Abstract
Type 2 diabetes is characterized by insulin resistance, elevated blood sugar levels, oxidative stress, chronic inflammation, dyslipidemia, increased angiogenesis, and a multitude of associated complications. The use of herbs in the treatment of diabetes has been a longstanding practice, proving effective in preventing and treating diabetic symptoms. However, the specific molecular mechanisms underlying their effectiveness remain largely unexplored. A versatile class of phytochemicals, the chalcone, serves as precursor to a wide range of flavonoids, obtained naturally and derived synthetically as well as semi synthetically found effective in treating Type 2 diabetes mellitus. Natural chalcones and its plant metabolites were reported effective in treating diabetes and related complications by virtue of their interaction with diverse metabolic targets involved in glucose homeostasis. This review specifically explores studies on such natural chalcones, its plant metabolites and phyto-complexes conducted within the last decade on drug targets of Type 2 diabetes mellitus like α-glucosidase, PTP1B, α-amylase, DPP-IV, aldose reductase, PPARγ.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-insulin dependent diabetes mellitus, often referred to as Type 2 diabetes mellitus (T2DM), is a chronic disease that causes hyperglycemia, hyperinsulinemia, and glycosuria. This condition is linked with other health problems, like obesity, dyslipidemia, and hypertension, which can lead to cardiovascular risks. According to the International Diabetes Federation (IDF), the global prevalence of diabetes among individuals aged 20–79 years was reported to be 10.5% in 2021. Furthermore, the IDF has projected that the number of people with diabetes will reach an estimated 783.2 million worldwide by the year 2045 (Sun et al. 2022). Based on the WHO 2019 Global Health Estimates, diabetes ranked among the top ten leading causes of death and disability globally. In the year 2017 alone, more than 1 million deaths were attributed to T2DM. It also ranked as the ninth leading cause of mortality (Khan et al. 2020). Extracts and phytoconstituents from medicinal plants have a variety of modes of action on several metabolic targets associated with glucose homeostasis. The phytoconstituents like flavonoids, alkaloids, coumarins, polypeptides, triterpenes, polyphenols, and many more have been reported as antihyperglycemics. Each of these groups, known for their effectiveness in managing hyperglycemia, could be regarded as a privileged scaffold. They are known to act by activation of peroxisome proliferator-activated receptor gamma (PPARγ); inhibition of enzymes such as α-glucosidase, protein tyrosine phosphatase 1B (PTP1B), dipeptidyl peptidase- IV (DPP-IV), aldose reductase, and α-amylase; and increased sensitivity and secretion of insulin. They also contribute to decreased insulin resistance, promotion of glucose uptake in adipose tissues and skeletal muscles, and downregulation of gluconeogenesis (Alam et al. 2022; Salehi et al. 2019; Zhang et al. 2021a).
Chalcone, the α, β-unsaturated ketone synthesized by shikimate pathway represents the principal intermediate in the flavonoid biosynthesis pathway. Chalcones exhibit a range of pharmacological effects, including anticancer (Michalkova et al. 2021; Bhilare et al. 2022), cardioprotective (Mahapatra and Bharti 2016), antioxidant (Kozlowski et al. 2007), antimicrobial (Nowakowska 2007), antiviral (Elkhalifa et al. 2021), antitubercular (Bukhari et al. 2013), anti-inflammatory (Mahapatra et al. 2017), immunosuppressant (Arshad et al. 2017; Rudrapal et al. 2021), anti-HIV (Wang et al. 2004), antifungal (Svetaz et al. 2007), antimalarial, (Narender et al. 2005), and many more.
Chemically they are (2E)-1,3-diphenylprop-2-en-1-one which consists of open-chain flavonoid skeleton, where aromatic A & B rings are separated by three carbon ketoethylenic group (Zhuang et al. 2017; Rauter et al. 2018). The three carbon containing chromophore, α, β-unsaturated carbonyl system (–CO–CH=CH–) is responsible for the colour associated with the chalcones. Cis-trans isomerisation of the olefinic bond is responsible for existence of chalcone in either Trans-(E) or Cis-(Z) form. The Cis-(Z) form is unstable compared to the thermodynamically stable Trans-(E) form due to the steric hindrance offered by ring B on the carbonyl group (Evranos Aksöz and Ertan 2011).
Flavonoids are discriminated chemically based on the carbon position of ring ‘C’ to which ring ‘B’ is attached as well as oxidation and unsaturation of ring ‘C’. Isoflavones is a special subclass where ring ‘B’ is attached at position 3 on the ring ‘C’. Rest of other classes like flavones, flavanones, flavonols, dihydroflavonols (flavanonols), and anthocyanins in which ring ‘B’ instead attached to position ‘2’ of the ring ‘C’ (Liu et al. 2021). A flavonoid subclass, aurones are structurally similar to the flavones and have little presence in nature. Aurone chemically are (2Z)-2-benzylidene-1-benzofuran-3(2H)-one and differs from other flavonoids with absence of 6:6 chromane core (Rauter et al. 2018; Mazziotti et al. 2022) (Fig. 1).
Biosynthesis of Chalcone in plants is brought about by a major precursor, phenylalanine and vital enzyme Chalcone synthase (CHS). Biosynthesis of key intermediate chalcone and other subgroups from the flavonoid class is depicted in Fig. 2. Apart from these well known biosynthetic products, numerous Diels–Alder adducts (DAs) derived from flavonoids have been documented in plant flora. In a ground-breaking advancement, recently the research teams led by Gao and Lei have successfully employed an innovative method to discover M. alba Diels-Alderase (MaDA). This enzyme, extracted from Morus alba cell cultures, exhibits the remarkable ability to facilitate the [4 + 2] cycloaddition of chalcones, including morachalcone A, along with diverse polyphenolic natural dienes to produce DAs (Gao et al. 2020). Chalcones as precursors to other metabolites belonging to the flavonoid family are known to be produced by certain plants with genus Alpinia, Angelica, Pongamia, Glycyrrhiza, Artemisia, Humulus, Cleistocalyx, Cedrelopsis, Mallotus, Flemingia, Dorstenia, Broussonetia, Solanum, Nardostachys, Maytenus, Lindera, and Scutellaria (Rozmer and Perjési 2016).
Recent reports have drawn attention to the potential of specific herbs and/or their extracts, which contain chalcones and metabolites, for the treatment of T2DM. These have been studied in in vivo and in vitro models including human volunteers (Table 1). Data available from the recent literature also shows that the majority of investigations have speculated on possible mechanisms for antihyperglycemic activity of the isolated chalcones and/ or their metabolites, using different in vivo and in vitro methods. In light of this, the present review aims to offer insights into natural chalcones and their natural metabolites, along with their proposed biological targets, that have been explored in the treatment of T2DM. A literature search for the present review was conducted using databases such as PubMed central, Scopus, Science Direct, and google scholar using keywords like ‘chalcones’, ‘chalcone metabolites’, ‘flavonoids’, ‘anthocyanidins’, ‘aurones’, ‘T2DM’, drug targets of T2DM, ‘phytocomplexes’, ‘diabetes complications’ and possible combinations of all these keywords, with search limited to last decade i.e. from 2012 onwards.
Before exploring the recent advancements regarding the effectiveness of flavonoids in treating T2DM, let’s briefly overview the diverse mechanisms through which they exert their actions.
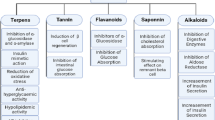
Numerous flavonoids have been documented to effectively manage T2DM through diverse mechanisms, including the management of insulin resistance, modulation of insulin secretion, maintenance of glycemic control, regulation of glucose absorption from the diet, alleviation of inflammation, and management of oxidative stress and associated complications.
Impairment in biological response to insulin stimulation and insulin secretion by the body tissues and cells are the foremost causes that lead to T2DM. Increase in the insulin secretion by insulin secretagogues is known to be associated with closure of K+-ATP channel, consisting of sulfonylurea receptor localized in pancreatic β-cells. When blood glucose levels rise, β cells in the pancreas release insulin, which attaches to its receptor to activate it. This prompts the translocation of glucose transporter-4 (GLUT-4) from storage vesicles to the cell surface, boosting the number of glucose transporters. Consequently, glucose uptake from the bloodstream into cells increases, enhancing insulin sensitivity. GLUT2 and GLUT4 are pivotal proteins involved in glucose homeostasis, each with unique locations and mechanism for maintaining blood glucose balance. GLUT4 facilitates insulin-regulated glucose uptake in insulin-sensitive tissues such as muscle and adipose tissue. GLUT2, on the other hand found in tissues such as the liver, pancreas, small intestine, and kidney, and involved in glucose sensing, regulation of insulin secretion, and absorption of glucose from the gut (Dhankhar et al. 2023; Choudhury et al. 2017; Soares et al. 2017).
The synthesis and release of insulin from pancreatic beta cells are also known to be affected by the endogenous metabolic hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide (GLP). GIP and GLP are incretin hormones that stimulate insulin secretion in response to elevated blood glucose levels. GLP-1, the most potent incretin hormone, is a natural metabolic hormone released by enteroendocrine cells located in the gastrointestinal tract. This hormone, released post meal consumption, augment the synthesis and release of insulin from pancreatic β cells, inhibit glucagon release, slows down gastric emptying, which can help reduce postprandial blood sugar spikes. DPP-IV, a ubiquitous enzyme rapidly metabolize the GLP-1 within 1–2 min after secretion and make it ineffective for its natural function. Thus, the new class of antidiabetics, DPP-4 inhibitors prevent the breakdown of GLP-1, thereby effectively mimicking the action of incretin hormones (Hinnen 2017).
Insulin sensitizers, on the other hand, improve liver and skeletal muscle’s sensitivity to natural insulin, decrease the production of glucose by liver, lower the glucose absorption from the intestine, and also reduce hepatic glucose output by stimulating glycolysis and reducing gluconeogenesis. Insulin sensitizers, also known as human peroxisome proliferator-activated receptor gamma (PPARγ) activators, offer an alternative method to enhance insulin sensitivity and regulate glucose homeostasis. Among the three subtypes of the receptor -α, γ and δ—PPARγ is specific subtype which enhances glucose uptake by skeletal muscles and suppresses the glucose production by delaying the gluconeogenesis. PPARγ is transcription factors that is member of nuclear hormone receptor superfamily, and important adipocyte differentiation marker, which maintain glucose homeostasis by regulating carbohydrate and protein metabolism. Through adipocyte differentiation, PPARγ plays a significant role in energy storage lipogenesis and glucose metabolism, which enhances insulin sensitivity and lowers fatty acid levels (Kozuharova et al. 2017). Dual agonists targeting both PPARα and PPARγ have gained significant recognition in recent diabetes therapy. The rationale behind employing these dual agonists is to mitigate the undesirable effects typically associated with PPARγ agonists, effectively control inflammation, achieve synergistic action, regulate lipid metabolism, and enhance insulin sensitivity (Qaoud et al. 2022; Lebovitz 2019; Balakumar et al. 2019). Similarly, modulation of other adipocyte differentiation markers such as C/EBPα, adiponectin, and Fatty Acid Binding Protein 4 (FABP4) may also contribute to improved insulin sensitivity and reduced inflammation, thereby potentially mitigating T2DM complications (Trojnar et al. 2019).
Insulin signaling is also known to be influenced by the activation of the transcription factor Sterol Regulatory Element-Binding Protein-1c (SREBP-1c), a key regulator of lipid metabolism, which contributes to the development of insulin resistance in T2DM. Inhibition of SREBP-1c activity can lead to beneficial effects by reducing lipid synthesis, improving insulin sensitivity, and mitigating inflammation (Langeveld and Aerts 2009).
Overloading of lipids and glucose, disordered secretion of insulin, oxidative stress, autophagy, adipokines and inflammation can all have an impact on insulin sensitivity. Several flavonoids have been shown to activate AMP- activated protein kinase (AMPK) pathway (via inhibition of IRS1 serine phosphorylation and mTOR-S6K signaling), promote GLUT-4 translocation to improve adipose insulin resistance, increase hepatic insulin signaling, alleviate inflammation pathways (by deactivation of NF-κB/IKK and reduction of JNK phosphorylation), and thereby ameliorate insulin resistance (Zhang et al. 2020; Kuzmenko and Klimentyeva 2016; Chang et al. 2015; Luo et al. 2015; Han et al. 2015; Soares et al. 2017). Glycosphingolipids, a class of lipid and its metabolites, have been documented to inhibit the phosphorylation of the insulin signaling mediator Akt/Protein Kinase B. This interference also contributes to the development of insulin resistance observed in T2DM and associated obesity (Langeveld and Aerts 2009).
PTP1B inhibitors have been reported to ameliorate the insulin resistance and obesity associated with the T2DM. PTP1B is one from the non-receptor protein tyrosine phosphatases (PTPs), ubiquitously expressed in the major target tissues of insulin action. PTP1B has received significant interest because of its crucial involvement in T2DM and obesity, acting as a suppressor of the insulin, and leptin signaling pathways. Thus its inhibitors are considered to be the potential agents for the treatment of T2DM and associated complications including obesity. In insulin resistant states, there is often an upregulation of PTPs, particularly PTP1B. Its negative regulatory actions on insulin signaling promote the dephosphorylation of tyrosine residues on the IR and IRSs, reducing the sensitivity of cells to insulin.
The leptin signaling pathway controls body weight by reducing appetite and increasing energy expenditure. PTP1B attenuates leptin signaling by dephosphorylating Janus kinase 2 (JAK2), a key downstream component of the leptin receptor signaling pathway. This downregulation of leptin signaling can contribute to obesity by reducing the responsiveness of hypothalamic neurons to leptin, leading to increased appetite and decreased energy expenditure (Abdelsalam et al. 2019; Maccari et al. 2007; Zhang et al. 2006; Chen et al. 2002).
The low-grade inflammation resulting from chronic high blood glucose levels is a major risk factor for type 2 diabetes. The association of inflammation in metabolic disease can be seen as altered upregulated expression of the pro-inflammatory cytokines in adipocytes of obese animals, with T2DM being no exception. In T2DM these cytokines are involved in several metabolic pathways related to insulin resistance, reactive oxygen species (ROS) production, lipoprotein lipase activity, and adipocyte function. Additionally, it has been documented that inflammatory cytokines can stimulate the expression of inducible nitric oxide synthase (iNOS), potentially leading to insulin resistance (Farzaei et al. 2019; Perreault and Marette 2001).
NF-κB, known as nuclear factor kappa-light-chain-enhancer of activated B cells, serves as a vital mediator in the inflammatory process, pivotal in regulating the expression of genes associated with inflammation. These genes consist of pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, chemokines like MCP-1, adhesion molecules such as ICAM-1, and anti-apoptotic proteins.
Exposure to TNF-α or elevated free fatty acids triggers the inhibitory serine phosphorylation of IRS-1, thereby reduces its tyrosine phosphorylation level in response to insulin and impairing its binding to the insulin receptor. This blocks downstream signaling and insulin’s effects. Recently, another pro-inflammatory cytokine, IL-6 was reported to inhibit insulin signal pathways in liver cells, which is known to be released by a number of tissues, mainly adipose tissue, and it causes insulin resistance by downregulating the expression of GLUT-4 and IRS-1. This is driven by IL-6-mediated activation of the JAK–STAT signaling pathway and increased level of suppressor of cytokine signaling 3 (SOCS-3). IL-6 causes inhibition of SOCS-3 auto-phosphorylation, phosphorylation of IRS-1 at its tyrosine residue, decreased binding affinity between the p85 subunit of phosphoinositide-3-kinase (PI3K) and IRS-1, and subsequently inhibits the activation of the Akt pathway.
Studies have also indicated that the activation of the Mitogen-activated protein kinases (MAPK) signaling pathway initiates the activation of transcription factors such as NF-κB, resulting in the elevation of pro-inflammatory mediators like IL-1β, IL-6, and TNF-α. Thus, suppression of the level of serum TNF-α and IL-6 could ameliorate the insulin resistance complications (Kerimi and Williamson 2016; Chen et al. 2015; Carey and Febbraio 2004).
Numerous studies have demonstrated that flavonoids inhibit the activity of digestive enzymes, such as α-glucosidase and α-amylase, thereby reducing glucose absorption through the gastrointestinal tract. Small intestinal brush-border membrane of enterocytes and saliva contains key enzymes like alpha-glucosidase and alpha-amylase respectively, which play vital role in carbohydrate metabolism. In the sequence of events after the carbohydrate meal, firstly glycogen and starch are hydrolyzed at α (1 → 4) bonds to form maltose. This hydrolysis is catalysed by salivary α-amylase in the mouth, followed by hydrolysis of non-abosorbable complex carbohydrate into glucose by the action of α-glucosidase in small intestine (Hanhineva et al. 2010). Oral anti-diabetic medications, functioning as inhibitors of α-glucosidase and α-amylase, work by impeding the absorption of glucose in the gastrointestinal tract, thereby reducing the surge in postprandial hyperglycaemia (Derosa and Maffioli 2012).
Flavonoids also have been reported to achieve the glycaemic control by inhibiting the human Sodium Glucose Co-transporters. Glucose uptake from the diet across the small intestinal cells to the blood is exhibited by the two major glucose transporters, sodium-coupled glucose transporter 1 (SGLT1) and GLUT2, at fasting and fed states respectively. Additionally, the sodium-coupled glucose transporter 2 (SGLT2), located on early proximal convoluted tubule of kidney actively facilitate tubular reabsorption of glucose from urine, contributing to the maintenance of glycaemic parameters (Ebbeling et al. 2003; Hsia et al. 2017). The presence of SGLT1 in the latter portion of the proximal tubule is responsible for reabsorbing the remaining filtered glucose. Thus, inhibition of SGLT2 and SGLT1 in the proximal tubule reverses the normal glucose reabsorption and fosters the glucose excretion in the urine, helping to maintain glucose levels in the blood (Tentolouris et al. 2019). Intestinal SGLT1 operates against a concentration gradient, in coordination with the facilitated diffusion via GLUT2. SGLT1 inhibitors in the initial segment of the intestine decreases glucose absorption (Kellett et al. 2008).
Hyperglycaemia and high free fatty acid induced ROS/reactive nitrogen species (RNS) accumulation play pivotal role in β-cell dysfunction. This leads to development of insulin resistance, obesity induced T2DM, and development of diabetes associated vascular complications. Thus one of the strategies to treat diabetes and related complications is the reduction of oxidative stress by suppressing formation of ROS, which may prevent endothelial inflammation, the major cause of complication of diabetes. The complications of chronic diabetes, which essentially cannot be controlled by insulin, include microvascular complications like retinopathy, neuropathy, and nephropathy, while macrovascular complications like peripheral, cerebrovascular, and ischemic heart disease (Styskal et al. 2012; Dai and Mumper 2010).
Flavonoids have also been found effective in treating T2DM related complications by multiple mechanisms. The formation of advanced glycation end-products (AGEs) constitutes a significant contributing factor to the pathogenesis of T2DM and its associated complications. The release of pro-inflammatory cytokines and the generation of free radicals are promoted by the accumulation of AGEs in tissues. Under hyperglycaemic conditions, glucose uptake in the retina, kidneys, peripheral nerves, and lens of the eye occurs independently of insulin action. AGEs are generated through nonenzymatic glycation via the polyol pathway, originating from the key intermediate 3-deoxyglucosone (3-DG). In this pathway, Aldose reductase (AR) catalyzes a NADPH-dependent reaction, using NADPH as a cofactor, to convert excess glucose into sorbitol. Since sorbitol being less permeable through cell membranes, it gets accumulated in the cells. In addition, the fructose, which is formed from oxidized sorbitol, also accumulates into the cells. This accumulation increases the NADH/NAD + ratio and thereby create pseudohypoxia condition. The accumulation of sorbitol and its metabolic end products may also result in increased cellular osmolarity, thereby causing cellular damage in diabetes. Thus, the use of inhibitors of AR is one strategy to treat diabetic complications (Mylari et al. 2005; Kawanishi et al. 2003; Mylari et al. 2003; Miyamoto 2002; Nishikawa et al. 2000).
NADPH oxidase (NoX), a membrane-bound enzyme, generates ROS in T2DM. Overexpression of NoX is linked to the activation of various pathways including advanced glycation end products, protein kinase C, polyol pathway, and the hexosamine pathway. It also stimulates the deposition of collagen activation of inflammatory cytokines, and endothelial growth factors. Consequently, overexpression of NoX contributes to diabetic complications such as cardiomyopathy, nephropathy, retinopathy, and neuropathy (Laddha and Kulkarni 2020).
Furthermore, the excessive activation of VEGFR-1 and VEGFR-2 by VEGF-A (Vascular Endothelial Growth Factor A) can indeed contribute to similar complications (Wirostko et al. 2008). ROS-induced protein carbonylation, the oxidative modification of protein is also implicated in various complications of diabetes (Althunibat et al. 2019).
Flavonoids are known for their ability to activate radical scavenging enzymes, not only alleviating inflammation but also exhibiting significant antioxidant activity by scavenging free radicals. This is attributed to their ability to increase levels of antioxidant defensive enzymes such as glutathione peroxidase (GPx), glutathione (GSH), serum catalase (CAT), glutathione reductase (GRx), and superoxide dismutase (SOD) in β-cells. ROS attack on lipids leads to their oxidative degradation, resulting in the generation of various byproducts such as malondialdehyde (MDA), thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides (LH), and 4-hydroxy-2-Nonenal (4-HNE). Among these, MDA and 4-HNE are significant aldehydic metabolites known for their harmful effects, contributing to the development of T2DM and atherosclerotic cardiovascular disease in individuals with T2DM. The antioxidant defensive enzymes present in adipose tissues, decrease the level of lipid peroxidation thereby reduce level of serum MDA, a product of fatty acid peroxidation (Shabalala et al. 2022; Matsuda and Shimomura 2013; Sajeeth et al. 2010).
A vital role in cellular antioxidant defense is played by the Nrf2/Keap1/ARE pathway. Dysregulation of this pathway is linked to the development of T2DM. In T2DM, impaired Nrf2 activation and reduced expression of its target genes increase susceptibility to oxidative stress and inflammation. Modulating the altered Nrf2/Keap1/ARE pathway shows promise as a therapeutic approach for managing T2DM and its complications. This modulation can enhance antioxidant defenses and decrease tissue damage associated with T2DM (David et al. 2017).
In T2DM, a combination of factors including aging, obesity, insulin resistance, inflammation, and oxidative stress can lead to the diminishment of Sirtuin 1 (SIRT1) activity. Restoration of SIRT1 plays a multifaceted role in T2DM and its complications by promoting glucose uptake in skeletal muscle and adipose tissue, reducing insulin resistance, exhibiting anti-inflammatory properties, and regulating oxidative stress (Kitada et al. 2019).
Overall, the antioxidant effect help repair the radical that causes biological damage associated with T2DM. The antioxidant effects discussed earlier do not exclusively target diabetes but rather alleviate the complications associated with Type 2 diabetes mellitus.
The subsequent sections of the review explore the potential of different flavonoids in addressing T2DM and its related complications through one or more of the mechanisms previously discussed.
Targets of T2DM on which chalcone and its metabolites act
Control over insulin resistance and regulation of insulin secretion
The chalcone 4′-dihydroxy-4-methoxydihydrochalcone (8), extracted from Artemisia dracunculus L., restored insulin sensitivity in a palmitic acid-treated insulin resistant rat skeletal muscle cell line. It acted by downregulating the accumulation of glycosphingolipid metabolites that are instrumental in the development of insulin resistance (Obanda et al. 2014).

