Key message
Short review focussing on the role and targeting of vacuolar substructure in plant immunity and pathogenesis.
Abstract
Plants lack specialized immune cells, therefore each plant cell must defend itself against invading pathogens. A typical plant defense strategy is the hypersensitive response that results in host cell death at the site of infection, a process largely regulated by the vacuole. In plant cells, the vacuole is a vital organelle that plays a central role in numerous fundamental processes, such as development, reproduction, and cellular responses to biotic and abiotic stimuli. It shows divergent membranous structures that are continuously transforming. Recent technical advances in visualization and live-cell imaging have significantly altered our view of the vacuolar structures and their dynamics. Understanding the active nature of the vacuolar structures and the mechanisms of vacuole-mediated defense responses is of great importance in understanding plant-pathogen interactions. In this review, we present an overview of the current knowledge about the vacuole and its internal structures, as well as their role in plant–microbe interactions. There is so far limited information on the modulation of the vacuolar structures by pathogens, but recent research has identified the vacuole as a possible target of microbial interference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant vacuole and its function in the cell
Unlike cells from other organisms, plant cells have a uniquely large and prominent organelle called the vacuole, occupying 90–95% of the cell’s volume (Owens and Poole 1979). The vacuole has important physiological functions, one of the main being the preservation of turgor pressure against the cell wall, thus supporting the structural stability of the cell and of the surrounding tissue (Marty 1999). It also serves as a storage tank holding many different materials needed by the cells, including but not limited to: sugars, metabolites, carbohydrates, lipids, amino acids, enzymes, proteins and anthocyanins (Marty 1999; Paris et al. 1996). In addition, the vacuole stores toxic ions and many other compounds that play a role in the defense against bacterial pathogens and herbivores (Martinoia et al. 2012; Van der Hoorn and Jones 2004).
Depending on tissue and cell types, vacuoles are diverse in their morphologies and functions (Swanson et al. 1998). Two main types are found in plants, the protein storage vacuoles (PSV) and the lytic vacuoles (LV) and they are functionally distinct (Hoh et al. 1995; Paris et al. 1996; Robinson et al. 1995). The typical storage compartment PSVs are most abundant in seeds (Epimashko et al. 2004; Hoh et al. 1995; Otegui et al. 2005; Paris et al. 1996; Swanson et al. 1998) and are formed during seed development and maturation. They accumulate large amounts of storage proteins, which are synthesized in the endoplasmic reticulum (ER) and delivered into vacuoles via the prevacuolar complex (Sansebastiano et al. 2017), where they remain stored until they are mobilized during germination and seedling growth (Bewley and Black 1994). PSVs also store defense proteins for the response against microbial pathogens and herbivores. After seed imbibition, PSVs are converted into lytic vacuoles (Bewley and Black 1994; Wang et al. 2007), which are the predominant compartments occurring in vegetative cells and are also called vegetative vacuoles. The identity of each vacuole is determined by its pH and by the presence of specific proteins known to be localized to PSVs or LVs, such as tonoplast intrinsic proteins (TIPs), which are integral membrane proteins found in specific vacuolar membranes (Jauh et al. 1999; Johnson et al. 1989). For example, the PSVs have a neutral pH and are defined by the presence of α- and δ-tonoplast intrinsic protein (TIP), whereas the LVs have an acidic pH and are marked by γ-TIP (Jiang et al. 2000; Jauh et al. 1999).
Vacuoles are increasingly recognized for their role in cellular signaling during growth (Zhang et al. 2014), the immune responses (Hatsugai and Hara-Nishimura 2010) and the regulation of cell death (CD) (Hara-Nishimura and Hatsugai 2011; Koyano et al. 2014). However, little is known about vacuolar structures and their roles in the defense response against pathogens. Several reviews have summarized the interactions between the plant vacuole and pathogenic microbes (Hara-Nishimura and Hatsugai 2011; Hatsugai et al. 2006). Here, the first section of this review focuses on the most up-to-date insights about the plant vacuolar structures and dynamics and vacuolar markers. The last section focuses on the role of the vacuole and vacuolar structures in plant-pathogen interactions.
Vacuolar structures
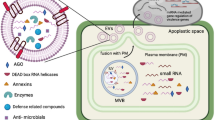
Recent advances in the visualization of the vacuole together with developments in image analysis has revealed the highly organized and complex morphology of the vacuole as well as its dynamics. The plant vacuole is surrounded by a membrane barrier known as the tonoplast, which separates the vacuolar content from the cytoplasm (Fig. 1). The semi-permeable tonoplast maintains a balance of nutrients and ions inside and outside of the vacuole, thus keeping a suitable turgor pressure in the plant cell.
Schematic diagram presents the vacuole and vacuolar structures in plant cells. The vacuole structures tonoplast, TVS, bulb, IVSP, nucleus, nucleolus, and plasma membrane are shown with arrowheads and the cytosolic flow with arrows. TVS transvacuolar structures, IVSP intravacuolar spherical structures
The tonoplast not only surrounds the typical large vacuole but also other transient and mobile structures such as transvacuolar strands (TVS) and bulbs, represented in Fig. 1 (Ruthardt et al. 2005; Uemura et al. 2002). TVSs are dynamic thin tubular structures that traverse the central vacuole, containing cytoplasm and even small organelles (Uemura et al. 2002). Moreover, TVSs provide a direct connection between the perinuclear cytoplasm and the cortical cytoplasm of the cell and as such they act as an important transport route for the distribution of the cytoplasmic content, including the smaller organelles (Grolig and Pierson 2000; Nebenführ et al. 1999). Indeed, it was observed that Golgi bodies (Nebenführ et al. 1999), mitochondria (Van Gestel et al. 2002), endosomes (Ovečka et al. 2005; Ruthardt et al. 2005), and amyloplasts (Saito et al. 2005) dynamically move through the TVSs. Additionally, they play a role in the positioning of the nucleus (Katsuta et al. 1990; Kumagai and Hasezawa 2001; Williamson 1993). The dynamics of the TVS depends on the actin cytoskeleton; consequently, the disruption of the actin filaments leads to the loss of the transvacuolar strands and inhibition of their movement (Kovar et al. 2000; Kutsuna et al. 2003; Tominaga et al. 2000).
