Abstract
Purpose
The pathogenesis of pituitary adenomas (PA) is complex. Ki-67, pituitary tumour transforming gene (PTTG), vascular endothelial growth factor (VEGF), cyclin D1, c-MYC and pituitary adenylate cyclase-activating peptide (PACAP) protein expression were analysed and correlated with tumour and patient characteristics.
Methods
74 pituitary tumour samples (48 non-functional PA, 26 functional PAs); Immunohistochemical analysis of protein expression, retrospective analysis of MR images and in vitro analysis of octreotide treatment was carried out on GH3 cells.
Results
PTTG expression was negatively associated with age and positively with PA size, regrowth and Ki-67 index. Cyclin D1 correlated with Ki-67 and tumour size. c-MYC negatively correlated with size of tumour and age; and correlated with PTTG expression. Somatostatin analogue treatment was associated with lower Ki-67, PTTG and Cyclin D1 expression while T2 hypointense PAs were associated with lower PTTG, cyclin D1, c-MYC and Ki-67. In vitro analyses confirmed the effect of somatostatin analogue treatment on Pttg and Cyclin D1 expression.
Conclusions
Interesting and novel observations on the differences in expression of tumour markers studied are reported. Correlation between Ki-67 expression, PTTG nuclear expression and recurrence/regrowth of PAs, emphasizes the role that Ki-67 and PTTG expression have as markers of increased proliferation. c-MYC and PTTG nuclear expression levels were correlated providing evidence that PTTG induces c-MYC expression in PAs and we propose that c-MYC might principally have a role in early pituitary tumorigenesis. Evidence is shown that the anti-proliferative effect of somatostatin analogue treatment in vivo occurs through regulation of the cell cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pathogenesis of pituitary adenoma (PA) development and progression has attracted considerable research interest over the last few decades. However, pathways involved in PA tumorigenesis have proven to be very intricate and complex [1]. The aim of this study was to analyse a sizeable group of PAs to identify potentially useful tumour markers of in vivo biological behaviour. We selected Ki-67 together with pituitary tumour transforming gene (PTTG), vascular endothelial growth factor (VEGF), cyclin D1, c-MYC and pituitary adenylate cyclase-activating peptide (PACAP). Ki-67 is a well-established proliferation marker extensively studied in pituitary tumours although certain associations have been the subject of controversy. PTTG has proven links to pituitary tumorigenesis and might have a role as a proliferation marker [2–4]. VEGF is a marker of angiogenesis [5] with possible links to other markers. Cyclin D1 and c-MYC, two important onco-proteins that have numerous roles in cell signalling cascades, have not been as extensively studied in pituitary tumours. PACAP is associated with increased cAMP levels in pituitary cells and has thus been conceptually implicated in pituitary tumorigenesis [6]. In this study protein expression of these potential markers and correlation with various tumour characteristics was undertaken.
Patients and methods
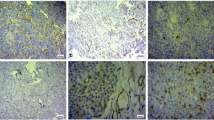
Immunohistochemistry was used to analyse the expression of proteins in samples of surgically removed pituitary tumours embedded in paraffin wax (Fig. 1). Details on the immunohistochemistry method used are found in supplemental methods and supplemental Table 1. The necessary patient consent and ethical approval was obtained.
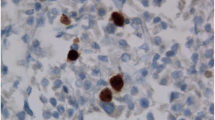
Immunohistochemical staining showing different staining patterns of the respective biomarkers and the associated % positivity of the particular case (representative section of whole slides shown) (magnification ×200) a Ki-67: Ki-67 Index 12.5%; b VEGF: 21% cytoplasmic positivity; c PTTG: 30% cytoplasmic positivity; d PTTG nuclear positivity: 0.8%; e cyclin D1: 28.3% nuclear positivity; f c-MYC: 35.3% nuclear positivity; g PACAP: 92% total positivity, 92% nuclear positivity, 1.5% cytoplasmic positivity; h PACAP: 82% positivity with no cytoplasmic positivity
Patients
In all 74 pituitary tumour samples were available. Criteria for classifying the functional status of PAs have been previously reported [7]. Pre-operative MR images were evaluated using the criteria and methods described previously [8]. Parameters determined included tumour volume, intensity of the PA on T2 images, cavernous sinus invasion, suprasellar extension, infrasellar extension and vertical extension index. The Hardy/Bates classification [9] and Knosp classification [10] were utilised to further characterise the PAs studied. Recurrence/regrowth-free survival was measured from the date of surgery to the date of recurrence, and the data were censored at the date of the end of follow up period.
