Abstract
Key message
The GAF1 transcription factor is shown to bind to the promoter of the Arabidopsis GA-biosynthetic enzyme GA20ox1 and, in association with DELLA protein, promotes GA20ox1 expression, thereby contributing to its feedback regulation and tissue specificity.
Abstract
Gibberellins (GAs) are phytohormones that promote plant growth and development, including germination, elongation, flowering, and floral development. Homeostasis of endogenous GA levels is controlled by GA feedback regulation. DELLAs are negative regulators of GA signaling that are rapidly degraded in the presence of GAs. DELLAs regulate several target genes, including AtGA20ox2 and AtGA3ox1, encoding the GA-biosynthetic enzymes GA 20-oxidase and GA 3-oxidase, respectively. Previous studies have identified GAI-ASSOCIATED FACTOR 1 (GAF1) as a DELLA interactor, with which DELLAs act as transcriptional coactivators; furthermore, AtGA20ox2, AtGA3ox1, and AtGID1b were identified as target genes of the DELLA-GAF1 complex. Among the five Arabidopsis GA20ox genes, AtGA20ox1 is the most highly expressed gene during vegetative growth; its expression is controlled by GA feedback regulation. Here, we investigated whether AtGA20ox1 is regulated by the DELLA-GAF1 complex. The electrophoretic mobility shift and transactivation assays showed that three GAF1-binding sites exist in the AtGA20ox1 promoter. Using transgenic plants, we further evaluated the contribution of the DELLA-GAF1 complex to GA feedback regulation and tissue-specific expression. Mutations in two GAF1-binding sites obliterated the negative feedback regulation and tissue-specific expression of AtGA20ox1 in transgenic plants. Thus, our results showed that GAF1-binding sites are involved in GA feedback regulation and tissue-specific expression of AtGA20ox1 in Arabidopsis, suggesting that the DELLA-GAF1 complex is involved in both processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioactive gibberellins (GAs) are tetracyclic diterpenoid phytohormones that regulate various aspects of plant growth and development, including seed germination, elongation of root and stem, leaf expansion, flower induction, and anther development (Richards et al. 2001). Biologically active GAs such as GA1 and GA4 are produced by GA biosynthetic enzymes (Yamaguchi, 2008). Endogenous levels of GAs are regulated through feedback control of GA biosynthetic enzymes in several steps (Yamaguchi, 2008; Fukazawa et al., 2011; Hedden and Thomas, 2012). Six biosynthetic enzymes are required for the conversion of geranylgeranyl diphosphate to bioactive GAs in Arabidopsis (Arabidopsis thaliana). Specifically, the final steps in GA biosynthesis are catalyzed by GA 20-oxidase (GA20ox) and GA 3-oxidase (GA3ox), and endogenous GA levels control the expression levels of some GA20ox and GA3ox paralogs. Further, the expression levels of AtGA20ox1, AtGA20ox2, AtGA20ox3, and AtGA3ox1 are increased in GA-deficient mutants, while they are decreased by GA treatment (Chiang et al., 1995; Phillips et al., 1995; Thomas et al., 1999; Rieu et al., 2008). GA feedback regulation has been shown to depend on GA signaling components, including GA receptor GIBBERELLIN INSENSITIVE DWARF1 (GID1), the F-box protein SLEEPY1 (SLY1), DELLA proteins, and SPINDLY (Sasaki et al., 2003; Ueguchi-Tanaka et al., 2005; Griffiths et al., 2006; Sun, 2011; Hauvermale et al., 2012). Five DELLAs have been found in Arabidopsis, including REPRESSOR OF ga1-3 (RGA), GIBBERELLIN INSENSITIVE (GAI), RGA-LIKE1 (RGL1), RGL2, and RGL3, which act as negative regulators in the GA signaling pathway (Tyler et al., 2004). An analysis of the phenotypes of each della mutant showed that although the functions of DELLAs are partially overlapping, they may become distinct during plant development. GAI and RGA are involved in the repression of GA-induced vegetative growth and floral initiation (Dill et al., 2001; King et al., 2001). In turn, RGL2 mainly represses GA-induced seed germination. Further, RGA, RGL1, and RGL2 are involved in flower and fruit development (Cheng et al., 2004; Tyler et al., 2004; Yu et al., 2004). DELLAs are rapidly degraded in the presence of GAs through the ubiquitin-26S proteasome pathway (Sun, 2011). Under conditions of GA-deficiency, DELLAs accumulate in the nucleus and are involved in regulating the transcription of several genes. Fourteen early GA-responsive genes, including AtGA20ox2 and AtGA3ox1, have been identified as the target genes of DELLA (Zentella et al., 2007). These genes are activated by the accumulation of DELLA, and a chromatin immunoprecipitation (ChIP) assay indicated that DELLA proteins are associated with the promoters of several genes. However, DELLAs do not have an apparent DNA-binding motif, and some DNA-binding proteins are necessary to regulate these genes directly. Many transcription factors have been identified as DELLA interactors (de Lucas et al., 2008; Feng et al., 2008; Bai et al., 2012). These transcription factors, including type-B ARABIDOPSIS RESPONSE REGULATORS1 (ARR1) (Marin-de la Rosa et al., 2015), ABSCISIC ACID INSENSITIVE 3 (ABI3), ABI5 (Lim et al., 2013), and SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 9 (SPL9) (Fukazawa et al., 2014), interact with DELLA and activate gene expression.
