Abstract
Purpose
Measurement of prolactin in clinical laboratories is an important component in the management of patients with pituitary adenoma. Prolactin measurement is known to be sensitive to the high-dose hook effect, in the presence of extremely high prolactin concentrations. This interference is referred to in most recent articles discussing prolactin assays and the management of prolactin-secreting pituitary adenomas. The objective of our study was to evaluate if the high-dose hook effect remains relevant in current practice, when using currently available assays.
Methods
Serum from a patient with a giant macroprolactinoma was assayed using all of the available prolactin assays in France in 2020, using native serum and after dilution. Technical inserts from assays were reviewed to assess the information on analytical principles, numbers of steps, and any reference to high dose hook effect.
Results
Fourteen assay kits were studied by 16 laboratories; all were two-site immunometric assays, mostly using one step (11/14). Results obtained after dilution varied from 17,900 µg/L to 86,900 µg/L depending on the assay used. One tested assay was sensitive to the high-dose hook effect leading to a falsely lower prolactin concentration when measuring native serum (150 µg/L compared to 17,900 µg/L after dilution).
Conclusion
The high-dose hook effect still exists in a very small minority of prolactin assays. The evolution of assay methods may lead to new assays that remain sensitive to this effect in the future. We therefore advise that the hook effect should still be mentioned in prolactin assay recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Measurement of prolactin in clinical laboratories is an important part of the management of patients with pituitary adenomas. Prolactin is known to be sensitive to various forms of analytical interference, such as macroprolactin [1], but also to the high-dose hook effect, similar to other biochemical markers that are known to undergo large variations in concentration. In the presence of extremely high concentrations of prolactin, assay antibodies can be saturated and fail to form a sandwich, leading to a lower than expected result for prolactin [2]. This uncommon analytical pitfall, which was not present in older competitive assays, has been described in sandwich assays, also known as two-site immunometric assays, when they are carried out in one step [3, 4]. This known phenomenon can be avoided by using sandwich assays that are run in two steps (including a wash step before the addition of the second antibody), or by diluting the serum samples when using sandwich assays that are run in a single step [2, 5].
The high-dose hook effect is referred to in most recent papers and book chapters that discuss prolactin assays and the management of prolactin-secreting pituitary adenomas [5,6,7,8]. However, articles describing case reports or series of high-dose hook effects in the prolactin assay are mostly old or poorly-documented [6, 9,10,11,12,13,14,15]. Although there have been few changes in the principles of immunoassays since these cases, most assays have evolved. The objective of our study was to evaluate if reference to the high dose hook effect is still relevant in current practice. In this study we aim to describe the different assay reagents currently available for the measurement of prolactin, examine the manufacturers’ recommendations concerning the high-dose hook effect, and test these reagents in order to establish whether the high-dose hook effect in the measurement of prolactin still exists.
Methods
Assays and protocol: A selection of French laboratories representative of the different assay techniques listed in the Probioqual (French association for the promotion of quality control in medical biology) survey report of December 2020 was made thanks to help from the specialized biochemistry working group of the French Society of Nuclear Medicine and Probioqual, in order to include an exhaustive list of prolactin assays used in France at the end of 2020. These laboratories agreed to provide the technical data sheets of the assay suppliers and to perform prolactin measurements on a sample that we provided, firstly on native serum and then after 1:10 (or greater) dilution as necessary, as practiced in their laboratory. Analyses were carried out in duplicates for the manual assays and in singulate on the analyzers.
Blood sample preparation
A single patient, diagnosed with a giant macroprolactinoma (80 × 50 × 40 mm) gave informed consent and agreement to participate in the study. Serum samples were aliquoted and stored at -20 °C until analysis. The volume of sample provided to participating laboratories was large enough (500 µL) to allow measurement on native serum and after dilution on all of the systems tested.
Data collected: Prolactin values obtained on native serum and after serum dilution were collected. The technical data sheets were reviewed to assess the information provided on analytical principles: competitive or two-site immunometric assays; number of steps for the two-site immunometric assays: either one step or two steps (with a washing step); linearity range; reference to a high dose hook effect if present; and the concentration above which the high dose hook effect could likely occur.