In vitro analysis, real-time PCR and western blotting
GH3 cells were purchased from ECACC and grown in DMEM with 10% FBS and 1% antibiotics. Cells were seeded in six well plates 24 h prior to treatment. At time of treatment, medium was replaced with DMEM alone and cells were treated either with vehicle (water), 50 or 500 nM octreotide (Sandostatin, Novartis). After 24 h cells were lysed either for RNA extraction using the SV Wizard RNA extraction kit (Promega, UK), or for western blotting in passive lysis buffer (Promega). cDNA synthesis was carried out using the GoScript cDNA kit (Promega, UK) following the manufacturer’s instructions. Real-time PCR of the Pttg and Ccnd1 genes was carried out using the ribonuclease inhibitor 1 (RI) and Gapdh genes as housekeeping genes in the ABI7300 real-time thermocycler (Applied Biosystems, USA) using the HighROX qPCR Master mix (Solis Biodyne, Estonia). Western blots were carried out on 12% SDS–PAGE to quantify the protein expression of Pttg and Cyclin D1 proteins using B-actin as loading control. Antibodies for Pttg and Cyclin D1 were obtained from Abcam (USA) while B-actin antibody was from Novus Biologicals (USA). The membrane was then analysed using the Odyssey® infra-red Li-Cor System. Analysis of band intensity was carried out using the ImageJ® software.
Statistical analysis
Kolmogorov–Smirnov test was carried out to determine the normal distribution of the data and then either T Test/ANOVA (for parametric data) or Mann–Whitney U/Kruskal–Wallis or χ2 (for non-parametric data) tests were used as appropriate. Correlation analyses of protein expression with radiological and clinical parameters were carried out using Spearman correlation. Receiver operator characteristics (ROC) curve analyses were used to obtain a threshold value of expression of a particular protein predicting a certain outcome. Linear regression analysis and COX proportional hazards regression analysis were also used to determine variables that independently predicted a particular outcome. All statistics were carried out using IBM SPSS® Statistics for Windows, Version 22.0, (IBM Corp. Armonk, NY, USA).
Results
Morphological findings
Of the 74 PAs studied, 48 were non-functional PAs and 26 were functional PAs consisting of 17 GH-secreting PAs, 5 ACTH-secreting PAs, 3 prolactinomas and a TSHoma. Median age at diagnosis was 50.5 (IQR 40–62) years, and 27 patients (36.5%) were male. Their mean (±SD) maximal tumour diameter before operation was 25.1 (±10.1) mm. At the time of surgery three patients were on dopamine agonist treatment while nine patients were on a SSA (Octreotide LAR®) with a median duration of SSA treatment prior to surgery of 6 months (Range 2–20 months). None of the subjects received radiotherapy prior to their operation. 44.4% of all PAs had cavernous sinus invasion. Up till the end of the follow up period (31st December 2014), 12 patients had had regrowth/recurrence of their PA after surgery, while 4 patients had passed away. The mean (±SD) duration of follow up was 6.0 (±3.9) years.
There was a positive correlation between age at diagnosis and maximal tumour diameter, suprasellar extension and infrasellar extension (Table 1). Analysis of the patients’ demographic and radiological data did not reveal any statistically significant clinical differences between males and females. Comparing functional and non-functional tumours, there were statistically significant differences in age at diagnosis, maximal tumour diameter, cavernous sinus invasion, suprasellar and infrasellar extension (Tables 2, 3).