Recently, we identified a DELLA-binding transcription factor, designated GAI-ASSOCIATED FACTOR1 (GAF1), and revealed the role of DELLAs as transcriptional coactivators (Fukazawa et al., 2014). GAF1 is a transcription factor belonging to the INDETERMINATE1 (ID1) (Colasanti et al., 1998) domain (IDD) family. Sixteen IDD proteins exist in the Arabidopsis genome. Further, Arabidopsis IDD family proteins and Maize ID1 bind to the consensus sequence TTTTGTCG (Kozaki et al., 2004). GAF1 also interacts with corepressor TOPLESS-RELATED (TPR). DELLAs and TPR act as coactivators and corepressors of GAF1, respectively (Fukazawa et al., 2014). Thus, GAs convert the GAF1 complex from a transcriptional activator to a repressor, whereby GAF1-target genes are downregulated in the presence of GA. This mechanism is suitable for explaining GA feedback regulation. Previously, we identified GA feedback-regulated genes, including GA20ox2, GID1b, and GA3ox1, as GAF1 target genes, and ChIP analysis indicated that the DELLA-GAF1 complex binds to promoters of AtGA20ox2 and AtGID1b (Fukazawa et al., 2014). Furthermore, we identified multiple GAF1-binding sites in the AtGA20ox2 promoter and examined the effect on GA feedback regulation of a mutation in each of these GAF1-binding sites. The results indicated that the DELLA-GAF1 complex acts as a main component of the GA feedback regulation of AtGA20ox2 (Fukazawa et al., 2017).
AtGA20ox1 and AtGA20ox2 act redundantly to promote hypocotyl and internode elongation, flowering time, elongation of anther filaments, and to determine the number of seeds. These genes have been reported to be highly expressed during vegetative and early reproductive development. Specifically, AtGA20ox1 is the most highly expressed GA20ox family member in the vegetative shoot and stem, and the ga20ox1 mutant has a semi-dwarf phenotype, whereas the ga20ox2 mutant is only slightly smaller than the wild-type. The ga20ox1 ga20ox2 double mutant exhibits a more severe dwarf phenotype, as well as a late-flowering phenotype under long day (LD) and short day (SD) conditions, indicating that AtGA20ox1 is most important for growth and development among five GA20ox genes. Similar to AtGA20ox2, AtGA20ox1 is also controlled by feedback regulation, but the contribution of the DELLA-GAF1 complex to GA feedback regulation of GA20ox1 remains unclear. To investigate whether the DELLA-GAF1 complex is involved in feedback regulation and tissue-specific expression of GA20ox1, we found multiple GAF1-binding sites in the promoter of AtGA20ox1 and investigated the effect of a mutation in each of these GAF1-binding sites on GA feedback regulation. We showed that the DELLA-GAF1 complex is the main component contributing to GA feedback regulation of AtGA20ox1.
Results
The DELLA-GAF1 complex activates the AtGA20ox1 promoter
The Arabidopsis genome contains five GA20ox genes, among which, AtGA20ox1, −2, and −3 function partially redundantly at several developmental stages, whereas AtGA20ox4 and −5 have very minor roles (Rieu et al., 2008; Plackett et al., 2012). The expression of AtGA20ox1, −2, and −3 is negatively regulated by GA, whereas the expression of AtGA20ox4 and −5 is not regulated by GA levels. Previously, we reported that DELLA-GAF1 complex is involved in feedback regulation of AtGA20ox2, AtGA3ox1, and AtGID1b (Fukazawa et al., 2014). Moreover, the DELLA-GAF1 complex is the main component of the GA feedback regulation of AtGA20ox2 (Fukazawa et al., 2017). Therefore, we postulated that AtGA20ox1 might also be regulated by the DELLA-GAF1 complex. To this end, we performed a transient assay using Arabidopsis cultured cells to investigate whether the DELLA-GAF1 complex directly regulates the expression of AtGA20ox1. Effector plasmids expressing GAF1 and/or DELLA under the control of the CaMV35S promoter were co-introduced with a reporter plasmid containing a LUC reporter gene driven by the AtGA20ox1 promoter (− 464 to + 45, + 1 indicates transcription start site) (Fig. 1A). DELLA-GAF1 activated the AtGA20ox1 promoter-LUC fusion (Fig. 1A), indicating that the DELLA-GAF1 complex regulates the AtGA20ox1 promoter.
