Abstract
Background
Phospholipid fatty acid (PLFA) analysis is an effective non-culture-based technique for providing information on the living soil microbial community. The coupling of 13C tracers with PLFA analysis can indicate the response of microbial populations to environmental change and has been widely used to trace C flux in soil-plant systems.
Scope
Based on studies applying 13C PLFA analysis, the current technological status, current applications and future opportunities are discussed and evaluated. First we describe some aspects of the labelling and analytical methodology. The approaches to study the incorporation of 13C substrate and rhizodeposition C into soil microbial communities are compared. We continue with the application of 13C-labelling to study soil microbial communities, including the utilization of soil mineralisation products, the C flux from plants into the soil microbial pool, the biodegradation of pollutants and on the application to a specific microbial group, i.e. methanotrophs. Additionally, some perspectives on the limitations of the 13C PLFA method and future research avenues are noted.
Conclusions
Although including some limitations and complications, the 13C PLFA method provides an excellent tool for understanding the relationship between microbial populations and soil biogeochemical cycling, thus providing a key to open the soil microbial black box.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
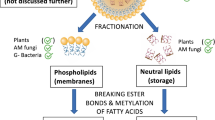
Soil microbial ecology has always been hampered by the difficulty in seeing the activities of microorganisms in their natural environment. A major breakthrough occurred nearly 40 years ago when Jenkinson and colleagues at Rothamsted and Anderson and Domsch in Braunschweig developed methods for estimating the soil microbial biomass (Anderson and Domsch 1978; Vance et al. 1987; Insam 2001). However, for many years the soil microbial biomass remained very much a black box until the advent of molecular methods began to reveal the vast diversity of the microbial community (Tiedje et al. 1999). At the same time, simpler methods based upon specific biomarkers were developed (Insam 2001). Among these was the use of phospholipid fatty acid (PLFA) analysis, first applied to soil by Frostegård et al. (1993).
The advantage of PLFAs over other biomarkers is that they are indicative of living organisms since they are rapidly hydrolysed upon cell death. Their disadvantage is that the taxonomic resolution, with a few notable exceptions (given below), is relatively coarse. We can distinguish bacteria and fungi, to some extent Gram negative and Gram positive organisms, actinomycetes and certain mycorrhizal fungi. Archaea are not included as they do not contain PLFAs; they can be typed however if phospholipid ether lipids are analysed (Gattinger et al. 2003). However to take analysis beyond just community structure, we can utilize stable isotope probing (PLFA-SIP) to link microbial populations to specific biogeochemical processes (Boschker et al. 1998). Another advantage is that the ability to perform PLFA-SIP is relatively easy compared with, say, DNA and RNA-SIP. Using 13C-labelled substrates (including 13C-CO2) we can follow their utilization by different microbial groups as the label is incorporated into their PLFAs. In longer term experiments this label may then enter the soil microbial food web and insights gained into the flow of soil carbon. In this review we have restricted ourselves to the application of 13C PLFAs to soil microbiology though clearly there are applications in other ecosystems.
From the late 1990’s there has been a continual increase in the number of studies applying PLFA-SIP to soil studies (based on ISI Web of Science search: a total of 174 references up until July 2014, excluding review articles). The majority of studies have used isotope labelling but about 16 % took advantage of natural abundance; this is where differences in the natural abundance of 13C, such as between C3 and C4 plants, can be exploited (Stemmer et al. 2007; Nottingham et al. 2009). About 70 % of studies have used some form of pulse labelling technique while 30 % have taken a continuous labelling approach. The most common substrate is plant material, either labelled beforehand or in situ with 13CO2, or using differences in natural abundance. The next most common substrate is actually 13CH4, followed by 13C-glucose. In terms of ecosystem, there has been a fairly even split between grassland, forest and arable land, with fewer studies on wetlands (including peatlands). The majority of studies report on the presence of bacteria and fungi with about half giving the Gram positive-Gram negative split. A minority report on actinomycetes but very few pick up protozoa. In the following, we describe briefly some aspects of the labelling methodology and analytical methodology. We continue with the application of 13C-labelling to study soil microbial communities, including the soil-plant ecosystem, the biodegradation of soil pollutants and with some detail on the application to methanotrophy in soil. We conclude with some perspectives on the limitations of the method and likely future opportunities and developments.
Labelling and analysis approaches
Labelling methodology
A wide range of possibilities exist both in potential labelling approaches and in potential labelling materials. In most labelling studies there is an intention to cause a shift in the δ13C signature of the PLFAs extracted from soil and from this infer the sources of carbon being used by soil microbes. To be useful as a labelling material it is necessary that the isotopic value of the label differs from that of the unlabelled material. Labelling can therefore be achieved using material which is either enriched or depleted in 13C compared to the unlabelled material. The scientific question being addressed should be the main driver for selecting both the labelling material and the approach to be used.
Potentially any carbon containing compound which can be labelled with 13C can be added to soil directly and the δ13C values of PLFAs followed to assess utilisation of the labelled carbon component of the compound by various soil microbe groups. The added 13C substrates include plant litter, root residue, root exudates and environmental pollutants. The use of 13C labelled CH4 or CO2 coupled with compound specific analysis of PLFAs has been also used to study specific microbial communities in soil-plant systems (Nazaries et al. 2013; Chowdhury and Dick 2013)
In soil-plant ecosystems, a common form of 13C labelling used in conjunction with compound specific isotope analysis of soil PLFAs is that where atmospheric CO2 is labelled. The 13CO2 is first fixed by plants in the process of photosynthesis; labelled plant inputs then enter the soil to be potentially taken up and assimilated by soil microbes, subsequently labelling their PLFAs. Two main approaches are used to deliver 13CO2 to the plants: either pulse or continuous steady state labelling. In pulse labelling, as its name suggests, the 13CO2 is delivered to plants over a discrete period of time, typically this period tends to be short measured in hours, after which the plant is returned to an atmosphere containing unlabelled CO2. In continuous steady state labelling the labelled CO2 is constantly maintained following a single switch from unlabelled CO2. In steady state labelling the labelling periods tend to be longer, ranging from days to years. Pulse and steady state labelling are two contrasting methods but other approaches have been developed which lie somewhere between these two extremes, using for example a series of repeated pulse labelling periods. This approach avoids the use of the complex equipment required for steady state labelling but is considered to give more homogenous labelling of plant pools than single pulse labelling, though it is accepted that such labelling will not be as homogeneous as that achieved using steady state labelling (Bromand et al. 2001; Subedi et al. 2006; Sangster et al. 2010).
