Abstract
Introduction
Diagnosing Cushing’s syndrome (CS) can be a challenge, especially in ACTH-dependent CS, when it comes to detecting the origin of ACTH secretion.
Materials and methods
Retrospective data were collected on 170 patients with ACTH-dependent CS (149 CD, 21 EAS) referring to two endocrinology units, focusing on three non-invasive tests: dexamethasone 8 mg overnight challenge (HDDST); corticotrophin-releasing hormone (CRH) assay and the desmopressin (DDAVP) test.
Results
Patients with EAS were slightly older and had higher ACTH, serum and urinary cortisol levels than patients with CD (p < 0.01). CD patients had a stronger ACTH and cortisol response after CRH injection (p < 0.0001), and a more pronounced reduction in cortisol levels after HDDST (p < 0.0001). A threshold percentage ACTH increase after CRH stimulation of 72.4 % was able to identify CD with a sensitivity (SE) of 76 % (95 % CI 68–83) and a specificity (SP) of 100 % (95 % CI 83–100). As for HDDST, a cortisol suppression >52.7 % below the basal level suggested a pituitary origin with a SE of 88 % (95 % CI 81–93) and a SP of 90 % (95 % CI 68–99). There were no cases of EAS with positive responses to both these tests. Increases in ACTH and cortisol levels after the DDAVP test were also higher in CD than in EAS (p < 0.01), though the SE and SP were lower.
Conclusions
Patients with CD showed a stronger response to HDDST and CRH, and the adopted cut-offs showed a good SE and SP in discriminating them from patients with EAS. Concordant tests indicated CD when positive, whereas no response to either test was highly suggestive of EAS. The DDAVP test was of limited utility in the diagnostic phase. In conclusion, the choice of tests may play an important part in the differential diagnosis of ACTH-dependent CS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corticotropin (ACTH)-related causes account for 80–85 % of cases of Cushing’s syndrome (CS). About 80 % of cases are pituitary-dependent CS (Cushing’s disease, CD), the remainder are due to an ectopic source (EAS) [1]. The most difficult issue is how to identify the origin of ACTH secretion, which should only be sought once a diagnosis of hypercortisolism has been established [2]. When seeking the source of ACTH, the gold standard for diagnostic purposes is bilateral inferior petrosal sinus sampling (BIPSS), and its performance is recommended in all patients who have ACTH-dependent CS with no visible lesions larger than 6 mm on pituitary MRI [3]. BIPSS might require hospitalization in some cases, however, and it is expensive, time-consuming and requires a dedicated team. Although the incidence of complications is reportedly low, the procedure can cause severe damage, such as sinus thrombosis, hemorrhage and deep vein thrombosis. It also carries a risk of false positive results [4–6]. Several other tests have been proposed for the differential diagnosis of ACTH-dependent forms of CS, but their performance has been far from satisfactory [7]. The lack of any wholly reliable diagnostic tests or imaging procedures clearly capable of revealing an ectopic source of ACTH suggests the need to adopt a step-by-step approach for its diagnosis and therapeutic management, bearing in mind that 12–50 % of patients with EAS undergo inappropriate transsphenoidal surgery (TSS) and the rates are even higher among those with the occult form [8, 9]. There are traditionally two tests most often used in clinical practice, i.e. the high-dose dexamethasone suppression test (HDDST) and the corticotropin-releasing hormone (CRH) test. The former has probably been the most widely used because it is easy to perform, and achieves an adequate sensitivity (65–100 %) and specificity (60–100 %). The latter was introduced more recently, but it has been increasingly used over time, even though there is still no consensus on how to interpret the results [7]. It was also suggested that combining the two tests could improve the diagnostic power of each one considered alone, but this assumption was not always supported by the results achieved [10, 11]. The desmopressin (DDAVP) test is also used to distinguish CD from EAS thanks to the expression of vasopressin receptors in corticotropinomas [12]. This test is widely adopted in the postoperative period as an early predictor of recurrent disease, but used for diagnostic purposes it has generated unsatisfactory results [13]. To the best of our knowledge, this is the largest series investigating the value of using all three tests together. Hence the present study, the aim of which was to assess the performance of three non-invasive tests performed in a large, consecutive series of patients with ACTH-dependent CS at two tertiary centers, with a view to identifying the best cut-off criteria for each test and assessing the utility of their combined interpretation in the diagnostic setting.