Dimethylchalcone (DMC), 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone (9), was investigated beyond its known role in inhibiting glucose absorption from the small intestine. It was assessed for its potential effects on insulin release and the viability of pancreatic islets using insulin secretion assays and MTT assays of MIN6 cells, respectively. Cells pretreated with compound 9, which was separated from Cleistocalyx operculatus (Roxb.) Merr. et Perry dried flower buds, demonstrated remarkable and dose-dependent amelioration of H2O2-induced impaired glucose responded insulin secretion. Additionally, it restored the secretion of insulin in simulated basal insulin secretion mode. Compound 9, at highest dose of 25 μM, showed 54% improvement in glucose-simulated insulin secretion and statistically significant MIN6 cell viability (Hu et al. 2012).

Further investigation of compound 9 was conducted for insulinotropic effect in glucotoxicity-induced RIN-5F β-cells. Compound 9 at 2 μM concentration, showed 63% improvement in insulin secretion and significantly expressed mRNA levels of GLP-1R, GLUT2-GCK, PRE-INS, and PDX-1 under the glucotoxicity condition of 33 mM glucose. RIN-5F cells, kept under the same glucose condition for 48 h, showed a dose-dependent increase in glucose-stimulated insulin secretion after treatment with 9 at 20 μM. This increase was attributed to a 3.3-fold rise in PDX-1 expression, as confirmed from western blotting analysis. Moreover, it was discovered that it notably reduced MCP-1 mRNA expression and iNOS mRNA expression, consequently mitigating oxidative stress and inflammatory damage induced by glucotoxicity. (Hu et al. 2014a, b).
Compound 9 also demonstrated to promote glucose uptake in L6 rat skeletal muscle cells and 3T3-L1 adipocytes. Only the insulin sensitive 3T3-L1 adipocytes reported to promote significantly (78%) the glucose uptake at 10 μM as compared with the insulin at concentration of 100 nM (102%). Compound 9 at 10 and 20 μM concentration did not show the effect on glycogen synthesis in HepG2 hepatocytes. Study also revealed that it demonstrated a paradoxical effect on both lipid accumulation and cell differentiation attributed to the regulated expression of PPAR-γ. At higher doses of 10 and 20 μM, compound 9 resulted in a notable reduction in lipid accumulation. However, at a lower dose of 2.5 μM, it significantly increased the gene expressions of adipocyte differentiation markers, namely C/EBP-α and PPAR-γ. This effect of DMC in 3T3-L1 adipocytes was further reported to be in line with enhanced adiponectin production (56%) during cell differentiation at 2.5 μM and comparable with positive control Pioglitazone (1 μM/ 59%). It was reported that the increase in lipid content takes place through the regulation of PPAR-γ, as confirmed by Western blotting analysis. At a low dose of 2.5 μM, a significant 40% enhancement in the protein expression of PPAR-γ was reported, which is comparable to a 30% increase observed with pioglitazone (Hu et al. 2014a, b).
Flavonoids from licorice were studied for their cellular activity on the insulin transduction pathway by evaluating Akt-phosphorylation level in HepG2 cells using Western blot technique. All the studied flavonoids were also studied for their potential to inhibit PTP1B (separately discussed under PTP1B inhibitors). All the PTP1B inhibitor flavonoids under study showed different cellular activity in the insulin-signaling pathway. Glycybenzofuran (10), a weak PTP1B inhibitor after insulin stimulation was found to be excellent anti-insulin-resistant candidate. It exhibited a concentration-dependent rapid rise in phosphorylated Akt levels. In contrast concentration-independent increase in the pAkt was noted for Licoagrone (27). Another flavonoid, Licoagroaurone (29) showed concentration-dependent decrease in pAkt level (Li et al. 2013).

Beer derived polyphenolic constituents, 8-prenylnaringenin (11) and xanthohumol (12), were investigated for their effect on the lipid and carbohydrate metabolism in a C57Bl/6 mice model of T2DM. With a decrease in lipogenesis and the prevention of weight gain, significant improvements in glucose tolerance and insulin resistance have been described for both the compounds. High Fat Diet (HFD) fed animals treated with compound 11 and 12 demonstrated enhanced insulin sensitivity and glucose tolerance as confirmed from the intraperitoneal insulin tolerance test and oral glucose tolerance test. This improvement resulted in reversal of insulin resistance and sensitivity indices when compared to the mice fed with HFD and ethanol. Both the compounds reported to activate AMPK, the crucial regulator of both glycolytic and lipid metabolism in the hepatic and skeletal muscles, as a result of inhibition of expression of SREBP-1c. Increased insulin sensitivity was reported to be in association with the activation of AMPK and associated SREBP-1c expression and its downstream lipogenesis enzyme inhibition. Modulation of insulin action was also found to be reported as a result of reduced hepatic and skeletal muscle PFKFB3 expression. This reduction was followed by increased phospho-AS160 expression due to the activation of PI3K/Akt signaling pathway. Ultimately, this signaling pathway led to the translocation of GLUT4 membrane and an improvement in glucose uptake. Apart from improving insulin sensitivity and modulation of insulin action, both the 11 and 12 ameliorated the T2DM associated metabolic complications by reducing the hepatic and muscle TG (triglycerides) and cholesterol content as a result of down-regulation of expression in VEGFR-1, VEFG-B, and/ or CD36 (Costa et al. 2017a, b).

