Abstract
Aims
This study aimed to determine the influence of tree species on soil microbial community structure.
Methods
We conducted a litter and root manipulation and a short-term nitrogen (N) addition experiment in 19-year-old broadleaf Mytilaria laosensis (Hamamelidaceae) and coniferous Chinese fir (Cunninghamia lanceolata) plantations in subtropical China. Phospholipid fatty acid (PLFA) analysis was used to examine treatment effects on soil microbial community structure. Redundancy analysis (RDA) was performed to determine the relationships between individual PLFAs and soil properties (soil pH, carbon (C) and N concentration and C:N ratio).
Results
Soil C:N ratio was significantly greater in M. laosensis (17.9) than in C. lanceolata (16.2). Soil C:N ratio was the key factor affecting the soil microbial community regardless of tree species and the litter, root and N treatments at our study site. The fungal biomarkers, 18:1ω9 and 18:2ω6,9 were significantly and positively related to soil C:N ratio and the abundance of bacterial lipid biomarkers was negatively related to soil C:N ratio. N addition for 8 months did not change the biomass and structure of the microbial community in M. laosensis and C. lanceolata soils. Soil nutrient availability before N addition was an important factor in determining the effect of N fertilization on soil microbial biomass and activity. PLFA analysis showed that root exclusion significantly decreased the abundance of the fungal biomarkers and increased the abundance of the Gram-positive bacteria. Rootless plots had a relatively lower Gram-positive to Gram-negative bacteria ratio and a higher fungi to bacteria ratio compared to the plots with roots under both M. laosensis and C. lanceolata. The response of arbuscular mycorrhizal fungi (16:1ω5) to root exclusion was species-specific.
Conclusions
These observations suggest that soil C:N ratio was an important factor in influencing soil microbial community structure. Further studies are required to confirm the long-term effect of tree species on soil microbial community structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil microorganisms regulate key processes that control soil carbon (C) and nutrient cycling. Tree species might influence soil microbial biomass and community structure through a variety of mechanisms, essentially by controlling quantity and quality of both above- and belowground litter production. Field studies comparing the soil microbial community beneath different tree species have found that a distinct soil microbial community is very often associated with different plant species, especially between broadleaf and coniferous species (Templer et al. 2003; Ushio et al. 2008; Weand et al. 2010). These studies suggest that the inter-species variation in litter chemistry, root exudates and soil physicochemical properties (soil pH or organic matter content) regulate the composition of the soil microbial community (Ushio et al. 2008; Strickland et al. 2009). However, these studies are often not carried out on the same soil, so other factors may have influenced soil microbial community structure. For example, some studies have shown that land-use history was a more important factor in determining microbial community structure than tree species or soil properties (Fraterrigo et al. 2006; Jangid et al. 2011). Land-use change can also have significant and long-lasting effects on soil C and nutrient contents, soil texture, and pH and thus influence soil microbial community structure. A clear understanding of which factors play an important role in the development of the soil microbial community will benefit our understanding of forest management impacts on soil C and nutrient cycling.
Litter and root exudates, which are the main sources of C for soil microorganisms, differ in quality and quantity between different tree species. Thus, the differences between species in litter and root substrate quality will influence the size and structure of the soil microbial community (Fisk and Fahey 2001; Myers et al. 2001). Laboratory incubation studies have demonstrated that addition of litter with high quality (low C:N ratio) (Bastian et al. 2009; Potthast et al. 2010) or litter with high soluble C content (Fanin et al. 2014) to soil led to an increase of microbial biomass and activated a copiotrophic microbial community (such as Gram-negative bacteria). However, our recent analysis of soil microbial community structure between broadleaf and coniferous species revealed that despite significant differences in litter C:N and lignin:N ratios, the soil microbial community structure did not differ significantly between the tree species (Huang et al. 2013a). This suggests that other factors influence microbial community structure that may act either independently or synergistically with the tree species.
It is generally believed that soil pH and/or C:N ratio is a major factor in determining soil microbial community structure (Bååth and Anderson 2003; Aciego and Brookes 2009; Rousk et al. 2010a, 2010b). Studies have shown that fungi are favored at low pH, and the composition of bacterial communities is influenced by soil pH in both arable and forest soils (Rousk et al. 2010a). In addition to soil pH, the C:N ratio has also been shown to be an important factor in regulating the soil microbial community composition (Högberg et al. 2007). There is a widespread perception that forest N addition will lead to an increase in C in the system by increasing the C content of the soil organic layer (Liu and Greaver 2010). Some N addition experiments estimated that N addition changed the composition of the soil microbial community (e.g., Demoling et al. 2008; Börjesson et al. 2012). From an incubation study, Allison et al. (2009) found that a low level of N addition can stimulate the abundance of fungal species in boreal forest. However, De Vries et al. (2006) found that N addition reduced the fungal biomass in grassland. Studies of Demoling et al. (2008) showed that the effects of N fertilization on soil microbial biomass and activity differed depending on the conditions at the site prior to fertilization.
Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) is a native conifer timber species and has been widely planted in subtropical China for more than 1000 years due to its fast growth and good timber quality. Cunninghamia lanceolata plantations now cover 9.11 million hectares and account for more than 18 % of all forest plantations in China and 5 % of all plantations in the world (Huang et al. 2013b). Field observations suggest that growth of C. lanceolata after harvest and replanting is significantly reduced and this problem occurs in many C. lanceolata growing regions (Fang 1987), which has drawn considerable attention (Zhang 1997; Huang et al. 2000a). The decrease of C. lanceolata growth in replanted woodlands has been attributed to the depletion of soil nutrients (Zhang et al. 2004; Luan et al. 2010), allelopathy (Huang et al. 2000a) and pathogenic fungi (Zhang and Zak 1994). As a result, plantings of native broadleaved tree species in C. lanceolata sites are currently encouraged in subtropical China for provision of multiple environmental outcomes including enhanced biodiversity and improved soil fertility. Mytilaria laosensis (Hamamelidaceae) is an evergreen broadleaf and commercial tree species which is increasingly planted following C. lanceolata harvest (Wu 2005).
The objective of this study was to examine the factor(s) influencing the microbial community structure beneath M. laosensis and C. lanceolata plantations planted on similar soil after harvest of C. lanceolata and with the same forest management history. We used a detritus input and removal treatment (DIRT) experiment, an inter-site study to examine the feedback between plant and soil microbes through manipulation of above- and belowground litter inputs to forest ecosystems (eg., Wang et al. 2013), to examine litter input factors influencing microbial community structure under the two tree species. We hypothesized that: (1) soil pH and (or) C:N ratio rather than tree species were the important factors in determining microbial community structure in M. laosensis and C. lanceolata soils and (2) N addition may decrease soil C:N ratio and then change soil microbial community structure under both tree species.
Materials and methods
Site description and experimental design
The site is located at Xiayang forest farm (26°48′N, 117°58′E), northwest Fujian Province, South Eastern China. The site has a deep red soil classified as a sandy clay loam Ferric Acrisol according to the FAO/UNESCO classification. The experimental site has a humid subtropical climate, with short and mild winters in January and February, and long, hot and humid summers between June and October. Spring and autumn are warm transition periods. Annual precipitation is concentrated in spring and summer. Mean annual rainfall was 1669 mm and average temperature was 19.3 °C in 2011.
In October 1992, a second rotation plantation of C. lanceolata with an area of 5 ha was harvested using chainsaws at age 29. The understory vegetation was slashed and all surface organic matter was burnt on the site. In April 1993, eight 20 m × 30 m plots were established on hill slopes (230–278 m elevation). The two species were then randomly planted in the eight plots with four plots of C. lanceolata seedlings and four plots of M. laosensis seedlings. The trees were spaced at 2 m × 2 m to make up 150 trees per plot (2500 stems ha−1). The plots were separated by more than 10 buffer tree rows. The plantations were 19-years-old in 2011.
In July 2011, tree height and diameter at breast height (DBH, measured at 1.3 m above-ground) were recorded for all trees within plots. The basal area of a tree was calculated as π × (DBH/2)2. Litterfall was measured monthly from five 0.5 m2 litter traps, systematically positioned within each plot from July 2011 for 12 months. Fine root (<2 mm) production was determined with the coring method. In brief, ten soil cores were extracted from each plot in September 2011 by using a 3.5-cm-diameter hole saw. The cores were 80 cm deep. Roots were picked from the cores first by hand, and then the soil was washed through a 590 μm sieve. The litter and fine root samples were oven-dried at 60 °C and weighed. A sub-sample of each litter and fine root type (C. lanceolata and M. laosensis) were finely ground for determination of C and N concentrations using an Elementar Vario EL III CN analyzer. Lignin concentrations in litter were determined using the Klason lignin procedure (Hatfield et al. 1994). All the features of tree, litter, root and soil are given in Table 1.
In July 2011, we conducted a DIRT experiment in the eight 20 m × 30 m plots. Three small plots (average size 2 m x 2 m) were laid out in each large plot. Each small plot received one of the following randomly allocated treatments: 1) control,no treatment; 2) litter exclusion, litter input was excluded with 1-mm-mesh screens placed 0.5 m above the ground; and 3) root exclusion, roots were excluded by trenching to 1 m followed by insertion of impenetrable barriers. Each treatment was replicated four times.
In July 2012, we also conducted a short-term N addition experiment. Twelve 2 m × 2 m plots were established with six plots in M. laosensis and six plots in C. lanceolata buffers. Two treatments, each with three replications, were applied as follows: 1) control, no N addition; 2) N addition, 5 g NH4NO3 (50 kg N ha−1 yr−1) was applied monthly for 8 months to the plot.