Results
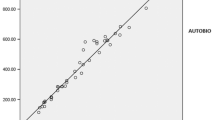
In a survey of data from December 2020, Probioqual received 464 results for prolactin measurements. The assay reagents used came from 11 manufacturers. Two manufacturers used the same assay reagents on different analyzers: Architect and Alinity from Abbott were combined in one analyzer; this was also the case for Cobas e601 and e801 from Roche. Probioqual combined the results from these different analyzers that used the same assay reagents (Fig. 1; Table 1) and thus produced a total of 14 different analytical systems. Samples with elevated prolactin concentration were sent to 16 French laboratories to test the 14 different assays (i.e. including testing the different analyzers from Abbott and Roche).
Careful analysis of the assay technical notices allowed an updated evaluation of the status of prolactin assay reagents in 2020:
-
All assays consisted of two-site immunometric assays with various labels (radioactivity, chemiluminescent, electrochemiluminescent etc.).
-
The reactions consisted of one step (78.6% = 11 reagents/14) or two step (21.4% = 3 reagents/14) reactions.
-
The linearity range varied from 190 to 470 µg/L depending on the manufacturer.
-
All of the one step assays referred to the high dose hook effect in their manufacturer’s notices for use (100%).
-
The theoretical concentration below which the high-dose hook-effect is not observed varied from 9,520 µg/L to 50,000 µg/L depending on the manufacturer.
Prolactin measurement: When performed on native serum, only one reagent was sensitive to the high dose hook effect and gave a result 150 µg/L below the linearity range of the assay, while all other assays reported the result as “above the upper limit of the assay”. Results obtained after dilution varied from 17,900 µg/L to 86,900 µg/L depending on the assay used (Table 1). The assay which was sensitive to the high dose hook effect gave a result of 17,900 µg/L after dilution, compared to 150 µg/L on native serum. This reagent is a two-site radio-immunometric assay performed in one step, manufactured by Beckman and used in France in only two laboratories (two results out of 464 in the Probioqual survey).
Aside from the value obtained with the Vista reagent, all results obtained after dilution were above the prolactin value indicated by the manufacturer as not being sensitive to the high dose hook effect. These results validated the absence of a high dose hook effect with the various assays. The result obtained using Vista (Siemens) was 30,800 µg/L, and the limit for sensitivity to the hook effect was indicated as 50,000 µg/L by the manufacturer.
Discussion
To our knowledge, this is the first study aimed at determining whether the high-dose hook effect still exists with current assays for measuring prolactin. In the guidelines covering the management of prolactinomas, this potential analytical interference is always mentioned since it could lead to misdiagnosis and subsequently to inadequate treatment [6], however this assertion is based on quite old publications.
A review of current manufacturers’ package inserts showed that the current methodologies are based on two-site immunometric assays, which are suitable for prolactin determination. These assays, which have developed with the widespread use of monoclonal antibodies, are more sensitive and specific than previous competitive assays, but are subject to interference that was not present with competitive techniques, high-dose hook effect for example. This interference can be avoided by using sandwich assays that are performed in two steps or by diluting the samples when using sandwich assays that are carried out in a single step [2, 5]. Two-site immunometric assays are supposed to offer wide linearity range compared to competitive assays. It is not understandable why for prolactin measurement the measurement range is so narrow not in accordance with concentrations observed in some pituitary adenoma. Dilution of samples with a suspected elevated concentration is not easy to handle under routine conditions. Communication between clinicians and biochemistry laboratories are very important; clinicians must be aware that the prolactin result may be affected by analytical interference. Some manufacturers have developed two-site immunometric assays performed in one step with no washing step, termed homogenous phase assays (for example TRACE® (Time-Resolved Amplified Cryptate Emission). These technologies are assumed to not be sensitive to the high-dose hook effect since the signal is measured several times during the reaction, however the supplier still mentions in the assay insert that the hook effect is not observed until an indicated prolactin concentration.
Our study is the first to test all of the currently available prolactin assays in France. It is reassuring to find that no hook effect occurred with modern assays. The Beckman assay, which was sensitive to high-dose hook effects, is an old immunoradiometric assay (IRMA) which is no longer recommended, since IRMA has been not found to improve the performance of prolactin measurement.