Materials and methods
We retrospectively recorded data on 170 consecutive patients (f/m = 133/37, mean age 43.24 ± 14.51 years) referred to two tertiary care centers in Italy—the endocrinology units at the University Hospital in Padova (100 patients), and the Ospedali Riuniti in Ancona (70 patients). The patients were diagnosed with ACTH-dependent CS (149 CD and 21 EAS) between 2003 and 2013. A diagnosis of CS was suspected on the grounds of clinical features and confirmed by at least two tests indicating high 24 h urinary free cortisol (UFC) levels, loss of circadian rhythm in plasma/salivary cortisol (at 8.00 a.m. and 11.00 p.m.), and lack of cortisol suppression after 1 mg of dexamethasone overnight (overnight suppression test; OST, normal values ≤50 nmol/L). The diagnosis of ACTH-dependent syndrome was confirmed on the strength of detectable ACTH levels (>10 ng/L), and an appropriate response to the HDDST and CRH stimulation tests and the DDAVP test. Pituitary MRI was performed if a pituitary origin was suspected. Patients underwent BIPSS when hormone tests were equivocal. The diagnosis was confirmed histologically in all cases of EAS except one, who had an occult tumor and whose BIPSS findings were consistent with an ectopic ACTH production. For patients with CD, the pituitary origin of their ACTH secretion was confirmed by biochemical remission after TSS, histology and/or temporary hypoadrenalism.
The DDAVP (10 µg iv) and CRH (100 µg iv) tests were performed in the morning with patients fasting and resting. Ovine CRH (oCRH) was used for 51 patients (from 2003 to 2007), and human CRH (hCRH) in the other 119 patients. The test methods have been reported elsewhere [14]. For the HDDST, a single dose of 8 mg of dexamethasone was administered at 23.00 h; serum cortisol levels were measured between 8.00 and 9.00 a.m. on the day before and after administering the dexamethasone and the levels were compared. No major side-effects were recorded; most patients reported only a mild sense of warmth, especially after the CRH injection, and a few patients experienced short-lived nausea.
All patients signed to give their informed consent before being tested. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Plasma cortisol and UFC levels were assessed by RIA using commercial kits (Diagnostic Products Corp., Los Angeles, CA, USA). From December 2011 onwards, UFC was measured with an Agilent HPLC series 1200, an Agilent 6430 triple quadrupole mass spectrometer equipped with an Electrospray Ionization source, operating in positive ion mode (Agilent Technologies, Palo Alto, USA). Plasma ACTH levels were measured by IRMA (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA). For patients referred to the endocrinology unit in Ancona, the following tests were used: serum cortisol and 24-h UFC were measured with the Access (ECLIA) automated electrochemiluminescent method (Beckman Coulter, Brea, CA, USA). Plasma ACTH was tested via ECLIA using the Elecsys ACTH reagent kit (Roche Diagnostics, Indianapolis, IN, USA). Saliva samples were collected in a commercial polyester-based sampling device, Salivette (Sarstedt, Numbrecht, Germany). Salivary cortisol levels were measured with the Access automated chemiluminescent method (Beckman Coulter, Brea, CA, USA). UFC values were expressed as the ratio between the value measured and the upper limit of normality.