Soil sampling
In the DIRT experiment plots, sampling was performed in May 2012 when the treatments had been in place for 10 months. In the N addition experiment, sampling was performed in March 2013 when the treatments had been in place for 8 months. Soil samples were collected at 0–5 cm depth beneath the litter layer. Three samples per plot were taken randomly and combined to one composite sample. Soil samples were transported to the laboratory and stored at 4 °C for less than 2 days prior to processing. The moist soils were sieved (2 mm sieve) to remove large pieces of organic debris prior to analysis.
Soil chemistry
Soil pH was measured at a 1:2.5 soil/water (w/w) ratio. Soil C and N concentrations were determined on finely ground (<0.20 mm) sub-samples using a LECO EPS-2000 CNS thermal combustion furnace (LECO Corp., St Jose, MI). Mineral N was extracted from field moist samples (10 g oven-dry equivalent) by shaking with 2 M KCl at a soil solution ratio of 1:10, followed by centrifuging at 2000 rpm for 20 min. The supernatant was filtered through Whatman 42 filter paper, and mineral N was determined in the supernatant using an automated ion analyzer (Quik Chem method 10–107-064-D for NH4 + and 10107–04-1-H for NO3 −). Soil dissolved organic C (DOC) and N (DON) were extracted in cold water. Water extracts were prepared by mixing 10 g (oven-dry equivalent) of field moist soil and 40 mL distilled water on an end-to-end shaker for 1 h. The mixture was then centrifuged at 3500 rpm for 20 min and filtered through a Whatman 42 paper and then a 0.45 μm filter membrane. The organic C and N concentrations in the water extracts were determined using a SHIMADZU TOC-VCPH/CPN analyzer (fitted with a TN unit). Total C and N were determined in the soil light density fraction by the method of Huang et al. (2011). In summary, 10 g air-dried soil was placed in a centrifuge tube with 40 mL NaI (Fisher Chemical, UK) with a density of 1.70 g cm−3. The tubes were shaken by hand for 3 min and then centrifuged at 1000 rpm for 15 min. The floating material was aspirated from the surface of tubes (about the top 20 mL) and then placed into a filter unit (in a funnel containing Whatman GF A/E filter paper). The shaking – centrifugation – aspiration process was repeated at least four times, until no floating material remained. The samples on the filter paper were rinsed thoroughly with deionized water and collected. The collected material, designated light fraction, was dried at 60 °C for 24 h, and finely ground in a mortar and pestle before analysis. The C and N concentrations in the soil light fraction were determined by an Elementar Vario EL III CN analyzer.
Microbial community structure
We analyzed soil microbial community structure in the 0–5 cm layer through determination of the phospholipid fatty acids (PLFA) from 10 g subsamples stored at −20 °C. The method we used was adapted from White et al. (1979) and slightly modified (after a pilot run) to maximize extraction of fatty acids from the soil. Fatty acids were extracted with a one phase solvent consisting of a 1:2:0.8 mixture of chloroform, methanol, and phosphate buffer (pH 7.4). Soil samples were extracted with 20 mL of the solvent in a shaker for 24 h. The samples were centrifuged at 1000 g for 10 min, and the supernatant was removed. The remaining soil was re-extracted with 10 mL of the same extraction solvent for a further 12 h. The supernatant was removed after centrifuging and the two extracts were combined and then evaporated under N2 to a volume of 1 mL. The phospholipids in the concentrated extract were separated on silicic acid columns by sequentially eluting with organic solvents of increasing polarity and amended with a non-adecanoic acid standard (100 μl). They were then saponified and methylated, forming fatty acid methyl esters (FAMEs). Individual FAMEs were identified by gas chromatography (Hewlett Packard 5890 GC, equipped with a 6890 series injector, a flame ionization detector and an Ultra 2 capillary column (25 m × 0.2 mm, 0.33 μm film thickness) based on their retention times and in combination with the MIDI Sherlock Microbial Identification System (MIDI Inc., Newark, DE).
Total lipid abundance was calculated as the sum of lipids of which chain length was from C10 to C20 and could be measured as microbial biomass. Gram-positive bacteria were represented by all iso and anteiso branch chain fatty acids (Denef et al. 2009; Landesman and Dighton 2010), whereas Gram-negative bacteria were represented by monounsaturated and cyclopropane fatty acids (Frostegård et al. 2011; Ushio et al. 2013). 18:2ω6,9 and 18:1ω9 were used as indicators of fungi biomass, while 16:1ω5 was used to indicate arbuscular mycorrhizal fungi (Swallow et al. 2009). The PLFAs 10 Me16:0, 10 Me17:0 and 10 Me18:0 were used to indicate soil actinomycetes (Supplemental Table 1). The abundance of individual PLFAs was calculated as the absolute amount of C (nmol PLFA-C g−1 soil) and then converted to mole percentage PLFA-C. Fungi:bacteria and Gram-positive: Gram-negative bacteria ratios were calculated as (18:2ω6,9 + 18:1ω9)/(sum of all bacterial lipid), and (sum of the branched lipids)/(sum of the mono-unsaturated and cyclopropyl lipids), respectively.