Several case reports have previously reported that IRMA assays are sensitive to the high-dose hook effect [9, 12, 15, 16]. However, we observed in this study that not all IRMA assays are sensitive to the hook effect. Indeed, among the assays which were tested in this study, two were based on IRMA methodology and one (Diasource) was not sensitive to the hook effect on the sample tested. Several other case reports using chemiluminescent technologies have also been reported [11, 13, 14].
Published case reports regarding hook effects often lack pertinent information. Indeed, the vast majority do not mention the assay used [10, 11, 14], and many cases concern old assays that are no longer available. These studies reported that high dose hook effects are more frequent in male subjects, due to the larger size of the adenoma at the time of diagnosis, and the larger amount of prolactin produced. In future publications describing the high dose hook effect, or other forms of analytical interference, specifying the assay used must be mandatory to allow better understanding of the current assay systems used.
This study allowed us to verify that analytical interference did not occur with assays that have a two-step protocol thanks to the washing step before the addition of the second antibody [15]. Although this is theoretically the easiest way to avoid the hook effect [2, 5], two-step reaction protocols are used in less than 25% of laboratories in France at the time of the survey. A major hindrance in this case is that the assay and analyzer are generally combined and it is not possible to run a two-step assay on an analyzer that is not suitable for this protocol.
A major strength of this study is the confirmation that the concentration of the studied sample was higher than the concentration indicated by the manufacturer as the limit below which there was no hook effect; except in the case of one assay (Vista, Siemens). This validates our study design which was to use a sample concentration that exceeded the concentration indicated in the manufacturer’s product inserts. Consequently, all manufacturer product data sheets could be modified to take into account this higher prolactin threshold, with the exception of the Vista assay.
A limitation of our work is that the Probioqual data reflects assays used in France at the time of the survey but not the proportion of data obtained using these assays in clinical activities. Laboratories working with clinicians from pituitary centers may have selected an assay that is not sensitive to the high-dose hook effect. In addition, this data reflects the present situation and we cannot predict the future in terms of new assays that may appear, and thus checking for analytical interference should continue [3].
An unexpected finding of our study was the diversity in results obtained for the same sample. Indeed, concentrations which varied from 17,900 to 86,880 µg/L were measured on a single sample despite adoption of the World Health Organization’s third international standard (IS) for prolactin 84/500, which has been recently replaced with the 4th IS 83/573 [17]. This lack of consistency between methods has already been reported [7]: it might come from a lack of commutability of the IS; and also different diluent used or variable specificity of assay antibodies; those causes are common in all immunoassays. This confirms the importance of following patient prolactin levels as they decrease during medical treatment using the same assay (in order not to misinterpret variations). Lastly, standardization of prolactin measurement is urgently needed and we must hope that manufacturers will follow these recommendations. Regardless of these difficulties, it is essential that the laboratory provides an absolute prolactin value by using as many dilutions as necessary to help in the management of these adenomas.
Conclusions
Although extremely rare, the high-dose hook effect should still be referred to in prolactin measurement, in order to remind clinicians and biochemists that it may be present. The risk is that if the effect is no longer mentioned in product inserts then it may no longer be taught to clinicians and biochemists. Given the development of new biochemistry technical platforms and the use of new assays and reagents (not tested here), inexperienced biochemists/clinicians run the risk of misdiagnosis and inappropriate treatment due to the high-dose hook effect, and it thus still needs to be considered.
