Abstract
Phytochemicals are widely known for the pharmacological effects in treating various human conditions and in recent years, new compounds are being discovered with substantial health benefits. Karanjin is a furanoflavonoid mainly isolated from Millettia pinnata L., emerging in the field of pharmacology and exerting potential therapeutic values in pre-clinical studies. The review aims to highlight the potential of karanjin as a neuroprotective agent with the significance of modulating the underlying molecular mechanistic pathways. Common neurodegenerative diseases reported globally include Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis. The main problem in the treatment of neurodegenerative diseases is the effect of the prescribed drugs for the underlying conditions is only momentary whereby a permanent solution is unavailable. Bioactive compounds under the class of flavonoids have largely been acknowledged for neuroprotection in pre-clinical studies and partial clinical trials through various mechanism of action such as modulation of NF-kB pathway, inhibition of oxidative stress, modulation of PI3K/Akt, and more. Molecular docking results of karanjin have proven the potential against Alzheimer’s and Parkinson’s disease through modulation of molecular targets adenosine A2A receptor, α-synuclein, catechol-O-methyltransferase, monoamine oxidase B, angiotensin converting enzyme, β-site APP cleaving enzyme, glycogen synthase kinase-3, TNF-α converting enzyme, and acetylcholinesterase involved in the disease progression, compared to commercial standard drugs. The review emphasizes the optimization method for the isolation of karanjin and the various impending mechanistic effects of karanjin in modulating neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies on phytochemicals have gained huge interest among researchers in the past decade. Plant derived bioactive compounds have been a reliable source of drug to treat human conditions and many such compounds and their derivatives were successfully commercialized into prescribed medicine (Nasim et al. 2022). Bioactive compounds received much attention in the field of medicinal chemistry and pharmacology since the compounds exerted therapeutic ability for multiple conditions but shown very minimal or no adverse effects. In fact, a single bioactive compound could possess numerous therapeutic values, which can be applied through different modes of administration and formulation (Riaz et al. 2023). Flavonoids are a large group of bioactive compounds in plants that are known for medicinal properties against human ailments. The class of compounds is mainly present in leaves, stem, seeds, fruits, flowers, and roots of plants either in the free form or in the form of flavonoid glycosides (Ekuadzi et al. 2013). The immense therapeutic values of flavonoids have brought into consideration of developing synthetic drugs to mimic the structure and electron donating efficacy of the compounds.
Karanjin (IUPAC: 3-methoxy-2-phenylfuro[2,3-h]chromen-4-one; C18H12O4) is a furanoflavonoid (Fig. 1) majorly isolated from the karanja tree species M. pinnata also known as Pongamia pinnata L. Pierre belonging to the family Fabaceae. The karanja tree is widely distributed in Asian countries such as Sri Lanka, Malaysia, Philippines, Burma, and especially in the southern region of India where it has a long history of traditional use. Each part of the tree were traditionally used as crude drug for wound healing and also reportedly possess medicinal effects for skin diseases, ulcers, piles, rheumatism, scabies, and many more (Sharma et al. 2020). Different parts of the tree such as the seeds, leaves, flower, roots, and bark were reported to possess different pharmacological effects (Sajid et al. 2012). The flower extract of P. pinnata has anti-hyperglycemic properties, its leaves are used for rheumatic pain and its roots are effective for gonorrhea (Akanksha and Maurya 2010). The alcoholic extract of the seed oil has significant anti-bacterial and anti-hyperglycemic properties. The seeds from P. pinnata has been utilised for its natural bioactive compounds as it contains about 33 to 36% essential oil and 20 to 28% protein (Belide et al. 2010). Karanjin was also reported to be majorly present in the seeds or legumes of several other plants belonging to the family Fabaceae such as Millettia pulchra Benth. Kurz, Fordia cauliflora Hemsl., Tephrosia purpurea L., Millettia pachycarpa Benth., Desmodium sequax Wall., and Lonchocarpus latifolious Willd (Kumar and Gehlot 2012; Fan et al. 2013; Singh et al. 2016, 2021; Zhang et al. 2020). Karanja seed oil was primarily used as biofuel and biopesticide in rural areas. Research shows that karanja oil has significant larvicidal and insecticidal activities, relating to its conventional use (Al Muqarrabun et al. 2013). The highest yield of karanjin (approximately 20%) was reported from the seeds of P. pinnata whereas the lowest yield was from the fruits of Millettia leucantha Kurz. at an insignificant value (Phrutivorapongkul et al. 2003; Vismaya et al. 2010; Sriphana et al. 2018). Accumulation of karanjin in the legumes or seeds can be related to the metabolism and survival of the trees whereby the legumes and seeds are prone to microbial infection or attack by herbivores. Bioactive compounds are commonly biosynthesized by plants as a protective mechanism to survive biotic and abiotic stress (Zabalza et al. 2017). The presence of bioactive compounds like karanjin in a mangrove tree species like P. pinnata is justifiable as the tree requires adaption to rough environmental conditions and salt water for instance. The tree is also reported to be growing at various climates from arid to cold, and geographical locations from low to high altitudes (Katekhaye et al. 2012b; Kesari et al. 2013). Moreover, the use of karanja oil as a biopesticide explains the natural role of the furanoflavonol in preventing the attack of insects and herbivores on the seeds of P. pinnata. Karanjin isolated from the seed oil of P. pinnata was also reported as a biopesticide, attributing to the insecticidal and larvicidal effects of karanja oil (Al Muqarrabun et al. 2013; Raghav et al. 2019). The pharmacological reports on karanjin states that it possesses anti-diabetic, anti-cancer, anti-tumor, anti-inflammatory, and anti-Alzheimer’s activities (Gnanaraj et al. 2022).
Neuron cells function to transmit information within the nervous system. Hippocampus region of the brain contains densely packed neuron cells which is important for memory and learning. Progressive degeneration of the neuron cells is commonly characterized as neurodegenerative disease implicated by various underlying reasons such as inheritance, trauma, and environmental factors. Neurodegenerative disease is an umbrella term collectively used for non-communicable neurological disorders (Anand and Dhikav 2012). The devastating diseases can impair a person’s physical movement, critical thinking, cognitive function, mental orientation, speech, memory, and motor skills (Bak and Chandran 2012). Among the most common neurodegenerative diseases are Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS). Recent statistics by the United Nations shows the number of patients with dementia have increased globally over the past two decades from 13.5 million to 21.2 million by 2025 as the world’s population had increased drastically (Zheng and Chen 2022). The number of patients diagnosed with Alzheimer’s and Parkinson’s disease are anticipated to be doubled by the year 2050 as the human population in the age group older than 65 years will increase. Particularly, the number of worldwide dementia cases are expected to reach an alarming 21.2 million by 2025, and further up to 36.7 million by 2050. Moreover, recent reports revealed the rate of death caused by Alzheimer’s disease is leveled with stroke-related deaths, accounting for the third highest cause of mortality in the world after heart disease and cancer (Lamptey et al. 2022). Parkinson’s disease being the second most common neurodegenerative disease after Alzheimer’s disease, is also doubling in numbers (Ding et al. 2022). Unlike other conditions, the neurodegenerative diseases are highly unpredictable neither no early diagnosis is available nor have any reliable biomarkers been depicted to trace early pathogenesis of them, especially in the cases of Alzheimer’s and Parkinson’s disease. In fact, the underlying pathogenesis for Alzheimer’s and Parkinson’s disease is mostly unknown whereby detection of pathological changes are only apparent upon recognition of symptoms, which is considered too late for reversal of the diseases (Emamzadeh and Surguchov 2018; Hampel et al. 2018). Blood–brain barrier (BBB) becomes the major obstacle in the treatment of neurodegenerative diseases as most of the favourable drugs could not penetrate the line, thus lowering the bioavailability of the drugs at the target site. Though surgery was a successful option is several cases, the concern for long-term defect including the damage to BBB became a drawback. Hence, use of potential drugs or formulation that could cross the BBB to prevent the progression of neurodegeneration became a target (Youdim et al. 2003; Hajialyani et al. 2019; Khan et al. 2020a, b). It is important to learn the underlying molecular mechanism in each of the disease progression and development since studies reported there are more than one pathophysiology involved. Upon crossing the BBB, the potential drug candidate should be compatible to modulate the molecular targets that mainly triggers the neuronal death. Flavonoids are known to fulfil these criteria likewise proven in several in vivo preclinical studies. Therefore, the review aims to emphasize the possibility of karanjin as a neuroprotective agent with the significance of modulating the underlying molecular mechanistic pathways. Relevant literature for the review were collected from common search engines, Google Scholar, Scopus, Science Direct, Wiley Online Library, Springer, etc. Inclusion criteria for the selection of content were full length articles, abstracts, and book chapters limited to the studies on karanjin, flavonoids with neuroprotective effects, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and ALS.
Karanjin as a bioactive compound
Origin of karanjin
The biosynthesis of flavonoids in plants is generally initiated by synthesis of aromatic amino acids through shikimate pathway. Phosphoenol pyruvate that enters from pentose phosphate pathway together with erythrose-4-phosphate forms shikimate through a series of reactions. Shikimate is further converted to chorismate upon undergoing a series of reactions. Chorismate acts as precursor for the aromatic amino acids phenylalanine and tyrosine, synthesized through a specific terminal pathway and tryptophan synthesized on another terminal pathway. The aromatic amino acids stand as the precursors for numerous secondary metabolites in plants, especially alkaloid and phenolic compounds (Maeda and Dudareva 2012; Tohge et al. 2013). Flavonoid biosynthesis further takes the phenylpropanoid pathway, which is required for the synthesis of lignin. Phenylalanine is the important precursor in this pathway where it is converted to cinnamate, then p-coumarate, further to chalcone, dihydroflavonols, and anthocyanins or tannins upon series of reactions. The conversion of phenylalanine to lignins, coumarins, flavonoids, or tannins all depends on the requirement of the plant or necessity at the time of biosynthesis due to abiotic or biotic stress. Each plant species have its own unique ways in deriving secondary metabolites that possess bioactivities, depending on the environmental factors, geographical location, genetic inheritance, and more. Similarly, it is hypothesized that karanjin could have been biosynthesized through two dominant pathways, namely chalcone pathway and acetophenone pathway (Singh et al. 2021). Chalcone pathway involves the formation of chalcone derivative scaffolds that are synthesized to form the backbone structure of flavonoids. The formation of flavonoids from chalcone scaffolds involves multiple enzymes from different classes such as transferases, isomerases, hydrolases, and reductases. The enzymes and chalcone derivatives were identified to be present in karanja tree through analysis of transcriptome sequencing of the whole plant (Sreeharsha et al. 2016). Addition of furan ring to the flavonoid backbone of karanjin is probably by cytochrome P450 through hydroxylation of methyl group to carbonyl group since a similar biosynthesis pathway was proposed and described for the formation of furanoterpenes (Gaikwad and Madyastha 2002). The chemistry was explained as the hydrogen bonds are formed with ketone, the molecule orientation at its active site in a way exposing methyl group to carbonyl group for hydroxylation by cytochrome P450 selectively to yield hydroxymethyl. Intramolecular non-enzymatic cyclization takes place in the subsequent step to yield hemiketal, which is further dehydrated to yield furan. On the other hand, acetophenone pathway hypothetically involves acetophenone originated from acetyl coenzyme A or derived from phenylalanine through phenyl propanoid intermediates, as the precursor for furan ring in karanjin (Trivedi et al. 2015). Isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), were considered the putative electrophiles for the formation of furan ring in this pathway and later chalcone scaffold and flavonoid biosynthesis would take over the finishing sequence of karanjin. Since karanjin is largely present in the seed oil of karanja tree, together with the other furanoflavonoid pongamol and fatty acids, the probable biosynthesis pathway could be acetophenone pathway to initiate the furan ring and later attached to chalcone backbone formation. The backbone structure of pongamol resembles furanochalcone, whereas karanjin is biosynthesized as the end product in the process. Hence, it is assumed that both the furanoflavonoids were initially synthesized by the furan ring formation from acetophenone pathway followed by attachment to the chalcone backbone formation specific for karanjin biosynthesis. The assumed biosynthesis pathway of karanjin and pongamol is depicted in Fig. 2.
