Abstract
Chronic exposure to ultraviolet radiation (UVR) leads to premature aging of the skin, with external manifestations of unsightly scars and internal molecular dysregulations that significantly reduce the protective function of the skin and increase the risk of cancer development. Photoprotection through daily application of sunscreen product is widely recommended to avoid UV-induced skin photodamage and to minimaze the risk for dermal malignancies. However, the environmental hazard that is a consequence of the use of traditional sunscreen products drives the increased interest in the investigation of alternative UVR blockers. Due to their structural diversity, modulation of multiple molecular mechanisms, and favorable safety profile, natural plant-derived compounds have become attractive candidates for skin photoaging prevention. This review summarizes the critical aspects of skin photoaging, from its pathological characteristics and current photoprotective options to the specific molecular players that emerge as therapeutic targets. Special emphasis has been placed on phytochemicals targeting the molecular hallmarks of UV-induced skin aging. The potential of plant molecules to control oxidative stress, inflammation, photo-senescence, DNA damage, extracellular matrix components degradation, and to manage different types of UV-trigerred cell death has been highlighted. Summarizing the molecular signalling pathways responsible for the photoprotective action of plant-derived molecules may provide meaningful outlook for development of new effective therapeutics options for prevention of skin photoaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The progressive development of aging hallmarks on skin is largely influenced by environmental factors such as pollutants, chemicals, solar radiation and detrimental lifestyle habits such as smoking. The most considerable alterations in the skin appearance and function occur upon direct exposure to ultraviolet radiation (UVR) to a degree that outreaches the capacity of skin defence mechanisms and is perceived as photoaging (Jin et al. 2020; Lee et al. 2022b; Wu et al. 2022).
The electromagnetic radiation of the solar spectrum that reaches the Earth’s surface includes UVB (280–320 nm), UVA (320–400 nm), visible (400–750 nm) and infrared (750–2500 nm) array. All other sunlight emissions with wavelength below 280 nm and above 2500 nm (gamma rays, UVC, micro-and radio waves) are absorbed within the atmosphere. The UV light comprises only about 2% of the solar irradiation that could interfere with human skin but it initiates significant biological response (Young et al. 2017; de Assis et al. 2021). Despite the fact that UVB has lower penetration depth than UVA, mostly affecting the epidermal basal layers, its action is about 1000 times more effective than UVA per unit dose (J/m2). In this regard, UVA waves reach deeper into the dermis but the chages induced on skin function are milder (Gęgotek et al. 2017; Young et al. 2017; Oliveira et al. 2019; Wang et al. 2019; de Assis et al. 2021; Fitsiou et al. 2021; Cui et al. 2022).
Notably, excessive exposure to solar UVR (UVA and UVB light) is the primary factor of extrinsic skin aging and is closely related to the pathogenesis of various injuries including sunburn, acute inflammation and skin neoplasia (Sand et al. 2018; Silva et al. 2020; Wang et al. 2021; Lee et al. 2022b; Li et al. 2022). The visible signs of photoaging that compromise the aesthetically pleasing appearance of the skin include leathery texture, erythema, edema, wrinkles, roughness, dryness and sagging, as well as irregularities in pigmentation, reduced recoil capacity, increased fragility and impaired wound healing (Natarajan et al. 2014; Young et al. 2017). More importantly, beyond the visual manifestations the photo-induced skin damage accelerates aging molecular mechanism and affects skin barrier function. The molecular hallmarks of photoaging consist of reduced dermal thickness resulting from expansion of the hypo/dermal white adipose tissue (WAT) layer; proteostasis failure associated with extracellular matrix (ECM) remodelling such as decreased collagen synthesis and increased elastin degradation and hyaluronan deficiency; diminished DNA damage response; apoptosis resistance; impaired autophagy regulation; histone modifications; chronic inflammation and premature senescence with elevated senescence-associated secretory phenotype (SASP) production. Therefore, the substantial damage that UVR exposure provokes on the skin motivates the increased interest in discovering potent ingredients for photoaging prevention that ensure skin beauty and health (Natarajan et al. 2014; Atanasov et al. 2021; Franco et al. 2021; Dańczak-Pazdrowska et al. 2023).
Natural products have long been exploited to treat a variety of skin disorders, such as wounds, burns, psoriasis and atopic dermatitis (Atanasov et al. 2021; Dańczak-Pazdrowska et al. 2023; Koycheva et al. 2023). Plant-derived molecules are used in various pharmaceutical products, cosmetics and in food industry due to their wide range of biological activities, including antioxidant, anti-inflammatory and anti-aging (Gęgotek et al. 2017; Garg et al. 2020; Domaszewska-Szostek et al. 2021; Merecz-Sadowska et al. 2021; Chaiprasongsuk and Panich 2022; Dańczak-Pazdrowska et al. 2023). Moreover, their relatively low toxicity and biodegradability expose phytochemicals as an attractive option for cosmetic and therapeutic agents than could limit the use of currently available sunscreen active ingredients (Gęgotek et al. 2017; Young et al. 2017; Garg et al. 2020; Merecz-Sadowska et al. 2021; Chaiprasongsuk and Panich 2022; Dańczak-Pazdrowska et al. 2023).
The intensified research on the molecular mechanism that drive UVR-induced skin aging during the past decade has formed the need of critical evaluation of the published data in the field. Keyword combinations used to obtain relevant articles were: “photodamage”, “photoaging”, “ultraviolet radiation”, “skin” and “natural compound”. The following databases have been accessed: Scopus, PubMed and ClinicalTrials.gov, or selected journals’ website. The inclusion criteria implied in the selection of articles were: (i) publications that investigated the modulation of skin aging induced by UVR; (ii) articles written in English; (iii) year of publication ≥ 2013.
In this review, we attempt to summarize the advancements in skin aging research with emphasis on the molecular mechanisms that drive UV-induced photodamage and strategies for prevention of photoaging. The therapeutic potential of bioactive phytochemicals for topical application as photoprotective and anti-photoaging ingredients has been highlighted.
Molecular hallmarks of UV-induced skin photoaging
The skin is a body sensor and a barrier against environmental factors such as external irritation, invasion of pathogens, and solar irradiation (Bustamante et al. 2020; de Assis et al. 2021; Lee et al. 2022b; Dańczak-Pazdrowska et al. 2023). The largest organ of the human body is comprised of epidermis, dermis and hypodermis (at the interchange with the dermal subcutaneous fat). The epidermal layer consists mainly from keratinocytes along with melanocytes and Langerhans cells (Koenig et al. 2020; Atanasov et al. 2021; Fitsiou et al. 2021). The further subdivision of the epidermis is based on the stage of differentiation of the keratinocytes and from the surface to the inner strata are as follows: conreum, lucidum, granulosum, spinosum and basale (Fig. 1). The inferior dermal skin segment is composed largely of fibroblasts that provide support and nutrition through regulation in ECM components production, such as collagen (the main ECM structural protein), elastic fibers, hyaluronic acid (essential for skin moisture), amino polysaccharides and glycoproteins (Kruglikov et al. 2019; Wong et al. 2022; Dańczak-Pazdrowska et al. 2023; Jenster et al. 2023). Within the dermis reside the hair follicles and glands as well as the major part of the skin vasculature and neurons. The hypodermal layer is often considered as shared region between the skin and subcutaneous white adipose tissue (sWAT) as it serves mainly as an energy storage depot rich in adipocytes. The dermal to sWAT communication is of critical importance for the regulation of the neuroendocrine function of the skin (Mariño et al. 2014; Young et al. 2017; de Assis et al. 2021; Wong et al. 2022).
Skin tissue architecture and representation of UVR photon energy to penetartion. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
Skin may adapt to UVR exposure by photoprotective mechanisms such as increasing keratinocyte cell division (stratum corneum thickening) and by tanning (melanogenesis). Acute exposure to high intensity UVA radiation induces immediate pigment darkening that is mediated by photo-oxidation of pre-existing melanin and redistribution of melanosomes (Moon et al. 2017; Martic et al. 2020; de Assis et al. 2021). This is followed by persistent pigment darkening that lasts 2–3 days. Then, delayed tanning is initiated, mainly by UVB-induced de novo melanin synthesis and an increased proliferation of active melanocytes (Moon et al. 2017). Melanogenic hormones are produced and secreted from epidermal keratinocytes. Following overexpression of the pro-opiomelanocortin (POMC) polypeptide, α-melanocyte-stimulating hormone (α-MSH) production is activated. In turn, α-MSH binds to melanocortin 1 receptor (MC1R) in melanocytes and mediates melanosome biogenesis (Moon et al. 2017; Choi et al. 2020; Martic et al. 2020). The microphthalmia-associated transcription factor (MITF) is involved in the transcriptional regulation of the melanogenic process including tyrosol activity and MC1R expression (Choi et al. 2020; Yun et al. 2020). The paired box gene 3 (Pax3) and cAMP responsive element-binding protein (CREB) directly interact with MITF and regulate its transcriptional activity (Hurbain et al. 2018; Choi et al. 2020; Yun et al. 2020). Despite the fact that melanogenesis is a protective mechanism against UVR-induced skin damage, it is generally insufficient to prevent UVR-mediated mutagenic effects and premature skin aging (Moon et al. 2017; Hurbain et al. 2018; Bustamante et al. 2020; Choi et al. 2020; Yun et al. 2020). Moreover, prolonged overproduction of melanin thus has unfavorable consequences such as dysregulated pigmentation and elevated oxidative stress burden (Natarajan et al. 2014; Moon et al. 2017; Bustamante et al. 2020; Martic et al. 2020). Therefore, when considering modulation in the process of melanogenesis as a physiological photoprotective mechanism, a balance should be kept between overactivation and suppression.
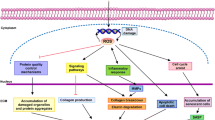
Different paradigms are proposed to explain the molecular basis for UVR-induced skin aging including the theory of premature cellular senescence, that include accumulation of DNA damage, decreased DNA repair capacity, telomere attrition; the theory of oxidative stress accumulation and the theory of chronic skin inflammation (Natarajan et al. 2014; Correia-Melo et al. 2016; Chen et al. 2018; de Pedro et al. 2018; Fenini et al. 2018; Victorelli et al. 2019; Alafiatayoa et al. 2020; Martic et al. 2020; de Assis et al. 2021; Katayoshi et al. 2021; Dańczak-Pazdrowska et al. 2023; Jenster et al. 2023; Zhang et al. 2023). Despite that none of this is conclusive, there is a solid data on the molecular mechanisms involved in photoaging (Correia-Melo et al. 2016; Chen et al. 2018; de Pedro et al. 2018; Fenini et al. 2018; Victorelli et al. 2019; Alafiatayoa et al. 2020; Bustamante et al. 2020; de Assis et al. 2021; Fitsiou et al. 2021; Katayoshi et al. 2021; Zhang et al 2023). Overview of the molecular and extrinsic hallmarks of photoaging is outlined in Fig. 2.
Hallmarks of photoaging. Excessive prolonged exposure of the skin to the effects of ultraviolet radiation leads to premature skin aging. Characteristic of the photoaging process are external manifestations on the skin as erythema, epidermal thickness, dysregulated pigmentation, tan darkening, sunburn, as well as transepidermal water loss and compromised skin elasticity, deep wrinkle formation, delayed wound healing and risk of non-melanoma skin cancer development. All these visible signs develop as a result of an accumulation of molecular changes in skin function characterized by excessive reactive oxygen species (ROS) generation, depletion of endogenous antioxidant enzymes, extracellular matrix (ECM) elements degradation as results of matrix metalloproteinases (MMPs) overexpression, dysregulated proteostasis, abnormal lipid oxidation, DNA damage, premature senescence, inflammation, mitochondrial dysfunction, reinforced cell death and deregulated autophagy. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
The aged epidermis is characterized by physiological and genetic perturbations, such as impaired balance in nicotinamide adenine dinucleotide (NAD+) levels, diminished activity of sirtuins (SIRT) and activation of the "guardian of the genome" tumor protein p53 due to oxidative stress and telomere shortening (Natarajan et al. 2014; Wong et al. 2022; Dańczak-Pazdrowska et al. 2023). Oxidative stress induced by UVR is a major contributor to the process of skin aging and inflammation through the activation of signalling pathways such as the mitogen-activated protein kinase (MAPK)/activator protein-1 (AP-1), the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), the nuclear factor kappa B (NF-κB), the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, and kelch-like ECH-associated protein 1(KEAP1)/nuclear factor erythroid 2-related factor 2 (NRF2) in epidermal keratinocytes and dermal fibroblasts (Fig. 3). Overproduction of reactive oxygen and nitrogen species (ROS and RNS, respectively) induced by UVR also plays a role in the regulation of melanogenesis via MAPK/AP-1, PI3K/Akt and MC1R/MITF signalling. Keratinocyte growth factor 2 (KGF2), also known as fibroblast growth factor 10 (FGF10), protects the skin from the harmful effects of UVR by regulating tissue regeneration and antioxidant defence systems (Feng et al. 2022; Robinson et al. 2022). The mechanism by which KGF2 reduces ROS levels, restores the cell cycle, protects cells from mitochondrial dysfunction and activates antioxidant defence mechanisms is expressed in the activation of aryl hydrocarbon receptor and NRF2 (Natarajan et al. 2014; Xu et al. 2014; Alafiatayoa et al. 2020; Hegedűs et al. 2020; Lone et al. 2020; Katayoshi et al. 2021; Feng et al 2022; Lee et al. 2022b; Robinson et al. 2022; Wong et al. 2022; Li et al. 2023).
Molecular signalling pathways involved in UV-induced skin photoaging. Exposure to UVR causes excessive ROS accumulation and activation of molecular signalling pathways in the skin aiming at prevention of photodamage. Among the major UV-initiated molecular cascades involved in photoaging process are MAPKs/AP-1, KEAP1/NRF2, TGF-β/Smad, PI3K/Akt/mTOR, AMPK/SIRT and Wnt/β-catenin signalling pathways. Overactivation of these molecular networks by intensive or prolonged UVR exposure results in decreased collagen synthesis, ECM degradation, DNA damage, mitochondrial dysfunction, skin inflammation, premature senescence, impared cell proliferation, migration and regeneration as well as increased cell death and apoptosis. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
Cellular senescence is a physiological response to accumulated stress, characterized with durable cell-cycle arrest of previously replication-competent cells, overexpression of anti-proliferative molecules such as the cyclin-dependent kinase inhibitor 1 (p21) or 2A (p16INK4a) and activation of damage sensing signalling pathways (e.g., p38/MAPK and NF-κB). These molecular changes result in elevated expression of a number of senescence-associated transcripts that define the SASP of the aged skin cells, namely senescence-associated-β-galactosidase (SA-β-Gal), matrix metalloproteinases (MMP)-1, -3 and -9, interleukin 1 beta (IL-1β), IL-6 and IL-8, chemokines and growth factors (Childs et al. 2015; Victorelli et al 2019; Alafiatayoa et al. 2020; Fitsiou et al. 2021). Epidermal senescence disrupts skin barrier permeability by loosened tight junctions, excessive water loss and increased production of SASP-associated molecules (Victorelli et al. 2019). The senescence state initiates shift in the morphology and functionality of cellular components. Specifically, in fibroblasts UV-triggered photo-senescence promotes the secretion of ECM degrading MMPs. With regard to melanocytes, senescence mechanism has not been fully understood but recent investigations have proposed that aged melanocytes contribute to the skin aging process by impairing proliferation of the neighboring keratinocytes through a paracrine signalling (Moon et al. 2017; Hurbain et al. 2018; Martic et al. 2020).