One common element of pulse, repeat pulse and steady state labelling is that they all use plants to deliver the labelled materials into the soil and subsequently into soil microbial PLFAs. Other methodologies such as leaf labelling and stem injection have been proved to be successful in introducing labelled carbon materials into plants. These are usually low molecular weight compounds such as bicarbonate, urea and aspartic acid (Putz et al. 2011; Powers and Marshall 2011; Churchland et al. 2012; Rasmussen et al. 2013). Churchland et al. (2012) successfully traced 13C-labelled aspartic acid injected into the stems of 22 year old Sitka spruce trees into the soil PLFA pool. An advantage of these techniques is that they can be applied to trees where 13CO2 labelling would require very large chambers (Powers and Marshall 2011; Churchland et al. 2012) and that they allow individual plants within natural communities to be labelled (Putz et al. 2011).
13C PLFA analysis
The main components of a typical GC-C-IRMS system are shown in Fig. 1. In order to make PLFAs amenable for GC analysis they are derivatised to their fatty acid methyl esters (FAMEs) prior to analysis. A mixture of FAMEs is injected into the instrument prior to the GC column; on their passage through the GC column the FAME mix is separated into a series of individual FAME peaks which enter the oxidation (or combustion) column. On passing through the oxidation column all the carbon present in the FAMEs is converted into CO2. A series of CO2 peaks therefore leaves the oxidation column. These CO2 peaks are subsequently separated into peaks of 12CO2 and 13CO2, and quantified in the isotope ratio mass spectrometer. The δ13C values determined by GC-C-IRMS are therefore those of the methyl esters (δ13C FAME). A mass balance equation is used to account for the one carbon added in the methyl group during the derivatisation process (Esperschütz et al. 2009) to determine the isotope ratio of the PLFAs themselves (δ13C PLFA).
Application of 13C-PLFAs to investigate soil microbial communities
Microbial utilization of added substrates
One important use of 13C-labelling is for the investigation of microbial utilization of soil organic matter mineralization products. Often the labelled compounds added to soil are chosen to mimic soil organic matter mineralization products or plant inputs. A range of 13C enriched low molecular weight compounds typical of those lost by plants in rhizodeposition, such as glucose, glycine, acetate, amino acid mixtures, and water extracts of plants, have all been successfully traced into soil PLFA pools (Paterson et al. 2008a; Rinnan and Bååth 2009; Dungait et al. 2011, 2013; Andresen et al. 2014; Lemanski and Scheu 2014). More recalcitrant fractions typical of those entering soil through leaf litter and root residues have been added to soil and traced into soil PLFA pools. Pure compounds of starch and vanillin were traced into the PLFA pools of tundra soils by Rinnan and Bååth (2009). The incorporation of 13C-labelled crimson clover, ryegrass root and straw residues into PLFAs was studied by Williams et al. (2006). Their results confirmed that a subset of the soil biomass was primarily responsible for assimilating residue-derived C, and residue properties and soil conditions influenced which members of the community were assimilating residue-derived C. Stemmer et al. (2007) traced C4 maize straw added to soil developed under C3 vegetation into the soil PLFA pools. Elfstrand et al. (2008) successfully traced decomposing 13C enriched green clover manure into PLFAs associated with actinomycetes and Gram-positive bacteria. While Paterson et al. (2008a), in individually applying both labelled labile and recalcitrant fractions to soil and compound specific analysis of the soil PLFAs, was able to show that distinct microbial communities utilised the different fractions. Where recalcitrant plant inputs have been controlled, either through plant exclusion (Paterson et al. 2011), or by litter removal/addition treatments (Brant et al. 2006), the capacity of the microbial community to utilise recalcitrant substrates is increased where the soil has a history of these as inputs, with δ13C-PLFA identifying a central role for the fungal component. The capacity of δ13C-PLFA to link microbial identity with function capacity has also been applied to identify groups potentially involved in decomposition of native soil organic matter (Garcia-Pausas and Paterson 2011). However, for fungal communities the utility of δ13C-PLFA is limited by the availability of discrete biomarkers. For example, although it is likely that distinct fungal populations are involved in the group’s strong utilisation of both labile and recalcitrant substrates, this cannot be resolved with PLFA biomarkers.
As mentioned, the labelled C compounds added to soil do not necessarily relate to those mimicking plant losses. Wang et al. (2014) followed the incorporation of urea derived 13C into the soil microbial pools of four different arable soils. The distribution and 13C PLFA composition derived from a weathering profile developed on a black shale suggested that some assimilation of shale organic matter was carried out by anaerobic microorganisms (Petsch et al. 2003). 13C-labelled biochar has been used to monitor its degradation and metabolism by microbial communities; the actinomycetal PLFA 18:0(10Me) and Gram-negative bacterial PLFAs (16:1ω7c, 16:1ω5c, 18:1ω7c) were found to link with its degradation (Watzinger et al. 2014). A few studies have followed the fate of 13C-labelled microbial cultures added to soil (Kindler et al. 2009; Murase et al. 2011). Recently, the use of position-specific 13C-labelled substrates, coupled with quantification of 13CO2 efflux from parallel incubations of substrate isotopomers, has been demonstrated as a means of characterising the relative activity of metabolic pathways in soil (Dijkstra et al. 2011). Apostel et al. (2013) extended this concept to also quantify fate of position-specific 13C-label into PLFA fractions, which offers an exciting opportunity to further link microbial identity with C-cycling processes.
Carbon flux in soil-plant systems
In pulse labelling the relatively short labelling period followed by a chase period results in the label being biased towards tracking carbon which has been recently fixed by the plants. In these instances the labelled plant inputs entering the soil tend to be in the form of rhizodeposition comprising of root exudates (a range of generally low molecular weight compounds), sloughed cells, etc. Pulse labelling techniques as applied to studying carbon allocation in trees have recently been reviewed (Epron et al. 2012). Considering the supply of carbon to plant meristems, de Visser et al. (1997) state that the accurate determination of the contribution of recently fixed and older carbon requires homogenous labelling and knowledge of the specific activity of the assimilate pools. Such homogenous labelling is not achieved in short term pulse labelling experiments (De Visser et al. 1997) and the mixing of labelled and non-labelled C pools in transport to plant carbon sinks is not accounted for (Schnyder 1992). The same constraints apply to attempts to quantify the contribution of recently fixed and older plant carbon to rhizodeposition and subsequently into soil microbes. On the other hand, pulse labelling approaches are very well suited to study the temporal dynamics of the transfer of the carbon fixed by plants into rhizodeposition and subsequently into soil microbial pools. In PLFAs extracted from soil following a pulse chase experiment using CO2 enriched in 13C, it would be expected that their 13C enrichment would immediately, or following a lag period due to plant metabolization and re-allocation of store carbon pools, increase to a maximum value after which they would fall again to approach the δ13C values of unlabelled controls. The shape of the tracer time course can provide information on both the extent to which the label has mixed with unlabelled pools before arrival in microbial PLFAs and the turnover rate of the various PLFA pools (Epron et al. 2012).
