Abstract
Background and aims
Soluble boron (B) sources pose a risk of B toxicity to seedlings just after planting and leaching losses after application and before plant uptake. Boron phosphate (BPO4) has low water solubility and slowly releases B, and hence could be safe for seedlings. Therefore, we investigated the toxicity of several B sources co-granulated with mono-ammonium phosphate (MAP) or co-compacted with potassium chloride referred to as muriate of potash (MOP) on canola seedlings.
Methods
Ulexite, borax, colemanite and BPO4 compounds synthesized at 500 or 800 °C for 1 h were co-granulated with MAP or co-compacted with MOP at inclusion rates of 0.5, 1.0 and 2.0 % B. The seedling toxicity of these products was evaluated by placing a fertilizer granule in the centre of a soil-filled Petri dish in which canola was seeded. The area of the non-vegetated zone around the granule application site was evaluated after 7 and 12 days of growth.
Results
Application of ulexite, borax and colemanite co-granulated with MAP resulted in toxicity symptoms at the lowest concentration of 0.5 % B, and the area of the affected zone increased with increasing concentrations of B in the granule, whereas no toxicity symptoms were observed with the application of co-granulated BPO4 products even at 2.0 % B content. Similar results were observed for the MOP fertilizers, except for colemanite which showed no toxicity when combined with MOP. Hot water-soluble B concentrations were measured in concentric sections around the granule application site and were in agreement with the toxicity results, with concentration in the toxic range close to the granule for the most soluble B sources.
Conclusions
BPO4 is potentially a seedling-safe B fertilizer source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Boron (B) is an essential micronutrient required for normal plant growth and development (Gupta 1979). Deficiency and toxicity of B are widespread problems (Shorrocks 1997), and may limit the yield and reduce the quality of crops (Camacho-Cristobal et al. 2008; Marschner 1986). Many studies on B fertilization indicate that the concentration range between deficiency and toxicity is narrow and application of B can be extremely toxic to plants at concentrations only slightly above optimum levels (Gupta 1983; Keren and Bingham 1985).
The application of fertilizer to soil at the same time as seed planting has become an increasingly common approach to supply crop nutrients, as this gives newly emerged seedlings early access to nutrients (Qian and Schoenau 2010). This strategy was found to be effective for phosphorus (P) fertilizers due to the low mobility of P in the soil (Qian and Schoenau 2010). However, this technique may not be suitable with fertilizers that contain a soluble B source, as toxicity is likely to occur when fertilizer is banded with the seed (Miller et al. 1971).
Soluble B sources such as sodium borate or borax (Na2B4O7.10H2O) and boric acid (H3BO3) are the most commonly used B fertilizer sources to maintain plant growth (Wear and Wilson 1954). However, not only may these soluble B sources pose a risk of B toxicity to seedlings just after planting, leaching losses after application and before plant uptake may result in low fertilizer use-efficiency in high rainfall areas (Mortvedt 1994). Slow-release B sources would reduce both the risk of seedling toxicity and of leaching losses. It has been suggested that colemanite (Ca2B6O11.5H2O) and ulexite (NaCaB5O9.8H2O) may serve as slow-release B sources (Wear and Wilson 1954). Byers et al. (2001) found that B concentrations in alfalfa were lower with colemanite than with granubor or ulexite as the B source. Broschat (2008) found that B release in sand columns followed the order: borax > ulexite > colemanite. However, we found that co-granulation of ulexite and colemanite with mono-ammonium phosphate (MAP) increases the solubility of these slow-release sources, thus counteracting their slow-release characteristics (submitted) and potentially making them toxic to seedlings in close contact to the fertilizer. In contrast with colemanite or ulexite, boron phosphate (BPO4) maintains its slow-release characteristics when co-granulated with phosphate fertilizers like MAP (submitted). The relatively high B content (10 %) in BPO4 and its low solubility should make it an efficient slow-release B fertilizer source (Magda et al. 2010; Ray 1972).
The aim of this study was to compare seedling toxicity of BPO4 and other B sources (ulexite, borax and colemanite) in B-enriched macro-fertilizers. The B sources were co-granulated or compacted with MAP or MOP (muriate of potash) and the toxicity to canola seedlings was assessed using a newly developed seedling toxicity test. Canola (Brassica napus L.) was used as test species because of its high B requirement.