Collagen as the most abundant structural protein in the skin is responsible for its stability and tensile strength. Collagen fibers predominantly consist of type I (85%–90%) and type III (10%–15%) collagen. The ECM structure is fine-tunned by the MMPs family that is comprised of more than 23 proteases, including MMP-1 (collagenase-1), MMP-3 (stromelysin-1), MMP-7 (matrilysin), MMP-9 (gelatinase B), MMP-10 (stromelysin-2), MMP-12 (metalloelastase), and MMP-13 (collagenase-3) (Natarajan et al. 2014; Lee et al. 2022a; Alafiatayoa et al. 2021; Fitsiou et al. 2021; Dańczak-Pazdrowska et al. 2023). Among these, MMP-1 is responsible for the breakdown of the dominant subtypes of fibrillar collagens in dermal ECM—type I and III that are further cleaved by MMP3 and MMP9 (Natarajan et al. 2014; Oliveira et al. 2019; Wang et al. 2019; de Assis et al. 2021; Gao et al. 2021; Xiao et al. 2022). Additionally, MMPs activity is regulated by tissue inhibitors of metalloproteinases (TIMPs) and intracellular level of Ca2+ and Zn2+ ions (Xuan et al. 2019; Xiao et al. 2022). Overproduction of ROS is a trigger of UV-stimulated MMPs expression in human keratinocytes and fibroblasts that is associated with skin photoaging process (Hseu et al. 2018; Muzaffer et al. 2018; Hao et al. 2019; Xue et al. 2022). Consequently, exploring natural molecules that diminish ROS production, inhibit MMPs expression, reduce collagen degradation or to promote collagen synthesis could be an approach to ameliorate UV-induced skin photodamage.
Mitochondria are the major source of ROS in UVB-irradiated keratinocytes. As the inflammatory response is a crucial innate protective mechanism, it is promptly triggered after epidermal damage forced by UV irradiation. Senescent cells typically develop mitochondria with abnormal characteristics, such as point mutations and deletions of mitochondrial DNA (mtDNA) (Correia-Melo et al. 2016; Baek et al. 2017; Zhang et al. 2020, 2023; Feng et al. 2022). Defective mitochondria produce insufficient ATP and often are source of more ROS that exacerbates oxidative stress. Feed-forward loop exists between regulated cell death (RCD) and inflammation. Consequently, UVR-induced chronic inflammation and stratification of the epidermis could be attributed to mitochondrial dysfunction resulting from inflammasome NLR pyrin domain containing (NLRP) inflammasome activation and cell death induction (Sand et al. 2018; Robinson et al. 2022; Jenster et al. 2023; Lee et al. 2022b; Li et al. 2023).
Collectively, the solid mechanistic data on the complex photoaging process generated through the past 2 decades provide options for development of novel therapeutic strategies targeted against skin photodamage. Activation of the intrinsic cellular defence mechanisms to resist UV-induced skin aging would complement the currently used photoprotective agents or even replace them at certain point with more effective and environmentally friendly active compounds.
Current photoprotective and therapeutic options against photoaging
Prolonged and acute direct exposure to solar irradiation intensifies the skin aging process and leads to photodamage and deleterious effects on human skin (Gęgotek et al. 2017; Fitsiou et al. 2021; Franco et al. 2021). Limiting the time of direct sunlight exposure especially when the UVR intensity is high during the day is critical to reduce the risk of accelerated skin photoaging and development of skin malignancies. The primary step for skin protection that remains the most effective approach against UV-induced damage is the use of sunscreen products, which in addition minimize acute skin injury (Gęgotek et al. 2017; Young et al. 2017; Garg et al. 2020; Dańczak-Pazdrowska et al. 2023). Sunscreen products vary by their UV absorbtion range (UVA, UVB or both UVA/UVB) and by sun protection factor (SPF) value (Young et al. 2017; Bustamante et al. 2020; de Assis et al. 2021; Dańczak-Pazdrowska et al. 2023). The SPF is adopted as a measure to guide the consumers of the level of protection and represents the amount of UVR required to produce sunburn on protected skin relative to the solar energy that initiates sunburn on unprotected skin. However, various factors impact the amount of solar radiation that initiate sunburn (i.e. skin tone, amount and frequency of sunscreen application, time spent at direct sun light, daily and seasonal solar cycles) that are not reflected by the SPF value (Young et al. 2017; Duan et al. 2019; Bustamante et al. 2020; Dańczak-Pazdrowska et al. 2023). The photoprotective ingredients currently approved for use in sunscreen products are regulated as over-the-counter (OTC) drugs by the Food and Drug Administration agency (FDA) in the United States of America (USA) and as cosmetics within the European Union (Table 1). The FDA regulations restrict the use of UVR blockers to several active ingredients while in Europe the list of compounds that absorb or reflect UVA and UVB light has greater variety. Depending on their mechanisms of action, to absorb or to scatter/reflect UV light, sunscreens are classified as chemical or physical, respectively. Prolonged repeated use of UVR blockers-containing cosmetic products is recommended as primary skin prevention from sunburn (Yamada et al. 2020; Ferreira et al. 2023). Protection by sunscreens inhibits many of the acute and chronic effects of UVR exposure and can delay the development of photoaging and photocarcinogenesis (Young et al. 2017; Yamada et al. 2020). Usually, the recommendation for the use of sunscreen products is about 2 mg/cm2, evenly applied every 2 h (Young et al. 2017; Yamada et al. 2020). Their safety for human health under normal conditions of use is secured by legislative restrictions on the content of UV filters or blockers in the sunscreen products (Table 1). However, several major concenrs about their toxicity in long-term use remain a subject of discussion (Garg et al. 2020). Chemical sunscreens are the most commonly marketed sunscreens, typically containing a combination of active ingredients such as oxybenzone, avobenzone, octisalate, octocrylene, homosalate, benzophenone-3, para-amino benzoi acid (PABA) and octinoxate in alcohol-based or lipophilic formulations, that delivery of actives into the stratum corneum to prevent UV radiation. The oxybenzone is one of the most commonly used sunscreen ingredient. Due to reported toxicity and adverse effects such as causing oxidative stress and cell death it is permitted in formulations up to 6% by FDA. A negative impact of sunscreens on environment such us death of coral reefs, has led to a ban on the use of sunscreens containing oxybenzone by states like Hawaii (Yamada et al. 2020; de Assis et al. 2021). Benzophenone-3 has been reported to exhibit allergenic properties (Garg et al. 2020).
The main active ingredients with optical properties that act as physical sunscreens are the minerals, titanium dioxide and zinc oxide. Despite their effectiveness, safety and broad-spectrum protection, the physical UV filters, their use were limited until nano-sized versions were developed. A main risk in their use is increased penetration due to decreased size (Yamada et al. 2020). The TiO2 is widely accepted because it does not pass through the stratum corneum and is effective in UVB penetration, but simultaneously failing to prevent UVA penetration, while better UV protector catalyses the production of a large number of free radicals, leading to the damage of DNA and proteins (Garg et al. 2020). Another disadvantage of inorganic UV filters is that UV irradiation of metal oxides in sunscreen products can lead to the generation of ROS and degradation of organic compounds may occur and there is no data to support their long-term safety (Garg et al. 2020; Ferreira et al. 2023). Although a number of studies have shown that nanoparticles do not penetrate the dermis and there are no systemic effects, thera are upcoming questions about their effects on the skin, and whether nanoparticles cause cellular stress. Despite proven sunscreen prevention of skin cancer and photoageing an amount of researches in vitro, in vivo, and clinical studies focused on the toxicity and delivery of sunscreens, reported existance of an important knowledge gap in sunscreen-skin interactions (Yamada et al. 2020). In conjunction with toxicity of approved sunscreen ingredients is growing interest to reveal the therapeutic potential of bioactive phytochemicals as alternative topical photoprotective and anti-photoaging candidates (Ferreira et al. 2023).Apart from the sunscreen products, the treatment options for skin photodamage are limited to drugs for topical application, optical therapy, stem cell-based therapy, cytokine injections. The available drugs to treat the consequences of UV-induced photodamage include topical retinoids, chemical peels, antioxidants, corticosteroids and 5-fluorouracil. Their mechanism of action and common side effects are summarized in Table 2. Alternatively, laser therapy and different phototherapies (carbon dioxide, ablative and photodynamic therapies), fractional and nonabrasive cutaneous resurfacing radiofrequency techniques (microdermabrasion, microneedling), surgical rejuvenation procedures (dermabrasion) are also available to treat the visible signs of photoaging (Xu et al. 2014; Young et al. 2017; Dhaliwal et al. 2019; Rusu et al. 2020; de Assis et al. 2021; Hur et al. 2022; Zou et al. 2022; Dańczak-Pazdrowska et al. 2023; Takaya et al. 2023). Considerable interest has been focused on stem cells as therapeutic option in skin regeneration during the past 2 decades (Xu et al. 2014; Dhaliwal et al. 2019; Zou et al. 2022; Takaya et al. 2023). Solid experimental evidence demonstrate that the combinatorial treatment with adipose-derived stem cells and fractional carbon dioxide laser therapy improves the healing process of UVB-induced photoaging skin via canonical Wnt/β-catenin signalling pathway of dermal fibroblast activation (Xu et al. 2014).
Recent research has demonstrated compromised effectiveness and safety of certain UV filters due to their absorption through the skin and risk of reaching systemic circulation, which can causes detrimental effects such apoptosis induction or elevated mutagenesis. Moreover, UV filters are becoming emergent contaminants in the surface waters as mentioned above (Ferreira et al. 2023).
Notably, it is challenging to develop effective, photostable and safe products that protect from the harmful impact of UVR on skin. Although the efficacy of the available photoprotective agents has been proven, the necessary repeated long-term use to achieve results, limited safety profile, as well adverse effects they can cause and the environmental risk entails the discovery of novel photoprotective agents (Garg et al. 2020; de Assis et al. 2021; Dańczak-Pazdrowska et al. 2023; Ferreira et al. 2023). The use of plant-derived natural products to attenuate the manifestation of UV-induced skin aging has progressively gained attention. Natural compounds of plant origin like polyphenols (resveratrol), flavonoids (kaempferol and quercetin) and phenolic acids (gallic acids) are promissing candidates to becoming an alternative to typical synthetic UV filters (Ferreira et al. 2023).
Phytochemicals emerging as photoprotective agents
Plants are utilized as a source of bioactive molecules to preserve human health from centuries (Atanasov et al. 2021). Several plant secondary metabolites classes, including phenolic compounds, alkaloids and carotenoids, are well known for their relevant photoprotective activity, ability to prevent photoaging and related skin diseases (Garg et al. 2020; Domaszewska-Szostek et al. 2021; Merecz-Sadowska et al. 2021; Chaiprasongsuk and Panich 2022; Dańczak-Pazdrowska et al. 2023; Koycheva et al. 2023). Analysis of the contemporary photoaging research has provided evidence for natural compounds that have merged as UVR blockers and photoprotective agents (Fig. 4). In this section, an attempt has been made to classify the natural compounds with photoprotective potential according to their molecular mechanisms of action (Table 3).
Modulation of the molecular mechanisms of UVR-induced cell death
The current view on cell death of keratinocytes defined their specific form of uncontrolled cell death as cornification. During cornification, the terminally differentiating keratinocytes in the upper layers of the epidermis rise up through the granular layer, they are replaced by newly formed granular cells that fill the space of the previous and uncontrolled cell death occurs (de Pedro et al. 2018, 2021; Koenig et al. 2020). Regulated cell death (RCD) is one of the main responses of skin cells against UVR-induced oxidative stress (Natarajan et al. 2014; Orioli and Dellambra 2018; Rice and Rompolas 2020; Zhang et al. 2020; de Assis et al. 2021; Barresi et al. 2022; Chen et al. 2022a, b, c; Feng et al. 2022; Song et al. 2023). In contrast to biologically uncontrolled cell death, RCD is controlled under structured signalling cascades and defined molecular mechanisms. During RCD process, damage-associated molecular patterns and proinflammatory cytokines could be released from necroptotic cells to activate the immune response. Recently, the RCD types have been re-defined into different modalities including intrinsic apoptosis, extrinsic apoptosis, pyroptosis, necroptosis, and feroptosis (Mariño et al. 2014; Baek et al. 2017; Chen et al. 2018; de Pedro et al. 2018; Orioli and Dellambra 2018; Koenig et al. 2020).
Apoptosis
Increased apoptosis rate caused by UVR is a major molecular hallmark of photoaging. Apoptosis is the most common type of RCD and is associated with increased outer mitochondrial membrane (OMM) permeability, DNA fragmentation and caspase activation. Upon prolonged exposure to high intensity UVR the intracellular calcium levels in the endoplasmic reticulum (ER) are depleted, thereby increasing the expression of the pro-apoptotic caspases 3 and 9, Bcl-2 and Bax, hence, provoking increase in ROS generation and accelerating apoptotic death in its final stage (Alafiatayoa et al. 2020; Lone et al. 2020; Feng et al. 2022).
Poly (ADP-ribose) polymerase (PARP) is a multifunctional zinc finger protein that is activated in UVB-induced apoptosis and regulates DNA damage-related genes, such as the serine/threonine kinase ATM activation and the tumor suppressor protein p53, and interacts with adenoside monophosphate-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling. Inhibition of PARP1 which constitutes 85–90% to total PARP, reduces the amount of cyclopyrimidine dimers (CPDs) formed after UV irradiation, which promotes the activation of the nucleotide excision repair (NER) pathway (Li et al. 2023). Pharmaceutical inhibition of PARP1 and PARP2 is a future prospect to prevent loss of mitochondrial activity resulting from NAD+ and ATP depletion (Mariño et al. 2014; Li et al. 2023). Given that UVB cleaves caspase-3 and activates PARP both can be used as molecular markers for UV-induced apoptosis in keratinocytes (Lin et al. 2019). In UVR-induced chronic inflammation, PARP expression is upregulated upon cell cycle arrest, which induces the activation of apolipoprotein D (ApoD). The ApoD is a marker involved in oxidative stress-induced fibroblast senescence and mitochondria are its main source (Correia-Melo et al. 2016; Chen et al. 2018; Fitsiou et al. 2021). By regulating pathways related to mitochondrial biogenesis, activated ApoD could be exploited as a target against skin aging (Takaya et al. 2023). In intrinsic apoptosis endonuclease G enzyme is released upon OMM permeabilization that translocates into the nucleus and accelerates the process of chromosomal DNA fragmentation. In response to DNA damage, PARP1 is hyperactivated and mitochondria-associated apoptosis-inducing factor 1 is synthesized, leading to mitochondrial depolarization, resulting in stimuli causing lysosomal permeabilization to release cathepsin proteases into the cytosol. This mechanism of action is linked to a new form of regulated caspase-independent cell death called parthanatos and apoptosis-inducing factor 1 has been linked as a marker. Released cathepsins can also increase OMM permeability, thereby stimulating intrinsic apoptosis (Mariño et al. 2014; Lin et al. 2019).