Statistical analysis
Statistical analyses were performed using SPSS 11.5 for Windows or Microsoft Excel 2003. One way ANOVA was used to determine the impact of treatments on soil microbial and chemical properties. The concentrations of the individual PLFAs (expressed as mol%) were subjected to principal component analysis (PCA). Before conducting this analysis, % mole abundance of individual lipids were subjected to the arcsine square root transformation to ensure normality in the lipid data set. For microbial community structure, the PCA axis that explained the largest variation in PLFA data was used. We also performed redundancy analysis (RDA) to determine which soil properties (pH, soil C, soil N and C:N ratio) were related to soil microbial community structure. The RDA analysis was based on a covariance matrix, where % mol abundance of individual lipids was centered. The ordination axes representing aggregates of the environmental factors were tested for significance (P < 0.05) to explain the variation in the PLFA data using a Monte Carlo permutation test. We used Spearman correlation analysis to study the significance of relationships between PLFA signatures and soil C and N properties in the DIRT experiment plots. Throughout the text, differences were considered significant if P ≤ 0.05.
Results
Soil chemistry
Our study shows tree species had a significant (P < 0.01, Supplemental Table 2) effect on soil C concentration in the DIRT experiment where soil C was greater under M. laosensis than C. lanceolata in all treatments (Table 2). A similar pattern was evident in the N addition experiment although the species main effect was not significant (P = 0.06, Supplemental Table 2). Because of the greater C concentrations, C:N ratios were also greater in M. laosensis soil (Table 2). Litter or root exclusion (DIRT experiment) and N addition did not significantly affect soil C concentrations (Table 2). In the DIRT experiment, root exclusion significantly increased the soil N concentration and reduced the C:N ratio in M. laosensis soil (P < 0.05, Supplemental Table 2), but not in C. lanceolata soil. N addition did not significantly affect soil N concentrations. Soil pH was not significantly affected by species or treatment in either experiment.
In the DIRT experiment plots, soil NO3 − and DON were significantly greater under C. lanceolata than under M. laosensis (P < 0.01, Table 3). Soil DOC, light fraction organic C and N were greater in M. laosensis soil than in C. lanceolata soil (P < 0.01, Table 3). Root exclusion significantly increased the NH4 + and total mineral N concentrations, however did not significantly change the DOC, DON or light fraction organic C and N in M. laosensis soil (Table 3). Under C. lanceolata, litter and root exclusion did not affect the concentrations of any of these parameters (P > 0.05, Table 3).
Microbial community structure
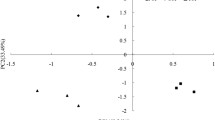
In the DIRT experiment, root exclusion had a greater influence on soil microbial community structure than litter exclusion (Fig. 1 and 2). The % mol abundance of individual lipid biomarkers was subjected to a PCA and the PC1 and PC2 components (x- and y-axis, respectively) together accounted for 80.2 % of the variation (PC1 56.4 % and PC2 23.8 %, Fig. 1a and b). The samples from the root exclusion plots were found to the left, clearly separated from the control and no litter input plots under both M. laosensis and C. lanceolata (PC1 scores subjected to one-way ANOVA, P = 0.00).
Principal component analysis of the signature phospholipid fatty acids used for examining the general soil microbial community structure in the 0–5 cm soil layer sampled in the detritus input and removal treatment (a and b) and N addition (c and d) experiments under M. laosensis (a and c) and C. lanceolata (b and d)
Selected soil microbial community characteristics, based on signature lipid biomarkers (PLFA) in the 0–5 cm soil layer sampled in the detritus input and removal treatment experiment in 19- year-old M. laosensis and C. lanceolata plantations. For the same parameter, means with the same letter are not significantly different at P < 0.05 owing to treatment effects. Histograms show the mean of four replicates with the standard deviation
Litter and root exclusion did not significantly affect the microbial biomass estimated by total PLFAs under M. laosensis and C. lanceolata (Fig. 2a and b) although individual fungal and bacterial biomarkers were affected. After 10 months of root exclusion, the abundance of the two fungal biomarkers 18:1ω9 and 18:2ω6,9 was reduced by 2.26 and 3.78 mol% in M. laosensis soil (P < 0.01) and by 1.73 and 1.15 mol% in C. lanceolata soil (P < 0.05), respectively (Fig. 2c and d). Root exclusion significantly increased the abundance of the Gram-positive bacteria in both species (Fig. 2e and f) and the Gram-positive to Gram-negative bacteria ratio (P < 0.05). The fungi to bacteria ratio was also significantly lower in root exclusion plots than control plots in the soils of both tree species (Fig. 2g and h). The abundance of 16:1ω5, indicative of arbuscular mycorrhizal fungi, did not differ significantly between root exclusion (3.27 ± 0.87 mol%) and control (3.16 ± 0.24 mol%) plots in M. laosensis soil. However, root exclusion caused an increase of about 27 % in the PLFA 16:1ω5 in C. lanceolata soil (2.30 ± 0.15 mol% in control and 2.93 ± 0.38 mol% in root exclusion plots, P = 0.03).