Materials and methods
Boron fertilizer sources
The B sources were borax (BDH Analar), ulexite (ChemSupply), colemanite (Active Micronutrient Fertilizer) or BPO4 compounds. The BPO4 compounds were synthesized in the laboratory by mixing analytical reagent H3BO3 and solution phosphoric acid (H3PO4) and heating at 500 or at 800 °C for 1 h (Abat et al. 2014a). These B sources were co-granulated with MAP (submitted) or co-compacted with MOP at a rate of 0.5, 1.0 or 2.0 % B. The co-granulated MAP + B fertilizers were produced by thoroughly mixed and ground MAP and B source (<250 μm) in a grinder. The ground mixture (~20 g) was then transferred into a stainless steel laboratory scale pan granulator. A binder solution, lignosulfonate (~1 mL) was pumped using a peristaltic pump to the nebulizer and directed toward the tumbling materials rather than onto the pan surface while the pan granulator was rotating. A heat gun, set at a distance from the drum, was used to slowly and evenly dry the granules. The materials were rotated for about 15 to 20 min. Fines (≤1 mm) were reground and fed back into the drum, after which the granules produced were poured into a container and dried overnight in an oven at 40 °C.
To make the co-compacted MOP fertilizer, mixtures of MOP and the B source (ground to <150 μm) were homogenized and 2 mL of water was pipetted onto a 7 g subsample to form a tacky paste. The paste was compacted to a pellet using a 30 mm-diameter disc in a hydraulic press, by applying a pressure of 89.5 kg force cm−2. The pellet produced was circa 4 mm thick. The pellets were then cut into ‘chips’ of about ±2.4–4.0 mm in size while still pliable, prior to air drying. Analysis of the MOP co-compacted B fertilizers indicated that the B concentration was 15 % lower than the nominal rate for the MOP co-compacted colemanite samples and 32 % lower for the MOP co-compacted ulexite samples. This was likely related to water absorption by the colemanite and ulexite during and/or after grinding.
Toxicity test
The soil was a sandy loam from Bordertown (South Australia), with a pH in 0.01 M calcium chloride (CaCl2) of 5.12. The concentration of available B was determined using hot-water extraction (Bingham 1982) and was 0.26 mg/kg, which is considered deficient for most crops (Sims and Johnson 1991). Other physical and chemical properties of the soil are given in Table 1.
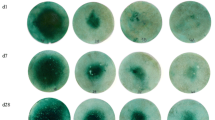
A Petri dish with an internal diameter of 9 cm was filled with about 50 g of soil moistened to field capacity. Canola seeds were sterilized by soaking in 0.04 M sodium hypochlorite (NaOCl) for 15 min, rinsed three times with deionized water and air-dried at room temperature. About one g of the sterilized canola seeds were weighed into a container. Another 10 g of the moistened soil was added to the seeds and mixed thoroughly. The soil and seed mixture was then spread evenly onto the soil in the Petri dish. A 30-mg co-granulated MAP fertilizer granule was placed at a depth of about 0.5 cm in the centre of the dish and the weight of the Petri dish recorded. The lid was then put on and the Petri dish transferred to a controlled environment room. The temperature of the room was 23–24 °C during the day and 15–16 °C during the night. The light was kept on for a period of 12 h per day and the light intensity was 232 μmol m−2 s−1. A blank (no fertilizer) and MAP-only treatment were also included. All treatments were replicated five times. The soil was consistently kept moist near field capacity to ensure seed germination. When the seedlings started to emerge (on the third day), the Petri dish lid was removed. Soil water content was maintained near field capacity by watering twice daily. On the seventh and twelfth day, photographs of the growing seedlings were taken. The percentage of vegetation coverage was determined with image analysis software (GIMP 2.8). The original images were decomposed (YCbCr ITU 470) and a threshold function was applied to the redness layer to convert the images to black (vegetated) and white (non-vegetated), after which the area of the vegetated zone was quantified (Fig. 1).
The experiment with the MOP fertilizers was carried out in the same way, but using a 40-mg co-compacted MOP granule and with four replicates per treatment.
Soil analysis
At the end of the 12-day experiment, the soils were air-dried and the B concentrations in soil sections around the granule application site were determined in hot-water extracts for the treatments with the granules with 2 % B, the MAP- or MOP-only and the blank (no fertilizer) treatment. The soil in the Petri dish was sampled in three concentric sections: less than 7.5 mm (corresponding to 1.7 g soil), between 7.5 and 15 mm (corresponding to 5 g soil) and further than 15 mm from the fertilizer application site (corresponding to 53 g soil). Five grams of soil (less for the inner section) were weighed into a centrifuge tube and 20 mL of boiling deionized water was added and shaken for 1 h. The suspension was filtered through Whatman No. 42 filter paper. Boron concentration in the filtrate was then analyzed using inductively coupled plasma—optical emission spectroscopy (ICP-OES; Optima 7300 DV, Perkin Elmer).