On the other hand, the bulbs are highly dynamic spherical structures between 1 and 22 µm of diameter located in the vacuolar lumen (Madina et al. 2018; Saito et al. 2002). 3-D reconstruction of electronic microscopic images indicates that the bulbs are formed of a double membrane and cytoplasmic material is detected between the two lipid bilayers (Saito et al. 2002). This double membrane of the bulbs is responsible for their brighter fluorescence signal compared to that of the tonoplast membrane (Saito et al. 2002). Similar to the dynamics of the TVSs, the movement of the bulbs is dependent on actin (Beebo et al. 2009; Uemura et al. 2002). However, whether bulbs are naturally occurring structures has recently been questioned by Segami et al. (2014), as they proposed that some bulbs are artifacts due to the dimerization of the GFP moiety of tagged tonoplastic proteins, while intravacuolar spherical structures (IVSP) form naturally. The IVSPs are different from bulbs in florescence intensity (twofolds the fluorescence intensity of the tonoplast vs. 3 or more folds for the bulbs) and thickness of the double membranes (Segami et al. 2014). These structures are also less abundant in cells and are believed to temporarily store membrane components, however, whether IVSPs are independent structures remains to be resolved (Segami et al. 2014). This model also needs to be further explored as GFP dimerization alone cannot explain the accumulation of bulbs in the YFP-2xFYVE A. thaliana line, as this marker binds to the membrane via a protein-lipid interaction and not a transmembrane domain (Saito et al. 2011). Two functions have been proposed for bulbs; first they may be involved in tonoplastic proteins degradation inside of the vacuole (Maîtrejean et al. 2011; Saito et al. 2002). On the other hand, they may be useful for rapid cell expansion by serving as membrane reservoirs (Saito et al. 2002). Recently, Han et al. (2015) even proposed that bulbs may not have specific functions other than the transient accumulation of tonoplast and cytoskeletal proteins until the bulb membrane is reabsorbed back into the tonoplast. Thus, the definitive function and biogenesis mechanism of bulbs still need to be fully elucidated.
Markers to study the vacuolar structures
The large central vacuole can be easily detected as a large transparent region in the plant cell as seen in light microscopy (Marty 1999), whereas the fine structure of the tonoplast and the intravacuolar compartments had mostly been studied by electron microscopy (Gaffal et al. 2007; Gao et al. 2015; Morita et al. 2002; Saito et al. 2002). The development of chemicals and fluorescent protein markers made possible the use of live-cell imaging to study the vacuole in a more detailed manner, which allows a greater understanding of its structure and dynamics under different growth conditions and various stress types (Reisen et al. 2005). Two different approaches for visualizing the plant vacuolar structures are presented here in below: chemical and protein markers. For selecting a particular technique, it is important to be aware of its advantages and limitations.
Chemical markers
To date, a number of fluorescent/chemical dyes have been identified for staining the tonoplast and vacuolar membranous structures (presented in Table 1). For example, the well-known amphiphilic styryl dyes, FM1-43 (N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl) pyridinium dibromide) and FM4-64 (N-(3-triethylammoniumpropyl)-4-(4-diethylaminophenylhexatrienyl) pyridinium dibromide), are valuable and frequently used chemical dyes for vital staining of the tonoplast (i.e. staining in live cells) (Kim et al. 2001; Kutsuna and Hasezawa 2002; Kutsuna et al. 2003; Leshem et al. 2006; Okubo-Kurihara et al. 2008; Parton et al. 2001; Silady et al. 2008; Tanaka et al. 2007). The FM4-64 has also been reported for staining transvacuolar strands (Kutsuna and Hasezawa 2002; Kutsuna et al. 2003; Silady et al. 2008; Tanaka et al. 2007) and bulbs (Kim et al. 2001; Silady et al. 2008; Tanaka et al. 2007). The FM dyes initially stain the plasma membrane, then small cytoplasmic compartments, and finally reach the tonoplast in a process that is time, temperature, and energy-dependent. In general, to label the tonoplast with FM dyes, the cells are pulsed labeled for several minutes and then chased for several hours in fresh medium. The optimal duration of the chase period depends on the trafficking activity of the cells. In the initial stage of the chase period, the dyes simultaneously label the tonoplast and the other endomembrane components, including the endosomal organelles and developing cell plates (Higaki et al. 2008; Kutsuna and Hasezawa 2002; Ovečka et al. 2005; Parton et al. 2001; Tanaka et al. 2007; Ueda et al. 2001; Vermeer et al. 2006), whereas a longer chase period is required to visualize only the tonoplast (Emans et al. 2002; Kutsuna and Hasezawa 2002; Ueda et al. 2001). In addition to the FM dyes, BCECF (2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein) fluorescently labels the vacuolar lumen, the tonoplast, the transvacuolar strands, and the bulbs (Higaki et al. 2007; Kutsuna and Hasezawa 2002; Mitsuhashi et al. 2000; Swanson et al. 1998; Toyooka et al. 2006). Vital staining using dyes is rapid, simple to perform, and compatible with the concomitant visualization of fluorescently tagged protein markers. However, this method has several limitations, in either sensitivity or specificity, and vital dyes themselves can sometimes introduce artifacts that must be taken care of during sample preparation or live cells imaging (Melan 1999; Schnell et al. 2012).
Protein markers
In addition to chemical markers, many marker proteins tagged with fluorescent proteins are used to label the tonoplast and the vacuolar structures in living cells (Table 1). Most of those markers are integral membrane proteins, with the exception of YFP-2xFYVE which lacks a transmembrane domain and binds to phosphatidylinositol 3-phosphate (Saito et al. 2011). As YFP-2xFYVE labels bulbs more intensely than the tonoplast, it suggests that PI3P is more concentrated in the bulbs (Vermeer et al. 2006; Saito et al. 2011). However, not all tonoplast markers can also label bulbs, showing that bulb membranes are qualitatively different than tonoplast membranes (Saito et al. 2002). For example, γ-TIP-GFP was found to be concentrated in tonoplast and bulbs, whereas GFP-AtRAB7c, another tonoplast marker, did not give any fluorescent signal in bulbs from transgenic plants, although the presence of bulbs in these lines was confirmed by transmission electron microscopy (Saito et al. 2002). Some aquaporin isoforms also specifically label tonoplast or bulbs, for example TIP2;1-GFP exclusively localizes in the tonoplast but does not label the bulbs in salt-treated root cells, whereas TIP1;1 relocalized into intracellular spherical structures hypothesized to be tonoplast compartmentalization domains specialized for degradation of this isoform (Boursiac et al. 2005). In addition, two candidate effectors of pathogens have been observed to target the tonoplast and tonoplast-derived structures (TVSs and bulbs): HaRxLR17 from the oomycete Hyaloperonospora arabidopsidis (Hpa) (Bozkurt et al. 2015; Caillaud et al. 2012) and Mlp124357 from the fungus Melampsora larici-populina (Mlp) (Madina et al. 2018), and they can be used to study those organelles. The GFP-tagged marker proteins allow the visualization of the vacuole and vacuolar structures without further experimental manipulations once the transgenic lines are constructed. Although this is a powerful technique, it is laborious, time-consuming, and often not practical for many laboratories (Melan 1999; Schnell et al. 2012).