DELLA-GAF1 and TPR-GAF1 complexes regulate the AtGA20ox1 promoter. (A) Schematic representation of the reporter and effector. The reporter plasmid consists of A 464 bp fragment of the AtGA20ox1 promoter and a LUC reporter gene. The effector plasmids consist of CaMV 35S promoter, a Ω translation enhancer sequence, the coding region of the GAF1, GAI, or TPR tested. Transactivation assay of GAF1, GAI, and TPR. The reporter, effector, and internal control constructs were co-introduced into protoplasts of T87 Arabidopsis cultured cell. The transfected cells were incubated for 20 h, and then Luc and rLUC activities were measured. The results are shown as Luc/rLUC activity. Error bars indicate SD of three biological replicates (n = 3). Asterisks represent Student’s t-test significance compared with mock-treated control (*P < 0.05). (B) Transactivation assay of GAF1 and GAI. The effector, reporter, and internal control constructs were co-introduced into protoplasts of T87 Arabidopsis cultured cell. The transfected cells were incubated for 20 h, and then Luc and rLUC activities were measured. The results are shown as Luc/rLUC activity. Error bars indicate SD of three biological replicates (n = 3). Asterisks represent Student’s t-test significance compared with mock-treated control (*P < 0.05)
Identification of the cis-acting elements of the AtGA20ox1 promoter for the DELLA-GAF1 complex
To identify the cis-acting elements responsible for the regulation by the DELLA-GAF1 complex in the AtGA20ox1 promoter, we constructed a series of 5′-deletions of the AtGA20ox1 promoter fused to a LUC reporter gene (Δ − 364, Δ − 264, Δ − 164, or Δ − 64) and investigated whether these promoters are activated by the DELLA-GAF1 complex in a transient assay using Arabidopsis T87 cultured cells. The effector plasmids expressing GAF1 and DELLA driven by the CaMV35S promoter were co-introduced with a reporter plasmid containing a LUC reporter gene under the control of the deletions series of the GA20ox1 promoter (Fig. 1B). The DELLA-GAF1 complex activated Δ − 464, Δ − 364, Δ − 264, and Δ − 164, but not Δ − 64, suggesting that at least one functional cis-element for the DELLA-GAF1 complex exist in the AtGA20ox1 promoter between − 464 and − 64 (Fig. 1B).
Identification of GAF1-binding sites in the AtGA20ox1 promoter
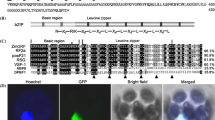
The IDD transcription factor family proteins, including GAF1, bind to ID1-cis, a consensus sequence TTTTGTCG (Kozaki et al., 2004; Fukazawa et al., 2014). Three sequences similar to ID1-cis, GACAAAGACAAAA, TTTTGTCA, and ACAAAAacaACAAAA, were found between region − 330 to − 100 of the AtGA20ox1 promoter (+ 1, indicated transcription start site). Then, an electrophoretic mobility shift assay (EMSA) was performed to investigate whether GAF1 binds to these sequences. The recombinant GAF1 protein bound to the GACAAAGACAAAA, TTTTGTCA, and ACAAAAacaACAAAA sequences, named “−330”, “−250”, and “−100” respectively (Fig. 2A-D). Subsequently, the results of the ChIP analysis used to examine the binding of GAF1 to the AtGA20ox1 promoter in plants showed that GAF1 binds to” −330 and/or −250″ and “−100″ regions of the AtGA20ox1 promoter. However, GAF1 did not bind to the AtGA20ox1coding region (Fig. 2E). As the binding sites are in close proximity, the ChIP assay did not distinguish between the -330 and -250 regions; however, these results unequivocally indicate that GAF1 binds directly to the AtGA20ox1 promoter in vivo.
Identification of GAF1-binding sites in the AtGA20ox1 promoter. (A) EMSA assay using recombinant GAF1 protein. Oligonucleotides containing AtGA20ox1 promoter (GA20ox1-330, GA20ox1-250, and GA20ox1-100) were used as probes. Arrows indicate regions of primers used in the ChIP assay. (B–D) EMSA using the recombinant GAF1 protein. Oligonucleotides containing sequences 330 (WT, lanes 1–4; mt, lane 5) (B), 250 (WT, lanes 1–4; mt, lane 5) (C), and 100 (WT, lanes 1–4; mt, lane 5) (D) were used as probes. Red characters indicate mutated bases. WT and mt indicate competition with a 100-fold excess of unlabeled WT and mutated probe, respectively. The specific GAF1-DNA complexes are indicated by an arrowhead. + indicates an addition to the reaction mixtures, while – indicates omission from the reaction mixtures. (E) GAF1 binds to two regions of the AtGA20ox1 promoter in vivo. ChIP assays were performed with anti-GST or anti-myc in 35S:myc-tag-GAF1 transgenic plants. The co-precipitated level of each DNA fragment was quantified by real-time PCR and normalized to the input DNA. Error bars indicate the SD of three biological replicates (n = 3). Asterisks represent Student’s t-test significance compared with anti-GST control (*P < 0.05)
Mutations in the GAF1-binding sites of the AtGA20ox1 obliterates transcriptional activation by DELLA-GAF1
To investigate whether the GAF1-binding sites identified in vitro are functional in plants for activating the AtGA20ox1 promoter by the DELLA-GAF1 complex, we generated a series of mutant promoters fused to the Luc reporter gene, in which mutations were introduced to eliminate the GAF1-binding sequences (Fig. 3). We determined whether the three GAF1-binding sites (-330, -250, and -100) in the AtGA20ox1 promoter are required for the transcriptional activation by DELLA-GAF1. AtGA20ox1 promoters carrying a single cis-element mutation (mt100, mt250, or mt330) in the three GAF1-binding sites were activated by the DELLA-GAF1 complex, but the transactivation activity was lower than that of the AtGA20ox1 promoter (Fig. 3B). To investigate the effect of the combinations of mutations in GAF1-binding sites, we compared the transactivation activity of double or triple mutants of the GAF1-binding site in AtGA20ox1 promoter coupled to a GUS reporter (Fig. 3C). DELLA-GAF1 activated the double mutant (mt330/250) promoter, but not the triple mutant (3mt) or double mutant (mt330/100) promoters, thereby indicating the involvement of multiple cis-elements in transactivation of AtGA20ox1 (Fig. 3C). Therefore, two GAF1-binding sites (-330 and -100) are involved in the DELLA-GAF1complex-mediated activation of AtGA20ox1.