Among the 74 pituitary adenomas analysed, tumour progression after surgery was greater for Hardy/Bates Grade III–IV tumours compared to Grade I–II tumours where no regrowth or recurrence was observed. Similarly, outcomes were worse for tumours with a higher Knosp classification (Supplemental Table 2). All the tumours that regrew had cavernous sinus invasion on preoperative MRI. 36.4% of all PAs that had cavernous sinus invasion, eventually had regrowth/recurrence of their PA.
Ki-67
Ki-67 immunoreactivity among the PAs studied ranged from 0.02 to 9.83%. There were no statistically significant differences in the Ki-67 index between functional and non-functional PAs or between the different subtypes of PAs. PAs that regrew were shown to have a significantly higher Ki-67 index than those that did not (P < 0.001) (Table 4). Using ROC curve analysis a Ki-67 index of 2.0% or greater had a sensitivity of 75% and a specificity of 90% for predicting recurrence/regrowth of the tumour. Cox regression showed that a Ki-67 index greater than 2% was an independent predictor of tumour regrowth or recurrence (P < 0.001). Other variables that were included in the analysis (radiotherapy after operation, age at diagnosis, maximal tumour diameter and cavernous sinus invasion) were excluded by the stepwise method. Statistically significant positive correlations were noted between Ki-67 index and PTTG expression, PTTG nuclear expression and Cyclin D1 expression (Table 1).
PTTG
We report two separate indices for PTTG expression; one was the percentage of cells that showed any PTTG expression (nuclear or cytoplasmic) and another index based on those cells that specifically showed nuclear positivity (Fig. 1). Both indices were positively correlated (P < 0.001). Cytoplasmic staining of PTTG was much commoner than nuclear staining and ranged from 0 to 30.4% and was present to a varying degree in 99% of PAs studied. Nuclear staining was detected in fewer cells ranging from 0 to 1.1% and was detected in 76% of PAs studied.
There was a negative correlation between either total PTTG or nuclear PTTG expression and age at diagnosis with younger patients having higher levels. Similar to Ki-67 index, PTTG nuclear positivity was associated with a higher risk of regrowth or recurrence of the PA (P = 0.026), an association which was not significant when total PTTG expression levels were analysed (Table 4). Nuclear PTTG % positivity was positively correlated with tumour volume, suprasellar extension and suprasellar extension values.
PTTG expression was correlated with the Ki-67 index of the tumours. PTTG nuclear expression was correlated with Ki-67 index and c-MYC positivity (Table 1).
VEGF
VEGF expression was primarily located in the cytoplasm or in the cell membrane and its expression ranged from 0 to 22.0% of cells in the PAs studied. Both VEGF % positivity and VEGF total score showed a negative correlation with tumour recurrence (Table 4). No statistically significant correlations were noted between total VEGF % positivity and any clinical characteristics or other immunohistochemical markers (Table 1).
Cyclin D1
Cyclin D1 expression was mainly found in the nucleus ranging from 0 to 47.3% of cell positivity. Males had a higher Cyclin D1% positivity (P = 0.040) compared to females (Table 2) and non-functional tumours had a higher expression than functional tumours (P < 0.001) (Table 3).
There was positive correlation between Cyclin D1% positivity and maximal tumour diameter, tumour volume and suprasellar extension (Table 1) indicating that the larger the tumour, the higher the level of Cyclin D1 expression. Also a higher level of Cyclin D1 expression was detected in those tumours that had cavernous sinus invasion (Table 4). Statistically significant positive correlations were noted between Cyclin D1 expression and Ki-67 index (Table 1).
c-MYC
c-MYC expression was located in the nucleus and it ranged widely from 0 to 67.2% of cells in the PAs studied. Males had a lower expression of c-MYC compared to females while there was no significant difference between functional and non-functional PAs (Tables 2, 3). c-MYC % positivity was positively correlated with PTTG % nuclear positivity and negatively correlated with age at diagnosis meaning that patients who were diagnosed at a younger age and underwent surgery had tumours which had higher c-MYC expression levels than patients diagnosed at an older age (Table 1). A negative correlation was noted between c-MYC % positivity and maximal tumour diameter, tumour volume and suprasellar extension, showing that the larger the tumour, the lower the expression of c-MYC (Table 1).