Protein distribution and motility of tonoplast and bulbs
Protein distribution
The complexity and dynamic changes in vacuolar lumen content raise the question of how cells regulate the movement of these materials between the cytosol and the vacuole. A controlled transport across the tonoplast is essential for appropriate plant responses to environmental conditions and for adequate intracellular signaling. The tonoplast contains numerous proteins that facilitate the transport of water, ions and metabolic products across the membrane (Martinoia et al. 2012; Zhang et al. 2014). In response to variation in the cytoplasmic environments, the activity of tonoplast enzymes, transporters, and channels is changed and thus they regulate the material exchange between the cytoplasm and the vacuolar lumen, maintaining cellular homeostasis. For example, H+ -ATPase (V-ATPase), H+ -pyrophosphatase (V-PPase), and water channels (aquaporins) have been characterized and their roles in the regulation of transport across the membrane have been discussed (Hedrich 2012; Martinoia et al. 2012; Neuhaus and Trentmann 2014).
On the other hand, the lipids of the tonoplast provide an essential molecular environment for the activity of the membrane proteins and serve as a barrier between the cytoplasm and the vacuolar lumen. Interestingly, lipids and proteins in the tonoplast are not always uniformly distributed and tend to be enriched in particular regions, termed membrane micro-domains, that depend on sphingolipids and sterols (Kusumi et al. 2005; Lillemeier et al. 2006; Minami et al. 2009). For example, the tonoplast of Arabidopsis suspension cultured cells contains micro-domains with higher ratios of the saturated phospholipids phosphatidylcholine (PC) and phosphatidylethanolamine (PE) in which the vacuolar-type proton ATPase (V-ATPase) was more abundant in detergent-resistant microdomains and appeared to be unevenly distributed in the tonoplast, whereas the vacuolar-type proton pyrophosphatase (V-PPase) was distributed evenly (Yoshida et al. 2013). Another study showed a similar non-uniform distribution of the V-ATPase in the tonoplast of isolated maize root cells (Kluge et al. 2004) and detergent-resistant micro-domains containing a high percentage of sphingolipids, free sterols and saturated fatty acids were described in the tonoplast of sugar-beet roots (Ozolina et al. 2011). The causes of this precise distribution of lipids and proteins in the tonoplast membrane are not fully elucidated yet; however, the environmental conditions seem to act as important regulators. For example, under phosphate deficiency the Arabidopsis phospholipase D PLDζ2 adopts an uneven distribution in which the higher concentrations of PLDζ2 were preferentially positioned close to mitochondria and chloroplasts and thus facilitated transfer between them and the tonoplast (Yamaryo et al. 2008).
Recently, it was observed that an overexpression in Arabidopsis of tonoplast intrinsic protein 1;1 fused with GFP (AtTIP1;1-GFP) labels both the tonoplast and the bulbs of the central vacuole but that the distribution of the GFP fusion protein was uneven along the tonoplast (Beebo et al. 2009). We also observed an uneven distribution of a fluorescently tagged protein on the bulb membrane (Video S1). Our recent report showed that the candidate effector protein Mlp124357-eGFP localized in the tonoplast, the TVS, and the bulbs of the vacuolar lumen in Arabidopsis (Madina et al. 2018). With the help of high-resolution 3-D imaging, we observed two different distribution patterns on the bulb membranes for both Mlp124357-eGFP and the well-known tonoplast marker γ-TIP-YFP. This suggests the existence of two distinct bulb types in these cells, some with a regular marker protein distribution and some with exclusion spots.
Protein mobility
Previous description of bulbs and prevailing models indicate they are connected to the tonoplast (Saito et al. 2002). On the other hand, a report pointed out the qualitative differences in protein content between the bulb membranes and the tonoplast (Saito et al. 2011). To investigate the connection between the two membranes, protein mobility between the tonoplast and the bulb membrane have recently been assessed using fluorescence recovery after photobleaching (FRAP) of young Arabidopsis leaf epidermal cells expressing Mlp124357-eGFP or γ-TIP-YFP. We observed the fluorescence of the bleached region of tonoplast recovered completely within 1 min, whereas the bleached area of the bulb membrane fluorescence did not recover (Madina et al. 2018). This observation can be explained by two mechanisms: (1) something restricts the movement of proteins from the tonoplast to the bulb membrane even though they are still attached or (2) the connection between the bulbs and the tonoplast is severed. Both explanations provide a new perception of the biology of tonoplast-derived substructures, but further investigation is required to validate the molecular mechanisms implicated. Furthermore, as the vacuole plays a crucial role in plant defense, the role of protein distribution and motility in tonoplast and bulbs should also be the focus of more research.
The vacuole in plant-pathogen interaction
The immune system of plants lack antibodies or phagocytosis. As an alternative, they have evolved numerous layers of active defense responses against pathogens including the production of reactive oxygen species (ROS) (Alvarez et al. 1998; Zhang et al. 2003) and of many other defense compounds such as phytoalexins (Neuhaus et al. 1991). Attempted attacks by avirulent pathogens may result in cell death (CD) in the tissues, a reaction known as the hypersensitivity response (HR), which is effective in preventing the spread of pathogens (Mur et al. 2007). These defense responses largely depend on the plant’s vacuole because it constitutes a reservoir for many secondary metabolites, hydrolytic enzymes and defense proteins (Marty 1999; Hara-Nishimura and Hatsugai 2011).
Secondary metabolites
Plant vacuoles accumulate a variety of secondary metabolites including cyanogenic glycosides, benzoxazinoids, and phenolics, some of which are thought to function as direct defenses against pathogens by reducing their performance, survival, and reproduction (Shitan 2016; Steppuhn et al. 2004). For example, the well-known cyanogenic glycosides are stored in the plant vacuole as inactive precursors and are able to form toxic hydrocyanic acid (HCN) in response to tissue damage by different phytopathogens (Vetter 2000; Freeman and Beattie 2008). A recent study reported that mutation of the cyanogenic 4-hydroxyindole-3-carbonyl nitrile (4-OH-ICN) pathway increases susceptibility to the bacterial pathogen Pseudomonas syringae in Arabidopsis, suggesting a role in inducible pathogen defense (Rajniak et al. 2015). Benzoxazinoids are among the most important plant defense compounds for grasses (Poaceae) and are also stored as inactive glucosides in the vacuole to avoid toxicity to the plant itself (Niemeyer 2009; Handrick et al. 2016; Zhou et al. 2018). While some benzoxazinoids are constitutively present, others are only synthesised following pathogen infection. Upon tissue damage, they undergo enzymatic and chemical degradation to become the active benzoxazinoid form (Niemeyer 2009). Benzoxazinoids have also been shown to act as defense signaling molecules and to induce callose deposition in response to the pathogenic fungal elicitor chitosan in maize (Ahmad et al. 2011). Another large group of secondary metabolites implicated in defense are the flavonoids, which are widely distributed in terrestrial plants and serve as defense compounds in the plant–microbe interaction (Harborne and Williams 2000; Taylor and Grotewold 2005; Grotewold 2006). For example, silencing of a G-type ABC transporter of M. truncatula (MtABCG10) results in a lower concentration of isoflavonoids in the roots which in turns results in increased growth of Fusarium oxysporum, indicating that flavonoids play an important role in the defense against root-infecting pathogens (Banasiak et al. 2013). Finally, the seed coat accumulates flavonoids to protect the embryo and the endosperm from external stresses such as UV radiation and pathogen infections (Lepiniec et al. 2006; Shimada et al. 2006, 2018).
