Abstract
Even after a century of substantial advancements in oncology medicines, cancer still ranks as one of the top causes of death worldwide. As a result, there is an ongoing and pressing need for the development of new cancer drugs. Natural compounds and their semi-synthetic derivatives continue to show promise as potential therapeutic leads, due to their high chemical diversity, biochemical specificity, significant molecular activities, and pharmacological properties. They serve as excellent starting points for the discovery of new drugs, inspiring new breakthroughs in biology, chemistry, and medicine. In recent years, scientists have made significant advancements in the development of naturally occurring or partly synthesized analogues with improved bioactivity, simpler synthetic targets and reduced toxicity. This review aims to summarize the importance and biological actions of natural compounds identified form plants and their role in prevention of cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is one of the most serious health issues in the world and the leading cause of death in the twenty-first century, according to the World Health Organisation (Ma and Yu 2006). In 2020, almost 10 million individuals succumbed to cancer worldwide, with breast, lung, rectum, prostate, colon, skin and stomach cancer (https://www.who.int/news-room/fact-sheets/detail/cancer). Particularly, the expanding global population and rising life expectancy will cause an annual rise in the number of cancer cases. Therefore, the potent anticancer medications is essential. Chemotherapeutic medicines are often used in cancer treatment, and they have become a crucial component of cancer medication therapy.
Several strategies have been used to reduce the side effects of cancer therapy medications, including avoiding causing damage to adjacent cells and tissues, enhancing drug accumulation and efficacy in the lesion, and developing innovative drug delivery and targeting systems (Xia et al. 2021). There are numerous additional approaches to treating cancer, such as surgical removal of the tumor, cancer vaccines, stem cell transformation, chemotherapy, immunotherapy, photodynamic therapy, radiotherapy, or an assortment of these methods, which typically come with detrimental side effects (Chu et al. 2020). These adverse effects include limited metastasis, toxicity, and lack of specificity, low bioavailability, and rapid clearance(Lichota et al. 2018). Treatment options for cancer are determined by the location, stage, and type of the cancer. Chemotherapy drugs, such as alkylating agents (e.g., cyclophosphamide, oxaliplatin, carboplatin, and melphalan), topoisomerase inhibitors (e.g., doxorubicin and irinotecan), and microtubule-acting agents (e.g., vincristine), may have side effects such as pulmonary, cardiotoxicity, gastrointestinal toxicity, cardiovascular toxicity, and nephrotoxicity, hematologic toxicity (Kuroda et al. 2014).
After thorough investigation, it was shown that natural compounds present in a variety of medicinal plants had chemopreventive potential. These phytochemicals, which can be directly used or modified through physico-chemical processes, provide a significant source of potentially effective anticancer compounds (Park et al. 2016).
Finding new anticancer drugs is crucial due to the worldwide prevalence of cancer (Satheesh et al. 2011). The use of plants to treat cancer has a long-standing history, and numerous clinical studies have shown the effectiveness of various natural chemicals, including alkaloids, terpenes, phenols, and flavonoids (Nakahata et al. 2011). Drugs with a natural origin, or natural products, have been very important in treating human diseases (Kumar et al. 2023). The use of willow salicylates for pain relief and cinchona quinine for the treatment of malaria constitute two examples of how natural products have been essential in the treatment of sickness. In India, it is estimated that there will be 679,421 cancer cases (94.1 per 100,000) among men and 712,758 (103.6 per 100,000) among women in 2020, with lung cancer affecting 1 in 68 men, breast cancer affecting 1 in 29 women, and 1 in 9 Indians expected to experience cancer during their lifetimes (Mathur et al. 2020). Chemoprevention employs drugs, either synthetic or natural, to stop, slow down, or reverse the carcinogenesis process (Paul et al 2021). In addition to conventional treatments, the utilization of natural products for therapeutic purposes is still the most common.
Role of plants in cancer treatment
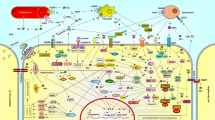
More than 60% of today's anticancer medications were in some manner derived from natural sources. Natural substances with active chemical components are abundant in nature and may be used to treat cancer (Fig. 1) (Rayan et al. 2017). One approach for using natural product medicines to treat cancer is described below:
Extract
Plant extracts are sometimes used in cancer treatment, particularly in Ayurveda therapy, although this method is less common in allopathic medicine, where the active ingredient must be identified and labeled.
Isolate
To comply with FDA (Food and Drug Administration) regulations, most extracts are isolated to their active ingredient for cancer treatment.
Semi-synthetic modification
After isolation, the active ingredients undergo semi-synthetic modification to create a semi-synthetic natural product for cancer treatment.
Synthetic modification
Recent advancements in synthetic chemistry and drug design have made it possible to modify natural products synthetically, which can be useful when the natural product is in limited supply.
Formulation enhancements
Since many natural products are poorly soluble and difficult to absorb through the GI tract, formulation enhancements are being developed to make these drugs more effective.
Conjugates
It is common to tag natural substances with monoclonal antibodies, and this method has been shown to be effective in treating tumors and identifying their epitopes.
Combination therapy
Effective cancer chemotherapy often involves a combination of several drugs, including those derived from both natural and synthetic sources.
Plant derived cancer-prevention agents
The use of plant-derived medicines such as Vinblastine (VBL), Vincristine (VCR), etoposide, paclitaxel, docetaxel, topotecan, and irinotecan have become highly sought after and are among the most potent cancer chemotherapeutics available in the market (Mishra et al. 2014). Despite their toxicities, side effects, and formulation issues like low solubility, these medicines have been successful in treating cancer (Narvekar et al. 2014). The quest for anti-cancer medications derived from plants was sparked in the 1950s by the discovery and development of vinblastine, vincristine, and podophyllotoxins. Since it took around 30 years for novel chemotypes like taxanes and camptothecins to develop into therapeutically useful medications, the NCI (National Cancer Institute) of the United States began a massive plant collection effort in 1960 as a result of this (Ojo et al 2022).
Chemical diversification of natural products
The process of chemical diversification involves modifying natural product extracts, which are composed of complex molecules with various ring systems and functional groups (Mishra et al. 2011). Functional groups and new skeletons in a single step, increasing the chemical diversity of natural goods (Grigalunas et al. 2022). (Figs. 2, 3).
Anticancer drugs derived from plants in clinical development
Vinca alkaloid
These are a vital category of drugs used to treat cancer. These drugs work by disrupting the dynamics of microtubules during cell division, leading to a specific block in mitosis, which ultimately results in cell death (Mukhtar et al. 2014). The Catharanthusroseus G. Don (Apocynaceae), Madagascar periwinkle, is a source of several Vinca alkaloids, including Vindesine (VDS), Vincristine (VCR), Vinorelbine (VRLB), Vinblastine (VBL), and Vincamine (Moudi et al. 2013).
Vinblastine
Vinblastine (VLB) (Fig. 4a) is a naturally existing potent substance present in the Catharanthus roseus (L.) G. Don plant. It acts as a stathmokinetic oncolytic drug, impeding the dynamic movement of microtubules during cell division, and is employed for the treatment of diverse cancer types and other medical ailments (Dhyani et al. 2022). VLB is known to have both suppressive and destructive effects on microtubules, which can lead to side effects such as thinning or brittle hair, sunburn, nausea, vomiting, stomach discomfort, constipation, diarrhoea, jaw pain, headaches, and other aches. VLB is employed in the treatment of various tumor types, encompassing head, breast, Letterer–Siwe disease, testicular cancer, Kaposi's sarcoma, Hodgkins and non-Hodgkins lymphoma, as well as non-small cell lung cancer, mycosis fungoides, bladder, neck, and cervical cancer.
Vincristine
Vincristine (Fig. 4b), formerly referred to as leurocristine and marketed as Oncovin, is a derived from the Catharanthus roseus plant, which is also recognized as Madagascar periwinkle or Vincarosea. It is a chemotherapy drug that inhibits cell division by blocking the formation of microtubules, which are part of the cytoskeleton and mitotic spindle. This leads to cell cycle arrest in metaphase, affecting Cells that are undergoing rapid division including intestinal epithelium, bone marrow, and cancer cells (Barrales-Cureño et al., 2019). Vincristine commonly used in various chemotherapy regimens to treat acute nephroblastoma, Hodgkin's lymphoma, lymphoblastic leukemia, non-Hodgkin's lymphoma, and other cancers (Skubnik et al. 2021).
Vinorelbine
Vinorelbine (Fig. 4c) is a partially synthetic vinca alkaloid that is derived from Catharanthus roseus, marketed as Navelbine by Abbott Healthcare in India. It works by suppressing mitosis during metaphase through tubulin-mediated interaction with microtubular proteins in the mitotic spindle (Klotz et al. 2012). Like other vinca alkaloids, vinorelbine can affect various cellular processes, including amino acid and nucleic acid synthesis, respiration, and Ca2+-transport ATPase activity. Vinorelbine is approved for the management of non-small cell lung cancer, metastatic breast cancer, and rhabdomyosarcoma (Bhambhani et al. 2021).
Vincamine
Vincamine (Fig. 4d) belongs to the class of monoterpenoidindole alkaloids. Although Vincamine has pharmacological effects on both the cardiovascular and central nervous systems, its primary impact is on the blood vessels in the brain, and it is commonly used to prevent and treat cerebrovascular diseases, improve brain metabolism and immune function, and aid in wound healing, among other uses (Zhang et al. 2018).
Vindesine
Vindesine (Fig. 4e), a Vinca alkaloid derived from vinblastine, is utilized in the treatment of acute lymphocytic leukemia, a prevalent type of cancer. Its mechanism of action involves inhibiting the mitotic activity of tubulin, thereby preventing cells from entering metaphase mitosis. Vindesine demonstrates significant efficacy in the treatment of non-small cell lung cancer and resistant cases of juvenile chronic lymphocytic leukemia, where vincristine is not effective. In vitro tests have shown that vindesine is about three times more potent than vincristine and ten times more potent than vinblastine (Dhyani et al. 2022). Vindesine exhibits a limited production of post-metaphase cells and displays potential in the treatment of patients who have experienced a relapse after receiving vincristine within a multi-agent therapy regimen (Qin et al. 2022).