Statistical analysis
Quantitative data are presented as medians and ranges, compared between groups with Wilcoxon’s rank sum test followed by the Dunn test for pairwise comparisons in the event of a statistically significant result when more than two groups were involved. Categorical data are summarized as counts and percentages of subjects in each category and compared with the Chi square or Fisher’s exact test. A logistic regression model was employed to identify an optimal cut-off for delta ACTH when performing the DDAVP and CRH tests. Sensitivity (SE) and specificity (SP) were calculated with 95 % confidence intervals (CI) using the exact method. Estimates of the area under the curve (AUC) for the DDAVP and CRH tests, separately and combined, were accompanied by the 95 % CI and compared with Wilcoxon’s rank sum test. OST was correlated with HDDST using McNemar’s test and considering the cut-off obtained with the ROC analysis. Using said cut-off, the sensitivity of the HDDST alone, of the HDDST plus the CRH test, and of the HDDST plus the CRH and the DDAVP tests in detecting ectopic patients was considered serially, and estimated with the 95 % CI calculated with the exact binomial method. Statistical significance was assumed for a p value <0.05. The data were analyzed with SAS 9.2 (SAS Institute Inc., Cary, NC, USA) for Windows.
Results
Baseline characteristics and first-line tests
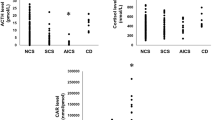
No difference in gender distribution emerged between the two groups, though the prevalence of female gender was less evident in the EAS group (the male/female ratio was 1:4 in the CD group versus 1:2 in the EAS group; p = 0.0845). Patients with CD were 10 years younger at the time of the disease’s presentation than those with EAS, although there was a significant overlap between the two groups (p = 0.007); Table 1. The patients’ BMI was similar for the two groups (p = 0.132). The levels of UFC (166 patients), morning and midnight serum cortisol (157 patients), and cortisol after 1 mg dexamethasone (155 patients) were significantly higher in the patients with EAS (p = 0.0004 for UFC; and p < 0.0001 for the others).
HDDST
The percentage reduction in serum cortisol after the HDDST was higher in CD than in EAS patients (p < 0.0001). Using the ROC analysis, a cut-off of 52.7 % for the decrease vis-à-vis baseline cortisol levels was identified as achieving the best performance in diagnosing patients with CD, with a SE of 88 % (95 % CI 81–93) and a SP of 90 % (95 % CI 68–99); the AUC for this test was 0.9307 (95 % CI 0.8877–0.9737). The SP could be increased to 100 % by changing the cut-off to a 75 % decrease -with a concomitant drop in the SE, of course, to 76 %; Fig. 1. Overall, cortisol after OST correlated with the cortisol levels after HDDST (p < 0.0001), but 47/137 patients with levels suppressed to below 30 % of the baseline values with OST showed a greater than 53 % reduction after the HDDST. The utility of OST in diagnosing the origin of ACTH secretion was significantly lower than HDDST (p < 0.0001; 95 % CI 0.1383–0.43), and its addition to CRH test did not give further information to HDDST alone (p = 0.1110; 95 % CI −0.0649–0.00669).
CRH test
No differences emerged between CD and EAS patients in peak ACTH levels using this test, whereas the absolute and percentage increases in ACTH were both significantly higher in cases of CD (p < 0.0001 for both). The same trend was found for cortisol, with a higher increment in the CD group (p < 0.0001); only the peak cortisol levels remained higher in EAS, reflecting these patients’ higher basal levels.
After the ROC curve analysis, the percentage increase in ACTH emerged as the best predictor of a pituitary origin when the increase was >72.4 % above the baseline, achieving a 76 % SE and 100 % SP; the AUC for this test was 0.9329 (95 % CI 0.8921–9737); Fig. 2. No differences were seen between peak ACTH levels and their percentage increases after the two different types of CRH were injected (p = 0.1403 and p = 0.0789, respectively), whereas the absolute increase was slightly higher after stimulation with oCRH (p = 0.0255). Otherwise the rise in cortisol after oCRH was significantly higher, and its peak, absolute and percentage increases were all higher than after hCRH. Since we only considered the percentage increase in ACTH, the difference between the two types of CRH did not matter.
DDAVP test
The DDAVP injection produced a higher ACTH peak and percentage increase in the CD group than in the EAS group (p = 0.0178 and 0.0015, respectively), whereas there was no difference in terms of the absolute increase (p = 0.8572). The same applied to cortisol, with higher peak and percentage increases (p < 0.0001, in both cases) in the CD patients and a comparable absolute cortisol increase (p = 0.3444). A pituitary origin was suggested in the presence of an ACTH percentage >32.3 % above the baseline value, achieving a SE of 83 % and a SP of 62 %; the AUC for this test was 0.7138 (95 % CI 0.5945–0.8351), Fig. 2.
