Abstract
The Lemnaceae, known as duckweed, the smallest flowering aquatic plant, shows promise as a plant bioreactor. For applying this potential plant bioreactor, establishing a stable and efficient genetic transformation system is necessary. The currently favored callus-based method for duckweed transformation is time consuming and genotype limited, as it requires callus culture and regeneration, which is inapplicable to many elite duckweed strains suitable for bioreactor exploitation. In this study, we attempted to establish a simple frond transformation system mediated by Agrobacterium tumefaciens for Lemna minor, one of the most widespread duckweed species in the world. To evaluate the feasibility of the new transformation system, the gene CYP710A11 was overexpressed to improve the yield of stigmasterol, which has multiple medicinal purposes. Three L. minor strains, ZH0055, D0158 and M0165, were transformed by both a conventional callus transformation system (CTS) and the simple frond transformation system (FTS). GUS staining, PCR, quantitative PCR and stigmasterol content detection showed that FTS can produce stable transgenic lines as well as CTS. Moreover, compared to CTS, FTS can avoid the genotype constraints of callus induction, thus saving at least half of the required processing time (CTS took 8–9 months while FTS took approximately 3 months in this study). Therefore, this transformation system is feasible in producing stable transgenic lines for a wide range of L. minor genotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant bioreactors were exploited after conventional microbial fermentation systems and mammalian cell reactors. In recent years, the use of plants as bioreactors has emerged as an exciting area of research and the significant advances have created new opportunities (Desai et al. 2010; Saveleva et al. 2016). The most attractive point of plant bioreactors is the economical large-scale production and the versatility of the transcription-translation apparatus of plants as eukaryotes (Cox et al. 2006; He et al. 2011). For the efficient production of recombinant product, selection of the host species is very important. The life cycle, biomass yield, containment and scale-up costs are factors should be considered (Sharma and Sharma 2009).
The Lemnaceae, known as duckweed, a monocotyledonous group of flowering aquatic plant, comprises the smallest angiosperms in the plant kingdom and is classified into five genera and 37 species (Appenroth et al. 2013; Wang et al. 2014). The potential application of duckweed in plant bioreactors, in addition to wastewater treatment and energy material use, has drawn increasing attention (Lam et al. 2014; Stomp 2005; Zhao et al. 2012). As a potential expression system, duckweed is a better candidate than most plants presently applied (Stomp 2005), which can be explained by the following reasons: (1) As an aquatic non-crop plant, duckweed suits the current social and resource circumstances, as its use neither competes with human for food needs, nor competes with grain crops for land. (2) Being the smallest angiosperm with the fastest doubling time, duckweed proliferates rapidly in a nearly exponential growth manner analogous to that of microorganisms. It has been documented that an annual yield of 55 t/h/y dry biomass could be realized under appropriate conditions (Oron 1994). Undoubtedly, this higher rate of reproduction will greatly shorten the bioreactor production cycle. (3) Simple and inexpensive culture conditions make duckweed suitable for large-scale production. (4) Laboratory-cultured duckweed undergoes completely asexual reproduction without disturbance by pollen, which allows the research materials to be genetically stable (Stomp 2005).
Lemna minor, a Lemnaceae family member, as one of the most widespread duckweed species in the world, has all the advantage above. Moreover, L. minor has been well studied due to its rapid growth and high nutritional value (Ge et al. 2012; Iatrou et al. 2015). Therefore, L. minor was chosen as the plant bioreactor material in this study. At present, several proteins have already been expressed in L. minor by genetic transformation based on the conventional callus induction approach (hereafter called callus transformation system, CTS) (Bertran et al. 2015; Ko et al. 2011; Popov et al. 2006). However, two main problems with this transformation method are hindering current research. One is that tissue culture, especially callus induction and the subsequent frond regeneration, is time consuming and laborious (Chang and Chiu 1976; Khvatkov et al. 2015; Stefaniak et al. 2002). The other bottleneck is that the tissue culture conditions of duckweed vary among species and even ecotypes; some ecotypes cannot achieve callus induction or regeneration, limiting the transformation of some elite strains (Moon and Stomp 1997). Therefore, it is necessary to establish a prompt and efficient genetic transformation method without the use of callus.
To establish a simple and efficient transformation system for L. minor, a frond transformation system mediated by A. tumefaciens (hereafter called frond transformation system, FTS) was employed. To evaluate the feasibility of this system, the sterol 22-desaturase gene (CPY710A11) of tomato was overexpressed to improve the yield of stigmasterol in L. minor. Stigmasterol isolated from plants has been proven to have multiple medicinal values, including antioxidant, antitumor, hypoglycemic, hypocholesterolemic, and anti-inflammatory effects (Batta et al. 2006; Gabay et al. 2010; Ghosh et al. 2011; Jamaluddin et al. 1994; Panda et al. 2009). We hope that the FTS can pave the way for further large-scale production of target products via L. minor bioreactor and provide a reference for other species of duckweed or other plants.