PACAP
PACAP expression was mainly nuclear ranging from 15 to 100% positivity while cytoplasmic expression was much rarer ranging from 0 to 11.8% (Fig. 1) and both correlated negatively to each other. Hence we report the three separate indices for PACAP expression (nuclear, cytoplasmic and both). There was negative correlation between PACAP % total or nuclear positivity and PTTG % positivity. PACAP % total and nuclear positivity was negatively correlated with tumour volume and infrasellar extension. Cytoplasmic PACAP % positivity was negatively correlated with tumour volume and was statistically significantly associated with gender (Table 2) and functional status of the PA (Table 3).
Immunohistochemical analysis and somatostatin analogue treatment
Analyses of both all the PAs together and only functional tumours revealed that tumours treated with SSA before surgery had a lower Ki-67 index, PTTG% positivity, PTTG % nuclear positivity, and Cyclin D1% positivity compared to those that did not.
In vitro analysis and somatostatin analogue treatment
In order to verify the effect of somatostatin analogue treatment on protein expression in pituitary adenoma cells, a common in vitro model was used. GH3 cells were treated with physiological (nmolar) concentrations of octreotide and changes in the expression of Pttg and Cyclin D1 were observed both at the RNA and protein level. As observed in Fig. 2, Pttg gene and protein expression were significantly down regulated at both 50 and 500 nM concentrations of octreotide treatment, while Cyclin D1 RNA expression was significantly down-regulated at 500 nM octreotide and a reduction in protein expression of approximately 20% was achieved according to Image J imaging software.
In vitro analysis of Pttg and Cyclin D1 RNA and protein expression with somatostatin analogue treatment. a Real-time expression of the Pttg and Ccnd1 genes in GH3 cells at 24 h after treatment with 50 or 500 nM octreotide. Control value set at 1. qPCR was carried out in duplicate and results shown represent averages of three separate experiments. (*P < 0.05; **P < 0.01). b Western blot indicating protein expression of Pttg and Cyclin D1 proteins from GH3 cell lysates 24 h after treatment with 50 or 500 nM octreotide. Western blots were repeated twice for three separate experiments. c Image J graph results for the western blot obtained for Pttg antibody after treatment with 50 or 500 nM of Octreotide after 24 h. Readings from Image J analyser display the area under the graph for each peak obtained from the western blot image
Immunohistochemical analysis and T2 MR image intensity
As detailed in Table 2, there were statistically significant differences in the immunohistochemical analyses of tumours grouped according to their T2 MR image intensity. Hypointense tumours had consistently the lowest levels of PTTG % positivity, PTTG nuclear % positivity, Cyclin D1% positivity, c-MYC % positivity and Ki-67 index. For PTTG % positivity, PTTG nuclear % positivity and c-MYC % positivity the highest levels were seen in the isointense group while for Cyclin D1% positivity and Ki-67 index the highest levels of expression were seen in the hyperintense group of PAs. The hypointense group of PAs were the smallest tumours while the hyperintense group were the largest PAs.
Discussion
Establishing expression of particular biomarkers of PAs can facilitate decision making after surgery. This study provides evidence for the use of three different markers in the earlier detection of tumours with higher rates of regrowth/recurrence, namely tumours with high Ki-67, nuclear PTTG and low VEGF levels, together with specific radiological characteristics such as larger tumour diameter, volume, and suprasellar extension. We also confirm the known in vitro effects of PTTG on c-MYC expression in human pituitary tumours and provide novel data supporting somatostatin induced inhibition of the cell cycle.
We showed a clear association between tumour recurrence/regrowth and a higher Ki-67 index with a threshold index of 2% or more in our study as compared to 1.3% [11], 1.5% [12]; 2% [13] and 2.9% [14] in other studies. These variations could reflect differing methodologies. Interestingly there was a significant positive correlation between Ki-67 index and the vertical extension index [suprasellar extension–infrasellar extension]. In contrast to other reports [15, 16], no statistically significant difference in Ki-67 index was observed between functional and non-functional tumours.