It is well-known that karanjin belongs to the subclass of flavonol under flavonoids, characterized by a backbone structure of cinnamoyl with methoxy substituent groups and benzoyl with a furan ring. Chalcone as the precursor for the backbone of karanjin, is an open-chain flavonoid consisting two aromatic rings merged by a three carbon α,β-unsaturated carbonyl system. It is also known that compounds biosynthesized using chalcone backbone have profound pharmacological properties such as anti-cancer, anti-inflammatory, and anti-microbial activities (Saito et al. 2015). Furan is a five membered aromatic heterocyclic structure with high reactivity and has important biological properties. Though furan by itself could be carcinogenic to humans, evidence shows the effectiveness of furan as immunomodulatory agent, also with anti-microbial and anti-inflammatory abilities (Nevagi et al. 2015; Alizadeh et al. 2020). Hence, furans are more likely described with bioactive nature with therapeutic credentials. Compounds that contain furan ring has significant pharmacological properties whereby most pharmaceutical drugs contains furan or tetrahydrofuran ring. The presence of furan readily facilitates the absorption of drugs across almost all of the biological membranes, especially the gastrointestinal and pulmonary systems (Saeid et al. 2023). Although furan fused with chalcone is not a common finding in plant secondary metabolites but furanoflavonoids were discovered in several plant species belonging to the genus Millettia, Derris, Tephrosia, Desmodium, Fordia and Lonchocarpus. It is noteworthy that furan fused chalcone derivatives are found in legume containing plants or trees under the family Fabaceae or previously known as Leguminosae. The seeds or legumes of trees are highly vulnerable to be attacked by herbivores and insects thus the furan ring, with natural insecticidal and antimicrobial properties are assumed to be synthesized alongside the flavonoid precursor chalcones (Saito et al. 2015; Singh et al. 2016; Saeid et al. 2023).
Isolation of karanjin
Karanja tree has been reported to contain more than 115 flavonoids and their derivatives, comprising of flavones, flavans, and chalcones. More than 42 miscellaneous compounds of various classes of terpenes, fatty acids, sterols and aromatic esters were reported to be discovered in extracts of P. pinnata (Al Muqarrabun 2013). The flavonoids and their derivatives were reportedly present in the leaves, flowers, fruit, stem bark, root, and seed of P. pinnata. Isolation of flavonoids is generally done through various extraction and fractionation methods (Yin et al. 2013). Similarly, there are several reported methods for optimized isolation of karanjin from P. pinnata. Karanjin was successfully isolated for the first time in 1925 by Prof. Dr. Limaye, from the seed oil of P. glabra, making it a brand compound of the tree (Limaye 1925). Isolation and characterization of karanjin has been successfully performed from the seeds, leaves, root, stem bark, and flowers of karanja tree. According to past reports, seeds of karanja tree contains the highest concentration of karanjin, therefore the isolation process is mostly directed towards the seeds and not the other parts of the tree that also contains karanjin in low concentrations (Katekhaye et al. 2012b; Dhanmane and Salih 2018; Rekha et al. 2020; Singh et al. 2021). Conventional extraction method involves extraction using solvents and isolation using chromatographic techniques. An attempt to extract and isolate karanjin using distillation technique by precipitating alcohol fractions of karanja oil that was obtained through mechanical pressing of the seeds yielded very little amount (0.99%) of karanjin though the time taken for the process was lengthy (30 h) (Rao et al. 1939). Another extraction method used petroleum ether to extract the bark of P. pinnata and separated the products using fractional crystallization and chromatography acquiring five new flavones, where karanjin as one of the flavone was extracted with concentrated hydrochloric acid to obtain a yield of 0.08% (Row 1952). Apart from conventional extraction, isolation of karanjin was also performed through liquid–liquid extraction on callus cultures of in vitro propagated P. glabra, followed by column chromatography (Sreelakshmi and Janardhan Reddy 2012). The study was merely validating the presence of karanjin in the nodules of P. glabra plantlets, hence no significance was given for the yield. Various solvents viz. methanol, ethyl acetate, petroleum ether, and hexane were used to prepare crude extract through liquid–liquid extraction with the seed oil of P. pinnata, and the extracts were concentrated and subjected to chromatographic techniques for the isolation of pure karanjin (Raghav et al. 2019). Chromatographic techniques such as TLC, HPLC, and column chromatography were commonly used for the purification of karanjin from the isolates of P. pinnata, with a mobile phase comprising methanol, water and acetic acid (Lee et al. 2009; Katekhaye et al. 2012a; Doke et al. 2021).
Modern chromatographic techniques such as high-performance thin layer chromatography (HPTLC) and high-speed counter-current chromatography were also employed in isolating karanjin from the bark of P. pinnata, in a pure form at a rapid duration (Lanjhiyana et al. 2012; Yin et al. 2013). Although the purity of karanjin was high (> 95%) in these methods, the yield however was considerably lower as compared to the conventional isolation. Another modern technique demonstrated to isolate karanjin with high purity, rapid duration and increased yield using energy-efficient sonochemical extraction of karanjin from P. pinnata leaves (Doke et al. 2021). Ultrasonic-assisted extraction initiated the isolation of karanjin from karanja leaves within 30 min for a better yield (5.46 mg/g) compared to the yield (2.5 mg/g) produced by alcoholic extraction using soxhlet for 24 h. The extraction method also proved that karanjin has higher affinity towards polar solvents such as methanol and ethyl acetate where the extraction yield could be increased with increased solute–solvent ratio up to 1:8 (w/v). Increase in carbon chain of non-polar solvents causes steric hindrance, leading to poor hydrogen bonding with the ketone group of karanjin which explains the low yield (Sajid et al. 2012). The researchers also reported that increase in the ultrasonic assisted extraction (UAE) time could increase the yield of karanjin but the maximum yield was obtained at 30 min, and it became constant after up to 120 min. It was clearly noticed that karanjin was heat sensitive and could deteriorate upon accumulation of heat or increase in temperature during the extraction process (Damle and Choudhari 2018; Doke et al. 2021). Therefore, any extraction or isolation method of karanjin that involves excessive heat is not suitable to obtain desired yield. Likewise, increasing the energy of UAE would accumulate heat in the process due to the energy being supplied. A stability study on isolated karanjin from the methanol extract of P. pinnata seeds using HPTLC and HPLC showed that karanjin was not degraded at photolytic, thermal, neutral, and oxidative stress conditions but were degraded by acidic and alkaline conditions (Damle and Choudhari 2018). This indicates extraction of P. pinnata should be maintained in a neutral condition to isolate pure karanjin.
Considering the factors that influence the yield of karanjin extract, a combination of conventional and modern isolation method could possibly give a higher yield. Seeds of P. pinnata contains highest concentration of karanjin in the hull and minimal concentration in the kernel. The seed oil of P. pinnata is a rich source of karanjin that can be isolated and purified using modern chromatographic tools. An optimized method developed by Vismaya et al. (2010) explained the isolation of karanjin from the seed oil of P. pinnata obtaining 20% yield, which is considered the highest of all reported. The researchers extracted the seed oil using petroleum ether in a ratio of 1:5 (w/v), concentrated the extract and subjected to liquid–liquid extraction using methanol in a ratio of 1:2 (v/v). The concentrated methanol extract was subjected to neutral activated alumina column chromatography, eluted with methanol and the eluent was collected and distilled to obtain a partially purified fraction. Adsorption chromatography using alumina has shown better recovery of karanjin as compared to silica which commonly has poor recovery of the compound. The partially purified fraction essentially contained karanjin with approximately 80% purity was recovered using preparative HPLC (silica column C5 250 mm × 21.2 mm) in a water-acetonitrile mobile phase 20–80% linear gradient (4 mL/min flow rate, 110 min). Karanjin in the partially purified fraction was also purified using crystallization technique by adding acetonitrile and water in a ratio of 7:3 (v/v) at 7 °C for a week. Preparative HPLC offers the purified karanjin in a short duration whereas crystallization takes a longer time but both techniques are conducive in attaining high yield of pure karanjin. The purity of karanjin recovered from preparative HPLC and crystallization method was above 95% as validated by spectroscopic (UV, NMR) and chromatographic (HPLC, LC–MS) analysis (Vismaya et al. 2010; Yi et al. 2014; Singh et al. 2016; Ghosh and Tiwari 2018). Therefore, the validated method is considered simple and viable in getting high yield of karanjin from the seeds of P. pinnata to analyze the pharmaceutical applications of the compound. The reported extraction yields of karanjin from different parts of karanja tree is given in Table 1, references of studies on isolation of karanjin without reporting the yield were excluded.