The compound Phloretin (13) exhibited a hypoglycemic effect in STZ-induced type 2 diabetes rats after a four-week treatment regimen, employing a dosage of 100 mg/kg. Additionally, in vitro studies conducted on L6 myotubes also supported this finding.The treatment was found to increase the expression levels of GLUT4, Insulin Receptor substrate (IRS-1), PI3K, and Akt, as confirmed by the results of Western blot analysis. GLUT4 translocation in L6 myotubes was confirmed using confocal laser scanning fluorescence microscopy assay. The compound 13 reported to induce insulin sensitization by promoting increased glucose consumption through GLUT4 translocation and activation of the Akt/PI3K pathway, as a result of the compound’s ability to activate IRS-1 (Shen et al. 2017).

Extracts derived from black currant and green currant demonstrated a dose-dependent inhibition of glucose transport across the small intestine when tested with CaCo-2 cells. Potential impact of phenolics, anthocyanin glycosides, and their aglycone counterparts on the mRNA expression levels of sodium-independent GLUT5, Glucose Transporter Type-2 (GLUT2), and SGLT1 was examined. Anthocyanins or anthocyanidins were found ineffective in inhibition of glucose transporter mRNA expression (Barik et al. 2020).
The antihyperglycemic and antihyperlipidemic activity of Licochalcone E (49e) was examined after isolating it from the roots of Glycyrrhiza inflata. In in vitro studies 3T3-L1 preadipocyte and C3H10T1/2 multipotent stem cell lines treated with the different concentration of Lico E resulted in induction of adipocyte differentiation at concentration upto 10 μM, as confirmed from the microscopic examination and spectrophotometric quantification of the stains. Histopathological studies further confirmed marked reduction in size of epididymal white adipose tissue (eWAT) or perirenal white adipose tissue (PWAT) in 49e treated five week old male C57BL/6 J mice. RT-PCR studies confirmed that both the adipocyte differentiation and decrease in size of WAT is associated with the increased PPARγ mRNA expression with 1.72 -fold and 1.78-fold in PWAT and eWAT respectively. PPARγ transactivation was further studied using luciferase activity to prove the agonistic behaviour of compound 49e. Studies confirmed that 49e exhibits weak partial PPARγ agonistic activity, with almost 10% transactivation, comparable to that of rosiglitazone used as a control. The Western blotting results demonstrated a significant increase of 3.4-fold in Akt signaling in eWAT. The observed increase in Akt signaling in eWAT is linked to the up-regulation of PPARγ mRNA expression, facilitation of adipogenic differentiation, and an increase in the number of small adipocytes. It has been reported that these effects play a role in regulating hyperlipidemia and hyperglycemia (Park et al. 2012).
Protein tyrosine phosphatase-1B (PTP1B) inhibitors
Prenylated phenolics isolated from Glycyrrhiza inflata, namely 14 (licoagrochalcone A), 15 (kanzonol C), 16 (2’-hydroxyisolupalbigenin), 17 (gancaonin Q), 18 (glisoflavanone), and 19 (glabrol) exhibited remarkable PTP1B inhibition, with IC50 values ranging between 0.31 and 0.97 µM.
The phenolic compounds with the highest inhibitory potency against PTP1B, namely 14, 15, 16, 17, 18, and 19, all share a common characteristic of having at least one isoprenyl group on ring A and/ ring B of the respective flavonoid core. These compounds, when compared to their non-prenylated derivatives, exhibit relatively stronger inhibition. (Lin et al. 2017). The conclusions drawn by Lin and co-workers supports the SAR of chalcones as studied earlier for PTP1B inhibitory chalcones and semisynthetic derivatives of licochalcone A (Goo Yoon et al. 2009).

Chalcone derivatives, specifically morusalbins A − D and albanin T, along with various phenolics from the root bark of Morus alba L. (Moraceae), demonstrated PTP1B inhibitory activity. Among the isolated compounds, Morusalbin D (20) was identified as a potent PTP1B inhibitor, exhibiting significantly greater potency with an IC50 value of 1.90 ± 0.12 μM. This was in comparison to the positive control, ursolic acid, which had an IC50 value of 9.47 ± 0.47 μM. Non-competitive PTP1B inhibitory potential of morusalbin D (20), macrourin G (21), and albasin B (22) was reported to be confirmed from kinetic studies with Ki values of 1.09, 1.00 and 0.33, and μM, respectively. Negative binding energies of these DAs as a output of molecular docking studies, when posed with PTP1B receptor in the same pocket, confirmed the tight binding and high affinity to its active site. Strong PTP1B inhibitory potential of compound 20 in comparison to 21 and 22 was noted because of strong hydrogen H-bonding with a key allosteric residue, Asn193, at the hetero oxygen of ring E, confirming the potency of ketalized DA. All the three compounds interacted with same allosteric residues of PTP1B, Phe280 (in the α7 helix) and Arg199, Phe196, Pro188, Leu192, and Ala189 (in α3 helix) through hydrophobic interactions. In the same study, the compounds mentioned earlier were examined for their inhibitory activities against α-glucosidase, which are discussed in a separate section dedicated to α-glucosidase inhibitors (Ha et al. 2018).

Ketalized DAs (Morbilisin A-H) and 2-arylbenzofurans (chalcomoracin) from leaves of Morus notabilis were examined for their inhibitory activity against PTP1B, providing insights into their potential as inhibitors for this enzyme. Morbilisin A (23), Morbilisin E (24), Morbilisin G (25a), Morbilisin H (25b), and chalcomoracin (26) were reported as more potent inhibitors having IC50 values ranging between 1.5—2.3 µM, comparable to reference standard oleanolic acid (3.0 µM). Compound 23 and 26 were found to be most potent amongst the isolates (Wang et al. 2015).

The flavonoids obtained from the hairy root cultures of Glycyrrhiza pallidiflora, G. glabra, G. uralensis, as well as from the leaves and roots of G. uralensis, specifically biflavonoids, licoagrone (27), licoagrodin (28), prenylated chalcone isobavachalcone (30), prenylated aurone licoagroaurone (29), and glycybenzofuran (10) were reported to have PTP1B inhibitory potential. Their IC50 values ranged between 6.0 µM and 23.9 µM when compared with positive control RK- 682 (IC50 4.6 µM). Based on kinetic analysis using different concentrations of potent flavonoids, compound 27 and compound 29 were identified as non-competitive and mixed-type inhibitors of PTP1B, respectively. This determination was made based on their Ki values of 24.0 µM and 6.3 µM, respectively, using the p-nitrophenyl phosphate (pNPP) substrate. Glycybenzofuran (10), a weak inhibitor reported as competitive PTP1B inhibitor with excellent cellular activity and inhibitory selectivity. Compound 27 and 28 were reported as most potent inhibitors amongst all isolates. PTP1B inhibition demonstrated by licorice flavonoids 10, 27, 28, 29 and 30 were reported to be attributed to the presence of prenyl groups and hydroxy moieties in the ortho position (Li et al. 2013).

Amongst the isolated flavonoids from Glycyrrhiza uralensis Fisch, flavonols and chalcones were found to be more potent than the isoflavones in inhibiting PTP1B and α-glucosidase. Among the isolates, licoflavone B (31) exhibited the highest potency with an IC50 value of 15.62 ± 0.20 µM. This IC50 value was lower than that of the standard NaVO4 (29.10 ± 0.20 µM), indicating its superior inhibitory activity. Interestingly the total flavonoid extract displayed comparable activity with an IC50 value of 15.84 µg/mL, showcasing its significant inhibitory potential. Isoflavones were less potent in comparison to flavonols and chalcones for PTP1B inhibition. The prenyl or variant prenyl function on ring B is reported to be responsible for strong PTP1B inhibition by potent chalcones and flavonols (Guo et al. 2015). In a subsequent section, the α-glucosidase inhibitory activity of the flavonoids is addressed separately, providing detailed insights into their potential as inhibitors of this enzyme.

NPs that inhibits the digestive enzymes: α-glucosidase and α-amylase inhibitors
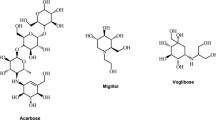
The potential of Bauhinia pulla wood and leaf extracts, along with eight isolated compounds belonging to the flavonoid, chalcone, steroid, and steroidal glycoside classes, was investigated for their ability to inhibit α-glucosidase. Bioactives were reported to be isolated by bioassay guided fractionation after screening the extracts for inhibitory potential. Among the isolated compounds, quercetin (32) was strong inhibitor of enzyme with an IC50 value of 5.41 µg/mL, compared to acarbose, which had IC50 of 124.11 µg/mL. The chalcone 4-methyl ether isoliquiritigenin (33) showed better inhibition in comparison to the 5-deoxyluteolin (34) and 3, 2’,4’-trihydroxy-4-methoxychalcone (35). Molecular docking studies were performed to compare the molecular interactions of flavonoids and its precursor chalcone. It was confirmed from the docking studies that the binding site for both chalcone and flavonoid from Bauhinia pulla is same. It was reported further that the number and the location of the hydroxy group affects the alpha- glucosidase activity. This confirmation was reported based on the observed reduction in inhibitory potential when modifying or removing the -OH group from positions 5, 7, or 8 of the ring A and position 3 of the ring C in flavonoids. Conversely, the inclusion of a hydroxy group on the 3- or 5-positions of B ring in chalcones resulted in a decrease in α- glucosidase activity (Dej-Adisai et al. 2021).

Ethanolic extracts of Syzygium aqueum leaf and the six individual flavonoids demonstrated significantly stronger inhibition of α-amylase and α-glucosidase compared to the positive control acarbose. The six compounds isolated are namely myricetin-3-O-rhamnoside (36a), europetin-3-O-rhamnoside (36b), phloretin (37a), myrigalone-B (37b), myrigalone-G (37c), and 4-hydroxybenzaldehyde (38). Amongst the isolated flavonoids, compound 36a and 36b showed highest α-glucosidase and α-amylase inhibitory activity with EC50 values of 1.9 µM and 1.1 µM against α-glucosidase, while 2.3 µM and 1.9 µM against α-amylase, respectively. Amongst the isolated dihydrochalcones, 37c showed highest α-glucosidase inhibition activity (7 ± 1.4 µM), and 37a showed good α-glucosidase inhibition with EC50 of 20 ± 2.2 µM, when compared to quercetin (27 ± 9.9). Compound 37b exhibited significant inhibition of α-amylase with an IC50 value of 8.3 ± 1.3 µM, whereas the standard quercetin demonstrated an IC50 value of 17 ± 7.3 µM (Manaharan et al. 2012).

Flavonoids, specifically morusalbin D (20), albasin B (22), macrourin G (21), and yunanensin A (39), isolated from the root bark of Morus alba L. (Moraceae), demonstrated inhibitory activity against α-glucosidase. Contrary to the observed noncompetitive inhibition displayed by the DAs towards PTP1B (as discussed under PTP1B inhibitors), all of the studied DAs exhibited competitive inhibition of α-glucosidase. Competitive inhibition of α-glucosidase by all these compounds was confirmed from the kinetic studies with Ki values of 1.19, 0.64, 2.42, and 0.42 μM, respectively. In-silico studies disclosed that these DAs exhibit tight binding and high affinity for α-glucosidase. This was confirmed from binding interactions with vital catalytic residues such as His280, Asp215, Gln279, Glu277, His112, Asp352, and Asp242 (Ha et al. 2018).

Ethyl acetate extract and 25 individual phenolic compounds obtained from mulberry fruit (Morus alba L.) showed α-glucosidase inhibitory activity. Isolated polyphenols include eight flavonoid glycoside, three chalcones, three derivatives of flavanonols or flavanones, eight derivatives of phenolic acids, pyrocatechol, 5,7-dihydroxychromone, and tyrosol. Chalcone derivatives, flavanones, flavanols, quercetin (32), and isobavachalcone (30) were claimed as potential candidates compared to acarbose (IC50 = 119.15 ± 4.90 µM) as reference standard. Most potent compound 32 inhibited the enzyme with IC50 of 8.57 ± 57 µM. Morachalcone (40) and compound 30 from chalcone class (structure as under PTP1B inhibitors) were reported to be less potent inhibitors than compound 32 but significant inhibitors than positive control acarbose, with IC50 value of 49.96 ± 18 µM and 67.30 ± 51 µM, respectively. Weaker inhibitory potential was observed with compounds 41, 42a-42 h, and 43a-43b than acarbose. It was further concluded that hydroxyl on the 3- and/ or 7- position on ring ‘C’ and ‘A’ respectively, and more number of hydroxyl group on ring ‘B’ of flavonoid core increases inhibitory potential. On the contrary at the same positions glycosylation of one or both the hydroxyls reverse the inhibitory potential (Wang et al. 2013).

Polyphenols such as huperolides A, B, and C (Compounds 44–46, respectively), which are derivatives of dihydrochalcones, were isolated from the Malus hupehensis (Pamp.), a plant native to China. Additionally, known phytoconstituents from crabapple tea were also separated and inhibitory activity of these compounds against α-glucosidase was studied, with acarbose used as a positive control. Newly isolated dihydrochalcone derivative, huperolides C (45b) showed equivalent inhibitory potential as compared to acarbose, while phlorizin, a 2′-glucoside of phloretin (60) and 3-hydroxyphloridzin (46), significantly inhibited α- glucosidase in concentration-dependent manner. Excellent inhibitory activity was found with compound 46 amongst the all isolates and even better than acarbose with IC50 of 39.03 µg/ mL. On the other hand huperolides A (44) and atropisomer huperolides B (45a) were reported as weak inhibitors (Lv et al. 2019).

C-glycosylbioflavonoids, isovitexin (47) and vitexin (48) from the butanolic leaves extract of Ficus deltoideav Jack (Moraceae), showed the in vivo α-glucosidase inhibition in sucrose loaded STZ-induced male Sprague–dawley rats, with IC50 of 6.7 and 4.1 µg/mL respectively, compared to acarbose (IC50 = 4.3 × 10–2 µg/ml) as reference standard. Both the flavonoids reported to be effective in reducing glucose level in the blood of sucrose loaded diabetic rats at oral dose of 100 mg/kg and 200 mg/kg, respectively. Butanolic extract and the fractionated butanolic extract showed 55 and 76.9% inhibition of α-glucosidase, from which active constituents were isolated (Choo et al. 2012).