In accordance with other studies [17, 18], we identified a much higher cytoplasmic expression of PTTG compared to nuclear expression and demonstrated a strong correlation between the two. This contrasts with the studies by Filippella et al. [3] and Ramirez et al. [13] where nuclear expression was seen in 89 and 99% respectively with no reference to cytoplasmic PTTG expression. The utilisation of different antibodies and protocols in the various studies could explain these conflicting reports. PTTG is an oncogenic protein with numerous functions and can promote transformation towards tumorigenesis in numerous settings [19]. In contrast to previous reports [3, 13] we demonstrated a significant negative correlation of PTTG with age, such that tumours from younger patients had higher PTTG expression (Table 1) and a positive correlation between PTTG % nuclear positivity and tumour volume or suprasellar extension. Given that age at diagnosis and tumour size were positively correlated (Table 1), the presence of higher PTTG expression in younger patients and larger tumours assumes greater significance. Furthermore, higher PTTG expression was observed in functional versus non-functional PAs after having excluded those tumours that were pre-treated with SSA or DA (Table 3).
Ki-67 positivity and PTTG nuclear expression, were strongly positively correlated and both correlated positively with tumour regrowth/recurrence. Two studies [3, 14] also report an association between nuclear PTTG expression and tumour recurrence while one study found no such association [13]. Our data support the use of both Ki-67 and nuclear PTTG (as opposed to total PTTG) as markers for proliferation and regrowth. Nuclear PTTG has also been identified as a potent marker for proliferation in breast cancer, with an abundance of nuclear PTTG in cancerous tissue as opposed to predominantly cytoplasmic PTTG in normal breast tissue suggesting that nuclear localization of PTTG could be integral to its oncogenic function [20].
Interestingly, we found a positive correlation between c-MYC positivity and PTTG nuclear expression. MYC is a transcription factor with numerous roles in cell cycle initiation and cell transformation and a general marker of “stemness” [21]. Over-expression of c-MYC increases the intrinsic rate of mutations leading to aberrant DNA synthesis and chromosomal instability in tumorigenesis [22]. In vitro studies in cell lines suggested that PTTG induces the expression of c-myc gene and that PTTG binds to the c-myc promoter region [23]. To our knowledge, the association we report in human pituitary adenomas is novel and provides further species specific evidence to support the in vitro findings reported to date. This together with our observations that tumours from younger patients and smaller tumours had higher c-MYC expression levels, seems to support a role for c-MYC in early pituitary tumorigenesis.
In contrast cyclin D1 appears to play a role in later pituitary tumour growth and dedifferentiation [24]. Cyclin D1 is an important regulator of cell cycle progression and plays an important role as an oncoprotein in most tumorigenic processes [25]. Dividing cells need to maintain high levels of cyclin D1 to sustain tumour growth [26]. Data from this study corroborates earlier reports [27, 28] of higher levels of cyclin D1 in non-functional PAs compared to functional tumours with a positive correlation between Ki-67 index and cyclin D1% positivity.
PACAP is a pleiotropic neuropeptide that regulates gene expression of pituitary hormone and influences growth and differentiation of pituitary cells [6]. Despite observing almost universally high levels of nuclear PACAP expression in the PAs we studied, correlations with tumour volume, infrasellar extension and PTTG % positivity were negative suggesting a protective role for PACAP in PAs [29]. Cytoplasmic PACAP expression was positively associated with functionality of the tumours which is in line with its role as a hypophysiotropic hormone in maintaining differentiated cell function. In summary, given the conflicting in vivo and in in vitro reports and the demonstration that its effects on secretion are cell and context specific [6], a definite role for PACAP in tumour formation or behaviour remains to be established.
The relationship between VEGF and pituitary tumorigenesis has also been the subject of controversy with conflicting reports regarding VEGF expression in the different subtypes [30–33]. We did not find any association between VEGF % positivity and the other immunohistochemical markers analysed in this study or with any patient/tumour characteristic except tumour regrowth. There was a negative correlation of VEGF % positivity with tumour recurrence/regrowth which supports the hypothesis that angiogenesis and cell proliferation in PAs might not be directly linked. Turner et al. [34] did not find an association between vascular density and Ki-67. Similarly, there was no correlation between VEGF or CD31 (an endothelial marker) expression and Ki-67 index, again suggesting that VEGF might be augmenting tumour vascularity through complex mechanisms rather than a direct effect on tumour cell proliferation [31].