Physicochemical properties of karanjin
Karanjin is a needle shaped white crystalline solid, with the molecular weight 292.3 g/mol and a zero net charge. The ultraviolet-visual absorption (UV/Vis λmax) values for karanjin in methanol shows two peak bands at 260 and 308 nm, indicating the presence of cinnamoyl and benzoyl structures, respectively (Zsila et al. 2003). The molar extinction coefficient of karanjin was estimated at 7380 M−1 cm−1. Karanjin is known to exhibit solvatochromic property, fluorescing color according to polarity of the extracting solvent that is commonly expressed in chromatographic panes (Singh et al. 2021). Detection of the florescence and UV-visual absorption by karanjin can be employed to estimate the solvent–solute characteristics. The photophysical and photochemical properties of karanjin has potential to be developed into a probe or a coloring dye, targeting biological interactions in animal models. The bioavailability studies of karanjin can be simplified by this measure since the ability of karanjin in crossing the BBB can be assured through detection of its florescence at the target site i.e. brain tissues or other specific protein targets related to neurodegenerative diseases. Likewise, the pharmacokinetics and pharmacodynamics of karanjin in an in vivo model can be easily understood, provided an optimized method for the detection of florescence in targeted tissues is developed. Solubility of karanjin is influenced by the functional groups of the furanoflavonoid, projecting a semi-polar and hydrophobic behavior. Karanjin is highly soluble in organic solvents viz. methanol, ethyl acetate, ethanol, dimethyl sulfoxide, benzene, and petroleum ether (Arshad et al. 2013; Voicescu et al. 2014). Karanjin is not reactive towards light in its crystal form and can be stored at room temperature, however it is best to store it air tight, away from direct light, at temperature below 8 °C for a longer shelf-life. Nonetheless, karanjin is formed as orange solution in methanol and is more reactive compared to its solid form. The melting point of karanjin crystals is reported to be ranging from 157 to 161 °C (Noor et al. 2020; Singh et al. 2021). Karanjin was assessed for its drug-likeliness through in-silico approach using Biovia Discovery Studio 19.0 (open-source software), and found to be obeying the Lipinski’s rule of five (molecular mass less than 500 Da, less than five hydrogen bond donors, less than ten hydrogen bond acceptors, octanol–water partition co-efficient log P less than 5) (Gnanaraj et al. 2022). There are four hydrogen bond acceptors in the chemical structure of karanjin but there are no hydrogen bond donors, whereas the partition co-efficient predicted log P value was 2.54 or 3.43. Karanjin was also predicted by the in-silico software to successfully permeate the BBB and inhibit the membrane transporter protein, p-glycoprotein to reach brain cells. Presence of furan ring in karanjin could possibly facilitate the drug permeability across BBB to reach the brain tissues. For instance, preladenant is a neuroprotective drug under clinical trial that also contains a furan ring in its structure. The drug preladenant was reported with anti-Parkinson’s effect in Phase II of clinical trials. The drug was administered orally, and could reach the brain cells effectively to exert anti-Parkinson’s effect (Hauser et al. 2011). The in-silico ADMET studies on karanjin also showed no toxicity towards liver and the classes of cytochromes (CYP) P450, hence could be safe for oral uptake and also through pulmonary circulation. The maximum recommended therapeutic dose of karanjin was estimated to be 157 mg per day. Since the gastrointestinal uptake was computed to be high, the bioavailability of karanjin was also calculated to have a score of 0.55, which again falls under the obedience of Lipinski’s rule of five as a suitable drug candidate.
Detection of karanjin in different chromatographic and spectroscopic instruments for standardization studies have been described by past researchers. The Rf value for karanjin in thin layer chromatography was 0.3–0.8 for a mobile phase of toluene:ethyl acetate (7:3). The limit of detection and limit of quantification of isolated karanjin compared to its standard (10 ng µL−1) was reported to be in the range of 10–25 and 50–80 ng, respectively (Katekhaye et al. 2012a; Damle and Choudhari 2018). FTIR spectrum of karanjin was reported with specific functional groups, methoxy group stretching (=C–H) at 3052 and 3016, (–C–H) at 2927 and 2852, keto group stretching (–C=O) at 1744, and (C=C) at 1622 and 1492 cm−1, respectively (Rekha et al. 2021). Mass spectrum analysis on ESI–MS (70 eV) showing signals of protonated molecular ions of karanjin at m/z 293.2 [M + H]+ and m/z 315.0 indicating the adducts of sodium salts for karanjin thus confirming the molecular weight to be 293. The nuclear magnetic resonance report was, 1H NMR (400 MHz, CDCl3) δ 8.22 (1H, d, J = 9 Hz), 8.16 (2H, dd, J1 = 9 Hz, J2 = 2 Hz), 7.55–7.59 (m, 4H), 7.19 (1H, d, 480 J = 2 Hz), 3.94 (3H, s) and 13C NMR (100 MHz, DMSO) δ 60.9 (-OCH3), 109.82 (C-A), 104.09 (C-B), 154.55 (C-2), 157.86 (C-3), 174.48 (C-4), 145.57 (C-5), 146.82 (C-6), 128.20 (C-7), 116.82 (C-8), 149.64 (C-9), 141.58 (C-10), 119.47 (C-1´), 130.73 (C-2´), 128.18 (C-3´), 121.64 (C-4´), 128.48 (C-5´), 130.52 (C-6´) (Patel and Trivedi 2015; Noor et al. 2020). The high performance liquid chromatography (HPLC) analysis of karanjin using C18 column under isocratic mobile phase (methanol: water: acetic acid; 85:13.5:1.5) for 20 min with a flow rate of 1 mL/min at a detection wavelength of 300 nm, shows a significant single peak at approximately 6 min retention time (Katekhaye et al. 2012a). Recently, the chemical synthesis method of karanjin derivatives were reported and three major derivatives were successfully synthesized namely karanja ketone, karanja ketone oxime, and karanja oxazole. The purity of the derivatives was determined and the bioactivities of the derivatives showed good antioxidant effects in ketone and oxime (Rekha et al. 2021).
Pharmacological reports and mechanism of action of karanjin
Phytochemical evaluation and pharmacological reports on P. pinnata has been well documented. Traditional uses of the tree and its pharmacological relevance somehow has connection to the presence of numerous bioactive compounds, especially flavonoids. Karanjin being a major bioactive compound of karanja tree has significant amount of reports on its pharmacology in various models. To begin with, karanjin has strong antioxidant properties that were tested against DPPH, ABTS, FRAP, and nitric oxide scavenging activities (Ghosh and Tiwari 2018; Rekha et al. 2020; Ansari et al. 2021). Several in-vitro studies reported that karanjin has anti-cancer properties against human hepatocellular carcinoma cell lines (HepG2), human lung adenocarcinoma cell lines (A549), human leukemia cell lines (HL-60) (Guo et al. 2015), human cervical cancer cells (HeLa) (Raghav et al. 2019), and human breast cancer cells (MCF-7, MDA-MB-231) (Yu et al. 2022; Bhatt et al. 2022). The inhibitory concentration at 50% (IC50) values for karanjin were different for different cancer cell lines, whereby most of the concentrations were below 20 µM. It was learned that karanjin could induce anti-cancer effect through inducing apoptosis via inducing cell cycle arrest at G2/M phase, inducing apoptosis and scavenging reactive oxygen species (ROS) (Guo et al. 2015; Mrudul and Pravin 2020; Roy et al. 2021). Karanjin is reported to modulate the cytochrome P450 enzymes specifically CYP1A, which can be found in higher numbers in cancer cells compared to healthy cells (Joshi et al. 2018; Dwivedi and Shastry 2023). CYP1A is responsible for the biotransformation of carcinogens leading to chromosomal aberrations, formation of ROS, and DNA damage. CYP1A also mediates carcinogenesis and development in epithelial, lung, oral, and gastrointestinal cancers. In a separate in vitro study, karanjin was able to inhibit the CYP1A enzyme in recombinant human embryonic kidney cells (HEK293) and CYP1A sacchrosomes. Karanjin was also assumed to have blocked the heme iron-oxo intermediate formation by interacting with heme atoms at the substrate binding site of CYP1B, thus preventing the hydroxylation reaction (Joshi et al. 2018). These findings were supported by molecular docking analysis that exhibited the effectiveness of karanjin in modulating CYP1 enzymes and other cytochrome P450 targets to prevent carcinogenesis (Dwivedi and Shastry 2023). ATP binding cassette (ABC) transporters are ATP-dependent channels that are highly expressed on cancer cells. The ABC transports xenobiotics, drugs, and food through cellular membranes and tissue barriers. It is known that the transporters are responsible for drug-resistance in cancer cells. Even at a low concentration of 1 µM, karanjin was reported to interfere with the ABC transporters ABCC1 (multidrug resistance associated protein 1), ABCB1 (P-glycoprotein-multidrug resistance gene 1), and ABCG2 (multidrug resistance protein 1) in neuroblastoma cells (UKF-NB-3) and glioblastoma cells (G62), by enhancing the ATPase activity. It was discovered that karanjin sensitized the ABC transporters to anti-cancer drugs vincristine and mitoxantrone, enabling cytotoxic effect to the cancer cells (Michaelis et al. 2014). The ability to inhibit CYP enzymes and ABC transporters hails karanjin as a potential anti-cancer drug. Karanjin triggered apoptosis in cancer cells through suppression of Bcl2, the apoptosis regulator gene in the Bax/Bcl2 ratio, thus leading to caspase-dependent cell death during the cell cycle arrest at G2/M. Moreover, the translocation of nuclear factor kappa-B (NF-kB) was inhibited by karanjin in cancer cells, hence preventing the survival of the cells from survival genes expressed by the NF-kB pathway (Roy et al. 2019).
Karanjin exhibited anti-diabetic property in an in vitro study by stimulating the translocation of insulin-sensitive glucose transporter 4 (GLUT4) and facilitated the uptake of glucose across a plasma membrane in skeletal muscles. The mechanism involved in the process was the activation of 5’ adenosine monophosphate-activated protein kinase (AMPK) enzyme that is responsible for the regulation of cellular metabolism. It was discovered that karanjin activated the AMPK pathway for translocation of GLUT4 to plasma membrane of skeletal muscles in L6 myotubes, without the involvement of phosphoinositide (PI) 3-kinase (PI3K)/ protein kinase B (Akt) which was verified through western blot analysis to check the protein expression of phosphorylated Akt (Ser-473) (Jaiswal et al. 2011). However, a recent in-silico molecular docking study suggests that karanjin is exerting anti-cancer property against breast cancer through activation of PI3K/Akt pathway (Dwivedi and Shastry 2023). The variation suggests that karanjin is capable of modulating the right pathway either proliferation or metabolism, depending on the necessity in the cellular environment. Apart from that, karanjin has potentially exhibited anti-HIV activity by expressing half-maximal cytotoxic concentration (> 693.15 µM) in C8166 cells (Pandey et al. 2014). Nitric oxide scavenging activity of karanjin was associated with a potential against psoriasis through a molecular docking analysis against the disease related target receptors, interleukin (IL17-A, IL17-F, IL23), retinoid related orphan receptor (ROR), and toll-like receptor (TLR-7) (Ghosh and Tiwari 2018). Karanjin is confidently potent as a skin care drug since another report on the formulated gel of karanjin proved to be suitable in treating acne by reducing the pore size of sebaceous glands and inflammation induced by carrageenan (Ansari et al. 2021).
