Abstract
Purpose
Papillary craniopharyngiomas can cause considerable morbidity due to mass effect and potential surgical complications. These tumors are known to harbor BRAF V600 mutations, which make them exquisitely sensitive to BRAF inhibitors.
Methods
The patient is a 59 year old man with a progressive suprasellar lesion that was radiographically consistent with a papillary craniopharyngioma. He was consented to an Institution Review Board-approved protocol, which permits sequencing of cell free DNA in plasma and the collection and reporting of clinical data.
Results
The patient declined surgical resection and was empirically treated with dabrafenib at 150 mg twice daily. Treatment response was demonstrated after 19 days, confirming the diagnosis. After achieving a near complete response after 6.5 months on drug, a decision was made to deescalate treatment to dabrafenib 75 mg twice daily with subsequent tumor stability for 2.5 months.
Conclusion
Patients with a suspected papillary craniopharyngioma can be challenged with dabrafenib as a potentially effective diagnostic and therapeutic strategy, given that rapid regression with dabrafenib is only observed in tumors harboring a BRAF V600 mutation. Further work is needed to explore the optimal regimen and dose of the targeted therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas are rare, slow-growing epithelial tumors that cause considerable morbidity due to their location and adherence to surrounding critical structures, including the optic apparatus, the hypothalamus, the ventricular system, and major intracranial vessels. Standard primary treatment of craniopharyngiomas, both papillary and adamantinomatous, consists of maximal resection followed by radiation if the resection is subtotal. Though surgical cure can be theoretically accomplished with a gross total resection, surgical efforts to achieve a gross total resection can result in substantial hormonal and neurologic deficits. Hypothalamic injury may result in obesity, disturbed circadian rhythm, mood and behavioral changes [1]. Additionally, craniopharyngiomas can cause arginine vasopressin deficiency either due to suprasellar involvement or infundibular damage during surgery; they can also cause blindness due to damage to the optic nerves or chiasm. Surgery can be highly morbid when the tumor abuts and invades the hypothalamus or the optic nerve/chiasm, and/or when the tumor capsule is highly adherent to surrounding tissues; in these instances, more limited interventions, such as biopsy, cyst decompression, and partial debulking followed by radiotherapy, are often favored [2].
Craniopharyngiomas are classified as papillary and are BRAF V600 mutant in up to 95% of cases, or adamantinomatous and associated with CTNNB1 mutations [3]. Papillary craniopharyngiomas primarily occur in patients 40–55 years of age, whereas adamantinomatous craniopharyngiomas have a bimodal age distribution, primarily occurring between the ages of 5–15 and 45–60 [4]. Several studies have shown that radiographic features can differentiate these two tumor types [5, 6]. Unlike their adamantinomatous counterparts, papillary craniopharyngioma are predominantly solid, less commonly lobulated, and less commonly calcified. When papillary craniopharyngiomas are cystic, the cyst fluid is typically hypointense on T1 weighted imaging, in contrast to adamantinomatous tumors where the cyst fluid is most commonly T1 hyperintense. Another potentially distinguishing feature is the finding that papillary tumors tend to exhibit higher fluorodeoxyglucose uptake on positron emission tomography, compared to adamantinomatous craniopharyngiomas [7].
Dabrafenib is a small molecular inhibitor of some mutated forms of BRAF, including BRAF V600E, BRAF V600K, and BRAF V600D [8]. BRAF V600 mutations are gain of function mutations that result in constitutive activity of the BRAF kinase resulting in stimulation of tumor growth. Combination treatment with the BRAF inhibitor, dabrafenib, and the MEK inhibitor, trametinib, is currently approved for the treatment of any solid tumors carrying a BRAF V600 mutation, based on two trials showing a high response rate agnostic of tumor type [9, 10]. It is known that BRAF inhibitors, often in combination with MEK inhibitors, are active in papillary craniopharyngioma based on case reports and preliminary results of a trial investigating vemurafenib/cobimetinib which resulted in an objective response in 15 out of 16 patients [11]. Of note, the one non-responder was treated for only 2 days prior to discontinuing therapy. In these publications, BRAF inhibitors were tried after pathologic diagnosis was confirmed with surgery. In the trial investigating vemurafenib and cobimetinib, the median tumor reduction was 83% at a follow-up of 22 months.
It has been shown that combination BRAF and MEK inhibition in melanoma results in longer progression free survival by achieving greater inhibition of the MAPK signaling pathway. Additionally, the combination has been shown to cause less skin toxicity by limiting the paradoxical MAPK pathway activation that can lead to squamous skin cancer [12]. However, the addition of a MEK inhibitor does result in added toxicity, such as an increased risk of pyrexia and cardiotoxicity, and it is unknown whether a grade 1 tumor like a papillary craniopharyngioma, that does not undergo malignant transformation, requires combination treatment or the recommended phase 2 dose for dabrafenib [8].
