Abstract
Purpose
To report the effects of pegvisomant (PEGV) treatment on patient-reported outcomes in acromegaly patients.
Methods
We conducted an extension study of an open-label, multinational, non-interventional study (ACROSTUDY) evaluating the long-term safety and efficacy of PEGV for acromegaly in routine clinical practice. Enrolled patients were rollover patients from ACROSTUDY, or treatment naïve/semi-naïve (NSN; no PEGV within 6 months of enrollment). Exploratory efficacy endpoints were changes in symptoms with the Patient-Assessed Acromegaly Symptom Questionnaire (PASQ) and quality of life with the Acromegaly Quality of Life questionnaire (AcroQoL) analyzed by controlled or uncontrolled IGF-I levels. Results were analyzed in all patients, in NSN patient subgroup, and by diabetes status.
Results
A total of 544 patients with acromegaly were enrolled, including 434 rollover subjects from ACROSTUDY and 110 NSN patients. Mean PEGV treatment duration was 7.8 years (range, 0–19.6 years). Overall, the majority of PASQ scores improved over time, but there was no significant difference between IGF-I controlled or uncontrolled groups. In the NSN subgroup, most PASQ and AcroQoL scores remained similar to baseline up to 1 year, regardless of IGF-I control. Patients with diabetes reported better PASQ scores over time with PEGV treatment, regardless of IGF-I control. IGF-I normalization increased from 10% of patients at baseline to more than 78% at year 10, with a mean daily PEGV dose of 18.7 mg.
Conclusions
Overall, patients treated with PEGV had small improvements in PASQ. While IGF-I normalization increased with PEGV treatment, IGF-I control had no effects on PASQ and AcroQoL scores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is caused by excess circulating growth hormone (GH) and insulin-like growth factor I (IGF-I), most often due to a GH-secreting pituitary adenoma [1]. Objective features of acromegaly may be subtle or severe, and can include excessive growth of hands and feet, coarsening of facial features, as well as prognathism [2,3,4]. Due to delayed detection, patients often have many complications at the time of diagnosis, including rheumatologic, cardiovascular, respiratory, neoplastic, neurological, and metabolic manifestations, which can negatively impact quality of life (QoL) [3, 5, 6]. Acromegaly treatment is multimodal, and may include surgery, medical therapy, radiation, or a combination of one or more of these treatments [7]. Medications include somatostatin receptor ligands (SRLs), dopamine agonists (DA), and the growth hormone receptor blocker, pegvisomant (PEGV), a pegylated GH receptor (GHR) antagonist. Goals of treatment include biochemical normalization, tumor control, prevention of complications, and normalization or improvement of QoL [8]; however, several studies show that QoL is still impaired even when biochemical control is reached [9,10,11,12].
PEGV binds to the human GHR and blocks signal transduction, resulting in a decline in circulating concentrations of IGF-I [13]. PEGV was approved by the European Medicines Agency (EMA) in 2002 [14] and by the US Food and Drug Administration (FDA) in 2003 [15]. Endocrine Society guidelines and consensus statements recommend using PEGV when patients have an inadequate response to surgery and/or radiation and fail to respond to other medical treatments, or as a primary therapy after failed surgery in selected patients [16, 17]. Clinical studies of PEGV have not reported any unexpected safety concerns, but found IGF-I normalization rates lower than those reported in initial clinical trials [18, 19], prompting the need for longer-term data in routine clinical practice.
ACROSTUDY was a non-randomized, open-label, multinational, non-interventional, post-authorization safety study (PASS) conducted to provide safety data for 1000 patients monitored over 5 years, as requested by the EMA. Initiated in 2004, the study monitored long-term safety and clinical outcomes; these data were submitted to the EMA in 2012, and in 2013 the EMA declared that the PASS commitment was fulfilled. However, the PASS study was voluntarily extended to follow-up a smaller patient subgroup, and to enroll a treatment naïve/semi-naïve to PEGV population of approximately 100 patients to analyze long-term safety as well as patient-reported outcomes (PROs) for health-related QoL.
This paper reports the effects of IGF-I control on treatment outcomes in terms of symptom scores on the Patient-Assessed Acromegaly Symptom Questionnaire (PASQ) and QoL on the Acromegaly Quality of Life questionnaire (AcroQoL) [20, 21] during long-term treatment with PEGV.
Methods and material
Study design
ACROSTUDY was an open-label, multinational, non-interventional study of the long-term safety and efficacy of PEGV used in the treatment of acromegaly in routine clinical practice. While the primary ACROSTUDY monitored long-term safety for at least 5 years, the current analysis, a voluntary long-term follow-up extension of the ACROSTUDY, was designed to include a subgroup of rollover patients (approximately 400), and a new subgroup of patients who were treatment naïve or semi-naïve (NSN; planned approximately 100). Both rollover and NSN subgroups formed the full analysis population (FAP); only patients in the newly enrolled NSN subgroup formed the NSN population.