Several in vivo studies involving animals were reported on the pharmacological potentials of karanjin. The initial report on the oral administration of karanjin (50 and 100 mg/kg) for 10 days in streptozotocin-induced diabetic Sprague–Dawley rats reported that karanjin has significant anti-hyperglycemic and anti-hyperlipidemia activities. It was deduced by the researchers that the anti-hyperglycemic activity of karanjin could be due to inhibition of protein-tyrosine phosphatase 1B, which is a mediator of insulin resistance in cells (Tamrakar et al. 2008). Oral administration of karanjin (10 and 20 mg/kg) for 14 days was reported to exhibit gastroprotective and anti-ulcer activity in male Wistar rats that were stressed with ethanol and forced to swim. The ethanol administration and stress induction in rats significantly caused gastric mucosal membrane damage and gastric ulcer formations which were ameliorated by karanjin through inhibition of oxidative stress and proton pump (H+, K+ -ATPase) inhibition (Vismaya Belagihally et al. 2011). Anti-inflammatory property of karanjin was described in a study involving paw edema formation and arthritis due to complete Freund’s adjuvant (CFA)-administration in male Wistar rats. The rats were orally dosed with karanjin (10 and 20 mg/kg) for 13 days upon administration with CFA. The results exhibited anti-inflammatory reaction by karanjin that was mediated by suppression of pro-inflammatory cytokines, reduced paw edema and erythema, prevention of cartilage and joint injury, and inhibition of nitric oxide ROS formations by preventing nuclear translocation of NF-kB p65. Therefore, it was presumed that the inhibition of inflammatory reaction was overall orchestrated by karanjin through suppression of NF-kB pathway, accompanied by oxidative stress prevention (Bose et al. 2014). Karanjin was also reported to have significantly prevented colitis in 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced BALB/c mice. Karanjin (100 and 200 mg/kg) was orally administered for 7 days in TNBS-induced mice, and was reported to inhibit oxidative stress markers, suppress inflammatory reactions, and prevent shortening of colon (Patel and Trivedi 2017). Previously, the same authors also described the anti-colitis effect of karanjin (100 and 200 mg/kg) oral administration in dextran sulfate sodium (DSS)-induced colitis in female C57BL6 mice and similar parameters were assessed hence describing inhibition of oxidative stress and prevention of inflammation (Patel and Trivedi 2015). Another study was reported on oral administration of karanjin (50, 100 and 200 mg/kg) for 18 weeks consecutively for the prevention of dimethylhydrazine-induced colon carcinoma in male Wistar rats. It was noted that karanjin could prevent colon carcinoma through reversal of oxidative stress and regulation of apoptosis marker proteins, therefore suggesting the alteration of the mitochondrial p53 and its related downstream pathway by karanjin to induce apoptosis in colon cancer (Zhang et al. 2020). A recent study exhibited the potential of karanjin in preventing Alzheimer’s disease using a diazepam-induced Swiss albino mice model. Karanjin (25 and 50 mg/kg) was orally administered for 8 days in diazepam-induced mice and the behavioral studies were assessed (Saini et al. 2017). Although the study showed memory impairment of karanjin treated mice in the maze tests, there were no biochemical or histological studies to significantly proof the neuroprotective mechanism of karanjin. Therefore, the study could not provide a direction for further neuroprotective mechanism of karanjin. In our recent in-silico study, it was proven that karanjin has the potential to treat Alzheimer’s and Parkinson’s disease through binding to targeted proteins involved in the progression of the neurodegenerative diseases (Gnanaraj et al. 2022). The summary of pharmacological reports of karanjin is given in Table 2. In all the animal studies, karanjin was administered orally and the highest dose was 200 mg/kg, which indicates the bioavailability of karanjin in the gastrointestinal system is high, which can be related back to the in-silico results. The lethal dose (LD50) of karanjin was reported to be more than 600 mg/kg in BALB/c mice model (Zhang et al. 2020).
The distribution of karanjin in mammalian cells was studied in a manner of understanding its effect on humans and other organisms if applied as a biopesticide. The findings were clear that karanjin could penetrate the mammalian cells easily but is not harmful to the healthy cells nor it could cause adverse effects (Raghav et al. 2019). Binding of karanjin to bovine serum albumin (BSA) was performed to evaluate the toxicity of the compound towards the protein structure that mimics human serum albumin. Other chemical pesticides tend to cause severe toxicity to BSA upon binding. Toxicological studies on karanjin proved its safety in physiological conditions that were validated using cell lines and animal models. Pharmacokinetic studies on karanjin in male and female Sprague–Dawley rats were performed by different researchers to learn the absorption and elimination pattern of the compound. In the first study involving female rats, karanjin was administered at 10 mg/kg and the mean maximal plasma concentrations (Cmax) was 0.498 ± 0.01 µg/mL, whereas the elimination half-time (T1/2) was 3.78 h, and karanjin was eliminated from the systemic circulation at 24 h (Shejawal et al. 2014). In the second study on male rats, karanjin was available in plasma at 1.5 h post oral administration and T1/2 was in the range of 1.9–2.2 h. Karanjin at the doses 5, 10, and 20 mg/kg showed Cmax of 0.187 ± 0.03 µg/mL, 0.347 ± 0.04 µg/mL, and 0.772 ± 0.12 µg/mL, respectively (Yi et al. 2014). These findings demonstrate the effectiveness of karanjin as a safe and potential drug candidate in its estimated dose range, adding together the pharmacological reports to support the claim. The mechanism of action exerted by karanjin in every in vitro and in vivo studies can be mapped to understand the targeted pathways of karanjin, which could be useful in construing the potential effect of karanjin on neurodegenerative diseases.
Mechanism of neurodegenerative disease development
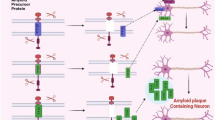
Neurodegenerative diseases are commonly linked with the pathophysiological change in brain regions due to loss of neurons, protein aggregation, disturbance in neuronal network or synaptic failure, aberrant proteostasis, defected DNA and RNA, cytoskeletal abnormalities, altered energy homeostasis, or neuronal inflammation (Van Schependom and D’haeseleer 2023; Wilson et al. 2023). There are few risk factors associated with the development of the diseases primarily age, individual genetic makeup and environmental factors. Progression and spreading of neurodegenerative diseases within the brain tissues are connected to many underlying mechanisms. People affected by neurodegenerative disease display impaired cognitive and motor functions, especially movement, speech, stability, and bowel movements (Ding et al. 2022). Most of the current treatments are targeted on improvement of symptoms and not focused on recovery of the damaged brain cells. Alzheimer’s disease is described with the accumulation of amyloid-β peptide (Aβ) and tau proteins in the brain. A typical Alzheimer’s condition is characterized by accumulation of tau proteins causing an increase of neurofibrillary tangles composed of accumulated insoluble filaments of hyperphosphorylated tau (Pickett et al. 2019). As a consequence, the patient undergoes extensive neuronal loss, synaptic failure, and damaged neurotransmitters leading to impaired memory. Patients diagnosed with Alzheimer’s disease at the early stage are prone to short-term memory loss due to disrupted hippocampal circuitry functions as an effect of plaque accumulation. In the advanced stages, patients with Alzheimer’s disease will exhibit major functional impairment, loss of problem-solving abilities, and apparent behavioral changes, which leads to dementia (Long and Holtzman 2019; Scheltens et al. 2021). The mechanistic pathway of Alzheimer’s disease progression is not completely elucidated but a recent review emphasizes the involvement of mitochondrial oxidative stress due to toxicity caused by Aβ accumulation and regulation of tau protein hyperphosphorylation by glycogen synthase kinase 3 (GSK3β) and cyclin dependent kinase 5 (CDK5) (Tiwari et al. 2019). Apart from GSK3β and CDK5, other kinases such as ERK2, caspases 3 & 9, serine/threonine kinase, and protein kinases A & C are also stimulants of tau protein hyperphosphorylation, activated by Aβ. Hence, in can be deduced that the PI3K/Akt/GSK3β signaling pathway and oxidative stress are relevant in the pathogenesis of Alzheimer’s disease (Fig. 3).
Mechanism of Alzheimer’s disease progression. The accumulation of extracellular senile plaques and intracellular neurofibrillary tangles (NFT) are the hallmarks of AD. The hyperphosphorylation of tau results in microtubule (MT) destabilization, MT disassembly and aggregation of tau into NFT. The amyloid beta (Aβ) is produced from the proteolytic cleavage of amyloid precursor protein (APP), which shows the tendency to aggregate into Aβ oligomers and eventually forming senile plaques. Both Aβ oligomers and senile plaques activate microglia and astrocytes, thus resulting in the release of pro-inflammatory cytokines, chemokines. This leads to lipid peroxidation, oxidative stress, inflammation and eventually neuronal death. Soluble Aβ oligomers are believed to cause mitochondrial dysregulation and eventual mitochondrial-associated cell death
Parkinson’s disease is a condition commonly related to aging factor, regarded with tremors, imbalance, walking disabilities, muscle stiffness, olfactory dysfunctions. The disease can be inherited genetically or it can be stimulated by other factors (Ascherio and Schwarzschild 2016; Poewe et al. 2017). Apart from age, other factors that contribute to Parkinson’s disease are habitual smoking, excessive caffeine consumption, and chronic exposure to environmental chemicals and toxins. The underlying mechanism for the development of Parkinson’s disease is unknown but studies suggest that morphological alterations in the disease are recognized by death of dopaminergic neuromelanin-containing neurons in the substantia nigra, leading to the loss of pigmentation in the locus coeruleus. Decrease of the dopaminergic neurons in the striatum of basal ganglia consequently leads to cardinal motor symptoms (Stoessl et al. 2014). An important mechanism in the progression of Parkinson’s disease is the misfolding and aggregation of α-synuclein which causes the death of nigrostriatal neurons and formation of Lewy bodies. Moreover, oxidative stress again plays an important role in progression of the disease coupled with mitochondrial stress and dysfunction, ferroptosis of dopaminergic neurons, defected autophagy lysosomal system, failure of ubiquitin–proteasome system, and ultimately neuroinflammation (Schulz-Schaeffer 2010; Li et al. 2023). It is well-learned that PARK genes are involved in the regulation of oxidative stress and neuroinflammation in Parkinson’s disease progression (Park et al. 2014; Tsunemi and Krainc 2014). A recent review suggests the involvement of gut microbiota in the pathogenesis of Parkinson’s disease whereby the whole scenario is depicted from gut inflammation, triggering the release of lipopolysaccharides (LPS) by specific microbiota and pro-inflammatory cytokines that crosses the plasma membrane, causes aggregation of α-synuclein in the enteric nervous system (Dong-Chen et al. 2023). Accumulation of α-synuclein as a damage-associated molecular pattern (DAMP) in microglial cells, prompts neuroinflammation while entering the cells through toll-like receptors (TLRs), subsequently initiating an innate immune response. NF-kB is upregulated in the process, hence NLRP3 inflammasome is activated to maximize the generation of pro-inflammatory cytokines that further aggravates oxidative stress and damage, causing ferroptosis or death of dopaminergic neurons, which explains the pathophysiology of Parkinson’s disease progression (Fig. 4).