Hydrolytic enzymes
Like animal lysosome, plant vacuole contains hydrolytic enzymes (e.g. aspartate proteinases, cysteine proteinases, and nucleases) that play an important role in the crucial events of plant cell death to prevent the spread of biotrophic pathogens (Boller and Kende 1979; Wada 2013). As first reported by Jones almost two decades ago, the plant vacuole play an important role in the programmed cell death that occurs in response to biotrophic pathogens (Jones 2001). It was also reported that the vacuolar processing enzyme (VPE) is up-regulated during cell death associated with leaf senescence and lateral root formation in Arabidopsis (Hatsugai et al. 2004). The same group confirmed that during the defense response, plants use the vacuole content in both a destructive and a non-destructive way. The destructive pathway is effective against virus infection, during which the tonoplast collapses and releases vacuolar hydrolytic enzymes called vacuolar processing enzymes (VPE) to suppress virus proliferation in the host cytosol (Hatsugai et al. 2004). The non-destructive way is effective against extracellular bacterial infections and involves the fusion of the plasma membrane to the tonoplast, which allows the discharge of the vacuolar content, including the proteasome subunit PBA1, in the apoplast. This leads to a hypersensitive cell death which suppresses the bacterial proliferation (Hatsugai et al. 2009). Interestingly, although structurally unrelated to caspases, both the vacuolar processing enzyme and the proteasome subunit PBA1 exhibit a caspase-like activity (Hatsugai et al. 2009). As it has been observed that proteasome defects impair the vacuole membrane fusion and VPE deficiency prevents virally induced hypersensitive cell death (Hatsugai et al. 2004, 2009), the identification of PBA1 and VPE substrates would help to unravel the molecular mechanisms of tonoplast breakdown or fusion with the PM.
Defense proteins
The defense proteins, including pathogenesis related proteins (PR proteins) (Neuhaus et al. 1991), myrosinases (Ueda et al. 2006), and lectins (Bowles et al. 1986), are located in the vacuole and act as an effective second line of defense when a pathogen causes tissue damage. For example, overexpression of the pathogen-inducible PR1 genes in tobacco enhances resistance to several fungi, including Peronospora tabacina and Phytophthora parasitica f.sp. nicotianae, and to the bacteria Pseudomonas syringae pv. tabaci (Broekaert et al. 2000). The association between PR-1 proteins and enhanced resistance against oomycetes has also been noted when PR1 expression was transiently silenced by double-stranded RNA interference in barley (Schultheiss et al. 2003). The myrosinases accumulate mainly in the vacuoles of idioblastic myrosin cells, a cell type specific to the abaxial side of the leaf known to accumulate myrosinases (Höglund et al. 1991, 1992; Thangstad et al. 1990, 1991). When plants experience tissue damage, the myrosinases are released from the collapsed vacuoles of the myrosin cells and start the hydrolysis of their glucosinolates substrates to produce isothiocyanates, which are toxic for bacteria. This chemical defense system is known as the myrosinase-glucosinolate system, which is also called the mustard oil bomb (Fuji et al. 2016; Grubb and Abel 2006; Halkier and Gershenzon 2006; Hopkins et al. 2009; Kissen et al. 2009; Rask et al. 2000; Wittstock and Halkier 2002). Moreover, overexpression of Ta-JA1, a jacalin-related lectin gene that resides in vacuole, has been found to increase resistance to bacteria, fungal, and viral pathogens in tobacco plant (Ma et al. 2010).
Vacuole dynamics
The relationship between vacuole dynamics and cell death during the defense response has been discussed for over a decade (Jones 2001). For example, during cell death of the tracheary element in Zinnia elegans, disintegration of the tonoplast was observed (Obara et al. 2001). Recent studies have demonstrated that during cell death induced by a pathogenic signal such as the oomycete elicitor cryptogein from Phytophthora cryptogea or the bacteria Erwinia carotovora, the complex vacuole of BY-2 cells simplified and then ruptured (Higaki et al. 2007; Hirakawa et al. 2015). This seem to be regulated by the vacuolar-localized protease called VPE (Higaki et al. 2011). The structural simplification of vacuoles is also observed in various processes involving program cell death, such as gibberellin-mediated cell death in central aleurone cells (Gao et al. 2015), embryogenesis of gymnosperms (Smertenko et al. 2003), and leaf formation in lace plants (Gunawardena 2007). However, it remains elusive whether simplification of the vacuole is a typical process in defense-related CD.
Tonoplast, TVS, and bulbs in plant-pathogen interaction
Although the vacuole plays an important role in plant defense, little is known about the manipulation of plant vacuolar structures by pathogens. To date, only two effector proteins, one from H. arabidopsidis and the other from M. larici-populina, have been found to reside in the host tonoplast, TVS, and bulbs (Bozkurt et al. 2015; Caillaud et al. 2012; Madina et al. 2018). Such localization of effector proteins may indicate a pathogenic strategy to modulate the host vacuole and vacuolar structures to suppress the vacuole-mediated defense response. For example, dynamic changes of the vacuolar structures are observed during cryptogein-induced PCD in tobacco BY-2 cells (Higaki et al. 2007). As illustrated in Fig. 2, immediately after cryptogein-treatment, no prominent structural changes of the cells were observed, rather, a complex vacuole structures featuring many transvacuolar strands were seen and the nuclei localizes in the central region of the cell (Fig. 2a). However, after some time the TVSs are gradually decreased in number and complexity and bulb structures appeared (Fig. 2b). Subsequently, bulb structures disappeared resulting the simpler vacuolar structure before the cell death (Fig. 2c, d). The simple-shaped vacuole weakened the tonoplast, leading to its ruptures, which sequentially would be lethal for the cell since it would dilute and acidify the cytosol and release cellular reactive secondary metabolites and hydrolytic enzymes (Higaki et al. 2011). A similar kind of vacuolar simplification was observed in BY-2 cells treated with culture filtrates of Erwinia carotovora, a plant pathogenic bacterium (Hirakawa et al. 2015), but in that situation the lysis of the plasma membrane seemed to occur while the vacuole was still intact. It is believed that the simplification of the vacuole is crucial for the vacuolar breakdown, but how these phenomena are orchestrated remains to be elucidated.