In the N addition experiment, PCA analysis of all the PLFA data showed that the PC1 and PC2 components (x- and y-axis, respectively) together accounted for 87.4 % of the variation (PC1 63.1 % and PC2 24.3 %, Fig. 1c and d). There were no major differences in soil microbial community structure between the control and N addition treatment in either of the M. laosensis or the C. lanceolata soils (PC1 scores subjected to one-way ANOVA on ranks P > 0.05, Supplemental Table 3). Compared with the control, N addition also had no effect on the content of bacteria, actinomycetes or fungi as measured in nmol g−1 in the soil of either species (P > 0.05, data not shown).
Correlations among potential PLFA signatures and measured soil chemistry properties
Redundancy analysis showed that soil microbial community structure in the DIRT experiment plots was related to soil pH and C:N ratio; together, all of the environmental data explained 49.4 % of the variance, with axis 1 explaining 38.8 % of the variance and axis 2 explaining another 10.6 % (Fig. 3a). In the N addition experiment plots, soil microbial community structure was significantly related to soil C, soil N and C:N ratio; together, all of the environmental data explained 57.6 % of the variance, with axis 1 explaining 54.1 % of the variance and axis 2 explaining another 3.5 % (Fig. 3b). The abundance of the fungal lipid biomarkers, 18:1ω9 and 18:2ω6,9 was significantly and positively related to soil C:N ratio and the abundance of bacterial community was significantly and negatively related to soil C:N ratio (Fig. 4).
In addition, soil NO3 − was significantly and negatively correlated with the content of total PLFAs, while soil DOC was significantly and positively correlated with the content of total PLFAs (P < 0.05, Table 4). The abundance of Gram-positive bacteria, arbuscular mycorrhizal fungi (16:1ω5) and total fungi showed significant correlations with soil NO3 −, mineral N and DOC in the DIRT experiment plots (Table 4). The abundance of Gram-negative bacteria and arbuscular mycorrhizal fungi were significantly related to soil light fraction organic C and N.
Discussion
Overall, our results showed that soil C:N ratio has a strong relationship with the microbial community structure in our subtropical broadleaf M. laosensis and coniferous C. lanceolata plots. Although this result is based on a single date of sampling and microbial community are temporally sensitive (Landesman and Dighton 2010), we note that it agrees with the results of Högberg et al. (2007) and Ushio et al. (2008), who found that soil C:N ratio and the effect of tree species on this ratio, were the important factors in influencing soil microbial community structure. Tree species specific effects on soil microbial community structure are due to several mechanisms, including the inter-specific differences in the quality and quantity of above- and belowground inputs to soil, and the indirect influence on soil pH and C:N ratio (Leckie et al. 2004; Ushio et al. 2008; Iovieno et al. 2010). Recent evidence shows that site factors, such as pH, texture, organic matter content and C:N ratio of the soil, and the influence of tree species on these factors, plays an important role in determining microbial community structure (Fierer et al. 2009; Rousk et al. 2010a, 2010b). Our previous study showed that although the litter chemistry (C:N and lignin:N ratio) varies significantly between coniferous C. lanceolata and broadleaf M. laosensis, it did not change soil microbial community structure in the mineral soil (Huang et al. 2013a). The results of the present study confirmed our first hypothesis that soil C:N ratio rather than tree species was the important factor in determining soil microbial community structure.
Soil C:N ratio can reflect the substrate quality for soil microorganism growth (Myrold 1999). In general, the biomass and activity of microbes is constrained by the availability and quality of C and nutrients (Wardle 1992; Demoling et al. 2007). Our observations are consistent with other studies that have demonstrated that fungal biomass decreases and bacterial biomass increases with increasing pH and decreasing soil C:N ratio (Högberg et al. 2007; Lauber et al. 2008). Previous studies have shown that land-use change has significant effects on soil properties such as pH and C and N content and the changes of these soil properties may shift the structure of bacterial and fungal communities (Fierer and Jackson 2006; Lauber et al. 2008). In our study, the tree species transition from coniferous C. lanceolata to broadleaf M. laosensis significantly increased soil C:N ratio due to the input of large amounts of high quality above-ground litter. We also found that the soil labile organic C and N under M. laosensis were greater than under C. lanceolata in previous research at this site (Wan et al. 2014). Microorganisms would find this more labile organic matter easier to use than less labile substrates. In the DIRT experiment, we found that soil NO3 −, mineral N, DOC, DON, and light fraction organic C and N concentrations were related to the content of total PLFAs and the abundance of bacterial and fungal communities, suggesting that soil organic matter and nutrient quality may play a critical role in mediating the size and structure of the microbial community in forest soils.