The accumulation of Lewy bodies (aggregates of misfolded α-synuclein) and the loss of dopaminergic neuron is the hallmark of Parkinson’s disease. A Aggregates of α-synuclein exert lipid peroxidation, ferroptosis and mitochondrial damage in neuron cells. B The presence of protofibrils, α-synuclein oligomer and Lewy bodies activates astrocyte and microglia to result in increased expressions of pro-inflammatory cytokines, chemokines and adhesion molecules as well as oxidative stress
Huntington’s disease is a genetically inherited progressive neurodegenerative disease caused by early synaptic losses. Degradation and death of neurons in the specific voluntary movement controlling areas in brain, is characterized as the progressed state of Huntington’s disease (Milnerwood and Raymond 2010). The disease is passed down to progeny from their parents and symptoms will arise at the age range of 30–40, often ending in death. Patient’s suffering from Huntington’s disease will experience cognitive disturbances such as loss of thinking abilities, emotional problems, motor and dysfunctions such as uncontrolled and involuntary movements (Bates et al. 2015; Jimenez-Sanchez et al. 2017). Gene mutation and aggregation of huntingtin protein carrying an abnormally elongated polyglutamine (polyQ) is the basal pathophysiology of the disease progression, yet the underlying mechanism is not fully discovered. The damage of central dopaminergic system in the brain leads to excessive dopamine production in Huntington’s disease, which are thought is be the causative agent for chorea (unusual jerking and writhing) (Bhatt et al. 2015). Oxidative stress is imparted as the pathogenesis of synaptic loss in the early stages of Huntington’s disease, therefore targeting the oxidative stress markers, autophagy and Nrf2-ARE pathway could be a possible approach to overcome the disease (Cordeiro et al. 2020; Lamptey et al. 2022). Neuroinflammation is also associated with the progression of Huntington’s disease whereby the role of NF-kB pathway is prominent in upregulating the production of pro-inflammatory cytokines (Lum et al. 2023) (Fig. 5).
Simplified overview of pathogenesis of Huntington’s disease. The mutation of huntingin causes conformational change that leads to protein misfolding to result in mutant toxic huntingtin. Mutant toxic huntingtin is cleaved by proteases and targeted to proteasome for degradation and elimination. The proteasome was impaired in cases of Huntington’s disease. Mutant toxic huntingtin could, be translocated to nucleus to cause altered gene expression, cause dysfunctional calcium signaling and homeostasis, alter neurotransmitter receptor activity and release, and cause mitochondrial damage
ALS is a motor neuron disease that progresses aggressively on nerve cells and the spinal cord, ensuing paralysis and muscle weakness. Deterioration of motor neurons happens gradually before the neurons are completely dead, thus preventing signal delivery to the brain (Morris 2015; Taylor et al. 2016). Mutation of four major genes (SOD1, FUS, C9orf72, TARDBP), are accountable for more than 70% of cases ALS, though there are many other genes involved (Hardiman et al. 2017). The four genes are responsible for major motor functions such as homeostasis, DNA repair, glial cell and mitochondrial functions. Impairment of the major motor functions leads to the progression of ALS. In the case of ALS, the pathophysiology is directed to aggregation of intraneuronal proteins such as TAR DNA, SOD1 and neurofilament (Kim et al. 2020). The neurodegenerative disease is also not defined of its detailed pathogenesis since there are very limited pre-clinical studies on the type of disease. But based on the few studies using transgenic mice model of ALS, the NF-kB pathway can be highlighted for the disease progression since it involves oxidative stress through SOD1 suppression (Wang et al. 2018a, b). SOD is an antioxidant enzyme, responsible for the alleviation of oxidative stress (Stephenie et al. 2020). Hence, the inclusion of NF-kB pathway is crucial in the progression of oxidative stress related ALS (Fig. 6).
The mutation of several genes is implicated in the pathophysiology of amyotrophic lateral sclerosis (ALS) Mutation of fused in sarcoma (FUS), superoxide dismutase 1 gene (SOD1) and CHCHD10 is believed to contribute to mitochondrial-associated oxidative stress dysfunction and eventual neuronal death. The formation of SOD1 aggregates contribute to alteration of cytoskeletal dynamics as well as dysfunctional axonal transport
Synaptic and neuronal dysfunctions are common findings in all neurodegenerative diseases described earlier. Core physiological processes in external and internal cellular environment are required to function optimally to interact with the hallmarks of neurodegenerative diseases to overcome synaptic dysfunction. Factors like oxidative stress, defective energy metabolism, organelle degradation, protein degradation, loss of cytoskeletal dynamics, and neuronal death are the causatives of synaptic failure (Henstridge et al. 2016; Verma et al. 2022; Wilson et al. 2023). Another hallmark of neurodegenerative diseases are aggregation of proteins (Aβ, Tau, α-synuclein, polyQ, TAR DNA, SOD1, etc.) at the neuronal cells implicates aberrant proteostasis. Ubiquitin–proteasome system and autophagy-lysosomal pathway are major mechanisms involved in the protein homeostasis of neuronal cells. It was learned that autophagy-lysosomal pathway plays a crucial role in the amelioration of neurodegenerative diseases especially by removing protein aggregation in Alzheimer’s and Parkinson’s disease (Schulz-Schaeffer 2010; Spires-Jones et al. 2017; Dong-Chen et al. 2023). Excessive protein aggregation causes adverse effect on autophagy-lysosomal pathway, whereby the pathway transfers the aggregation prone proteins such as misfolded prions, Tau, and α-synuclein to neighboring cells. Autophagy and lysosomal dysfunction in neurodegenerative diseases could be caused by oxidative stress and mitochondrial dysfunction. DNA damage is a common hallmark in neurodegenerative diseases. Denatured DNA or mutations are progenitors for the progression of neurodegenerative disorders, whereby oxidative DNA damage influences the mitochondrial functions that leads to protein aggregation (Devine and Kittler 2018; Wang et al. 2018a, b; Tiwari et al. 2019). Although there are environmental factors, stress and genotoxic influences for DNA mutation and denaturation, ROS is fundamentally involved in the process. In other words, oxidative stress again plays a part in pathogenesis of neurodegenerative diseases. Neuroinflammation is a pathological hallmark of neurodegenerative diseases that is triggered upon encountering of aggregated protein by microglial cells and astrocytes. It is best known that neuroinflammation is correlated with oxidative stress as the process is controlled by the similar mechanistic pathways.
Potential intervention of karanjin as a neuroprotective drug
Phenolic compounds especially flavonoids have a strong influence in mitigating oxidative stress related diseases and its underlying pathways. The intervention of polyphenols in neurodegenerative diseases are widely studied and suggested to have sufficient capabilities to become a therapeutic drug for treatment of neurodegenerative disorders, or as a nutritional health supplement to prevent and protect against developing neurodegenerative ailments (Ayaz et al. 2019; Devi et al. 2021). Early epidemiological studies identified that uptake of fruit or vegetable rich diet and frequent consumption of tea or coffee reduced the risk of developing Parkinson’s disease, indicating the presence of phenolic compounds in minimizing the risk factor associated with neurodegenerative diseases (Yang et al. 2018). Being the largest class of phenolic compounds, flavonoids have a versatile nature in terms of pharmacological properties. Recent pre-clinical studies on animal models have shown the effectiveness of several flavonoids in preventing the pathophysiological progression of Alzheimer’s disease (Dourado et al. 2020; Khan et al. 2020a, b). Numerous pre-clinical studies were reported on different flavonoids against Alzheimer’s and Parkinson’s disease in the past ten years. Examples of the flavonoids that were reported with neuroprotective activity are naringin, baicalein, quercetin, rutin, kaempferol, morin, anthocyanin, hesperidin, hesperitin, silibinin, silibilin, naringenin, apigenin, myricitrin, chrysin, tanshinone I, epigallocatechin gallate (EGCG) and more. Studies show that flavonoids exert modulatory effects at the neuronal sites where accumulation of peptides such as amyloid-β peptide (Aβ) and α-synuclein in the brain, which suppress the development of neurodegenerative diseases (Teles et al. 2018; Cho et al. 2020). Flavonoids could act as an inhibitor of monoamine oxidase (MAO), which is used as a method of treatment in Parkinson’s and Alzheimer’s disease. MAO acts as a remover of neurotransmitter hormones such as serotonin, dopamine, and norepinephrine thus inhibition of MAO could suppress the development of neurodegenerative diseases (Yamada 2004; Schapira 2011). Studies also suggest that flavonoids are capable to counter act with the oxidation products of MAO such as 5-S-cysteinyl-dopamine and dihydrobenzothiazine-1 that acts as neurotoxins involved in the death of nigral neurons of substantia nigra (Ayaz et al. 2019; Dong-Chen et al. 2023). Recent evidence suggests that flavonoids have tendencies to inhibit the TNF-α and other pro-inflammatory cytokine production as well as blocking oxidative stress-induced neuronal injuries (Devi et al. 2021). Flavonoids have been reported to improve cognitive and behavioral changes in animal model of Huntington’s disease. Mitochondrial activity were enhanced, cellular stress markers and inflammatory markers were reportedly reduced by flavonoids for the Huntington’s disease animal models (Sandhir and Mehrotra 2013; Rehman et al. 2016). The effects of flavonoids on ALS were reported using transgenic mice model SOD1-G93A, where flavonoids reportedly delayed the onset of the disease progression and improved motor performance by preserving the motor neurons and dendritic cells thus extending the lifespan of animals (Wang et al. 2018a, b).