Our data highlights a number of novel effects brought about by pre-treatment with SSA. Lower levels of Ki-67, total and nuclear PTTG expression and cyclin D1were found in functional tumours pre-treated with SSA compared to those that were not. While among all PAs studied, SSA treated tumours were noted to be generally smaller with less suprasellar and infrasellar extensions (Table 5), there was no statistically significant difference in the PA size of SSA treated functional adenomas compared to non-treated functional PAs. Our in vitro studies using the GH3 cell line and physiological concentrations of somatostatin analogue correlate well with these findings, indicating that octreotide does influence the expression of cell cycle regulators, namely it decreased the expression of PTTG and cyclin D1. Few studies document the in vivo effect of SSA on PA tissue [35]. Jaffrain-Rea et al. [16] report lower Ki-67 index in GH-secreting PA pre-treated with Octreotide and PRLomas pre-treated with a DA. Similarly Cap et al. [36] and Losa et al. [37] reported significantly lower Ki-67 index in SSA pre-treated GH secreting PAs. The authors suggested that this effect is mediated by a lower cell replication rate rather than increased cell apoptosis. Here we specifically report lower expression of nuclear PTTG and cyclin D1 confirmed in vitro using RT-PCR and Western blot techniques, together with low Ki-67 in the SSA pre-treated functional PAs. Both PTTG nuclear expression and cyclin D1 expression levels were correlated with Ki-67 expression in our study and furthermore PTTG nuclear expression itself was associated with regrowth/recurrence of PA. This is further proof that the anti-proliferative effect of SSA in vivo occurs through regulation of the cell cycle.
Another novel observation reported in our study is the correlation between expression of tumour markers and T2 MR image intensity. We report lower levels of Ki-67, PTTG total and nuclear, cyclin D1 and c-MYC expression in the hypointense PA group which were statistically significantly the smallest tumours and with the least extrasellar extensions (Table 2). Hagiwara et al. [38] noted that most hypointense PAs on T2 MR imaging were densely granulated tumours histologically. Cuevas-Ramos et al. [39] and Larkin et al. [40] reported that densely-granulated GHomas had significantly lower proliferation indices, were smaller and less invasive than sparsely-granulated cells. Our data confirm these observations and provide new insights regarding the relation between the tumours’ radiological characteristics and the respective biomarkers and cell-cycle regulators. T2 intensity on MRI can therefore be of use as a prognostic indicator with our data suggesting that T2 hypointense tumours seemingly having a better prognosis.
In conclusion we demonstrate a clear correlation between Ki-67 expression, PTTG nuclear expression and recurrence/regrowth of PAs, further highlighting the role that Ki-67 and PTTG expression have as markers of increased proliferation and potential for regrowth. Identifying those PAs with the biomarker and radiological characteristics indicated can help support decision making regarding the requisite frequency and intensity of biochemical and radiological follow up of these patients. Furthermore the adoption of a lower threshold for repeat surgical/radiotherapeutic intervention, intensification of medical treatment or introduction of newer agents after appropriate risk benefit analysis could be of benefit to patients with such PAs. We provide evidence that PTTG induces c-MYC expression in human PAs and c-MYC possibly plays a major role in early pituitary tumorigenesis as opposed to cyclin D1. We further characterise somatostatin induced changes in specific biomarkers and cell cycle regulators in human PAs and in vitro, further elucidating the post receptor effects of somatostatin. The effects of the different cell cycle regulators and the association with three putative tumorigenic peptides are summarised in Fig. 3.