N addition can be an effective measure for improving plant productivity, N availability and substrate quality in forest ecosystems. Many studies have found that increased N tends to decrease total microbial biomass and the fungi:bacteria ratio (Rousk et al. 2011). The fungal community has been found to be more sensitive to N addition than the bacterial community (Demoling et al. 2008; Allison et al. 2009; Börjesson et al. 2012). However, in our study, 8 monthly N additions did not change the biomass and structure of the soil microbial community under M. laosensis or C. lanceolata. Therefore, our second hypothesis was not confirmed. This may be because of the short time period of our N addition experiment (Demoling et al. 2008; Weand et al. 2010). We found that the soil C:N ratio was significantly higher under M. laosensis than under C. lanceolata at the site prior to fertilization. The soil C:N ratio was significantly and positively related to fungal lipid biomarkers and negatively related to bacterial community biomarkers. These results lead us to conclude that the soil nutrient availability before N addition was an important factor in determining the effect of N fertilization on soil microbial biomass and community structure.
The PLFA analysis of the soils from the DIRT plots showed that root exclusion significantly changed soil microbial community structure under M. laosensis and C. lanceolata. This result is in accordance with previous studies (Brant et al. 2006a; Kramer et al. 2010; Wang et al. 2013), where researchers found that root C input had a greater influence on soil microbial community structure than above-ground litter input. Some studies have found that root exclusion decreased fungal biomass and changed bacterial community structure in the organic layer, but not in the mineral horizons of forest soil (Siira-Pietikäinen et al. 2003; Subke et al. 2004). However, our findings were consistent with the studies of Brant et al. (2006a) in which root exclusion significantly decreased fungal biomarker abundance and changed the bacterial community structure in the mineral soil. Root exclusion, preventing the flow of photosynthates from canopy to soil, should lead to a reduction in plant belowground C allocation (Li et al. 2004; Feng et al. 2009; Leff et al. 2012). Root-derived C is the predominant source of mineral soil organic matter and the major (>60 %) source of C for microbes in forest soils (Brant et al. 2006b; Kramer et al. 2010). Therefore, inhibition of root C input will cause a reduction of the biomass and activity of soil microbes and may change the structure of the soil microbial community. Kramer and Gleixner (2006) found that Gram-negative bacteria preferentially utilize recent plant material as a microbial C source while Gram-positive bacteria use substantial amounts of soil organic matter C. In this study, root exclusion increased the abundance of Gram-positive bacteria, which supports the argument that root exclusion probably shifted the soil microbial community towards species tending to utilize more recalcitrant C.
An interesting result we found is that root exclusion did not change the abundance of arbuscular mycorrhizal fungi (16:1ω5) in M. laosensis soil, however, it caused an increase of 27 % in C. lanceolata soil. In M. laosensis plots, we observed abundant mycorrhizae and fine roots beneath the litter layer. Studies have shown that leaf litter may provide a readily available nutrient source via superficial fine roots and mycorrhizal hyphae (Sayer et al. 2006; Luizao et al. 2007). This result leads us to conclude that the mycorrhizal fungal community used litter-derived C when root C input was reduced in M. laosensis soil. Studies in subtropical China suggest that growth of Chinese-fir in replanted woodlands could lead to an increase in the concentrations of allelochemicals such as phenolics in soil (Huang et al. 2000b). Plant allelochemicals not only affect plant growth (Huang et al. 2000b), but may also reduce soil microbial biomass and activity and alter the community composition (Li and Xiao 2012). For example, Souto et al. (2000) reported that plant-produced phenolic compounds inhibited soil microbial activity in French subalpine Norway spruce (Picea abies) forests. In our study, the increase in abundance of arbuscular mycorrhizal fungi in C. lanceolata soil implies that root exclusion caused an increase in C quality in contrast to C. lanceolata soil. The changes in the abundance and composition of arbuscular mycorrhizal fungi could have a large influence on soil C processing rates and C input quality (Langley and Hungate 2003).
Conclusions
Many studies have examined the effect of tree species on the structure of the soil microbial community, but often such studies do not clearly separate the effect of other factors, such as soil properties and land-use history that may affect the community, from the tree species itself. Here, we have shown that the effect of tree species on soil C:N ratio rather than the effect of tree species directly on the microbial community was the main factor in determining community structure. Soil nutrient availability before N addition was an important factor in determining the effect of N fertilization on soil microbial biomass and community structure. Although the quality of above-ground litter differed significantly between broadleaf M. laosensis and coniferous C. lanceolata, root C input had a greater influence on soil microbial community structure than above-ground litter input. Root exclusion decreased the abundance of fungal biomarkers and changed the bacterial community structure in the mineral soil.