Materials and methods
Materials and culture conditions
L. minor strain ZH0055 was originally collected from Ya’an, Sichuan Province, China, then was sent to Elias Landolt for species identification. L. minor strain D0158 (6580) was originally collected from Bergen, New Jersey, USA. L. minor strain M0165 (6591) was originally collected from Escalon, California, USA. They were conserved in the Duckweed Germplasm Bank, Chengdu Institute of Biology, Chinese Academy of Science. They were preserved on Hoagland solid medium (Hoagland and Arnon 1950) supplemented with 1.5% (w/v) sucrose and 0.7% (w/v) agar at pH 5.8, and grown at 25 ± 1 °C with a light intensity of 40 µmol/m2/s under a 16 h/day photoperiod. Prior to use, the preserved fronds were transferred to Hoagland liquid medium containing 1.5% (w/v) sucrose to preculture for 7 days at 25 ± 1 °C with a light intensity of 100 µmol/m2/s under a 16 h/day photoperiod.
Binary vector construction
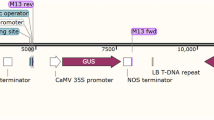
The open reading frame of CYP710A11 (GenBank: JN388603.1) behind the CaMV35S (cauliflower mosaic virus 35S) promoter followed by the terminator of the nopaline synthase gene was inserted into the binary vector pCAMBIA2301. The binary vector pCAMBIA2301, which contains a neomycin phosphotransferase gene (NPTII) and a β-glucuronidase gene (GUS) gene (uidA), both driven by the CaMV35S promoter was obtained from the Center for Application of Molecular Biology to International Agriculture (CAMBIA), Australia. The uidA gene contains an intron in the coding region (Supplementary Material Fig. 1) to ensure that the observed GUS activity occurs in the plant cell and is not due to the presence of endogenous residual A. tumefaciens (Chhabra et al. 2011). The binary vector pCAMBIA2301-CYP710A11 was transferred into the A. tumefaciens strain EHA105 using the freeze–thaw method (R Höfgen 1988).
Determination of the phytotoxic level of the antibiotic
To determine the concentration of antibiotic use, the phytotoxic level of G418 was tested. The concentration gradient was set as 0 mg/L, 5 mg/L, 10 mg/L, and 20 mg/L according to a preliminary result that L. minor tolerance of G418 was mostly lower than 20 mg/L. Sterile L. minor was cultured in an artificial climate incubator (GZ-300-GSII, Guangzhi Tech, Shaoguan, China) for two weeks with Hoagland liquid medium at 25 ± 1 °C with a light intensity of 100 µmol/m2/s under a 16 h/day photoperiod for a preculture period of 7 days. Then, five fronds were inoculated into each well of sterile 12-well plates, which contained 5 mL Hoagland medium with various G418 concentrations. Each concentration treatment was repeated three times. The plates were cultured in an artificial climate incubator under the same conditions as preculture. The culture medium was replaced every 5 days, and the number of fronds per well was recorded 15 days later.
The increased number of fronds was calculated as follows:
INF, increased number of fronds after culture for 15 days; Nt, total number of fronds after culture for 15 days; N0, initial number of fronds, N0 = 5.
Duckweed transformation
Two approaches were used to transform L. minor in this research: one is the CTS, and the other is FTS. The media and solutions used in the transformation processes are shown in Table 1.
For CTS, callus induction and transformation followed the protocol described by Chhabra et al. (2011) with minor modification. Sterile fronds were inoculated on the callus induction medium to obtain embryogenic callus. The callus was rapidly submerged in A. tumefaciens suspension (OD600 = 0.5) and infiltrated (− 0.08 MPa) for 10 min at room temperature. Then, the callus was cocultured for 4 days in the dark at 25 °C on coculture medium. Afterward, the callus was transferred to callus selection medium. Callus that remained green was subcultured on fresh callus selection medium every 2 weeks. Finally, frond regeneration from callus was induced on frond regeneration medium. Regenerated fronds were transferred to Hoagland liquid medium containing 15 g/L sucrose to expand the culture. Callus induction efficiency, regeneration efficiency, and frond number per callus were calculated using the methods described by Huang et al. (2016). The minimum diameter of callus accepted was approximately 2 mm.
For FTS, the daughter frond was removed without damaging the underlying meristematic zone of mother frond by a plucking motion using forceps. The wounded mother frond with the meristematic zone exposure was transferred to a new sterile conical flask. Then, 100 mL of pretreatment solution was added. The conical flask was sealed with aseptic breathable film and kept in an ice bath for 20 min. Then, A. tumefaciens suspension (OD600 = 0.5) was added to the conical flask and vacuum infiltrated (− 0.08 MPa) for 10 min at room temperature. Finally, the fronds were removed and placed on a sterile 90*15 mm petri dish with three pieces of filter paper, and 1.5 mL liquid coculture medium was added to coculture at 22 °C in darkness for 4 days. Filter paper with only 1.5 mL liquid coculture medium was used to avoid the overgrowth of A. tumefaciens. After coculture, all fronds were directly inoculated on selection culture without washing. The newly produced fronds were subcultured on fresh selection medium every 2 weeks and selected for approximately 3 months.
GUS (β-glucuronidase) staining and GUS-positive rate
Histochemical GUS staining was conducted and analyzed as described by Jefferson (1987). Fronds or calli after coculture with A. tumefaciens for 4 days were stained by GUS-solution. The GUS-positive rate was calculated as follows:
GR, GUS-positive rate (%); Ns, number of blue calli or fronds after GUS staining; Nt, total number of calli or fronds.
The transgenic lines obtained were also identified by the same staining method.
DNA extraction, PCR analysis and Tail-PCR analysis
The genomic DNA of L. minor was extracted from GUS-positive and nontransformed control L. minor using the Plant Genomic DNA Miniprep Kit (Sigma–Aldrich, St. Louis MO, USA) following the protocol provided by the manufacturer.