During UVB irradiation, mitochondria-generated H2O2 can circulate freely in the cytosol, further increasing cellular H2O2 levels and causing the accumulation of oxidative stress associated with the activation of apoptosis. The enzyme peroxiredoxin III (PrxIII), expressed in all layers of the epidermis, extensively reduces H2O2 levels, thereby protecting skin cells from photodamage. Subsequently PrxIII is reduced by thioredoxin, thioredoxin reductase, and reduced nicotinamide adenine dinucleotide phosphate (NADPH) and drives the cells to apoptosis through loss of OMM permeability, activation of caspases and release of cytochrome C (Baek et al. 2017). Damaged mitochondria resulting from an imbalance or mutations between mitofusin 1 and 2 proteins, responsible for the process of fusion and fission in mitochondrial dynamics, affects the innate immune system by detachment of multiple pro-inflammatory signals, like ROS, which trigger the NLRP inflammasome and cyclic GMP/AMP synthase (cGAS)/stimulator of IFN genes (STING) pathways (Katayoshi et al. 2021; Lee et al. 2022a; Jenster et al. 2023; Li et al. 2023). It is assumed that exposure of HaCaT cells to UVB light increases the number of mitochondria, due to activation of mitochondrial fission and/or inhibition of mitochondrial fusion resulting in ROS generation and activation of the NLRP3 inflammasome and cGAS-STING molecular pathway, ultimately inducing skin inflammation and cell apoptosis. The link between mitochondrial dynamics and NLRP3/cGAS-STING inflammasome pathways/apoptosis in HaCaT cells sheds a new insight for understanding UVB irradiation-caused skin injury. In photodamaged skin, STING exerts a regulatory role by activating the NF-κB inflammatory pathway and causing stimulation of interferron gamma (IFNγ) secretion, leading cells to undergo apoptosis. The dynamin-related protein 1 (DRP1) mediates the OMM fusion of neighboring mitochondria and is proposed to participate in UV-induced skin damage. During fission events, the DRP1 is recruited to the OMM where it binds to outer membrane-anchored adaptor proteins to mediate membrane constriction. Targeted DRP1 inhibition in UV-exposed keratinocytes has been found to counteract mitochondrial fission and inflammatory skin aging (Zhang et al. 2020; Li et al. 2022, 2023).
The UVR damages the antioxidant defence system, leading to dermo-epidermal stress and generation of phototoxic products that accelerate skin aging. In human fibroblasts, genes encoding antioxidant enzymes are suppressed under the influence of ROS. Prostaglandinendoperoxide synthase 2 (PTGS2) gene is encoding cyclooxigenase 2 (COX-2) enzyme that governs the response to UVR. For this reason, UV light is also used in the treatment of skin diseases by promoting apoptotic cell death. Activated tumour necrosis factor alpha (TNF-α) communicates with epithelial and keratinocyte cells, promoting the synthesis of an inflammatory response and leading to skin photodamage (Alafiatayoa et al. 2020; Rice and Rompolas 2020; Silva et al. 2020).
Properly functioning ER and balanced intracellular Ca2+ levels are of critical importance for the physiological keratinocyte differentiation and apoptotic signalling. To maintain proper disulfide bond formation and protein folding, the ER lumen maintains higher Ca2+ concentrations (de Pedro et al. 2021; Dańczak-Pazdrowska et al. 2023). As a result, ER release Ca2+ ions that fuse into mitochondria and promote cell death in different phases of the cell cycle. Excessive UV-mediated oxidative stress in the ER lumen leads to the accumulation of misfolded proteins, followed by activation of the unfolded protein response (UPR). To protect skin cells against ER stress, glucose-related protein 78 (GRP78)/Bip is significantly important as it promotes protein folding and participates in the restoration of proteostasis. Together with hydroxymethylglutaryl coenzyme A reductase degraded protein 1 (Hrd1), which is able to degrade misfolded or unfolded endoplasmic reticulum accumulation to protect skin cells against ER stress (Natarajan et al. 2014; Jin et al. 2020; Lone et al. 2020; Dańczak-Pazdrowska et al. 2023). The activation of UPR is regulated by ER transmembrane sensor proteins that are inositol-requiring enzyme 1 alpha, double-stranded RNA-dependent protein kinase-like ER kinase (PERK), and activating transcription factor 4 (ATF4) while their up-regulation is prevented by GRP78/Bip under physiological conditions. Unresolved and prolonged ER stress activates PERK/eIF2α/ATF4 signalling pathway that shifts the adaptive response towards cell death via up-regulation of CAAT/enhancer-binding protein homologous protein (CHOP) that on its turn increases oxidative stress, energy depletion, and finally leads to apoptosis (Jin et al. 2020; Lone et al. 2020).
Interaction between the myelocytomatosis oncogene (MYC) and the transcriptional yes-associated protein (YAP) protein results in regulation of cell growth, differentiation, focal adhesion and apoptosis in skin cells (de Pedro et al. 2018, 2021; Silva et al. 2020). The YAP transcription factor could also modulate the expression of other major cell cycle regulating genes including p73, Peroxisome proliferator-activated receptor gamma (PPAR-γ), member of the mothers against decapentaplegic homolog (Smad) family, Forkhead box O 1 (FOXO1), and TEA domain transcription factor 1. The YAP translocation into the nucleus is prevented by phosphorylation at Ser127, which is inhibited during UVB irradiation. Doxorubicin- and cisplastin-induced or UV-mediated DNA damage is mediated by the p73 pathway and YAP promotes a proapoptotic response. As a consequence of UVB irradiation the transcriptional levels of Bax and p21 are increased, which are target genes of the YAP/p73 axis, leading to cell death (Natarajan et al. 2014; Moya and Halder 2019; Liu et al. 2022a; Dańczak-Pazdrowska et al. 2023). Pretreatment with silibinin at a 200 μM concentration, a flavonolignan isolated from Silybum marianum, affects the increased expression of YAP/p73-related genes including Bax, p21 and RING finger protein 71, inhibiting UVB-induced apoptosis and increasing growth ability (Liu et al. 2022a).
There is a growing interest in the role of microRNAs (miRNAs) in regulating UVB-induced cell death. For instance, in UVB-mediated extrinsic apoptosis, activation of the TNF-α receptor signalling leads to cleavage of caspase-8 and caspase-3, which downregulates miR-133a-3p expression (Song et al. 2023). Despited being a synthetic anti-diabetic drug, metformin chemical structure has been inspired by natural biguinide compounds from Galega officinalis and its anti-aging properties has been a subject of intense reseach during the past decade. Topical application of metformin 0.6% in keratinocytes and hairless mice, has been reported to prevent UVB-trigerred apoptosis through increased expression of miR-133a-3p and the cylindromatous lysine 63 deubiquitinase (CYLD) thus being a potential therapeutic application to prevent photodamage (Chen et al. 2022a, b, c; Song et al. 2023).
Substantial amount of research reported that natural compounds hold photoprotective potential mediated through inhibition of apoptosis (Cui et al. 2022; Xue et al. 2022). The sesquiterpene zerumbone (2.5–10 μM) reversed UVA-induced excessive ROS accumulation, DNA single-strand breaks, apoptotic DNA fragmentation and the dysregulated Bax/Bcl-2 ratio in skin keratinocytes (Yang et al. 2018; 2023). The purified polysaccharide fucoidan (25–100 μM) and the O-methylated isoflavone tectorigenin (0.1–10 μM) also regulated caspase-3, Bax and Bcl-2 in UVB-irradiated HaCaT and zebrafish models (Noh et al. 2018; Su et al. 2020). The increase of cell viability of the keratinocytes as well as the decrease of caspase-3 and caspase-9 expression as a result of the stilbenoid resveratrol application (20–40 μM) in UVB-stimulated HaCaT cells and ICR mice, contributes to resveratrol photoprotective action (Cui et al. 2022). Theaflavin-3′-gallate (0.1–40 μM) that is an oxidated polyphenol protects HaCaT cells from UVB-induced apoptosis and necrosis (Zheng et al. 2021). The polyphenol-rich fraction of Houttuynia cordata Thunb. extract (25–100 μg/mL) exhibited protective effects on UVB-induced apoptosis markers capase-3 and caspase-9 and its downstream substrate cleaved PARP-1 and also the cell apoptosis in UVB-induced HaCaT. These effects have been associated to the high content of the polyphenolic compounds quercitrin and hyperoside and the modulation of cell death mechanisms through p38/MAPK/ERK and PI3K/Akt signalling pathways (Charachit et al. 2022). Further, quercitrin (100 μM) restored the catalase expression and glutathione (GSH) level, leading to reduction of oxidative DNA damage, regulation of cleaved PARP1 and cleaved caspase-3 and NF-κB caused by UVB exposure in vitro (Yin et al. 2013). Chlorogenic acid (0.1–10 μM) regulated UVA-induced apoptosis in human dermal fibroblasts (HDFs) through downregulation of cleaved-PARP (Xue et al. 2022).
Cryptotanshinone (0.02–0.1 μM) is a diterpenoid antraquinone that has been reported to effectively inhibit apoptosis through suppression of caspase-3 and -9 activity in UVB- and UVA- induced in human epidermal keratinocytes and dermal fibroblasts (Guo et al. 2022). Pretreatment with dihydrocaffeic acid (7–35 μM) attenuated UVB-mediated cellular damage, evidenced by decreased cell death, lipid peroxidation, apoptosis/necrosis and its markers (loss of mitochondria membrane potential, DNA condensation, and cleaved caspase 9 expression) in UVB-exposed L929 fibroblasts (Oliveira et al. 2019).
Taken altogether, UV-induced photodamage initiates abnormal increase in apoptosis rate in skin tissue. Several emerging molecular target pathways that participate in the regulation of this response have been highlighted including PARP/ApoD axis, DRP1/AMPK/SIRT signalling and YAP/p73 pathway, along with the well-defined p53, Bcl, Bax, caspase-3 and -9. Compounds that modulate UV-mediated apoptosis through one or more of these molecular pathways represent potential candidates for novel photoprotective agents.
Autophagy
In aging skin, autophagy provides a cytoprotective defence by targeting impaired proteins and organelles to lysosomal degradation. It is important to acknowledge that autophagy is a dynamic process with dual role that is maintaining both cell viability and epidermal stratification (Mariño et al. 2014; Correia-Melo et al. 2016; Chen et al. 2018; Koenig et al. 2020; Barresi et al. 2022). Autophagy operates as a pro-survival stress response by a cascade of conversion steps leading to the formation of a phagophore and recycling damaged cellular components (Mariño et al. 2014; Koenig et al. 2020). On the other hand, it can facilitate cell death through activation of lysosomal proteases, such as cathepsin D that has functional relevance for cornification. Suppression of autophagy causes detrimental changes in the differentiation and aging-associated features of epithelial skin cells (Childs et al. 2015; Koenig et al. 2020; Wang et al. 2021). Replicative DNA damage and disrupted cellular homeostasis in UVR-exposed keratinocytes can induce autophagy deficiency and lead to premature senescence of fibroblasts closely related to aging (Mariño et al. 2014; Chen et al. 2018; Narzt et al. 2019; Barresi et al. 2022; Kim et al. 2022a, b). Further, autophagy is crucial to melanosome recycling and prevention of pigmentation disorders. The exact role of the autophagosome-lysososmal activity in melanocytes turnover—especially in response to UVR exposure is not completely understood. Excessive UVB irradiation impairs the proteasome and increases autophagic activity in melanocytes (Mariño et al. 2014; Chen et al. 2018; Martic et al. 2020; Barresi et al. 2022). The UV-induced cellular damage affects autophagy usually through changes in autophagy adaptor sequestosome 1 (SQSTM1)/p62 receptor complex. Oxidative stress induced as a result of UVR is regulated by the p38/MAPK activation and is closely related to the regulation of unc-51-like kinase 1 (ULK1) by mTOR signalling. The ULK1 participates in a complex with the autophagy-related (Atg) genes that is involved in the maturation and initiation of autophagosome formation (Chen et al. 2018; Narzt et al. 2019; Koenig et al. 2020). Activated autophagy protein Atg7 plays an important role in the post-translational modification of microtubule-associated protein 1 light chain 3 (LC3) adaptor protein and serves as autophagy marker (Chen et al. 2018; Barresi et al. 2022; Gu et al. 2022). Its deletion blocks autophagy and impairs the resistance of epidermal keratinocytes to intrinsic and environmental oxidative stress and sensitized keratinocytes to apoptosis and stress-induced senescence (Chen et al. 2018; Barresi et al. 2022).
Low-dose UVB exposure may cause an inductive effect on autophagy due to hormetic effect on DNA damage response and ROS generation especially in mitochondria. Thus, the autophagy response runs in a privileged mechanism to promote the survival of cells in which UVB damage can be repaired (Chen et al. 2018; Kruglikov et al. 2019; Gu et al. 2022). The ULK1 has been found as a specific regulator of autophagy in keratinocytes as it integrates upstream signals of AMPK or mTOR and transduce them to the downstream autophagy effector proteins. In sunlight exposed skin the expression of ULK1 complex, Atg5 and Atg7 genes is reduced and the autophagy flux is inhibited via mTOR-independent pathway indicating that UVB selectively inhibits AMPK signalling that may be targeted to prevent photodamage (Chen et al. 2018; Lin et al. 2019).
Caveolin-1 is a protein structural component of lipid rafts that form caveolae localized in the plasma membrane and various cellular compartments. Both overexpression and deficiency of caveolin-1 lead to accelerated skin aging. In stratified models, caveolin-1 demonstrates differential expression during skin barrier formation and participates in cell-to-cell communication, but acts as negative regulator of autophagy genes. Caveolin-1 directly influences autophagy through competitive binding with the Atg12/Atg5 complex and modulation in gene expression of Atg5, Atg12, and Atg16 that obstructs autophagosome formation (Kruglikov et al. 2019).