Steady state labelling (Fig. 2) has the potential to quantify the flux of carbon from plants into the soil microbial pool. With steady state labelling of 13CO2 the labelling periods used have varied greatly. In laboratory experiments periods of days to weeks have been utilised, whilst in free air CO2 enrichment (FACE) experiments the labelling periods are typically much longer (Esperschütz et al. 2009; Grams et al. 2011). For example, Esperschütz et al. (2009) used a steady state approach to trace 13CO2 into soil PLFA pools following the labelling of young beech trees in open top chambers over an entire growing season. As the labelling period increases, the labelled plant inputs will not just contain recent photosynthate but will additionally contain material derived from carbon fixed by the plant considerably earlier – from the start of the labelling period until the time of sampling. It will therefore start to include material derived from tissue turnover such as root and leaf litter. Comparing results from pulse with steady state labelling techniques and considering not just soil microbes but additionally other soil animal groups, Högberg et al. (2010) noted “the longer the labelling period and time elapsed after labelling, the greater the numbers of individuals and animal groups that are likely to be labelled”.
Another technique which utilises the plant to deliver carbon to soil is where advantage is taken of the differing discrimination against 13C in the photosynthetic pathways used by C3 and C4 plants; resulting in them having different δ13C signatures. Typically C3 plants and the soil organic matter beneath them have δ13C values of around −27 ‰, whilst C4 plants and their associated soil organic matter both have values around −13 ‰ (Boutton 1996). In effect a single switch from C3 to C4 plants, or vice versa, is equivalent to steady state labelling. In conjunction with compound specific analysis of PLFAs it allows discrimination of the use of new carbon fixed after the vegetation switch from the use of older carbon fixed prior to the switch (Kramer and Gleixner 2006, 2008).
The presence of plant roots is known to affect both the abundance and composition of microbial communities (Paterson et al. 2007), but application of δ13C-PLFA allows direct identification of components of communities active in utilisation of plant-derived C, concurrent with assessment of community shifts (Tavi et al. 2013). Butler et al. (2003) were first to apply 13CO2 pulse-labelling coupled with δ13C-PLFA to identify microbial groups active in utilisation of plant-derived C. Their greenhouse experiment tracked recent assimilate from Lolium perenne and identified fungal and Gram negative groups as being dominant in utilisation of plant-derived C, and that the partitioning of C-flow through these groups was affected by plant growth stage. Treonis et al. (2004) applied a similar approach, under field conditions, and found consistent results, confirming that recent plant assimilate is predominantly channelled through fungal and Gram negative components, but to a much lesser extent through abundant Gram positive biomarkers. The application of continuous labelling to establish uniform 13C-enrichment of root inputs to soil extends this approach to allow quantification of the relative use of plant- and SOM-sources by rhizosphere microbial groups. Taking this approach has identified that free-living microbial groups, including fungi and Gram negatives active in utilisation of root-derived C, obtain a large proportion (>40 %) of C from SOM sources (Paterson et al. 2007, 2011). An implication of this is that environmental factors (e.g. elevated atmospheric CO2) that increase root-derived C-flow may also increase microbial utilisation of SOM, i.e. priming effects (Paterson et al. 2008b). The importance of root-derived C for sustaining soil food webs was demonstrated by Pollierer et al. (2012) in an elegant experimental design exploiting long-term FACE labelling coupled with litter substitutions with paired, unlabelled plots. Isotopic analyses of soil animal populations indicated that the majority of decomposer biomass was derived from root inputs to soil, as opposed to above ground litter. For experiments utilising FACE sites or C3/ C4 vegetation shifts, microbial utilisation of C-sources can be extended to consider plant C that has been incorporated into SOM pools. That is, microbial substrate can be partitioned into that derived from plant inputs from the onset of FACE treatments or vegetation change, relative to SOM present prior to these treatments. Kramer and Gleixner (2008) quantified the contribution of C derived from 40 years of maize cultivation on a soil previously cultivated with C3 vegetation to bulk SOM and to microbial PLFA. The contribution of maize C to SOM declined with depth, but at each depth the total microbial PLFA was relatively enriched indicating preferential use of maize inputs, relative to SOM >40 years old. They further identified a greater preference for maize-derived C in Gram negative biomarkers, relative to Gram positives, supporting that these groups have distinct roles in soil C-cycling processes. Streit et al. (2014) utilised a FACE site incorporating a warming treatment to demonstrate that, despite no effect on community structure, soil warming resulted in greater microbial (specifically fungal) use of older SOM (>9 years), highlighting potential impacts of changing climate on stabilised SOM pools.
Mycorrhizal hyphae represent an important route for C-transfer to soil, being both abundant and having rapid rates of turnover (Godbold et al. 2006). Van Aarle and Olsson (2003) identified (in axenic systems), that AM fungi (Scutellospora calospora and Glomus intraradices) associated with Plantago lanceolata could be quantified by the abundance of PLFA 16:1ω5 and that the corresponding neutral lipid accumulated as a storage compound. Subsequent studies have highlighted that PLFA 16:1ω5 is also abundant in Gram negative biomass, limiting its utility as an AM biomarker (van Aarle and Olsson 2003). However, studies applying 13CO2 pulse-labelling have been successful in demonstrating rapid transfer of plant assimilate to neutral lipid 16:1ω5, consistent with it being a marker of AM fungal biomass (Olsson and Johnson 2005). While continuous labelling offers the opportunity to quantify the extent to which it is a biomarker unique to AM fungi, as if it is assumed that the AM biomass is derived exclusively from plant assimilate (Ho and Trappe 1973), the extent of biomarker 13C-enrichment should match that of the plant C-source.
Pollution biodegradation
PLFA analysis combined with 13C-labelled tracers has been used recently as an effective technique for distinguishing the specific microbial populations involved in soil pollutant biodegradation (Evershed et al. 2006). Many studies (Hanson et al. 1999; Pelz et al. 2001; Mauclaire et al. 2003) have focused on 13C-labelled toluene as a microbial substrate, due to its wide distribution as part of the BTEX (Benzene-Toluene-Ethylbenzene-Xylene) set of petroleum pollutants and its rapid biodegradation. Hanson et al. (1999) compared the incorporation of 13C-toluene and 13C-glucose into soil microbial communities. They found that 27 and 91 % of the total soil PLFA species were labelled in the 13C-toluene and 13C-glucose treatments, respectively, confirming the advantage of coupling 13C tracers with PLFA analysis for investigating pollutant biodegradation in complex environments. However, the shift in PLFA profile should be explained carefully when using the 13C-labelled technique, since the high amount 13C-labelled pollutant generally applied can exert an inhibitory effect on fatty acid biosynthesis and change the microbial community structure (Fang et al. 2004).