References
Melmed S (2011) Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7(5):257–266
Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S (1999) Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med 5:1317–1321
Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G, Colao A, Meduri G, Chanson P (2006) Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol 65:536–543
McCabe CJ, Khaira JS, Boelaert K, Heaney AP, Tannahill LA, Hussain S, Mitchell R, Olliff J, Sheppard MC, Franklyn JA, Gittoes NJL (2003) Expression of pituitary tumour transforming gene (PTTG) and fibroblast growth factor-2 (FGF-2) in human pituitary adenomas: relationships to clinical tumour behaviour. Clin Endocrinol 58:141–150
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52(2):269–324
Gruppetta M, Mercieca C, Vassallo J (2013) Prevalence and incidence of pituitary adenomas: a population based study in Malta. Pituitary 16(4):545–553
Gruppetta M, Vassallo J (2016) Epidemiology and radiological geometric assessment of pituitary macroadenomas: population based study. Clin Endocrinol 85:223–231
Bates AS, Farrell WE, Bicknell EJ, McNicol AM, Talbot AJ, Broome JC, Perrett CW, Thakker RV, Clayton RN (1997) Allelic deletion in pituitary adenomas reflects aggressive biological activity and has potential value as a prognostic marker. J Clin Endocrinol Metab 82(3):818–824
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–618
Gejman R, Swearingen B, Hedley-Whyte ET (2008) Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol 39:758–766
Honegger J, Prettin C, Feuerhake F, Petrick M, Schulte-Mönting J, Reincke M (2003) Expression of Ki-67 antigen in nonfunctioning pituitary adenomas: correlation with growth velocity and invasiveness. J Neurosurg 99(4):674–679
Ramírez C, Cheng S, Vargas G, Asa SL, Ezzat S, González B, Cabrera L, Guinto G, Mercado M (2012) Expression of Ki-67, PTTG1, FGFR4, and SSTR 2, 3, and 5 in nonfunctioning pituitary adenomas: a high throughput TMA, immunohistochemical study. J Clin Endocrinol Metab 97:1745–1751
Noh TW, Jeong HJ, Lee MK, Kim TS, Kim SH, Lee EJ (2009) Predicting recurrence of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 94:4406–4413
Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER Jr (1996) Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38:99–106
Jaffrain-Rea ML, Di Stefano D, Minniti G, Esposito V, Bultrini A, Ferretti E, Santoro A, Faticanti Scucchi L, Gulino A, Cantore, G (2002) A critical reappraisal of MIB-1 labelling index significance in a large series of pituitary tumours: secreting versus non-secreting adenomas. Endocr Relat Cancer 9:103–113
Minematsu T, Suzuki M, Sanno N, Takekoshi S, Teramoto A, Osamura RY (2006) PTTG overexpression is correlated with angiogenesis in human pituitary adenomas. Endocr Pathol 17:143–153
Saez C, Japon MA, Ramos-Morales F, Romero F, Segura DI, Tortolero M, Pintor-Toro JA (1999) Hpttg is overexpressed in pituitary adenomas and other primary epithelial neoplasias. Oncogene 18:5473–5476
Bradshaw C, Kakar SS (2007) Pituitary tumor transforming gene: an important gene in normal cellular functions and tumorigenesis. Histol Histopathol 22(2):219–226
Ogbagabriel S, Fernando M, Waldman FM, Bose S, Heaney AP (2005) Securin is overexpressed in breast cancer. Mod Pathol 18(7):985–990
Gabay M, Li Y, Felsher DW (2014) MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med 4(6):a014241
Prochownik EV (2008) c-Myc: linking transformation and genomic instability. Curr Mol Med 8(6):446–458
Pei L (2001) Identification of c-myc as a down-stream target for pituitary tumor-transforming gene. J Biol Chem 276:8484–8491
Formosa R, Gruppetta M, Falzon S, Santillo G, DeGaetano J, Xuereb-Anastasi A, Vassallo J (2012) Expression and clinical significance of Wnt players and survivin in pituitary tumours. Endocr Pathol 23(2):123–131
Fu M, Wang C, Li Z, Sakamaki T, Pestell RG (2004) Minireview: cyclin D1: normal and abnormal functions. Endocrinology 145:5439–6477