ACROSTUDY was conducted in 15 countries (Austria, Belgium, Denmark, France, Germany, Greece, Hungary, Italy, Netherlands, Portugal, Slovakia, Spain, Sweden, UK, and the US), although not all countries were involved in the study extension (Table 1). The study was conducted according to the International Conference for Harmonisation Good Clinical Practices and in compliance with all legal and regulatory requirements of US, European, and international professional organizations. All patients (or informed legal representatives) gave written informed consent before study enrollment.
Inclusion criteria
ACROSTUDY primarily included adults (≥ 18 years) with acromegaly who were already receiving or began therapy with PEGV. All patients had undergone pituitary imaging within 6 months before enrollment. Pediatric patients could be enrolled in Europe but not in the US.
For the extension study, rollover patients continued treatment from the primary study after informed consent, and after verification of treatment compliance. NSN patients were defined, respectively, as never having received PEGV (naïve) or not being treated with PEGV during the 6 months before enrollment (semi-naïve); these patients began PEGV therapy during the study. Inclusion criteria for NSN patients were similar to those for rollover patients, except that these patients had to be enrolled within 5 days of the first dose of PEGV, and had to complete baseline evaluations (including PROs and laboratory tests) within 5 days of the first dose.
Exclusion criteria were discontinuation from the original ACROSTUDY, inability of patient (or representative) to understand the study and sign consent, recent (within 6 months) enrollment in another investigational drug trial for acromegaly, pregnancy or breastfeeding, allergy to PEGV, or tumor complications (need for surgical tumor decompression, treatment for visual field loss, cranial nerve palsies, or intracranial hypertension).
Endpoints
Data collected from all patients in ACROSTUDY included acromegaly diagnosis, pituitary function, physical examination, previous and/or current therapies for acromegaly, PEGV dosage, IGF-I measurement, liver enzymes, adverse events, and concomitant medications/comorbidities. Every IGF-I measurement was defined as normal, above the upper limit of normal (ULN), or below the lower limit of normal (LLN) for the laboratory used by each investigational site. Fasting blood glucose (elevated, > 200 mg/dL) and glycosylated hemoglobin (HbA1c; elevated, > 6.5%) were measured to determine diabetes status. IGF-I and HbA1c data were also analyzed separately for patients in the FAP, and the NSN subgroup.
Exploratory efficacy endpoints were changes in symptoms with the PASQ and QoL with the AcroQoL questionnaire. Paper questionnaires were autonomously completed by each patient. Responses were also analyzed by achievement of IGF-I normalization, which was reassessed at each timepoint. The AcroQoL questionnaire was only administered during the extension and not during the primary ACROSTUDY.
The PASQ is an acromegaly-specific questionnaire that includes 6 questions evaluating headache, excessive sweating, joint pain, fatigue, soft-tissue swelling, and numbness or tingling in the extremities, as well as a total score [22, 23]. Each item was scored from 0 (no symptoms) to 8 (severe, incapacitating symptoms), and lower scores indicated improvement. A final question asked the patient to judge overall health status (scored 0–10). Absolute value and change from baseline were analyzed at each visit (month 6, year 1, year 2, etc.) for patients in the FAP, the NSN subgroup, and by diabetes and IGF-I status.
AcroQoL includes 22 questions on 3 subscales denoted as Physical, Psychological/Appearance, and Psychological/Personal Relationship, plus a dimension for a global score [20]. The 22 items were scored on a Likert scale of 1–5 for occurrence frequency (1 = always; 2 = most of the time; 3 = sometimes; 4 = rarely; or 5 = never) or level of agreement (1 = completely agree; 2 = moderately agree; 3 = neither agree nor disagree; 4 = moderately disagree; or 5 = completely disagree). The subscales all had different point ranges, so they were standardized on a scale from 0 (worst QoL) to 100 (best QoL) before adding them together for the global score. A score <40 was considered severe impairment; ≥ 40 to < 60 moderate impairment; and 60 to 100 mild or no impairment [24]. Absolute value and change from baseline were analyzed at each visit (month 6, year 1, year 2, etc.) for patients in the NSN subgroup, and by diabetes and IGF-I status. In contrast to the PASQ, an improvement in AcroQoL is denoted by an increase in score.
Statistical analyses
No formal sample size calculation was performed. Estimates of approximately 400 rollover patients and 100 NSN patients were judged sufficient to evaluate symptoms and QoL between patients who achieved or did not achieve normalization of IGF-I.
There were no pre-specified statistical tests of hypotheses in ACROSTUDY. Timing of outcome assessments was measured during visit time, which was at the discretion of the investigator (e.g. baseline, month 6, and yearly thereafter). Patients had different follow-up durations depending on their enrollment status; rollover patients had >5 years of data, whereas NSN patients had a maximum of 3 years of data. Missing values were not imputed. Analyses were run at timepoints with a sample size of at least 50.