Combinations of tests
When the previously-identified criteria were applied to patients who underwent both the CRH test and the HDDST, a pituitary origin was correctly identified in 76 % of the CD patients in the case of a concordant positive response. Conversely, both tests were negative in only 5.6 % of the CD patients, but in 89.5 % of the patients with EAS (Fig. 3).
The tests were also assessed in series, considering the same previously-found cut-offs, in 142 patients who underwent all three tests. The HDDST alone showed a good SE of 89.5 % (95 % CI 67–99 %) in identifying EAS. When only the 31 cases with a negative response to the HDDST (17 EAS and 14 CD) had the CRH test, we obtained a SE of 100 % (95 % CI 81–100 %) for the combination of the two tests (HDDST + CRH test) in diagnosing EAS.
The DDAVP test was assessed in patients with a positive response to the CRH test and an insufficient suppression in the HDDST: a positive response to both the CRH and the DDAVP tests correctly identified CD patients in 5/6 cases. In patients with a negative CRH test result, a positive HDDST and DDAVP test result indicated a pituitary origin. When the HDDST and the CRH test were concordant, performing the DDAVP test did not contribute to the diagnosis: the SE for the HDDST + CRH + DDAVP combination performed in series was only 64.7 % (95 % CI 38–86 %).
The concomitant presence of an adequate suppression after the HDDST and a positive response in the CRH test was able to diagnose CD, correctly identifying 93/123 patients. None of the EAS patients were found positive in both tests. The DDAVP test showed a good concordance with the other tests, proving positive in 79 of the 92 cases of CD.
Imaging
All patients with CD underwent pituitary MRI and adenoma was detected in 73.2 % of cases; it was a microadenoma in the majority (81.7 %) of cases. Thirteen patients with EAS also underwent pituitary gland MRI, and an image compatible with pituitary adenoma was found in 5 cases (all microadenomas <6 mm in size).
Macroadenomas were more frequent in males even though the difference was only close to statistical significance (p = 0.0666). Macroadenomas displayed basal ACTH levels and peak after DDAVP injection higher than patients with microadenomas or with not visible lesion (p = 0.0004).
On the contrary, patients with macroadenomas showed lower ACTH response to CRH injection. Cortisol response to both DDAVP and CRH tests did not differ between these two groups of patients even though patients with microadenomas had a tendency to present enhanced cortisol response to CRH than those with bigger pituitary lesions. No differences were highlight for cortisol levels after HDDST between micro and macroadenomas.
BIPSS
This procedure was performed in 32 cases (26 CD and 6 EAS), and was conclusive in 30/32 patients. Among the 2 inconclusive procedures, one generated a false positive result due to cyclical hypercortisolism in a patient with a pulmonary neuroendocrine tumor discovered later on, and the procedure had been stopped in the other case due to an anatomical variant coming to light.
