Scientific Conference. IFOAM, Zurich, pp 73–75
Regehr A (2013) Evaluation of maize and soybean intercropping on soil quality and nitrogen transformations in the Argentine Pampa. M.Sc. Thesis, University of Waterloo
Sakala WD, Cadisch G, Giller KE (2000) Interactions between residues of maize and pigonpea and mineral N fertilizers during decomposition and N mineralization. Soil Biol Biochem 32:679–688
Sanchez JE, Paul EA, Wilson TC, Smeen J, Harwood RR (2002) Corn root effects on the nitrogen-supplying capacity of a conditional soil. Agronomy 94:391–396
Sharma AR, Behera UK (2009) Recycling of legume residues for nitrogen economy and higher productivity in maize (Zea mays)-wheat (Triticum aestivum) cropping system. Nutr Cycl Agroecosyst 83:197–210
Sørensen P, Jensen ES (1991) Sequential diffusion of ammonium and nitrate from soil extracts to a polytetraethylene trap for 15N determination. Anal Chim Acta 252:201–203
Steel GD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics: a biometrical approach. McGraw-Hill, New York
Studdert G, Echeverría HE (2000) Crop rotations and nitrogen fertilization to manage soil organic carbon dynamics. Soil Sci Soc Am J 64:1496–1503
Tracy BF, Frank DS (1998) Herbivore influence on soil microbial biomass and nitrogen mineralization in a northern grassland ecosystem: Yellowstone National Park. Oecologia 114:556–562
Tolon-Becerra A, Tourn M, Botta GF, Lastra-Bravo X (2011) Effects of different tillage regimes on soil compaction, maize (Zea mays L.) seedling emergence and yields in the eastern Argentinean Pampas region. Soil Tillage Res 117:184–190
Vachon KE, Oelbermann M (2011) Crop residue input and decomposition in a temperate maize-soybean intercrop system. Soil Sci 175:157–163
Vandermeer J (1992) The ecology of intercropping. Cambridge University Press, Cambridge
Verchot LV, Holmes Z, Groffman PM, Lovett GM (2001) Gross vs net rates of N mineralization and nitrification as indicators of functional differences between forest types. Soil Biol Biochem 33:1889–1901
Vervaet H, Boeckx P, Boko AMCV, Cleemput O, Hofman G (2004) The role of gross and net N transformation processes and NH4 + and NO3 − immobilization in controlling the mineral N pool of a temperate mixed deciduous forest soil. Plant Soil 264:349–357
Videla C Del Carmen (2004) Mineralização bruta de nitrogênio em um Mollisol do sudeste da Provincia de Buenos Aires (Argentina). Ph.D. Thesis, Centro de Energía Nuclear na Agricultura, Piracicaba, Brazil
Zaman M, Di HJ, Cameron KC, Frampton CM (1999) Gross nitrogen mineralization and nitrification rates and their relationships to enzyme activities and the soil microbial biomass in sols treated with dairy effluent and ammonium fertilizer at different water potentials. Biol Fertil Soils 29:178–186
Zhang F, Li L (2003) Using competitive and facilitative interactions in intercropping systems enhances crop productivity and nutrient-use efficiency. Plant Soil 284:305–312
Acknowledgments
Funding for this study was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Foundation for Innovation (CFI). This work was supported by the University of Waterloo, the Instituto Nacional de Tecnologia Agropecuaria (INTA), Universidad Nacional de Mar del Plata, the National Research Council of Argentina (CONICET), and a scholarship to A. Regehr from NSERC, the Inter-American Institute for Co-operation on Agriculture (IICA-Canada) and the University of Waterloo. We also thank J. Schimel for his constructive editorial comments, and the anonymous reviewers that helped improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Liz Shaw.
Rights and permissions
About this article
Cite this article
Regehr, A., Oelbermann, M., Videla, C. et al. Gross nitrogen mineralization and immobilization in temperate maize-soybean intercrops. Plant Soil 391, 353–365 (2015). https://doi.org/10.1007/s11104-015-2438-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2438-0