Statistical analysis
Statistical analysis was conducted using Genstat 15th Edition SP2. Analysis of variance was performed to assess the effect of fertilizer source or B rate on the affected area. The differences between the means were evaluated using the Duncan Test for multiple comparisons.
Results
Seed emergence
Clear differences in the toxicity symptoms were observed between the fertilizer treatments (Fig. 1). In the treatments using MAP co-granulated with ulexite, borax and colemanite, toxicity symptoms (yellowing and stunted growth) were already evident even in the 0.5 % B treatments and became more severe with increasing B content in the fertilizer (Fig. 1). The canola seedlings treated with co-granulated BPO4 products displayed healthy growth without obvious visual toxicity symptom observed even at 2.0 % B content (Fig. 1). Also for the MOP fertilizers co-compacted with ulexite and borax, toxicity symptoms were already observed at 0.5 % B and became more prominent with increasing B contents. On the other hand, the canola seedlings in the MOP with colemanite treatment showed healthy growth, similar to that observed for the blank and BPO4 treatments. Only at 2 % B as BPO4 or colemanite, there was a small affected zone close to the fertilizer application site at day 7 (Fig. 1), but this had disappeared by day 12.
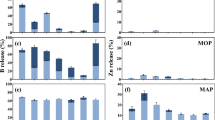
The non-vegetated area was derived through image analysis (Table 2). In the blank treatment, there was a small non-vegetated area due to incomplete coverage. The non-vegetated area was larger for the MAP (without B) treatment than for the blank treatment, indicating that the MAP fertilizer had a slight toxic effect on the seedlings. For the MAP + B treatments, the non-vegetated area generally followed the order: ulexite ≥ borax ≥ colemanite > BPO4 and increased with increasing B concentration in the fertilizer. The non-vegetated area was significantly larger for MAP co-granulated with ulexite and colemanite at 1 and 2 % B and co-granulated with borax at 2 %B than for the control MAP (Table 2). However, there was no significance difference between control MAP and MAP co-granulated with BPO4 at any of the B levels (Table 2).
For the MOP + B treatments, the non-vegetated area generally followed the order: borax ≥ ulexite > colemanite ~ BPO4. Thus, colemanite showed less toxicity when combined with MOP than with MAP, indicating that the ranking in toxicity between the B sources depends on the macro-fertilizer. The non-vegetated area was significantly larger for the ulexite and borax than for the colemanite and BPO4 treatments for all B rates, with the exception of ulexite vs BPO4 at 0.5 % B. There was no significance difference between MOP -only and MOP co-compacted with colemanite and BPO4 at any of the B contents (Table 2).
Hot-water extractable B concentrations
Hot-water extractable B concentrations around the fertilizer application site were higher for the MAP products with ulexite, borax and colemanite than for those with BPO4 (Fig. 2a). For the MOP fertilizers, the B concentrations at >7.5 mm from the fertilizer application site were higher for the products with ulexite and borax than for those with colemanite and BPO4 (Fig. 2b). In the inner section (<7.5 mm from the application site), however, the measured hot-water extractable concentration was much larger for the MOP + colemanite treatment (100 mg B/kg) than for the other treatments (≤25 mg B/kg), which can be explained by the large liquid: solid ratio used during extraction, resulting in solubilization of colemanite that was not solubilized under in situ conditions and hence did not reach the middle section.
Hot-water extractable B concentration for the fertilizers with 2 % B at 12 days after fertilizer application at 7.5–15 or >15 mm from the fertilizer application sites, for the MAP + B fertilizers (a) and the MOP + B fertilizers (b). The dashed line indicates a concentration of 5 mg B/kg, which is close to the toxicity threshold for most crops
The total amount of added B recovered with the hot-water extraction was calculated by summing the hot-water extractable B amount recovered in the three sections (soil mass multiplied by the hot-water extractable concentrations) (Table 3). For the MAP treatments, between 42 and 56 % of the added B was recovered in hot-water extractable form for the soluble B sources (ulexite, borax and colemanite) compared to 16 % for the BPO4 500 °C and 3 % for BPO4 800 °C. For the MOP treatments, >70 % of B was recovered in hot-water extractable form for ulexite and borax compared to <50 % for the less soluble sources. The difference in recovery between colemanite and the BPO4 sources co-compacted with MOP was due to the higher recovery for the colemanite treatment in the inner section, while there was little difference in recovery between colemanite and BPO4 500 °C in the middle and outer section. This indicates that the solubility of colemanite was higher than that of the BPO4 products under the extraction conditions (high liquid: solid ratio) but not under in situ conditions (Table 3).