Discussion
It has always been difficult to differentiate between CD and EAS origin due to a considerable overlap between the two forms in terms of their clinical presentation, but a correct diagnosis is crucial in order to give patients appropriate treatment options. In this study, we examined the hormonal features of patients with CD and EAS, paying special attention to second-line diagnostic tests. The baseline characteristics of the two groups differed: EAS patients had more severe disease with significantly higher levels of ACTH, morning and midnight serum cortisol and UFC, and a weaker cortisol suppression overnight after the administration of 1 mg of dexamethasone. Although such features can point in the direction of an appropriate diagnosis, these first-line tests are not enough to establish the source of ACTH production, so more specific tools are needed. BIPSS is recommended in all cases of ACTH-dependent CS with no pituitary adenoma at least 6 mm in size [3]. Bearing in mind that approximately 30 % of pituitary MRIs are negative, and that a sizable proportion of patients have a small microadenoma (<6 mm), this means that a great many procedures can be avoided (to some degree at least) if the second-line tests are interpreted properly. Although plasma ACTH levels >20 ng/L suggest an ACTH-dependent condition, this parameter is insufficient for discriminating between CD and EAS. Although ACTH and serum cortisol levels tend to be higher in EAS than in CD, the considerable overlap between the two conditions makes it difficult to discriminate between them [8]. That is why different tests are used to diagnose ACTH-dependent CS. The rationale behind the most widely used test, the HDDST, lies in that the corticotroph tumor cells retain some responsiveness to the negative feedback effects of glucocorticoids in CD, but not in EAS. Several variants of this test exist, including the standard usage of 2 mg every 6 h for 2 days, or the administration of 8 mg orally overnight [1]. The latter is easier to perform and only takes 1 day to complete; previous works found a drop in serum cortisol of >50 % with respect to the basal level suggestive of CD, with a sensitivity ranging from 60 to 80 % and a high specificity, which can be further improved by adopting a cortisol suppression cut-off of >80 % [15–17]. The utility of the HDDST has been questioned, however, as it seems to add little or no information to the OST. In fact, some authors have reported that a suppression >50 % vis-à-vis the baseline would predict an adequate suppression in response to the HDDST [18]. In our series, a suppression >52.7 % performed well, and raising this cut-off to 75 % brought the SP of the test up to 100 %, since none of the EAS patients reached such a suppression level. Unlike Isidori et al., we did not find a complete agreement between the results obtained with 1 and 8 mg of dexamethasone: a number of patients with a next to nothing suppression (<30 %) after 1 mg responded well to 8 mg, achieving a reduction of >52.7 % from their baseline cortisol levels. We are aware of the potential pitfalls of this test, however, such as in cyclical CS, in which case cortisol levels can vary widely during the day of the test. Another important potential confounder may relate to the effects of medication commonly used in patients with CS, including antidepressants, antihypertensives, and lipid-lowering agents: these classes of drugs have the potential to interfere with the CYP3A4 enzyme system, which regulates dexamethasone metabolism, potentially altering test findings and diagnostic accuracy [2].
The CRH test has been suggested as the best non-invasive tool for diagnosing CD. Pituitary corticotrophs continue to express CRH receptors, leading to an increase in ACTH and cortisol after the injection of CRH [9, 10, 19]. In our patients, the percentage increase in ACTH represented the most reliable parameter for the differential diagnosis of ACTH-dependent CS: patients with EAS tended not to respond to CRH, while those with CD showed a stronger increase in ACTH and cortisol (although some patients with a pituitary origin had a negative response, probably due to high levels of basal cortisol) [20]. Consistently with these studies, we found that this test performed well, but no better than the HDDST in terms of the AUC, as reported in [16, 21]; the addition of OST to CRH test did not prove to be more accurate than HDDST alone. Previous works had shown a similar response to hCRH and oCRH, although the latter is able to evoke a stronger and more prolonged response in ACTH and cortisol levels [20]. In our series, the peak ACTH and its percentage increase did not differ between patients stimulated with hCRH and oCRH. As we built the ROC curves considering the percentage increase in ACTH, the difference between the two types of CRH could not influence the outcome of our statistical analyses.
Interestingly patients with macroadenomas tended to present a lower increase of ACTH after CRH injection compared to microadenomas; this finding might account for the false negative responses to CRH test in CD patients in our series.
Desmopressin is a preferential vasopressin receptor V2 and V3 agonist that causes a significant rise in ACTH and cortisol levels in most patients with CD, but only occasionally in those with EAS [13, 22]. This response is based on overexpression of the V3 receptor in human corticotroph adenomas [12], and that is why its use has been suggested as an alternative to the CRH test in the differential diagnosis of ACTH-dependent CS [23, 24]. The DDAVP test has also proved useful in differentiating between CD and pseudo-Cushing’s [22, 25], as well as in the postoperative period after neurosurgery to enable the prompt detection of patients at high risk of recurrent CD [14, 24, 26–28]. Though promising, its overall sensitivity and specificity are lower than those of the CRH test [29]—but combining it with the CRH test seems to achieve a greater discriminatory power than either of the tests used alone [25, 30].