Epipodophyllotoxin
Epipodophyllotoxin is a cytotoxic chemical compound with notable research focusing on podophyllotoxin and its semi-synthetic derivatives, such as teniposide, etoposide phosphate, etoposide, and among lignans. These derivatives have garnered significant interest due to their therapeutic potential, particularly in the treatment of lung cancer(Ma et al. 2006). Podophyllotoxin, which is plentiful inpodophyllin, obtained from Podophyllumpeltatum L. or P. emodi Wall, plays a crucial role in this context (Ardalani et al. 2017).
Etoposide
Etoposide phosphate (Fig. 5a) is an anti-cancer medication that hinders the activity of the topoisomerase II, responsible for DNA unwinding and re-ligation. It achieves this by forming a ternary complex with both topoisomerase II and DNA, thereby impeding the re-ligation of DNA strands. As a result, DNA breakage occurs (Montecucco et al. 2015). This drug is commonly used in the treatment of various types of cancer, including, lung cancer, Ewing's sarcoma, testicular cancer, lymphoma, Kaposi's sarcoma and glioblastoma multiforme, nonlymphocytic leukemia. Etoposide also finds application in a conditioning regimen prior to a bone marrow or blood stem cell transplant (Chakarov et al. 2014).
Teniposide
Teniposide (Fig. 5b), a semi-synthetic derivative of podophyllotoxin, possesses anticancer characteristics by inhibiting DNA synthesis. It achieves this by binding to both topoisomerase II and DNA, resulting in the formation of a complex that triggers double-stranded DNA breaks and impedes their repair. As a consequence, cells are unable to complete the mitotic phase, ultimately leading to cell death. The drug's efficacy is particularly notable during the G2 and S stages of the cell cycle (Liscano et al. 2020). Teniposide has been found to cause myelosuppression when used in conjunction with other chemotherapy agents, and common adverse effects encompassing, gastrointestinal toxicity, alopecia and hypersensitivity reactions. Teniposide is frequently used in chemotherapy for tumors such as paediatric acute lymphocytic leukaemia (Skok et al. 2019).
Taxanes
Paclitaxel, which is derived from the Pacific Yew tree's bark and commonly known as Taxol, is a natural substance that serves as the model taxane. Docetaxel is a semi-synthetic form of paclitaxel. Taxanes, including docetaxel, enhance the stability of microtubules, leading to the prevention of chromosomal separation during the anaphase stage (Mikuła-Pietrasik et al. 2019).
Paclitaxel
Paclitaxel (Fig. 6a) is a chemotherapy agent that affects the usual functioning of microtubule growth by binding to the tubulin subunit. This interaction leads to the formation of a stable complex between paclitaxel and microtubules, preventing their disassembly. This negatively impacts how cells operate since microtubules must shorten and lengthen to serve as the cell's transportation system. Additionally, paclitaxel has demonstrated the ability to induce programmed cell death, known as apoptosis, in cancer cells. It achieves this by binding to the apoptosis inhibitor protein Bcl-2, inhibiting its normal activity and promoting apoptosis in the cancer cells (Leung and Cassimeris 2019). Paclitaxel is approved in the United kingdom for the treatment of lung malignancies, Kaposi's sarcoma, breast, and ovarian, and should be available after anthracyclic chemotherapy fails to cure advanced breast cancer (Farrar and Jacobs 2019).
Docetaxel
Docetaxel (Fig. 6b) is a chemotherapy medication that acts by impeding the disassembly of microtubules within cells. This disruption leads to the accumulation of microtubules, ultimately initiating the process of apoptosis, or programmed cell death (Mukhtar et al. 2014). Docetaxel exhibits more cytotoxicity than paclitaxel and is more effective against various cancer cells when used alongside other anti-cancer drugs. It is known to have negative outcomes on hematology, such as neutropenia, anemia, febrile neutropenia, and thrombocytopenia. Clinical studies have provided evidence of the cytotoxic effects of docetaxel against specific targets liver, head, breast, lung, melanoma, prostate, renal, gastric, ovarian, neck and colorectal cancers (Whitaker and Placzek 2019). It is marketed as Taxotere and is primarily used to treat non-small cell malignancies of the breast, prostate, and other organs.
Camptothecins
Camptothecins are cytotoxic quinoline alkaloids that exhibit inhibitory effects on the DNA enzyme topoisomerase I (topo I). The discovery of camptothecin was made by M. E. Wall and M. C. Wani in 1966 during their exploration for potential anticancer compounds derived from natural sources. It was isolated from the Chinese tree Camptotheca acuminata, commonly referred to as the "happy tree," which has been traditionally utilized in Chinese medicine for the treatment of cancer (Martino et al. 2017).
Topotecan
A camptothecin derivative that is commonly used in chemotherapy is topotecan (Fig. 7a), also known as Hycamtin. Topotecan is water-soluble and shares the same mode of action as irinotecan. The cytotoxic effects of camptothecins occur specifically during the S-phase by blocking the repair of single-strand breaks in DNA that are caused by topoisomerase I (Amjad et al. 2020). Consequently, the inability of mammalian cells to repair the double-strand breaks caused by camptothecins ultimately triggers apoptosis, a process of programmed cell death.
Irinotecan-HCL
Irinotecan (Fig. 7b) is an anti-cancer medication utilized for the treatment of colorectal cancer. It functions by inhibiting enzymes that have anti-tumor effects. It is sold under different brand names including Camptosar, CP0, IRINOTECAN, and CPT-11. Its mode of action involves binding to topoisomerase I and preventing it from functioning, resulting in the creation of a ternary complex that interferes with the replication fork as it moves. This causes double-stranded DNA breakage and replication arrest, leading to programmed cell death due to ineffective DNA damage repair (Kciuk et al. 2020). The drug may cause side effects such as gastrointestinal issues, including infection, diarrhea, abdominal cramps, nausea, and vomiting. In combination with cisplatin, irinotecan is employed to treat advanced small cell lung cancer. Patients who are hypersensitive to the medication or its ingredients should avoid using irinotecan hydrochloride trihydrate for injection (Cinausero et al. 2017).
Cephalotaxanes
Cephalotaxanes (Fig. 8) refer to a versatile group of phytochemicals that have been found to be effective against various types of cancer such as stomach and lung carcinoma. These compounds function by hindering the synthesis of proteins and targeting specific molecular processes. They do not affect the elongation of new peptide chains. Harringtonine, a potential anti-cancer alkaloid, is derived from the Cephalotaxus plant species, including C. harringtonia and C. fortune (Lichota and Gwozdzinski 2018).
Homoharringtonine
Homoharringtonine, an alkaloid extracted from Cephalotaxus harringtonia, is being studied as a treatment for haematological tumours. Also known as omacetaxine mepesuccinate or Omapro, ChemGenex is currently developing irinotecan, which has been granted orphan drug status in both the United States and Europe. Omacetaxine works by preventing the production of specific proteins, especially Mcl-1, which leads to apoptosis. It is different from tyrosine kinase inhibitors like imatinib and may be more effective in individuals who have become resistant to this type of treatment. Nausea, diarrhea, fever vomiting, and reversible heart muscle toxicity has been observed in some cases. Omacetaxine is used to treat various cancers, including sarcoma, breast cancer, endometrial and ovarian cancer, solid tumors, acute promyelocytic blood cancer, myelodysplastic syndrome, chronic myeloid blood cancer (Lü and Wang 2014).
Flavones
Flavones have gained significant attention in recent years as a potential therapy for various cancers such as prostate, ovarian, breast, cervical, and pancreatic cancers, among others, through extensive research and the development of synthetic equivalents (Batra and Sharma 2013).
Flavopiridol
Rohitukine, a chromane alkaloid originally discovered in Amoorarohituka (Roxb.) Wight & Arn. And Dysoxylum binectariferum Hook.f. plants belonging to the Meliaceae family, has been extensively investigated for its therapeutic potential. Ongoing clinical trials are assessing the efficacy of alvocidib, also known as flavopiridol (Fig. 9) or HMR-1275, a cyclin-dependent kinase inhibitor, in the treatment of chronic lymphocytic leukemia. Additionally, flavopiridol has been studied as a potential therapy for arthritis. Encouraging results were observed in a phase I/II clinical trial where flavopiridol demonstrated positive outcomes for treating diffuse large B-cell lymphoma or recurrent mantle cell lymphoma. Its mode of action involves the inhibition of cyclin-dependent kinases, leading to apoptosis and impeding the division of non-small lung cancer cells. The main adverse effects under investigation include secretory diarrhea and a proinflammatory syndrome associated with hypotension. Flavopiridol is currently being researched for potential application in the treatment of lymphoid leukemia, lung and liver carcinoma, and lymphoma (Cuneo et al. 2019).
Stilbenes
Stilbenes are employed in human adenocarcinoma cells to induce apoptosis and modulate multidrug resistance (Wesołowska et al. 2010).
Combretastatin
A subclass of natural stilbenoid phenols are the combretastatins. The South African Bush Willow plant, Combretumcaffrum, produces a range of natural combretastatin compounds in its bark. The capacity of combretastatin family members to impair tumour vascularity varies. In reference to the previously identified vascular disrupting substance colchicine, combretastatin attaches to the -subunit of tubulin at a location known as the colchicine site. Microtubule production in cancer cells is suppressed by tubulin polymerization (Karatoprak et al., 2020). Several clinical trials are currently underway to investigate the therapeutic potential of combretastatin A-4, which is regarded as the most potent naturally occurring compound in the combretastatin family. These trials also include the phosphate prodrug of combretastatin A-4 (CA-4-P) and other analogues such as ombrabulin. One notable phase III clinical trial involving 180 patients is evaluating the effectiveness of CA-4-P in combination with carboplatin for the treatment of anaplastic thyroid cancer was started by the pharmaceutical company OXiGENE in July 2007. There is presently no therapy for this type of cancer that the FDA has completely authorised. High blood pressure appears to be combretastatin's primary adverse impact. Drugs were used to treat this in the ovarian cancer study. Low blood counts and hair loss are other effects. Women of reproductive age should not take combretastatin A-2 (Randall and Monk 2010).