The ranking in hot-water B concentrations relates well to the toxicity results: for MAP co-granulated, colemanite is nearly as soluble and toxic as ulexite and borax. However, when co-compacted with MOP, colemanite is less soluble and less toxic than ulexite and borax.
Discussion
The results of this study showed that the application of fertilizers with ulexite or borax as B sources at seeding adversely affected germination and early growth of canola seedlings. On the other hand, canola seedlings treated with BPO4 products did not display these negative effects. For colemanite, the effect depended on the macrofertilizer. Toxic effects were similar to those of ulexite and borax when colemanite was co-granulated with MAP, but there was no apparent toxicity when colemanite was co-compacted with MOP.
Toxicity thresholds of around 5 mg B/kg (hot-water extraction) have been published for most crops (Cayton 1985; Nable et al. 1997). Mortvedt and Osborn (1965) found that root growth of oat and alfalfa was markedly decreased at hot-water B concentrations above 10 mg/kg. In this study, we found that the hot-water B concentrations were ≥5 mg B/kg in all three sections for MAP co-granulated with borax, ulexite and colemanite and for MOP co-compacted with ulexite and borax (Fig. 2). Thus, the toxic effects of the fertilizers could be related to their solubility under in situ conditions. The higher toxicity of colemanite when combined with MAP can be explained by the solubilizing effect of MAP on colemanite, due to a decrease in pH and Ca2+ concentration because of Ca-P precipitation around the granule while this is not the case for MOP (submitted).
Toxic effects of soluble B fertilizers in the field have frequently been observed. The application of borax fertilizer, a soluble B source, has caused severe injury to crops grown in coarse-textured soils (Winsor 1950). Ozturk et al. (2010) found that the application of borax fertilizer broadcast at 15 kg B/ha in field experiments reduced the seed yield of eight canola cultivars by 31 % on average. Banding of B fertilizer can be advantageous if the concentrations are within the acceptable range and the seed is placed adequately away from the band (Hughes-Games 1991), as it can be more effective in delivering B to roots. However, the risk of B toxicity is higher with banded B fertilizer. For instance, considering 30-mg granules with 2 % soluble B, an application rate of 1.5 kg B/ha corresponds to 2.5 granules per dm2 or an average distance between granules of circa 6.3 cm. However, when banded with the seed, the distance between granules would be much smaller, and the likelihood of seeds being within a distance that would harm the seedling would be much higher.
The use of less soluble B sources is expected to eliminate or reduce the risk of B toxicity to crop seedlings. Colemanite applied in pure form has been regarded as a slow-release B fertilizer reducing risk of toxicity. Wear and Wilson (1954) reported that water soluble B extracted from colemanite was five times less than from borate, and it required twice as much colemanite as borate to produce the same toxicity effect. Because it is difficult to separately apply low rates of pure B fertilizers, B fertilizer is usually applied either blended or co-granulated with macronutrient fertilizers. Co-granulated fertilizer allows for a better field distribution of the B. However, when co-granulated with MAP, the low pH and high P concentrations (reducing Ca activities) render colemanite almost as soluble as ulexite and borax. Here, we showed that the toxicity of MAP co-granulated with B was similar for colemanite, ulexite and borax. In contrast, BPO4 still acted as a slow-release B source when co-granulated with MAP, and did not increase toxicity compared to MAP without B. In a recent pot trial study, we assessed the response of canola to these co-granulated MAP fertilizers under leaching conditions in two consecutive crops (Abat et al. 2014b). While the more soluble B sources induced B toxicity in the first crop and B deficiency in the second crop, the fertilizers with BPO4 provided adequate B supply for both crops.
The application of fertilizers with soluble B sources at planting clearly has disadvantages for crop seedlings and could be potentially toxic even at low application rates. Predicting the rates at which toxicity may occur is difficult, as this depends on soil characteristics, environmental conditions and crop species. Use of BPO4 as a B source in NPK fertilizer is likely to be a seedling-safe alternative. Further studies in field experiments are required to assess the effectiveness of BPO4 as a slow-release B fertilizer.