In our series, patients with CD showed a greater response to DDAVP, but the test did not perform as well as the other two tests. Its utility in differentiating between CD and EAS proved to be very limited, even though CD patients had a stronger response in terms of both ACTH and cortisol, particularly when the results were concordant [11, 13, 25, 30, 31]. We hypothesized a role for DDAVP in confirming the pituitary origin of ACTH secretion in patients with an incomplete suppression in response to the HDDST and a positive response to the CRH test. We found the combination of the CRH test and the HDDST very informative in cases with a positive response, where as almost 90 % of the EAS patients and only 5 % of those with CD failed to respond to either the CRH test or the HDDST. Similar results were reported by other groups studying smaller samples of patients [21, 32]. These data suggest that performing the BIPSS could be avoided at least in patients with concordant results in second-line tests. In addition, the remission rate seen in previous studies was comparable in patients who did or did not undergo BIPSS prior to surgery [33].
MRI represents a fundamental step in the workup for diagnosing CD, but its low sensitivity and the chances of pituitary incidentalomas being detected in cases of EAS restrict its diagnostic value; in a large Italian multicenter study, a pituitary adenoma was found in 73.2 % of patients with CD, but also in 38 % of those with EAS [34]. The presence of a microadenoma is therefore not enough for hypercortisolism to be labeled as pituitary-dependent.
BIPSS is the procedure with the best performance in discriminating CD from EAS, but even this test is not foolproof, and false negative results are possible. The main reason for the absence of an ACTH gradient in patients harboring a pituitary ACTH-secreting tumor are unilateral or bilateral anatomical variants in the petrosal venous system, but cyclicity can also give rise to false positive results. The procedure can also be burdened with severe complications, albeit rarely in experienced hands [32], and we need to remember that it is expensive too. In the presence of a clear-cut response to both the HDDST and the CRH test, the likelihood of EAS is very low, so the need to perform BIPSS could be reconsidered even in the absence of a visible lesion on pituitary MRI. In the case of discordant test results, on the other hand, BIPSS should be performed to avoid any misdiagnoses and unnecessary pituitary surgery, especially in patients with a negative CRH test result.
The limitations of this study include the different size of the two samples, a fact that may have weakened our statistical analysis. On the other hand, this distribution is typical of real clinical practice because CD is far more common than EAS. Another limitation concerns the use of two different types of CRH. Although oCRH could evoke a stronger and more prolonged response, we found no significant differences in the percentage increase in ACTH, which is the parameter that we used to build the ROC curves, and this consequently limited the impact of having used two different types of CRH on the accuracy of this test. A major concern regarding this report is the presence of two distinct populations of patients at two different centers, but potential limitations due to the use of different hormone assays were minimized by using the ULN for UFC or considering the percentage variation for the cortisol and ACTH measurements. The strengths of the study include the size of our sample and the number of tests available for statistical analysis.
In conclusion, our data further stress the role of CRH and HSDDT in the differential diagnosis of ACTH-dependent CS. They are easy to perform in outpatients, with virtually no side effects. The criteria for diagnosing CD are an ACTH increment >72.4 % during the CRH test and a cortisol suppression >52.7 % after HDDST. Patients with these features can avoid undergoing BIPSS irrespective of their MRI findings. Patients with two negative responses have a very strong chance of having an ectopic tumor, especially in cases with severe disease and high ACTH and cortisol levels. DDAVP test may play a support role only in case of discordant results of the other two tests.