The wide application of herbicides, fungicides, pesticides and other agrochemicals has resulted in serious pollution in soil-plant ecosystem. Since soil microorganisms are the main drivers for the biodegradation of these mainly organic pollutants, 13C-labelled pollutants, e.g., polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), pentachlorophenols (PCPs), are usually amended to soil samples and then the 13C labelling of PLFAs used for characterizing the pollutant-degrading microbial communities (Kamashwaran and Crawford 2001; Johnsen et al. 2002; Tillmann et al. 2005; Antizar-Ladislao et al. 2008). Changes in Gram-positive to Gram-negative bacterial PLFAs (Mellendorf et al. 2010), dominating degradation species (Tillmann et al. 2005) and total microbial biomass (Tomco et al. 2013) have been all been demonstrated in herbicide polluted soils. The application of 13C-labeling PLFAs combined with gene probe methods to study biocide-degrading microbial populations suggested a shift in PLFA profile where the phylum Acidobacteria and the genera Phenylobacterium and Comamonas were the main assimilators during Cu-N-cyclohexyldiazenium dioxide degradation (Jakobs-Schönwandt et al. 2010).
Methanotrophs
The application of 13C-labelling of PLFAs to the study of methanotrophy in soils has several advantages over other areas of interest. First, we are dealing with a single substrate, methane, which as a gas, is easily applied to soil. Second, it is fortuitous that most known methanogens possess some very specific PLFAs that are not found in other organisms (Bodelier et al. 2009; Chowdhury and Dick 2013). Third, we also understand much about the unique methane monooxygenase (MMO) genes in these organisms such that a number of studies have benefited from the combination of labelling of PLFAs with genetic analysis (Knief et al. 2006; Mohanty et al. 2006).
The impetus behind most studies on methanotrophy is to understand that part of the methane cycle responsible for removing atmospheric methane or for mitigating emissions from methanogenesis in flooded soils in the context of methane as a major greenhouse gas. Hence there has been considerable interest in using 13C-labelling of PLFAs to the study of methanotrophy.
In the real world, methane is available at two levels: either at 1.8 ppmv as atmospheric methane or at much greater concentrations in environments where there is active methanogenesis. This has led to the speculation that there are two physiological groups: high affinity methanotrophs, which can take up methane at 1.8 ppmv, and low affinity methanotrophs, which are only operative at >40 ppmv (Chowdhury and Dick 2013). To date, only low affinity methanotrophs have been isolated in pure culture so we only know the PLFA profiles for this group.
Based primarily upon their metabolic pathways, the arrangement of their intracytoplasmic membranes, G + C content and pmoA genotype, methanotrophs have been divided into two major groups, Type I and Type II (Chowdhury and Dick 2013; Nazaries et al. 2013). Type I and II organisms are also described as Gammaproteobacteria and Alphaproteobacteria, respectively (Nazaries et al. 2013). The differences between these two groups are also reflected in their PLFA profiles (Table 1). Generally, Type I methanotrophs have been described as containing primarily 16:1 PLFAs and Type II methanotrophs as having 18:1 PLFAs (Chowdhury and Dick 2013) but this is an over-simplification. As Table 1 shows, Type I organisms may contain 18:1 PLFAs and Type II organisms may contain 16:1 PLFAs. However, it is generally accepted that 16:1ω8c and 16:1ω5t are particularly valuable biomarkers for Type I and 18:1ω8c for Type II methanotrophs since they have not been found in non-methanotrophs (Bodelier et al. 2009) while 16:1ω6c and 18:1ω7c may also be useful biomarkers (Chowdhury and Dick 2013).
The pattern of 13C labelling of PLFAs in soil exposed to 13CH4 will give an indication of the methanotroph type. A primary question is whether there are characteristic patterns found in similar ecosystems? Looking at studies on various grasslands, we find the prime PLFA to be 18:1ω7c with lesser amounts in either 16:1ω5c or 16:0 (Maxfield et al. 2006, 2011; Menyailo et al. 2008; Singh et al. 2009). While 18:1ω7c may be found in Type I methanogens, it is always accompanied by 16:1ω7c so the strong indication is of Type II methanogens in grasslands. An exception was in New Zealand pasture which was dominated by 16:1ω7c, 16:1ω11c and 16:1ω11t, indicating a Type I community (Singh et al. 2007).
Forest systems follow a similar pattern to grassland with 18:1ω7c being the prime PLFA but with lesser amounts of 16:0 and/or some form of 17:0 (Bengtson et al. 2009; Bull et al. 2000; Crossman et al. 2006; Knief et al. 2003; Maxfield et al. 2011; Menyailo et al. 2010; Nazaries et al. 2011; Singh et al. 2007). Occasionally other PLFAs were seen to be present in large amounts, i.e. 18:0 (Maxfield et al. 2011), br18:0 (Bengtson et al. 2009; Bull et al. 2000) and cyc19:0 (Nazaries et al. 2011). In contrast to grasslands, 16:1ω5c was absent from those forest soils studied. Again the overall pattern points to Type II methanogens dominating in forest ecosystems. However, there were some exceptions. Mohanty et al. (2006) looking at a German beech forest report label in 16:1ω8c, 16:1ω5t and 18:1ω8c, indicating the presence of both Type I and Type II organisms. Knief et al. (2003), studying four forest sites in Germany found three sites, a mixed forest and two deciduous forests, to have large amounts of 16:0, some 14:0 and 16:1ω7c but only traces of 18:1ω7c, suggesting a Type I methanotrophic community.
Only one study has examined non-flooded arable soils. Maxfield et al. (2011), using some of the Broadbalk plots in England, found label in 16:0, 18:0 and 18:1ω7c on mineral-fertilized plots. On plots with farmyard manure additions there was also label in 16:1ω7c, 14:0 and other minor PLFAs. The indication is of Type II in the former plots but also the possibility of Type I organisms in the manured plots. Three studies have looked at rice soils. Here the general pattern is of label in 16:1ω7c, 16:0 and 16:1ω6c with minor labelling of 18:1ω7c and 18:1ω9c (Qiu et al. 2008; Shrestha et al. 2008), indicating a Type I community. Mohanty et al. (2006) only reported the presence of 16:1ω8c, 16:1ω5t and 18:1ω8c, indicating a mixture of Type I and II organisms.
Wetland habits, i.e. peatlands and flooded soils, generally show dominance by 16:1ω7c, 16:0, 18:1ω7c, other 16:1 PLFAs and sometimes 18:1ω9c (Bodelier et al. 2012; Knief et al. 2006). The dominance of 16:1 over 18:1 would indicate a largely Type I community. Knief et al. (2006) suggest that in their gleysols the dominance of 18:1ω7c and 16:0 indicates a Type II community but these PLFAs are equally found in Type I isolates (Bodelier et al. 2009). Bodelier et al. (2012), studying a series of wet soils, found traces of 18:1ω8c in their more flooded soils, suggesting the presence of Type II in addition to a majority of Type I organisms.