PCR analysis of putative transgenic plants was performed using the primer set CYP-F and CYP-R (Supplementary Material Table 1), amplifying gene CYP710A11 (1506 bp). Meanwhile, the RNR2B (ribonucleotide reductase 2B, 895 bp) gene, as a reference gene, was amplified by the primer set RNR2B-F and RNR2B-R (Supplementary Material Table 1). RNR2B is the ribonucleotide reductase 2B gene in the genome of L. minor (https://genomevolution.org/r/ik6h). Transgenic lines were also tested for the absence of A. tumefaciens contamination using the primer set RepA-F and RepA-R (Supplementary Material Table 1) which amplified a specific sequence (1000 bp) of the vector, which is located close to the pVS1-REP (replication origin from pVS1) of pCAMBIA2301.
The T-DNA insertion sites were determined by the Tail-PCR method (Liu and Whittier 1995). Primers used were shown in Supplementary Material Table 1. All Tail-PCR reactions were carried out in iCycler iQTM (Bio-Rad, Hercules, California, USA). The specific PCR products from the tertiary TAIL-PCR reactions were extracted from agarose gel with TAKARA MiniBEST Agarose Gel Extraction Kit Ver. 4.0 (TAKARA, Tokyo, Japan). The DNA fragments were sequenced by Sangon Biotech Co., Ltd (Shanghai, China). L. minor genome assembly used in the TAIL PCR analysis was obtained from CoGe database (vs1, genome id40199, https://genomevolution.org/r/ik6). L. minor clone 5500 was used in the assembly (Van Hoeck et al. 2015).
RNA extraction and qPCR (quantitative polymerase chain reaction) analysis
The total RNA of L. minor was extracted using Total RNA Isolation Solution (Generay, Shanghai, China) following the protocol supplied by the manufacturer. First-strand cDNA synthesis and reverse-transcriptase PCR were conducted by using the Rayscript cDNA Synthesis Kit (Generay, Shanghai, China). For qPCR analysis, the gene-specific primer set CYP-F1 and CYP-R1 (Supplementary Material Table 1) was used to amplify the 841 bp specific sequence of CYP710A11. The primer set 18S-F and 18S-R (Supplementary Material Table 1) was used to amplify the 124 bp specific sequence of 18S rRNA, which is stably expressed in L. minor tissues, as an internal control gene. All reactions were replicated at least three times. Gene expression was analyzed by qPCR using iQ SYBR-Green (Bio-Rad, Hercules, California, USA). The threshold cycle (Ct) values of the qPCR reactions were obtained from three independent biological replicates with three technical replicates each.
Stigmasterol content determination
Five grams of fresh L. minor fronds was dried at 60 °C in a hot-air oven (ZRD-A7230, ZHICHENG, Shanghai, China) to a constant weight. The dried samples were milled using a mortar. One hundred milliliters of methanol was added to extract the stigmasterol for 60 min with 500 W ultrasonic treatment. After extraction, the liquid fraction was collected via filtration using filter paper, and the collected liquid fraction was concentrated to approximately 10 mL at 45–55 °C. Then, methane was added to a final volume of 25 mL to obtain the stigmasterol extract. Prior to determination, the stigmasterol extract was filtered using a 0.45 µm syringe filter. A high-performance liquid chromatograph (HPLC) system (Agilent 1260; Agilent Tech, USA) was used for stigmasterol analysis. Ten milliliters of stigmasterol extract was injected and separated by a C-18 column (Capcell Pak, Shiseido, Japan) at 25 °C using a mixture of 85% acetonitrile and 15% methanol as the mobile phase. Stigmasterol was detected by a DAD detector (G1315B, Agilent Tech, USA) at 240 nm. A stigmasterol solution of 1 mg/mL was used as the external standard, which was prepared by dissolving 1 mg of stigmasterol (Sigma–Aldrich, St. Louis, MO, USA) to 1 mL methanol at 40 °C with 500 W ultrasonic treatment for 5 min. All results were obtained from three independent biological replicates with three technical replicates each.
Statistical analysis
All results are expressed as the mean ± standard error in the figures. Statistical analysis was performed using SPSS software (Version 22.0, SPSS Inc., Chicago, IL, USA). ANOVA followed by Student’s t test was used to determine significant differences, and P < 0.05 was considered statistically significant.
Results
Phytotoxic level of antibiotic
To determine the antibiotic dosage for selection, the concentration of G418 was set as 0 mg/L, 5 mg/L, 10 mg/L, and 20 mg/L. Based on the increase in number of fronds after 15 days of treatment, 20 mg/L G418 was used to ZH0055 selection, 15 mg/L G418 was used for D0158 selection, and 10 mg/L G418 was used for M0165 selection (Fig. 1). The addition of antibiotics to the callus and frond selection media was remarkably effective in this study.