Colchicine
Colchicine, a bioactive compound found in the Colchicum autumnale plant family, has been researched for its potential to treat various conditions including cirrhosis, gout, and crystal arthritis (Dasgeb et al. 2018). Its mechanism of action involves promoting apoptosis, stabilizing microtubule production, binding permanently to tubulin, and arresting the cell cycle. However, its lack of selectivity can lead to unwanted effects on normally dividing cells. Semi-synthetic derivatives of colchicine have been developed to target a wider range of malignancies, but caution is advised due to the hazards associated with its use in cancer treatment. Gloriosasuperba has been identified as a significant source of colchicine in tropical regions (Ben-Chetrit 2019).
Elliptine
Ellipticine, a naturally occurring anticancer agent obtained from various parts of plants such as Bleekeriavitensis and Ochrosiaelliptica, along with isoreserpiline, formerly known as elliptine. The alkaloids are present in other members of the Apocynaceae family such as Aspidosperma and Ochrosia. Ellipticine exerts its effects on DNA primarily through two mechanisms: inhibition of topoisomerase II activity and intercalation with DNA, thereby impeding its proliferation. It is an effective treatment for various cancers, including ependymoblastoma, leukaemia, myeloma, melanoma, breast, and colon. Ellipticine also inhibits CDK2 kinase and prevents the phosphorylation of the p53 protein, making it useful in treating human lung and colon cancer. Elliptinium, a derivative of ellipticine, is currently undergoing clinical trials in France to assess its potential as an anticancer agent (Mazumder et al. 2022).
Berberine
Berberine, a potent anti-cancer agent, is sourced from the roots and rhizomes of plants such asBerberis vulgaris, Tinosporacordifolia, Rhizomacoptidis and Berberisaquifolium. Its therapeutic effectiveness has been demonstrated in various cancer types, including breast, prostate, and colorectal cancers. It achieves this through multiple mechanisms, including the promotion of apoptosis and cell cycle arrest in the G2/M phase. Furthermore, berberine inhibits anti-apoptotic proteins such as c-IAP1 and Bcl-2 while activating pro-apoptotic proteins like p21, p53, caspase-3, and caspase-9 (Rauf et al. 2021).
Terpenoid acid
Terpenoid acid, a natural phytomolecule, possesses significant anticancer properties. Extensive studies have demonstrated its efficacy in the treatment of leukemia, pancreatic cancer, and breast cancer both In-vitro and In-vivo. Additionally, other anticancer drugs like CDDO (2-cyano-3, 12-dioxoolean-1, 9-dien-28-oic acid) and its methyl ester have exhibited promising responsiveness against ovarian cancer. Another triterpenoid called betulinic acid has been identified in betulaceous plants such as Ziziphusmauritiana, Ziziphusrugosa, Ziziphusoenoplia, and Betula sp. This compound has shown cytotoxic effects against various types of cancer, including human melanoma (Lee et al. 2019).
Capsaicin
Capsaicin, a natural compound found in red peppers, has demonstrated powerful anti-metastatic, anti-angiogenic, antimutagenic, chemopreventive and anticancer effects on various types of cancer cells, including bladder, pancreatic, endothelial, prostatic, skin, leukaemia, lung, liver, and colon cells. Capsaicin has demonstrated the ability to modulate several molecular targets associated with breast cancer, such as Caspase-3, ROS (reactive oxygen species), Rac1, and HER-2. Its effectiveness is further enhanced when apoptosis is induced in the presence of the p53 gene product, commonly referred to as the "Grandfather of the Genome". In breast cancer, capsaicin triggered apoptosis for activating the Rac1, ROS pathways. The proteins c-Jun N-terminal protein kinase-1 and p38 are primarily responsible for inducing these pathways (Sarkar et al. 2015).
Cyanidin glycosides
Cyanidin glycosides, organic compounds present in a variety of fruits and vegetables like grapes, blackberries, and red cabbage, possess notable antioxidant properties and the ability to hinder cell growth and division. Extensive studies have revealed that cyanidin glycosides can induce apoptosis in prostate cancer cells, suppress the expression of the COX-2 enzyme in colon cancer, and decrease the production of enzymes like MMP-9 and Erk in gastric cancer cells. Another potent natural substance, saffron, contains carotenoids such as crocin and crocetin, which have demonstrated inhibitory effects on iNOS and COX-2 enzymes, the ability to induce apoptosis, and control various cellular factors associated with cancer growth. Epigallocatechin, a polyphenolic compound found in green tea, has been found to restore tumor suppression genes expression in breast cancer and inhibit the activity of PI3K kinase domain in various cancers (Jain et al. 2022).
Saffron
Saffron, also known as Crocus sativus L., is a spice containing abundant carotenoids such as crocin, crocetin, and safranal, which possess powerful biological properties. Additionally, saffron demonstrates inhibitory effects on iNOS (inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) enzymes, which are associated with inflammation and cancer promotion. These actions contribute to saffron's potential as a valuable agent in cancer prevention and treatment, reducing serum levels of cytokines like IL- and TNF-, regulating the cell cycle by controlling cyclins and cdks, upregulating the Bax/Bcl-2 ratio, controlling the expression of caspases, downregulating the expression of MMPs, inducing apoptosis, targeting microtubules, and inhibiting invasion and metastasis (Ahmed et al. 2020).
Epigallocatechin
Epigallocatechin, a prominent polyphenolic compound found in green tea, possesses remarkable properties in restoring the expression of tumour suppressor genes like retinoid X receptor alpha. This is achieved by its interaction with several high-affinity target proteins, including Zap-70. Consequently, epigallocatechin contributes to the inhibition of breast cancer. Moreover, it demonstrates ATP-competitive activity against the active site of the PI3K kinase domain in different cancer types, such as MDA-MB-231, cervical cancer, brain cancer, and bladder cancer. Molecular docking studies have provided validation for these findings, highlighting the potential of epigallocatechin as a therapeutic agent in the battle against various cancers (Negri et al. 2018).
Gingerol
Ginger is a natural source of gingerol, a group of bioactive compounds that exhibit potent anti-cancer effects against breast, colon, pancreatic, and ovarian cancers. These compounds function by inhibiting the nuclear translocation of NF-B and the phosphorylation of IB, which subsequently leads to a decrease in the expression of iNOS and TNF-alpha. Furthermore, gingerol induces apoptosis in leukemia cells through the mitochondrial pathway. Among the different types of gingerol, (Ashraf 2020)-gingerol stands out for its remarkable anti-cancer potential, particularly in the treatment of breast cancer. Its inhibitory effects on cancer cells are attributed to several factors, including the suppression of cell division, cell cycle arrest, increased apoptosis, and the release of pro-apoptotic mitochondrial cytochrome c. Numerous studies have shed light on the mechanisms of gingerol's action on various cancer cells, including K562 cells and MOLT4 cells with heightened levels of reactive oxygen species (Mao et al. 2019).
Lycopene
Lycopene, a vibrant red pigment found in tomatoes, watermelons, red papayas, and red carrots, possesses notable anticancer properties. It exhibits remarkable effectiveness in targeting the PI3K/Akt signaling pathway, specifically in pancreatic and stomach cancer. In addition, lycopene enhances the activity of antioxidant enzymes such as GSH, GST, and GPxn, which play a crucial role in eliminating oxidative damage caused by carcinogens in breast, endometrial, prostate, and colon cancer. Lycopene influences multiple cellular signaling pathways, including NF-B and JNK, which affect cell proliferation and progression. This has been demonstrated in HT-29 colorectal cancer cells and animal models. By inhibiting NF-B and JNK activation, lycopene not only impedes invasion, metastasis, and proliferation in human SW480 colorectal cancer cells but also exhibits anti-inflammatory effects by suppressing the production of COX-2, iNOS, IL-1, IL-6, and TNF-α. (Trejo-Solís et al. 2013).
Vitamin D from mushrooms
Mushrooms that have been exposed to ultraviolet B (UVB) light are a rich source of vitamin D, which has been shown to possess anti-cancer properties against colon, breast, pancreatic, and ovarian cancer. Vitamin D targets various proteins, enzymes, and signaling pathways involved in cancer development and progression. Furthermore, vitamin D derived from UVB-exposed mushrooms has been found to inhibit tumor growth and metastasis while promoting apoptosis, the programmed cell death of cancer cells (Cardwell et al. 2018).
Mushroom polysaccharides
Mushrooms are known to contain biologically active polysaccharides in both their fruiting bodies and mycelial mass. These polysaccharides have demonstrated immune cell-mediated anticancer properties and the ability to inhibit carcinogenesis. Specific mushroom polysaccharides, such as glucans, lentinan, tegafur, tegafur combined with lentinan, and schizophyllan, have been utilized in the treatment of lung, breast, and gastric cancers. These polysaccharides have shown various beneficial effects, including boosting cellular immunity, inducing apoptosis, preventing invasion and metastasis, activating macrophages, and acting as T-cell adjuvants. Moreover, they can influence gene expression of cytokines and improve survival rates in patients with head and neck cancer. Mushroom dietary fibers have also been observed to exhibit potent anticancer effects against different types of cancer by targeting diverse cellular pathways (Jeon and Shin 2018). The cell walls of mushrooms are rich in high molecular weight components that have the ability to absorb chitin, homo- and heteropolysaccharides, heavy metals, and other cancer-causing compounds. These components have demonstrated potent anticancer properties against various types of cancer and are currently being investigated for their potential applications in pharmaceuticals and protein engineering. Among the bioactive components found in mushrooms, mushroom proteins have garnered significant research attention. Several mushroom species, including Polyporusadusta, Ganodermacarpense, Pleurotusostreatus, Pleurotuseryngii, Pleurotusnebrodensis, Amanita phalloides, and Calvatiacaelata, have been identified as containing effective anticancer substances, particularly for the treatment of lung cancer. For instance, extracts from Ganodermalucidum have shown significant reductions in the production of MMP-2, MMP-9, IL-6, and IL-8 in triple negative breast cancer cells. Chemical compounds derived from mushrooms, such as polysaccharopeptide, have also exhibited anticancer properties by increasing the ratio of CD4+/CD8+/CD14+/CD16 T lymphocytes, enhancing the quantity and percentage of B lymphocytes and CVP, and inducing apoptosis and cell cycle arrest (Panda et al. 2022).