A schematic illustration of the reorganization of the vacuolar structures during pathogen-induced cell death in BY-2 cells. a After cryptogein treatment at 0 h, the vacuole contains many transvacuolar strands and the nucleus localizes at the central region of the cell. b After 12 h, the nucleus moves from the center to the periphery of the cell and bulb-like structures appear within the vacuole while transvacuolar strands are gradually disrupted. c After 18 h, the bulb-like structures disappear, and small spherical vacuoles appear. d After 24 h, the VM and PM lose their integrity. Dashed lines indicate broken membranes
Conclusions and future perspectives
Although the role of the vacuole in plant-pathogen interactions has recently attracted much attention, our knowledge of the pathogen manipulation of host vacuolar membrane structures and trafficking remains minimal. As we have reviewed here, plant vacuolar structures are highly organized and dynamic, and they are involved in many cellular processes including plant-pathogen interactions. However, further studies are necessary to characterize the biogenesis mechanisms of bulbs and transvacuolar strands and to elucidate which physiological roles they play in the environmental responses of plants. Live-cell imaging using chemical dyes or fluorescently tagged proteins will undoubtedly serve as a critical technique to reveal the underlying mechanisms between vacuolar structural trafficking and plant-pathogen interactions and the use of plants defective in specific transport steps in vacuolar trafficking will help to elucidate the intracellular itinerary of still uncharacterized bulb membrane proteins.
References
Ahmad et al (2011) Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol 157:317–327
Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Banasiak J, Biała W, Staszków A, Swarcewicz B, Kępczyńska E, Figlerowicz M, Jasiński M (2013) A Medicago truncatula ABC transporter belonging to subfamily G modulates the level of isoflavonoids. J Exp Bot 64:1005–1015
Beebo A et al (2009) Life with and without AtTIP1; 1, an Arabidopsis aquaporin preferentially localized in the apposing tonoplasts of adjacent vacuoles. Plant Mol Biol 70:193–209
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum Press, New York
Boller T, Kende H (1979) Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol 63:1123–1132
Bottanelli F, Foresti O, Hanton S, Denecke J (2011) Vacuolar transport in tobacco leaf epidermis cells involves a single route for soluble cargo and multiple routes for membrane cargo. Plant Cell 23:3007–3025
Boursiac Y, Chen S, Luu D-T, Sorieul M, Van den Dries N, Maurel C (2005) Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol 139:790–805
Bowles DJ, Marcus SE, Pappin D, Findlay J, Eliopoulos E, Maycox PR, Burgess J (1986) Posttranslational processing of concanavalin A precursors in jackbean cotyledons. J Cell Biol 102:1284–1297
Bozkurt TO, Belhaj K, Dagdas YF, Chaparro-Garcia A, Wu CH, Cano LM, Kamoun S (2015) Rerouting of plant late endocytic trafficking toward a pathogen interface. Traffic 16:204–226
Broekaert WF, Terras FRG, Cammue BPA (2000) Induced and preformed antimicrobial proteins. In: Slusarenko AJ, Fraser RSS, van Loon LC (eds) Mechanisms of resistance to plant diseases. Kluwer Academic Publishers, Dordrecht, pp 371–477
Caillaud MC, Piquerez SJ, Fabro G, Steinbrenner J, Ishaque N, Beynon J, Jones JD (2012) Subcellular localization of the Hpa RxLR effector repertoire identifies a tonoplast-associated protein HaRxL17 that confers enhanced plant susceptibility. Plant J 69:252–265
De Angeli A, Monachello D, Ephritikhine G, Frachisse J, Thomine S, Gambale F, Barbier-Brygoo H (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442:939
Emans N, Zimmermann S, Fischer R (2002) Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14:71–86
Endler A et al (2006) Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141:196–207
Epimashko S, Meckel T, Fischer-Schliebs E, Lüttge U, Thiel G (2004) Two functionally different vacuoles for static and dynamic purposes in one plant mesophyll leaf cell. Plant J 37:294–300
Escobar NM, Haupt S, Thow G, Boevink P, Chapman S, Oparka K (2003) High-throughput viral expression of cDNA-green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell 15:1507–1523
Foresti O, Luis L, Denecke J (2006) Overexpression of the Arabidopsis syntaxin PEP12/SYP21 inhibits transport from the prevacuolar compartment to the lytic vacuole in vivo. Plant Cell 18:2275–2293
Freeman BC, Beattie GA (2008) An overview of plant defenses against pathogens and herbivores. Plant Health Instr 149
Fuji K, Shirakawa M, Shimono Y, Kunieda T, Fukao Y, Koumoto Y, Takahashi H, Hara-Nishimura I, Shimada T (2016) The adaptor complex AP-4 regulates vacuolar protein sorting at trans-Golgi network by interacting with VACUOLAR SORTING RECEPTOR 1. Plant Physiol 170:211–219
Gaffal KP, Friedrichs GJ, El-Gammal S (2007) Ultrastructural evidence for a dual function of the phloem and programmed cell death in the floral nectary of Digitalis purpurea. Ann Bot 99:593–607
Gao C, Zhao Q, Jiang L (2015) Vacuoles protect plants from high magnesium stress. Proc Natl Acad Sci USA 112:2931–2932
Gattolin S, Sorieul M, Hunter PR, Khonsari RH, Frigerio L (2009) In vivo imaging of the tonoplast intrinsic protein family in Arabidopsis roots. BMC Plant Biol 9:133
Grolig F, Pierson ES (2000) Cytoplasmic streaming: from flow to track. In: Actin: a dynamic framework for multiple plant cell functions, vol 89. Springer, New York, pp 165–190
Grotewold E (2006) The genetics and biochemistry of floral pigments. Ann Rev Plant Biol 57:761–780
Grubb CD, Abel S (2006) Glucosinolate metabolism and its control. Trends Plant Sci 11:89–100
Gunawardena AH (2007) Programmed cell death and tissue remodelling in plants. J Exp Bot 59:445–451
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Ann Rev Plant Biol 57:303–333
Han SW, Alonso JM, Rojas-Pierce M (2015) REGULATOR OF BULB BIOGENESIS1 (RBB1) is involved in vacuole bulb formation in Arabidopsis. PLoS ONE 10:e0125621
Handrick V et al (2016) Biosynthesis of 8-o-methylated benzoxazinoid defense compounds in maize. Plant Cell 28:1682–1700