Callus transformation system
Three L. minor strains (ZH0055, D0158 and M0165) were transformed using the CTS as controls in this study. However, only ZH0055 was induced to form callus successfully, indicating that tissue culture of duckweed is limited by genotype. The callus induction and regeneration of ZH0055 are presented in Fig. 2. Yellow fragile callus was induced by repeated subculture every 2 weeks (Fig. 2a). Microscopic observation revealed that the yellow fragile callus was composed of mainly meristematic cells. The cells are approximately 20 µm and always uniform, densely packed, and highly regenerative (Fig. 2b). A part of the meristematic callus of ZH0055 regenerated successfully (Fig. 2c) and formed normal fronds after expanding culture (Fig. 2d). The callus induction efficiency of ZH0055 reached up to 92%, while the regeneration efficiency was relatively low at only 30%. In addition, the frond number per callus was 4.67 ± 1.16. The expression of the GUS gene was detected after callus coculture with A. tumefaciens for 4 days (Fig. 2e, f). Nine months later, all transgenic lines of ZH0055 could be passaged stably by vegetative propagation (Fig. 2g, h). The rate of stable transgenic line formation from infected ZH0055 callus was 4%. Twenty-four transgenic lines were obtained from ZH0055 by CTS.
Tissue culture and callus transformation of Lemna minor ZH0055. a Yellow fragile callus. b Microscopical investigation of the callus, callus was embedded in paraffin and cut into slices, and then stained with safranin and fast green. c Fronds regeneration; black arrows indicate the regenerated fronds. d Fully regenerated fronds in Hoagland medium. e GUS staining of wild-type callus. f GUS staining of callus after coculture with Agrobacterium tumefaciens for 4 days. g GUS staining of the wild type. H GUS staining of a stable transgenic line
Frond transformation system
Three L. minor strains (ZH0055, D0158 and M0165) were transformed successfully by FTS. Individual fronds were separated from one L. minor plant, and the meristematic regions of the frond were scratched with a scalpel (Fig. 3a). After coculture with A. tumefaciens for 4 days (Supplementary Material Fig. 2d), all ZH0055, D0158 and M0165 showed expression of the GUS gene (Supplementary Material Fig. 2a–c). One plant was separated into three fronds and transferred to a new conical flask, and all wounded fronds were alive and green in the beginning (Fig. 3b). Then, most of the fronds became bleached and died, while new fronds emerged from a few of the meristematic zones of the wounded fronds (Fig. 3c; Supplementary Material Fig. 2e; Supplementary Material Fig. 2f). The new fronds were subcultured on new frond selection medium every 2 weeks. Stable transgenic lines were obtained (Fig. 3d–h) and showed normal phenotypes (Supplementary Material Fig. 2g). The rates of stable transgenic lines formation from the infected fronds of ZH0055, D0158 and M0165 were 2%, 6% and 2%, respectively. The numbers of transgenic lines obtained from ZH0055, D0158 and M0165 by FTS were 12, 35 and 13, respectively. Stable transgenic lines of L. minor strains ZH0055, D0158 and M0165 were achieved in 3 months by FTS, much faster than by CTS (Fig. 4).
Frond transformation of Lemna minor ZH0055. a The daughter frond was removed without damaging the underlying meristematic zone of mother frond by a plucking motion using forceps. b At the beginning of selection culture, all fronds were green. c During selection culture, red arrows indicate the new fronds that were generated from some fronds, while most of the fronds became bleached. d, e GUS staining of the wild type. f, g GUS staining of a stable transgenic line. h Microscopy of a stable transgenic frond
GUS staining
To compare A. tumefaciens infection rate of the CTS and FTS, GUS-positive rates were detected after coculture with A. tumefaciens. The GUS-positive rate of FTS was relatively lower than that of CTS, but it was still between 30% and 40%. Moreover, the GUS-positive rates of ZH0055, D0158 and M0165 were very similar, which indicated that A. tumefaciens infection rate is varies little among different genotypes when applied as part of FTS (Fig. 5).
To confirm positive transgenes, GUS was used as a reporter gene. The expression and location of the GUS gene in transgenic lines can be accurately identified by GUS staining because of its high sensitivity. GUS staining showed that the GUS gene was stably expressed in transgenic L. minor from both CTS and FTS (Fig. 2g, h; Fig. 3d–g). Microscopic observation revealed that almost every cell of the transgenic L. minor expressed the GUS gene (Fig. 3h).
Detection of the CYP710A11 gene
To determine whether the gene CYP710A11 was integrated into the genome of L. minor, PCR detection was carried out. Genomic DNA isolated from GUS-positive and wild-type L. minor was used for PCR amplification. DNA electrophoresis showed that only bands representing RNR2B were detected in the wild type, while bands representing both RNR2B and CYP710A11 were clearly detected in the transgenic lines, which suggested that all transgenic lines contained CYP710A11. In addition, bands representing CYP710A11 and RepA were detected in the plasmid, while no RepA band was detected in the transgenic lines, which suggested that contamination by A. tumefaciens was not detected in the analyzed samples (Fig. 6). The results of the experiment indicated that CYP710A11 was successfully transferred into L. minor by both CTS and FTS.
PCR analysis of CYP710A11 in the genomes of transgenic duckweed lines. M, 2000 bp marker DNA; Wild types, lanes from left to right represent wild-type Lemna minor ZH0055 for callus transformation, wild-type L. minor ZH0055, wild-type L. minor D0158 and wild-type L. minor M0165 for frond transformation, respectively; P, plasmid of Agrobacterium tumefaciens; Transgenic lines, stable transgenic duckweed; CT, transgenic duckweed by callus transformation; FT, transgenic duckweed by frond transformation; 1, 2, and 3, three transgenic lines of each duckweed strain
To determine the T-DNA insertion sites, L. minor genomic DNA flank and the inserted T-DNA left border was cloned by Tail-PCR using T-DNA specific primers together with five arbitrary degenerate primers (Supplementary Material Table 1). Two transgenic lines (D0158-FT3 and M0165-FT3) were chosen to perform Tail-PCR. When the PCR products were sequenced, only one sequence of each transgenic line was obtained, suggesting T-DNA likely integrated into L. minor genome in manner of single copy. The T-DNA insertion sites in L. minor genome were the 226 bp of contig_5064 and the 10,174 bp of contig_13868 in the transgenic line D0158-FT3 and M0165-FT3, respectively (Fig. 7).