Vitamin E
Vitamin E is a group of compounds consisting of tocotrienols and tocopherols, known for their anti-tumor properties. These fat-soluble antioxidants can be found in oils derived from wheat, safflower, sunflower, and germ. Scientific research has provided evidence that both tocopherols and tocotrienols possess anti-cancer effects, demonstrating proapoptotic and anti-proliferative actions in both in vitro and in vivo experiments (Rizvi et al. 2014).
Fisetin
Fisetin, a flavonoid naturally occurring in various plant species like grapes, strawberries, apples, and onions, has been extensively studied for its potential as an anticancer agent. Scientific investigations have revealed that fisetin exhibits several beneficial effects, including the induction of apoptosis and inhibition of proliferation and migration in human colon cancer cells. It has also demonstrated promising results in the treatment of human lung cancer by blocking the PI3K and Akt signaling pathways and inhibiting the growth of different cancer cell types. Furthermore, fisetin has shown the ability to induce apoptosis in human lung cancer cells by inhibiting the MAPK signaling pathway and reducing the production of reactive oxygen species (ROS) in human oral cancer. In human renal carcinoma Caki cells, fisetin has been found to increase the expression of DR5, leading to apoptosis (Rizvi et al. 2014).
Resveratrol
Resveratrol, a polyphenol naturally found in sources like grapes, blueberries, bilberries, peanuts, and mulberries, has been extensively studied for its potential in treating various cancers, including breast, colorectal, liver, pancreatic, prostate, and lung carcinoma. Its therapeutic effects are attributed to its ability to modulate the expression of several proteins involved in cancer progression. Resveratrol up-regulates p53 and Bcl-2 associated X proteins while down-regulating proteins such as MMPs, NF-B, AP-1, Bcl-2, cyclins, cyclin-dependent kinases, cytokines, and COX-2. Additionally, it has been observed to suppress the activity of VEGF protein by reducing MAP kinase phosphorylation (Shrikanta et al. 2015).
Anti-cancer agents from blue green algae
Blue-green algae, commonly found in marine environments, offer a diverse range of anticancer compounds that can activate various signaling pathways, including protein kinase-c enzymes, NF-B, MAPK kinases, p53, cytokine release, and ROS creation, or induce apoptosis. Many of these algae have been extensively studied for their chemical constituents with potential anticancer properties. For instance, Curacin-A from Lyngbya majuscule and Calothrixin A (I) and B (II) from Calothrix cell extracts exhibit strong antiproliferative effects and have shown promise in inhibiting colon, kidney, and breast cancers. Another compound, cryptophycin 1 (GSV 224), isolated from a species of Nostoc, has been tested against human solid tumors and yielded encouraging results. Edible seaweeds, including Padinaboergeseni, Ulvareticulata, Gracilariafoliifera, Palmariapalmata, Acanthophoraspicifera, Sargassumthunbergii, Ascophyllumnodosum, and Eclonia cava, have also demonstrated anticancer properties and have been utilized in the treatment of kidney cancer, amyloid adenocarcinoma, and colon adenocarcinoma, human nasopharyngeal and colorectal cancer, among others. Additionally, cyanobacteria like Spirulinaplatensis produce beneficial compounds such as phycobiliproteins (including c-phycocyanin, phycocyanobilin, and allophycocyanin) and have shown potential in combating various human diseases, including liver, lung, stomach, and breast cancer (He et al. 2022).
Apigenin
Apigenin (APG) is a flavonoid that is naturally present in a variety of fruits and vegetables, including celery, parsley, and chamomile. It exhibits potent anti-cancer properties while demonstrating low toxicity and non-mutagenicity. APG has been shown to specifically target the leptin/leptin receptor pathway in lung cancer. In addition, it inhibits the STAT3 signaling pathway, promotes caspase-dependent extrinsic apoptosis, and disrupts the phosphorylation of JAK2 and STAT3 pathways. These effects have been observed in the treatment of MDA-MB-453 breast cancer cells (Javed et al. 2021).
Elemene
Elemene, a sesquiterpene derived from Curcuma wenyujin, has emerged as a promising anticancer agent capable of exerting multiple effects on drug-resistant cancers. This natural compound, a key component of traditional Chinese medicine, exhibits inhibitory activity against various types of cancers, induces apoptosis and cell death, reduces Akt phosphorylation and CD34 expression, suppresses the PI3K/Akt/mTOR and MAPK pathways, and attenuates angiogenesis, and upregulates E3 ubiquitin ligases Cbl-b and c-Cbl in human gastric cancer. Furthermore, it has been shown to inhibit the expression of VEGF, a crucial factor in cancer progression (Li et al. 2022).
Chalcone
Chalcone, a naturally occurring flavonoid found in a variety of fruits and vegetables, exhibits potent anticancer properties. It functions by activating multiple caspases, including caspase-8, 9, and 12, which play crucial roles in the apoptotic pathway. Moreover, chalconeupregulates the expression of pro-apoptotic proteins like Bid, Bax, and Bak, while downregulating the expression of anti-apoptotic Bcl-2 genes. These mechanisms contribute to its effectiveness in treating various cancers, including colon, lung, breast, liver, and prostate cancer. Chalcone specifically targets nuclear and cellular components such as Bax, Bid, and Bak proteins, Bcl-2 proteins, as well as caspase-8, 9, and 12 enzymes to induce apoptosis and inhibit cancer growth (Michalkova et al. 2021).
Sesquiterpene lactones
Sesquiterpene lactones are naturally occurring bioactive compounds that can be found in various plant families, with a notable presence in the Asteraceae family. These compounds have shown significant potential in the treatment of different types of cancers, including liver, lung, breast, prostate, and esophageal cancers. Among the sesquiterpene lactones, deoxyelephantopin and isodeoxyelephantopin, derived from Elephantopuscarolinianus and Elephantopusscaber, have exhibited remarkable apoptotic effects through diverse mechanisms. These mechanisms include the generation of reactive oxygen species (ROS), disruption of mitochondrial function, modulation of Bcl-2 family proteins, cell cycle arrest, inhibition of NF-B signaling pathway, and activation of STAT3. By inducing ROS, deoxyelephantopin and isodeoxyelephantopin promote oxidative stress, leading to apoptosis in cancer cells. Furthermore, they interfere with mitochondrial function, affecting the energy metabolism and promoting apoptotic pathways. The modulation of Bcl-2 family proteins by these sesquiterpene lactones also plays a significant role in apoptosis induction. They can alter the balance between pro-apoptotic and anti-apoptotic proteins, triggering the apoptotic cascade (Moujir et al. 2020).
Chrysin
Chrysin, also referred to as 5, 7-dihydroxyflavone, is a powerful anti-cancer compound naturally present in various sources, including propolis, honey, blue passion flower, and chamomile. Studies have shown that chrysin exhibits remarkable anti-cancer effects with minimal adverse reactions in cancer cell lines such as DU145 and PC-3. The efficacy of chrysin in combating cancer is attributed to several mechanisms. It has been observed to induce DNA fragmentation and cell death specifically in SW480 colorectal cancer cells. Moreover, chrysin has the ability to arrest the cell cycle at the G2/M phase, thereby impeding cancer cell proliferation. Additionally, chrysin reduces lipid peroxidation, protecting cells from oxidative damage. Another key mechanism through which chrysin exerts its anti-cancer effects is the activation of MAPK signaling pathways, including ERK1/2 and P38 proteins, in prostate cancer cells (Abotaleb et al. 2018).
Scutellarin
Scutellarin, a potential anti-cancer medication, is naturally present in Scutellaria barbata and Scutellaria altissima, two medicinal plants renowned for their anti-tumor properties against various malignancies, including colon, liver, and prostate cancer. In prostate cancer, scutellarin has been demonstrated to impede cancer cell growth, induce cell cycle arrest specifically in the G2/M phase, and enhance the expression of caspase-3, caspase-9, and the Bax/Bcl-2 ratio. These effects collectively promote apoptosis and hinder the progression of prostate cancer cells. Similarly, in liver cancer (HepG2 cells), scutellarin exhibits its anti-cancer potential by activating the caspase-3 enzyme and initiating the STAT3 signaling cascade, which ultimately leads to apoptosis. By modulating these cellular pathways, scutellarin effectively combats liver cancer cells through the regulation of the p53 gene product. This demonstrates its efficacy in targeting colorectal cancer and influencing critical cellular processes involved in cancer progression (Sun et al. 2022).
Oroxylin
Oroxylin A, a flavone compound, shows promise as an anti-cancer agent with its ability to regulate gene expression and inhibit key cellular pathways. It achieves its anticancer effects by downregulating the expression of COX-2 and iNOS genes, both of which are associated with cancer development and progression. Furthermore, Oroxylin A acts by blocking NF-B and preventing the degradation of I-B, which effectively inhibits the activation of NF-B induced by LPS (lipopolysaccharide). This inhibition of NF-B activity is crucial in suppressing the inflammatory response and other processes involved in cancer growth and metastasis. Notably, Oroxylin A is often used in combination with 5-FU (5-fluorouracil), a commonly used chemotherapy drug for colorectal cancer. This combination treatment approach has shown promise in reducing the required dosage of 5-FU and minimizing associated adverse effects in in vivo studies (Chen et al. 2000).