All statistics were descriptive, and any inferential statistics (e.g. P-values) were considered exploratory. The effects of IGF-I control (normalized versus not normalized) were analyzed with a longitudinal repeated-measures model with control (yes/no) as a time-varying factor, visit window (e.g. month 6, year 1) as a continuous variable, and baseline measurement as covariate using mixed procedure in SAS v9.4.
For PASQ and AcroQoL, differences in the change from baseline for individual and total scores between IGF-I controlled and IGF-I uncontrolled status were calculated with 95% confidence interval (CI). The longitudinal repeated-measures model was used to summarize the effects of IGF-I control over time on these PROs.
Complete data is available upon request to corresponding author.
Results
Patient disposition and characteristics
Patient disposition is shown in Fig. 1. Overall, the ACROSTUDY population included 2221 patients, of which 2090 had at least 5 years of follow-up including 1624 (77.7%) patients classified as non-active (i.e., terminated, exited, or died) and 466 (22.3%) patients classified as active. For this extension study, 434 of the 466 active patients were enrolled with 110 newly enrolled NSN patients (96 naïve and 14 semi-naïve) forming the FAP (n = 544). Overall, 450 (83%) patients completed the study, including 366 rollover patients and 84 NSN patients; however, only 9 subjects in the NSN subpopulation had reached year 3 before the study was closed. Discontinuations for rollover (n = 68) versus NSN patients (n = 26), respectively, were due to treatment discontinuation (54.4% vs. 61.5%), patient death (8.8% vs. 0%), withdrawal of informed consent (1.5% vs. 7.7%), and other reasons (35.3% vs. 30.8%). PASQ results were available for 203 FAP patients (37.3%), which included 84 patients (76.4%) from the NSN subgroup. AcroQoL results were available for 84 patients from the NSN subgroup.
Patient characteristics are summarized in Table 1. Overall, most patients were male (55.5%) and white (93.9%), with mean age of 42.8 years at acromegaly diagnosis. Patients began treatment at a mean age of 49.5 years and had a mean treatment duration of 7.8 years. Before the start of PEGV treatment, the majority of patients were on SRL (64.4%) or SRL/DA (30.9%). At the start of PEGV treatment, PEGV-alone was used in 45.6% of patients.
Patient-reported outcomes
PASQ
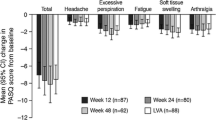
PASQ data were gathered from baseline to year 15 for the FAP and to year 3 for the NSN subgroup; however, because sample sizes were small at later timepoints, the FAP was analyzed to year 5 and the NSN was analyzed to year 1. Total scores and overall health status by IGF-I control, as well as individual scores for each sign and symptom of PASQ for FAP and NSN are presented in Fig. 2. For subjects in the FAP, mean total PASQ scores were 16.2 at baseline (n = 196), and improved to 13.6 by year 5 (n = 57), while mean overall health status scores were 4.1 at baseline (n = 201), and improved to 3.6 by year 5. Overall, improvements in mean scores from baseline to year 5 were observed in all individual PASQ domains including fatigue (3.5–2.9), soft tissue swelling (2.3–1.8), headache (2.2–1.8), and numbness/tingling of limbs (2.2–1.8).
Mean PASQ scores for A Total; B Headache; C Excessive sweating; D Joint pain; E Fatigue; F Soft tissue swelling; G Numbness/tingling of limbs; H Overall health status for the FAP and NSN subgroup. Each item is scored from 0 (no symptoms) to 8 (severe, incapacitating symptoms). Overall health status was scored from 0 to 10. P-values comparing IGF-I controlled vs. uncontrolled over time (up to 5 years for FAP and up to 1 year for NSN) were all non-significant (P>0.05). FAP full analysis population (rollover and naïve/semi-naïve patients), IGF-I insulin-like growth factor I, IGF-IC IGF-I controlled, IGF-IU IGF-I uncontrolled, NSN naïve/semi-naïve analysis population, LLN lower limit of normal, PASQ Patient-Assessed Acromegaly Symptom Questionnaire. *Does not include subjects with IGF-I < LLN
When the FAP was analyzed by IGF-I control, mean total PASQ scores in the IGF-I−controlled group improved from 15.3 at baseline to 12.4 after 5 years of treatment, while mean scores in the uncontrolled IGF-I group changed from 16.2 to 16.4, respectively, (Fig. 2A). In the IGF-I−controlled group, improvements from baseline to year 5 were observed in the overall (3.8–3.3), headache (2.4–1.5), excessive sweating (2.5–2.1), and fatigue (3.5–2.7) domains; results remained similar over time in the other domains. In the uncontrolled IGF-I group, results were similar from baseline to year 5 in all domains. Overall, there were no significant differences between the two groups for any of the domains; however, scores tended to be better with IGF-I control.