It is worth noting that those studies using near ambient methane concentrations during their labelling, presumably to label “high affinity” methanogens, have usually obtained most label in 18:1ω7c with the exception of Maxfield et al. (2011) who obtained most in 16:0. This would point to a Type II assignment. Chowdhury and Dick (2013) have stated that Type II methanotrophs prefer a methane-rich environment while Type I methanotrophs prefer a methane-limited environment. The evidence from the above survey would suggest the reverse. Those environments generally exposed to only ambient methane, grasslands, forests and non-flooded arable land, appear to be dominated by Type II methanotrophs while those environments potentially exposed to much larger methane concentrations, wetland soils and paddy soils, appear to be dominated by Type I methanotrophs. There are some exceptions where the opposite is true, situations where both Type I and Type II methanotrophs coexist and several cases where it is not entirely clear. It is perhaps important to bear in mind that we are still limited by the number of methanotrophs that have been obtained in culture and had their PLFA profiles characterised. The evidence from 13C labelling is that that there are many uncultured methanotrophs out there that have rather different PLFA profiles.
Other specific groups
Specific lipid biomarkers have been ascribed to both sulphur-oxidizing and sulphate-reducing bacteria (Green and Scow 2000). However, there are few examples of PLFA-SIP being applied to these groups in soils. Lipski (2006) describes some results of 13CO2 uptake (as carbonate or bicarbonate) into the PLFAs of both sulphur and iron-oxidizing bacteria in sediments and gleysols.
Perspectives
Limitations of the 13C PLFA method
The approach of combining lipid biochemistry and carbon isotope labelling provides greater analytical capabilities for biogeochemical systems than the sum of the two approaches applied separately. However, the combined technique also includes the potential limitations of the two approaches. We generally assume that certain PLFAs are markers for a particular group or at least indicative of changes in that group when evaluating the change in soil microbial community due to a specific treatment (Frostegård et al. 2011). However, PLFAs are not perfect markers. Up to now, no PLFA is exclusive to one species/group of soil microbes except perhaps for methanotrophs. There are several examples where the same PLFAs are stated to indicate very different groups. The PLFA 16:1ω 5c was used as a marker of arbuscular mycorrhizal fungi when a non-mycorrhizal control can be included that accounts for the background levels originating from bacteria (Olsson 1999), but is also found in high relative abundance in Gram-negative bacteria (Butler et al. 2003; Yao et al. 2012). The PLFA 18:1ω7c, usually considered to be indicator of Gram-negative bacteria (Butler et al. 2003) is also found in large amounts in arbuscular mycorrhizal fungi (Sakamoto et al. 2004).
Plant roots contain some PLFAs; these PLFAs generally have an even number of carbon atoms and are unbranched (McMahon et al. 2005). The most common PLFAs in plants include 16:0, 18:1ω9c, and 18:2ω6,9c, which also exist in many other eukaryotic microorganisms, including fungi. Most of the 13C-labelled plant rhizodeposition carbon in some studies was distributed into 16:0, 18:1ω7c, and 18:1ω9c and their rates of increase in labelled C were high (Lu et al. 2004; Yao et al. 2012). Since these PLFAs can be derived from plant roots, the effect of contaminating fine roots on soil PLFA pattern cannot be ruled out as a reason for the high relative abundance of these PLFAs.
Cross-feeding is a universal complication in stable isotope probe techniques. During the labelling period, soil microorganisms will use the labelling substrate C in soil. On the other hand, secondary assimilation may have occurred. After long incubation periods, microbes that feed on either metabolites produced by the primary utilizer of the substrate, or on the dead primary utilizer itself, may be detected (Uhlík et al. 2009). The direct assimilation of respired 13CO2 is a distinct possibility that some experimenters have tried to avoid by increasing the ambient concentration of 12CO2. The incorporation of labelled CH4-derived carbon into bacteria which do not grow on CH4 confirmed that the cross-feeding of methane carbon occurred (Qiu et al. 2009).
Future opportunities and developments
The application of 13C-labelling PLFAs is mainly based on the fact that certain PLFAs are indicative of particular microbial groups. Up to now, several characteristic PLFAs, such as 16:1ω8c and 18:1ω8c, can be used as indicative of methanotrophic bacteria (Sundh et al. 2000). The PLFA 18:2ω6,9 of casing soil demonstrated the change of microbial community structure during mushroom cropping stage (Cai et al. 2009). An expansion of characteristic PLFAs will enhance the stable carbon isotopic analysis of microbial community structure. Some new potential PLFA biomarkers, e.g., thermophilic, stress and starvation indicators, have been reviewed and discussed (Ruess and Chamberlain 2010; Frostegård et al. 2011). Some neutral lipids or non-lipids were also used as indicators recently, e.g. the neutral lipid fatty acid 16:1ω5c as an indicator arbuscular mycorrhiza (Olsson et al. 1995; Olsson and Johnson 2005) and glycerol dialkyl glycerol tetraethers (GDGT) as indicators of soil archaea (Leininger et al. 2006).
In order to reduce the cross-feeding effect in the 13C PLFA method, ideally a short sampling time should be chosen, but this is difficult to optimize in practice. The rate of label incorporation by primary autotrophic assimilation may be slower than secondary heterotrophic incorporation if sampling is too late but if sampling is too early there may be insufficient 13C label in PLFAs for accurate detection. Generally, the 13C-PLFA patterns for the same soil are analysed at a series of time points, and then the rates of primary assimilation and secondary heterotrophic incorporation can be compared (Wang et al. 2014).
The 13C PLFA analysis method can identify the living microbial biomass due to the rapid degradation of PLFAs following cell death. The method is more sensitive in detecting shifts in microbial community structure when compared to DNA/RNA based methods (Ramsey et al. 2006). However, the incorporation of 13C-labelled substrate into soil microbial communities cannot give the information on detailed species composition or phylogenetic resolution using only 13C PLFA analysis. On the other hand, DNA/RNA stable isotope probing (SIP) methods provide little information on the activity of soil microorganism in the environment. Consequently, the combined use of PLFA-SIP and DNA/RNA-SIP methods can complement one another and expand our understanding of who is doing what within the soil ecosystem, thus providing keys to open the soil microbial black box.