Distribution of upstream and downstream genes at the insertion sites of transgenic lines D0158-FT3 and M0165-FT3. a Distribution of upstream and downstream genes at the insertion sites of transgenic line D0158-FT3. Lminor_020917, DEAD-box ATP-dependent RNA helicase 21. b Distribution of upstream and downstream genes at the insertion sites of transgenic lines M0165-FT3. Lminor_003163, Serine/threonine-protein phosphatase PP-X isozyme 2 (PPX2); Lminor_003164, unknown function; Lminor_003165, Fimbrin-5(FIM5); Lminor_003166, Fasciclin-like arabinogalactan protein 7(FLA7); Lminor_003167, Rho GDP-dissociation inhibitor 1 (GDI1); Lminor_003168, Probable calcium-binding protein CML22 (CML22)
Expression of the CYP710A11 gene in Lemna minor
To study the expression of CYP710A11, qPCR for wild-type and transgenic L. minor was performed. As shown in Fig. 8, differential expression levels of CYP710A11 mRNA were detected in all transgenic lines but not in wild types. A paired t-test of the relative expression levels of CYP710A11 indicated that all transgenic lines showed significantly (P < 0.01) higher expression than the wild types did. In addition, the ZH0055 transgenic lines transformed via each system showed no significant (P > 0.05) difference, while the relative expression levels of CYP710A11 among ZH0055, D0158 and M0165 showed significant (P < 0.05) differences. Among them, M0165 showed the highest expression of CYP710A11, D0158 showed an intermediate expression level, and ZH0055 showed the lowest expression of CYP710A11.
Quantitative PCR analysis of CYP710A11 mRNA levels in transgenic duckweed. W wild types; CT transgenic duckweed by callus transformation; FT transgenic duckweed by frond transformation; 1, 2, and 3, three transgenic lines of each duckweed strain. Columns, average relative expression of three repetitions from each RNA sample; bars, standard deviation in each case, asterisks (*P < 0.05, **P < 0.01) indicate significant differences from the wild type
Stigmasterol content of transgenic lines
To test the effects of introducing CYP710A11 into L. minor, the content of the downstream product stigmasterol was measured by HPLC. Under the same culture condition, three stable transgenic lines and the wild type of each L. minor material were used for HPLC measurement. The results showed that the stigmasterol contents of all transgenic lines were significantly (P < 0.01) higher than those of the wild types, reaching 397.08% higher than that of the wild type in M0165. Moreover, the content of stigmasterol and the expression of CYP710A11 showed positive correlations. Interestingly, the stigmasterol content of ZH0055 transgenic lines obtained from the two different transformation systems showed no significant (P > 0.05) difference (Fig. 9) in this study. The results of stigmasterol detection showed that overexpression of CYP710A11 in L. minor can increase the production of stigmasterol.
Stigmasterol content of transgenic duckweed. W, wild types; CT, transgenic duckweed by callus transformation; FT, transgenic duckweed by frond transformation; 1, 2, and 3, tree transgenic lines of each duckweed strain; FW, fresh weight. Columns, average stigmasterol content of three repetitions from each sample; bars, standard errors in each case; asterisks (*P < 0.05, **P < 0.01) indicate significant differences from the wild type
Discussion
Transformation mediated by A. tumefaciens is a standard technique for genetic modification of higher plants, a term that includes both dicotyledonous and monocotyledonous species (Komari and Kubo 1999). Most techniques that introduce foreign genes to generate transgenic plants depend on the dedifferentiation of organ and redifferentiation of transformed cells. Several studies on genetic engineering of duckweed have been performed based on the CTS. However, callus induction and regeneration depend on genotypes, presenting a major bottleneck (Bertran et al. 2015; Ko et al. 2011; Popov et al. 2006; Huang 2016; Moon and Stomp 1997). The Duckweed Germplasm Bank in our laboratory contains all 37 species of duckweed resources and nearly 800 different genotype strains. However, not all of these duckweed strains could be induced to form callus. The FTS, which avoids this restriction will allow better use of these elite duckweed resources. To evaluate the effects of the FTS, we transformed three different genotypes of the same species L. minor (ZH0055, D0158 and M0165). Due to geographical isolation, these strains have different genotypes. In this study, only ZH0055 was induced to form callus, while D0158 and M0165 were failed at callus induction. Furthermore, we compared the results of CTS and FTS. Using the new system, transgenic plant lines from the three L. minor strains were obtained efficiently, and the process was not subject to genotype restriction. GUS staining, PCR, qPCR and stigmasterol detection demonstrated that the function of FTS was as efficient as that of CTS. Although the A. tumefaciens infection rate of CTS is higher than that of FTS (Fig. 5), the regeneration rate of ZH0055 was low, at only 30%. In addition, the callus induction and regeneration of ZH0055 took a long time, nearly threefold longer than the time taken by FTS to obtain stable transgenic lines. The latest report showed that callus induction and regeneration still takes 9 weeks and is only suitable for several strains of Landoltia punctata (Huang et al. 2016). Therefore, FTS can avoid the genotype restriction of callus induction. Moreover, it saves time.