Beclin-1 is a member of the class III PI3K complex and is activated in response to various stimuli such as nutrient deprivation, AMPK overexpression and starvation to initiate autophagy (Mariño et al. 2014; Natarajan et al. 2014; Wang et al. 2021). Plant flavonoids could regulate skin aging through autophagy by increase in beclin-1 expression and reduction of p62 and LC3I/II conversion (Gu et al. 2022). Isoorientin (10–40 μM) is a flavone C-glycoside (luteolin-6-C-glucoside) that induces autophagy by overexpression of LC3II in UVB-stimulated HDFs (Zheng et al. 2019).
In chronic UVA/UVB irradiation oxidized phospholipids generated by oxidative stress can be detected in photo-aged skin (Vats et al. 2021). Autophagy is important to the degradation of lipids and proteins modified by photodamage and limited NRF2 activity in keratinocytes (Lin et al. 2019; Narzt et al. 2019). The nuclear protein 1 (NUPR1), possibly in coordination with NRF2, has been associated with autophagy and MAPK signalling which are both activated by oxidized phospholipids. For instance, the induction of autophagy by resveratrol is related to p38 MAPK signalling (Narzt et al. 2019; Cui et al. 2022). Interestingly, the fatty acid amide sanshool (10–40 μM) protects UVB-exposed HDFs via induction of autophagy and prevention of apoptosis by a MAPK-mediated mechanism (Hao et al. 2019).
The selective destruction of damaged mitochondria by mitophagy as a specialized form of macroautophagy is regulated by the PTEN-induced kinase 1 (PINK1)/Parkin signalling pathway (Baek et al. 2017; Li et al. 2018; Lee et al. 2022a; Gu et al. 2022; Zhang et al. 2023). As with autophagy, activation of the PI3K/Akt/mTOR signalling resulting from UVA irradiation causes suppression of mitophagy. Inhibition of mTOR, respectively, is associated with enhanced mitochondrial recycling and upregulation of PINK1 and Parkin genes (Correia-Melo et al. 2016; Chen et al. 2022a, b, c; Lee et al. 2022a). Metformin at 100 μM has been described as an AMPK activator and Akt/mTOR inhibitor with pro-mitophagic and photoprotective potential (Chen et al. 2022a, b, c). Alternatively, ginsenoside Rh2 (1–50 μM) that is a steroid saponin and one of the main bioactive components in ginseng, shows an antimitophagic effect by reducing the level of PINK1/Parkin signalling pathway, promoting OMM regeneration and restoring ATP expression in human fibroblasts (Lee et al. 2022a).
Regulation of autophagy is crucial for sustaining skin tissue homeostasis and proper function. Overactivation of PI3K/Akt/mTOR/ULK1 signalling and suppression of AMPK/SIRT1 pathways are the main involved in autophagy dysregulations associated with UVR-induced photodamage. Exploration of bioactive molecules that interact with autophagy should be done with caution as a balance need to be kept between suppression and stimulation.
Pyroptosis
Skin inflammation caused by UVB irradiation leads to morphological and histological disturbances, NLRP inflammasomes activation, infiltration of immune cells, and expression of anti-inflammatory proteins. The UVB-induced cytotoxicity in keratinocytes cleaves gasdermin proteins, which generate amino-terminal fragments and promote inflammatory cell death, called pyroptosis. The membrane pore formed by gasdermin as well as some pro-inflammatory cytokines such as IL-1β and IL-18 are involved in the signalling of the inflammatory response resulting from oxidative stress. Pyroptosis is defined as an inflammatory form of cell death mediated by NLRP3/gasdermin D which can disrupt the cell immune defence and activate NK cells, triggering immunogenic death (Zhang et al. 2020; Chen et al. 2022a, b, c; Lee et al. 2022b; Li et al. 2022; Robinson et al. 2022; Jenster et al. 2023; Mei et al. 2023).
Infiltrating neutrophils are involved in the pathogenesis of photoaging by activating important enzymes regulating inflammation and tissue remodelling, as well as, the enzyme elastase. Overexpression of IL-8 and TNF-α is associated with neutrophil accumulation and gasdermin E activation following UVB irradiation (Chen et al. 2022a, b, c; Li et al. 2022).
The inflammasomes NLRP1 and NLRP3 are overexpressed in the UVB-exposed keratinocytes that represent a hyperactivated inflammatory response in sunburn involved in the cascade of some apoptosis-associated proteins (ASC), procaspase-1, as well as IL-1β and IL-18, which are expressed in human keratinocytes but not in mice (Lee et al. 2022b; Robinson et al. 2022). Salidroside (applied at 333 μM) is a phenylpropanoid and a characteristic secondary metabolite for Rhodiola rosea L., which exerts an anti-inflammatory potential in UVB-induced pyroptosis via reduction of the NLRP3/gasdermin/caspase-1 signalling pathway (Mei et al. 2023).
Taken altogether, the inflammasome complex interaction with gasdermin proteins has been defined as critical in the UV-mediated pyroptosis. Modulation of these molecular signalling pathways that determine the UV-induced inflammatory response and survival of skin cells is a promising new avenue for development of anti-photodamage agents.
Ferroptosis
Cells that have lost their ability to regenerate and self-replicate, following damage by replicative stress under UV irradiation, reach cell death. The subtypes of cell death described above interact and differ depending on which signalling mechanisms are activated or not. Ferroptosis is classified by the Cell death nomenclature committee 2018 as RCD, which is stimulated by oxidized phospholipid peroxidation and controlled by the enzyme glutathione peroxidase 4 (GPX4) part of the glutathione peroxidase family (Mariño et al. 2014; Vats et al. 2021; Feng et al. 2022; Galluzzi et al. 2023). The involvement of ferroptosis in skin physiology is not completely studied, but it is suggested as the primary response in UVA- and UVB-induced cellular inflammation (Mariño et al. 2014; Vats et al. 2021; Galluzzi et al. 2023). Substantial experimental data describe the cytoprotective activity of some ferroptosis inhibitors, emphasizing the potential of ferrostatin-1 and liproxstatin-1 that are acting as lipid radical acceptors and 12/15-lipoxygenase inhibitors. The sensitivity of keratinocytes to UVB-induced ferroptosis depends on the extent of proferroptosis death signal generation and dysregulation of the glutathione balance and can be inhibited by iron chelators and lipophilic antioxidants (Mariño et al. 2014; Natarajan et al. 2014; Vats et al. 2021).
The formation of phospholipids in cellular metabolism, as well as in the construction of tissues, takes place through enzymatic and non-enzymatic reactions, and at the end of the process they are oxidized with the formation of phospholipid peroxide groups. Proapoptotic and proferroptotic phospholipids can be used as signalling markers induced by primary hydroperoxy-lipids. Accumulation of increased numbers of lipid hydroperoxides was observed in response to increased ROS levels induced by UV irradiation that disturbs the GSH balanceby reduced synthesis of GPX4. In the biosynthesis of GSH, the NADPH is involved in protecting cells from ferroptosis by driving the GSH/GPX4 axis (Victorelli et al. 2019; Feng et al. 2022). Depletion of NADPH by cytosolic NADPH phosphatase promotes ferroptosis in some cancer cells. Irradiation of keratinocytes with an UV light degrades the nicotinamide mononucleotide (NMN), which is a direct precursor of NAD+ and a balancer in the redox balance between NAD+/NADH. Inactivation of NAD+ activity results in the inhibition of the SIRT family NAD+-dependent histone deacetylases that are involved in the regulation of various cellular processes associated with aging. Active GPX4 cannot completely block the propagation of lipid peroxides due to disruption of the NAD+/NADH chain and the GSH recruitment process. In cases where there is too much accumulated iron, GPX4 fails to reduce lipid ROS levels and leads the cells to ferroptotic death. Supplementation with NMN or other NAD+-boosters supports cell regeneration in certain aging-related dysfunctions. In mouse models, its protective role is expressed by reducing mtROS through a SIRT-dependent pathway and preventing mitochondrial dysfunction. Administration of NAD+ precursors reduces UV-induced skin damage by significantly facilitating the NAD+/NADH system, supports GSH biosynthesis, and exhibits anti-ferroptotic potential in a GPX4-dependent manner. Glutathione promotes the resorbtion of reactive electrophilic molecules, such as the level of 4-hydroxynonenal (4-HNE), which is activated in the mechanism of ferroptosis and is a marker of lipid peroxidation (Childs et al. 2015; Victorelli et al. 2019; Feng et al. 2022; Dańczak-Pazdrowska et al. 2023).
Regulating UVR-triggered ECM remodelling mechanisms
The balance between MMPs expression and the biosynthesis of the major ECM components plays an important role in skin aging. The UV-induced MAPK signalling activation has a key role in the regulates apoptosis and MMPs expression (Natarajan et al. 2014; Alafiatayoa et al. 2020; Jin et al. 2020; Dańczak-Pazdrowska et al. 2023). Besides, active MAPKs induce phosphorylation and nuclear translocation of transcriptional complex AP-1 (consisting of c-Fos and c-Jun proteins), which in turn is leading to overactivation of MMP promoters (Natarajan et al. 2014; Kruglikov et al. 2019; Wang et al. 2019; Jin et al. 2020; Oh et al. 2020a, 2020b, 2020c; de Assis et al. 2021; Fernando et al. 2021; Liu et al. 2022a; Nisar et al. 2022). Additionally, TGF-β/Smad pathway is closely associated with collagen synthesis regulation (Natarajan et al. 2014; Young et al. 2017; Oh et al. 2020a, 2020b, 2020c, 2021). The formation of wrinkles, epidermal thickening and reduced elasticity of the skin accompanied by damage to the ECM and chronic inflammation are hallmarks of UVA-induced skin photoaging (Natarajan et al. 2014; Young et al. 2017; Kim et al. 2018; Dhaliwal et al. 2019; Shin et al. 2019a, 2019b; Ding et al. 2020; Kandan et al. 2020; Ahn et al. 2021; Kim et al 2022a, b).
Many plant-derived compounds have been reported to exert dermatoprotective effects improving UVA-induced premature skin aging by the suppression of MMPs and ECM components (mainly collagen and elastin) degradation (Dhaliwal et al. 2019; Oh et al. 2020a, 2020b, 2020c; de Assis et al. 2021; Domaszewska-Szostek et al. 2021; Fernando et al. 2021; Chaiprasongsuk and Panich 2022; Liu et al. 2022a; Nisar et al. 2022). For instance, bakuchiol is a meroterpenoid that targets several cellular pathways, including the modulation of retinoic acid receptors genes and upregulation of collagen and ECM synthesis enzymes. Additionally, the topical application of 0.5% bakuchiol cream has been found to modulate oxidative stress response in keratinocytes through NRF2 activation and to prevent UV-induced mitochondrial lipid peroxidation (Dhaliwal et al. 2019). Ginsenoside Rh2 destabilized reduced levels of elastin but not collagen (Lee et al. 2022a), while zerumbone inhibited collagen III degradation by inhibiting c-Fos and c-Jun translocation (Yang et al. 2018). The phenolic compound protocatechuic aldehyde (0.5–3 μg/mL) also suppressed MMP1 while stimulated procollagen in UVA-induced HDFs (Ding et al. 2020). The application of santamarine (10 μM), a sesquiterpene lactone isolated from Artemisia scoparia Waldst. & Kitam., reduced MMP1, MMP3, MMP9 expression and alleviated type I procollagen production in UVA-exposed HDFs (Oh et al. 2021). Santamarine suppressed UVA-induced activation of MAPK/AP-1 pathway and restored collagen biosynthesis through enhanced TGF-β/Smad signalling. Furthermore, santamarine demonstrates antioxidant activity in UVA-irradiated HDFs, as it reduces ROS generation and stimules NRF2-mediated expression of HO-1 and SOD (Oh et al. 2021).
Atractyligenin (a diterpenoid extracted from coffee silverskin) has also been reported to inhibit MMP1, MMP2 and MMP3 and restore loss of collagen absorbtion-related receptor Endo180, hence, altered UVA-induced morphological changes in fibroblasts. Atractyligenin (2.5–100 μM) regulation of MMPs was observed due to significant reduction of ROS generation and modulation of MAPK/AP-1 signalling pathway in UVA-induced HDFs (Xuan et al. 2019). Chlorogenic acid regulated collagen biosynthesis in UVA-exposed HDFs evidenced by increased expression of COL1A1 and COL1A2 and suppressed MMP1 and MMP3 in primary HDFs via inhibition of ROS production and influence of TGF-β/Smad signalling (Xue et al. 2022). The UVB-induced MMP1 production was significantly reduced simultaneously with the remarkable increas in procollagen type I production by neochlorogenic acid (50–200 μM) in UVB-exposed photoaged human fibroblasts and keratinocytes through mechanism of inhibition of MAPK/AP-1 signalling pathway (Ahn et al. 2021). The treatment with 3,5-dicaffeoyl-epi-quinic acid (1–20 μM) reduces phosphorylation of p38, ERK and activation of the part of transcriptional complex AP-1 c-Fos while increases TGF-β/Smad complex (Smad2/3 and Smad4) protein expressions (Oh et al. 2020c). Shin et al. (2019a) reported that quercetin and caffeic acid phenethyl ester protect from UVA/UVB-induced photoaging in ex vivo human skin tissues. Quercetin (20–40 μM) reduces MMP1 and COX-2 protein expressions, and preventes collagen degradationby AP-1, NF-κB and phosphorylation of MAPKs and STAT3 inhibition in UVA/UVB-exposed ex vivo, leading to relieve of skin aging and inflammation (Shin et al. 2019a). The supression of MMP1 levels in both primary HDFs and ex vivo human skin tissues were also observed after caffeic acid phenethyl ester (2.5–5 μM) treatment via inhibition HAT activity, leading to the attenuation of non-histone proteins (acetyl-lysine) and histone H3K9 acetylation (Shin et al. 2019b).
The flavanone eriodictyol (2.5–40 μM) up-regulated TIMP-1 and COL1A1 activity in a dose-dependent manner in UVA-exposed HaCaT and FEK-4 cells (Nisar et al. 2022). Similarly, syringaresinol (1–20 μM) that is a lignan and is commonly refered as a phytoestrogen, reversed UVA-mediated type 1 procollagen decreased gene and protein expressions in HaCaT and HDFs by regulation of the MAPK/AP-1 signalling pathway (Oh et al. 2020a).
The trans-cinnamic acid (20–100 μM) significantly suppressed UVA-mediated upregulation of MMP1 and MMP3 and inhibited type I procollagen degradation in human foreskin-derived fibroblast (Hs68) and nude BALB/c−nu mice. It exerted these effects through complex mechanism including inhibition of ROS generation and c-Fos and c-Jun translocation in parallel with NRF2 activation (increased HO-1, γ-GCLC expression) mediated by PKC, AMPK, CKK and ROS signalling (Hseu et al. 2019). Cryptotanshinone treatment also inhibited UVA-induced MMP1 and MMP3 mRNA expressions in HFF-1 cells (Guo et al. 2022).