In NSN subjects, most of the PASQ scores remained similar over time. Mean total PASQ scores were 18.2 at baseline, improving to 15.6 at year 1; and mean overall health status scores were 4.5 at baseline, improving to 4.0 at year 1. Improvements from baseline to year 1 were observed for excessive sweating (3.1–2.4), joint pain (3.1–2.4), soft tissue swelling (2.6–2.2) and numbness/tingling of the limbs (2.2–1.8). When analyzed according to IGF-I control, the mean total PASQ score in patients with controlled IGF-I was 19.5 at baseline, which improved to 16.6 at year 1. Patients with uncontrolled IGF-I had a mean total PASQ score of 17.7 at baseline (lower, but not significantly, than the baseline value of patients with controlled IGF-I), which improved to 12.4 at year 1. In patients with controlled IGF-I, four of the individual PASQ domains showed improvements from baseline to year 1, while all PASQ domains showed improvements in patients with uncontrolled IGF-I. Similar to the FAP, no significant differences between the controlled and uncontrolled IGF-I groups were observed for any of the PASQ domains, but in this NSN subgroup, patients with uncontrolled IGF-I tended to have better results.
Patients with diabetes (n = 48) had higher total mean PASQ scores at baseline than those reported from the FAP (17.5 vs. 16.2, respectively). Total mean PASQ scores in patients with diabetes improved to 16.2 at year 1 (n = 33), 12.9 at year 2 (n = 21), and 10.8 at year 5 (n = 12). Mean overall health status scores also improved from 4.6 at baseline (n = 49), to 4.1 at year 1 (n = 33) and 3.3 at year 2 (n = 21). No significant differences were observed between IGF-I controlled and uncontrolled groups for patients with diabetes for any PASQ domains.
AcroQoL
Since the AcroQoL questionnaire was only administered to NSN patients, data were only available up to year 3; however, only year 1 data are presented as sample sizes at years 2 and 3 were small.
Individual scores for each AcroQoL dimension and by level of IGF-I control, are presented in Fig. 3 (higher scores indicate improvement). Improvements occurred between baseline and 1 year for all AcroQoL dimensions. For the IGF-I−controlled and uncontrolled groups, there was no significant differences between the AcroQoL scores over the course of the study.
Mean AcroQoL A Physical; B Psychological/appearance; C Psychological/personal relationship; and D Global scores by level of IGF-I control for the NSN population. AcroQoL subscales were standardized on a scale from 0 to 100 from worst to best QoL, so higher scores indicate improvement (a score <40 was considered severe impairment; ≥40 but <60 was moderate impairment; and 60 to 100 was mild or no impairment). P-values comparing IGF-I controlled vs. uncontrolled over time were all non-significant (P>0.05)
AcroQoL Acromegaly Quality of Life questionnaire, IGF-I insulin-like growth factor I, NSN naïve/semi-naïve analysis population
For patients with diabetes, mean global AcroQoL scores were 56.1 at baseline (n = 26), 61.4 at month 6 (n = 18), and returned to baseline levels at year 1 (55.6; n = 20). Again, no significant differences were observed between IGF-I controlled and uncontrolled groups over time for all four AcroQoL dimensions.
IGF-I normalization
Results for IGF-I (normal, <LLN, >ULN) by mean daily PEGV dose from baseline to 15 years are presented in Table 2; Fig. 4A. Percentages of patients with normal IGF-I increased over time in the FAP and NSN subgroup. IGF-I normalization increased from 10.3% at baseline up to 78.6% at year 10 at a mean PEGV dose of 18.7 mg/day in the FAP; at year 10, about half of the patients were on PEGV alone (54.2%). Overall, IGF-I remained normal in >65% of patients from year 2 up to year 14 (range, 65.5–79.3%). In the NSN subgroup, patients with IGF-I normalization increased from 13.1% at baseline to 64.3% at year 2 at a mean PEGV dose of 14.8 mg/day.
Normalization of A IGF-I and B HbA1c by IGF-I control with pegvisomant in the FAP and the NSN subgroup. HbA1c hemoglobin A1c, FAP full analysis population, IGF-I insulin-like growth factor I, IGF-I C IGF-I controlled, IGF-I U IGF-I uncontrolled, LLN lower limit of normal, NSN naïve/semi-naïve analysis population, ULN upper limit of normal
Normal HbA1c levels (Fig. 4B) were consistently observed more often in patients with controlled IGF-I then in those with uncontrolled IGF-I over time (>82% vs. >70%, respectively). In patients with controlled IGF-I, mean percent HbA1c levels was 6.1% at baseline and ranged from 5.7 to 6.6% over the course of the study. In those with uncontrolled IGF-I, mean percent HbA1c levels were 6.6% at baseline, and ranged from 5.9 to 8.2% over the course of the study. In the NSN subgroup, subjects with controlled IGF-I showed a mean percent HbA1c level of 5.8% at baseline, which remained the same at year 2; in IGF-I uncontrolled patients, HbA1c was 7.5% at baseline and improved to 6.5% at year 2.
Discussion
This long-term follow up of rollover patients and newly enrolled naïve/semi-naïve PEGV patients with acromegaly in ACROSTUDY evaluated patient-rated symptoms and health-related QoL measures.