Reference list
Parlant-Pinet L, Harthe C, Roucher F, Morel Y, Borson-Chazot F, Raverot G, Raverot V (2015) Macroprolactinaemia: a biological diagnostic strategy from the study of 222 patients. Eur J Endocrinol 172:687–695. doi:https://doi.org/10.1530/EJE-14-1073
Haddad RA, Giacherio D, Barkan AL (2019) Interpretation of common endocrine laboratory tests: technical pitfalls, their mechanisms and practical considerations. Clin Diabetes Endocrinol 5:12. doi:https://doi.org/10.1186/s40842-019-0086-7
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA, Endocrine S (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288. doi:https://doi.org/10.1210/jc.2010-1692
Samperi I, Lithgow K, Karavitaki N, Hyperprolactinaemia (2019) J Clin Med 8. doi:https://doi.org/10.3390/jcm8122203
Vilar L, Vilar CF, Lyra R, Freitas MDC (2019) Pitfalls in the Diagnostic Evaluation of Hyperprolactinemia. Neuroendocrinology 109:7–19. doi:https://doi.org/10.1159/000499694
Petersenn S, Giustina A (2020) Diagnosis and management of prolactinomas: current challenges. Pituitary 23:1–2. doi:https://doi.org/10.1007/s11102-019-01025-y
Saleem M, Martin H, Coates P (2018) Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues. Clin Biochem Rev 39:3–16
Chanson P, Maiter D (2022) Prolactinoma. In The Pituitary, Melmed, S., Ed. Elsevier: ; pp. 495–543
St-Jean E, Blain F, Comtois R (1996) High prolactin levels may be missed by immunoradiometric assay in patients with macroprolactinomas. Clin Endocrinol (Oxf) 44:305–309. doi:https://doi.org/10.1046/j.1365-2265.1996.663486.x
Yener S, Comlekci A, Arda N, Men S, Yesil S (2008) Misdiagnosis due to the hook effect in prolactin assay. Med Princ Pract 17:429–431. doi:https://doi.org/10.1159/000141512
Unnikrishnan AG, Rajaratnam S, Seshadri MS, Kanagasapabathy AS, Stephen DC (2001) The ‘hook effect’ on serum prolactin estimation in a patient with macroprolactinoma. Neurol India 49:78–80
Petakov MS, Damjanovic SS, Nikolic-Durovic MM, Dragojlovic ZL, Obradovic S, Gligorovic MS, Simic MZ, Popovic VP (1998) Pituitary adenomas secreting large amounts of prolactin may give false low values in immunoradiometric assays. The hook effect. J Endocrinol Invest 21:184–188. doi:https://doi.org/10.1007/BF03347299
Schofl C, Schofl-Siegert B, Karstens JH, Bremer M, Lenarz T, Cuarezma JS, Samii M, von zur Muhlen A, Brabant G (2002) Falsely low serum prolactin in two cases of invasive macroprolactinoma. Pituitary 5:261–265. doi:https://doi.org/10.1023/a:1025334001748
Barkan AL, Chandler WF (1998) Giant pituitary prolactinoma with falsely low serum prolactin: the pitfall of the “high-dose hook effect”: case report. Neurosurgery 42, 913–915; discussion 915–916, doi:https://doi.org/10.1097/00006123-199804000-00126
Fleseriu M, Lee M, Pineyro MM, Skugor M, Reddy SK, Siraj ES, Hamrahian AH (2006) Giant invasive pituitary prolactinoma with falsely low serum prolactin: the significance of ‘hook effect’. J Neurooncol 79:41–43. doi:https://doi.org/10.1007/s11060-005-9108-7
Comtois R, Robert F, Hardy J (1993) Immunoradiometric assays may miss high prolactin levels. Ann Intern Med 119:173. doi:https://doi.org/10.7326/0003-4819-119-2-199307150-00029
Ferguson J (2016) WHO International collaborative study of the proposed 4th International Standard for Prolactin, Human. World Health Organization. In World Health Organization.,
Acknowledgements
We thank Probioqual who allowed us to use their data and Drs Marc Barba; Florence Boux de Casson; Anne-Sophie Gauchez; David Guenet; Jean Guibourdenche; Isabelle Lacroix; Cindy Lauro; Mathieu Pecquet; Antoine Pilon; Christel Pissier; Caroline Richet; Rémi Segues; Séverine Trabado and Alice Veauville who performed prolactin concentration measurements on the analyzers available in their laboratories.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by VR and PP. The first draft of the manuscript was written by VR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval - Consent to participate
Informed consent was obtained from the participant included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raverot, V., Perrin, P., Chanson, P. et al. Prolactin immunoassay: does the high-dose hook effect still exist?. Pituitary 25, 653–657 (2022). https://doi.org/10.1007/s11102-022-01246-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-022-01246-8