The benzofuran loliolide, extracted from the edible brown seaweed Sargassum horneri, in concentration range between 50 and 200 μM dose-dependently attenuates intracellular ROS production and mRNA expressions of implicated in UV-induced photoaging MMP1, MMP2, MMP3, MMP8, MMP9, MMP13 along with inhibiting collagenase and elastase activity, and reduced pro-inflammatory factors (IL-1β, IL-6, IL-8, IL-33 and TNF-α) comparable to vitamin C in UVB-induced HDFs and this activity was due to suppression of NF-κB and MAPK signalling pathways (Fernando et al. 2021). Similarly, purified polysaccharide fraction isolated from edible algae Sargassum fusiforme suppressed ROS, mRNA relative expression of MMP1, MMP3, MMP9 and cytokines (IL-1β, IL-6, TNF-α) in a dose-dependent manner compared to hyaluronic acid in UVB-photoaged HaCaT cells suggesting its beneficial photoprotective properties along with reduction of oxidative stress and inflammation (Hu et al. 2022). The C-Mc ginsenoside (1–20 μM) suppressed the secretion of MMP1, MMP3 and inflammatory cytokines (TNF-α, IL-6) inhibiting NF-κB/IκB-α expression and MAPK/AP-1 signalling pathway along ameliorated pro-collagen type I synthesis through TGF-β/Smad in UVB-irradiated NHDFs. In addition, the C-Mc ginsenoside reduced MMPs targeting ROS and kept a balance of endogenous oxidation enhancing HO-1, NAD(P)H quinone oxidoreductase 1 (NQO1) protein expression and NRF2/ARE signalling in vitro (Liu et al. 2022b).
The UVB-induced MMP1, MMP3 and MMP9 overexpressions were also remarkably reversed by treatments with capsaicin, ishigoside, dieckol and decanal (Kang et al. 2020; Kim et al. 2022a, b; Wu et al. 2022; Xiao et al. 2022). Pretreatment with the glyceroglycolipid ishigoside (10–100 μM), isolated from brown algae Ishige okamurae, also reduced intracellular Ca2+ level and up-regulated TIMP1 secretion (that both affect the regulation of MMPs) in UVB-exposed HaCaT by blocking the MAPK/AP-1 pathway (Xiao et al. 2022). Additionally, the mRNAs expressions of MMP1a, MMP3, and MMP9 were also remarkably reduced in UVB-exposed murine dermal fibroblasts (MDFs) in result of capsaicin (1–10 μM) treatment in a dose-dependent manner accompanied with inhibition of ROS generation. Pretreatment with capsaicin (an alkaloid) promoted collagen synthesis ex vivo in dorsal skin of mice and in MDFs through supression of UVB-stimulated MAPK/ERK and c-Jun phosphorylation (Wu et al. 2022). The MMP1 and MMP3 expressions elevated by UVB radiation were also effectively decreased after decursin and sanshool treatments (Hwang et al. 2013; Hao et al. 2019). Sanshool diminished intracellular ROS generation triggering MMPs and protein expression of MMP1 and MMP3 in UVB-exposed HDFs (Hao et al. 2019). Protocatechuic acid and its esters ethyl protocatechuate and heptyl protocatechuate downregulated matrix MMP-1 and MMP-9 in UVB-irradiated L929 murine fibroblasts and alleviated oxidative biomarkers (Dare et al. 2020). The sesquiterpenes eurylosesquiterpenol, 1(R),4β-dihydroxy-trans-eudesm-7-ene-1-O-β-D-glucopyranoside and (1R,4S,10R)10,11-dimethyl-dicyclohex-5(6)-en-1,4-diol-7-one, isolated from Oplopanax elatus Nakai. in concentration range betwenn 10 and 50 μM inhibited MMP1 protein expression via suppression of phosphorylation of MAPKs/p38/ERK (Yan et al. 2021).
Decanal (25–100 μM) has been reported to attenuate collagen degradation and to enhance hyaluronic acid accumulation in UVB-exposed Hs68 dermal fibroblasts. The mRNA expressions of COL1A1, COL1A2, COL3A1 and hyaluronic acid synthase 2 (HAS2) increased upon decanal application. The treatment with this aldehyde also resulted in increase of the intracellular cyclic adenosine monophosphate (cAMP)/PKA levels along with suppression of MAPK/AP-1 signalling pathways (Kang et al. 2020). Dieckol is a phlorotanin found to decrease epidermal thickness and wrinkle formation by diminishing collagen degradation, and increasing hyaluronic acid content in UVB-exposed HR-1 hairless mice. The pro-collagen levels and COL1A1 mRNA expression have been restored by dieckol (5–10 mg/kg b.w.) administration, as well as enzymes responsible for cellular synthesis and degradation of hyaluronic acid, compared to UVB control under MAPKs/AP-1 and TGF-β/Smad2/3 regulation (Kim et al. 2022a, b).
The terpenoid glucoside of apigenin, vicenin-2 (10–60 μM) supresses MMP-2, MMP-9 and MMP-12 in UVB-exposed HDFs to levels similar to PABA by inhibition of MAPK/AP-1 signalling pathway (Duan et al. 2019). Similarly, tectorigenin (0.1–10 μM) UVB photoprotective effects have been attributed to prevention of MMP1-mediated collagen type I degradation, the induction of oxidative enzyme expressions in human keratinocytes (Noh et al. 2018). Similarly, Choi et al. (2023) have reported that kaempferide (O-methylated flavonol from Alpinia officinarum Hance rhizome) in concentration range applied from 250 to 500 μM remarkably reduces wrinkle formation, epidermal thickening and elevated collagen content in UVB-irradiated C57BL/6 mice. Moreover, kaempferide treatment modulates UVB-induced expression of related to collagen synthesis and inflammation genes including COL1A1, MMP1a, MPP3, IL-6, IL-8, and MPC-3 in NIH-3T3 skin fibroblasts (Choi et al. 2023). Topical application of the common natural flavone luteolin (60–120 mg/kg b.w.) significantly reduced UVB-induced oxidative stress, reducing erythema and wrinkle formation in rat skin. The TGF-β/Smad3 signalling has been found as responsible for the collagen type I expression elevation in HDFs (Mu et al. 2021). The photoprotective effects induced by kaempferide, vicenin-2, tectorigenin and luteolin treatments inhibit UVB-induced ROS generation, while antioxidant enzymes depletion is recovered upon application of vicenin-2, luteolin and tectorigenin.
Curcumin was used as photosensitizer in chlorin-e6-curcumin conjugate mediated photodynamic therapy that demonstrated anti-wrinkle formation potential against UVB/LED radiation in vitro and in vivo. The conjugate application suppressed protein expression of MMP1, MMP2 and MMP9 as well as promoted type I procollagen in Hs68 fibroblast and hairless SKH1 mice achieved by inhibition of COX-2, iNOS, NF-κB/p65 and p38(MAPK) phosphorylation (Hur et al. 2022).
Alleviating UVR-triggered inflamatory skin response
Many studies have reported that at a molecular level skin photoaging induced by UVB light leads to ROS triggered excessive oxidative and inflammatory responses (Natarajan et al. 2014; Bustamante et al. 2020; Li et al. 2022; Chen et al. 2022a, b, c). Inflammasome activation in response to environmental stress stimuli occurs in human keratinocytes that is not observed in murine model systems (Sand et al. 2018). Ribotoxic stress induced by UVB activates NLRP1 inflammasome complex mediated by the Zipper sterile-alpha-motif and p38-MAPK kinases (Fenini et al. 2018; Lee et al. 2022b; Jenster et al. 2023). The NPLR1 has been identified as critical component in the UVB sensing by human skin keratinocytes and the subsequent inflammasome-driven pyroptosis (Sand et al. 2018; Robinson et al. 2022).
Immunomodulation is another critical aspect of UVR-induced skin inflammation. Neutrophil infiltration into the epidermis is being among the earliest events in response to UVR overexposure that eventually leads to a neutrophil cell death with release of neutrophil extracellular traps (NET). The Rho kinase has been found to regulated the NET formation through modulation of protein kinase C alpha (PKCα)-mediated cytoskeletal remodelling in UVB-irradiated skin inflammation in mice (Li et al. 2022).
Topical application of decursin, opuntiol or acacetin mitigate UVA-stimulated histopathological changes, inflammatory response and epidermal hyperplasia. Besides, they modulate MMPs and collagen levels in UVA-induced in vivo models of photoaging (Hwang et al. 2013; Kandan et al. 2020; Feng et al. 2022; Mu et al. 2022). The UVA-induced depletion of collagen I and collagen III and UVA-activated expressions of MMP2, MMP9, MMP12 proteins along with expression of pro-inflammatory markers including iNOS, TNF-a, IL-6, COX-2 and VEGF, were all remarkably reversed by sesquiterpenoid opuntiol (50 mg/kg b.w.) application in mouse skin under MAPK/AP-1 and NF-κB signalling (Kandan et al. 2020). Opuntiol treatment significantly decreased the overexpression of phosphorilated ERK1, JNK, p38 and the nuclear translocation of NF-κB p65 and AP-1 at a protein level in the mouse skin (Kandan et al. 2020). Decursin, a coumarin compound isolated from the Angelica gigas Nakai roots, suppressed MMPs secretion and mRNA and protein expressions by inhibition of inflammatory NF-κB pathway in UVB-mediated HDFs. The phosphorylation of p50 and p65 NF-κB subunits, pIKKαβ, pIkBα were remarkable downregulated by decursin treatment (Hwang et al. 2013).
Syringaresinol treatment decreased phosphorylation of MAPKs/pERK, pJNK, p-p38 and nuclear levels of activated c-Fos and c-Jun as a mechanism to inhibit UVA-induced MMP-1 production in HaCaT keratinocytes and HDFs. The downregulation of pro-inflammatory markers IL1-β, IL-6, iNOS, COX-2, TNF-α in UVA-stimulated keratinocytes and fibroblasts indicated that syringaresinol could reduce skin inflamation (Oh et al. 2020a). The protocatechuic aldehyde MMP1/procollagen synthesis modulation was accompanied by reduction of ROS, NO and PGE2 levels, also decreased TNF-α, IL-1β, IL-6 cytokines expression and COX-2 and iNOS protein expression by mechanism of suppression of MAPK/AP-1 and NF-κB signalling (Ding et al. 2020). Eriodictyol (5–40 μM) is a flavanone that has been reported to suppress the phosphorylation of JNK, ERK, and p38 and inflammatory responses by downregulating cytokines IL-1β, IL-6, COX-2, TGFβ, TNF-α expressions in UVA-exposed human keratinocytes and fibroblasts (Nisar et al. 2022). Photoprotective properties of topical application of sanshool in vivo were demonstrated by reduction of inflammation through inhibition of JAK2/STAT3 signalling (Hao et al. 2019). Ishigoside inhibited inflammation, decreased IL-6 and IL-8 release and NF-κB p65 expression, ROS generation and DNA damage, and elevated antioxidant enzymes HO-1, SOD, GPx expression in human keratinocytes (Xiao et al. 2022).
Managing oxidative stress response
Oxidative stress caused by the accumulation of ROS generated by mitochondria or UVR can directly destroy lipids, proteins, and nucleic acids, by protein carbonylation and the production of 8-hydroxy-20-deoxyguanosine (8-OHdG). The DNA damage response and senescence of autophagy-deficient keratinocytes are increased abnormally after oxidative stress (Natarajan et al. 2014; Childs et al. 2015; de Pedro et al. 2018; Bustamante et al. 2020). Therefore, the mitochondrial photoaging theory is associated with the free radical theory. It has been reported that SIRT1 and PGC-1α promote mitochondrial regeneration and maintain energy metabolism (Mariño et al. 2014; de Pedro et al. 2018; Katayoshi et al. 2021; Li et al. 2020). Under UVR stress conditions the activation of antioxidant defence mechanisms including NRF2-mediated upregulation of the antioxidant enzymes such as HO-1, NQO1, SOD, GPX, catalase, and COX-2 along suppresion of NF-κB pathway, plays an essential role in skin prevention from photodamage (Duan et al. 2019; Chaiprasongsuk and Panich 2022; Dańczak-Pazdrowska et al. 2023).
Plant-derived phenolic compounds are well known antioxidants due to their ROS scavenger properties (Sirerol et al. 2015; Kim et al. 2019; Merecz-Sadowska et al. 2021; Chaiprasongsuk and Panich 2022; Chen et al. 2022a, b, c). Correspondingly, dihydrocaffeic acid significantly decreased oxidative damage by elevation of endogenous antioxidant enzymes (SOD, CAT, GSH) and MMP1 protein expression in UVB-stimulated murine fibroblast cells (Oliveira et al. 2019). For instance, vicenin has been found to absorb UV photons and effectively reduce the UVB-induced ROS formation, and act as effective sunscreens (Duan et al. 2019). Zerumbone showed an antioxidant potential against ROS-mediated oxidation in epidermal cells by activating the KEAP/NRF2/ARE signalling pathway (Yang et al. 2018). Resveratrol is an example of natural polyphenol with a wide range of pharmacological properties that could mitigate UVB-induced photoaging. Attenuation of MMPs expression, inflammatory factors (IL-6, TNF-α, COX-2) and apoptosis, along with inhibition of ROS-mediated MAPK signalling pathway and modulation of oxidative stress via NRF2 signalling activation have been found upon resveratrol application in UVB-irradiated HaCaT cells and in the skin of photodamaged ICR mice (Cui et al. 2022). The histological analysis of the skin revealed that the structural disorders e.g., epidermal hyperkeratosis, infiltration of inflammatory cells and collagen fibers damage in the dermis have been improved by resveratrol. Besides, its therapeutic effect of resveratrol to alleviate the loss of collagen in the dermis of UVB-photoaged skin of ICR mice was confirmed by increased type III collagen immunoreactivity observed via immunohistochemistry analysis (Cui et al. 2022). Pretreatment with penta-1,2,3,4,6-O-galloyl-β-d-glucose (10–20 μM), a gallotannin polyphenolic compound that occurs naturally in fermented Rhus verniciflua Stokes, suppresses UVB-induced MMP1 secretion in HaCaT cells and MMP13 in murine skin, while up-regulates type I collagen protein expression in vivo by MAPK/JNK1 signalling (Kim et al. 2019).
Acacetin (5–20 μM), a O-methylated flavone derived from Acacia farnesiana (Linn.) Willd., was found to be effective in ameliorating UVA-induced oxidative stress and cell death. The skin damage (erythema and wrinkles) were alleviated by acacetin in UVA-irradiated rat skin. Mitochondrial membrane potential was also restored after topical application of acacetin in UVA-exposed rats (Mu et al. 2022). Phytonutrients such as pectin activate KEAP/NRF2/ARE signalling and suppress the UVB-induced IL-6 release and NF-κB transcriptional activity upon topical application in BALB/c murine model (Chen et al. 2022a, b, c).