The α-glucosidase inhibitory activity of eighteen flavonoids, derived from Glycyrrhiza uralensis Fisch, belonging to the classes of flavones, chalcones, and isoflavones, were examined in separate study. In vitro studies on the isolated flavonoids revealed that twelve compounds showed strong α-glucosidase inhibition than positive control acarbose having IC50 value of 5.42 ± 0.10 µg/mL. Chalcones out of all the flavonoids, were reported as strong inhibitor at the dose of 5 µg/mL, in contrast to the isolated isoflavones and flavones. It is pertinent to note that the tested total flavonoid extract also showed up to 95% inhibitory activity, which is equivalent to that of the isolated flavonoids at same concentration. The compounds that showed promising inhibition are licochalcone A (49a), licochalcone B (49b), licochalcone C (49c), licochalcone D (49d), licochalcone E (49e), genistein (50a), eurycarpin A (50b), luteone (50c), 4′,7-dihydroxyflavone (51a), licoflavone C (51b), licoflavone B (51c), and glabrol (52). The findings from the in vitro study of these compounds indicated that the combined presence of hydroxy and prenyl functional groups contributed to the observed inhibitory activity, rather than solely relying on either the hydroxy or prenyl group alone. Isoflavones and flavonols, although found active, were reported weaker inhibitors than chalcones (49a-49e). This is particularly pertinent to the formation of ring by prenyl and the position of hydroxy group, specifically at C-5 for isoflavones and flavonols and C-2’ for chalcones (Guo et al. 2015).

Compound 9, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone from the Cleistocalyx operculatus (Roxb.) Merr. et Perry (Myrtaceae) dried flower buds, was separated and screened for α-glucosidase and α-amylase inhibitory potential. It showed strong pacreatic α-amylase inhibition by noncompetitive way in dose dependent manner with IC50 of 43 μM, as compared to acarbose and showed merely 20% inhibition against intestinal α-glucosidase (Hu et al. 2012).
Anthocyanins extracted from blueberries, blue honeysuckle, and blackcurrants, as well as the corresponding anthocyanidins obtained through acid hydrolysis were investigated for their α-glucosidase inhibitory potential. Blackcurrant anthocyanidins and anthocyanins reported with highest α-glucosidase inhibitory potential than others. Reported IC50 values of purified anthocyanidin and anthocyanin rich extract from blackcurrant were 0.005 ± 0.00 and 0.152 ± 0.05 mg/mL, which were significantly stronger than blue honeysuckle (0.025 ± 0.00 and 0.188 ± 0.10 mg/mL respectively). Anthocyanidin-rich extract containing cyanidin (53a) and delphinidin (53b) from blackcurrant, and Cyanidin (53a) from blue honeysuckle showed greater α-glucosidase inhibitory potential than corresponding cyanidin glycoside (53c) and delphinidin glycoside (53d) (Zhang et al. 2019).

Pelargonidin-3-O-rutinoside (54a), isolated from the strawberries, was reported as potent inhibitor of α-glucosidase and found to suppress the postprandial hyperglycemia, as confirmed from the in vivo oral maltose and oral sucrose tolerance tests using ICR mice model. Eighteen monomeric anthocyanins containing anthocyanidins like pelargonidin, malvidin, cyanidin, delphinidin, peonidin, and petunidin linked with the glycosyl function like glucoside, arabinoside, sambubioside, galactoside, and rutinoside were isolated using combination of HPLC and high-speed countercurrent chromatography (HSCCC). Compound 54a was reported as active amongst all, and inhibited the α-glucosidase with IC50 of 1.69 mM, in comparison to the positive control acarbose with IC50 356.26 mM. Other anthocyanins like pelargonidin-3-O-glucoside (54b), delphinidin-3-O-galactoside (54c), delphinidin-3-O- arabinoside (54d), delphinidin-3-O-glucoside (54e), delphinidin-3-O-rutinoside (54f), petunidin-3-O-galactoside (54 g), petunidin-3-O-arabinoside (54 h), petunidin-3-O-glucoside (54i), malvidin-3-O-galactoside (54j), malvidin-3-O-arabinoside (54 k), malvidin-3-O- glucoside (54 l), peonidin-3-O- galactoside (54 m), and peonidin-3-O- arabinoside (54n) also showed better α-glucosidase inhibition than positive control with IC50 ranging between 10.35 to 234.40 mM. Circular dichroism (CD) spectroscopic analysis revealed that active 54b, 54a, and 54 k are responsible for the loss of the α-helix structure of α-glucosidase, which in turn inhibits normal substrate enzyme binding. Weak α-glucosidase inhibitor delphinidin-3-O-sambubioside, with sambubioside as glycosyl moiety at R3 position showed slight alteration in α-helix structure.
The activity data presented in the study establish a clear structure–activity relationship, indicating that inhibitory potential is influenced by the presence of anthocyanidins, with the order of effectiveness being pelargonidin > malvidin > peonidin > delphinidin > petunidin > cyanidin (Xu et al. 2019).

In a study aimed at confirming the role of phenolics in managing postprandial glycaemia, inhibitory activities of green currant, black currant extract, and phenolics isolated from them were studied against salivary α-amylase, intestinal α-glucosidase, and sugar transporters. Cyanidin (53a) reported to be potent α-amylase inhibitor amongst the all anthocyanins and anthocyanidins with IC50 of 0.47 μg/mL, further proved the potency of anthocyanin aglycones than glycosides.
The isolated cyanidin (53a) and malvidin (71), a anthocyanin aglycones showed to inhibit enzyme with 19.1% and 12.5% inhibition at 66 μg/mL, while the glycoside form cyanidin-3-glucoside (6b) showed 15.8% inhibition at 33 μg/mL (Barik et al. 2020).
Major anthocyanins isolated from five Hungarian sour cherry varieties of Prunus cerasus L., were studied for their inhibitory potential against the HSA. HSA catalyzed hydrolysis was described to be competitively inhibited by all the studied extracts including anthocyanins. Malvidin-3,5-O-diglycoside (55), having lowest IC50 value (80 ± 10 μM) was confirmed as potent inhibitor of HSA in comparison to cyanidin-3-O-glucoside (6b) and cyanidin 3-O-rutinoside (56). Both the compounds 6b and 56 were reported to be better inhibitors than malvidin-3-O-glucoside (54 l) (Homoki et al. 2016).

DPP-IV inhibitors or GLP-1 mimetics
Compounds from flavonoid class like lanceolin (57a), lanceoletin (57b), 4-methoxylanceoletin (57c), 3,2’-dihydroxy-4–3’-dimethoxychalcone-4’-glucoside (57d), (2R)-8-methoxybutin (58), luteolin (1c), quercetin (32), and leptosidin (59) were reported to be isolated from Coreopsis lanceolata flowers. Dose-dependent inhibition of DPP-IV was noted with compounds 57b, 57c, 57d, 58, and 59. The compound lanceoletin (57b) showed highest activity amongst all, having IC50 of 9.6 µM, when compared with sitagliptin (IC50 of 0.071 µM). It was reported further that 57b becomes less effective as DPP-IV inhibitor, when substituted with more than one methoxy and/ or in its glycosidic form (Kim et al. 2020).

The antihyperglycemic activity of the methanolic extract derived from the flowers of Helichrysum arenarium L. Moench (Asteraceae) was investigated in sucrose loaded Mice. Further, the methanol extract, ethyl acetate fractions, and methanol eluted fraction were subjected to in vitro testing to assess their DPP-4 inhibitory activity. The methanol extract demonstrated promising in vitro DPP-4 inhibition with an IC50 value of 41.2 µg/ml. This result directed the bioassay-guided isolation of three new dimeric dihydrochalcone glycosides, namely arenariumosides V–VII (from the methanol-eluted fraction), in addition to several flavonoid constituents. The compounds that reported to inhibit DPP-4 are arenariumosides V–VII (60, 61 and 62 respectively), arenariumosides III (63a), chalconeringenin 2’,4’-di-O-β-D-glucopyranoside (63b), chalconeringenin 2’-O-β-D-glucopyranoside (63c), 6-hydroxy-3’-O-methylluteolin-7-O-β-D-glucopyranoside (64a), apigenin-7-O-gentiobioside (64b), apigenin-7-O-β-D-glucopyranosiduronic acid methyl ester (64c), luteolin-7-O-β-D-glucopyranoside (64d), apigenin-7-O-β-D-glucopyranoside (64e), 6-hydroxyluteolin-7-O-β-glucopyranoside (64f), quercetin 3-O-β-D-glucopyranoside (65a), quercetin 3,3’-di-O-β-D-glucopyranoside (65b), kaempferol-3,7,-di-O-β-D-glucopyranoside (65c), kaempferol-3-O-β-D-glucopyranosyl-(1 → 3)-β-D-glucopyranoside (65d), kaempferol-7-O-β-D-glucopyranoside (65e), kaempferol-3,4’-di-O-β-D-glucopyranoside (65f), kaempferol 3-O-gentiobioside (65 g), and aureusidin 6-O-β-D-glucopyranoside (66). Isolated thirty flavonoids from previous report including arenariumosides I–IV, apigenin 7-O-gentiobioside, luteolin 7-O-b-D-glucopyranoside, and aureusidin 6-O-β-D-glucopyranoside (66) were also screened for DPP-4 inhibitory activity. All the studied compounds showed DPP-4 inhibition, but chalconaringenin 2’-O-β-D-glucopyranoside (63c, IC50 = 23.1 µM) and aureusidin 6-O-β-D-glucopyranoside (66, 24.3 µM) showed relatively strong DPP-4 inhibitory activities with mixed type inhibition, in comparison to the positive control alogliptin (0.0018 µM) and diprotin A (2.3 µM) (Morikawa et al. 2015).

Inhibitors of human Sodium Glucose Co-Transporters (SGLT1)
In vivo and in vitro intestinal SGLT1 inhibition mediated decrease in glucose uptake by an apple extract and its polyphenols was reported. Apple extract and polyphenols like phlorizin (67), phloretin (13), kaempferol (68), and quercetin (32), isolated from it, reduced postprandial blood sugar level as confirmed in C57BL/6N mice jejunal segments and in Xenopus leavis oocytes. Compound 67 was reported to be potent amongst the polyphenols, inhibiting human SGLT1 with IC50 of 0.46 ± 0.19 μM in oocytes and 4.1 ± 0.6 µM in mice jejunal segments. Similar results as obtained with C57BL/6N mice were noted, when human volunteers which were previously administered the apple extract were tested for an oral glucose tolerance. It also reduced venous blood glucose and plasma insulin concentration while increasing renal glucose excretion in human volunteers, similar to the results observed in mice (Schulze et al. 2014).

Compound 9, known as 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone, has been reported to exhibit significant inhibition of glucose transport in both fasting and fed states, as confirmed through the Caco-2 Cell monolayer glucose transport assay. Dose-dependent reduced transport of glucose across the cell monolayers in the simulated fasting state was shown by 9. At concentration of 40 μM, it reported to exhibit 18% of glucose transport. This is in comparison to the 77% (300 μM) exhibited by the known SGLT1 inhibitor phloridzin dehydrate, which was used as a positive control. During the simulated fed state, no significant dose effect was observed. However, at the same concentration, 9 exhibited 52% glucose transport. This is in comparison to the known GLUT2 inhibitor phloretin, which showed 54% inhibition at a concentration of 150 μM (Hu et al. 2012).
Chalcones and other flavonoids in treatment of diabetes and related complications
The antidiabetic properties of specific compounds found in the resinous inflorescences of Humulus lupulus, namely Xanthohumol (12) and prenylflavanones such as 8-prenylnaringenin (11) and isoxanthohumol (69), were evaluated. Prenylated chalcone compounds 12, 11, and 69 were reported as potent tight-binding and noncompetitive inhibitors of human aldose reductase, AKR1B1 with Ki values 15.08 µM, 0.71 µM, and 0.34 µM, respectively (Seliger et al. 2018).

Oral administration of isoliquiritigenin (7), a flavonoid from the root of licorice, ameliorated diabetes retinal injury. This was achieved through the restoration of retinal SIRT-1 level, down-regulation of miR-195, reduction of oxidative stress and endothelial damage, as well as inflammation and reduced NF-κB gene expression. Further histopathological and electron microscopy studies proved that it also helped in the preservation of retinal normal histology and ultrastructure. It has been documented that treatment with compound 7 has shown improvement in diabetes-induced retinal injury by reducing oxidative stress, inflammation, and endothelial damage through the involvement of the miR-195/SIRT-1/NF-κB pathway (Alzahrani et al. 2020).
Beer-derived polyphenols Xanthohumol (12) and 8-prenylnaringenin (11) were reported to play a role in impaired lipid and carbohydrate metabolism in T2DM. These polyphenols were also found to contribute to the angiogenic paradox resulting from neovascularization imbalance in the heart and kidneys of diabetic C57Bl/6 mice. Modulation of T2DM angiogenic paradox by the 12 was correlated with the decreased angiogenesis, expression of VEGFR-2, VEGF-A level, and enzyme PFKFB3 in the endothelial cells of kidney in studied animals, as confirmed from ELISA assay. Compound 11 on the other hand showed inverse effects in the left ventricle of the T2DM mice than that of 12 and improve the impaired angiogenesis. Interestingly, 11 and 12 decreased the VEGF-B level, which plays a vital role in mediating the transport of lipids in endothelial cells. It also plays a role in the uptake of dietary lipids to peripheral tissues through endothelial cells (Costa et al. 2017a, b).
The effect of apigenin (70) on obesity and associated metabolic disorders was reported to be investigated. The study was conducted using C57BL/6 J mice with HFD-induced obesity, and apigenin (70) was administered at a dose of 0.005% w/w. The study reported that apigenin (70) was able to lower the fasting blood glucose levels. This activity was reported to be attributed to the suppression of gluconeogenesis. This was confirmed by the decreased expression and activities of PEPCK and G6Pase, the vital hepatic gluconeogenic enzymes in glucose homeostasis. This is further reported to be involved in the improvement of insulin resistance by decrease in the homeostatic index of insulin resistance and insulin level. Amelioration of plasma glucose levels, insulin resistance, and decreased fat accumulation in obese mice supplemented with apigenin was correlated with the significant decrease in plasma levels of pro-inflammatory chemokines such as MCP-1. Additionally, it led to a reduction in cytokines like IL-6, TNF-α, IFN-γ, further proving its protective effect against dyslipidemia and hepatic steatosis. This protective effect was characterized by reduced total plasma cholesterol, plasma free fatty acids, cytokine-induced plasma apoB/apoA1 ratio, and the apoB levels. Apigenin, along with the glycemic control and amelioration of insulin resistance, was found to reduce the lipogenesis and lipolysis in the liver by downregulation of mRNA expression of PPARγ in C57BL/6 J mice with HFD induced obesity (Jung et al. 2016).