The proportion of patients with normalized IGF-I increased throughout the study from 10% at baseline to 78% by year 10 in the FAP, and from 13% at baseline to 64% by year 2 in the NSN subgroup, demonstrating a lack of tachyphylaxis. Numeric improvements in some of the PASQ scores were noted in the first 5 years in the FAP and in the first year in the NSN subgroup. AcroQoL scores remained similar to baseline by year 1 for the NSN subgroup. Overall, no significant differences were observed in PASQ and AcroQoL scores when stratified by IGF-I control; however, those with controlled IGF-I in the FAP tended to have somewhat better scores, without reaching statistical significance. Surprisingly, NSN patients with uncontrolled IGF-I had better PASQ baseline scores than those with controlled IGF-I. Patients with diabetes had improved total mean PASQ scores from baseline to year 5. AcroQoL scores remained stable from baseline to year 1 in patients with diabetes, which was not unexpected since AcroQoL does not include diabetes among the items evaluated. Finally, HbA1c improved over time with PEGV, despite not achieving IGF-I normalization, consistent with improved glucose metabolism previously reported in acromegaly patients [13, 25, 26].
While the correlation between PROs and laboratory evaluation requires more studies, we highlight the possibility that acromegaly symptoms and decreased HRQoL could persist even after achieving biochemical disease control. The role of complete or partial biochemical control on QoL score improvement has been previously studied in prospective clinical trials [27], but the impact of changes in a real-life study is less known. A recent large meta-analysis focused on QoL and PASQ showed that total PASQ score decreased by 2.3 points (95% CI, –1.3 to –3.3) and AcroQoL increased by 2.9 points (95% CI, 0.5 to 5.3) with treatment in 24 studies [28]. As expected, treatment-naïve patients saw a larger effect size compared with other patients. The authors suggested that, even if not validated, PASQ should be used in addition to biochemistry for monitoring patients, as was done in a subset of patients in ACROSTUDY as shown in the present report.
Commonly reported breakthrough symptoms of patients on chronic injectable SRLs were joint pain, fatigue, snoring, excessive sweating, and headaches despite biochemical control [29], and adding PEGV to SRLs or switching to PEGV has been shown in some studies to improve QoL. Here, we found that addition of PEGV (up to 5 years) improved PASQ scores in at least 3 of 6 domains, as well as the total and overall score, while other domains score remained similar in IGF-I controlled patients. In IGF-I uncontrolled patients, all 6 PASQ domains as well as the total and overall scores remained similar to baseline after 5 years. PASQ scores in patients with diabetes also improved with treatment over time.
Though treatment of acromegaly may affect QoL, biochemical control does not always correlate with degree of QoL impairments; QoL may still be impaired despite biochemical control [29,30,31,32,33]. Even with biochemical remission, patients treated with medical therapy have lower QoL compared with patients cured by surgery, which may possibly be related to their negative perception of having a persistent disease and/or still require chronic, life-long medication [34,35,36]. Several studies on how different types of medical therapy affect QoL have been published [20, 37, 38]. A recent French study showed that better IGF-I control in patients is associated with higher survival and quality of life, generating more quality-adjusted life years [39]. In this real-life international study, overall biochemical control (i.e., normal IGF-I) was achieved with PEGV in more than two-thirds of patients in the FAP and the NSN subgroup. Overall, the degree of control of IGF-I was lower than that reported in several controlled, clinical PEGV trials, likely representing the lack of adequate titration seen in the real-world setting. We cannot rule out that use of a higher PEGV dose, which may have further lowered IGF-I, could have further improved the QoL scores. Furthermore, some symptoms, especially joint pain, could be irreversible and could worsen despite biochemical normalization of acromegaly. Severity of the disease at baseline and long-term duration of the disease can also negatively impact recovery of some subscale scores. Finally, it must be emphasized that alleviation of symptoms is not the only goal of acromegaly treatment, and that normalization of biochemical parameters has been shown to be associated with improvements of morbidity and mortality in these patients. Our PRO data from 84 treatment naïve/semi-naïve patients represents one of the largest PRO datasets (from baseline) in patients with acromegaly. The small improvements in PROs indicate that appropriately treating patients with PEGV may improve their QoL, as well as signs and symptoms, as shown by the observed positive trends.
In addition to the initial description of AcroQoL in 2002, in which good psychometric properties of the questionnaire were described [21], sensitivity to change of the score was shown in several longitudinal studies, in which clinical improvement with treatment was associated with increased AcroQoL scores [38, 40, 41]. Furthermore, AcroQoL scores have been successfully mapped to the well-established generic EuroQoL-5D questionnaire, showing that utility variables can be derived from AcroQoL for use in pharmacoeconomic studies [42]. However, clinical experience in reference centers who follow patients with life-long acromegaly has demonstrated that having been diagnosed with acromegaly negatively affects QoL despite having a “cure.” A delay in diagnosis may be one of the main reasons for reduced QoL, resulting in long-term, irreversible morbidity (i.e. chronic pain due to arthropathy, changed appearance, etc.) [43].