References
Aciego PJ, Brookes P (2009) Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol Biochem 41:1396–1405
Allison SD, LeBauer DS, Ofrecio MR, Reyes R, Ta AM, Tran TM (2009) Low levels of nitrogen addition stimulate decomposition by boreal forest fungi. Soil Biol Biochem 41:293–302
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Bastian F, Bouziri L, Nicolardot B, Ranjard L (2009) Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol Biochem 41:262–275
Börjesson G, Menichetti L, Kirchmann H, Kätterer T (2012) Soil microbial community structure affected by 53 years of nitrogen fertilisation and different organic amendments. Biol Fert Soils 48:245–257
Brant JB, Myrold DD, Sulzman EW (2006a) Root controls on soil microbial community structure in forest soils. Oecologia 148:650–659
Brant JB, Sulzman EW, Myrold DD (2006b) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232
De Vries FT, Hoffland E, Van Eekeren N, Brussaard L, Bloem J (2006) Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol Biochem 38:2092–2103
Demoling F, Figueroa D, Bååth E (2007) Comparison of factors limiting bacterial growth in different soils. Soil Biol Biochem 39:2485–2495
Demoling F, Ola Nilsson L, Bååth E (2008) Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol Biochem 40:370–379
Denef K, Roobroeck D, Manimel Wadu MC, Lootens P, Boeckx P (2009) Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol Biochem 41:144–153
Fang Q (1987) Effects of continued planting of Chinese fir on the fertility of soil and the growth of stands. Sci Silvae Sinicae 23:389–397 (In Chinese, Abstract in English).
Fanin N, Hättenschwiler S, Fromin N (2014) Litter fingerprint on microbial biomass, activity, and community structure in the underlying soil. Plant Soil 379:79–91
Feng WT, Zou XM, Schaefer D (2009) Above- and belowground carbon inputs affect seasonal variations of soil microbial biomass in a subtropical monsoon forest of southwest China. Soil Biol Biochem 41:978–983
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. PNAS 103:626–631
Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009) Global patterns in belowground communities. Ecol Lett 12:1238–1249
Fisk MC, Fahey TJ (2001) Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young northern hardwood forests. Biogeochemistry 53:201–223
Fraterrigo JM, Balser TC, Turner MG (2006) Microbial community variation and its relationship with nitrogen mineralization in historically altered forests. Ecology 87:570–579
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Hatfield RD, Jung HJG, Ralph J, Buxton DR, Weimer PJ (1994) A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J Sci Food Agr 65:51–58
Högberg MN, Högberg P, Myrold DD (2007) Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150:590–601
Huang Z, Liao L, Wang S, Cao G (2000a) Allelopathy of phenolics from decomposing stump-roots in replant Chinese fir woodland. J Chem Ecol 26:2211–2219
Huang Z, Liao L, Wang S, Liu Y (2000b) Dynamics of phenolics content of Chinese fir stump-roots and the rhizosphere soil and it’s allelopathy. Chin J Appl Ecol 11:190–192, In Chinese, Abstract in English
Huang Z, Clinton PW, Davis MR, Yang Y (2011) Impacts of plantation forest management on soil organic matter quality. J Soils Sediments 11:1309–1316
Huang Z, Wan X, He Z, Yu Z, Wang M, Hu Z, Yang Y (2013a) Soil microbial biomass, community composition and soil nitrogen cycling in relation to tree species in subtropical China. Soil Biol Biochem 62:68–75
Huang Z, He Z, Wan X, Hu Z, Fan S, Yang Y (2013b) Harvest residue management effects on tree growth and ecosystem carbon in a Chinese fir plantation in subtropical China. Plant Soil 364:303–314
Iovieno P, Alfani A, Bååth E (2010) Soil microbial community structure and biomass as affected by Pinus pinea plantation in two Mediterranean areas. Appl Soil Ecol 45:56–63
Jangid K, Williams MA, Franzluebbers AJ, Schmidt TM, Coleman DC, Whitman WB (2011) Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol Biochem 43:2184–2193
Kramer C, Gleixner G (2006) Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol Biochem 38:3267–3278
Kramer C, Trumbore S, Fröberg M, Cisneros Dozal LM, Zhang D, Xu X, Santos GM, Hanson PJ (2010) Recent (<4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol Biochem 42:1028–1037
Landesman WJ, Dighton J (2010) Response of soil microbial community and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol Biochem 42:1751–1758
Langley JA, Hungate BA (2003) Mycorrhizal controls on belowground litter quality. Ecology 84:2302–2312
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415
Leckie S, Prescott C, Grayston S (2004) Forest floor microbial community response to tree species and fertilization of regenerating coniferous forests. Can J For Res 34:1426–1435
Leff JW, Wieder WR, Taylor PG, Townsend AR, Nemergut DR, Grandy AS, Cleveland CC (2012) Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Global Change Biol 18:2969–2979
Li Q, Xiao H (2012) The interactions of soil properties and biochemical factors with plant allelopathy. Ecol Envir Sci 21:2031–2036