To determine the selective agent dosages for the different L. minor materials, the phytotoxic level of the antibiotic was tested. Kanamycin was the most commonly used selective agent for duckweed in previous studies. However, high level of kanamycin was used during selection (Ko et al. 2011; Vunsh et al. 2007). This lower sensitivity may allow the regeneration of untransformed plant cells on kanamycin-containing medium. Generally, kanamycin is very effective for transgenic dicot species but not for many monocots (Wilmink and Dons 1993), while the antibiotic G418 was tested as an alternative selectable marker shown to be more effective in various monocots, such as Gramineae (Dekeyser et al. 1989; Ozias-Akins et al. 1988), perhaps due to more effective binding to ribosomes. Therefore, G418 was chosen as the selective agent for L. minor, a monocotyledon. Finally, the G418 dosage for the lines ZH0055, D0158 and M0165 were 20 mg/L, 15 mg/L and 10 mg/L in selection culture, respectively. The results of cellular GUS staining of L. minor cells showed that G418 is remarkably effective for L. minor selection.
The existence of chimerism may be the main problem to be worried about when applying this simple FTS. The bother of chimerism seems to be more frequent than originally thought and it has been reported in several plants, including tobacco (Schmülling and Schell 1993), soybean (Christou 1990), potato (Rakosy et al. 2007), rice (Christou and Ford 1995), and strawberry (Mathews et al. 1995). In this study, the problem of chimerism can be settled by repeatedly subculturing on frond selection medium containing the selective agent. This issue is also the reason we selected the more effective antibiotic (G418) and its concentration. Low sensitivity to antibiotics may lead to the formation of chimeras (Dominguez et al. 2004). After selection for 3 months, stable transgenic lines were generated, and almost all cells of the transgenic lines were found to show the expression of the GUS reporter gene (Fig. 3h). One possible explanation is that duckweed usually reproduces asexually. The leaves of duckweed are commonly termed as fronds and are not considered leaves by the strict botanical definition. Unlike the ordinary leaves of most plants, new fronds emerge from meristematic zones of their parent fronds and generate a whole plant (Les et al. 2002). The FTS is effective because the meristematic cells were transformed when the meristems were wounded, and then the transgenic daughter fronds arose on the selective culture medium. The expression of the GUS gene was observed at the wounded meristems by GUS staining after 4 days of coculture with A. tumefaciens (Supplementary Material Fig. 2a–c). After multiple passages, stable transgenic lines were obtained (Fig. 3d–h). This phenomenon is similar to that of stable wheat transformation based on shoot apical meristems (SAMs) which avoiding the problems arise in tissue culture (Hamada et al. 2017).
Plants as bioreactors are the most attractive objects in the biotechnology field, and a major advantage of plants is their autotrophic type of nutrition (Saveleva et al. 2016). In contrast, most heterologous expression systems based on bacteria, yeast, and cell cultures require high-cost propagators and media for growth (Itakura et al. 1977; Moreira 2007; Wildt and Gerngross 2005). The utilization of transgenic L. minor as a bioreactor makes it possible to produce large amounts of biomass with low labor costs and little capital investment, which benefits commercial-scale production. Phytosterols, secondary metabolites including stigmasterol, possess many therapeutic properties. To date, stigmasterol isolated from plants has been proved to have antioxidant, antitumor, hypoglycemic, hypocholesterolemic, and anti-inflammatory effects (Batta et al. 2006; Gabay et al. 2010; Ghosh et al. 2011; Jamaluddin et al. 1994; Khanna et al. 2007; Panda et al. 2009; Pandith et al. 2013). It also serves as a precursor for the synthesis of synthetic progesterone, adrenocortical hormone and ecdysteroids (Kovganko and Survilo 2000; Sundararaman and Djerassi 1977). Fortunately, the biosynthetic pathway of stigmasterol is very well understood. In brief, β-sitosterol is catalyzed by sterol 22-desaturase to produce stigmasterol. Previously, overexpressing the sterol 22-desaturase gene had been reported to enhance stigmasterol production in tomato (Raksha et al. 2016; Whitaker and Gapper 2008). Similarly, stigmasterol production in all transgenic lines significantly increased after the sterol 22-desaturase gene (CYP710A11) was transferred into L. minor in this study (Fig. 9). Therefore, FTS will allow rapid exploitation of the L. minor bioreactor system, because it is not restricted by genotypes amenable to callus induction. In the future, L. minor bioreactor can be used to express additional useful products, such as monoclonal antibodies and cytokines.
Conclusion
In summary, we established a rapid, simple, efficient and stable frond transformation system mediated by A. tumefaciens for L. minor. This method has advantages over the CTS. The FTS overcomes the genotype restriction of L. minor callus induction, and shortens the time required for genetic transformation via simplify the complex regeneration progress. By frond transformation, the foreign gene was expressed effectively and the product yield increased significantly in transgenic L. minor. This study could be utilized to transform a wide range of L. minor genotypes and will pave the way for the exploitation of L. minor as a bioreactor.