In this report, we demonstrate that a 2-to-3-week trial of a BRAF inhibitor can be used to diagnose papillary craniopharyngioma without a biopsy by inducing rapid tumor regression, which is only expected in the presence of a BRAF V600 mutation. This strategy permits the non-surgical management of this tumor type. A course of dabrafenib could also be used as a neoadjuvant treatment to reduce tumor volume prior to the first surgery, potentially resulting in a single safer, more complete resection.
Methods
The patient provided written informed consent to an Institutional Review Board-approved protocol, which permits sequencing of tumoral cell free DNA in plasma and collection and reporting of clinical data (NCT01775072). Clinical sequencing was performed through MSK-ACCESS, a next generation sequencing assay on plasma that can detect low frequency somatic alterations in 129 genes including BRAF as previously described [13].
Results
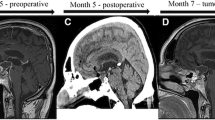
The patient is a 59 year old man who presented with headache and underwent a brain MRI that identified a mixed nodular and cystic lesion that was deforming his optic chiasm. He had intact visual fields. Clinical and laboratory evaluation revealed no evidence of arginine vasopressin deficiency or anterior pituitary dysfunction (Table 1), and he was referred for surgery. Neither biopsy nor resection could be safely performed through a transsphenoidal approach due to bulbous anterior cerebral arteries which limited access to the tumor. Because he was asymptomatic from the tumor apart from headaches, he was observed for 6 months over which time the cystic tumor demonstrated growth, resulting in further mass effect on the optic nerve and chiasm (Fig. 1).
During the observation period, the patient underwent MSK-ACCESS testing, which is designed to detect tumoral cell-free DNA mutation in plasma. The assay involves deep sequencing of 129 cancer associated genes including BRAF; this testing returned negative for pathogenic somatic mutations.
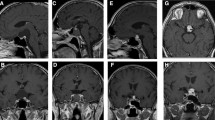
Resection by transcranial approach was offered but the patient declined. It was noted that the patient’s age and tumor features supported the diagnosis of a papillary craniopharyngioma. Specifically, on MRI the cyst fluid was T1 hypointense, the enhancing nodule was solid, there was an absence of calcification on CT, and the solid nodule was hypermetabolic (Fig. 2). A decision was made to start treatment with dabrafenib 150 mg twice daily and trametinib 2 mg once daily without pathologic confirmation. After 10 days on treatment with dabrafenib and trametinib, the trametinib was discontinued due to pyrexia. 19 days after starting the BRAF inhibitor, while on single agent dabrafenib 150 mg twice daily, he was reimaged (Fig. 3) showing regression of both the solid and the cystic component of the tumor.
Radiographic characteristics of the presumed papillary craniopharyngioma. The T1 post contrast sequence demonstrates a solid enhancing nodule with a cyst, which is hypointense on T1 and hyperintense on T2. The CT shows that the tumor is non-calcified, and the FDG-PET demonstrates that the solid nodule is hypermetabolic
The patient was continued on dabrafenib 150 mg twice daily for 6.5 months with a near complete treatment response (Fig. 3). Following a discussion on discontinuing the dabrafenib and pursuing radiation to the small residual, the patient decided to remain indefinitely on dabrafenib, assuming the absence of progression, on a lower dose, i.e. 75 mg twice daily. Follow-up imaging after 2 months of follow-up showed stable disease on the lower dose.
The patient tolerated treatment with dabrafenib with minimal side effects. Besides the pyrexia, which resolved after discontinuation of the trametinib, he had no side effects except an increase in longstanding palpitations. The palpitations were evaluated with a 4-day Holter and were found to be caused by paroxysmal atrial flutter, with the atrial flutter accounting for 1% of the record. He was evaluated and managed by cardiology without anticoagulation. Upon discussion with cardiology, it was not felt that the atrial flutter necessitated dose reduction, given that dabrafenib has not been clearly associated with atrial arrhythmias. He currently remains under surveillance for dermatologic and ophthalmologic complications by respective specialists.
Discussion
Craniopharyngiomas can be surgically cured but due to their challenging location and local adherence, radical resection can be morbid. For this reason, there is increasing recognition that limited interventions such as excisional biopsy or cyst drainage, followed by radiation may result in better patient outcomes.
In this report, we demonstrate that empirical treatment with dabrafenib for 2–3 weeks can be used to diagnose a papillary craniopharyngioma without surgery, as the regression that occurs in BRAF mutant tumors with BRAF inhibitors is rapid. For patients who are diagnosed with a papillary craniopharyngioma using empirical treatment with dabrafenib, options include discontinuation of the drug and pursuit of surgery or radiation on the tumor remnant, or continuation of the BRAF inhibitor.
Dabrafenib was extremely well tolerated in the reported patient with the only possible adverse event being an increase in pre-existing palpitations. The palpitations were not worked up until after treatment with dabrafenib was initiated. The palpitations were found to be due to atrial flutter; it remains unclear whether dabrafenib contributed to this atrial flutter, which is managed with rate control. Important side effects of dabrafenib include alopecia, hyperkeratosis, xeroderma, constipation, fever, interstitial nephritis, retinal detachment, and uveitis [14]. Prolonged use has been associated with the development of squamous cell carcinomas and basal cell cancers. Patients on dabrafenib require monitoring for complications of treatment; however, long term treatment is known to be feasible based on treatment of other disease types harboring BRAF V600 mutations.