Mutation of two GAF1-binding sites completely obliterates DELLA-GAF1 activation of the GA20ox1 promoter. (A) Comparison between ID1-cis and GAF1-binding sequences in AtGA20ox1 promoter. Blue characters indicate conserved sequences among these GAF1-binding sequences. Arrows show the direction of each GAF1-binding sequence. The inset box displays the sequence comparison of GAF1-binding regions of −330 (complementary), −250, and −100 (complementary), with maize ID1-binding sequence as the control. (B), (C) Transient assays of GAF1 and GAI, respectively. Reporter constructs of each AtGA20ox1 promoter mutant fused to LUC used in the assay are shown in the left panels. The red cross indicates mutations in GAF1-binding sequences of the AtGA20ox1. The reporter, effector, and internal control plasmids were co-introduced into protoplasts of T87 Arabidopsis cultured cells. The transfected cells were incubated for 20 h, and then Luc and rLUC activities were measured. The results are shown as Luc/rLUC activity. Error bars indicate SD of three biological replicates (n = 3). Asterisks represent Student’s t-test significance compared with mock-treated control (*P < 0.05)
GAF1-binding sites are involved in the GA feedback regulation of AtGA20ox1
To examine whether the two GAF1-binding sites are involved in the GA negative feedback regulation of AtGA20ox1, we generated transgenic plants carrying mutant promoter fused with GUS, in which mutations were introduced to eliminate the GAF1-binding sites (Fig. 4), and the GUS activity of each transgenic plant was measured by fluorometric GUS assay. To investigate the effect of GA feedback regulation on induced GUS expression, paclobutrazol treatment was used to reduce endogenous GA content in transgenic plants. As expected, the mutation abolished the negative feedback regulation of AtGA20ox1. Mutations in two GAF1-binding sites (mt330/100) completely inhibited GA feedback regulation of the promoter (Fig. 4). Thus, these GAF1-binding sites in the AtGA20ox1 promoter are involved in GA negative feedback, indicating that the DELLA-GAF1 complex is the main component in GA feedback regulation of AtGA20ox1. The decrease in GUS activity following GA treatment was not detected in both AtGA20ox1 promoter:GUS and mutated AtGA20ox1 (mt330/100) promoter:GUS transgenic plants, owing to the low basal GUS activity under untreated conditions.
Mutation of two GAF1-binding sites completely obliterates GA response of the AtGA20ox1 promoter. Fluorometric GUS assay for GA feedback in transgenic plants carrying 464 bp of AtGA20ox1 promoter:GUS or mtGA20ox1 promoter:GUS (mt330/100). The green and yellow bars indicate the GUS activities of plants treated for 1 week with GA3 and PAC, respectively. The black bars indicate the GUS activity of mock-treated control plants, which was arbitrarily set to 1.0 (control = 1). Mean activities of eight independent transgenic lines for each construct are shown. Asterisks represent Student’s t-test significance compared with mock-treated control (*P < 0.05). Error bars indicate SD of four biological replicates (n = 3)
GAF1-binding sites are involved in tissue-specific expression of AtGA20ox1 in the leaf vein and flower buds
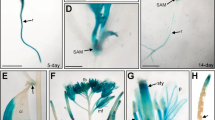
To investigate the contribution of the GAF1-binding sites of the AtGA20ox1 promoter to the tissue-specific expression of AtGA20ox1, the GUS expression patterns in transgenic plants carrying AtGA20ox1 promoter:GUS and mutant AtGA20ox1 promoter:GUS constructs were compared. The GUS staining assay indicated that AtGA20ox1 was expressed mainly in leaf veins, flower buds (Fig. 5A-E), consistent with the expression pattern of the GAF1 promoter:GUS construct (Fukazawa et al., 2014). Expression of the mutant AtGA20ox1 promoter:GUS (mt330/100) in the leaf veins disappeared (Fig. 5F, H), and the expression in flower buds are decreased (Fig. 5G). In contrast, GUS expression was observed in the roots of both AtGA20ox1 promoter:GUS (Fig. 5D, E) and mutant AtGA20ox1 (mt330/100) promoter:GUS transgenic plants (Fig. 5I, J). Furthermore, it indicated that mutation of the GAF1-binding site in the AtGA20ox1 promoter did not disrupt basal transcription. These results indicate that the binding of GAF1 to the AtGA20ox1 promoter participates in the GA feedback regulation of AtGA20ox1 and its tissue-specific expression in leaf veins and flower buds.