Diverse group of phytochemicals that modulate the NRF2 transcription factor have been identified to prevent photoaging, skin inflammation and hyperpigmentation. Therefore, natural plant-derived molecules targeting the oxidative stress that are able to reduce ROS formation are used in cosmetics to maintain skin homeostasis and prevent UV-induced oxidative damage (Noh et al. 2018; Li et al. 2020; Domaszewska-Szostek et al. 2021; Chaiprasongsuk and Panich 2022; Cui et al. 2022).
Phytochemicals that modulate cellular senescence in the context of photoaging
Cellular senescence induces aberrant cell differentiation, disrupts the ECM, stimulates tissue inflammation and induces senescence in neighboring cells (Childs et al. 2015; Bustamante et al. 2020; Fitsiou et al. 2021; Dańczak-Pazdrowska et al. 2023). Excessive UVR exposure as a potent stress environmental factor accelerates skin aging phenotypes by causing premature accumulation of senescent cells that are in state of permanent growth arrest, but remain metabolically active and secrete a specific set of pro-inflammatory mediators (Fitsiou et al. 2021; Guo et al. 2022). The senescence response depends on the activation of the p16INK4a/pRB and p53/p21WAF1 tumor suppressor pathways in response of DNA damage that prevent skin cancer development. The most common senescence-associated biomarkers in human UV-exposured skin cells include elevated p16INK4a, p21, p53 and SA-β-Gal, H2A histone family member X, and depleted lamin B1 (Childs et al. 2015; de Pedro et al. 2018; Guo et al. 2022; Li et al. 2023). In addition, senescent cells are characterized by chromatin reorganization, DNA damage, and telomere damage foci, deletion of base pairs in mitochondrial DNA. Secretion of SASP exert pleiotropic effects on tissue aging and regeneration, inflammation, wound healing and tumorogenesis. Skin senescent cells promote tissue dysfunction through SASP, which causes senescence in neighboring cells by a paracrine mechanism. The role of paracrine cross talk between keratinocytes, fibroblasts and melanocytes, mitochondrial dysfunction and autophagy are also essential in the senescence response (Fitsiou et al. 2021; Dańczak-Pazdrowska et al. 2023). In addition, cellular senescence is often accompanied by changes in chromatin structure and the appearance of heterochromatin structure, which is known as senescence associated heterochromatin foci (SAHF). Lamin B1 is involved in the formation of SAHF and is also one of the biomarkers of senescence and aging (Gu et al. 2022). Therefore, targeting skin senescent cells/senescence or SASP production by natural senotherapeutics (senolytics/senostatics) such as flavonoids (apigenin, luteolin, quercetin, fisetin, kaempferol, genistein, naringenin) could be an attractive approach to delay skin photoaging (Childs et al. 2015; Domaszewska-Szostek et al. 2021; Dańczak-Pazdrowska et al. 2023).
The increased expression of SA-β-Gal in skin cells is one of the most widely used biomarker to evaluate senescent cells (Natarajan et al. 2014; Childs et al. 2015). Natural phytochemicals including zerumbone, astragaloside, luteolin, acacetin, and shikimic acid alleviated UVA- and UVB-induced SA-β-Gal activity in human fibroblasts (Yang et al. 2018; Martínez-Gutiérrez et al. 2021; Mu et al. 2021, 2022). Cryptotanshinone (fat-soluble diterpenoid anthraquinone compound) protected against UVB- and UVA-induced senescence in both HaCaT and HFF-1 cells, respectively reducing SA-β-Gal leves and down-regulating the continuous secretion of SASP factors IL-6 and IL-8 (Guo et al. 2022). Shikimic acid (a cyclohexane carboxylic acid) applied between 10 and 50 μM acts as a senescence inhibitor that prevented SA-β-Gal accumulation, HAS2 overexpression, and decrease in p16INK4A expression in UVB-irradiated HDFs through specific NAD+/SIRT1-dependent mechanism (Martínez-Gutiérrez et al. 2021). Caffeine (10–20 mg/kg b.w.) has been reported to effectively reduce the UVA/UVB-induced SA-β-Gal activity and epidermis thickness, and increase collagen fiber content as well as supress both p21 and p53 phosphorylation, hence, providing anti-photoaging activity by activation the A2AR/SIRT3/AMPK-mediated autophagy (Li et al. 2018).)
Targeting UVR-induced DNA damage response in photoaging through phytochemicals
The extreme ROS production increases MMPs accumulation and collagen depletion destructing ECM, it also evokes DNA damage and apoptosis of skin cells (Natarajan et al. 2014; de Pedro et al. 2018, 2021; Bustamante et al. 2020; Katayoshi et al. 2021; Li et al. 2023). The UVR in the sunlight affects indirectly skin, promoting DNA damage by/mediated by excessive ROS generation (leading to oxidative stress/damage) and directly synthesizing cyclobutane pyrimidine dimmers (CPDs) and pyrimidine-pyrimidone (6–4) photoproducts formation by UVB photons absorption to cellular DNA, that lead in acute and chronic consequences on the skin (Yin et al. 2013; Carpenter et al. 2018; Sánchez-Marzo et al. 2020; Tanveer et al. 2023). Prevention of the mutations formation and acceleration of repair of photodamage is critical for skin health (Carpenter et al. 2018; Sánchez-Marzo et al. 2020; Tanveer et al. 2023). The NER pathway is closely associated with the UVA and UVB-induced p53 overexpression that causes excision repair-associated DNA strand breaks, cell cycle arrest, and apoptosis in damaged human skin tissue. The ERKs, p38, ATM, ATR, and JNK-1 kinases are involved in the p53 phosphorylation in response to UVR exposure (de Pedro et al. 2018; Bustamante et al. 2020; Garg et al. 2020; Katayoshi et al. 2021; Li et al. 2023). The DNA repair capacity is also linked to mitigation of UV-induced chronic inflammation and cellular defence systems, including the inhibition of ROS and RNS that cause DNA damage. Photoprotective agents support the DNA repair enzymes and antioxidants, prevent DNA damage and provide protection from UV-induced mutations and immunological effects (Natarajan et al. 2014; de Pedro et al. 2018; Bustamante et al. 2020; Garg et al. 2020; Katayoshi et al. 2021; Li et al. 2023).
The CPDs are the most abundant UVR-induced DNA photolesions. Oxidation reaction may contribute to the formation 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxoG) that is identified as a ubiquitous biomarker of DNA oxidation in human skin (Carpenter et al. 2018; Sánchez-Marzo et al. 2020; Tanveer et al. 2023). The photoprotective effects of hydroxytyrosol (10–50 μg/mL) pretreatment in LED‐blue light-irradiated keratinocytes and fibroblasts were also observed by inhibition of 8‐OHdG (consistent with ROS levels suppression; ROS-mediated DNA damage) formation and PCNA protein expression (Avola et al. 2019).
Cryptotanshinone impacts skin cell oxidative damage by NRF2-mediated activation of HO-1, NQO1 and attenuates DNA damage, namely, CPD and γ-H2AX were markedly reduced in UV-irradiated cells. Further, it has reduced UVB- and UVA- induced mitochondrial dysfunction in human epidermal keratinocytes and fibroblasts. Cryptotanshinone also was able to promote mitochondrial biosynthesis by activating the AMPK/SIRT1/PGC-1α signalling pathway in both UV-exposed skin cells (Kim et al. 2022a, b). Chlorogenic acid treatment improved of the DNA double-strand breaks damage by significantly reduced γH2AX levels, and promoted/elevated Rad51 expressions as well as reduced ROS generation in UVA-irradiated HDFα (Xue et al. 2022).
The glucosinolate derivatives 3-methoxybenzyl isothiocyanate and 3-methoxyphenyl acetonitrile (25–50 μM) from Limnanthes alba Hartw. ex Benth. exibited their photoprotective effects by reduced UVB-induced epidermal and total DNA damage markers—epidermal CPDs, γH2AX, and PCNA (Carpenter et al. 2018). Protocatechuic acid and its esters (ethyl protocatechuate and heptyl protocatechuate) demonstrated photoprotective activity and anti-wrinkle effects by inhibition of oxidative stress, inflammation and DNA damage in L929 fibroblast cells. Further, they attenuated UVB-induced macromolecular damages via decrease in lipoperoxidation (by approximately 22%), increase mitochondrial membrane potential and DNA condensation along reduction of DNA fragmentation compared with the UVB control and N-acetylcysteine. Additionally, the derivatives markedly inhibited UVB-stimulated the nuclear translocation of NF-κB p65 and COX-2 expression (Dare et al. 2020).
A wide variety of phytochemical constituents such as green tea polyphenols (-)-epicatechin, (-)-epigallocatechin-3-gallate, proanthocyanidins from grape seed, oxyresveratrol, apigenin, and diterpenes rich rosemary extract (Yin et al. 2013; Carpenter et al. 2018; Dare et al. 2020; Sánchez-Marzo et al. 2020; Tanveer et al. 2023) have demonstrated great potential to protect the skin from the adverse effects of UVR through improvement of the DNA damage response mechanisms.
Collectively, natural molecules hold great potential as UVR blockers that could replace or complement the available sunscreen agents. However, they have certain characteristics such as limited bioavailability and easy degradation upon UVR exposure or phototoxicity that obstructs their development in topical skin products. These are the reasons that fuel the increased interest to nanotechnology for applications such as topical transdermal delivery system, thus can enhance their stability, efficacy, and skin permeation (Wang et al. 2022; Zhu et al. 2022; Dare et al. 2023). The development of proper topical products that target molecular mechanisms of photoaging by plant derived molecules are promising alternative to the available therapy.
Conclusions
Multiple interrelated molecular signalling pathways are dysregulated from excessive or repeated UV irradiation that drive the progression of premature skin aging. Skin cells react directly to both external and internal stress factors that uncover them as an attractive model for investigating novel molecular targets as well as small molecules relevant to UV-induced photoaging. Validating the clinical relevance of molecular players such as YAP, endonuclease G, ApoD proteins for targeting photoaging development will allow more precise management of photoprotection and aging skin rejuvenation.
Based on their biological activity to modulate molecular pathways involved in UV-induced photodamage, plant-derived natural compounds have emerged as a promising option for development of photoprotective or anti-photoaging products. Experimental data is limited mostly to cell-based or in vivo pre-clinical studies that lack unified model of UV-irradiation in regards to the exposure duration, energy of the UVR dose or distance from the light source to the object. The diversity of the photoaging models might be due to the presumption that they mimic different seasonal or age-related parameters, but it becomes a challenge to drive solid conclusion on the generated data. Therefore, fundamental research that could aid the definition of more accurate and reproducible photoaging models is needed. Furthermore, mechanistic clinical studies are necessary to validate the anti-photoaging potential of promising phytochemicals in terms of effective concentrations, skin permeability, tolerability, safety and proper delivery systems. Nevertheless, the characteristic features of natural compounds that make their clinical implementation laborious do not diminish the significance of their photoprotective and skin rejuvenating potential.