Diehl JA, Zindy F, Sherr CJ (1997) Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Gene Dev 11(8):957–972
Elston MS, Gill AJ, Conaglen JV, Clarkson A, Shaw JM, Law AJJ, Cook RJ, Little NS, Clifton-Bligh RJ, Robinson BG, McDonald KL (2008) Wnt pathway inhibitors are strongly down-regulated in pituitary tumors. Endocrinology 149:1235–1242
Jordan S, Lidhar K, Korbonits M, Lowe DG, Grossman AB (2000) Cyclin D and cyclin E expression in normal and adenomatous pituitary. Eur J Endocrinol 143:R1–R6
Oka H, Jin L, Reubi JC, Qian X, Scheithauer BW, Fujii K, Kameya T, Lloyd RV (1998) Pituitary adenylate-cyclase-activating polypeptide (PACAP) binding sites and PACAP/vasoactive intestinal polypeptide receptor expression in human pituitary adenomas. American J Pathol 153(6):1787–1796
McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, Hussain S, Sheppard MC, Franklyn JA, Gittoes NJ (2002) Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J Clin Endocrinol Metab 87:4238–4244
Cristina C, Perez-Millan MI, Luque G, Dulce RA, Sevlever G, Berner SI, Becu-Villalobos D (2010) VEGF and CD31 association in pituitary adenomas. Endocr Pathol 21:154–160
Shan B, Gerez J, Haedo M, Fuertes M, Theodoropoulou M, Buchfelder M, Losa M, Stalla GK, Arzt E, Renner U (2012) RSUME is implicated in HIF-1-induced VEGF-A production in pituitary tumour cells. Endocr Relat Cancer 19(1):13–27
Sánchez-Ortiga R, Sánchez-Tejada L, Moreno-Perez O, Riesgo P, Niveiro M, Picó Alfonso AM (2013) Over-expression of vascular endothelial growth factor in pituitary adenomas is associated with extrasellar growth and recurrence. Pituitary 16:370–377
Turner HE, Nagy Z, Gatter KC, Esiri MM, Wass JAH, Harris AL (2000) Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: relationship to tumor behaviour. Br J Cancer 82:1441–1445
Zatelli MC, Piccin D, Ambrosio MR, Bondanelli M, degli Uberti EC (2006) Antiproliferative effects of somatostatin analogs in pituitary adenomas. Pituitary 9(1):27–34
Cap J, Cerman J, Nemecek S, Marekova M, Hana V, Frysak Z (2003) The influence of treatment with somatostatin analogues on morphology, proliferative and apoptotic activity in GH-secreting pituitary adenomas. J Clin Neurosci 10:444–448
Losa M, Ciccarelli E, Mortini P, Barzaghi R, Gaia D, Faccani G, Papotti M, Mangili F, Terreni MR, Cavnanni F, Giovanelli M (2001) Effects of octreotide treatment on the proliferation and apoptotic index of GH-secreting pituitary adenomas. J Clin Endocrinol Metab 86:5194–5200
Hagiwara A, Inoue Y, Wakasa K, Haba T, Tashiro T, Miyamoto T (2003) Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology 228(2):533–538
Cuevas-Ramos D, Carmichael JD, Cooper O, Bonert VS, Gertych A, Mamelak AN, Melmed S (2015) A structural and functional acromegaly classification. J Clin Endocrinol Metab 100(1):122–131
Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O (2013) Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 168(4):491–499
Acknowledgements
The authors would like to acknowledge all the medical specialists and other healthcare staff at Mater Dei Hospital who gave invaluable support during this study.
Funding
This study was funded by two independent research funds awarded to JV (University of Malta Research Fund: MEDRP02-05 and the Dean’s Research Fund, Faculty of Medicine and Surgery: MDSIN08-22). RF is funded by the REACH HIGH Scholars Programme—Post-Doctoral Grant. The Research work disclosed in this publication is partially funded by the REACH HIGH Scholars Programme—Post Doctoral Grants. The grant is part-financed by the European Union, Operational Programme II—Cohesion Policy 2014–2020 “Investing in human capital to create more opportunities and promote the wellbeing of society”—European Social Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gruppetta, M., Formosa, R., Falzon, S. et al. Expression of cell cycle regulators and biomarkers of proliferation and regrowth in human pituitary adenomas. Pituitary 20, 358–371 (2017). https://doi.org/10.1007/s11102-017-0803-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-017-0803-0