Mutation of two GAF1-binding sites partially abrogates the tissue-specific expression of AtGA20ox1. GUS staining patterns in transgenic Arabidopsis plants carrying the ∆464 AtGA20ox1pro:GUS (A-E) and mt330/100 AtGA20ox1 pro:GUS (E-J). Whole plants (A and F), flower buds (B and G), true leaf (C and H), root (D and I), and root tip (E and J) were observed 3 weeks after seed germination. Plants were grown under LD condition for 3 weeks. Scale bar indicates 1 mm
Discussion
Difference between AtGA20ox1 and AtGA20ox2
GA biosynthesis occurs in different tissues, including leaf and shoot apices. AtGA20ox1 and AtGA20ox2 are mainly expressed in the leaf and shoot apex, respectively. The tissue-specific expression patterns of these genes are different, but their expression is controlled by GA feedback regulation, and the GA responsiveness is similar. DELLA-GAF1 contributes not only to GA feedback regulation but also to tissue-specific expression. As to why these genes are expressed in different tissues, other transcription factors might contribute to a tissue-specific expression. Previously, we showed that GUS activity of GA20ox2 promoter:GUS is increased by PAC–a GA inhibitor–treatment, but this increase in GUS activity is restricted in the shoot apex and the root tip. Although GAF1 and DELLA are expressed in other tissues, including leaf veins and trichomes, we could not observe GUS expression in these tissues, suggesting that some tissue-specific transcriptional repressors might contribute to this expression pattern. A ga20ox1 mutant exhibits a semi-dwarf phenotype, whereas a ga20ox2 mutant is slightly smaller than the wild type, indicating that AtGA20ox1 plays the most important role in vegetative growth. The expression levels of AtGA20ox1 are the highest among the five GA20ox genes during vegetative growth (Rieu et al., 2008). GA20ox2 is most highly expressed in the flower tissues, especially in the carpel and siliques. A mutation in the GAF1-binding site of each GA20ox promoter disrupts tissue-specific expression, indicating that GAF1 is involved in tissue-specific expression. The difference in tissue expression between AtGA20ox1 and AtGA20ox2 might depend on the specific combinations among the five DELLA and GAF1 proteins.
The DELLA-GAF1 complex is involved in the GA feedback regulation of AtGA20ox1
In this study, we identified three GAF1-binding sites in the AtGA20ox1 promoter (Figs. 2 and 3), and two of the three GAF1-binding sites were found to be involved in the GA feedback regulation and the tissue-specific expression of AtGA20ox1 (Figs. 4 and 5). Previously, we had shown that the DELLA-GAF1 complex is involved in the GA feedback regulation of AtGA20ox2 and acts as a main component of this regulation (Fukazawa et al., 2017). Further, we propose a model in which DELLA accumulates under GA deficient conditions, and accordingly, the DELLA-GAF1 complex binds to the AtGA20ox2 promoter in plants. In contrast, the application of GA accelerates the degradation of DELLAs, thus reducing the amount of DELLA available to bind to the GAF1-binding site in the AtGA20ox2 promoter. The DELLA-GAF1 complex is also involved in the feedback regulation of GA20ox1 and acts as a main component of the regulation of AtGA20ox1.
GAF1-binding sequences in the promoter of GA feedback-regulated genes
Maize ID1 and the Arabidopsis IDD family proteins, including GAF1, bind to the consensus sequence TTTTGTCG (Kozaki et al., 2004; Fukazawa et al., 2014). Partially identical sequences for this consensus sequences were identified in the promoter of AtGA20ox1 and they include a mutation at position 7 or 8 from the first T. The C at position 7 is particularly important for binding ID1. EMSA analysis using a mutated probe showed that ID1 could not bind to the mutated sequence of TTTTGTAG (Kozaki et al., 2004). In the present study, two GAF1-binding sites were identified in the promoter of AtGA20ox1, which are tandem repeats of the TTTTGTC or TTTTGT sequences. GAF1 bound to both the tandem repeat sequences (Fig. 2B, D). Therefore, the affinity of GAF1 and the partially identical sequence to the ID1-binding sequence might be enhanced by the tandem repeats.