Over the last several years, many other instruments have been developed to capture acromegaly disease activity and impact of treatment more holistically, including SAGIT [7], ACRODAT [22], and ACRO TSQ [44]. While biochemical control (i.e. controlled circulating IGF-I), is essential and should be the main focus of therapy [12, 16], it may not reflect normalization of IGF-I in all tissues, and this may be perceived as persistent morbidity by the patient. PROs should also play an important role to assess endpoints of therapy and further individualize treatment, and ideally should be part of the on-going clinical evaluation of the patient. However, as we have shown here, there are limitations in quantifying the improvement for whole groups with available questionnaires.
The strength of this study is that the data represent the real-life scenario of treating patients with acromegaly in routine clinical practice across many countries. Inherent limitations of this study include patient enrollment at variable times relative to initiation of PEGV treatment (except for those in the NSN subgroup), lack of uniform titration of the PEGV dose to normalize IGF-I in all patients, variability in IGF-I measurements, AcroQoL results being available only for the NSN subgroup, and lack of detailed medical history and severity of disease for all patients. Since some data were collected as part of routine practice, more systematic coordination of study data collection was lacking. Furthermore, it was not possible to differentiate between the possible effects of other drugs (i.e., DA/SRL) on PROs in patients who were on combination therapy. However, few studies have addressed sequential changes in medications and their impact on QoL. One study showed improvement in QoL but without significant change in IGF-I after the addition of 40 mg pegvisomant weekly to monthly SRL therapy in patients with acromegaly who had normalized IGF-I on SRL monotherapy [45]. Finally, 5-fold more NSN patients withdrew from the study than rollover patients, which might be expected for newer than more experienced patients.
In summary, we have shown herein a large group of patients with acromegaly treated with PEGV (alone or in combination with other drugs) that when PRO improvements occurred, they were mostly in the first 5 years, with some symptoms of acromegaly improving in the IGF-I controlled subgroup, but others worsening/remaining similar despite IGF-I control. These data confirm that clinical evaluation and careful symptom assessment is an important aspect of the care of acromegaly patients, which should not be limited to measurement of biochemical parameters.
References
Melmed S (2006) Medical progress: acromegaly. N Engl J Med 355(24):2558–2573
Akirov A, Asa SL, Amer L, Shimon I, Ezzat S (2019) The clinicopathological spectrum of acromegaly. J Clin Med 8(11):1962
Vilar L, Vilar CF, Lyra R, Lyra R, Naves LA (2017) Acromegaly: clinical features at diagnosis. Pituitary 20(1):22–32
Melmed S (2009) Acromegaly pathogenesis and treatment. J Clin Invest 119(11):3189–3202
Maione L, Brue T, Beckers A, Delemer B, Petrossians P, Borson-Chazot F, Chabre O, Francois P, Bertherat J, Cortet-Rudelli C et al (2017) Changes in the management and comorbidities of acromegaly over three decades: the French Acromegaly Registry. Eur J Endocrinol 176(5):645–655
Gadelha MR, Kasuki L, Lim DST, Fleseriu M (2019) Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev 40(1):268–332
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, Bolanowski M, Bonert V, Bronstein MD, Casanueva FF et al (2019) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab 105(4):dgz096
Fleseriu M, Biller BMK, Freda PU, Gadelha MR, Giustina A, Katznelson L, Molitch ME, Samson SL, Strasburger CJ, van der Lely AJ et al (2021) A Pituitary Society update to acromegaly management guidelines. Pituitary 24(1):1–13
Strasburger CJ, Karavitaki N, Stormann S, Trainer PJ, Kreitschmann-Andermahr I, Droste M, Korbonits M, Feldmann B, Zopf K, Sanderson VF et al (2016) Patient-reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinol 174(3):355–362
Geer EB, Sisco J, Adelman DT, Ludlam WH, Haviv A, Liu S, Mathias SD, Gelbaum D, Shi L (2020) Patient reported outcome data from acromegaly patients treated with injectable somatostatin receptor ligands (SRLs) in routine clinical practice. BMC Endocr Disord 20(1):117
Fleseriu M, Molitch M, Dreval A, Biermasz NR, Gordon MB, Crosby RD, Ludlam WH, Haviv A, Gilgun-Sherki Y, Mathias SD (2021) Disease and treatment-related burden in patients with acromegaly who are biochemically controlled on injectable somatostatin receptor ligands. Front Endocrinol (Lausanne) 12:627711