Kaempferol
Kaempferol, a naturally occurring compound, exhibits strong anticancer properties and can be derived from different sources like propolis, black tea, grapefruit, and broccoli. It has demonstrated notable potential in combating various types of cancer cells, including colorectal cancer and HT-29 cancer cells. One of the mechanisms through which kaempferol exerts its antitumor effects is by increasing the expression of the caspase-3 enzyme, which plays a crucial role in promoting apoptosis, or programmed cell death. Moreover, kaempferol also upregulates the p53 gene and its associated products, which are known for their tumor-suppressive functions (Imran et al. 2019).
Genistein
Genistein, an isoflavone found in soy, lentils, beans, and chickpeas, has emerged as a powerful compound in the fight against cancer. Extensive research has highlighted its significant anticancer properties, particularly in the context of colorectal cancer. One of its primary mechanisms of action is the promotion of apoptosis, or programmed cell death. Genistein exerts its anticancer effects by upregulating the production of pro-apoptotic proteins, including Bax and p21. These proteins play crucial roles in triggering the apoptotic process, leading to the elimination of cancer cells. Additionally, genistein acts by inhibiting NF-κB, a transcription factor involved in inflammation and cancer progression. By blocking NF-κB, genistein helps suppress the growth and survival of cancer cells (Banerjee et al. 2008).
Silymarin
Silymarin, a flavonoid derived from Sylibummarianum, is composed of various flavolignans, including Silydianin, Silychrystin, Silybin A and B, and Isosilybin A and B. When used in conjunction with paclitaxel and doxorubicin, silymarin has demonstrated effectiveness in treating colorectal cancer. Its mode of action involves inducing cell cycle arrest and facilitating apoptosis by inhibiting the activity of cyclin-dependent kinases (CDKs) (Bijak 2017).
Ursonic acid
Ursonic acid, a triterpene present in various plants like rosemary and basil, is known for its antioxidant properties. Notably, it displays a unique characteristic of exerting pro-oxidative effects specifically on cancer cells while maintaining the redox balance in normal cells. Moreover, ursonic acid has been found to exhibit pro-apoptotic properties in colorectal cancer, particularly in the HCT116 cell line. It achieves this by reducing the expression levels of pro-inflammatory NF-B cytokines, survival factors such as Bcl-2, and pro-metastatic MMP-9 matrix metalloprotease (Son and Lee 2020).
Ginsenosides
The medicinal properties of ginseng root are attributed to a group of active compounds called ginsenosides. Extensive research has revealed that these ginsenosides possess cancer-preventive effects against various types of cancer, including colon, liver, breast, ovarian, gastric, and melanoma. The major ginsenosides, namely Rb1, Rb2, Rc, Rd, Re, and Rg1, constitute approximately 80% of the ginsenosides found in ginseng root. On the other hand, minor ginsenosides such as Rg3(S), Rh2(S), F2, compound K (C-K), Rg2(S), Rh1(S), F1, protopanaxatriol, and gypenoside XVII have shown antagonistic interactions. Recently, a potent anti-cancer compound called panaxadiol has been identified in Panax ginseng and Panaxpseudoginseng. This compound exhibits significant anti-cancer activity in multiple cancer cell lines and targets various signaling pathways. Additionally, ginsenosides like Rg3 and Rg5 have been found to induce apoptosis in the HCT116 cell line by inhibiting the expression of NF-B. Furthermore, the metabolite of protopanaxadiol has been shown to enhance the effects of 5-FU on HCT116 cells by arresting the cell cycle at the G1 phase and promoting apoptosis (Redondo-Blanco et al., 2017).
Celastrol
Celastrol, derived from the bark of Tripterygiumwilfordii, is a potent and highly effective anticancer compound. Its mechanism of action involves the inhibition of heat shock proteins, which leads to apoptosis mediated by the caspase-3 enzyme in various cancers such as ovarian (OVCAR-8), colon (SW620), lung (95-D), and prostate cancer. In acute myelogenous leukemia 1-ETO/C-KIT, celastrol induces apoptosis and reduces the expression of oncoproteins. Furthermore, celastrol has shown promise in the treatment of lung cancer cells by inducing caspase-dependent pathways and degrading Hsp90 client proteins. Studies conducted on different cancer types and animal models have demonstrated that celastrol induces apoptosis, cell cycle arrest, and autophagy by activating the ROS/c-JNK signaling pathway. It also exhibits anti-angiogenic effects, inhibits invasion, and upregulates death receptors (Chang et al. 2023).
Gossypol
Gossypol, a natural phytochemical present in Thespesiapopulnea and cotton seeds (Gossypium), has demonstrated promising results in phase II clinical trials as a treatment for breast and prostate cancer. Its effectiveness as an anticancer agent has been evaluated across different tumor types, including lymphoid, hematologic, and solid tumors. In particular, gossypol has shown inhibitory effects on the growth of colorectal cancer cell lines such as HT-29, HCT116, and RKO. Additionally, it has been observed to induce autophagy and promote cell death in these cell lines (Pal et al. 2022).
Plant polysaccharides
For many years, plant-derived polysaccharides have been employed in the treatment of various cancer types, including liver cancer (HepG2 cells), lung cancer (A549 and HL-60 cells), human lymphatic endothelial cancer (HLEC), ovarian cancer, and lung cancer (H157 cells). The potent anticancer properties of polysaccharides extracted from medicinal plants have attracted significant interest due to their non-toxic nature. Both in vitro and in vivo studies have consistently shown that these plant polysaccharides can induce cell cycle arrest and apoptosis. Moreover, they have demonstrated efficacy in suppressing the growth of different types of cancers (Xie et al. 2018).
Isothiocyanates
Isothiocyanates, bioactive compounds commonly found in cruciferous vegetables such as broccoli and watercress, have been extensively investigated for their potential as anti-cancer agents. They offer promising therapeutic benefits in the treatment of various cancers, including cervical, prostate, lung, and colorectal cancer, without causing adverse effects. These natural compounds possess the ability to inhibit cancer cell proliferation, invasion, and progression through different mechanisms, including apoptosis induction and ROS-mediated processes. They also have the capacity to arrest the cell cycle at the G2/M phase, modulate signaling pathways, and alter epigenetic modifications. For example, sulforaphane, an isothiocyanate present in dietary sources, has been found to induce G2/M arrest, downregulate cyclinB1, and upregulate the protein GADD45, which is associated with anti-cancer activity in cervical cancer cells (Esteve 2020).
Genipin
Genipin, a natural compound derived from the Gardenia jasminoides plant, has demonstrated potential as a treatment for breast cancer. Its effectiveness is attributed to its ability to modulate various proteins and enzymes involved in breast cancer, including caspase-3, Bax, Bcl-2, JNK, p38, and MAPK. Through its action of upregulating pro-apoptotic signaling pathways like Bax and caspase-3, and downregulating Bcl-2 expression, as well as activating JNK and p38 MAPK, genipin has been found to exhibit anti-proliferative effects in MDA-MB-231 breast cancer cells (Cho 2022).
Denbinobin
Denbinobin is a powerful phytochemical with notable anti-cancer, anti-angiogenic, and apoptosis-inducing properties. Originally derived from the stems of Dendrobium moniliforme and Ephemeranthalonchophylla, this natural compound has demonstrated the ability to hinder metastasis by decreasing NF-B activation in human breast cancer cells. Additionally, denbinobin effectively reduces the activity of iNOS and COX-2 in a concentration-dependent manner (Hoseinkhani et al. 2020). The general mechanism of action of some anticancer derivatives were given in Fig. 10 highlights the antimetabolites, alkylating agents, and asparaginase involved in anticancer mechanism and Table 1 depicts some important plants and their constituents with potential benefits against different types of cancer.
Conclusion
In a world where the burden of cancer is steadily increasing and conventional therapies fall short, the search for alternative and effective strategies is of utmost importance. The potential of plant-based phytocompounds in cancer therapy shines through as a beacon of hope. Their eco-friendly nature, cost-effectiveness, and proven efficacy make them a compelling option for further exploration.
As we venture into the twenty-first century, drug-based approaches will continue to dominate the landscape of cancer treatment. However, the need for novel drugs that can combat resistant malignancies is urgent. This is where nature's medicine cabinet comes into play. The vast array of bioactive substances found in medicinal plants holds the key to unlocking groundbreaking treatments.
By delving deeper into the toxicological and genotoxic profiles of phytochemicals, we can ensure their safety and tailor their usage to different types of cancers. These natural wonders have already demonstrated their medicinal potential and serve as invaluable templates for the development of new chemical compounds.
In this era of advanced drug discovery techniques, it would be unwise to overlook the power of natural products. They remain an essential source for uncovering and creating novel drugs that can tackle the complexities of cancer. Embracing phytochemicals in our fight against this devastating disease is a forward-thinking and progressive approach.
In conclusion, the future of cancer therapy lies in the hands of these remarkable plant-based compounds. They offer a promising and sustainable solution to the challenges we face. Let us invest in further research, unravel their full potential, and revolutionize cancer treatment. Together, we can make a difference and bring renewed hope to those affected by this formidable adversary.