GAF1-binding sequences in the AtGA20ox1 promoter are involved in the tissue-specific expression of AtGA20ox1 in leaf veins and flower buds
The expression of AtGA20ox1 and AtGA20ox2 was elevated in ga1-3 GA-deficient mutants and decreased by GA treatment in the mutant or wild type (Phillips et al., 1995; Xu et al., 1999). DELLAs are negative regulators of GA signaling and act as major plant growth repressors (Dill and Sun, 2001; King et al., 2001); in fact, GAI and RGA are expressed in various tissues during plant growth and development (Silverstone et al., 1998; Lee et al., 2002; Tyler et al., 2004). Rieu et al. (2008) examined the expression levels of AtGA20ox1 and AtGA20ox2 in a GA deficient mutant ga1-3 and the triple mutant gai-t6 rga-24 ga1-3. Although endogenous GA levels were deficient in gai-t6 rga-24 ga1-3, the expression levels of AtGA20ox1 and AtGA20ox2 significantly decreased in this mutant, suggesting that GAI and RGA are involved in the GA feedback regulation of AtGA20ox1 and AtGA20ox2. However, the expression patterns of AtGA20ox1 and AtGA20ox2 in seedlings are different from those of GAI and RGA. The expression of AtGA20ox2 is restricted to meristematic tissues (Plackett et al., 2012), whereas RGA and GAI are ubiquitously expressed. GUS staining analysis using AtGA20ox1 or AtGA20ox2 promoter:GUS transgenic plants showed that AtGA20ox1 is expressed in leaf veins and flower buds (Fig. 5), while AtGA20ox2 is expressed in meristematic tissues in shoot and root apices (Fukazawa et al., 2017). As the expression pattern of GAF1 partially overlaps with that of AtGA20ox1 and AtGA20ox2 (Fukazawa et al., 2014), we hypothesized that GAF1 might play a role in the tissue-specific expression of AtGA20ox1 and AtGA20ox2 under GA-deficiency conditions. Among five GA20ox genes in Arabidopsis, the expression levels of AtGA20ox1 and AtGA20ox2 are high during vegetative and early reproductive development, and both genes act redundantly to promote hypocotyl elongation and determine flowering time. Because the mutants gaf1 gaf2, ga20ox1, and ga20ox2 exhibit similar phenotypes as short hypocotyl and late-flowering (Rieu et al., 2008; Plackett et al., 2012; Fukazawa et al., 2014), the DELLA-GAF1 complex might contribute to the transcriptional regulation of AtGA20ox1 in the leaf vein and flower bud and to that of AtGA20ox2 in the hypocotyl and shoot apex (Fukazawa et al., 2017), respectively. GAF1-overexpressing plants exhibit early flowering, while gaf1 idd1 exhibit late-flowering phenotypes, indicating that GAF1 is involved in flowering. Recently, we identified novel GAF1-target genes, including ELF3, TEM1, TEM2, and SVP, which are expressed in leaves and/or shoot apices (Fukazawa et al., 2021).
The levels of bioactive GAs in plants are maintained through feedback regulation. In Arabidopsis, AtGA20ox1, AtGA20ox2, AtGA20ox3, and AtGA3ox1 are downregulated by GA treatment. Feedback regulation of these genes is not observed in the penta della mutant (Livne et al., 2015), suggesting that DELLAs are involved in the GA feedback regulation. We showed that the DELLA-GAF1 complex is involved in GA feedback regulation of AtGA20ox1 and AtGA20ox2 (Fukazawa et al., 2014, 2017). Other transcription factors, including YABBY1, YABBY4, AT-hook protein of GA feedback1 (AGF1), and REPRESSION OF SHOOT GROWTH (RSG), are also involved in GA feedback regulation. (Dai et al., 2007; Matsushita et al., 2007; Yamaguchi, 2008; Fukazawa et al., 2010, 2011; Yang et al., 2016). In general, endogenous GA levels are maintained by feedback regulation; however, it remains unclear how plants overcome this feedback regulation when endogenous GA levels increase before germination and flowering. Further investigation of how the DELLA-GAF1 complex and other regulators control endogenous GA levels will help to reveal the molecular mechanism of GA accumulation in plants before germination and flowering.
Experimental procedures
Plant material and growth conditions
All transgenic lines were derived from A. thaliana ecotype Col-0 (wild-type). The generation of the transgenic plants overexpressing MYC-tagged GAF1 has been described previously (Fukazawa et al., 2014). To generate mutant plants, the AtGA20ox1 promoter and mutated AtGA20ox1 promoters were cloned into the vector pBI101 between the SalI and BglII sites. The primers used are listed in Supplemental Table S1. Agrobacterium-mediated Arabidopsis transformation was carried out using the floral dip method (Clough and Bent, 1998). The primer sets used for cloning are listed in Supplemental Table S1. Plants were grown in a controlled growth chamber at 22 °C under white light illumination (16/8 h light/dark).
Application of GA 3 to AtGA20ox1 or mtAtGA20ox1 promoter:GUS transgenic plants
To investigate the GA sensitivity, AtGA20ox1 promoter:GUS and mtAtGA20ox1 promoter:GUS transgenic plants were treated with PAC or GA3. Seven-day-old seedlings of transgenic plants grown on 1/2 MS agar media were transferred to 1/2 MS agar plates containing 1 μM PAC or 10 μM GA3 and grown for an additional week. Each 2-week-old transgenic plant was homogenized in the extraction buffer as described by Jefferson et al. (1987). After removing the cell debris by centrifugation at 15 000 × g for 10 min at 15 °C, GUS activities were determined at 37 °C using 1 mM 4-methylumbelliferyl glucuronide as the substrate.