A topic of further study is whether it is a viable option to continue BRAF inhibitor at a lower dose to reduce drug toxicity. The doses of BRAF/MEK inhibitors that have been used for papillary craniopharyngiomas are based on dose escalation studies that sought to define the recommended phase 2 dose [8]. Given the benign nature of papillary craniopharyngioma and the low to absent potential for malignant transformation, whether the high doses of BRAF inhibitors studied are needed for tumor control is an important question. It is also unclear whether the addition of a MEK inhibitor to a BRAF inhibitor, which improves progression free survival in cancer, improves progression free survival in the treatment of craniopharyngioma.
In conclusion, this report offers a potential new treatment paradigm, in which select patients with characteristic clinical and radiographic features consistent with papillary craniopharyngioma, are treated with a short course of a BRAF inhibitor. If regression occurs, this treatment can be diagnostic as well as therapeutic. This approach may lead to better patient outcomes by decreasing surgical morbidity, or even avoiding the risk of surgery altogether. The patient in this report will need long term follow-up to ensure continued treatment response and close monitoring for toxicity, given that the treatment plan is maintenance treatment with low dose dabrafenib as long as the drug remains well tolerated.
Data and materials availability
Not applicable.
References
Muller HL (2016) Craniopharyngioma and hypothalamic injury: latest insights into consequent eating disorders and obesity. Curr Opin Endocrinol Diabetes Obes 23(1):81–89. https://doi.org/10.1097/MED.0000000000000214
Muller HL (2014) Craniopharyngioma. Endocr Rev 35(3):513–543. https://doi.org/10.1210/er.2013-1115
Brastianos PK et al (2014) Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet 46(2):161–165. https://doi.org/10.1038/ng.2868
Muller HL et al (2019) Craniopharyngioma. Nat Rev Dis Primers 5(1):75. https://doi.org/10.1038/s41572-019-0125-9
Chen X et al (2019) Noninvasive molecular diagnosis of craniopharyngioma with MRI-based radiomics approach. BMC Neurol 19(1):6. https://doi.org/10.1186/s12883-018-1216-z
Huang ZS et al (2021) Machine learning-based multiparametric magnetic resonance imaging radiomic model for discrimination of pathological subtypes of craniopharyngioma. J Magn Reson Imaging 54(5):1541–1550. https://doi.org/10.1002/jmri.27761
Mukada N et al (2021) Subtype-dependent difference of glucose transporter 1 and hexokinase II expression in craniopharyngioma: an immunohistochemical study. Sci Rep 11(1):126. https://doi.org/10.1038/s41598-020-80259-4
Falchook GS et al (2012) Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 379(9829):1893–1901. https://doi.org/10.1016/S0140-6736(12)60398-5
Salama AKS et al (2020) Dabrafenib and trametinib in patients with tumors with BRAF(V600E) mutations: results of the NCI-MATCH trial subprotocol H. J Clin Oncol 38(33):3895–3904. https://doi.org/10.1200/JCO.20.00762
Subbiah V et al (2023) Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med 29(5):1103–1112. https://doi.org/10.1038/s41591-023-02321-8
Jannelli G et al (2023) Current advances in papillary craniopharyngioma: state-of-the-art therapies and overview of the literature. Brain Sci 13(3):515. https://doi.org/10.3390/brainsci13030515
Flaherty KT et al (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367(18):1694–1703. https://doi.org/10.1056/NEJMoa1210093
Rose Brannon A et al (2021) Enhanced specificity of clinical high-sensitivity tumor mutation profiling in cell-free DNA via paired normal sequencing using MSK-ACCESS. Nat Commun 12(1):3770. https://doi.org/10.1038/s41467-021-24109-5
Hauschild A et al (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380(9839):358–365. https://doi.org/10.1016/S0140-6736(12)60868-X
Funding
This research was funded in part through NIH awards P30 CA008748 and 1 R21 CA280469-01 (to Andrew L. Lin).
Author information
Authors and Affiliations
Contributions
All authors contributed to the acquisition, analysis, or interpretation of the data. AL: wrote the main manuscript text and prepared the figures. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
Andrew L. Lin reports that he has received funding from Bristol-Myers Squibb. Robert J. Young reports that he has consulted for Olea Sphere and ICON plc, unrelated to this work. The remaining authors have nothing to disclose.
Ethical approval
This patient provided written informed consent to a protocol, which was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB) and was conducted in accordance with International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws (NCT01775072). This protocol permits sequencing of tumoral cell free DNA in plasma and collection and reporting of clinical data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, A.L., Tabar, V., Young, R.J. et al. Dabrafenib as a diagnostic and therapeutic strategy for the non-surgical management of papillary craniopharyngioma. Pituitary 26, 482–487 (2023). https://doi.org/10.1007/s11102-023-01339-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-023-01339-y