References
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10(3):215–221
Andresen LC, Dungait JAJ, Bol R, Selsted MB, Ambus P, Michelsen A (2014) Bacteria and fungi respond differently to multifactorial climate change in a temperate heathland, traced with 13C-glycine and FACE CO2. PLoS One 9:e85070
Antizar-Ladislao B, Spanova K, Beck AJ, Russell NJ (2008) Microbial community structure changes during bioremediation of PAHs in an aged coal-tar contaminated soil by in-vessel composting. Int Biodeterior Biodegrad 61:357–364
Apostel C, Dippold M, Glaser B, Kuzyakov Y (2013) Biochemical pathways of amino acids in soil: assessment by position-specific labelling and 13C-PLFA analysis. Soil Biol Biochem 67:31–40
Bengtson P, Basiliko N, Dumont MG, Hills M, Murrell JC, Roy R, Grayston SJ (2009) Links between methanotroph community composition and CH4 oxidation in a pine forest soil. FEMS Microbiol Ecol 70:356–366
Bodelier PLE, Bar-Gillisen MJ, Hordijk K, Damste JSS, Rijpstra WIC, Geenevasen JAJ, Dunfield PF (2009) A reanalysis of phospholipid fatty acids as ecological biomarkers for methanotrophic bacteria. ISME J 3:606–617
Bodelier PLE, Bar-Gilissen MJ, Meima-Franke M, Hordijk K (2012) Structural and functional response of methane-consuming microbial communities to different flooding regimes in riparian soils. Ecol Evol 2:106–127
Boschker HTS, Nold SC, Wellsbury P, Bos D, de Graaf W, Pel R, Parkes RJ, Cappenberg TE (1998) Direct linking of microbial populations to specific biogeochemical processes by 13C-labelling of biomarkers. Nature 392:801–805
Boutton TW (1996) Stable Carbon Isotope Ratios of Soil Organic Matter and Their Use as Indicators of Vegetation and Climate Change. In: Boutton TW (ed) Mass Spectrometry of Soils. Marcel Dekker Inc, New York, pp 47–82
Brant JB, Sulzman EW, Myrold DD (2006) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232
Bromand S, Whalen JK, Janzen HH, Schjoerring JK, Ellert BH (2001) A pulse-labelling method to generate 13C- enriched plant materials. Plant Soil 235:253–257
Bull ID, Parekh NR, Hall GH, Ineson P, Evershed RP (2000) Detection and classification of atmospheric methane oxidizing bacteria in soil. Nature 405:175–178
Butler JL, Williams MA, Bottomley PJ, Myrold DD (2003) Microbial community dynamics associated with rhizosphere carbon flow. Appl Environ Microbiol 69(11):6793–6800
Cai WM, Yao HY, Feng WL, Jin QL, Liu YY, Li NY, Zheng Z (2009) Microbial community structure of casing soil during mushroom growth. Pedosphere 19(4):446–452
Chowdhury TR, Dick RP (2013) Ecology of aerobic methanotrophs in controlling methane fluxes from wetlands. Appl Soil Ecol 65:8–22
Churchland C, Weatherall A, Briones MJI, Grayston SJ (2012) Stable-isotope labeling and probing of recent photosynthates into respired CO2, soil microbes and soil mesofauna using a xylem and phloem stem-injection technique on Sitka spruce (Picea sitchensis). Rapid Commun Mass Spectrom 26:2493–2501
Crossman ZM, Wang ZP, Ineson P, Evershed RP (2006) Investigation of the effect of ammonium sulfate on populations of ambient methane oxidising bacteria by 13C-labelling and GC/C/IRMS analysis of phospholipid fatty acids. Soil Biol Biochem 38:983–990
de Visser R, Vianden H, Schnyder H (1997) Kinetics and relative significance of remobilized and current C and N incorporation in leaf and root growth zones of Lolium perenne after defoliation: assessment by 13C and 15 N steady-state labelling. Plant Cell Environ 20:37–46
Dijkstra P, Thomas SC, Heinrich PL, Koch GW, Schwartz E, Hungate BA (2011) Effect of temperature on metabolic activity of intact microbial communities: evidence for altered metabolic pathway activity but not for increased maintenance respiration and reduced carbon use efficiency. Soil Biol Biochem 43:2023–2031
Dungait JAJ, Kemmitt SJ, Michallon L, Guo SL, Wen Q, Brookes PC, Evershed RP (2011) Variable responses of the soil microbial biomass to trace concentrations of 13C-labelled glucose, using 13C-PLFA analysis. Eur J Soil Sci 62:117–126
Dungait JAJ, Kemmitt SJ, Michallon L, Guo SL, Wen Q, Brookes PC, Evershed RP (2013) The variable response of soil microorganisms to trace concentrations of low molecular weight organic substrates of increasing complexity. Soil Biol Biochem 64:57–64
Elfstrand S, Lagerlof J, Hedlund K, Martensson A (2008) Carbon routes from decomposing plant residues and living roots into soil food webs assessed with 13C labelling. Soil Biol Biochem 40:2530–2539
Epron D, Bahn M, Derrien D, Lattanzi FA, Pumpanen J, Gessler A, Högberg P, Maillard P, Dannoura M, Gérant D, Buchmann N (2012) Pulse-labelling trees to study carbon allocation dynamics: a review of methods, current knowledge and future prospects. Tree Physiol 32:776–798
Esperschütz J, Gattinger A, Buegger F, Lang H, Munch JC, Schloter M, Winkler JB (2009) A continuous labelling approach to recover photosynthetically fixed carbon in plant tissue and rhizosphere organisms of young beech trees (Fagus sylvatica L.) using 13C depleted CO2. Plant Soil 323:21–29
Evershed RP, Crossman ZM, Bull ID, Mottram H, Dungait JAJ, Maxfield PJ, Brennand EL (2006) 13C-labelling of lipids to investigate microbial communities in the environment. Curr Opin Biotech 17:72–82
Fang JS, Lovanh N, Alvarez PJJ (2004) The use of isotopic and lipid analysis techniques linking toluene degradation to specific microorganisms: applications and limitations. Water Res 38:2529–2536
Frostegård Å, Bååth E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Garcia-Pausas J, Paterson E (2011) Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol Biochem 43:1705–1715
Gattinger A, Gunther A, Schloter M, Munich JC (2003) Characterisation of Archaea in soils by polar lipid analysis. Acta Biotechnol 23:21–28
Godbold DL, Hoosbeek MR, Lukac M, Cotrufo MF, Janssens IA, Ceulemans R, Polle A, Velthorst EJ, Scarascia-Mugnozza G, de Angelis P et al (2006) Mycorrhizal hyphal turnover as a dominant process for carbon input into soil organic matter. Plant Soil 281:15–24
Grams TEE, Werner H, Kuptz D, Ritter W, Fleischmann F, Anderson CP, Matyssek R (2011) A free-air system for long term stable carbon isotope labelling of adult forest trees. Trees 25:187–198
Green CT, Scow KM (2000) Analysis of phospholipid fatty acids (PLFA) to characterize microbial communities in aquifers. Hydrogeol J 8:126–141
Hanson JR, Macalady JL, Harris D, Scow KM (1999) Linking toluene degradation with specific microbial populations in soil. Appl Environ Microbiol 65(12):5403–5408
Ho I, Trappe JM (1973) Translocation of 14C from Festuca plants to their ectomycorrhizal fungi. Nature 244:30–31