Fleseriu M, Fogelfeld L, Gordon MB, Sisco J, Crosby RD, Ludlam WH, Haviv A, Mathias SD (2020) An evaluation of the Acromegaly Treatment Satisfaction Questionnaire (Acro-TSQ) in adult patients with acromegaly, including correlations with other patient-reported outcome measures: data from two large multicenter international studies. Pituitary 23(4):347–358
Buchfelder M, van der Lely AJ, Biller BMK, Webb SM, Brue T, Strasburger CJ, Ghigo E, Camacho-Hubner C, Pan K, Lavenberg J et al (2018) Long-term treatment with pegvisomant: observations from 2090 acromegaly patients in ACROSTUDY. Eur J Endocrinol 179(6):419–427
Hayes LD, Sculthorpe N, Herbert P, Baker JS, Spagna R, Grace FM (2015) Six weeks of conditioning exercise increases total, but not free testosterone in lifelong sedentary aging men.Aging Male:1–6
SOMAVERT (pegvisomant (for injection, for subcutaneous use Prescribing Information. Pfizer. New York, NY. 2013)
Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, Strasburger CJ, Luger A, Clemmons DR, Giustina A (2018) A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol 14(9):552–561
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, Bolanowski M, Bonert V, Bronstein MD, Casanueva FF et al (2020) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update.J Clin Endocrinol Metab105(4)
van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, Klibanski A, Herman-Bonert V, Melmed S, Vance ML et al (2001) Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet 358(9295):1754–1759
Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML et al (2000) Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med 342(16):1171–1177
Badia X, Webb SM, Prieto L, Lara N (2004) Acromegaly Quality of Life Questionnaire (AcroQoL). Health Qual Life Outcomes 2:13
Webb SM, Prieto L, Badia X, Albareda M, Catala M, Gaztambide S, Lucas T, Paramo C, Pico A, Lucas A et al (2002) Acromegaly Quality of Life Questionnaire (ACROQOL) a new health-related quality of life questionnaire for patients with acromegaly: development and psychometric properties. Clin Endocrinol (Oxf) 57(2):251–258
Schreiber I, Buchfelder M, Droste M, Forssmann K, Mann K, Saller B, Strasburger CJ, German Pegvisomant I (2007) Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol 156(1):75–82
Sievers C, Brübach K, Saller B, Schneider HJ, Buchfelder M, Droste M, Mann K, Strasburger CJ, Stalla GK (2010) Change of symptoms and perceived health in acromegalic patients on pegvisomant therapy: a retrospective cohort study within the German Pegvisomant Observational Study (GPOS). Clin Endocrinol (Oxf) 73(1):89–94
van der Lely AJ, Gomez R, Pleil A, Badia X, Brue T, Buchfelder M, Burman P, Clemmons D, Ghigo E, Jorgensen JOL et al (2017) Development of ACRODAT((R)), a new software medical device to assess disease activity in patients with acromegaly. Pituitary 20(6):692–701
Feola T, Cozzolino A, Simonelli I, Sbardella E, Pozza C, Giannetta E, Gianfrilli D, Pasqualetti P, Lenzi A, Isidori AM (2019) Pegvisomant improves glucose metabolism in acromegaly: a meta-analysis of prospective interventional studies. J Clin Endocrinol Metab 104(7):2892–2902
Brue T, Lindberg A, van der Jan A, Akerblad AC, Koltowska-Haggstrom M, Gomez R, Droste M, Hey-Hadavi J, Strasburger CJ, Camacho-Hubner C (2019) Diabetes in patients with acromegaly treated with pegvisomant: observations from acrostudy. Endocrine 63(3):563–572
Biermasz NR, van Thiel SW, Pereira AM, Hoftijzer HC, van Hemert AM, Smit JW, Romijn JA, Roelfsema F (2004) Decreased quality of life in patients with acromegaly despite long-term cure of growth hormone excess. J Clin Endocrinol Metab 89(11):5369–5376
Broersen LHA, Zamanipoor Najafabadi AH, Pereira AM, Dekkers OM, van Furth WR, Biermasz NR (2021) Improvement in symptoms and health-related quality of life in acromegaly patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 106(2):577–587
Webb SM (2011) Pituitary tumors: coping with ‘cured’ pituitary tumors. Nat Rev Endocrinol 7(5):251–252
Ben-Shlomo A, Sheppard MC, Stephens JM, Pulgar S, Melmed S (2011) Clinical, quality of life, and economic value of acromegaly disease control. Pituitary 14(3):284–294
Kyriakakis N, Lynch J, Gilbey SG, Webb SM, Murray RD (2017) Impaired quality of life in patients with treated acromegaly despite long-term biochemically stable disease: results from a 5-years prospective study. Clin Endocrinol (Oxf) 86(6):806–815
Tiemensma J, Kaptein AA, Pereira AM, Smit JW, Romijn JA, Biermasz NR (2011) Affected illness perceptions and the association with impaired quality of life in patients with long-term remission of acromegaly. J Clin Endocrinol Metab 96(11):3550–3558
Geraedts VJ, Andela CD, Stalla GK, Pereira AM, van Furth WR, Sievers C, Biermasz NR (2017) Predictors of quality of life in acromegaly: no consensus on biochemical parameters. Front Endocrinol (Lausanne) 8:40