Flavonoid-rich extract and flavonoids isolated from the root extract of Sophora davidii (Franch.) Skeels were reported to be investigated for their role in the management of hyperlipidemia and further involvement in the expression of PPARγ and WAT size. The study was conducted against hepatic steatosis and the suppression of adipogenesis in KK-Ay Mice. Reversal of hepatic steatosis and inhibition of PPARγ expression confirms the antiobesity effect of flavonoids in KK-Ay mice (Huang et al. 2018a, b).
Anthocyanin extract from Vaccinium ashei (Blueberry), along with its constituent malvidin-3-galactoside (54j), malvidin-3-glucoside (54 l), and malvidin (71), exhibited a protective effect on eye, attributed to their anti-inflammatory and antioxidant mechanisms. The extract and isolated constituents were found to prevent diabetic retinopathy through various mechanisms. These included enhancing cell viability, increasing catalase and superoxide dismutase activity, reducing ROS levels, and decreasing the expression of Nox4. Furthermore, the anti-angiogenic effect was confirmed by reduction of VEGF levels and inhibition of Akt pathway. Additionally, they exhibited inhibitory effects on ICAM-1 and NF-κB, which are involved in inflammation during the development of diabetic retinopathy (Huang et al. 2018a).

Isolated flavonoids from Eysenhardtia polystachya bark, were reported to ameliorate oxidative stress by in vitro and in vivo methods. The compounds, 9-hydroxy-3,8-dimethoxy-4-prenylpterocarpan (72), 7-hydroxy-5,8’-dimethoxy-6’α-l-rhamnopyranosyl-8-(3-phenyl-trans-acryloyl)-1-benzopyran-2-one (73), α,3,2’,4’-tetrahydroxy-4-methoxy-dihydrochalcone-3’-C-β-glucopyranosy-6’-O-β-d-glucopyranoside (74), 6’,7-dihydroxy-5,8-dimethoxy-8 (3-phenyl-trans-acryloyl)-1-benzopyran-2-one (75), and 2’,4’-dihydroxychalcone-6’-O-β-d-glucopyranoside (76) reduced oxidative stress. The compound 75 was reported to be most potent compound from the series. Compound 75 exhibits potent free radical scavenging effects in various in vitro assays, including DPPH, metal chelating, TEAC, NO scavenging, hydrogen peroxide radical scavenging, BSA oxidation, superoxide, and hydroxyl radical scavenging, outperforming the reference ascorbic acid in most cases. Conversely, compound 77 demonstrates lower scavenging activity.
In vivo assays on STZ-induced hyperglycemic male CD1 mice reported to increase radical scavenging enzymes glutathione peroxidase (CSH-Px), (CAT), SOD, and glutathione reductase (GSH) in Serum, liver, pancreas, and kidney. Reduction in the level of enzymes responsible for hepatic damage like TB, ALP, SGOT, and SGPT supported the hepatoprotective effect of compounds. Overall, in vivo assay results conclude that compounds 69–73 alleviate complications of diabetes, by preventing and/or delaying the onset of pancreatic, renal, and hepatic damage. These compounds impart antioxidant properties, lower lipid peroxidation, and elevate radical scavenging enzyme activity. From the results of all in vitro antioxidant assays, it was reported that the electron donating group on ring A of all chalcones 73, 75, and 76 are orthro to the carbonyl group. Glycosidation reported to show decrease in activity, as confirmed by the moderate inhibitory activity of 76, while compound 74 showed low activity (Perez-Gutierrez et al. 2016).

Antioxidant properties of quercetin (32) was observed when administered to STZ induced diabetic rats, at a dose of 50 mg/kg. This resulted in an increase in insulin secretion in both the control saline group and the diabetic saline group. Additionally, the concentration of triglycerides was found to be decreased. Antioxidant effect of 32 was studied by quantifying the biomarkers of oxidative stress like SOD, TBARS, and CAT. It was reported that at 50 mg/kg dose, there was significant decrease in levels of TBARS in serum (16.0 ± 10.7 nmol MDA/mg protein) in comparison to the control saline group (21.0 ± 7.8 nmol MDA/mg protein). At same dose, no significant reduction in SOD activity was reported in treated diabetic rats, suggests the reversal of the effect caused by oxidative stress. There were no significant differences in CAT activities reported between the liver and kidney (Maciel et al. 2013).
8-prenylnaringenin (11) and Xanthohumol (12) have been reported to downregulate the expression of oxidative biomarkers in the liver and the kidneys of high-fat fed DMC57Bl/6 mice model. These biomarkers include Galectin-3 (Gal3), a protein associated with oxidative stress in the diabetic state, and 3-nitrotyrosine (3NT), a marker of cell damage. Moreover it promoted advanced glycation end products (AGEs) production, and reduced inflammation, and NO production (Luís et al. 2019).
Nobiletin (78), a flavone isolated from the peel of citrus fruits, provide protection against cardiac cardiomyopathy in male C57BL mice with STZ-induced diabetes. Hemodynamic measurements and echocardiography in compound 78 treated mice showed protective effect on cardiac function. Decrease in oxidative stress and weakened mRNA expression of isoforms of NoX like p22phox, p91pho, and p67phox was noted in treated mice. Attenuation of cardiac fibrosis was reported in treated subjects. Additionally, a decrease in the expressions of collagen Iα, transforming growth factor TGF-β1, fibronectin, and connective tissue growth factor CTGF was observed. Its treatment in diabetic mice inhibited activation of P38, NF-κB, and c-Jun NH2-terminal kinase (JNK), mitigating interstitial fibrosis and cardiac dysfunction (Zhang et al. 2016).

Naringin (79) and hesperidin (80) were found effective in attenuating the hyperglycemia-mediated oxidative stress of rats with HFD/STZ-induced type 2 diabetes. Both the 79 and 80 treated rats significantly decreased the production of pro-inflammatory cytokines, like IL- 6, and TNF-α. Apart from these effects, it also lowered the glucose level, glycosylated hemoglobin (HbA1c %), liver MDA, and NO (Mahmoud et al. 2012).

A citrus flavanone, hesperetin (81) was studied for its protective effect in diabetes -associated testicular injury in the STZ-rat. Beneficial effects observed after the hesperetin treatment were body weight loss prevention, improvement of serum testosterone and reduced serum glucose. Inhibition of apoptosis and lower activity of oxidative stress specific biomarkers such as ROS, caspase 3, DNA fragmentation, MDA, and protein carbonyl was observed in diabetic group treated with hesperetin.
Treatment with compound 81 enhanced the testicular antioxidant system not only by increasing GSH, mitochondrial membrane potential (MMP), and ferric reducing antioxidant power (FRAP) levels, but also improved the activities of enzymes such as SOD, GPx, and CAT. Improvement in the sperm counts, motility, and viability are some of the beneficial effects reported in hesperetin-treated diabetic rats. Additionally, attenuation of testicular indices of inflammation TNF-α and IL-17, along with prevention of seminiferous tubules damage, was observed in diabetic rats (Samie et al. 2018).

In diabetic rats, kaempferol (68) exhibited potent hypoglycemic and insulin-releasing effects, while also reducing oxidative, inflammatory, and fibrotic damage to the left ventricles (LVs) in STZ-diabetic rats. It significantly preserved the systolic and diastolic functioning of the LVs, which was associated with suppression of cardiac fibrosis and decreased ventricular collagen deposition, infiltration of inflammatory cells, and protein expression of Bcl2-associated X protein (Bax), cleaved caspase-3, and cytochrome-C. The protective mechanism was attributed to its anti-inflammatory and antioxidant actions, mediated through the activation of SIRT1 (Alshehri et al. 2021).
Research explored the alleviating effects of scutellarin (82) on Type 2 diabetic cardiomyopathy using a model of STZ-induced type 2 diabetic cardiomyopathy in adult male SD rats fed with a high-fat and high-sugar diet. It was observed to alleviate symptoms of diabetic cardiomyopathy by enhancing the levels of autophagy-associated proteins in cardiomyocytes, such as LC3-II and Beclin-1, while reducing those associated with apoptosis, such as Cyt-C, Bax, and caspase-3 (Su et al. 2022).

Additionally, scutellarin has been found to effectively protect against cardiac injury in type 2 diabetic mice. It demonstrated this effect by diminishing inflammation, oxidative stress, and apoptosis. The observed mitigations are attributed to its modulation of the TLR/MYD88/NF-κB pathway, Nrf2/Keap1/ARE pathway, and mitochondrial apoptotic pathway (Huo et al. 2021).
Scutellarin (82) relieved damage to the blood-retinal barrier (BRB) by inhibiting retinal inflammatory responses and subsequent oxidative stress injury initiated by microglia cells in STZ-induced diabetic mice. It has been documented to safeguard against BRB damage during the early stages of diabetic retinopathy. This protective effect is attributed to its inhibition of the ERK1/2-NFκB inflammatory signaling pathway, leading to reduced TNFα expression in microglia cells. Additionally, it has been reported to alleviate oxidative stress injury by inducing Nrf2 activation (Mei et al. 2019).
In STZ-induced diabetic rats, administration of a 2.5 mg/kg dose of Fisetin (83) over six weeks resulted in decreased development of diabetic cardiomyopathy. Fisetin mitigated cardiac damage by improving circulating levels of CK-MB, LDH, and cTnI (all are biomarkers employed in the evaluation of cardiovascular complications, being released into the bloodstream in response to damage occurring in heart muscle cells), as well as enhancing serum lipids and cardiovascular risk indices. It also reduced oxidative stress by increasing the activities of cardiac GSH, SOD, and CAT, while decreasing levels of MDA and protein carbonyl. Furthermore, studies reported that it inhibits the production of pro-inflammatory cytokines in diabetic rats by blocking cardiac NF-κB pathways (Althunibat et al. 2019).

Luteolin (1c) has been shown to provide protection to the kidneys in a mouse model of Diabetic nephropathy (DN), specifically the C57BL/6 J db/db mice. It decreased the concentration of MDA and increased SOD levels in db/db mice. Additionally, it was noted to reverse the elevated levels of IL-6, IL-1β, IL-17A, and TNF-α in DN mice. The observed protective effect of luteolin was linked to a reduction in glomerular sclerosis and interstitial fibrosis, which was found to be correlated with the inhibition of STAT3 expression (Zhang et al. 2021a, b).
Baicalin (84) alleviated diabetic nephropathy in a spontaneous mouse model by diminishing oxidative stress and inflammation. It mitigated oxidative stress through enhancements in the activities of antioxidant enzymes GSH-PX, SOD, and CAT, while reducing MDA levels. Additionally, it decreased the levels of pro-inflammatory cytokines IL-1β, IL-6, MCP-1, and TNFα. Thus, its primary mechanisms were reported to be associated with activating the Nrf2-mediated antioxidant signaling pathway and suppressing the MAPK-mediated inflammatory signaling pathway. Inhibition of the MAPK-mediated inflammatory signaling pathway was corroborated by the inhibition of MAPK family proteins, Erk1/2, JNK, and P38 (Ma et al. 2021).

Diosmetin (85), at dose of 25–100 mg/kg/day for 8 weeks in STZ induced diabetic nephropathy mice, decreased the levels of TNF-a, IL-6, and IL-1b in the serum of the diosmetin-treated group. It also attenuated oxidative stress by altered levels of MDA and NO and the activity of SOD and Myeloperoxidase (MPO) in the tissue homogenate of STZ-induced DN mice. It reduced the expression of NF-kB protein and thus the level of inflammatory cytokines in the serum of DN mice. Modulation of Akt/NF-kB/iNOS signaling pathway was reported to prove renoprotective, anti-diabetic, anti-inflammatory and anti-oxidant effects of Diosmetin (Jiang et al. 2018).

Biochanin A (86), an isoflavone, improved retinopathy in diabetic rats induced by STZ. It was observed to exert its effects by lowering blood sugar levels, modulating inflammation by reducing IL-1β and TNF-α, and suppressing angiogenesis through the inhibition of vascular endothelial growth factor in retinal tissues (Mehrabadi et al. 2018).

Phytocomplexes as antidiabetic agents
Diabetes Mellitus is treated now days using classical drugs acting on multiple molecular and cellular targets. In contrast with single ligand acting at a single receptor, herbal extracts in the form of phyto-complexes act as multi-target ligands, where numerous phytoconstituents bind to the diverse targets of DM.
Scutellariae Radix (SR) was reported to contain the baicalin, baicalein, wogonin, and wogonoside, and showed the suppression of gluconeogenesis and augmentation of insulin resistance. The alkaloid berberine, from herb Coptidis Rhizoma (CR), is known to lower blood glucose and promote the insulin secretion. Interestingly, the combined extract of both of these herbs in equal proportion work synergistically and is known as traditional Chinese medicine to treat the T2DM (Artasensi et al. 2020).
This phyto-complex approach not only validates the synergistic effect but also help improve the poor bioavailability of herbal drugs. Phyto-complex technology, a novel drug delivery system, where aqueous soluble or standardized plant extracts are incorporated into phospholipids, which thereby produce lipid compatible molecular complex (Mathur 2013).
Various reports on the DESIGNER (Deplete and Enrich Select Ingredients to Generate Normalized Extract Resources) have been published to generate the Phytocomplexes for the treatment of T2DM. In this process, Knockout Extract (KOE) is prepared using appropriate separation techniques. KOE is then utilized for in vivo and in vitro pharmacological studies, which can potentially produce synergistic effects and reduce undesirable effects. KOE derived from the bioactive extracts are known to act on multiple molecular and cellular targets. (Fig. 3a).
a General Multi-target drug approach for development of antidiabetic drugs from herbs b illustrating the multitarget mechanism of action of ethanolic extract from Artemisia dracunculus and its knockout extracts, KOE (i) and KOE (ii) containing chalcones and their natural metabolites on various targets in T2DM. ( +) represents activation and ( −) inhibition effects
One such study, where DESIGNER approach was employed, to identify the isomeric bioactive components from the mixture of phytoconstituents in an ethanolic extract of Artemisia dracunculus with hypoglycemic properties. The experiments conducted in vitro and in vivo, using the knockout extract, revealed that both 4’-O-methyldavidigenin (87) and 4-O-methyldavidigenin (88) possess the capacity to enhance insulin signaling in skeletal muscle. This finding suggests that these compounds could potentially contribute to a synergistic effect, leading to improved insulin secretion (Yu et al. 2019).
Artemisia dracunculus contains additional constituents from the flavonoid class, which have been demonstrated to act on various targets associated with T2DM (Eisenman et al. 2011; Vandanmagsar et al. 2021). The representative illustration of phytocomplexes derived from the ethanolic extract of Artemisia dracunculus using the DESIGNER approach is shown in Fig. 3b. The figure showcases the impact of these phytocomplexes on diverse targets associated with T2DM.