References
Appenroth KJ, Borisjuk N, Lam E (2013) Telling duckweed apart: genotyping technologies for the lemnaceae. Chin J Appl Environ Biol 19:1–10
Batta AK, Xu G, Honda A, Miyazaki T, Salen G (2006) Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism 55:292–299
Bertran K, Bertran K, Thomas C, Guo X, Bublot M, Pritchard N, Regan JT, Cox KM, Gasdaska JR, Dickey LF, Kapczynski DR, Swayne DE (2015) Expression of H5 hemagglutinin vaccine antigen in common duckweed (Lemna minor) protects against H5N1 high pathogenicity avian influenza virus challenge in immunized chickens. Vaccine 33:3456–3462
Chang WC, Chiu PL (1976) Induction of callus from fronds of duckweed (Lemna gibba L.). Bot Bull Acad Sin 17:106–109
Chhabra G, Chaudhary D, Sainger M, Jaiwal PK (2011) Genetic transformation of Indian isolate of Lemna minor mediated by Agrobacterium tumefaciens and recovery of transgenic plants. Physiol Mol Biol Plants 17:129–136
Christou P (1990) Morphological description of transgenic soybean chimeras created by the delivery, integration and expression of foreign DNA using electric discharge particle acceleration. Ann Bot-London 66:379–386
Christou P, Ford TL (1995) Recovery of chimeric rice plants from dry seed using electric discharge particle acceleration. Ann Bot-London 75:449–454
Cox KM, Sterling JD, Regan JT, Gasdaska JR, Frantz KK, Peele CG, Black A, Passmore D, Moldovan-Loomis C, Srinivasan M, Cuison S, Cardarelli PM, Dickey LF (2006) Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol 24:1591–1597
Dekeyser R, Claes B, Marichal M, Van MM, Caplan A (1989) Evaluation of selectable markers for rice transformation. Plant Physiol 90:217
Desai PN, Shrivastava N, Padh H (2010) Production of heterologous protein in plants: strategies for optimal expression. Biotechnol Adv 28:427–435
Dominguez A, Cervera M, Perez RM, Romero J, Fagoaga C, Cubero J, Lopez MM, Juarez JA, Navarro L (2004) Characterisation of regenerants obtained under selective conditions after Agrobacterium-mediated transformation of citrus explants reveals production of silenced and chimeric plants at unexpected high frequencies. Mol Breeding 14:171–183
Gabay O, Sanchez C, Salvat C, Chevy F, Breton M, Nourissat G, Wolf C, Jacques C, Berenbaum F (2010) Stigmasterol: a phytosterol with potential anti-osteoarthritic properties. Osteoarthr Cartil 18:106–116
Ge X, Zhang N, Phillips GC, Xu J (2012) Growing Lemna minor in agricultural wastewater and converting the duckweed biomass to ethanol. Bioresour Technol 124:485–488
Ghosh T, Maity TK, Singh J (2011) Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient Pharm Exp Med 11:41–49
Hamada H, Linghu Q, Nagira Y, Miki R, Taoka N, Imai R (2017) An in planta biolistic method for stable wheat transformation. Sci Rep 7:11443
He Y, Ning T, Xie T, Qiu Q, Zhang L, Sun Y, Jiang D, Fu K, Yin F, Zhang W, Shen L, Wang H, Li J, Lin Q, Sun Y, Li H, Zhu Y, Yang D (2011) Large-scale production of functional human serum albumin from transgenic rice seeds. Proc Natl Acad Sci USA 108:19078–19083
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32
Huang M, Fu LL, Sun XP, Di R, Zhang JM (2016) Rapid and highly efficient callus induction and plant regeneration in the starch-rich duckweed strains of Landoltia punctata. Acta Physiol Plant 38:122
Iatrou EI, Stasinakis AS, Aloupi M (2015) Cultivating duckweed Lemna minor in urine and treated domestic wastewater for simultaneous biomass production and removal of nutrients and antimicrobials. Ecol Eng 84:632–639
Itakura K, Hirose T, Crea R, Riggs AD, Heyneker HL, Bolivar F, Boyer HW (1977) Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science 198:1056–1063
Jamaluddin F, Mohamed S, Lajis MN (1994) Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of β-sitosterol and stigmasterol. Food Chem 49:339–345
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB (2007) Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol 7:344–351
Khvatkov P, Chernobrovkina M, Okuneva A, Shvedova A, Chaban I, Dolgov S (2015) Callus induction and regeneration in Wolffia arrhiza (L.) Horkel ex Wimm. Plant Cell Tiss Org 120:263–273
Ko SM, Sun HJ, Oh MJ, Song IJ, Kim MJ, Sin HS, Goh CH, Kim YW, Lim PO, Lee HY, Kim SW (2011) Expression of the protective antigen for PEDV in transgenic duckweed, Lemna minor. Hortic Environ Biote 52:511–515
Komari T, Kubo T (1999) Methods of genetic transformation: Agrobacterium tumefaciens. Molecular improvement of cereal crops. Springer, Dordrecht 5:43–82
Kovganko NV, Survilo VL (2000) New synthesis of ecdysteroids based on stigmasterol. Chem Nat Compd 36:377–380
Lam E, Appenroth KJ, Michael T, Mori K, Fakhoorian T (2014) Duckweed in bloom: the 2nd international conference on duckweed research and applications heralds the return of a plant model for plant biology. Plant Mol Biol 84:737–742
Les DH, Crawford DJ, Landolt E, Gabel JD, Kimball RT (2002) Phylogeny and systematics of Lemnaceae, the duckweed family. Syst Bot 27:221–240