References
Newell-Price J, Bertagna X, Grossman AB, Nieman LK (2006) Cushing’s syndrome. Lancet 367(9522):1605–1617
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93(5):1526–1540
Boscaro M, Arnaldi G (2009) Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab 94(9):3121–3131
Obuobie K, Davies JS, Ogunko A, Scanlon MF (2000) Venous thrombo-embolism following inferior petrosal sinus sampling in Cushing’s disease. J Endocrinol Invest 23(8):542–544
Lefournier V, Gatta B, Martinie M, Vasdev A, Tabarin A, Bessou P, Berge J, Bachelot I, Chabre O (1999) One transient neurological complication (sixth nerve palsy) in 166 consecutive inferior petrosal sinus samplings for the etiological diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 84(9):3401–3402
Bonelli FS, Huston J 3rd, Meyer FB, Carpenter PC (1999) Venous subarachnoid hemorrhage after inferior petrosal sinus sampling for adrenocorticotropic hormone. AJNR Am J Neuroradiol 20(2):306–307
Kola B, Grossman AB (2008) Dynamic testing in Cushing’s syndrome. Pituitary 11(2):155–162
Alexandraki KI, Grossman AB (2010) The ectopic ACTH syndrome. Rev Endocr Metab Disord 11(2):117–126
Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK (2005) Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab 90(8):4955–4962
Reimondo G, Paccotti P, Minetto M, Termine A, Stura G, Bergui M, Angeli A, Terzolo M (2003) The corticotrophin-releasing hormone test is the most reliable noninvasive method to differentiate pituitary from ectopic ACTH secretion in Cushing’s syndrome. Clin Endocrinol (Oxf) 58(6):718–724
Suda T, Kageyama K, Nigawara T, Sakihara S (2009) Evaluation of diagnostic tests for ACTH-dependent Cushing’s syndrome. Endocr J 56(3):469–476
Luque RM, Ibáñez-Costa A, López-Sánchez LM, Jiménez-Reina L, Venegas-Moreno E, Gálvez MA, Villa-Osaba A, Madrazo-Atutxa AM, Japón MA, de la Riva A, Cano DA, Benito-López P, Soto-Moreno A, Gahete MD, Leal-Cerro A, Castaño JP (2013) A cellular and molecular basis for the selective desmopressin-induced ACTH release in Cushing disease patients: key role of AVPR1b receptor and potential therapeutic implications. J Clin Endocrinol Metab 98(10):4160–4169
Tsagarakis S, Tsigos C, Vasiliou V, Tsiotra P, Kaskarelis J, Sotiropoulou C, Raptis SA, Thalassinos N (2002) The desmopressin and combined CRH-desmopressin tests in the differential diagnosis of ACTH-dependent Cushing’s syndrome: constraints imposed by the expression of V2 vasopressin receptors in tumors with ectopic ACTH secretion. J Clin Endocrinol Metab 87(4):1646–1653
Barbot M, Albiger N, Koutroumpi S, Ceccato F, Frigo AC, Manara R, Fassina A, Gardiman MP, Scanarini M, Mantero F, Scaroni C (2013) Predicting late recurrence in surgically treated patients with Cushing’s disease. Clin Endocrinol (Oxf) 79(3):394–401
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88(12):5593–5602
Aron DC, Raff H, Findling JW (1997) Effectiveness versus efficacy: the limited value in clinical practice of high-dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab 82(6):1780–1785
Aytug S, Laws ER Jr, Vance ML (2012) Assessment of the utility of the high-dose dexamethasone suppression test in confirming the diagnosis of Cushing disease. Endocr Pract 18(2):152–157
Isidori AM, Kaltsas GA, Mohammed S, Morris DG, Jenkins P, Chew SL, Monson JP, Besser GM, Grossman AB (2003) Discriminatory value of the low-dose dexamethasone suppression test in establishing the diagnosis and differential diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 88(11):5299–5306
Newell-Price J, Morris DG, Drake WM, Korbonits M, Monson JP, Besser GM, Grossman AB (2002) Optimal response criteria for the human CRH test in the differential diagnosis of ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab 87(4):1640–1645
Pecori Giraldi F, Invitti C, Cavagnini F (2001) Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-pituitary-adrenal axis. The corticotropin-releasing hormone test in the diagnosis of ACTH-dependent Cushing’s syndrome: a reappraisal. Clin Endocrinol (Oxf) 54(5):601–607