Hara-Nishimura I, Hatsugai N (2011) The role of vacuole in plant cell death. Cell Death Differ 18:1298–1304
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochem 55:481–504
Hatsugai N, Hara-Nishimura I (2010) Two vacuole-mediated defense strategies in plants. Plant Signal Behav 5:1568–1570
Hatsugai N et al (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305:855–858
Hatsugai N, Kuroyanagi M, Nishimura M, Hara-Nishimura I (2006) A cellular suicide strategy of plants: vacuole-mediated cell death. Apoptosis 11:905–911
Hatsugai N et al (2009) A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev 23:2496–2506
Hawes C, Saint-Jore CM, Brandizzi F, Zheng H, Andreeva AV, Boevink P (2001) Cytoplasmic illuminations: in planta targeting of fluorescent proteins to cellular organelles. Protoplasma 215:77–88
Hedrich R (2012) Ion channels in plants. Physiol Rev 92:1777–1811
Hicks GR, Rojo E, Hong S, Carter DG, Raikhel NV (2004) Geminating pollen has tubular vacuoles, displays highly dynamic vacuole biogenesis, and requires VACUOLESS1 for proper function. Plant Physiol 134:1227–1239
Higaki T, Kutsuna N, Okubo E, Sano T, Hasezawa S (2006) Actin microfilaments regulate vacuolar structures and dynamics: dual observation of actin microfilaments and vacuolar membrane in living tobacco BY-2 cells. Plant Cell Physiol 47:839–852
Higaki T et al (2007) Elicitor-induced cytoskeletal rearrangement relates to vacuolar dynamics and execution of cell death: in vivo imaging of hypersensitive cell death in tobacco BY-2 cells. Plant Cell Physiol 48:1414–1425
Higaki T, Kutsuna N, Sano T, Hasezawa S (2008) Quantitative analysis of changes in actin microfilament contribution to cell plate development in plant cytokinesis. BMC Plant Biol 8:80
Higaki T, Kurusu T, Hasezawa S, Kuchitsu K (2011) Dynamic intracellular reorganization of cytoskeletons and the vacuole in defense responses and hypersensitive cell death in plants. J Plant Res 124:315–324
Hirakawa Y, Nomura T, Hasezawa S, Higaki T (2015) Simplification of vacuole structure during plant cell death triggered by culture filtrates of Erwinia carotovora. J Integr Plant Biol 57:127–135
Höglund A-S, Lenman M, Falk A, Rask L (1991) Distribution of myrosinase in rapeseed tissues. Plant Physiol 95:213–221
Höglund A-S, Lenman M, Rask L (1992) Myrosinase is localized to the interior of myrosin grains and is not associated to the surrounding tonoplast membrane. Plant Sci 85:165–170
Hoh B, Hinz G, Jeong B-K, Robinson DG (1995) Protein storage vacuoles form de novo during pea cotyledon development. J Cell Sci 108:299–310
Hopkins RJ, van Dam NM, van Loon JJ (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Ann Rev Entomol 54:57–83
Hunter PR, Craddock CP, Di Benedetto S, Roberts LM, Frigerio L (2007) Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol 145:1371–1382
Inada N, Ueda T (2014) Membrane trafficking pathways and their roles in plant-microbe interactions. Plant Cell Physiol 55:672–686
Jaquinod M, Villiers F, Kieffer-Jaquinod S, Hugouvieux V, Bruley C, Garin J, Bourguignon J (2007) A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol Cell Proteomics 6:394–412
Jauh G-Y, Phillips TE, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11:1867–1882
Jiang L, Phillips TE, Rogers SW, Rogers JC (2000) Biogenesis of the protein storage vacuole crystalloid. J Cell Biol 150:755–769
Johnson KD, Herman EM, Chrispeels MJ (1989) An abundant, highly conserved tonoplast protein in seeds. Plant Physiol 91:1006–1013
Jones AM (2001) Programmed cell death in development and defense. Plant Physiol 125:94–97
Kamiya T, Akahori T, Ashikari M, Maeshima M (2006) Expression of the vacuolar Ca2+/H+ exchanger, OsCAX1a, in rice: cell and age specificity of expression, and enhancement by Ca2+. Plant Cell Physiol 47:96–106
Kasaras A, Melzer M, Kunze R (2012) Arabidopsis senescence-associated protein DMP1 is involved in membrane remodeling of the ER and tonoplast. BMC Plant Biol 12:54
Katsuta J, Hashiguchi Y, Shibaoka H (1990) The role of the cytoskeleton in positioning of the nucleus in premitotic tobacco BY-2 cells. J Cell Sci 95:413–422
Kim DH et al (2001) Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13:287–301
Kissen R, Rossiter JT, Bones AM (2009) The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem Rev 8:69–86
Kluge C et al (2004) Subcellular distribution of the V-ATPase complex in plant cells, and in vivo localisation of the 100 kDa subunit VHA-a within the complex. BMC Cell Biol 5:29
Kobae Y, Uemura T, Sato MH, Ohnishi M, Mimura T, Nakagawa T, Maeshima M (2004) Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant Cell Physiol 45:1749–1758
Kovar DR, Staiger CJ, Weaver EA, McCurdy DW (2000) AtFim1 is an actin filament crosslinking protein from Arabidopsis thaliana. Plant J 24:625–636
Kovermann P et al (2007) The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J 52:1169–1180
Koyano T, Kurusu T, Hanamata S, Kuchitsu K (2014) Regulation of vacuole-mediated programmed cell death during innate immunity and reproductive development in plants. In: Sawada H, Inoue N, Iwano M (eds) Sexual reproduction in animals and plants. Springer, Tokyo, pp 431–440
Küfner I, Koch W (2008) Stress regulated members of the plant organic cation transporter family are localized to the vacuolar membrane. BMC Res Notes 1:43
Kumagai F, Hasezawa S (2001) Dynamic organization of microtubules and microfilaments during cell cycle progression in higher plant cells. Plant Biol 3:4–16
Kusumi A et al (2005) Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Ann Rev Biophys Biomol Struct 34:351–378
Kutsuna N, Hasezawa S (2002) Dynamic organization of vacuolar and microtubule structures during cell cycle progression in synchronized tobacco BY-2 cells. Plant Cell Physiol 43:965–973
Kutsuna N, Kumagai F, Sato MH, Hasezawa S (2003) Three-dimensional reconstruction of tubular structure of vacuolar membrane throughout mitosis in living tobacco cells. Plant Cell Physiol 44:1045–1054
Lepiniec L, Debeaujon I, Routaboul J-M, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Ann Rev Plant Biol 57:405–430