Recent patents on plant based anti-diabetics (Table 2)
Conclusion
Recent reports from the WHO and the IDF sound an alarming bell regarding the increasing prevalence of T2DM in the near future and thus highlight the urgent need for the development of optimized treatments that are effective and free from side effects. Conventional antidiabetics are effective in T2DM, but suffer from higher incidences of side effects, thereby natural alternative or an adjunct therapy to conventional drugs is the choice of treatment nowadays. Though the reports have suggested the identification of chalcone and its natural metabolites acting on multiple targets of T2DM with antidiabetic effect, very few attempts have been found to be made for optimization of these leads for improving therapeutic efficacy with reduced side effects. Apart from reducing the side effects of recent T2DM treatment, the amelioration of the diabetic related complications is major challenge for the T2DM drug development. Recent Studies reported the use of phyto-complexes working same as multi-target ligands in treating T2DM. To tackle the multifaceted nature of diabetes and its related complications, there is a growing interest in developing multitarget drugs derived from natural sources. In particular, the utilization of optimized scaffolds based on chalcone and its metabolites from flavonoid class show promise in this regard. Such approaches have the potential to target multiple pathways and provide effective relief from the various aspects of diabetes and its associated complications.
While numerous studies have demonstrated the inhibitory effects of flavonoids including chalcones on α-amylase, there are conflicting reports also suggesting that neohesperidin dihydrochalcone can activate mammalian α-amylase through chloride-mediated allosteric activation (Kashani-Amin et al. 2013). The dual behaviour of flavonoids in both activating and inhibiting α-amylase requires further investigation, particularly in terms of anionic-dependent allosteric activation and its relationship with structural features.
PTP1B inhibitors have gained attention not only for their antidiabetic and antiobesity properties but also for their potential positive effects on the development and advancement of cancers (Lessard et al. 2012). The review emphasizes the potential significance of flavonoid scaffolds, which are known inhibitors of PTP1B, and suggests that further attention should be given for exploring their potential role in the development of novel natural products for cancer therapy, which is commonly associated with diabetes. Several investigations have examined the utilization of anthocyanin-rich foods or isolated anthocyanins and anthocyanidins as dietary supplements. However, there remains an opportunity to optimize these molecules further in order to enhance their absorption. It has been suggested that compared to the chalcones and other natural metabolites discussed earlier, the anthocyanin family of dietary flavonoids exhibits the least bioavailability (Lila et al. 2016).
In conclusion, this review builds on newly reported plant-derived chalcones and their metabolites to the existing pool of plant-based antidiabetic natural products. It provides a valuable resource for researchers seeking to optimize antidiabetic leads in the future.
Abbreviations
- AGEs:
-
Advanced glycation end products
- AMPK:
-
AMP-activated protein kinase
- BRB:
-
Blood-Retinal Barrier
- CAT:
-
Serum catalase
- CK-MB:
-
Creatine Kinase-MB
- cTnI:
-
Cardiac Troponin I
- DPP-IV:
-
Dipeptidyl Peptidase- IV
- Das:
-
Diels-Alder adducts
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GLP:
-
Glucagon-like peptide
- GLUT2:
-
Glucose Transporter Type -2
- GLUT4:
-
Glucose Transporter Type-4
- GPx:
-
GSH peroxidase
- HAS:
-
Human salivary α-amylase
- HFD:
-
High Fat Diet
- HOMA-IR:
-
Homeostasis Model Assessment of Insulin Resistance
- iNOS:
-
Inducible nitric oxide synthase
- IRS-1:
-
Insulin Receptor substrate
- KOE:
-
Knock Out Extract
- LDH:
-
Lactate Dehydrogenase
- MAPK:
-
Mitogen-activated protein kinases
- MDA:
-
Mmalondialdehyde
- NO:
-
Nitric oxide
- NoX:
-
NADPH oxidase
- PCT:
-
Proximal Convoluted Tubule
- PI3K:
-
Phosphoinositide-3-kinase
- PPARγ :
-
Peroxisome Proliferator-Activated Receptor Gamma
- PTP1B:
-
Protein Tyrosine Phosphatase 1B
- SIRT1:
-
Sirtuin 1
- SOCS-3:
-
Suppressor of cytokine signaling 3
- ROS:
-
Reactive Oxygen Species
- SREBP-1c:
-
Sterol Regulatory Element-Binding Protein-1c
- SOD:
-
Superoxide dismutase
- SGLT:
-
Sodium-coupled Glucose Transporter
- STZ:
-
Streptozotocin
- TBARS:
-
Thiobarbituric Acid Reactive Substances
- T2DM:
-
Type 2 diabetes mellitus
- WAT:
-
White adipose tissue
References
Abdelsalam SS, Korashy HM, Zeidan A, Agouni A (2019) The role of protein tyrosine phosphatase (PTP)-1B in cardiovascular disease and its interplay with insulin resistance. Biomolecules 9:286. https://doi.org/10.3390/biom9070286
Alam S, Sarker MM, Sultana TN, Chowdhury MN, Rashid MA, Chaity NI, Zhao C, Xiao J, Hafez EE, Khan SA, Mohamed IN (2022) Antidiabetic Phytochemicals from Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front Endocrinol 13:800714. https://doi.org/10.3389/fendo.2022.800714
Alshehri AS, El-Kott AF, Eleawa SM, El-Gerbed MSA, Khalifa HS, El-Kenawy AE, Albadrani GM, Abdel-Daim MM (2021) Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J Physiol Pharmacol 72:3
Althunibat OY, Al Hroob AM, Abukhalil MH, Germoush MO, Bin-Jumah M, Mahmoud AM (2019) Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci 221:83–92. https://doi.org/10.1016/j.lfs.2019.02.017
Alzahrani S, Ajwah SM, Alsharif SY, Said E, El-Sherbiny M, Zaitone SA, Al-Shabrawey M, Elsherbiny NM (2020) Isoliquiritigenin downregulates miR-195 and attenuates oxidative stress and inflammation in STZ-induced retinal injury. Naunyn Schmiedebergs Arch Pharmacol 393:2375–2385. https://doi.org/10.1007/s00210-020-01948-5
Arshad L, Jantan I, Bukhari SN, Haque MA (2017) Immunosuppressive Effects of Natural α, β-Unsaturated Carbonyl-Based Compounds, and Their Analogs and Derivatives, on Immune Cells: A Review. Front Pharmacol 8:22. https://doi.org/10.3389/fphar.2017.00022
Artasensi A, Pedretti A, Vistoli G, Fumagalli L (2020) Type 2 diabetes mellitus: A review of multi-target drugs. Molecules 25:1–20. https://doi.org/10.3390/molecules25081987
Azam AA, Pariyani R, Ismail IS, Ismail A, Khatib A, Abas F, Shaari K (2017) Urinary metabolomics study on the protective role of Orthosiphon stamineus in Streptozotocin induced diabetes mellitus in rats via 1H NMR spectroscopy. BMC Complement Altern Med 17:1–13. https://doi.org/10.1186/s12906-017-1777-1
Azofeifa G, Quesada S, Navarro L, Hidalgo O, Portet K, Pérez AM, Vaillant F, Poucheret P, Michel A (2016) Hypoglycaemic, hypolipidaemic and antioxidant effects of blackberry beverage consumption in streptozotocin-induced diabetic rats. J Funct Foods 26:330–337. https://doi.org/10.1016/j.jff.2016.08.007
Balakumar P, Mahadevan N, Sambathkumar R (2019) A Contemporary Overview of PPARα/γ Dual Agonists for the Management of Diabetic Dyslipidemia. Curr Mol Pharmacol 12:195–201. https://doi.org/10.2174/1874467212666190111165015
Barik SK, Russell WR, Moar KM, Cruickshank M, Scobbie L, Duncan G, Hoggard N (2020) The anthocyanins in black currants regulate postprandial hyperglycaemia primarily by inhibiting α-glucosidase while other phenolics modulate salivary α-amylase, glucose uptake and sugar transporters. J Nutr Biochem 78:108325. https://doi.org/10.1016/j.jnutbio.2019.108325
Ben Younes A, Ben Salem M, El Abed H, Jarraya R (2018) Phytochemical screening and antidiabetic, antihyperlipidemic, and antioxidant properties of anthyllis henoniana (Coss.) flowers extracts in an alloxan-induced rats model of diabetes. Evidence-Based Complement Altern Med. https://doi.org/10.1155/2018/8516302
Bhilare NV, Marulkar VS, Shirote PJ, Dombe SA, Pise VJ, Salve PL, Biradar SM, Yadav VD, Jadhav PD, Bodhe AA, Borkar SP (2022) Mannich bases: centrality in cytotoxic drug design. Med Chem 18:735–756. https://doi.org/10.2174/1573406418666211220124119
Bukhari SN, Franzblau SG, Jantan I, Jasamai M (2013) Current prospects of synthetic curcumin analogs and chalcone derivatives against mycobacterium tuberculosis. Med Chem 9:897. https://doi.org/10.2174/1573406411309070002
Carey AL, Febbraio MA (2004) Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia 47:1135–1142. https://doi.org/10.1007/s00125-004-1447-y
Chang WC, Wu JS, Chen CW, Kuo PL, Chien HM, Wang YT, Shen SC (2015) Protective effect of vanillic acid against hyperinsulinemia, hyperglycemia and hyperlipidemia via alleviating hepatic insulin resistance and inflammation in high-fat diet (HFD)-fed rats. Nutrients 7:9946. https://doi.org/10.3390/nu7125514
Chen RM, Hu LH, An TY, Li J, Shen Q (2002) Natural PTP1B inhibitors from Broussonetia papyrifera. Bioorganic Med Chem Lett 12:3387–3390. https://doi.org/10.1016/S0960-894X(02)00757-6
Chen L, Chen R, Wang H, Liang F (2015) Mechanisms linking inflammation to insulin resistance. Int J Endocrinol 2015:508409. https://doi.org/10.1155/2015/508409
Choo CY, Sulong NY, Man F, Wong TW (2012) Vitexin and isovitexin from the Leaves of Ficus deltoidea with in vivo α-glucosidase inhibition. J Ethnopharmacol 142:776–781. https://doi.org/10.1016/j.jep.2012.05.062
Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, Pichika MR, Gorain B, Kesharwani P (2017) An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Tradit Complement Med 8:361. https://doi.org/10.1016/j.jtcme.2017.08.012
Costa R, Rodrigues I, Guardão L, Lima JQ, Sousa E, Soares R, Negrão R (2017a) Modulation of VEGF signaling in a mouse model of diabetes by xanthohumol and 8-prenylnaringenin: Unveiling the angiogenic paradox and metabolism interplay. Mol Nutr Food Res 61:1600488. https://doi.org/10.1002/mnfr.201600488
Costa R, Rodrigues I, Guardão L, Rocha-Rodrigues S, Silva C, Magalhães J, Ferreira-de-Almeida M, Negrão R, Soares R (2017b) Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J Nutr Biochem 45:39. https://doi.org/10.1016/j.jnutbio.2017.03.006
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 10:7313–7352. https://doi.org/10.3390/molecules15107313
David JA, Rifkin WJ, Rabbani PS, Ceradini DJ (2017) The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res 2017:4826724. https://doi.org/10.1155/2017/4826724
Dej-Adisai S, Rais IR, Wattanapiromsakul C, Pitakbut T (2021) Alpha-glucosidase inhibitory assay-screened isolation and molecular docking model from bauhinia pulla active compounds. Molecules 26:5970. https://doi.org/10.3390/molecules26195970
Derosa G, Maffioli P (2012) α-Glucosidase inhibitors and their use in clinical practice. Arch Med Sci 8:899. https://doi.org/10.5114/aoms.2012.31621
Dhankhar S, Chauhan S, Mehta DK, Nitika SK, Saini M, Das R, Gupta S, Gautam V (2023) Novel targets for potential therapeutic use in Diabetes mellitus. Diabetol Metab Syndr 15:17. https://doi.org/10.1186/s13098-023-00983-5
Du Preez R, Wanyonyi S, Mouatt P et al (2020) Saskatoon berry amelanchier alnifolia regulates glucose metabolism and improves cardiovascular and liver signs of diet-induced metabolic syndrome in rats. Nutrients 12:1–16. https://doi.org/10.3390/nu12040931
Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS (2003) A reduced-glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med 157:773–779. https://doi.org/10.1001/archpedi.157.8.773
Eisenman SW, Poulev A, Struwe L, Raskin I, Ribnicky DM (2011) Qualitative variation of anti-diabetic compounds in different tarragon (Artemisia dracunculus L.) cytotypes. Fitoterapia 82:1062–1074. https://doi.org/10.1016/j.fitote.2011.07.003
Elkhalifa D, Al-Hashimi I, Al Moustafa AE, Khalil A (2021) A comprehensive review on the antiviral activities of chalcones. J Drug Target 29:403. https://doi.org/10.1080/1061186X.2020.1853759
El-Newary SA, Afifi SM, Aly MS et al (2021) Chemical profile of launaea nudicaulis ethanolic extract and its antidiabetic effect in streptozotocin-induced rats. Molecules 26:1–27. https://doi.org/10.3390/molecules26041000
Evranos Aksöz B, Ertan R (2011) Chemical and structural properties of chalcones I. Fabad J Pharm Sci 36:223–242