References
Abdul Aziz MY, Omar AR, Subramani T, Yeap SK, Ho WY, Ismail NH, Ahmad S, Alitheen NB (2014) Damnacanthal is a potent inducer of apoptosis with anticancer activity by stimulating p53 and p21 genes in MCF-7 breast cancer cells. Oncol Lett 7(5):1479–1484
Abedini MR, Erfanian N, Nazem H, Jamali S, Hoshyar R (2016) Anti-proliferative and apoptotic effects of Ziziphus Jujube on cervical and breast cancer cells. Avicenna J Phytomed 6(2):142
Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, Büsselberg D (2018) Flavonoids in cancer and apoptosis. Cancers 11(1):28
Ahmed S, Khan H, Aschner M, Mirzae H, Küpeli Akkol E, Capasso R (2020) Anticancer potential of furanocoumarins: mechanistic and therapeutic aspects. Int J Mol Sci 21(16):5622
Akhouri V, Kumari M, Kumar A (2020) Therapeutic effect of Aegle marmelos fruit extract against DMBA induced breast cancer in rats. Sci Rep 10(1):18016
Ali I, Braun DP (2014) Resveratrol enhances mitomycin C-mediated suppression of human colorectal cancer cell proliferation by up-regulation of p21WAF1/CIP1. Anticancer Res 34(10):5439–5446
Amjad MT, Chidharla A, Kasi A (2020) Cancer chemotherapy
Ardalani H, Avan A, Ghayour-Mobarhan M (2017) Podophyllotoxin: a novel potential natural anticancer agent. Avicenna J Phytomed 7(4):285
Ashraf MA (2020) Phytochemicals as potential anticancer drugs: time to ponder nature’s bounty. BioMed Res Int 2020:1–7
Augustin Y, Staines HM, Krishna S (2020) Artemisinins as a novel anti-cancer therapy: targeting a global cancer pandemic through drug repurposing. Pharmacol Ther 216:107706
Awale S, Linn TZ, Li F, Tezuka Y, Myint A, Tomida A, Yamori T, Esumi H, Kadota S (2011) Identification of chrysoplenetin from Vitex negundo as a potential cytotoxic agent against PANC-1 and a panel of 39 human cancer cell lines (JFCR-39). Phytother Res 25(12):1770–1775
Babykutty S, Padikkala J, Sathiadevan P, Vijayakurup V, Azis T, Srinivas P, Gopala S (2009) Apoptosis induction of Centella asiatica on human breast cancer cells. Afr J Tradit Complement Altern Med 6(1):9–16
Bahadori M, Baharara J, Amini E (2016) Anticancer properties of chrysin on colon cancer cells, in vitro and in vivo with modulation of Caspase-3,-9, Bax and Sall4. Iran J Biotechnol 14(3):177
Banerjee S, Li Y, Wang Z, Sarkar FH (2008) Multi-targeted therapy of cancer by genistein. Cancer Lett 269(2):226–242
Bao M-F, Yan J-M, Cheng G-G, Li X-Y, Liu Y-P, Li Y, Cai X-H, Luo X-D (2013) Cytotoxic indole alkaloids from Tabernaemontana divaricata. J Nat Prod 76(8):1406–1412
Barrales-Cureño HJ, Reyes CR, García IV, Valdez LGL, De Jesús AG, Cortés Ruiz JA, Sánchez Herrera LM, Calderón Caballero MC, Magallón JAS, Espinoza Perez J, et al. (2019) Alkaloids of pharmacological importance in Catharanthus roseus, vol 1. Intech Open Ltd., London
Batra P, Sharma AK (2013) Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech 3:439–459
Benarba B, Meddah B (2014) Ethnobotanical study, antifungal activity, phytochemical screening and total phenolic content of Algerian Aristolochia longa. J Intercult Ethnopharmacol 3(4):150
Ben-Chetrit E (2019). Colchicine. In: Textbook of autoinflammation, pp 729–749
Bhambhani S, Kondhare KR, Giri AP (2021) Diversity in chemical structures and biological properties of plant alkaloids. Molecules 26(11):3374
Bijak M (2017) Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—chemistry, bioavailability, and metabolism. Molecules 22(11):1942
Bode AM, Dong Z (2015) Chemopreventive effects of licorice and its components. Curr Pharmacol Rep 1:60–71
Borah P, Banik BK (2020) Medicinal plants and their compounds with anticancer properties. In: Green approaches in medicinal chemistry for sustainable drug design, pp 759–776. Elsevier.
Brahmachari G, Gorai D, Roy R (2013) Argemone mexicana: chemical and pharmacological aspects. Rev Bras 23:559–567
Caccialupi P, Ceci LR, Siciliano RA, Pignone D, Clemente A, Sonnante G (2010) Bowman-Birk inhibitors in lentil: heterologous expression, functional characterisation and anti-proliferative properties in human colon cancer cells. Food Chem 120(4):1058–1066
Cardwell G, Bornman JF, James AP, Black LJ (2018) A review of mushrooms as a potential source of dietary vitamin D. Nutrients 10(10):1498
Chakarov S, Petkova R, Russev GC (2014) DNA repair systems. Biodiscovery 13:e8961
Chang H-W, Liu P-F, Tsai W-L, Hu W-H, Hu Y-C, Yang H-C, Lin W-Y, Weng J-R, Shu C-W (2019) Xanthium strumarium fruit extract inhibits ATG4B and diminishes the proliferation and metastatic characteristics of colorectal cancer cells. Toxins 11(6):313
Chang C, Tang X, Woodley DT, Chen M, Li W (2023) The distinct assignments for Hsp90$α$ and Hsp90$β$: more than skin deep. Cells 12(2):277
Cheah KY, Howarth GS, Bindon KA, Kennedy JA, Bastian SEP (2014) Low molecular weight procyanidins from grape seeds enhance the impact of 5-Fluorouracil chemotherapy on Caco-2 human colon cancer cells. PLoS ONE 9(6):e98921
Checker R, Sharma D, Sandur SK, Subrahmanyam G, Krishnan S, Poduval TB, Sainis KB (2010) Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J Cell Biochem 110(5):1082–1093
Chen Y-C, Yang L-L, Lee TJF (2000) Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-$κ$B activation. Biochem Pharmacol 59(11):1445–1457
Chen M, Wu J, Luo Q, Mo S, Lyu Y, Wei Y, Dong J (2016) The anticancer properties of Herba Epimedii and its main bioactive componentsicariin and icariside II. Nutrients 8(9):563
Cho YS (2022) Genipin, an inhibitor of UCP2 as a promising new anticancer agent: a review of the literature. Int J Mol Sci 23(10):5637
Chu D-T, Nguyen TT, Tien NLB, Tran D-K, Jeong J-H, Anh PG, Thanh VV, Truong DT, Dinh TC (2020) Recent progress of stem cell therapy in cancer treatment: molecular mechanisms and potential applications. Cells 9(3):563
Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, Parisi G, Calvetti L, Sonis ST (2017) New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front Pharmacol 8:354
Cragg GM, Pezzuto JM (2016) Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract 25(Suppl. 2):41–59
Cuneo A, Barosi G, Danesi R, Fagiuoli S, Ghia P, Marzano A, Montillo M, Poletti V, Viale P, Zinzani PL (2019) Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: a multidisciplinary position paper. Hematol Oncol 37(1):3–14
da Alvares ACM, Schwartz EF, Amaral NO, Trindade NR, Pedrino GR, Silva LP, De Freitas SM (2014) Bowman-Birk protease inhibitor from Vigna unguiculata seeds enhances the action of bradykinin-related peptides. Molecules 19(11):17536–17558
Dasgeb B, Kornreich D, McGuinn K, Okon L, Brownell I, Sackett DL (2018) Colchicine: an ancient drug with novel applications. Br J Dermatol 178(2):350–356
Deep G, Agarwal R (2010) Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev 29:447–463
Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, Szopa A, Sharifi-Rad J, Docea AO, Mardare I et al (2022) Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int 22(1):1–20
Ding Y, He J, Huang J, Yu T, Shi X, Zhang T, Yan G, Chen S, Peng C (2019) Harmine induces anticancer activity in breast cancer cells via targeting TAZ. Int J Oncol 54(6):1995–2004
Dutta R, Khalil R, Green R, Mohapatra SS, Mohapatra S (2019) Withania somnifera (Ashwagandha) and withaferin A: potential in integrative oncology. Int J Mol Sci 20(21):5310
El-Shemy HA, Aboul-Soud MAM, Nassr-Allah AA, Aboul-Enein KM, Kabash A, Yagi A (2010) Antitumor properties and modulation of antioxidant enzymes’ activity by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr Med Chem 17(2):129–138
Esteve M (2020) Mechanisms underlying biological effects of cruciferous glucosinolate-derived isothiocyanates/indoles: a focus on metabolic syndrome. Front Nutr 7:111
Fallah M, Davoodvandi A, Nikmanzar S, Aghili S, Mirazimi SMA, Aschner M, Rashidian A, Hamblin MR, Chamanara M, Naghsh N et al (2021) Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer. Biomed Pharmacother 142:112024
Farrar MC, Jacobs TF (2019) Paclitaxel
Fei Fang E, Abd Elazeem Hassanien A, Ho Wong J, Shui Fern Bah C, Saad Soliman S, Bun Ng T et al (2011) Isolation of a new trypsin inhibitor from the Faba bean (Vicia faba cv. Giza 843) with potential medicinal applications. Protein Peptide Lett 18(1):64–72
Fiqri H, Arda AG, Adrebi K, Proborini WD, Widyanto RM (2020) Evaluation of total phenolic, total flavonoid, and in vitro cytotoxic activity of Syzygium cumini extract in cervical cancer cell. J Phys Conf Ser 1665(1):12033
Formagio ASN, Vieira MC, Volobuff CRF, Silva MS, Matos AI, Cardoso CAL, Foglio MA, Carvalho JE (2015) In vitro biological screening of the anticholinesterase and antiproliferative activities of medicinal plants belonging to Annonaceae. Braz J Med Biol Res 48:308–315