Li Y, Xu M, Sun OJ, Cui W (2004) Effects of root and litter exclusion on soil CO2 efflux and microbial biomass in wet tropical forests. Soil Biol Biochem 36:2111–2114
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828
Luan J, Xiang C, Liu S, Luo Z, Gong Y, Zhu X (2010) Assessments of the impacts of Chinese fir plantation and natural regenerated forest on soil organic matter quality at Longmen mountain, Sichuan, China. Geoderma 156:228–236
Luizao RCC, Luizao FJ, Proctor J (2007) Fine root growth and nutrient release in decomposing leaf litter in three contrasting vegetation types in central Amazonia. Plant Ecol 192:225–236
Myers RT, Zak DR, White DC, Peacock A (2001) Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Sci Soc Am J 65:359–367
Myrold DD (1999) Transformations of nitrogen. In: Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DS (eds) Principles and Applications of Soil Microbiology. Prentice-Hall, N.J., pp 259–294
Potthast K, Hamer U, Makeschin F (2010) Impact of litter quality on mineralization processes in managed and abandoned pasture soils in Southern Ecuador. Soil Biol Biochem 42:56–64
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010a) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME 4:1340–1351
Rousk J, Brookes PC, Bååth E (2010b) The microbial PLFA composition as affected by pH in an arable soil. Soil Biol Biochem 42:516–520
Rousk J, Brookes PC, Bååth E (2011) Fungal and bacterial growth responses to N fertilization and pH in the 150‐year‘Park Grass’UK grassland experiment. FEMS Microbiol Ecol 76:89–99
Sayer EJ, Tanner EVJ, Cheesman AW (2006) Increased litterfall changes fine root distribution in a moist tropical forest. Plant Soil 281:5–13
Siira-Pietikäinen A, Haimi J, Fritze H (2003) Organisms, decomposition, and growth of pine seedlings in boreal forest soil affected by sod cutting and trenching. Biol Fert Soils 37:163–174
Souto XC, Chiapusio G, Pellissier F (2000) Relationships between phenolics and soil microorganisms in spruce forests: significance for natural regeneration. J Chem Ecol 26:2025–2034
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology 90:441–451
Subke JA, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF (2004) Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 139:551–559
Swallow M, Quideau S, MacKenzie M, Kishchuk B (2009) Microbial community structure and function: the effect of silvicultural burning and topographic variability in northern Alberta. Soil Biol Biochem 41:770–777
Templer P, Findlay S, Lovett G (2003) Soil microbial biomass and nitrogen transformations among five tree species of the Catskill Mountains, New York, USA. Soil Biol Biochem 35:607–613
Ushio M, Wagai R, Balser TC, Kitayama K (2008) Variations in the soil microbial community composition of a tropical montane forest ecosystem: does tree species matter? Soil Biol Biochem 40:2699–2702
Ushio M, Balser TC, Kitayama K (2013) Effects of condensed tannins in conifer leaves on the composition and activity of the soil microbial community in a tropical montane forest. Plant Soil 365:157–170
Wan X, Huang Z, He Z, Hu Z, Yu Z, Wang M, Yang Y, Fan S (2014) Effects of tree species transfer on soil dissolved organic matter pools in a reforested Chinese fir (Cunninghamia lanceolata) woodland. Chin J Appl Ecol 25:12–18, In Chinese, Abstract in English
Wang Q, He T, Wang S, Liu L (2013) Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agr Forest Meteorol 178:152–160
Wardle D (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Weand MP, Arthur MA, Lovett GM, McCulley RL, Weathers KC (2010) Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol Biochem 42:2161–2173
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ (1979) Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51–62
Wu QZ (2005) Study of Mytilaria laosensis Plantation Biomass. J Fujian For Sci Tech 32:125–129, In Chinese, Abstract in English
Zhang Q (1997) Effects of soil extracts from repeated plantation woodland of Chinese-fir on microbial activities and soil nitrogen mineralization dynamics. Plant Soil 191:205–212
Zhang Q, Zak JC (1994) Potential role of fungi and bacteria in Chinese fir replant soil. Can J Bot 72:73–78
Zhang XQ, Kirschbaum MU, Hou Z, Guo Z (2004) Carbon stock changes in successive rotations of Chinese fir (Cunninghamia lanceolata (lamb) hook) plantations. For Ecol Manage 202:131–147
Acknowledgment
The research was supported by a National Natural Science Foundation of China (41371269), 2011 Program for New Century Excellent Talents in the University of Ministry of Education of China, and the Science Foundation for Excellent Talents of Fujian Province, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Paul Bodelier.
Rights and permissions
About this article
Cite this article
Wan, X., Huang, Z., He, Z. et al. Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 387, 103–116 (2015). https://doi.org/10.1007/s11104-014-2277-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2277-4