Farzaei MH, Singh AK, Kumar R, Croley CR, Pandey AK, Coy-Barrera E, Kumar Patra J, Das G, Kerry RG, Annunziata G, Tenore GC (2019) Targeting inflammation by flavonoids: novel therapeutic strategy for metabolic disorders. Int J Mol Sci 20:4957. https://doi.org/10.3390/ijms20194957
Flores-Fernández JM, Barragán-Álvarez CP, Díaz-Martínez NE, Villanueva-Rodríguez S, Padilla-Camberos E (2017) In vitro and in vivo postprandial glycemic activity of Citrus limetta peel flour. Pharmacogn Mag 13:613. https://doi.org/10.4103/pm.pm_158_17
Gao L, Su C, Du X, Wang R, Chen S, Zhou Y, Liu C, Liu X, Tian R, Zhang L, Xie K, Chen S, Guo Q, Guo L, Hano Y, Shimazaki M, Minami A, Oikawa H, Huang N, Houk KN, Huang L, Dai J, Lei X (2020) FAD-dependent enzyme-catalysed intermolecular [4+2] cycloaddition in natural product biosynthesis. Nat Chem 12:620–628. https://doi.org/10.1038/s41557-020-0467-7
Gaur R, Yadav KS, Verma RK, Yadav NP, Bhakuni RS (2014) In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 21:415–422. https://doi.org/10.1016/j.phymed.2013.10.015
Guo Z, Niu X, Xiao T, Lu J, Li W, Zhao Y (2015) Chemical profile and inhibition of α -glycosidase and protein tyrosine phosphatase 1B (PTP1B) activities by flavonoids from licorice (Glycyrrhiza uralensis Fisch). J Funct Foods 14:324–336. https://doi.org/10.1016/j.jff.2014.12.003
Ha MT, Seong SH, Nguyen TD, Cho WK, Ah KJ, Ma JY, Woo MH, Choi JS, Min BS (2018) Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and α- glucosidase. Phytochemistry 155:114–125. https://doi.org/10.1016/j.phytochem.2018.08.001
Han Y, Jung HW, Park YK (2015) Effects of Icariin on insulin resistance via the activation of AMPK pathway in C2C12 mouse muscle cells. Eur J Pharmacol 758:60. https://doi.org/10.1016/j.ejphar.2015.03.059
Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, Poutanen K (2010) Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci 11:1365. https://doi.org/10.3390/ijms11041365
Hinnen D (2017) Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr 30:202–210
Homoki JR, Nemes A, Fazekas E, Gyémánt G, Balogh P, Gál F, Al-Asri J, Mortier J, Wolber G, Babinszky L (2016) Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem 194:222–229. https://doi.org/10.1016/j.foodchem.2015.07.130
Hsia DS, Grove O, Cefalu WT (2017) An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 24:73–79. https://doi.org/10.1097/med.0000000000000311
Hu YC, Luo YD, Li L, Joshi MK, Lu YH (2012) In vitro investigation of 2’,4’-dihydroxy-6’-methoxy-3’,5’-dimethylchalcone for glycemic control. J Agric Food Chem 60:10683. https://doi.org/10.1021/jf303078r
Hu YC, Hao DM, Zhou LX, Zhang Z, Huang N, Hoptroff M, Lu YH (2014a) 2’,4’-Dihydroxy-6’-methoxy-3’,5’-dimethylchalcone protects the impaired insulin secretion induced by glucotoxicity in pancreatic β-cells. J Agric Food Chem 62:1602. https://doi.org/10.1021/jf405365d
Hu YC, Zhang Z, Shi WG, Mi TY, Zhou LX, Huang N, Hoptroff M, Lu YH (2014b) 2’,4’-Dihydroxy-6’-methoxy-3’,5’-dimethylchalcone promoted glucose uptake and imposed a paradoxical effect on adipocyte differentiation in 3T3-L1 cells. J Agric Food Chem 62:1898. https://doi.org/10.1021/jf405368q
Huang W, Yan Z, Li D, Ma Y, Zhou J, Sui Z (2018a) Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid Med Cell Longev. https://doi.org/10.1155/2018/1862462
Huang Y, Hao J, Tian D, Wen Y, Zhao P, Chen H, Lv Y, Yang X (2018b) Antidiabetic activity of a flavonoid-rich extract from Sophora davidii (Franch.) Skeels in KK–Ay mice via activation of AMP-activated protein kinase. Front Pharmacol 9:1–15. https://doi.org/10.3389/fphar.2018.00760
Huang Y, Zhou T, Zhang Y et al (2021) Antidiabetic activity of a flavonoid-rich extract from flowers of Wisteria sinensis in type 2 diabetic mice via actiion of the IRS-1/PI3K/Akt/GLUT4 pathway. J Funct Foods 77:104338. https://doi.org/10.1016/j.jff.2020.104338
Huo Y, Mijiti A, Cai R, Gao Z, Aini M, Mijiti A, Wang Z, Qie R (2021) Scutellarin alleviates type 2 diabetes (HFD/low dose STZ)-induced cardiac injury through modulation of oxidative stress, inflammation, apoptosis and fibrosis in mice. Hum Exp Toxicol 40:S460–S474. https://doi.org/10.1177/09603271211045948
Jayachandran M, Vinayagam R, Ambati RR, Xu B, Chung SSM (2018) Guava Leaf Extract Diminishes Hyperglycemia and Oxidative Stress, Prevents beta-Cell Death, Inhibits Inflammation, and Regulates NF-kB Signaling Pathway in STZ Induced Diabetic Rats. BioMed Res Int 2018:4601649. https://doi.org/10.1155/2018/4601649
Jiang Y, Liu J, Zhou Z, Liu K, Liu C (2018) Diosmetin attenuates Akt signaling pathway by modulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)/inducible nitric oxide synthase (iNOS) in streptozotocin (STZ)-induced diabetic nephropathy mice. Med Sci Monit 24:7007–7014. https://doi.org/10.12659/MSM.910764
Kashani-amin E, Larijani B, Ebrahim-habibi A (2013) Neohesperidin dihydrochalcone: presentation of a small molecule activator of mammalian alpha-amylase as an allosteric effector. FEBS Lett 587:652–658. https://doi.org/10.1016/j.febslet.2013.01.022
Kawanishi K, Ueda H, Moriyasu M (2003) Aldose reductase inhibitors from the nature. Curr Med Chem 10:1353–1374. https://doi.org/10.2174/0929867033457304
Kellett GL, Brot-Laroche E, Mace OJ, Leturque A (2008) Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr 28:35–54. https://doi.org/10.1146/annurev.nutr.28.061807.155518
Kerimi A, Williamson G (2016) At the interface of antioxidant signalling and cellular function: key polyphenol effects. Mol Nutr Food Res 60:1770–1788. https://doi.org/10.1002/mnfr.201500940
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J (2020) Epidemiology of Type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health 10:107–111
Khan M, Manzoor Z, Rafiq M, Munawar SH, Waqas MY, Majeed H, Ali Shah SZ, Hussain R, Hussain HI, Tahir T, Kotwica-Mojzych K (2022) Phytochemical screening, anti-inflammatory, and antidiabetic activities of different extracts from caralluma edulis plant. Molecules 27:5346. https://doi.org/10.3390/molecules27165346
Kim BR, Paudel SB, Nam JW, Jin CH, Lee IS, Han AR (2020) Constituents of coreopsis lanceolata flower and their dipeptidyl peptidase IV inhibitory effects. Molecules 25:4370. https://doi.org/10.3390/molecules25194370
Kitada M, Ogura Y, Monno I, Koya D (2019) Sirtuins and Type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol 10:187. https://doi.org/10.3389/fendo.2019.00187
Kozlowski D, Trouillas P, Calliste C, Marsal P, Lazzaroni R, Duroux JL (2007) Density functional theory study of the conformational, electronic, and antioxidant properties of natural chalcones. J Phys Chem A 111:1138. https://doi.org/10.1021/jp066496+
Kozuharova E, Matkowski A, Woźniak D, Simeonova R, Naychov Z, Malainer C, Mocan A, Nabavi SM, Atanasov AG (2017) Amorpha fruticose—A noxious invasive alien plant in Europe or a medicinal plant against metabolic disease? Front Pharmacol 8:1–17. https://doi.org/10.3389/fphar.2017.00333
Kuzmenko DI, Klimentyeva TK (2016) Role of Ceramide in Apoptosis and Development of Insulin Resistance. Biochemistry (mosc) 8:913
Laddha AP, Kulkarni YA (2020) NADPH oxidase: a membrane-bound enzyme and its inhibitors in diabetic complications. Eur J Pharmacol 881:173206. https://doi.org/10.1016/j.ejphar.2020.173206
Langeveld M, Aerts JM (2009) Glycosphingolipids and insulin resistance. Prog Lipid Res 48:196–205. https://doi.org/10.1016/j.plipres.2009.03.002
Lebovitz HE (2019) Thiazolidinediones: the forgotten diabetes medications. Curr Diab Rep 19:151. https://doi.org/10.1007/s11892-019-1270-y
Lessard L, Labbé DP, Deblois G, Bégin LR, Hardy S, Mes-Masson AM, Saad F, Trotman LC, Giguère V, Tremblay ML (2012) PTP1B is an androgen receptor–regulated phosphatase that promotes the progression of prostate cancer. Cancer Res 72(6):1529–1537. https://doi.org/10.1158/0008-5472.CAN-11-2602
Li W, Li S, Higai K, Sasaki T, Asada Y, Ohshima S, Koike K (2013) Evaluation of licorice flavonoids as protein tyrosine phosphatase 1B inhibitors. Bioorganic Med Chem Lett 23:5836–5839. https://doi.org/10.1016/j.bmcl.2013.08.102
Lila MA, Burton-Freeman B, Grace M, Kalt W (2016) Unraveling anthocyanin bioavailability for human health. Annu Rev Food Sci Technol 7:375. https://doi.org/10.1146/annurev-food-041715-033346
Lin Y, Kuang Y, Li K, Wang S, Song W, Qiao X, Sabir G, Ye M (2017) Screening for bioactive natural products from a 67-compound library of Glycyrrhiza inflata. Bioorg Med Chem 25:3706–3713. https://doi.org/10.1016/j.bmc.2017.05.009
Luís C, Costa R, Rodrigues I, Castela Â, Coelho P, Guerreiro S, Gomes J, Reis C, Soares R (2019) Xanthohumol and 8-prenylnaringenin reduce type 2 diabetes–associated oxidative stress by downregulating galectin-3. Porto Biomed J 4:e23. https://doi.org/10.1016/j.pbj.0000000000000023
Luo C, Yang H, Tang C, Yao G, Kong L, He H, Zhou Y (2015) Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int Immunopharmacol 28:744. https://doi.org/10.1016/j.intimp.2015.07.018
Lv Q, Lin Y, Tan Z, Jiang B, Xu L, Ren H, Tai WC, Chan CO, Lee CS, Gu Z, Mok DK (2019) Dihydrochalcone-derived polyphenols from tea crab apple (Malus hupehensis) and their inhibitory effects on α-glucosidase in vitro. Food Funct 5:2881. https://doi.org/10.1039/c9fo00229d
Ma L, Wu F, Shao Q, Chen G, Xu L, Lu F (2021) Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Devel Ther 15:3207–3221. https://doi.org/10.2147/DDDT.S319260
Maccari R, Paoli P, Ottanà R, Jacomelli M, Ciurleo R, Manao G, Steindl T, Langer T, Vigorita MG, Camici G (2007) 5-Arylidene-2,4-thiazolidinediones as inhibitors of protein tyrosine phosphatases. Bioorg Med Chem 15:5137. https://doi.org/10.1016/j.bmc.2007.05.027
Maciel RM, Costa MM, Martins DB, França RT, Schmatz R, Graça DL, Duarte MM, Danesi CC, Mazzanti CM, Schetinger MR, Paim FC, Palma HE, Abdala FH, Stefanello N, Zimpel CK, Felin DV, Lopes ST (2013) Antioxidant and anti-inflammatory effects of quercetin in functional and morphological alterations in streptozotocin-induced diabetic rats. Res Vet Sci 2:389–397. https://doi.org/10.1016/j.rvsc.2013.04.028
Mahapatra DK, Bharti SK (2016) Therapeutic potential of chalcones as cardiovascular agents. Life Sci 148:154. https://doi.org/10.1016/j.lfs.2016.02.048
Mahapatra DK, Bharti SK, Asati V (2017) Chalcone derivatives: Anti-inflammatory potential and molecular targets perspectives. Curr Top Med Chem 17:3146. https://doi.org/10.2174/1568026617666170914160446
Mahmoud AM, Ashour MB, Abdel-moneim A, Ahmed OM (2012) Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proin fl ammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications 26:483–490. https://doi.org/10.1016/j.jdiacomp.2012.06.001
Manaharan T, Appleton D, Cheng HM, Palanisamy UD (2012) Flavonoids isolated from Syzygium aqueum leaf extract as potential antihyperglycaemic agents. Food Chem 132:1802–1807. https://doi.org/10.1016/j.foodchem.2011.11.147
Marques AM, Pereira SL, Paiva RA, Cavalcante CV, Sudo SZ, Tinoco LW, Moreira DL, Guimaraes EF, Sudo RT, Kaplan MA, Sudo GZ (2015) Hypoglycemic effect of the methanol flower extract of piper claussenianum and the major constituent 2′,6′-dihydroxy-4′-methoxychalcone in streptozotocin diabetic rats. Indian J Pharm Sci 77:237–243. https://doi.org/10.4103/0250-474x.156624
Mathur M (2013) Phyto-complex and their role in enhancing efficacy of herbal drugs. Med Plants 5:118–125. https://doi.org/10.5958/j.0975-6892.5.3.018
Matsuda M, Shimomura I (2013) Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 5:e330–e341. https://doi.org/10.1016/j.orcp.2013.05.004
Mazziotti I, Petrarolo G, La Motta C (2022) Aurones: golden resource for active compounds. Molecules 27:2. https://doi.org/10.3390/molecules27010002
Mehrabadi ME, Salemi Z, Babaie S, Panahi M (2018) Effect of biochanin a on retina levels of vascular endothelial growth factor, tumor necrosis factor-alpha and interleukin-1beta in rats with streptozotocin-induced diabetes. Can J Diabetes 42:639–644. https://doi.org/10.1016/j.jcjd.2018.03.008
Mei X, Zhang T, Ouyang H, Lu B, Wang Z, Ji L (2019) Scutellarin alleviates blood-retina-barrier oxidative stress injury initiated by activated microglia cells during the development of diabetic retinopathy. Biochem Pharmacol 159:82–95. https://doi.org/10.1016/j.bcp.2018.11.011