The underlying mechanism of neuroprotection is relatable to alleviation of oxidative stress, as reported by previous studies. Essentially, pathophysiology of neurodegenerative diseases are correlated to the regulation of inflammatory mediators (TNF-α, IL-6, iNOS, COX-2, NLPR3, etc.), non-enzymatic/enzymatic antioxidants and oxidative stress markers (ROS, GSH, LPO, GPx, CAT, SOD, Nrf2, etc.), immunomodulation (TLRs, STATs, etc.), and also apoptosis (p53, caspases, Bax, Bcl2, JNK, ERK, etc.). The mechanistic pathways involved can be narrowed to MAPK, AMPK, RAGE, PI3K/Akt and NF-kB cascade signaling within the brain cells, leading to progression and development of the diseases. Although molecular targets and proteins involved are unique for each neurodegenerative disease, yet the mechanistic pathways are somewhat intertwined, which could be explained by the event of how a same flavonoid compound i.e. quercetin, hesperidin, kaempferol, or baicalein could exert pharmacological effects in controlling Alzheimer’s, Parkinson’s, and Huntington’s disease. The justification perhaps can be directed to the mode of drug administration, whereby majority of the studies involving animals’ stated oral administration (intragastric) therefore, the relationship of gut-brain axis in the prevention of neurodegenerative disease comes into discussion. It is well-known that flavonoids are capable of altering the CYP enzymes to escape the gastrointestinal metabolism, however the bioavailability of the compounds is at question since most flavonoids do not have the ability to permeate BBB (Teles et al. 2018; Khan et al. 2020a, b). The presence of gut microbiota is explained to significantly enhance the metabolism of dietary flavonoids into fractions of bioactive metabolites that are permeable across BBB. Presence of gut microbiota or probiotics are helpful in producing novel metabolites known to enhance the intestinal health, subsequently uptake the metabolites into circulation entering the central nervous system (Socala et al. 2021; Zhang et al. 2023). The plasma concentration of the metabolites are considered sufficient to produce the desired effect on brain cells, as depicted by the findings of flavonoid treated neurodegenerative animal models.
Karanjin as a furanoflavonoid has the potential of permeating the BBB due to the presence of furan ring, as described in the earlier section. The oral bioavailability of karanjin was considered good, as verified previously through molecular docking and pharmacokinetic studies. Considering the antioxidant properties of karanjin and inhibition of oxidative stress in animal models, the pharmacological properties could be beneficial for the prevention or modulation of neurodegenerative diseases. Based on the pharmacological reports on karanjin, it has the ability to inhibit the translocation of NF-kB, thereby hindering the progression of oxidative damage and inflammation in a disease. Similarly, karanjin is capable of modulating the cellular metabolism pathway AMPK and cellular proliferation pathway PI3K/Akt and MAPK. Therefore, majority of the signaling pathways involved in the progression of neurodegenerative diseases could be controlled by karanjin, making it a suitable drug candidate for neurodegeneration. Experimental models of neurodegenerative diseases should be targeted to learn the effect of karanjin on AGE-RAGE signaling pathway that is directly involved as the ligand binding site on cell surface, initiating cellular cascade signaling in pathophysiology of neurodegeneration. TLRs have common ligands and pathways as RAGE since both are membrane receptors involved the signaling cascade for inflammation and crosstalk between TLRs-RAGE were identified in the progression of neurodegenerative disease (Gasiorowski et al. 2018). Karanjin was reported to inhibit TLR7 in an in-silico analysis, hence there is a high chance for the compound to inhibit the RAGE signaling cascade in neurodegeneration. Although permeability of karanjin on BBB is proven, it is wise to optimize alternate routes of administration to increase the plasma concentration of the drug and to improve the T1/2. Oral administration of karanjin could possibly have a significant effect on neurodegenerative diseases either in its original form of permeated drug, or upon biotransformation by gut microbiota. The interrelation of gut microbiota and progression of neurodegenerative disorders together with the prevalence of karanjin molecules in the brain tissues upon oral administration could be an interesting discovery in that aspect. Another potential method of administration to treat neurodegeneration is through the intranasal cavity, whereby aerosolized nano-formulated karanjin could be delivered to reach the brain tissues efficiently. This passage could overcome the hindrance of gut metabolism, thus increasing the plasma concentration of karanjin. Lipophilicity of karanjin would enable the compound to diffuse through the epithelial cells of nasal cavity into the olfactory nerves to be transported to the brain tissues. The mechanism will be a stepping stone for the development of potential drugs from natural products to treat neurodegenerative diseases as depicted in Fig. 7.
Postulated mechanism of action of karanjin against neurodegenerative diseases such as Ad and Parkinson’s disease. Black arrow denotes the pathological process of neuronal cells in response to senile plaque and oligomers. Red arrow shows the postulated therapeutic action of karanjin to counteract the toxic insults from senile plaque and oligomers. Karanjin is believed to regulate MAPK and P13/Akt pathways which results in upregulations of anti-oxidant activity and therefore inhibits oxidative stress and associated apoptotic events, inhibition of apoptosis, and inhibition of inflammation. In addition, karanjin could inhibit the activation of NF-kB
Conclusion and future prospects
As a conclusion, it can be ascertained that karanjin has the credentials of preventing the pathophysiology of neurodegenerative diseases. Drug-likeliness of karanjin was verified through molecular-docking analysis, obeying the Lipinski’s rule of five. The presence of furan ring in the structure of karanjin enhances the permeability of the compound across biological membrane barriers thus improving the bioavailability. Isolation of karanjin could be optimized by liquid/liquid extraction from the seed oils of P. pinatta coupled with alumina adsorption chromatography and preparative HPLC. Highlights on the effects of karanjin on signaling pathways involved in the progression of neurodegeneration viz. NF-kB, AMPK, PI3K/Akt, MAPK, and AGE-RAGE supports the neuroprotective effect of karanjin as reported in our molecular docking study. The development of a potential neuroprotective drug from natural products is a dream project for many researchers across the globe. Though there are numerous bioactive compounds that were studied and reported pre-clinically against diseases like Alzheimer’s, Parkinson’s and Huntington’s, none of them have passed the clinical trials. Therefore, the drug with high potential needs solid pre-clinical evidence of pharmacological, toxicological, pharmacokinetics and pharmacodynamics against a specified disease. Karanjin could be the drug candidate against Parkinson’s or Alzheimer’s disease, provided the studies are conducted and presented in-line with the requirements from the national drug registration and regulatory bodies. Future direction of karanjin should include the formulation methods to deliver an effective dose of the drug across BBB to treat neurodegenerative diseases. Detailed pharmacological aspects of karanjin such as the effect on liver metabolism, mechanism of drug transport, half-life, drug-drug interaction, adverse reactions, and more should be explored. To increase the commercial value of karanjin, chemical synthesis instead of conventional isolation, could be employed as an effort for mass scale production.
References
Akanksha SAK, Maurya R (2010) Antihyperglycemic activity of compounds isolated from Indian medicinal plants. Ind J Exp Biol 48:294–298
Al Muqarrabun LM, Ahmat N, Ruzaina SA, Ismail NH, Sahidin I (2013) Medicinal uses, phytochemistry and pharmacology of Pongamia pinnata (L.) Pierre: a review. J Ethnopharmacol 150:395–420
Alizadeh M, Moludi J, Khodaei H, Saber A, Kheirouri S, Pourteymour Fard Tabrizi F, Kamari N (2020) Recent updates on anti-inflammatory and antimicrobial effects of furan natural derivatives. J Inflam Res 13:451–463
Anand KS, Dhikav V (2012) Hippocampus in health and disease: an overview. Annal Ind Acad Neurol 15(4):239
Ansari SA, Qadir A, Warsi MH, Mujeeb M, Aqil M, Mir SR, Sharma S (2021) Ethosomes-based gel formulation of Karanjin for treatment of acne vulgaris: In vitro investigations and preclinical assessment. 3 Biotech 11:456
Arshad N, Rashid N, Abbasi MA, Saleem S (2013) UV-absorption studies of interaction of karanjin and karanjachromene with ds. DNA: evaluation of binding and antioxidant activity. Cent Eur J Chem 11:2040–2047
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15:1257–1272
Ayaz M, Sadiq A, Junaid M, Ullah F, Ovais M, Ullah I et al (2019) Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front Aging Neurosci 11:155
Bak TH, Chandran S (2012) What wires together dies together: verbs, actions and neurodegeneration in motor neuron disease. Cortex 48(7):936–944
Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR et al (2015) Huntington disease. Nat Rev Dis Primer 1:15005
Belide S, Sajjalaguddam RR, Paladugu A (2010) Cytokinin preconditioning enhances multiple shoot regeneration in Pongamia pinnata (L.) Pierre-A potential, non-edible tree seed oil source for biodiesel. Electron J Biotechnol 13:1–8
Bhatt R, Singh D, Prakash A, Mishra N (2015) Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv 22:931–939
Bhatt G, Gupta A, Rangan L, Mukund Limaye A (2022) Global transcriptome analysis reveals partial estrogen-like effects of karanjin in MCF-7 breast cancer cells. Gene 830:146507