References
Abat M, Degryse F, Baird R, McLaughlin MJ (2014a) Formulation, synthesis and characterization of boron phosphate (BPO4) compounds as raw materials to develop slow-release boron fertilizers. J Plant Nutr Soil Sci 177:860–868
Abat M, Degryse F, Baird R, McLaughlin MJ (2014b) Responses of canola to the application of slow-release boron fertilizers and their residual effect. doi:10.2136/sssaj2014.07.0280
Bingham FT (1982) Boron. In: Page AL, Miller RH, Keeney DR (eds) In methods of soil analysis part 2 - chemical and microbiological properties, 2nd edn. Soil Science Society of America, Madison, pp 431–448
Broschat TK (2008) Release rates of soluble and controlled-release boron fertilizers. Hort Techn 18:471–474
Byers DE, Mikkelsen RL, Cox FR (2001) Greenhouse evaluation of four boron fertilizer materials. J Plant Nutr 24:717–725
Camacho-Cristobal JJ, Rexach J, Gonzalez-Fontes A (2008) Boron in plant: deficiency and toxicity. J Integ Plant Biol 50:1247–1255
Cayton MTC (1985) Boron toxicity in rice. pp 1–11. International Rice Research Institute
Gupta UC (1979) Boron nutrition of crops. Adv Agron 31:273–307
Gupta UC (1983) Boron deficiency and toxicity symptoms for several crops as related to tissue boron levels. J Plant Nutr 6:387–395
Hughes-Games G (1991) Boron for field crops. Soil factsheet order no. 631.021-1. Ministry of Agriculture and Food British Columbia, Abbotsford, pp 1–3
Keren R, Bingham FT (1985) Boron in water, soil and plants. Adv Soil Sci 1:229–276
Magda A, Pode R, Muntean C, Medeleanu M, Popa A (2010) Synthesis and characterization of ammonium phosphate fertilizers with boron. J Serbian Chem Soc 75:951–963
Marschner H (1986) Mineral nutrition in higher plants. Academic, London
Miller MH, Bates TE, Singh D, Baweja AS (1971) Response of corn to small amounts of fertilizer placed with the seed: 1. Greenhouse studies. Agron J 63:365–368
Mortvedt JJ (1994) Needs for controlled-availability micronutrient fertilizers. Fertil Res 38:213–221
Mortvedt JJ, Osborn G (1965) Boron concentration adjacent to fertilizer granules in soil, and its effect on root growth. Soil Sci Soc Am Proc 29:187
Nable RO, Banuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 193:181–198
Ozturk O, Soylu S, Ada R, Gezgin S, Babaoglu M (2010) Studies on differential response of spring canola cultivars to boron toxicity. J Plant Nutr 33:1141–1154
Qian P, Schoenau J (2010) Effects of conventional and controlled release phosphorus fertilizer on crop emergence and growth response under controlled environment conditions. J Plant Nutr 33:1253–1263
Ray LF (1972) Boron phosphate as boron source for plant life. Ed. U S Patent. pp 1–4, United States
Shorrocks V (1997) The occurrence and correction of boron deficiency. Plant Soil 193:121–148
Sims JT, Johnson GV (1991) Micronutrient soil tests. In: Mortvedt JJ, Cox FR, Shuman LM, Welch RM (eds) Micronutrients in agriculture, 2nd edn. Soil Science Society of America, Madison
Wear JI, Wilson CM (1954) Boron materials of low solubility and their use for plant growth. Soil Sci Soc Am Proc 18:425–428
Winsor HW (1950) Boron sources of moderate solubility as supplements for sandy soils. Soil Sci 69:321–332
Acknowledgments
The first author thanks the University of Adelaide for the scholarship to enable her to pursue her PhD and the Sarawak State Government for a study leave. The authors also thank Bogumila Tomczak, Deepika Setia, Colin Rivers, Ashleigh Broadbent and the staff of CSIRO Land and Water for their advice and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ismail Cakmak.
Rights and permissions
About this article
Cite this article
Abat, M., Degryse, F., Baird, R. et al. Boron phosphates (BPO4) as a seedling-safe boron fertilizer source. Plant Soil 391, 153–160 (2015). https://doi.org/10.1007/s11104-015-2424-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2424-6