Transient assay
GA20ox1 promoters with a deletion from position − 464 to + 45 (+ 1, transcription start site) were cloned into the HindIII-BamHI site of the p-less LUC vector, which is a pUC18-based plasmid containing the LUC gene (Fukazawa et al., 2017). All primers used for transient assay analysis are shown in Supplemental Table S1. GAF1, GAI, and TPR were cloned into the NotI-XhoI site of the pJ4 vector, which carries the CaMV 35S promoter with a viral translation enhancer, the Ω sequence (Fukazawa et al., 2000), to be used as effectors. Protoplasts were prepared from T87 Arabidopsis cultured cells, and protoplasts were transfected as described previously. Transient assays were performed as described previously (Fukazawa et al., 2014). Relative LUC activity was calculated via normalization to rLUC activity. The data are presented as averages of three independent biological replicates.
Electrophoretic mobility shift assay
EMSA was performed following a previously described procedure (Fukazawa et al., 2000, 2010). Briefly, GAF1 was cloned into the NotI-XhoI site of the pET30b vector (Novagen, Madison, WI, USA); recombinant protein 6 × His-GAF1 was expressed and affinity-purified from Escherichia coli BL21 (DE3) pLysE using Ni+-resin (Novagen, Madison, WI). The nucleotide sequences of the double-stranded oligonucleotides used for the gel mobility shift assays are listed in Supplemental Table S1. The oligonucleotides were annealed and then labeled using (α-32P) dCTP and the Klenow fragment of DNA polymerase I. Binding mixtures contained 50 fmol of the labeled probe, 1 μg of purified recombinant GAF1 or 1 μg of control extract of E. coli, and 2 μg of poly (dI/dC). A DNA competitor was used at a 100-fold excess molar concentration. The binding buffer consisted of 20 mM Tris–HCl (pH 7.5), 3 mM MgCl2, 50 mM KCl, 1 mM EDTA, 10% (v/v) glycerol, and 2 μM ZnCl2. Reactions were incubated at 4 °C for 30 min and loaded onto 4% polyacrylamide gels containing 6.7 mM Tris–HCl, pH 7.5, 1 mM EDTA, and 3.3 mM sodium acetate.
Histochemical staining
Kanamycin-resistant transgenic plants in the Col-0 background were histochemically stained to detect GUS activity by immersing seedlings in a staining solution (100 mM sodium phosphate buffer, pH 7.0, with 50 mM NaCl, 1 mM potassium ferricyanide, 0.1% v/v Triton X-100, and 1 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronide) overnight at 37 °C. Samples were immersed in a fixing solution (5% v/v formaldehyde, 5% v/v acetic acid, 20% v/v ethanol) after staining, followed by dechlorophylation in 70% v/v ethanol. Photographs of GUS-stained plants were taken using a DVM6 Digital Microscope (Leica).
ChIP assay
The ChIP experiment was performed following a previously described procedure (Fukazawa et al., 2010) with some modifications. Briefly, 2-week-old 4 × myc-GAF1 transgenic and Col-0 plants were cross-linked in 1% (v/v) formaldehyde by vacuum infiltration for 10 min and incubated at 4 °C for 1 h. Aliquots of each protein sample were immunoprecipitated with anti-GST (Santa Cruz Biotechnology Inc. SC-138) and anti-myc (MBL International Corporation 562) for 12 h at 4 °C. Chromatin-antibody complexes were precipitated with salmon sperm DNA/protein-G Dyna beads at 4 °C for 2 h. The primers sets used for ChIP assay are listed in Supplemental Table S1. The level of each co-precipitated DNA fragment was quantified by real time-PCR using specific primer sets and normalized to the input DNA. The levels of co-precipitated anti-GST antibody (immunoprecipitated DNA/input DNA) were set to 1. The results are shown as relative DNA enrichment.