Bose M, Chakraborty M, Bhattacharya S, Bhattacharjee P, Mandal S, Kar M, Mishra R (2014) Suppression of NF-κB p65 nuclear translocation and tumor necrosis factor-α by Pongamia pinnata seed extract in adjuvant-induced arthritis. J Immunother 11(3):222–230
Cho I, Song H, Cho JH (2020) Flavonoids mitigate neurodegeneration in aged Caenorhabditis elegans by mitochondrial uncoupling. Food Sci Nut 8(12):6633–6642
Cordeiro LM, Machado ML, da Silva AF, Obetine Baptista FB, da Silveira TL, Soares FA, Arantes LP (2020) Rutin protects Huntington’s disease through the insulin/IGF1 (IIS) signaling pathway and autophagy activity: study in caenorhabditis elegans model. Food Chem Toxicol 141:111323
Damle M, Choudhari S (2018) Validated stability indicating method for Karanjin using HPTLC and HPLC. J Pharmacog Phytochem 7(2):2808–2815
de Teles ARB, Diniz TC, Costa Pinto TC, de Oliveira-Júnior RG, Gama-e-Silva, de Lavor ÉM et al (2018) Flavonoids as therapeutic agents in Alzheimer’s and Parkinson’s diseases: a systematic review of preclinical evidences. Oxid Med Cell Longev 2018:7043213
Devi S, Kumar V, Singh SK, Dubey AK, Kim JJ (2021) Flavonoids: potential candidates for the treatment of neurodegenerative disorders. Biomedicines 9(2):99
Devine MJ, Kittler JT (2018) Mitochondria at the neuronal presynapse in health and disease. Nat Rev Neurosci 19:63–80
Dhanmane SK, Salih FA (2018) Isolation of karanjin from Pongamia pinnata and its identification by difference analytical techniques. Kurd J Appl Res 3:156–160
Ding C, Wu Y, Chen X, Chen Y, Wu Z, Lin Z et al (2022) Global, regional, and national burden and attributable risk factors of neurological disorders: the global burden of disease study 1990–2019. Front Public Health 10:952161
Doke RB, Bhalerao MS, Paraskar PM, Patil PS, Kulkarni RD (2021) Energy-efficient sonochemical extraction of bioactive compound Karanjin from Pongamia pinnata leaves. Chem Pap 75(9):4935–4947
Dong-Chen X, Yong C, Yang X, Chen-Yu S, Li-Hua P (2023) Signaling pathways in Parkinson’s disease: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther 8(1):73
Dourado NS, dos Souza C, de Almeida MM, Bispo da Silva A, dos Santos BL, Silva VD et al (2020) Neuroimmunomodulatory and neuroprotective effects of the flavonoid apigenin in in vitro models of neuroinflammation associated with Alzheimer’s disease. Front Aging Neurosci 12:119
Dwivedi PSR, Shastry CS (2023) Anti-tumor potential and mode of action of karanjin against breast cancer; an in-silico approach. Arab J Chem 16(6):104778
Ekuadzi E, Dickson R, Fleischer T, Annan K, Pistorius D, Oberer L, Gibbons S (2013) Flavonoid glycosides from the stem bark of Margaritaria discoidea demonstrate antibacterial and free radical scavenging activities. Phytother Res 28(5):784–787
Emamzadeh FN, Surguchov A (2018) Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci 12:612
Fan L, Zhang Y, Huang R, Qin S, Yi T, Xu F (2013) Determination of five flavonoids in different parts of Fordia cauliflora by ultra performance liquid chromatography/triple-quadrupole mass spectrometry and chemical comparison with the root of Millettia pulchra var. laxior. Chem Cent J 7:126
Gaikwad NW, Madyastha KM (2002) Biosynthesis of β-substituted furan skeleton in the lower furanoterpenoids: a model study. Biochem Biophys Res Commun 290(11):589–594
Gasiorowski K, Brokos B, Echeverria V, Barreto GE, Leszek J (2018) Rage-TLR crosstalk sustains chronic inflammation in neurodegeneration. Mol Neurobiol 55(2):1463–1476
Ghosh A, Tiwari GJ (2018) Role of nitric oxide-scavenging activity of Karanjin and Pongapin in the treatment of psoriasis. 3 Biotech 8:338
Gnanaraj C, Sekar M, Fuloria S, Swain SS, Gan SH, Chidambaram K et al (2022) In silico molecular docking analysis of karanjin against Alzheimer’s and Parkinson’s diseases as a potential natural lead molecule for new drug design, development and therapy. Molecules 27(9):2834
Guo J, Chen QQ, Lam CW, Zhang W (2015) Effects of karanjin on cell cycle arrest and apoptosis in human A549, HepG2 and HL-60 cancer cells. Biol Res 48:40
Hajialyani M, Hosein Farzaei M, Echeverría J, Nabavi S, Uriarte E, Sobarzo-Sánchez E (2019) Hesperidin as a neuroprotective agent: a review of Animal and clinical evidence. Molecules 24(3):648
Hampel H, Mesulam MM, Cuello AC, Khachaturian AS, Vergallo A, Farlow MR (2018) Revisiting the cholinergic hypothesis in Alzheimer’s disease: emerging evidence from translational and clinical research. J Prev Alzheimer’s Dis 6:1–14
Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W et al (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Prim 3:1–19
Hauser RA, Cantillon M, Pourcher E, Micheli F, Mok V, Onofrj M et al (2011) Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol 10(3):221–229
Henstridge CM, Pickett E, Spires-Jones TL (2016) Synaptic pathology: A shared mechanism in neurological disease. Ageing Res Rev 28:72–84
Jaiswal N, Yadav PP, Maurya R, Srivastava AK, Tamrakar AK (2011) Karanjin from Pongamia pinnata induces GLUT4 translocation in skeletal muscle cells in a phosphatidyl inositol-3-kinase-independent manner. Eur J Pharmacol 670:22–28
Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC (2017) Huntington’s disease: mechanisms of pathogenesis and therapeutic strategies. Cold Spring Harb Perspect Med 7:a024240
Joshi P, Sonawane VR, Williams IS, McCann GJP, Gatchie L, Sharma R et al (2018) Identification of karanjin isolated from the Indian beech tree as a potent CYP1 enzyme inhibitor with cellular efficacy via screening of a natural product repository. MedChemComm 9:371–382
Katekhaye S, Kale M, Laddha K (2012a) Development and validation of an HPLC method for karanjin in Pongamia pinnata Linn. leaves. Ind J Pharma Sci 74(1):72–75
Katekhaye S, Kale M, Laddha K (2012b) A simple and improved method for isolation of karanjin from Pongamia pinnata Linn. seed oil. Ind J Nat Prod Resour 3(1):131–134
Kesari V, Ramesh AM, Rangan L (2013) Rhizobium pongamiae sp. nov. from root nodules of Pongamia pinnata. BioMed Res Int 2013:165198
Khan A, Ikram M, Hahm JR, Kim MO (2020a) Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: special focus on neurological disorders. Antioxidants 9(7):609
Khan H, Tundis R, Ullah H, Aschner M, Belwal T, Mirzaei H, Akkol EK (2020b) Flavonoids targeting Nrf2 in neurodegenerative disorders. Food Chem Toxicol 146:111817
Kim G, Gautier O, Tassoni-Tsuchida E, Ma XR, Gitler AD (2020) ALS genetics: gains, losses, and implications for future therapies. Neuron 108:822–842
Kumar M, Gehlot S (2012) Systemic effect of Tephrosia purpurea (Sarapunkha) on G.I.T.—an experimental study. Int J Ayur Herbal Med 2(2):328–335
Lamptey RN, Chaulagain B, Trivedi R, Gothwal A, Layek B, Singh J (2022) A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of Nanotherapeutics. Int J Mol Sci 23(3):1851
Lanjhiyana S, Patra K, Ahirwar D, Rana A, Garabadu D, Lanjhiyana SK (2012) Development and validation of a HPTLC method for determination of karanjin in Pongamia pinnata: a novel Indian medicinal plant. Der Pharm Sin 3(1):144–147
Lee ST, Davis TZ, Gardner DR, Stegelmeier BL, Evans TJ (2009) Quantitative method for the measurement of three benzofuran ketones in Rayless Goldenrod (Isocoma pluriflora) and White Snakeroot (Ageratina altissima) by high-performance liquid chromatography (HPLC). J Agri Food Chem 57(12):5639–5643
Li K, Wang M, Huang ZH, Wang M, Sun WY, Kurihara H et al (2023) ALOX5 inhibition protects against dopaminergic neurons undergoing ferroptosis. Pharmacol Res 193:106779
Limaye DB (1925) Karanjin part I: acrystalline constituent of the oil from Pongamia glabra. In: Proceedings of the 12th Indian academy and science congress, India, pp 118–125
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179:312–339
Lum PT, Sekar M, Seow LJ, Shaikh MF, Arulsamy A, Retinasamy T et al (2023) Neuroprotective potency of Mangiferin against 3-Nitropropionic acid induced Huntington’s disease-like symptoms in rats: Possible antioxidant and anti-inflammatory mechanisms. Front Pharmacol 14:1189957
Maeda H, Dudareva N (2012) The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol 63(1):73–105
Michaelis M, Rothweiler F, Nerreter T, Sharifi M, Ghafourian T, Cinatl J (2014) Karanjin interferes with ABCB1, ABCC1, and ABCG2. J Pharm Pharm Sci 17(1):92–105
Milnerwood AJ, Raymond LA (2010) Early synaptic pathophysiology in neurodegeneration: Insights from Huntington’s disease. Trends Neurosci 33:513–523
Morris J (2015) Amyotrophic lateral sclerosis (ALS) and related motor neuron diseases: an overview. Neurodiagn J 55:180–194
Mrudul V, Pravin T (2020) Investigation of anti-cancer potential of Karanjin in MCF-7 and MDA-MB-231 breast carcinoma cells. Int J Adv Sci Technol 29(7s):937–945
Nasim N, Sandeep IS, Mohanty S (2022) Plant-derived natural products for drug discovery: current approaches and prospects. Nucleus (calcutta) 65(3):399–411
Nevagi RJ, Dighe SN, Dighe SN (2015) Biological and medicinal significance of benzofuran. Eur J Med Chem 97:561–581
Noor AAM, Othman SNN, Lum PT, Mani S, Farooq Shaikh M, Sekar M (2020) Molecules of interest—karanjin—a review. Pharmacog J 12:938–945
Pandey AK, Bajpai AK, Kumar A, Pal M, Baboo V, Dwivedi A (2014) Isolation, identification, molecular and electronic structure, vibrational spectroscopic investigation, and anti-HIV-1 activity of karanjin using density functional theory. J Theor Chem 14:1–13
Park JS, Koentjoro B, Veivers D, Mackay-Sim A, Sue CM (2014) Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum Mol Gen 23:2802–2815
Patel PP, Trivedi ND (2015) Simple, efficient and economic method for isolation and analysis of karanjin and pongamol from karanja seed oil and screening of antimicrobial potential. Int J Pharm Pharm Sci 7:248–252
Patel PP, Trivedi ND (2017) Effect of karanjin on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in Balb/c mice. Ind J Pharmacol 49(2):161–167
Phrutivorapongkul A, Lipipun V, Ruangrungsi N, Kirtikara K, Nishikawa K, Maruyama S, Watanabe T, Ishikawa T (2003) Studies on the chemical constituents of stem bark of Millettia leucantha: isolation of new chalcones with cytotoxic, anti-herpessimplex virus and anti-inflammatory activities. Chem Pharm Bull 51:187–190