Declaration
References
Ahn H, Kim H, Na C et al (2021) The protective effect of Adenocaulon himalaicum Edgew. and its bioactive compound neochlorogenic acid against UVB-induced skin damage in human dermal fibroblasts and epidermal keratinocytes. Plants 10:1669. https://doi.org/10.3390/plants10081669
Alafiatayoa AA, Laib K-S, Ahmada S et al (2020) RNA-Seq analysis revealed genes associated with UV-induced cell necrosis through MAPK/TNF-α pathways in human dermal fibroblast cells as an inducer of premature photoaging. Genomics 112:484–493. https://doi.org/10.1016/j.ygeno.2019.03.011/j.ygeno.2019.03.011
Atanasov AG, Zotchev SB, Dirsch VM et al (2021) Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 20:200–216. https://doi.org/10.1038/s41573-020-00114-z
Avola R, Graziano ACE, Pannuzzo G et al (2019) Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J Cell Physiol 234:9065–9076. https://doi.org/10.1002/jcp.27584
Baek J, Park S, Park J et al (2017) Protective role of mitochondrial peroxiredoxin III against UVB-induced apoptosis of epidermal keratinocytes. J Invest Dermatol 137:1333–1342. https://doi.org/10.1016/j.jid.2017.01.027
Barresi C, Rossiter H, Buchberger M et al (2022) Inactivation of autophagy in keratinocytes reduces tumor growth in mouse models of epithelial skin cancer. Cells 11:3691. https://doi.org/10.3390/cells11223691
Bustamante M, Hernandez-Ferre C, Tewari A et al (2020) Dose and time effects of solar-simulated ultraviolet radiation on the in vivo human skin transcriptome. Br J Dermatol 182:1458–1468. https://doi.org/10.1111/bjd.18527
Carpenter E, Le M, Miranda C et al (2018) Photoprotective properties of isothiocyanate and nitrile glucosinolate derivatives from meadowfoam (Limnanthes alba) against UVB irradiation in human skin equivalent. Front Pharmacol 9:477. https://doi.org/10.3389/fphar.2018.00477
Chaiprasongsuk A, Panich U (2022) Role of phytochemicals in skin photoprotection via regulation of NRF2. Front Pharmacol 13:823881. https://doi.org/10.3389/fphar.2022.823881
Charachit N, Sukhamwang A, Dejkriengkraikul P et al (2022) (2022) Hyperoside and quercitrin in Houttuynia cordata extract attenuate UVB-induced human keratinocyte cell damage and oxidative stress via modulation of MAPKs and Akt signaling pathway. Antioxidants 11:221. https://doi.org/10.3390/antiox11020221
Chen X, Li L, Xu S et al (2018) Ultraviolet B radiation down-regulates ULK1 and ATG7 expression and impairs the autophagy response in human keratinocytes. J Photochem Photobiol B-Biol 178:152–164. https://doi.org/10.1016/j.jphotobiol.2017.08.043
Chen Q, Zhang H, Yang Y et al (2022a) Metformin attenuates UVA-induced skin photoaging by suppressing mitophagy and the PI3K/AKT/mTOR pathway. Int J Mol Sci 23:6960. https://doi.org/10.3390/ijms23136960
Chen Y, Lian N, Chen S et al (2022b) GSDME deficiency leads to the aggravation of UVB-induced skin inflammation through enhancing recruitment and activation of neutrophils. Cell Death Dis 13:841. https://doi.org/10.1038/s41419-022-05276-9
Chen Y, Liu X, Lei X et al (2022c) Premna microphylla Turcz pectin protected UVB-induced skin aging in BALB/c-nu mice via NRF2 pathway. Int J Biol Macromol 215:12–22. https://doi.org/10.1016/j.ijbiomac.2022.06.076
Childs BG, Durik M, Baker DJ et al (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21:1424–1435. https://doi.org/10.1038/nm.4000
Choi Y-P, Kim G, Kim SH (2020) Suppression of Pax3–MITF-M axis protects from UVB-induced skin pigmentation by tetrahydroquinoline carboxamide. Int J Mol Sci 21:9631. https://doi.org/10.3390/ijms21249631
Choi J-K, Kwon O-W, Lee S-H (2023) Kaempferide prevents photoaging of ultraviolet-B irradiated NIH-3T3 cells and mouse skin via regulating the ROS-mediated signalling. Antioxidants 12:11. https://doi.org/10.3390/antiox12010011
Correia-Melo C, Marques FDM, Anderson R (2016) Mitochondria are required for pro-ageing features of the senescent phenotype. Embo J 35:724–742. https://doi.org/10.15252/embj.201592862
Cui B, Wang Y, Jin J et al (2022) Resveratrol treats UVB-induced photoaging by anti-MMP expression, through anti-inflammatory, antioxidant, and antiapoptotic properties, and treats photoaging by upregulating VEGF-B expression. Oxidative Med Cell Longev 2022:6037303. https://doi.org/10.1155/2022/6037303
Dańczak-Pazdrowska A, Gornowicz-Porowska J, Polańska A et al (2023) Cellular senescence in skin-related research: targeted signaling pathways and naturally occurring therapeutic agents. Aging Cell 22:e13845. https://doi.org/10.1111/acel.13845
Dare R, Oliveira M, Truiti M et al (2020) Abilities of protocatechuic acid and its alkyl esters, ethyl and heptyl protocatechuates, to counteract UVB-induced oxidative injuries and photoaging in fibroblasts L929 cell line. J Photochem Photobiol B-Biol 203:111771. https://doi.org/10.1016/j.jphotobiol.2019.111771
Dare R, Kolanthai E, Neal C et al (2023) Cerium oxide nanoparticles conjugated with tannic acid prevent UVB-induced oxidative stress in fibroblasts evidence of a promising anti-photodamage agent. Antioxidants 12:190. https://doi.org/10.3390/antiox12010190
de Assis L, Tonolli P, Moraes M et al (2021) How does the skin sense sun light? An integrative view of lightsensing molecules. J Photochem Photobiol C-Photochem Rev 47:100403. https://doi.org/10.1016/j.jphotochemrev.2021.100403
de Pedro I, Alonso-Lecue P, Sanz-Gómez N et al (2018) Sublethal UV irradiation induces squamous differentiation via a p53-independent. DNA Damage-Mitosis Checkpoint Cell Death Dis 9:1094. https://doi.org/10.1038/s41419-018-1130-8
de Pedro I, Galán-Vidal J, Freije A et al (2021) p21CIP1 controls the squamous differentiation response to replication stress. Oncogene 40:152–162. https://doi.org/10.1038/s41388-020-01520-8
Dhaliwal S, Rybak I, Ellis S et al (2019) Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br J Dermatol 180:289–296. https://doi.org/10.1111/bjd.16918
Ding Y, Jiratchayamaethasakul C, Lee S-H (2020) Protocatechuic aldehyde attenuates UVA-induced photoaging in human dermal fibroblast cells by suppressing MAPKs/AP-1 and NF-κB signaling pathways. Int J Mol Sci 21:4619. https://doi.org/10.3390/ijms21134619
Domaszewska-Szostek A, Puzianowska-Kuznicka A, Kuryłowicz A (2021) Flavonoids in skin senescence prevention and treatment. Int J Mol Sci 22:6814. https://doi.org/10.3390/ijms22136814
Duan X, Wu T, Liu T et al (2019) Vicenin-2 ameliorates oxidative damage and photoaging via modulation of MAPKs and MMPs signaling in UVB radiation exposed human skin cells. J Photochem Photobiol B-Biol 190:76–85. https://doi.org/10.1016/j.jphotobiol.2018.11.018
Feng Z, Qin Y, Huo F et al (2022) NMN recruits GSH to enhance GPX4-mediated ferroptosis defense in UV irradiation induced skin injury. Biochim Biophys Acta-Mol Basis Dis 1868:166287. https://doi.org/10.1016/j.bbadis.2021.166287
Fenini G, Grossi S, Gehrke S et al (2018) Genome editing of human primary keratinocytes by CRISPR/Cas9 reveals an essential role of the NLRP1 inflammasome in UVB sensing. J Invest Dermatol 138:2644–2652. https://doi.org/10.1016/j.jid.2018.07.016
Fernando I, Heo S-J, Dias M et al (2021) (–)-Loliolide isolated from Sargassum horneri abate UVB-induced oxidative damage in human dermal fibroblasts and subside ECM degradation. Mar Drugs 19:435. https://doi.org/10.3390/md19080435
Ferreira SM, Gomes SM, Santos L et al (2023) A novel approach in skin care: by-product extracts as natural UV filters and an alternative to synthetic ones. Molecules 28:2037. https://doi.org/10.3390/molecules28052037
Fitsiou E, Pulido T, Campisi J et al (2021) Cellular senescence and the senescence-associated secretory phenotype as drivers of skin photoaging. J Invest Dermatol 141:1119–1126. https://doi.org/10.1016/j.jid.2020.09.031
Franco A, Aveleira C, Cavadas C (2021) Skin senescence: mechanisms and impact on whole-body aging. Trends Mol Med 28:2. https://doi.org/10.1016/j.molmed.2021.12.003
Galluzzi L, Kepp O, Hett E et al (2023) Immunogenic cell death in cancer: concept and therapeutic implications. J Transl Med 21:162. https://doi.org/10.1186/s12967-023-04017-6
Gao S, Guo K, Chen Y et al (2021) Keratinocyte growth factor 2 ameliorates UVB-induced skin damage via activating the AhR/Nrf2 signaling pathway. Front Pharmacol 12:655281. https://doi.org/10.3389/fphar.2021.655281
Garg C, Sharma H, Garg M (2020) Skin photo-protection with phytochemicals against photo-oxidative stress, photo-carcinogenesis, signal transduction pathways and extracellular matrix remodelling—an overview. Ageing Res Rev 62:101127. https://doi.org/10.1016/j.arr.2020.101127
Gęgotek A, Bielawska K, Biernacki M et al (2017) Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol 12:733–744. https://doi.org/10.1016/j.redox.2017.04.014
Gu Y, Xue F, Xiao H, Chen L, Zhang Y (2022) Bamboo leaf flavonoids suppress oxidative stress-induced senescence of HaCaT cells and UVB-induced photoaging of mice through p38 MAPK and autophagy signalling. Nutrients 14:793. https://doi.org/10.3390/nu14040793
Guo K, Liu R, Jing R et al (2022) Cryptotanshinone protects skin cells from ultraviolet radiation-induced photoaging via its antioxidant effect and by reducing mitochondrial dysfunction and inhibiting apoptosis. Front Pharmacol 13:1036013. https://doi.org/10.3389/fphar.2022.1036013
Hao D, Wen X, Liu L et al (2019) Sanshool improves UVB-induced skin photodamage by targeting JAK2/STAT3-dependent autophagy. Cell Death Dis 10:19. https://doi.org/10.1038/s41419-018-1261-y
Hegedűs C, Boros G, Fidrus E et al (2020) PARP1 inhibition mugments UVB-mediated mitochondrial changes-implications for UV-induced DNA repair and photocarcinogenesis. Cancers 12:5. https://doi.org/10.3390/cancers12010005
Hseu Y-C, Korivi M, Lin F-Y et al (2018) Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of NRF2-mediated antioxidant genes in human skin fibroblasts. J Dermatol Sci 90:123–134. https://doi.org/10.1016/j.jdermsci.2018.01.004
Hseu Y, Chang C-T, Gowrisankar YV (2019) Zerumbone exhibits antiphotoaging and dermatoprotective properties in ultraviolet A-irradiated human skin fibroblast cells via the activation of NRF2/ARE defensive pathway. Oxidative Med Cell Longev 2019:4098674. https://doi.org/10.1155/2019/4098674
Hu J, Yao W, Chang S et al (2022) Structural characterization and anti-photoaging activity of a polysaccharide from Sargassum fusiforme. Food Res Int 157:111267. https://doi.org/10.1016/j.foodres.2022.111267
Hur G-A, Ryu A-R, Kim Y-W et al (2022) The potential anti-photoaging effect of photodynamic therapy using chlorin e6-curcumin conjugate in UVB-irradiated fibroblasts and hairless mice. Pharmaceutics 14:968. https://doi.org/10.3390/pharmaceutics14050968
Hurbain I, Romao M, Sextius P et al (2018) Melanosome distribution in keratinocytes in different skin types: Melanosome clusters are not degradative organelles. J Invest Dermatol 138:647–656. https://doi.org/10.1016/j.jid.2017.09.039
Hwang BM, Noh EM, Kim JS et al (2013) Decursin inhibits UVB-induced MMP expression in human dermal fibroblasts via regulation of nuclear factor-κB. Int J Mol Med 31(2):477–483. https://doi.org/10.3892/ijmm.2012.1202
Jenster L-M, Lange K-E, Normann S et al (2023) P38 kinases mediate NLRP1 inflammasome activation after ribotoxic stress response and virus infection. J Exp Med 220:e20220837. https://doi.org/10.1084/jem.20220837
Jin Y, Cheng X, Huang X et al (2020) The role of Hrd1 in ultraviolet (UV) radiation induced photoaging. Aging-US 12:21. https://doi.org/10.18632/aging.103851
Kandan P, Balupillai A, Kanimozhi G et al (2020) Opuntiol prevents photoaging of mouse skin via blocking inflammatory responses and collagen degradation. Oxidative Med Cell Longev 2020:5275178. https://doi.org/10.1155/2020/5275178
Kang W, Choi D, Park T (2020) Decanal protects against UVB-induced photoaging in human dermal fibroblasts via the cAMP pathway. Nutrients 12:1214. https://doi.org/10.3390/nu12051214
Katayoshi T, Nakajo T, Tsuji-Naito K (2021) Restoring NAD+ by NAMPT is essential for the SIRT1/p53-mediated survival of UVA- and UVB-irradiated epidermal keratinocytes. J Photochem Photobiol B-Biol 221:112238. https://doi.org/10.1016/j.jphotobiol.2021.112238
Kim H, Jeong Y, Kim J et al (2018) 3,5,6,7,8,3/,4/-Heptamethoxyflavone, a citrus flavonoid, inhibits collagenase activity and induces type I procollagen synthesis in HDFn cells. Int J Mol Sci 19:620. https://doi.org/10.3390/ijms19020620
Kim J-A, Lee J-E, Kim JH (2019) Penta-1,2,3,4,6-O-galloyl-β-d-glucose inhibits UVB-induced photoaging by targeting PAK1 and JNK1. Antioxidants 8:561. https://doi.org/10.3390/antiox8110561
Kim J, Chung K, Yoon Y et al (2022a) Dieckol isolated from Eisenia bicyclis ameliorates wrinkling and improves skin hydration via MAPK/AP-1 and TGF-β/Smad signaling pathways in UVB-irradiated hairless mice. Mar Drugs 20:779. https://doi.org/10.3390/md20120779
Kim KS, Choi YJ, Jang DS et al (2022b) 2-O-β-D-Glucopyranosyl-4,6-dihydroxybenzaldehyde Isolated from Morus alba (Mulberry) fruits suppresses damage by regulating oxidative and inflammatory responses in TNF-α-induced human dermal fibroblasts. Int J Mol Sci 23:14802. https://doi.org/10.3390/ijms232314802
Koenig U, Robenek H, Barresi C et al (2020) Cell death induced autophagy contributes to terminal differentiation of skin and skin appendages. Autophagy 16:932–945. https://doi.org/10.1080/15548627.2019.1646552
Koycheva IK, Marchev AS, Stoykova ID et al (2023) Natural alternatives targeting psoriasis pathology and key signaling pathways: a focus on phytochemicals. Phytochem Rev. https://doi.org/10.1007/s11101-023-09886-9
Kruglikov IL, Zhang Z, Scherer PS (2019) Caveolin-1 in skin aging—from innocent bystander to major contributor. Ageing Res Rev 55:100959. https://doi.org/10.1016/j.arr.2019.100959
Lee H, Kong G, Park J et al (2022a) The potential inhibitory effect of ginsenoside Rh2 on mitophagy in UV-irradiated human dermal fibroblasts. J Ginseng Res 46:646–656. https://doi.org/10.1016/j.jgr.2022.02.001
Lee T, Huang Y-T, Hsiao P-F et al (2022b) Critical roles of irradiance in the regulation of UVB-induced inflammasome activation and skin inflammation in human skin keratinocytes. J Photochem Photobiol B-Biol 226:112373. https://doi.org/10.1016/j.jphotobiol.2021.112373
Li Y-F, Ouyang S-H, Tu L-F et al (2018) Caffeine protects skin from oxidative stress-induced senescence through the activation of autophagy. Theranostics 8:5713–5730. https://doi.org/10.7150/thno.28778
Li Q, Bai D, Qin L et al (2020) Protective effect of D-tetramannuronic acid tetrasodium salt on UVA-induced photo-aging in HaCaT cells. Biomed Pharmacother 126:110094. https://doi.org/10.1016/j.biopha.2020.110094
Li M, Lyu X, Liao J (2022) Rho kinase regulates neutrophil NET formation that is involved in UVB-induced skin inflammation. Theranostics 12:2133–2149. https://doi.org/10.7150/thno.66457
Li C, Zhu Y, Liu W et al (2023) Increased mitochondrial fission induces NLRP3/cGAS-STING mediated pro-inflammatory pathways and apoptosis in UVB-irradiated immortalized human keratinocyte HaCaT cells. Arch Biochem Biophys 738:109558. https://doi.org/10.1016/j.abb.2023.109558