Liu GY, Whittier RF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681
Mathews H, Wagoner W, Kellogg J, Bestwick R (1995) Genetic transformation of strawberry: stable integration of a gene to control biosynthesis of ethylene. In Vitro Cell Dev-Pl 31:36–43
Moon HK, Stomp AM (1997) Effects of medium components and light on callus induction, growth, and frond regeneration in Lemna gibba (duckweed). In Vitro Cell Dev-Pl 33:20–25
Moreira AR (2007) The evolution of protein expression and cell culture. Biopharm Int 20:56–68
Oron G (1994) Duckweed culture for wastewater renovation and biomass production. Agr Water Manage 26:27–40
Ozias-Akins P, Rogers SG, Fraley RT, Horsch RB, Vasil IK (1988) Evaluation of selectable markers for obtaining stable transformants in the Gramineae. Plant Physiol 86:602–606
Panda S, Jafri M, Kar A, Meheta BK (2009) Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 80:123–126
Pandith H, Zhang X, Thongpraditchote S, Wongkrajang Y, Gritsanapan W, Baek SJ (2013) Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-kappaB pathway. J Ethnopharmacol 147:434–441
Popov SV, Golovchenko VV, Ovodova RG, Smirnov VV, Khramova DS, Popova GY, Ovodov YS (2006) Characterisation of the oral adjuvant effect of lemnan, a pectic polysaccharide of Lemna minor L. Vaccine 24:5413–5419
R Höfgen LW (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Rakosy TE, Aurori CM, Dijkstra C, Thieme R, Aurori A, Davey MR (2007) The usefulness of the gfp reporter gene for monitoring Agrobacterium-mediated transformation of potato dihaploid and tetraploid genotypes. Plant Cell Rep 26:661–671
Raksha BR, Siva R, Vino S, Babu S (2016) Spatio-varietal differences in stigmasterol biosynthesis in tomato and overexpression of a sterol desaturase gene for enhanced stigmasterol production. In Vitro Cell Dev-Pl 52:571–579
Saveleva NV, Burlakovskiy MS, Yemelyanov VV, Lutova LA (2016) Transgenic plants as bioreactors to produce substances for medical and veterinary uses. Russ J Genet Appl Res 6:712–724
Schmülling T, Schell J (1993) Transgenic tobacco plants regenerated from leaf disks can be periclinal chimeras. Plant Mol Biol 21:705–708
Sharma AK, Sharma MK (2009) Plants as bioreactors: recent developments and emerging opportunities. Biotechnol Adv 27:811
Stefaniak B, Woźny A, Budna I (2002) Callus induction and plant regeneration in Lemna Minor L. Biol Plantarum 45:469–472
Stomp AM (2005) The duckweeds: a valuable plant for biomanufacturing. Biotechnol Annu Rev 11:69
Sundararaman P, Djerassi C (1977) A convenient synthesis of progesterone from stigmasterol. J Org Chem 42:3633–3634
Van Hoeck A, Horemans N, Monsieurs P, Cao HX, Vandenhove H, Blust R (2015) The first draft genome of the aquatic model plant Lemna minor opens the route for future stress physiology research and biotechnological applications. Biotechnol Biofuels 8:188
Vunsh R, Li J, Hanania U, Edelman M, Flaishman M, Perl A, Wisniewski JP, Freyssinet G (2007) High expression of transgene protein in Spirodela. Plant Cell Rep 26:1511–1519
Wang W, Haberer G, Gundlach H et al (2014) The Spirodela polyrhiza genome reveals insights into its neotenous reduction fast growth and aquatic lifestyle. Nat Commun 5:3311
Whitaker BD, Gapper NE (2008) Ripening-specific stigmasterol increase in tomato fruit is associated with increased sterol C-22 desaturase (CYP710A11) gene expression. J Agr Food Chem 56:3828–3835
Wildt S, Gerngross TU (2005) The humanization of N-glycosylation pathways in yeast. Nat Rev Microbiol 3:119–128
Wilmink A, Dons JJM (1993) Selective agents and marker genes for use in transformation of monocotyledonous plants. Plant Mol Biol Rep 11:165–185
Zhao H, Appenroth K, Landesman L, Salmean AA, Lam E (2012) Duckweed rising at Chengdu: summary of the 1st international conference on duckweed application and research. Plant Mol Biol 78:627–632
Acknowledgements
This study was supported by the National Key Technology R&D Program of China (2015BAD15B01), the National Natural Science for General Foundation of China (31770395), Key deployment projects of Chinese Academy of Sciences (ZDRW-ZS-2017-2-1), Science and Technology Service Network Initiative of Chinese Academy of Sciences (KFJ-STS-ZDTP-008); Science & Technology Program of Sichuan Province (2017NZ0018 and 2017HH0077), and the Key and Open Fund of Key Laboratory of Environmental and Applied Microbiology, Chinese Academy of Sciences (KLEAMCAS201501, KLCAS-2014-05 and KLCAS-2016-02).
Author information
Authors and Affiliations
Contributions
HZ, XRM, GLY and YF conceived the research. GLY and XRM performed experiments. GLY and XRM analyzed the data and wrote the manuscript. YLX, LT, QL, YL, FL, YLJ, APD, and KZH contributed partial experiments.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, GL., Fang, Y., Xu, YL. et al. Frond transformation system mediated by Agrobacterium tumefaciens for Lemna minor. Plant Mol Biol 98, 319–331 (2018). https://doi.org/10.1007/s11103-018-0778-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-018-0778-x