Michalkova R, Mirossay L, Gazdova M, Kello M, Mojzis J (2021) Molecular mechanisms of antiproliferative effects of natural chalcones. Cancers (basel) 13:2730. https://doi.org/10.3390/cancers13112730
Miyamoto S (2002) Molecular modeling and structure-based drug discovery studies of aldose reductase inhibitors. Chem-Bio Inform J 3:74–85. https://doi.org/10.1273/cbij.2.74
Morikawa T, Ninomiya K, Akaki J, Kakihara N, Kuramoto H, Matsumoto Y, Hayakawa T, Muraoka O, Wang LB, Wu LJ, Nakamura S (2015) Dipeptidyl peptidase-IV inhibitory activity of dimeric dihydrochalcone glycosides from flowers of Helichrysum arenarium. J Nat Med 69:494–506. https://doi.org/10.1007/s11418-015-0914-8
Mylari BL, Armento SJ, Beebe DA, Conn EL, Coutcher JB, Dina MS, O’Gorman MT, Linhares MC, Martin WH, Oates PJ, Tess DA, Withbroe GJ, Zembrowski WJ (2003) A highly selective, non-hydantoin, non-carboxylic acid inhibitor of aldose reductase with potent oral activity in diabetic rat models: 6-(5-chloro-3-methylbenzofuran-2-sulfonyl)-2-H-pyridazin-3-one. J Med Chem 12:2283–2286. https://doi.org/10.1021/jm034065z
Mylari BL, Armento SJ, Beebe DA, Conn EL, Coutcher JB, Dina MS, O’Gorman MT, Linhares MC, Martin WH, Oates PJ, Tess DA, Withbroe GJ, Zembrowski WJ (2005) A novel series of non-carboxylic acid, non-hydantoin inhibitors of aldose reductase with potent oral activity in diabetic rat models: 6-(5-chloro-3-methylbenzofuran-2-sulfonyl)-2H-pyridazin-3-one and congeners. J Med Chem 20:6326–6339. https://doi.org/10.1021/jm050462t
Narender T, Tanvir K, Rao MS, Srivastava K, Puri SK (2005) Prenylated chalcones isolated from Crotalaria genus inhibits in vitro growth of the human malaria parasite Plasmodium falciparum I. Bioorg Med Chem Lett 15:2453–2455. https://doi.org/10.1016/j.bmcl.2005.03.081
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790. https://doi.org/10.1038/35008121
Noratto GD, Chew BP, Atienza LM (2017) Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem 227:305–314. https://doi.org/10.1016/j.foodchem.2017.01.097
Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42:125. https://doi.org/10.1016/j.ejmech.2006.09.019
Obanda DN, Ribnicky DM, Raskin I, Cefalu WT (2014) Bioactives of Artemisia dracunculus L. enhance insulin sensitivity by modulation of ceramide metabolism in rat skeletal muscle cells. Nutrition 30:S59. https://doi.org/10.1016/j.nut.2014.03.006
Park HG, Bak EJ, Woo GH, Kim JM, Quan Z, Kim JM, Yoon HK, Cheon SH, Yoon G, Yoo YJ, Na Y (2012) Licochalcone E has an antidiabetic effect. J Nutr Biochem 23:759–767. https://doi.org/10.1016/j.jnutbio.2011.03.021
Perez-Gutierrez RM, Garcia-Campoy AH, Muñiz-Ramirez A (2016) Properties of flavonoids isolated from the bark of eysenhardtia polystachya and their effect on oxidative stress in streptozotocin-induced diabetes mellitus in mice. Oxid Med Cell Longev. https://doi.org/10.1155/2016/9156510
Perreault M, Marette A (2001) Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med 7:1138–1143. https://doi.org/10.1038/nm1001-1138
Pretz D, Heyward PM, Krebs J, Gruchot J, Barter C, Silcock P, Downes N, Rizwan MZ, Boucsein A, Bender J, Burgess EJ (2023) A dahlia flower extract has antidiabetic properties by improving insulin function in the brain. Life Metab 2:1. https://doi.org/10.1093/lifemeta/load026
Purnima S, Beena P, Mini R, Swaminathan P (2012) Study of the effect of Adhatoda zeylanica and some related synthesized chalcones on glucose diffusion in vitro. ARPB 2:259–263
Qaoud MT, Almasri I, Önkol T (2022) Peroxisome Proliferator-activated receptors as superior targets for treating diabetic disease, design strategies-review article. Turkish J Pharm Sci 19:353–370. https://doi.org/10.4274/tjps.galenos.2021.70105
Rauter AP (2018) Nomenclature of Flavonoids. Pure Appl Chem 90:1429–1486. https://doi.org/10.1515/pac-2013-0919
Rozmer Z, Perjési P (2016) Naturally occurring chalcones and their biological activities. Phytochem Rev 15:87–120. https://doi.org/10.1007/s11101-014-9387-8
Rudrapal M, Khan J, Dukhyil AA, Alarousy RM, Attah EI, Sharma T, Khairnar SJ, Bendale AR (2021) Chalcone scaffolds, bioprecursors of flavonoids: chemistry, bioactivities, and pharmacokinetics. Molecules 26:7177. https://doi.org/10.3390/molecules26237177
Sajeeth C, Manna P, Manavalan R, Jolly C (2010) Phytochemical investigation and antioxidant activity of a polyherbal formulation (ESF/AY/500) on streptozotocin induced oxidative stress in rats. Der Pharma Chem 2:184–189
Salehi B, Ata A, Anil Kumar N, Sharopov F, Ramirez-Alarcon K, Ruiz-Ortega A, Abdulmajid Ayatollahi S, Valere Tsouh Fokou P, Kobarfard F, Amiruddin Zakaria Z, Iriti M (2019) Antidiabetic potential of medicinal plants and their active components. Biomolecules 10:551. https://doi.org/10.3390/biom9100551
Samie A, Sedaghat R, Baluchnejadmojarad T, Roghani M (2018) Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci 210:132–139. https://doi.org/10.1016/j.lfs.2018.08.074
Schulze C, Bangert A, Kottra G, Geillinger KE, Schwanck B, Vollert H, Blaschek W, Daniel H (2014) Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol Nutr Food Res 58:1795–1808. https://doi.org/10.1002/mnfr.201400016
Seliger JM, Misuri L, Maser E, Hintzpeter J (2018) The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J Enzyme Inhib Med Chem 33:607–614. https://doi.org/10.1080/14756366.2018.1437728
Shabalala SC, Johnson R, Basson AK, Ziqubu K, Hlengwa N, Mthembu SX, Mabhida SE, Mazibuko-Mbeje SE, Hanser S, Cirilli I, Tiano L (2022) Detrimental effects of lipid peroxidation in type 2 diabetes: exploring the neutralizing influence of antioxidants. Antioxidants 11:2071. https://doi.org/10.3390/antiox11102071
Shen X, Zhou N, Mi L, Hu Z, Wang L, Liu X, Zhang S (2017) Phloretin exerts hypoglycemic effect in streptozotocin-induced diabetic rats and improves insulin resistance in vitro. Drug Des Devel Ther 11:313–324. https://doi.org/10.2147/DDDT.S127010
Soares JM, Pereira Leal AE, Silva JC, Almeida JR, de Oliveira HP (2017) Influence of flavonoids on mechanism of modulation of insulin secretion. Pharmacogn Mag 52:639. https://doi.org/10.4103/pm.pm_87_17
Stolf AM, Cardoso CC, de Morais H, de Souza CE, Lomba LA, Brandt AP, Agnes JP, Collere FC, Galindo CM, Corso CR, Spercoski KM (2018) Effects of silymarin on angiogenesis and oxidative stress in streptozotocin-induced diabetes in mice. Biomed Pharmacother 108:232–243. https://doi.org/10.1016/j.biopha.2018.09.042
Styskal J, Van Remmen H, Richardson A, Salmon AB (2012) Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med 52:46–58. https://doi.org/10.1016/j.freeradbiomed.2011.10.441
Su Y, Fan X, Li S, Li Z, Tian M, Li S (2022) Scutellarin improves type 2 diabetic cardiomyopathy by regulating cardiomyocyte autophagy and apoptosis. Dis Markers 2022:3058354. https://doi.org/10.1155/2022/3058354
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JC, Mbanya JC, Pavkov ME (2022) IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. J Diabetes Res 183:109119. https://doi.org/10.1016/j.diabres.2021.109119
Svetaz L, Agüero MB, Alvarez S, Luna L, Feresin G, Derita M, Tapia A, Zacchino S (2007) Antifungal activity of Zuccagnia punctata Cav.: evidence for the mechanism of action. Planta Med 73(1074–1080):1074. https://doi.org/10.1055/s-2007-981561
Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N (2019) SGLT2 Inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health 16:2965. https://doi.org/10.3390/ijerph16162965
Trojnar M, Patro-Małysza J, Kimber-Trojnar Ż, Leszczyńska-Gorzelak B, Mosiewicz J (2019) Associations between fatty acid-binding protein 4–A proinflammatory adipokine and insulin resistance, gestational and Type 2 diabetes mellitus. Cells 8:227. https://doi.org/10.3390/cells8030227
Tu L, Wang R, Fang Z, Sun M, Sun X, Wu J, Dang Y, Liu J (2022) Assessment of the hypoglycemic and hypolipidemic activity of flavonoid-rich extract from Angelica keiskei. Molecules 27:6625. https://doi.org/10.3390/molecules27196625
Van LV, Pham EC, Nguyen CV, Duong NT, Le Thi TV, Truong TN (2022) In vitro and in vivo antidiabetic activity, isolation of flavonoids, and in silico molecular docking of stem extract of Merremia tridentata (L.). Biomed Pharmacot 146:112611. https://doi.org/10.1016/j.biopha.2021.112611
Vandanmagsar B, Yu Y, Simmler C, Dang TN, Kuhn P, Poulev A, Ribnicky DM, Pauli GF, Floyd ZE (2021) Bioactive compounds from Artemisia dracunculus L. activate AMPK signaling in skeletal muscle. Biomed Pharmacot 143:112188. https://doi.org/10.1016/j.biopha.2021.112188
Wang Q, Ding ZH, Liu JK, Zheng YT (2004) Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antiviral Res 64:189. https://doi.org/10.1016/j.antiviral.2004.08.005
Wang Y, Xiang L, Wang C, Tang C, He X (2013) Antidiabetic and Antioxidant Effects and Phytochemicals of Mulberry Fruit (Morus alba L.) Polyphenol Enhanced Extract. PLoS ONE 8:e71144. https://doi.org/10.1371/journal.pone.0071144
Wang M, Gao LX, Wang J, Li JY, Yu MH, Li J, Hou AJ (2015) Diels-Alder adducts with PTP1B inhibition from Morus notabilis. Phytochemistry 109:140–146. https://doi.org/10.1016/j.phytochem.2014.10.015
Wirostko B, Wong TY, Simó R (2008) Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res 27:608–621. https://doi.org/10.1016/j.preteyeres.2008.09.002
Xu Y, Xie L, Xie J, Liu Y, Chen W (2019) Pelargonidin–3–O–rutinoside as a novel α-glucosidase inhibitor for improving postprandial hyperglycemia. Chem Commun (camb) 55:39–42. https://doi.org/10.1039/c8cc07985d
Yu Y, Simmler C, Kuhn P, Poulev A, Raskin I, Ribnicky D, Floyd ZE, Pauli GF (2019) The designer approach helps decipher the hypoglycemic bioactive principles of artemisia dracunculus (Russian Tarragon). J Nat Prod 82:3321–3329. https://doi.org/10.1021/acs.jnatprod.9b00548
Zhang W, Hong D, Zhou Y, Zhang Y, Shen Q, Li JY, Hu LH, Li J (2006) Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim Biophys Acta 60:1505–1512. https://doi.org/10.1016/j.bbagen.2006.05.009
Zhang N, Yang Z, Xiang SZ, Jin YG, Wei WY, Bian ZY, Deng W, Tang QZ (2016) Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: induced diabetic cardiomyopathy. Mol Cell Biochem 417:87–96. https://doi.org/10.1007/s11010-016-2716-z
Zhang W, Jin Q, Luo J, Wu J, Wang Z (2018) Phytonutrient and anti-diabetic functional properties of flavonoid-rich ethanol extract from Angelica Keiskei leaves. J Food Sci Technol 55:4406–4412. https://doi.org/10.1007/s13197-018-3348-y
Zhang J, Sun L, Dong Y, Fang Z, Nisar T, Zhao T, Wang ZC, Guo Y (2019) Chemical compositions and α-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chem 299:125102. https://doi.org/10.1016/j.foodchem.2019.125102
Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y (2020) Oxidative stress and diabetes: antioxidative strategies. Front Med 14:583–600. https://doi.org/10.1007/s11684-019-0729-1
Zhang M, He L, Liu J, Zhou L (2021a) Luteolin attenuates diabetic nephropathy through suppressing inflammatory response and oxidative stress by inhibiting STAT3 pathway. Exp Clin Endocrinol Diabetes 129:729–739. https://doi.org/10.1055/a-0998-7985
Zhang S, Xu M, Zhang W, Liu C, Chen S (2021b) Natural polyphenols in metabolic syndrome: protective mechanisms and clinical applications. Int J Mol Sc 11:6110. https://doi.org/10.3390/ijms22116110
Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z (2017) Chalcone: a privileged structure in medicinal chemistry. Chem Rev 117:7762. https://doi.org/10.1021/acs.chemrev.7b00020
Acknowledgements
We express our gratitude to Dr. H. N. More, Principal of Bharati Vidyapeeth College of Pharmacy, Kolhapur, for providing us with the necessary facilities to conduct our work. Authors are also thankful to Dr. Manish S. Bhatia, Vice-principal, Professor, and Head of the Department of Pharmaceutical Chemistry at Bharati Vidyapeeth College of Pharmacy, Kolhapur, and Dr. Neha V. Bhilare, Associate professor in the Department of Pharmaceutical Chemistry at Arvind Gavali College of Pharmacy, Satara, for their valuable inputs in this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marulkar, V.S., Bhatia, N.M. Chalcone and derived natural products: versatile scaffolds for multiple targets in treatment of Type 2 diabetes. Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-10007-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-10007-3