Lin S, Li L, Li M et al (2019) Raffinose increases autophagy and reduces cell death in UVB-irradiated keratinocytes. J Photochem Photobiol B-Biol 201:111653. https://doi.org/10.1016/j.jphotobiol.2019.111653
Liu W, Wang F, Li C et al (2022a) Silibinin relieves UVB-induced apoptosis of human skin cells by inhibiting the YAP-p73 pathway. Acta Pharmacol Sin 43:2156–2167. https://doi.org/10.1038/s41401-021-00826-x
Liu X-Y, Li H, Hwang E et al (2022b) Chemical distance measurement and system pharmacology approach uncover the novel protective effects of biotransformed ginsenoside C-Mc against UVB-irradiated photoaging. Oxidative Med Cell Longev 2022:4691576. https://doi.org/10.1155/2022/4691576
Lone NA, Malik TA, Naikoo SH et al (2020) Trigonelline, a naturally occurring alkaloidal agent protects ultraviolet-B (UV-B) irradiation induced apoptotic cell death in human skin fibroblasts via attenuation of oxidative stress, restoration of cellular calcium homeostasis and prevention of endoplasmic reticulum (ER) stress. J Photochem Photobiol B-Biol 202:111720. https://doi.org/10.1016/j.jphotobiol.2019.111720
Mariño G, Niso-Santan M, Baehrecke E et al (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15:84–94. https://doi.org/10.1038/nrm3735
Martic I, Wedel S, Jansen-Dürr P et al (2020) A new model to investigate UVB-induced cellular senescence and pigmentation in melanocytes. Mech Ageing Dev 190:111322. https://doi.org/10.1016/j.mad.2020.111322
Martínez-Gutiérrez A, Fernández-Duran I, Marazuela-Duque A et al (2021) Shikimic acid protects skin cells from UV-induced senescence through activation of the NAD+-dependent deacetylase SIRT1. Aging-US 13:12308–12333. https://doi.org/10.18632/aging.203010
Mei M, Cai R, Yu Q et al (2023) Salidroside alleviates UVB-induced skin damage by inhibiting keratinocytes pyroptosis via the AQP3/ROS/GSDMD-N signaling pathway. J Funct Foods 107:105647. https://doi.org/10.1016/j.jff.2023.105647
Merecz-Sadowska A, Sitarek P, Zajdel K et al (2021) The modulatory influence of plant-derived compounds on human keratinocyte function. Int J Mol Sci 22:12488. https://doi.org/10.3390/ijms222212488
Moon H, Donahue LR, Choi E et al (2017) Melanocyte stem cell activation and translocation initiate cutaneous melanoma in response to UV exposure. Cell Stem Cell 21:665–678. https://doi.org/10.1016/j.stem.2017.09.001
Moya IM, Halder G (2019) Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol 20:211–226. https://doi.org/10.1038/s41580-018-0086-y
Mu J, Ma H, Chen H et al (2021) Luteolin prevents UVB-induced skin photoaging damage by modulating SIRT3/ROS/MAPK signaling: an in vitro and in vivo studies. Front Pharmacol 12:728261. https://doi.org/10.3389/fphar.2021.728261
Mu J, Chen H, Ye M (2022) Acacetin resists UVA photoaging by mediating the SIRT3/ROS/MAPKs pathway. J Cell Mol Med 26:4624–4628. https://doi.org/10.1111/jcmm.17415
Muzaffer U, Paul VI, Prasad NR et al (2018) Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine 42:100–111. https://doi.org/10.1016/j.phymed.2018.03.024
Narzt MS, Nagelreiter IM, Oskolkova O et al (2019) A novel role for NUPR1 in the keratinocyte stress response to UV oxidized phospholipids. Redox Biol 20:467–482. https://doi.org/10.1016/j.redox.2018.11.006
Natarajan VT, Ganju P, Ramkumar A et al (2014) Multifaceted pathways protect human skin from UV radiation. Nat Chem Biol 10:542–551. https://doi.org/10.1038/nchembio.1548
Nisar MF, Liu T, Wang M et al (2022) Eriodictyol protects skin cells from UVA irradiation-induced photodamage by inhibition of the MAPK signaling pathway. J Photochem Photobiol B-Biol 226:112350. https://doi.org/10.1016/j.jphotobiol.2021.112350
Noh D, Choi JG, Huh E et al (2018) Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes. Nutrients 10:1998. https://doi.org/10.3390/nu10121998
Oh JH, Joo YH, Karadeniz F et al (2020a) Syringaresinol inhibits UVA-induced MMP-1expression by suppression of MAPK/AP-1 signaling in HaCaT keratinocytes and human dermal fibroblasts. Int J Mol Sci 21:3981. https://doi.org/10.3390/ijms21113981
Oh JH, Karadeniz F, Lee JI et al (2020b) Anticatabolic and anti-inflammatory effects of myricetin 3-O-β-d-galactopyranoside in UVA-irradiated dermal cells via repression of MAPK/AP-1 and activation of TGFβ/Smad. Molecules 25:1331. https://doi.org/10.3390/molecules25061331
Oh JH, Karadeniz F, Kong C-S (2020c) Antiphotoaging effect of 3,5-dicaffeoyl-epi-quinic acid against UVA-induced skin damage by protecting human dermal fibroblasts in vitro. Int J Mol Sci 21:7756. https://doi.org/10.3390/ijms21207756
Oh JH, Kim J, Karadeniz F et al (2021) Santamarine shows anti-photoaging properties via inhibition of MAPK/AP-1 and stimulation of TGF-β/Smad signaling in UVA-irradiated HDFs. Molecules 26:3585. https://doi.org/10.3390/molecules26123585
Oliveira MM, Ratti BA, Daré RG et al (2019) Dihydrocaffeic acid prevents UVB-induced oxidative stress leading to the inhibition of apoptosis and MMP-1 expression via p38 signaling pathway. Oxidative Med Cell Longev 2019:2419096. https://doi.org/10.1155/2019/2419096
Orioli D, Dellambra E (2018) Epigenetic regulation of skin cells in natural aging and premature aging diseases. Cells 7:268. https://doi.org/10.3390/cells7120268
Rice G, Rompolas P (2020) Advances in resolving the heterogeneity and dynamics of keratinocyte differentiation. Curr Opin Cell Biol 67:92–98. https://doi.org/10.1016/j.ceb.2020.09.004
Robinson KS, Toh GA, Rozario P et al (2022) ZAKα-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science 377:6603. https://doi.org/10.1126/science.abl6324
Rusu A, Tanase C, Pascu G-A et al (2020) Recent advances regarding the therapeutic potential of adapalene. Pharmaceuticals 13:217. https://doi.org/10.3390/ph13090217
Sánchez-Marzo N, Pérez-Sánchez A, Barrajón-Catalán E et al (2020) Rosemary diterpenes and flavanone aglycones provide improved genoprotection against UV-induced DNA damage in a human skin cell model. Antioxidants 9:255. https://doi.org/10.3390/antiox9030255
Sand J, Haertel E, Biedermann T et al (2018) Expression of inflammasome proteins and inflammasome activation occurs in human, but not in murine keratinocytes. Cell Death Dis 9:24. https://doi.org/10.1038/s41419-017-0009-4
Shin EJ, Lee JS, Hong S et al (2019a) Quercetin directly targets JAK2 and PKCδ and prevents UV-induced photoaging in human skin. Int J Mol Sci 20:5262. https://doi.org/10.3390/ijms20215262
Shin EJ, Jo S, Choi H-K (2019b) Caffeic acid phenethyl ester inhibits UV-induced MMP-1 expression by targeting histone acetyltransferases in human skin. Int J Mol Sci 20:3055. https://doi.org/10.3390/ijms20123055
Silva L, Perasolia F, Carvalhoa K et al (2020) Melaleuca leucadendron (L.) L. flower extract exhibits antioxidant and photoprotective activities in human keratinocytes exposed to ultraviolet B radiation. Free Radic Biol Med 159:54–65. https://doi.org/10.1016/j.freeradbiomed.2020.07.022
Sirerol JA, Feddi F, Mena S (2015) Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic Biol Med 85:1–11. https://doi.org/10.1016/j.freeradbiomed.2015.03.027
Song C, Zhang W, Xiao T et al (2023) Reduction of miR-133a-3p contributes to apoptosis and gasdermin E-mediated pyroptosis of keratinocytes in skin exposed to ultraviolet B radiation. J Photochem Photobiol B-Biol 238:112613. https://doi.org/10.1016/j.jphotobiol.2022.112613
Su W, Wang L, Fu X et al (2020) Protective effect of a fucose-rich fucoidan isolated from Saccharina japonica against ultraviolet B-induced photodamage in vitro in human keratinocytes and in vivo in zebrafish. Mar Drugs 18:316. https://doi.org/10.3390/md18060316
Takaya K, Asou T, Kishi K (2023) New senolysis approach via antibody–drug conjugate targeting of the senescent cell marker apolipoprotein D for skin rejuvenation. Int J Mol Sci 24:5857. https://doi.org/10.3390/ijms24065857
Tanveer MA, Rashid H, Nazir LA et al (2023) Trigonelline, a plant derived alkaloid prevents ultraviolet-B-induced oxidative DNA damage in primary human dermal fibroblasts and BALB/c mice via modulation of phosphoinositide 3-kinase-Akt-Nrf2 signalling axis. Exp Gerontol 171:112028. https://doi.org/10.1016/j.exger.2022.112028
Vats K, Kruglov O, Mizes A et al (2021) Keratinocyte death by ferroptosis initiates skin inflammation after UVB exposure. Redox Biol 47:102143. https://doi.org/10.1016/j.redox.2021.102143
Victorelli S, Lagnado A, Halim J et al (2019) Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. Embo J 38:101982. https://doi.org/10.15252/embj.2019101982
Wang P-W, Hung Y-C, Lin T-Y et al (2019) Comparison of the biological impact of UVA and UVB upon the skin with functional proteomics and immunohistochemistry. Antioxidants 8:569. https://doi.org/10.3390/antiox8120569
Wang M, Lei M, Chang L et al (2021) Bach2 regulates autophagy to modulate UVA-induced photoaging in skin fibroblasts. Free Radic Biol Med 169:304–316. https://doi.org/10.1016/j.freeradbiomed.2021.04.003
Wang Y, Ouyang Q, Chang X (2022) Anti-photoaging effects of flexible nanoliposomes encapsulated Moringa oleifera Lam. isothiocyanate in UVB-induced cell damage in HaCaT cells. Drug Deliv 29:871–881. https://doi.org/10.1080/10717544.2022.2039802
Wong W, Crane E, Zhang H et al (2022) Pgc-1α controls epidermal stem cell fate and skin repair by sustaining NAD+ homeostasis during aging. Mol Metab 65:101575. https://doi.org/10.1016/j.molmet.2022.101575
Wu Q, Bai P, Guo H et al (2022) Capsaicin, a phytochemical from chili pepper, alleviates the ultraviolet irradiation-induced decline of collagen in dermal fibroblast via blocking the generation of ROS. Front Pharmacol 13:872912. https://doi.org/10.3389/fphar.2022.872912
Xiao Z, Yang S, Liu Y et al (2022) A novel glyceroglycolipid from brown algae Ishige okamurae improve photoaging and counteract inflammation in UVB-induced HaCaT cells. Chem-Biol Interact 351:109737. https://doi.org/10.1016/j.cbi.2021.109737
Xu X, Wang H-Y, Zhang Y et al (2014) Adipose-derived stem cells cooperate with fractional carbon dioxide laser in antagonizing photoaging: a potential role of Wnt and β-catenin signalling. Cell Biosci 4:24. https://doi.org/10.1186/2045-3701-4-24
Xuan SH, Lee NH, Park SN (2019) Atractyligenin, a terpenoid isolated from coffee silverskin, inhibits cutaneous photoaging. J Photochem Photobiol B-Biol 194:166–173. https://doi.org/10.1016/j.jphotobiol.2019.04.002
Xue N, Liu Y, Jin J et al (2022) Chlorogenic acid prevents UVA-induced skin photoaging through regulating collagen metabolism and apoptosis in human dermal fibroblasts. Int J Mol Sci 23:6941. https://doi.org/10.3390/ijms23136941
Yamada M, Mohammed Y, Prow TW et al (2020) Advances and controversies in studying sunscreen delivery and toxicity. Adv Drug Deliv Rev 153:72–86. https://doi.org/10.1016/j.addr.2020.02.001
Yan J, Hao M, Han Y (2021) Sesquiterpenes from Oplopanax elatus stems and their anti-photoaging effects by down-regulating matrix metalloproteinase-1 expression via anti-inflammation. Front Chem 9:766041. https://doi.org/10.3389/fchem.2021.766041
Yang H-L, Lee C-L, Korivi M et al (2018) Zerumbone protects human skin keratinocytes against UVA-irradiated damages through NRF2 induction. Biochem Pharmacol 148:130–146. https://doi.org/10.1016/j.bcp.2017.12.014
Yang H-L, Chen S-J, Pandey S et al (2023) The skin hydration and anti-inflammatory potential of zerumbone, a natural sesquiterpene of Zingiber zerumbet, enhanced Src/ERK-mediated HAS-2/AQP-3 and inhibited NFκB/AP-1 expression in UVB-irradiated human keratinocytes. J Funct Foods 111:105890. https://doi.org/10.1016/j.jff.2023.105890
Yin Y, Li W, Son Y-O et al (2013) Quercitrin protects skin from UVB-induced oxidative damage. Toxicol Appl Pharmacol 269:89–99. https://doi.org/10.1016/j.taap.2013.03.015
Young AR, Claveau J, Rossi AB (2017) Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol 76:S100–S109. https://doi.org/10.1016/j.jaad.2016.09.038
Yun C-Y, Roh E, Kim S-H et al (2020) Stem cell factor-inducible MITF-M expression in therapeutics for acquired skin hyperpigmentation. Theranostics 10:340–352. https://doi.org/10.7150/thno.39066
Zhang Z, Zhang Y, Xia S et al (2020) Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579:415–420. https://doi.org/10.1038/s41586-020-2071-9
Zhang C, Gao X, Li M et al (2023) The role of mitochondrial quality surveillance in skin aging: focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res Rev 87:101917. https://doi.org/10.1016/j.arr.2023.101917
Zheng H, Zhang M, Luo H et al (2019) Isoorientin alleviates UVB-induced skin injury by regulating mitochondrial ROS and cellular autophagy. Biochem Biophys Res Commun 514:1133–1139. https://doi.org/10.1016/j.bbrc.2019.04.195
Zheng X, Feng M, Wan J et al (2021) Anti-damage effect of theaflavin-3′-gallate from black tea on UVB-irradiated HaCaT cells by photoprotection and maintaining cell homeostasis. J Photochem Photobiol B-Biol 224:112304. https://doi.org/10.1016/j.jphotobiol.2021.112304
Zhu J, Huang X, Yang T et al (2022) An eco-friendly zein nanoparticle as robust cosmetic ingredient ameliorates skin photoaging. Ind Crop Prod 177:114521. https://doi.org/10.1016/j.indcrop.2022.114521
Zou X, Zou D, Li L et al (2022) Multi-omics analysis of an in vitro photoaging model and protective effect of umbilical cord mesenchymal stem cell-conditioned medium. Stem Cell Res Ther 13:435. https://doi.org/10.1186/s13287-022-03137-y
Acknowledgements
This research received funding from the European Union’s Horizon 2020 research and innovation programme, project PlantaSYST (SGA No 739582 under FPA No. 664620), and the BG05M2OP001-1.003-001-C01 project, financed by the European Regional Development Fund through the “Science and Education for Smart Growth” Operational Programme.
Funding
This work was supported by the European Union’s Horizon 2020 research and innovation programme, project PlantaSYST (SGA No 739582 under FPA No. 664620), and the BG05M2OP001-1.003-001-C01 project, financed by the European Regional Development Fund through the “Science and Education for Smart Growth” Operational Programme.
Author information
Authors and Affiliations
Contributions
IDS: conceptualization, methodology, visualization, writing—original draft. IKK: conceptualization, methodology, visualization, writing—original draft. BKB: methodology, visualization, writing—original draft. LVM: conceptualization, methodology, visualization, writing—original draft, review and editing. MIG: conceptualization, supervision, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the work on this review paper was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stoykova, I.D., Koycheva, I.K., Binev, B.K. et al. Molecular approaches to prevent UV-induced premature skin aging: focus on phytochemicals as photo-protectants. Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-09952-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-09952-w