Leshem Y et al (2006) Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci USA 103:18008–18013
Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM (2006) Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci USA 103:18992–18997
Ma QH, Tian B, Li YL (2010) Overexpression of a wheat jasmonate-regulated lectin increases pathogen resistance. Biochimie 92:187–193
Madina MH, Zheng H, Germain H (2018) New insight into bulb dynamics in the vacuolar lumen of Arabidopsis cells. Botany 96:511–520
Maîtrejean M et al (2011) Assembly and sorting of the tonoplast potassium channel AtTPK1 and its turnover by internalization into the vacuole. Plant Physiol 156:1783–1796
Martinoia E, Meyer S, De Angeli A, Nagy R (2012) Vacuolar transporters in their physiological context. Plant Biol 63:183–213
Marty F (1999) Plant vacuoles. Plant Cell 11:587–599
Melan MA (1999) Overview of cell fixatives and cell membrane permeants. Methods Mol Biol 115:45–55
Minami A et al (2009) Alterations in detergent-resistant plasma membrane microdomains in Arabidopsis thaliana during cold acclimation. Plant Cell Physiol 50:341–359
Mitsuhashi N, Shimada T, Mano S, Nishimura M, Hara-Nishimura I (2000) Characterization of organelles in the vacuolar-sorting pathway by visualization with GFP in tobacco BY-2 cells. Plant Cell Physiol 41:993–1001
Morita MT, Kato T, Nagafusa K, Saito C, Ueda T, Nakano A, Tasaka M (2002) Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 14:47–56
Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E (2007) The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot 59:501–520
Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121:1127–1141
Neuhaus HE, Trentmann O (2014) Regulation of transport processes across the tonoplast. Front Plant Sci 5:460
Neuhaus J-M, Sticher L, Meins F, Boller T (1991) A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci USA 88:10362–10366
Niemeyer HM (2009) Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: key defense chemicals of cereals. J Agric Food Chem 57:1677–1696
Obara K, Kuriyama H, Fukuda H (2001) Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in Zinnia. Plant Physiol 125:615–626
Okubo-Kurihara E, Sano T, Higaki T, Kutsuna N, Hasezawa S (2008) Acceleration of vacuolar regeneration and cell growth by overexpression of an aquaporin NtTIP1;1 in tobacco BY-2 cells. Plant Cell Physiol 50:151–160
Opalski KS, Schultheiss H, Kogel KH, Hückelhoven R (2005) The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f. sp. hordei. Plant J 41:291–303
Otegui MS, Noh YS, Martínez DE, Vila PMG, Andrew SL, Amasino RM, Guiamet JJ (2005) Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J 41:831–844
Ovečka M, Lang I, Baluška F, Ismail A, Illeš P, Lichtscheidl I (2005) Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 226:39–54
Owens T, Poole RJ (1979) Regulation of cytoplasmic and vacuolar volumes by plant cells in suspension culture. Plant Physiol 64:900–904
Ozolina N, Nesterkina I, Nurminsky V, Stepanov A, Kolesnikova E, Gurina V, Salyaev R (2011) Recognition of lipid-protein rafts in vacuolar membrane. Biochem Biophys Mol Biol 438:120–122
Paris N, Stanley CM, Jones RL, Rogers JC (1996) Plant cells contain two functionally distinct vacuolar compartments. Cell 85:563–572
Parton R, Fischer-Parton S, Watahiki M, Trewavas A (2001) Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci 114:2685–2695
Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2 + -activated channel TPC1 regulates germination and stomatal movement. Nature 434:404
Rajniak J, Barco B, Clay NK, Sattely ES (2015) A new cyanogenic metabolite in Arabidopsis required for inducible pathogen defence. Nature 525:376–379
Rask L, Andréasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J (2000) Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol 42:93–114
Reisen D, Marty F, Leborgne-Castel N (2005) New insights into the tonoplast architecture of plant vacuoles and vacuolar dynamics during osmotic stress. BMC Plant Biol 5:1
Robinson DG, Hoh B, Hinz G, Jeong B-K (1995) One vacuole or two vacuoles: do protein storage vacuoles arise de novo during pea cotyledon development? J Plant Physiol 145:654–664
Ruthardt N, Gulde N, Spiegel H, Fischer R, Emans N (2005) Four-dimensional imaging of transvacuolar strand dynamics in tobacco BY-2 cells. Protoplasma 225:205–215
Saito C (2003) A “bulb” subregion in the vacuolar membrane. Plant Morphol 15:60–67
Saito C et al (2002) A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J 29:245–255
Saito C, Morita MT, Kato T, Tasaka M (2005) Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell 17:548–558
Saito C et al (2011) The occurrence of ‘bulbs’, a complex configuration of the vacuolar membrane, is affected by mutations of vacuolar SNARE and phospholipase in Arabidopsis. Plant J 68:64–73
Sansebastiano GPD, Barozzi F, Piro G, Denecke J, Lousa CDM (2017) Trafficking routes to the plant vacuole: connecting alternative and classical pathways. J Exp Bot 69:79–90
Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, Von Wirén N (2006) AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Biol Chem 281:25532–25540
Schnell U, Dijk F, Sjollema KA, Giepmans BN (2012) Immunolabeling artifacts and the need for live-cell imaging. Nat Methods 9:152–158
Schultheiss H, Dechert C, Kiraly L, Fodor J, Michel K, Kogel KH, Hückelhoven R (2003) Functional assessment of the pathogenesis-related protein PR-1b in barley. Plant Sci 165:1275–1280
Segami S, Makino S, Miyake A, Asaoka M, Maeshima M (2014) Dynamics of vacuoles and H + -pyrophosphatase visualized by monomeric green fluorescent protein in Arabidopsis: artifactual bulbs and native intravacuolar spherical structures. Plant Cell 26:3416–3434
Sheahan MB, Rose RJ, McCurdy DW (2007) Actin-filament-dependent remodeling of the vacuole in cultured mesophyll protoplasts. Protoplasma 230:141–152
Shimada C, Lipka V, O’Connell R, Okuno T, Schulze-Lefert P, Takano Y (2006) Nonhost resistance in Arabidopsis-Colletotrichum interactions acts at the cell periphery and requires actin filament function. Mol Plant-Microbe Interact 19:270–279
Shimada T, Takagi J, Ichino T, Shirakawa M, Hara-Nishimura I (2018) Plant vacuoles. Ann Rev Plant Biol 69:123–145