Ritzel K, Beuschlein F, Berr C, Osswald A, Reisch N, Bidlingmaier M, Schneider H, Honegger J, Geyer LL, Schopohl J, Reincke M (2015) ACTH after 15 min distinguishes between Cushing’s disease and ectopic Cushing’s syndrome: a proposal for a short and simple CRH test. Eur J Endocrinol 173(2):197–204
Malerbi DA, Mendonça BB, Liberman B, Toledo SP, Corradini MC, Cunha-Neto MB, Fragoso MC, Wajchenberg BL (1993) The desmopressin stimulation test in the differential diagnosis of Cushing’s syndrome. Clin Endocrinol (Oxf) 38(5):463–472
Findling JW, Raff H (1999) Newer diagnostic techniques and problems in Cushing’s disease. Endocrinol Metab Clin North Am 28(1):191–210
Castinetti F, Morange I, Dufour H, Jaquet P, Conte-Devolx B, Girard N, Brue T (2007) Desmopressin test during petrosal sinus sampling: a valuable tool to discriminate pituitary or ectopic ACTH-dependent Cushing’s syndrome. Eur J Endocrinol 157(3):271–277
Tirabassi G, Faloia E, Papa R, Furlani G, Boscaro M, Arnaldi G (2010) Use of the desmopressin test in the differential diagnosis of pseudo-Cushing state from Cushing’s disease. J Clin Endocrinol Metab 95(3):1115–1122
Valéro R, Vallette-Kasic S, Conte-Devolx B, Jaquet P, Brue T (2004) The desmopressin test as a predictive factor of outcome after pituitary surgery for Cushing’s disease. Eur J Endocrinol 151(6):727–733
Losa M, Bianchi R, Barzaghi R, Giovanelli M, Mortini P (2009) Persistent adrenocorticotropin response to desmopressin in the early postoperative period predicts recurrence of Cushing’s disease. J Clin Endocrinol Metab 94(9):3322–3328
Romanholi DJ, Machado MC, Pereira CC, Danilovic DS, Pereira MA, Cescato VA, Cunha Neto MB, Musolino NR, de Mendonça BB, Salgado LR (2008) Role for postoperative cortisol response to desmopressin in predicting the risk for recurrent Cushing’s disease. Clin Endocrinol (Oxf) 69(1):117–122
Terzolo M, Reimondo G, Alì A, Borretta G, Cesario F, Pia A, Paccotti P, Angeli A (2001) The limited value of the desmopressin test in the diagnostic approach to Cushing’s syndrome. Clin Endocrinol (Oxf) 54(5):609–616
Newell-Price J, Perry L, Medbak S, Monson J, Savage M, Besser M, Grossman A (1997) A combined test using desmopressin and corticotropin-releasing hormone in the differential diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 82(1):176–181
Salgado LR, Fragoso MC, Knoepfelmacher M, Machado MC, Domenice S, Pereira MA, de Mendonça BB (2006) Ectopic ACTH syndrome: our experience with 25 cases. Eur J Endocrinol 155(5):725–733
Vilar L, Freitas MC, Naves LA, Canadas V, Albuquerque JL, Botelho CA, Egito CS, Arruda MJ, Silva LM, Arahata CM, Agra R, Lima LH, Azevedo M, Casulari LA (2008) The role of non-invasive dynamic tests in the diagnosis of Cushing’s syndrome. J Endocrinol Invest 31(11):1008–1013
Pecori Giraldi F, Cavallo LM, Tortora F, Pivonello R, Colao A, Cappabianca P, Mantero F, on behalf of the Altogether to Beat Cushing’s Syndrome Group (2015) The role of inferior petrosal sinus sampling in ACTH-dependent Cushing’s syndrome: review and joint opinion statement by members of the Italian Society for Endocrinology, Italian Society for Neurosurgery, and Italian Society for Neuroradiology. Neurosurg Focus 38(2):E5
Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F (1999) Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the hypothalamic–pituitary–adrenal axis. J Clin Endocrinol Metab 84(2):440–448
Funding
This research received no specific Grants from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest to disclose relating to this paper.
Rights and permissions
About this article
Cite this article
Barbot, M., Trementino, L., Zilio, M. et al. Second-line tests in the differential diagnosis of ACTH-dependent Cushing’s syndrome. Pituitary 19, 488–495 (2016). https://doi.org/10.1007/s11102-016-0729-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-016-0729-y