Andela CD, Biermasz NR, Kaptein AA, Pereira AM, Tiemensma J (2015) More concerns and stronger beliefs about the necessity of medication in patients with acromegaly are associated with negative illness perceptions and impairment in quality of life. Growth Horm IGF Res 25(5):219–226
Crespo I, Valassi E, Webb SM (2017) Update on quality of life in patients with acromegaly. Pituitary 20(1):185–188
Yoshida K, Fukuoka H, Matsumoto R, Bando H, Suda K, Nishizawa H, Iguchi G, Ogawa W, Webb SM, Takahashi Y (2015) The quality of life in acromegalic patients with biochemical remission by surgery alone is superior to that in those with pharmaceutical therapy without radiotherapy, using the newly developed Japanese version of the AcroQoL. Pituitary 18(6):876–883
Webb SM, Badia X (2016) Quality of life in acromegaly. Neuroendocrinology 103(1):106–111
Caron PJ, Bevan JS, Petersenn S, Houchard A, Sert C, Webb SM, Group PI (2016) Effects of lanreotide autogel primary therapy on symptoms and quality-of-life in acromegaly: data from the PRIMARYS study. Pituitary 19(2):149–157
Brue T, Chanson P, Rodien P, Delemer B, Drui D, Marie L, Juban L, Salvi L, Henocque R, Raverot G (2021) Cost-Utility of Acromegaly Pharmacological Treatments in a French Context. Front Endocrinol (Lausanne) 12:745843
Sardella C, Lombardi M, Rossi G, Cosci C, Brogioni S, Scattina I, Webb SM, Gasperi M, Martino E, Bogazzi F (2010) Short- and long-term changes of quality of life in patients with acromegaly: results from a prospective study. J Endocrinol Invest 33(1):20–25
Webb SM, Badia X, Surinach NL, Spanish AcroQol Study G (2006) Validity and clinical applicability of the acromegaly quality of life questionnaire, AcroQoL: a 6-month prospective study. Eur J Endocrinol 155(2):269–277
Badia X, Trainer P, Biermasz NR, Tiemensma J, Carreno A, Roset M, Forsythe A, Webb SM (2018) Mapping AcroQoL scores to EQ-5D to obtain utility values for patients with acromegaly. J Med Econ 21(4):382–389
Sibeoni J, Manolios E, Verneuil L, Chanson P, Revah-Levy A (2019) Patients’ perspectives on acromegaly diagnostic delay: a qualitative study. Eur J Endocrinol 180(6):339–352
Fleseriu M, Fogelfeld L, Gordon MB, Sisco J, Colwell HH, Ludlam WH, Haviv A, Mathias SD (2019) Development of a novel patient-reported measure for acromegaly: the Acro-TSQ. Pituitary 22(6):581–593
Neggers SJ, van Aken MO, de Herder WW, Feelders RA, Janssen JA, Badia X, Webb SM, van der Lely AJ (2008) Quality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomant. J Clin Endocrinol Metab 93(10):3853–3859
Acknowledgements
The authors would like to thank all investigators, sub-investigators and study coordinators and patients for their participation and contributions to ACROSTUDY. This study was sponsored by Pfizer Inc. The authors also want to acknowledge the medical writing assistance of Hui Zhang, Ph.D., and Dominique Verlaan, Ph.D., CMPP of Precise Publications, LLC, which was supported by Pfizer Inc.
Funding
This study was sponsored by Pfizer. Editorial/medical writing support was provided by Hui Zhang, Ph.D., and Dominique Verlaan, Ph.D., CMPP at Precise Publications, LLC and was funded by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RS is an editor for Pituitary; MF and TB are on the Editorial Board of Pituitary. RS is a principal investigator with research support to Johns Hopkins University for clinical research studies with Crinetics, Novartis, and Chiasma and occasional scientific consultant for Ipsen and Recordati. PM has received honoraria as consultant/speaker, and is a principal investigator for research grants from CamurusAB, Ipsen, Novartis, Pfizer. SMW has received honoraria as consultant/speaker or is a principal investigator for research grants from: Pfizer, Novartis, Ipsen, Recordati, HRA, Crinetics and Corcept. TB has received honoraria as consultant/speaker, or is a principal investigator for research grants from: Pfizer, Novartis Pharma, Ipsen Pharma, Recordati, Merck-Serono, Sandoz, Novo-Nordisk, Advanz Pharma, and Corcept. JL, MPW, SRV, and RG are employees of Pfizer and are stockholders of Pfizer. MF is a principal investigator with research support at Oregon Health & Science University for clinical research studies with Crinetics, Novartis, Recordati, Chiasma, Ionis and occasional scientific consultant for Crinetics, Pfizer, Ipsen, Recordati, and Chiasma.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salvatori, R., Maffei, P., Webb, S.M. et al. Patient-reported outcomes in patients with acromegaly treated with pegvisomant in the ACROSTUDY extension: A real-world experience. Pituitary 25, 420–432 (2022). https://doi.org/10.1007/s11102-022-01206-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-022-01206-2