Pickett EK, Herrmann AG, McQueen J, Abt K, Dando O, Tulloch J et al (2019) Amyloid beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimer’s disease. Cell Rep 29(11):3592–3604
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J et al (2017) Parkinson disease. Nat Rev Dis Prim 3(1):17013
Raghav D, Mahanty S, Rathinasamy K (2019) Biochemical and toxicological investigation of Karanjin, a bio-pesticide isolated from Pongamia Seed Oil. Pest Biochem Physiol 157:108–121
Rao NVS, Veerabhadrarao J, Seshadri TR (1939) A note on the preparation and reactions of karanjin. Proc Nat Acad Sci Ind 10(2):65–70
Rehman SU, Shah SA, Ali T, Chung JI, Kim MO (2016) Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol Neurobiol 54(1):255–271
Rekha MJ, Bettadaiah BK, Sindhu Kanya TC, Govindaraju K (2020) A feasible method for isolation of Pongamol from Karanja (Pongamia pinnata) seed and its anti-inflammatory activity. Indus Crop Prod 154:112720
Rekha MJ, Bettadaiah BK, Muthukumar SP, Govindaraju K (2021) Synthesis, characterization and anti-inflammatory properties of Karanjin (Pongamia pinnata seed) and its derivatives. Bioorg Chem 106:104471
Riaz M, Khalid R, Afzal M, Anjum F, Fatima H, Zia S et al (2023) Phytobioactive compounds as therapeutic agents for human diseases: a review. Food Sci Nut 11(6):2500–2529
Row LR (1952) New flavones from Pongamia pinnata (L.) merr. Aust J Sci Res 5:754–759
Roy R, Pal D, Sur S, Mandal S, Saha P, Panda CK (2019) Pongapin and Karanjin, furanoflavanoids of Pongamia pinnata, induce G2/M arrest and apoptosis in cervical cancer cells by differential reactive oxygen species modulation, DNA damage, and nuclear factor kappa-light-chain-enhancer of activated B cell signaling. Phytother Res 33:1084–1094
Roy R, Mandal S, Chakrabarti J, Saha P, Panda CK (2021) Downregulation of Hyaluronic acid-CD44 signaling pathway in cervical cancer cell by natural polyphenols Plumbagin, Pongapin and Karanjin. Mol Cell Biochem 476:3701–3709
Saeid H, Al-sayed H, Bader M (2023) A review on biological and medicinal significance of furan. AlQalam J Med Appl Sci 6(1):44–58
Saha MM, Mallik UK, Mallik AK (1991) A chromenoflavone and two caffeic esters from Pongamia glabra. Phytochem 30:3834–3836
Saini P, Lakshmayya L, Bisht VS (2017) Anti-Alzheimer activity of isolated karanjin from Pongamia pinnata (L.) Pierre and embelin from Embelia ribes Burm.f. Ayu 38(1):76–81
Saito Y, Kishimoto M, Yoshizawa Y, Kawaii S (2015) Synthesis and structure–activity relationship studies of furan-ring fused chalcones as antiproliferative agents. Anticancer Res 35:811–818
Sajid ZI, Anwar F, Shabir G, Rasul G, Alkharfy KM, Gilani AH (2012) Antioxidant, antimicrobial properties and phenolics of different solvent extracts from bark, leaves and seeds of Pongamia pinnata (L.) Pierre. Molecules 17(4):3917–3932
Sandhir R, Mehrotra A (2013) Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-Nitropropionic acid: Implications in Huntington’s disease. Biochim Biophys Acta Mol Basis Dis 1832(3):421–430
Schapira AHV (2011) Monoamine oxidase B inhibitors for the treatment of Parkinsonʼs disease. CNS Drugs 25(12):1061–1071
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Che’telat G, Teunissen CE et al (2021) Alzheimer’s disease. Lancet 397:1577–1590
Schulz-Schaeffer WJ (2010) The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol 120(2):131–143
Sharma A, Kaushik N, Rathore H (2020) Karanja (Milletia pinnata (L.) Panigrahi): a tropical tree with varied applications. Phytochem Rev 19(3):643–658
Shejawal N, Menon S, Shailajan S (2014) Bioavailability of karanjin from Pongamia pinnata L. in Sprague dawley rats using validated RP-HPLC method. J Appl Pharm Sci 4(3):10–14
Singh A, Jahan I, Sharma M, Rangan L, Khare A, Panda A (2016) Structural characterization, in silico studies and in vitro antibacterial evaluation of a furanoflavonoid from Karanj. Planta Med Let 3:e91–e95
Singh A, Bhatt G, Gujre N, Mitra S, Swaminathan R, Limaye AM, Rangan L (2021) Karanjin Phytochem 183:112641
Socała K, Doboszewska U, Szopa A, Serefko A, Włodarczyk M, Zielińska A et al (2021) The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res 172:105840
Spires-Jones TL, Attems J, Thal DR (2017) Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 134:187–205
Sreeharsha RV, Mudalkar S, Singha KT, Reddy AR (2016) Unravelling molecular mechanisms from floral initiation to lipid biosynthesis in a promising biofuel tree species, Pongamia pinnata using transcriptome analysis. Sci Rep 6:34315
Sreelakshmi L, Janardhan Reddy K (2012) Detection of karanjin from callus cultures of Pongamia glabra. J Pharmacog 3(2):67–70
Sriphana U, Yenjai C, Siriporn Tungnoi S, Srirapa J, Junsongduang A (2018) Flavonoids from Milletia leucantha and their cytotoxicity. Nat Prod Commun 13(8):961–962
Stephenie S, Chang YP, Gnanasekaran A, Esa NM, Gnanaraj C (2020) An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J Funct Foods 68:103917
Stoessl AJ, Lehericy S, Strafella AP (2014) Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet 384:532–544
Talapatra SK, Mallik AK, Talapatra B (1980) Pongaglabol, a new hydroxyfuranoflavone, and aurantiamide acetate, a dipeptide from the flowers of Pongamia glabra. Phytochem 19:1199–1202
Tamrakar AK, Yadav PP, Tiwari P, Maurya R, Srivastava AK (2008) Identification of pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamia pinnata fruits. J Ethnopharmacol 118:435–439
Taylor JP, Brown RH Jr, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539:197–206
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M (2019) Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomed 14:5541–5554
Tohge T, Watanabe M, Hoefgen R, Fernie AR (2013) Shikimate and phenylalanine biosynthesis in the green lineage. Front Plant Sci 4:62
Trivedi MK, Branton A, Trivedi D, Nayak G, Singh R, Jana S (2015) Evaluation of physical, thermal and spectroscopic properties of biofield treated p-hydroxyacetophenone. Nat Prod Chem Res 3(5):190
Tsunemi T, Krainc D (2014) Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum Mol Gen 23:2791–2801
Van Schependom J, D’haeseleer M (2023) Advances in neurodegenerative diseases. J Clin Med 12(5):1709
Verma M, Lizama BN, Chu CT (2022) Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl Neurodegener 11:3
Vismaya EWA, Manjunatha JR, Srinivas P, Kanya TCS (2010) Extraction and recovery of karanjin: a value addition to karanja (Pongamia pinnata) seed oil. Ind Crops Prod 32(2):118–122
Vismaya Belagihally SM, Rajashekhar S, Jayaram VB, Dharmesh SM, Thirumakudalu SK (2011) Gastroprotective properties of karanjin from Karanja (Pongamia pinnata) seeds: role as antioxidant and H, K-ATPase inhibitor. Evid Based Complement Alternat Med 2011:747246
Voicescu M, Ionescu S, Gatea F (2014) Photophysical properties of some flavones probes in homogeneous media. J Fluores 24(1):75–83
Wang C, Telpoukhovskaia MA, Bahr BA, Chen X, Gan L (2018a) Endo-lysosomal dysfunction: a converging mechanism in neurodegenerative diseases. Curr Opin Neurobiol 48:52–58
Wang TH, Wang SY, Wang XD, Jiang HQ, Yang YQ, Wang Y et al (2018b) Fisetin exerts antioxidant and neuroprotective effects in multiple mutant hSOD1 models of amyotrophic lateral sclerosis by activating ERK. Neuroscience 379:152–166
Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I (2023) Hallmarks of neurodegenerative diseases. Cell 186(4):693–714
Yamada M (2004) Clinical pharmacology of MAO inhibitors: safety and future. NeuroToxicol 25(1–2):215–221
Yang J, Jia M, Zhang X, Wang P (2018) Calycosin attenuates MPTP-induced Parkinson’s disease by suppressing the activation of TLR/NF-ΚB and MAPK pathways. Phytother Res 33(2):309–318
Yi D, Wang Z, Yi L (2014) Development and validation of an LC–MS method for determination of karanjin in rat plasma: application to preclinical pharmacokinetics. J Chromatogr Sci 53(4):456–461
Yin H, Zhang S, Long L, Yin H, Tian X, Luo X et al (2013) The separation of flavonoids from Pongamia pinnata using combination columns in high-speed counter-current chromatography with a three-phase solvent system. J Chromatogr A 1315:80–85
Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C (2003) Interaction between flavonoids and the blood-brain barrier: In vitro studies. J Neurochem 85(1):180–192
Yu J, Yang H, Lv C, Dai X (2022) The cytotoxicity of Karanjin toward breast cancer cells is involved in the PI3K/akt signaling pathway. Drug Dev Res 83(7):1673–1682
Zabalza A, Orcaray L, Fernández-Escalada M, Zulet-González A, Royuela M (2017) The pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pest Biochem Physiol 141:96–102
Zhang J, Xie Y, Fan Q, Wang C (2020) Effects of karanjin on dimethylhydrazine induced colon carcinoma and aberrant crypt foci are facilitated by alteration of the p53/BCL2/Bax pathway for apoptosis. Biotech Histochem 96(3):202–212
Zhang W, Dong X, Huang R (2023) Antiparkinsonian effects of polyphenols: a narrative review with a focus on the modulation of the gut-brain axis. Pharmacol Res 193:106787
Zheng JC, Chen S (2022) Translational neurodegeneration in the era of fast growing international brain research. Transl Neurodegener 11(1):1–2
Zsila F, Bikadi Z, Simonyi M (2003) Probing the binding of the flavonoid, quercetin to human serum albumin by circular dichroism, electronic absorption spectroscopy and molecular modelling methods. Biochem Pharmacol 65(3):447–456
Acknowledgements
The authors Charles Gnanaraj, Mogana Govendan, Mahendran Sekar and Wing-Hin Lee would like to thank the Ministry of Higher Education (MOHE), Malaysia for the support provided via the Fundamental Research Grant Scheme [Ref: FRGS/1/2020/SKK06/UNIKL/03/2]. The chemical structures in Figs. 1 and 2 were drawn using ChemDoodle version 12.2.1 (iChemLabs LLC, Virginia, US).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gnanaraj, C., Govendan, M., Loo, CY. et al. Karanjin: a potential furanoflavonoid for neuroprotection. Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-09925-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-09925-z