Ghasemzadeh A, Jaafar HZE, Rahmat A (2015) Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology. BMC Complement Altern Med 15(1):1–10
Giri A, Lakshmi Narasu M (2000) Production of podophyllotoxin from Podophyllum hexandrum: a potential natural product for clinically useful anticancer drugs. Cytotechnology 34:17–26
Grigalunas M, Brakmann S, Waldmann H (2022) Chemical evolution of natural product structure. J Am Chem Soc 144(8):3314–3329
Guruvayoorappan C, Kuttan G (2007) Immunomodulatory and antitumor activity of Biophytum sensitivum extract. Asian Pac J Cancer Prev 8(1):27
He S, Li Q, Huang Q, Cheng J (2022) Targeting protein kinase C for cancer therapy. Cancers 14(5):1104
Hoseinkhani Z, Norooznezhad F, Rastegari-Pouyani M, Mansouri K (2020) Medicinal plants extracts with antiangiogenic activity: where is the link? Adv Pharm Bull 10(3):370
Imai M, Kikuchi H, Denda T, Ohyama K, Hirobe C, Toyoda H (2009) Cytotoxic effects of flavonoids against a human colon cancer derived cell line, COLO 201: a potential natural anti-cancer substance. Cancer Lett 276(1):74–80
Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, Imran A, Shahbaz M, Tsouh Fokou PV, Umair Arshad M, Khan H et al (2019) Kaempferol: a key emphasis to its anticancer potential. Molecules 24(12):2277
Jagetia GC, Baliga MS, Venkatesh P (2003) Effect of Sapthaparna (Alstonia scholaris Linn) in modulating the benzo (a) pyrene-induced forestomach carcinogenesis in mice. Toxicol Lett 144(2):183–193
Jain R, Hussein MA, Pierce S, Martens C, Shahagadkar P, Munirathinam G (2022) Oncopreventive and oncotherapeutic potential of licorice triterpenoid compound glycyrrhizin and its derivatives: molecular insights. Pharmacol Res 178:106138
Javed Z, Sadia H, Iqbal MJ, Shamas S, Malik K, Ahmed R, Raza S, Butnariu M, Cruz-Martins N, Sharifi-Rad J (2021) Apigenin role as cell-signaling pathways modulator: implications in cancer prevention and treatment. Cancer Cell Int 21:1–11
Jeon S-M, Shin E-A (2018) Exploring vitamin D metabolism and function in cancer. Exp Mol Med 50(4):1–14
Jung SK, Lee M-H, Kim JE, Singh P, Lee S-Y, Jeong C-H, Lim T-G, Chen H, Chi Y-I, Kundu JK et al (2014) Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J Biol Chem 289(52):35839–35848
Karatoprak GŞ, Küpeli Akkol E, Genç Y, Bardakcı H, Yücel Ç, Sobarzo-Sánchez E (2020) Combretastatins: an overview of structure, probable mechanisms of action and potential applications. Molecules 25(11):2560
Karmakar S, Roy Choudhury S, Banik NL, Ray SK (2011) Molecular mechanisms of anti-cancer action of garlic compounds in neuroblastoma. Anti-Cancer Agents Med Chem (former Curr Med Chem-Anti-Cancer Agents) 11(4):398–407
Kciuk M, Marciniak B, Kontek R (2020) Irinotecan—still an important player in cancer chemotherapy: a comprehensive overview. Int J Mol Sci 21(14):4919
Kim S, Kim N, Jeong J, Lee S, Kim W, Ko S-G, Kim B (2021) Anti-cancer effect of panax ginseng and its metabolites: from traditional medicine to modern drug discovery. Processes 9(8):1344
Klotz DM, Nelson SA, Kroboth K, Newton IP, Radulescu S, Ridgway RA, Sansom OJ, Appleton PL, Näthke IS (2012) The microtubule poison vinorelbine kills cells independently of mitotic arrest and targets cells lacking the APC tumour suppressor more effectively. J Cell Sci 125(4):887–895
Kumar P, Patel D (2023) Ocimum sanctum: an all-round treatment for cancer? Altern Ther Health Med 29(4):253–257
Kuroda S, Kagawa S, Fujiwara T (2014) Selectively replicating oncolytic adenoviruses combined with chemotherapy, radiotherapy, or molecular targeted therapy for treatment of human cancers. In: Gene therapy of cancer, pp 171–183. Elsevier
Lam S, Ng T (2009) Novel galactonic acid-binding hexameric lectin from Hibiscus mutabilis seeds with antiproliferative and potent HIV-1 reverse transcriptase inhibitory activities. Acta Biochim Pol 56(4):649–654
Lauritano C, Andersen JH, Hansen E, Albrigtsen M, Escalera L, Esposito F, Helland K, Hanssen Kine Øand Romano G, Ianora A (2016) Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front Mar Sci 3:68
Lechner JF, Stoner GD (2019) Gingers and their purified components as cancer chemopreventative agents. Molecules 24(16):2859
Lee D, Lee SR, Kang KS, Ko Y, Pang C, Yamabe N, Kim KH (2019) Betulinic acid suppresses ovarian cancer cell proliferation through induction of apoptosis. Biomolecules 9(7):257
Leung JC, Cassimeris L (2019) Reorganization of paclitaxel-stabilized microtubule arrays at mitotic entry: roles of depolymerizing kinesins and severing proteins. Cancer Biol Ther 20(10):1337–1347
Li M, Liu Q, Cui Y, Li D, Wang H, Ng TB (2017) Isolation and characterization of a Phaseolus vulgaris trypsin inhibitor with antiproliferative activity on leukemia and lymphoma cells. Molecules 22(1):187
Li Y, Li Y, Zhang J, Ji L, Li M, Sun X, Feng H, Yu Z, Gao Y (2022) Current perspective of traditional Chinese medicines and active ingredients in the therapy of hepatocellular carcinoma. J Hepatocell Carcinoma 9:41–56
Liang X, Hu C, Han M, Liu C, Sun X, Yu K, Gu H, Zhang J (2022) Solasonine inhibits pancreatic cancer progression with involvement of ferroptosis induction. Front Oncol 12:1211
Lichota A, Gwozdzinski K (2018) Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci 19(11):3533
Liscano Y, Oñate-Garzón J, Delgado JP (2020) Peptides with dual antimicrobial–anticancer activity: strategies to overcome peptide limitations and rational design of anticancer peptides. Molecules 25(18):4245
Liu Q, Chen L, Yin W, Nie Y, Zeng P, Yang X (2022) Anti-tumor effect of ginkgetin on human hepatocellular carcinoma cell lines by inducing cell cycle arrest and promoting cell apoptosis. Cell Cycle 21(1):74–85
Lü S, Wang J (2014) Homoharringtonine and omacetaxine for myeloid hematological malignancies. J Hematol Oncol 7:1–10
Ma X, Yu H (2006) Global burden of cancer. Yale J Biol Med 79(3–4):85–94
Madhuri S, Pandey G (2009) Some anticancer medicinal plants of foreign origin. Curr Sci 96:779–783
Mao Q-Q, Xu X-Y, Cao S-Y, Gan R-Y, Corke H, Beta T, Li H-B (2019) Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 8(6):185
Martino E, Della Volpe S, Terribile E, Benetti E, Sakaj M, Centamore A, Sala A, Collina S (2017) The long story of camptothecin: from traditional medicine to drugs. Bioorg Med Chem Lett 27(4):701–707
Mathur P, Sathishkumar K, Chaturvedi M, Das P, Sudarshan KL, Santhappan S, Nallasamy V, John A, Narasimhan S, Roselind FS et al (2020) Cancer statistics, 2020: report from national cancer registry programme, India. JCO Glob Oncol 6:1063–1075
Mazumder K, Aktar A, Roy P, Biswas B, Hossain ME, Sarkar KK, Bachar SC, Ahmed F, Monjur-Al-Hossain ASM, Fukase K (2022) A review on mechanistic insight of plant derived anticancer bioactive phytocompounds and their structure activity relationship. Molecules 27(9):3036
Michalkova R, Mirossay L, Gazdova M, Kello M, Mojzis J (2021) Molecular mechanisms of antiproliferative effects of natural chalcones. Cancers 13(11):2730
Mikuła-Pietrasik J, Witucka A, Pakuła M, Uruski Pawełand Begier-Krasińska B, Niklas A, Tykarski A, Książek K (2019) Comprehensive review on how platinum-and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell Mol Life Sci 76:681–697
Mishra T, Khullar M, Bhatia A (2011) Anticancer potential of aqueous ethanol seed extract of Ziziphus mauritiana against cancer cell lines and Ehrlich ascites carcinoma. Evidence-Based Complement Altern Med 26:765029. https://doi.org/10.1155/2011/765029
Mishra S, Aeri V, Gaur PK, Jachak SM (2014) Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. Biomed Res Int 14:808302. https://doi.org/10.1155/2014/808302
Montecucco A, Zanetta F, Biamonti G (2015) Molecular mechanisms of etoposide. EXCLI J 14:95
Motyka S, Jafernik K, Ekiert H, Sharifi-Rad J, Calina D, Al-Omari B, Szopa A, Cho WC (2023) Podophyllotoxin and its derivatives: potential anticancer agents of natural origin in cancer chemotherapy. Biomed Pharmacother 158:114145
Moudi M, Go R, Yien CYS, Nazre M (2013) Vinca alkaloids. Int J Prev Med 4(11):1231
Moujir L, Callies O, Sousa PMC, Sharopov F, Seca AML (2020) Applications of sesquiterpene lactones: a review of some potential success cases. Appl Sci 10(9):3001
Mukhtar E, Adhami VM, Mukhtar H (2014) Targeting microtubules by natural agents for cancer therapy microtubule-targeting agents for cancer chemotherapy. Mol Cancer Ther 13(2):275–284
Musial C, Kuban-Jankowska A, Gorska-Ponikowska M (2020) Beneficial properties of green tea catechins. Int J Mol Sci 21(5):1744
Nakahata AM, Mayer B, Ries C, de Paula CAA, Karow M, Neth P, Sampaio MU, Jochum M, Oliva MLV (2011) The effects of a plant proteinase inhibitor from Enterolobium contortisiliquum on human tumor cell lines. Biol Chem 392(4):327–336. https://doi.org/10.1515/bc.2011.031