Högberg MN, Briones MJI, Keel SG, Metcalfe DB, Campbell C, Midwood AJ, Thornton B, Hurry V, Linder S, Näsholm T, Högberg P (2010) Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol 187:485–493
Insam H (2001) Developments in soil microbiology since the mid 1960s. Geoderma 100:389–402
Jakobs-Schönwandt D, Mathies H, Abraham WR, Pritzkow W, Stephan I, Noll M (2010) Biodegradation of a biocide (cu-n-cyclohexyldiazenium dioxide) component of a wood preservative by a defined soil bacterial community. Appl Environ Microbiol 76(24):8076–8083
Johnsen AR, Winding A, Karlson U, Roslev P (2002) Linking of microorganisms to phenanthrene metabolism in soil by analysis of 13C-labeled cell lipids. Appl Environ Microbiol 68(12):6106–6113
Kamashwaran SR, Crawford DL (2001) Anaerobic biodegradation of pentachlorophenol in mixtures containing cadmium by two physiologically distinct microbial enrichment cultures. J Ind Microbiol Biotechnol 27:11–17
Kindler R, Miltner A, Thullner M, Richnow HH, Kästner M (2009) Fate of bacterial biomass derived fatty acids in soil and their contribution to soil organic matter. Org Geochem 40:29–37
Knief C, Lipski A, Dunfield PF (2003) Diversity and activity of methanotrophic bacteria in different upland soils. Appl Environ Microbiol 69:6703–6714
Knief C, Kolb S, Bodelier PLE, Lipski A, Dunfield PF (2006) The active methanotrophic community in hydromorphic soils changes in response to changing methane concentration. Environ Microbiol 8:321–333
Kramer C, Gleixner G (2006) Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol Biochem 38:3267–3278
Kramer C, Gleixner G (2008) Soil organic matter in soil depth profiles: distinct carbon preferences of microbial groups during carbon transformation. Soil Biol Biochem 40:425–433
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Lemanski K, Scheu S (2014) Incorporation of 13C labelled glucose into soil microorganisms of grassland: effects of fertilizer addition and plant functional group composition. Soil Biol Biochem 69:38–45
Lipski A (2006) Detection of autotrophic sulphur- and iron-oxidizing bacteria using labelled fatty acid methyl esters (FAMEs). In: Cooper JE, Rao JR (eds) Molecular approaches to soil, rhizosphere and plant microorganism analysis. Cabi, Wallingford, pp 132–145
Lu YH, Murase J, Watanabe A, Sugimoto A, Kimura M (2004) Linking microbial community dynamics to rhizosphere carbon flow in a wetland rice soil. FEMS Microbiol Ecol 48:179–186
Mauclaire L, Pelz O, Thullner M, Abraham WR, Zeyer J (2003) Assimilation of toluene carbon along a bacteria-protist food chain determined by 13C-enrichment of biomarker fatty acids. J Microbiol Methods 55:635–649
Maxfield PJ, Hornibrook ERC, Evershed RP (2006) Estimating high-affinity methanotrophic bacterial biomass, growth, and turnover in soil by phospholipid fatty acid 13C labeling. Appl Environ Microbiol 72:3901–3907
Maxfield PJ, Brennand EL, Powlson DS, Evershed RP (2011) Impact of land management practices on high-affinity methanotrophic bacterial populations: evidence from long-term sites at Rothamsted. Eur J Soil Sci 62:56–68
McMahon SK, Williams MA, Bottomley PJ, Myrold DD (2005) Dynamics of microbial communities during decomposition of carbon-13 labeled ryegrass fractions in soil. Soil Sci Soc Am J 69:1238–1247
Mellendorf M, Soja G, Gerzabek MH, Watzinger A (2010) Soil microbial community dynamics and phenanthrene degradation as affected by rape oil application. Appl Soil Ecol 46(3):329–334
Menyailo OV, Hungate BA, Abraham WR, Conrad R (2008) Changing land use reduces soil CH4 uptake by altering biomass and activity but not composition of high-affinity methanotrophs. Global Change Biol 14:2405–2419
Menyailo OV, Abraham WR, Conrad R (2010) Tree species affect atmospheric CH4 oxidation without altering community composition of soil methanotrophs. Soil Biol Biochem 42:101–107
Mohanty SR, Bodelier PLE, Floris V, Conrad R (2006) Differential effects of nitrogenous fertilizers on methane-consuming microbes in rice field and forest soils. Appl Environ Microbiol 72:1346–1354
Murase J, Hordijk K, Tayasu I, Bodelier PLE (2011) Strain-specific incorporation of methanotrophic biomass into eukaryotic grazers in a rice field soil revealed by PLFA-SIP. FEMS Microbiol Ecol 75:284–290
Nazaries L, Tate KR, Ross DJ, Singh J, Dando J, Saggar S, Baggs EM, Millard P, Murrell JC, Singh BK (2011) Response of methanotrophic communities to afforestation and reforestation in New Zealand. ISME J 5:1832–1836
Nazaries L, Murrell J, Millard P, Baggs L, Singh BK (2013) Methane, microbes and models: fundamental understanding of the soil methane cycle for future predictions. Environ Microbiol 15:2395–2417
Nottingham AT, Griffiths H, Chamberlain PM, Stott AW, Tanner EVJ (2009) Soil priming by sugar and leaf-litter substrates: A link to microbial groups. Appl Soil Ecol 42(3):183–190
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310
Olsson PA, Johnson NC (2005) Tracking carbon from the atmosphere to the rhizosphere. Ecol Lett 8(12):1264–1270
Olsson PA, Bååth E, Jakobsen I, Soderstrom B (1995) The use of phospholipid and neutral lipid fatty-acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol Res 99(5):623–629
Paterson E, Gebbing T, Abel C, Sim A, Telfer G (2007) Rhizodeposition shapes rhizosphere community structure in organic soil. New Phytol 173:600–610
Paterson E, Osler G, Dawson LA, Gebbing T, Sim A, Ord B (2008a) Labile and recalcitrant plant fractions are utilized by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol Biochem 40:1103–1113
Paterson E, Thornton B, Midwood AJ, Osborne SM, Sim A, Millard P (2008b) Atmospheric CO2 enrichment and nutrient additions to planted soil increase mineralisation of soil organic matter, but do not alter microbial utilisation of plant and soil C-sources. Soil Biol Biochem 40:1103–1113
Paterson E, Sim A, Osborne SM, Murray PJ (2011) Long-term exclusion of plant-inputs to soil reduces the functional capacity of microbial communities to mineralise recalcitrant plant-derived carbon sources. Soil Biol Biochem 43:1873–1880
Pelz O, Chatzinotas A, Andersen N, Bernasconi SM, Hesse C, Abraham WR, Zeyer J (2001) Use of isotopic and molecular techniques to link toluene degradation in denitrifying aquifer microcosms to specific microbial populations. Arch Microbiol 175:270–281
Petsch ST, Edwards KJ, Eglinton TI (2003) Abundance, distribution and δ13C analysis of microbial phospholipid-derived fatty acids in a black shale weathering profile. Org Geochem 34:731–743
Pollierer MM, Dyckmans J, Scheu S, Haubert D (2012) Carbon flux through fungi and bacteria into the forest soil animal food web as indicated by compound specific 13C fatty acid analysis. Funct Ecol 26:978–990