Shitan N (2016) Secondary metabolites in plants: transport and self-tolerance mechanisms. Biosci Biotechnol Biochem 80:1283–1293
Silady RA, Ehrhardt DW, Jackson K, Faulkner C, Oparka K, Somerville CR (2008) The GRV2/RME-8 protein of Arabidopsis functions in the late endocytic pathway and is required for vacuolar membrane flow. Plant J 53:29–41
Smertenko AP, Bozhkov PV, Filonova LH, Arnold S, Hussey PJ (2003) Re-organisation of the cytoskeleton during developmental programmed cell death in Picea abies embryos. Plant J 33:813–824
Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT (2004) Nicotine’s defensive function in nature. PLoS Biol 2:e217
Swanson SJ, Bethke PC, Jones RL (1998) Barley aleurone cells contain two types of vacuoles: characterization of lytic organelles by use of fluorescent probes. Plant Cell 10:685–698
Tanaka Y, Kutsuna N, Kanazawa Y, Kondo N, Hasezawa S, Sano T (2007) Intra-vacuolar reserves of membranes during stomatal closure: the possible role of guard cell vacuoles estimated by 3-D reconstruction. Plant Cell Physiol 48:1159–1169
Taylor LP, Grotewold E (2005) Flavonoids as developmental regulators. Curr Opin Plant Biol 8:317–323
Thangstad O, Iversen T-H, Slupphaug G, Bones A (1990) Immunocytochemical localization of myrosinase in Brassica napus L. Planta 180:245–248
Thangstad O, Evjen K, Bones A (1991) Immunogold-EM localization of myrosinase in Brassicaceae. Protoplasma 161:85–93
Thomine S, Lelièvre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34:685–695
Tominaga M, Yokota E, Vidali L, Sonobe S, Hepler PK, Shimmen T (2000) The role of plant villin in the organization of the actin cytoskeleton, cytoplasmic streaming and the architecture of the transvacuolar strand in root hair cells of Hydrocharis. Planta 210:836–843
Toyooka K, Moriyasu Y, Goto Y, Takeuchi M, Fukuda H, Matsuoka K (2006) Protein aggregates are transported to vacuoles by macroautophagic mechanism in nutrient-starved plant cells. Autophagy 2:96–106
Ueda T, Yamaguchi M, Uchimiya H, Nakano A (2001) Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J 20:4730–4741
Ueda H et al (2006) AtVAM3 is required for normal specification of idioblasts, myrosin cells. Plant Cell Physiol 47:164–175
Uemura T, Yoshimura SH, Takeyasu K, Sato MH (2002) Vacuolar membrane dynamics revealed by GFP-AtVam3 fusion protein. Genes Cells 7:743–753
Van der Hoorn RA, Jones JD (2004) The plant proteolytic machinery and its role in defence. Curr Opin Plant Biol 7:400–407
Van Gestel K, Köhler R, Verbelen JP (2002) Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. J Exp Bot 53:659–667
Vermeer JE et al (2006) Visualization of PtdIns3P dynamics in living plant cells. Plant J 47:687–700
Vetter J (2000) Plant cyanogenic glycosides. Toxicon 38:11–36
Voelker C, Schmidt D, Mueller-Roeber B, Czempinski K (2006) Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J 48:296–306
Wada Y (2013) Vacuoles in mammals: a subcellular structure indispensable for early embryogenesis. Bioarchitecture 3:13–19
Wang J, Li Y, Lo SW, Hillmer S, Sun SS, Robinson DG, Jiang L (2007) Protein mobilization in germinating mung bean seeds involves vacuolar sorting receptors and multivesicular bodies. Plant Physiol 143:1628–1639
Williamson RE (1993) Organelle movements. Ann Rev Plant Biol 44:181–202
Wittstock U, Halkier BA (2002) Glucosinolate research in the Arabidopsis era. Trends Plant Sci 7:263–270
Wormit A et al (2006) Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 18:3476–3490
Xu Y, Ishida H, Reisen D, Hanson MR (2006) Upregulation of a tonoplast-localized cytochrome P450 during petal senescence in Petunia inflata. BMC Plant Biol 6:8
Yamaryo Y, Dubots E, Albrieux C, Baldan B, Block MA (2008) Phosphate availability affects the tonoplast localization of PLDζ2, an Arabidopsis thaliana phospholipase D. FEBS Lett 582:685–690
Yoshida K et al (2013) Studies on vacuolar membrane microdomains isolated from Arabidopsis suspension-cultured cells: local distribution of vacuolar membrane proteins. Plant Cell Physiol 54:1571–1584
Zhang W, Wang C, Qin C, Wood T, Olafsdottir G, Welti R, Wang X (2003) The oleate-stimulated phospholipase D, PLDδ, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell 15:2285–2295
Zhang C, Hicks GR, Raikhel NV (2014) Plant vacuole morphology and vacuolar trafficking. Front Plant Sci 5:476
Zhou S, Richter A, Jander G (2018) Beyond defense: multiple functions of benzoxazinoids in maize metabolism. Plant Cell Physiol 59(8):1528–1537
Acknowledgements
We are very thankful to Melodie B. Plourde for critical review of the manuscript.
Funding
This work was supported by a NSERC Grant No. RGPIN/435870-2013 and Canada Research Chair number 950-231790.
Author information
Authors and Affiliations
Contributions
Conceptualization: MHM, HZ, HG. Data curation: MHM, MSR, HZ, HG. Funding acquisition: HG. Methodology: MHM, MSR, HG. Project administration: HZ, HG. Resources: HG. Software: MSR, HG. Supervision: HZ, HG. Validation: MHM, MSR, HZ, HG. Visualization: MHM, HG. Writing—original draft: MHM, MSR, HG. Writing—review & editing: HZ, HG.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 Video S1. Uneven distribution of a marker protein on the bulb surface in Arabidopsis. Video of 3-D confocal images of leaf epidermal cell expressing Mlp124357-GFP showing a clear view of an uneven protein distribution of markers on bulbs in a single cell. The Mlp124357, an effector of Melampsora larici-populina, was cloned in fusion with GFP and stably transformed into wild type (Col-0) Arabidopsis. Live-cell imaging was performed by laser scanning confocal microscopy of leaf epidermal cells of 4-days old plants. The GFP was excited at 488 nm and green fluorescence was collected at 505-525 nm. Scale bar = 10 µm. The color gradient reflect the depth. (MP4 4425 kb)
Rights and permissions
About this article
Cite this article
Madina, M.H., Rahman, M.S., Zheng, H. et al. Vacuolar membrane structures and their roles in plant–pathogen interactions. Plant Mol Biol 101, 343–354 (2019). https://doi.org/10.1007/s11103-019-00921-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-019-00921-y