Narvekar M, Xue HY, Eoh JY, Wong HL (2014) Nanocarrier for poorly water-soluble anticancer drugs—barriers of translation and solutions. AAPS PharmSciTech 15:822–833
Negri A, Naponelli V, Rizzi F, Bettuzzi S (2018) Molecular targets of epigallocatechin—gallate (EGCG): a special focus on signal transduction and cancer. Nutrients 10(12):1936
Ojo OA, Adeyemo TR, Rotimi D, Batiha GE, Mostafa-Hedeab G, Iyobhebhe ME, Elebiyo TC, Atunwa B, Ojo AB, Lima CMG, Conte-Junior CA (2022) Anticancer properties of curcumin against colorectal cancer: A review. Front Oncol 12:881641. https://doi.org/10.3389/fonc.2022.881641
Ooko E, Kadioglu O, Greten HJ, Efferth T (2017) Pharmacogenomic characterization and isobologram analysis of the combination of ascorbic acid and curcumin—two main metabolites of Curcuma longa—in cancer cells. Front Pharmacol 8:38
Pal D, Sahu P, Sethi G, Wallace CE, Bishayee A (2022) Gossypol and its natural derivatives: multitargeted phytochemicals as potential drug candidates for oncologic diseases. Pharmaceutics 14(12):2624
Panda SK, Sahoo G, Swain SS, Luyten W (2022) Anticancer activities of mushrooms: a neglected source for drug discovery. Pharmaceuticals 15(2):176
Pandurangan AK, Esa NM (2014) Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac J Cancer Prev 15(14):5501–5508
Pang S-Q, Wang G-Q, Lin J, Diao Y, Xu R (2014) Cytotoxic activity of the alkaloids from Broussonetia papyrifera fruits. Pharm Biol 52(10):1315–1319
Park KH, Yin J, Yoon KH, Hwang YJ, Lee MW (2016) Antiproliferative effects of new dimeric ellagitannin from Cornus alba in prostate cancer cells including apoptosis-related S-phase arrest. Molecules 21(2):137
Paul S, Roy D, Pati S, Sa G (2021) The adroitness of andrographolide as a natural weapon against colorectal cancer. Front Pharmacol 2(12):731492. https://doi.org/10.3389/fphar.2021.731492
Qin R, You F-M, Zhao Q, Xie X, Peng C, Zhan G, Han B (2022) Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets. J Hematol Oncol 15(1):133
Rahman S, Parvin R (2014) Therapeutic potential of Aegle marmelos (L.)-an overview. Asian Pac J Trop Dis 4(1):71–77
Randall LM, Monk BJ (2010) Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol 117(3):497–504
Rauf A, Abu-Izneid T, Khalil AA, Imran M, Shah ZA, Emran TB, Mitra S, Khan Z, Alhumaydhi FA, Aljohani ASM et al (2021) Berberine as a potential anticancer agent: a comprehensive review. Molecules 26(23):7368
Rayan A, Raiyn J, Falah M (2017) Nature is the best source of anticancer drugs: indexing natural products for their anticancer bioactivity. PLoS ONE 12(11):e0187925
Redondo-Blanco S, Fernández J, Gutiérrez-del-Río I, Villar CJ, Lombó F (2017) New insights toward colorectal cancer chemotherapy using natural bioactive compounds. Front Pharmacol 8:109
Renner O, Mayer M, Leischner C, Burkard M, Berger A, Lauer UM, Venturelli S, Bischoff SC (2022) Systematic review of Gossypol/AT-101 in cancer clinical trials. Pharmaceuticals 15(2):144
Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F (2014) The role of vitamin E in human health and some diseases. Sultan Qaboos Univ Med J 14(2):e157
Rungruangmaitree R, Jiraungkoorskul W (2017) Pea, Pisum sativum, and its anticancer activity. Pharmacogn Rev 11(21):39
Sakarkar DM, Deshmukh VN (2011) Ethnopharmacological review of traditional medicinal plants for anticancer activity. Int J Pharm Tech Res 3(1):298–308
Sarkar A, Bhattacharjee S, Mandal DP (2015) Induction of apoptosis by eugenol and capsaicin in human gastric cancer AGS cells-elucidating the role of p53. Asian Pac J Cancer Prev 16(15):6753–6759
Satheesh LS, Murugan K (2011) Antimicrobial activity of protease inhibitor from leaves of Coccinia grandis (L.) Voigt. Indian J Exp Biol 49(5):366–374
Sawadogo WR, Schumacher M, Teiten M-H, Dicato M, Diederich M (2012) Traditional West African pharmacopeia, plants and derived compounds for cancer therapy. Biochem Pharmacol 84(10):1225–1240
Sen S, Dutta SK (2012) Evaluation of anti-cancer potential of ragi bifunctional inhibitor (RBI) from Eleusine coracana on human chronic myeloid leukemia cells. Eur J Plant Sci Biotechnol 6(2):103–108
Seo W-G, Hwang J-C, Kang S-K, Jin U-H, Suh S-J, Moon S-K, Kim C-H (2005) Suppressive effect of Zedoariae rhizoma on pulmonary metastasis of B16 melanoma cells. J Ethnopharmacol 101(1–3):249–257
Sharifi-Rad J, Kamiloglu S, Yeskaliyeva B, Beyatli A, Alfred MA, Salehi B, Calina D, Docea AO, Imran M, Anil Kumar NV et al (2020) Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Front Pharmacol 11:571459
Shrikanta A, Kumar A, Govindaswamy V (2015) Resveratrol content and antioxidant properties of underutilized fruits. J Food Sci Technol 52:383–390
Singh S, Yadav S, Sharma P, Thapliyal A (2012) Betula utilis: a potential herbal medicine. Int J Pharm Biol Arch 3(3):493–498
Skok Z, Zidar N, Kikelj D, Ilaš J (2019) Dual inhibitors of human DNA topoisomerase II and other cancer-related targets. J Med Chem 63(3):884–904
Škubník J, Pavlíčková VS, Ruml T, Rimpelová S (2021) Vincristine in combination therapy of cancer: emerging trends in clinics. Biology 10(9):849
Son J, Lee SY (2020) Therapeutic potential of ursonic acid: comparison with ursolic acid. Biomolecules 10(11):1505
Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L (2011) Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother Pharmacol 68:1161–1172
Sun X, Zhong X, Ma W, Feng W, Huang Q, Ma M, Lv M, Hu R, Han Z, Li J et al (2022) Germacrone induces caspase-3/GSDME activation and enhances ROS production, causing HepG2 pyroptosis. Exp Ther Med 24(1):1–13
Tariq A, Mussarat S, Adnan M (2015) Review on ethnomedicinal, phytochemical and pharmacological evidence of Himalayan anticancer plants. J Ethnopharmacol 164:96–119
Trejo-Solís C, Pedraza-Chaverrí J, Torres-Ramos M, Jiménez-Farfán D, Cruz Salgado A, Serrano-García N, Osorio-Rico L, Sotelo J (2013) Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid-Based Complement Altern Med 2013:1–7
Tuorkey MJ (2016) Molecular targets of luteolin in cancer. Eur J Cancer Prev 25(1):65
Wesołowska O, Wiśniewski J, Bielawska-Pohl A, Paprocka M, Duarte N, Ferreira M-JU, DUŚ D, Michalak K (2010) Stilbenes as multidrug resistance modulators and apoptosis inducers in human adenocarcinoma cells. Anticancer Res 30(11):4587–4593
Whitaker RH, Placzek WJ (2019) Regulating the BCL2 family to improve sensitivity to microtubule targeting agents. Cells 8(4):346
Xia W, Tao Z, Zhu B, Zhang W, Liu C, Chen S, Song M (2021) Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int J Mol Sci 22(17):9118
Xie T, Mo L, Li L, Mao N, Li D, Liu D, Zuo C, Huang D, Pan Q, Yang L et al (2018) Identification of side population cells in human lung adenocarcinoma A549 cell line and elucidation of the underlying roles in lung cancer. Oncol Lett 15(4):4900–4906
Xu L-N, Lu B-N, Hu M-M, Xu Y-W, Han X, Qi Y, Peng J-Y (2012) Mechanisms involved in the cytotoxic effects of berberine on human colon cancer HCT-8 cells. Biocell 36(3):113–120
Yadav B, Bajaj A, Saxena M, Saxena AK (2010) In vitro anticancer activity of the root, stem and leaves of Withania somnifera against various human cancer cell lines. Indian J Pharm Sci 72(5):659
Zhang XQ, Yang CY, Rao XF, Xiong JP (2016) Plumbagin shows anti-cancer activity in human breast cancer cells by the upregulation of p53 and p21 and suppression of G1 cell cycle regulators. Eur J Gynaecol Oncol 37(1):30–35
Zhang F, Shi J-J, Thakur K, Hu F, Zhang J-G, Wei Z-J (2017) Anti-cancerous potential of polysaccharide fractions extracted from peony seed dreg on various human cancer cell lines via cell cycle arrest and apoptosis. Front Pharmacol 8:102
Zhang Y, Li J, Yan C (2018) An update on vinpocetine: new discoveries and clinical implications. Eur J Pharmacol 819:30–34
Zhao Y, Wang C-M, Wang B-G, Zhang C-X (2005) Study on the anticancer activities of the Clematis manshrica saponins in vivo. China J Chin Mater Med 30(18):1452–1453
Zhou L, Wu F, Jin W, Yan B, Chen X, He Y, Yang W, Du W, Zhang Q, Guo Y et al (2017) Theabrownin inhibits cell cycle progression and tumor growth of lung carcinoma through c-myc-related mechanism. Front Pharmacol 8:75
Acknowledgements
We extend our gratitude to the B. S. Abdur Rahman Crescent Institute of Science and Technology, Chennai, India and Simbioen Labs and Scientific Services Pvt. Ltd, Chennai, India for their invaluable support in facilitating the production of this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pavithra, R., Khan, M.R. & Khan, M.S. Recent advancements in natural compounds for cancer therapy and prevention. Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-09940-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-09940-0