Powers EM, Marshall JD (2011) Pulse labeling of dissolved 13C-carbonate into tree xylem: developing a new method to determine the fate of recently fixed photosynthate. Rapid Commun Mass Spectrom 25:33–40
Putz B, Drapela T, Wanek W, Schmidt O, Frank T, Zaller JG (2011) A simple method for in situ-labelling with 15 N and 13C of grassland species by foliar brushing. Methods Ecol Evol 2:326–332
Qiu QF, Noll M, Abraham WR, Lu YH, Conrad R (2008) Applying stable isotope probing of phospholipid fatty acids and rRNA in a Chinese rice field to study activity and composition of the methanotrophic bacterial communities in situ. ISME J 2:602–614
Qiu QF, Conrad R, Lu YH (2009) Cross-feeding of methane carbon among bacteria on rice roots revealed by DNA-stable isotope probing. Environ Microbiol Rep 1(5):355–361
Ramsey PW, Rillig MC, Feris KP, Holben WE, Gannon JE (2006) Choice of methods for soil microbial community analysis: PLFA maximizes power compared to CLPP and PCR-based approaches. Pedobiologia 50:275–280
Rasmussen J, Kusliene G, Jacobsen OS, Kuzyakov Y, Eriksen J (2013) Bicarbonate as tracer for assimilated C and homogeneity of 14C and 15 N distribution in plants by alternative labeling approaches. Plant Soil 371:191–198
Rinnan R, Bååth E (2009) Differential utilization of carbon substrates by bacteria and fungi in tundra soil. Appl Environ Microbiol 75:3611–3620
Ruess L, Chamberlain PM (2010) The fat that matters: Soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biol Biochem 42:1898–1910
Sakamoto K, Iijima T, Higuchi R (2004) Use of specific phospholipid fatty acids for identifying and quant.ifying the external hyphae of the arbuscular mycorrhizal fungus Gigaspora rosea. Soil Biol Biochem 36:1827–1834
Sangster A, Knight D, Farrell R, Bedard-Haughn A (2010) Repeat pulse 13CO2 labeling of canola and field pea: implications for soil organic matter studies. Rapid Commun Mass Spectrom 24:2791–2798
Schnyder H (1992) Long-term steady-state labelling of wheat plants by use of natural 13CO2/12CO2 mixtures in an open, rapidly turned-over system. Planta 187:128–135
Shrestha M, Abraham WR, Shrestha PM, Noll M, Conrad R (2008) Activity and composition of methanotrophic bacterial communities in planted rice soil studied by flux measurements, analyses of pmoA gene and stable isotope probing of phospholipid fatty acids. Environ Microbiol 10:400–412
Singh BK, Tate KR, Kolipaka G, Hedley CB, Macdonald CA, Millard P, Murrell JC (2007) Effect of afforestation and reforestation of pastures on the activity and population dynamics of methanotrophic bacteria. Appl Environ Microbiol 73:5153–5161
Singh BK, Tate KR, Ross DJ, Singh J, Dando J, Thomas N, Millard P, Murrell JC (2009) Soil methane oxidation and methanotroph responses to afforestation of pastures with Pinus radiata stands. Soil Biol Biochem 41:2196–2205
Stemmer M, Watzinger A, Blochberger K, Haberhauer G, Gerzabek MH (2007) Linking dynamics of soil microbial phospholipid fatty acids to carbon mineralization in a 13C natural abundance experiment: impact of heavy metals and acid rain. Soil Biol Biochem 39:3177–3186
Streit K, Hagedorn F, Hiltbrunner D, Portmann M, Saurer M, Buchmann N, Wild B, Richter A, Wipf S, Siegwolf RTW (2014) Soil warming alters microbial substrate use in alpine soils. Glob Chang Biol 20:1327–1338
Subedi KD, Ma BL, Liang BC (2006) New method to estimate root biomass in soil through root-derived carbon. Soil Biol Biochem 38:2212–2218
Sundh I, Börjesson G, Tunlid A (2000) Methane oxidation and phospholipid fatty acid composition in a podzolic soil profile. Soil Biol Biochem 32:1025–1028
Tavi NM, Martikainen PJ, Lokko K, Kontro M, Wild B, Richter A, Biasi C (2013) Linking microbial community structure and allocation of plant-derived carbon in an organic agricultural soil using 13CO2 pulse-chase labelling combined with 13C-PLFA profiling. Soil Biol Biochem 58:207–215
Tiedje JM, Asuming-Brempong S, Nusslein K, Marsh TL, Flynn SJ (1999) Opening the black box of soil microbial diversity. Appl Soil Ecol 13:109–122
Tillmann S, Strömpl C, Timmis KN, Abraham WR (2005) Stable isotope probing reveals the dominant role of Burkholderia species in aerobic degradation of PCBs. FEMS Microbiol Ecol 52:207–217
Tomco PL, Holmes WE, Tjeerdema RS (2013) Biodegradation of clomazone in a california rice field soil: carbon allocation community effects. J Agric Food Chem 61:2618–2624
Treonis AM, Olstle NJ, Stott AW, Primrose R, Grayston SJ, Ineson P (2004) Identification of groups of metabolically active rhizosphere microorganisms by stable isotope probing of PLFAs. Soil Biol Biochem 36:533–537
Uhlík O, Jecná K, Leigh MB, Macková M, Macek T (2009) DNA-based stable isotope probing: a link between community structure and function. Sci Total Environ 407:3611–3619
van Aarle IM, Olsson PA (2003) Fungal lipid accumulation and development of mycelial structures by two arbuscular mycorrhizal fungi. Appl Environ Microbiol 69:6762–6767
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19(6):703–707
Wang J, Thornton B, Yao HY (2014) Incorporation of urea-derived 13C into microbial communities in four different agricultural soils. Biol Fertil Soils 50:603–612
Watzinger A, Feichtmair S, Kitzler B, Zehetner F, Kloss S, Wimmer B, Zechmeister-Boltenstern S, Soja G (2014) Soil microbial communities responded to biochar application in temperate soils and slowly metabolized 13C-labelled biochar as revealed by 13C PLFA analyses: results from a short-term incubation and pot experiment. Eur J Soil Sci 65:40–51
Williams MA, Myrold DD, Bottomley PJ (2006) Carbon flow from 13C-labeled straw and root residues into the phospholipid fatty acids of a soil microbial community under field conditions. Soil Biol Biochem 38:759–768
Yao HY, Thornton B, Paterson E (2012) Incorporation of 13C-labelled rice rhizodeposition carbon into soil microbial communities under different water status. Soil Biol Biochem 53:72–77
Acknowledgments
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB15020300), the Ningbo Natural Science Foundation (Grant No. 2013A610185), and the Fujian Natural Science Foundation (Grant No. 2014I0007). The James Hutton Institute receives funding from the Scottish Government's Rural and Environment Science and Analytical Services Division.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Thom W. Kuyper.
Rights and permissions
About this article
Cite this article
Yao, H., Chapman, S.J., Thornton, B. et al. 13C PLFAs: a key to open the soil microbial black box?. Plant Soil 392, 3–15 (2015). https://doi.org/10.1007/s11104-014-2300-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2300-9