References
Bai MY, Shang JX, Oh E, Fan M, Bai Y, Zentella R, Sun TP, Wang ZY (2012) Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat Cell Biol 14:810–817
Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131:1055–1064
Chiang HH, Hwang I, Goodman HM (1995) Isolation of the Arabidopsis GA4 locus. Plant Cell 7:195–201
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal : for Cell and Molecular Biology 16:735–743
Colasanti J, Yuan Z, Sundaresan V (1998) The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 93:593–603
Dai M, Zhao Y, Ma Q, Hu Y, Hedden P, Zhang Q, Zhou DX (2007) The rice YABBY1 gene is involved in the feedback regulation of gibberellin metabolism. Plant Physiol 144:121–133
de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451:480–484
Dill A, Sun T (2001) Synergistic derepression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159:777–785
Dill A, Jung HS, Sun TP (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98:14162–14167
Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, Schafer E, Fu X, Fan LM, Deng XW (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451:475–479
Fukazawa J, Sakai T, Ishida S, Yamaguchi I, Kamiya Y, Takahashi Y (2000) Repression of shoot growth, a bZIP transcriptional activator, regulates cell elongation by controlling the level of gibberellins. Plant Cell 12:901–915
Fukazawa J, Nakata M, Ito T, Yamaguchi S, Takahashi Y (2010) The transcription factor RSG regulates negative feedback of NtGA20ox1 encoding GA 20-oxidase. The Plant Journal : for Cell and Molecular Biology 62:1035–1045
Fukazawa J, Nakata M, Ito T, Matsushita A, Yamaguchi S, Takahashi Y (2011) bZIP transcription factor RSG controls the feedback regulation of NtGA20ox1 via intracellular localization and epigenetic mechanism. Plant Signal Behav 6:26–28
Fukazawa J, Teramura H, Murakoshi S, Nasuno K, Nishida N, Ito T, Yoshida M, Kamiya Y, Yamaguchi S, Takahashi Y (2014) DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell 26:2920–2938
Fukazawa J, Mori M, Watanabe S, Miyamoto C, Ito T, Takahashi Y (2017) DELLA-GAF1 complex is a main component in gibberellin feedback regulation of GA20 oxidase 2. Plant Physiol 175:1395–1406
Fukazawa J, Ohashi Y, Takahashi R, Nakai K, Takahashi Y (2021) DELLA degradation by gibberellin promotes flowering via GAF1-TPR-dependent repression of floral repressors in Arabidopsis. The Plant cell 33(7):2258–2272
Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, Thomas SG (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414
Hauvermale AL, Ariizumi T, Steber CM (2012) Gibberellin signaling: a theme and variations on DELLA repression. Plant Physiol 160:83–92
Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. Biochem J 444:11–25
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
King KE, Moritz T, Harberd NP (2001) Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 159:767–776
Kozaki A, Hake S, Colasanti J (2004) The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res 32:1710–1720
Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16:646–658
Lim S, Park J, Lee N, Jeong J, Toh S, Watanabe A, Kim J, Kang H, Kim DH, Kawakami N, Choi G (2013) ABA-insensitive3, ABA-insensitive5, and DELLAs Interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell 25:4863–4878
Livne S, Lor VS, Nir I, Eliaz N, Aharoni A, Olszewski NE, Eshed Y, Weiss D (2015) Uncovering DELLA-Independent Gibberellin Responses by Characterizing New Tomato procera Mutants. Plant Cell 27:1579–1594
Marin-de la Rosa, N., Pfeiffer, A., Hill, K., Locascio, A., Bhalerao, R.P., Miskolczi, P., Gronlund, A.L., Wanchoo-Kohli, A., Thomas, S.G., Bennett, M.J., Lohmann, J.U., Blazquez, M.A., and Alabadi, D. (2015). Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins. PLoS genetics 11, e1005337
Matsushita A, Furumoto T, Ishida S, Takahashi Y (2007) AGF1, an AT-hook protein, is necessary for the negative feedback of AtGA3ox1 encoding GA 3-oxidase. Plant Physiol 143:1152–1162
Phillips AL, Ward DA, Uknes S, Appleford NE, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P (1995) Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol 108:1049–1057
Plackett AR, Powers SJ, Fernandez-Garcia N, Urbanova T, Takebayashi Y, Seo M, Jikumaru Y, Benlloch R, Nilsson O, Ruiz-Rivero O, Phillips AL, Wilson ZA, Thomas SG, Hedden P (2012) Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 24:941–960
Richards DE, King KE, Ait-Ali T, Harberd NP (2001) How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol 52:67–88
Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, Phillips AL, Hedden P (2008) The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. The Plant Journal : for Cell and Molecular Biology 53:488–504
Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, Matsuoka M (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299:1896–1898
Silverstone AL, Ciampaglio CN, Sun T (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10:155–169
Sun TP (2011) The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Current Biology : CB 21:R338-345
Thomas SG, Phillips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2- oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA 96:4698–4703
Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun TP (2004) Della proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol 135:1008–1019
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, Matsuoka M (2005) Gibberellin insensitive DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698
Xu YL, Li L, Gage DA, Zeevaart JA (1999) Feedback regulation of GA5 expression and metabolic engineering of gibberellin levels in Arabidopsis. Plant Cell 11:927–936
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yang C, Ma Y, Li J (2016) The rice YABBY4 gene regulates plant growth and development through modulating the gibberellin pathway. J Experimental Botany 67(18):5545–5556
Yu H, Ito T, Zhao Y, Peng J, Kumar P, Meyerowitz EM (2004) Floral homeotic genes are targets of gibberellin signaling in flower development. Proc Natl Acad Sci USA 101:7827–7832
Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, Sun TP (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19:3037–3057
Acknowledgements
This study was partially supported by grants from the Japan Society for the Promotion of Science (JSPS) to J.F. (20K06688).
Author information
Authors and Affiliations
Contributions
J.F. and Y.T. designed the research; J.F., C.M., H.A., and K.M. performed the experiments; J.F. and Y.T. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Accession numbers Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: GAF1 (At3g50700), GAI (At1g14920), AtGA20ox1 (At4g25420), and PRR9 (At2g46790).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fukazawa, J., Miyamoto, C., Ando, H. et al. DELLA-GAF1 complex is involved in tissue-specific expression and gibberellin feedback regulation of GA20ox1 in Arabidopsis. Plant Mol Biol 107, 147–158 (2021). https://doi.org/10.1007/s11103-021-01195-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-021-01195-z