Abstract
The inherent capability and increased efficiency of microalgae to convert sunlight into solar chemical energy are further enhanced by the higher amount of oils stored in microalgae compared to other land-based plant species. Therefore, the widespread interest in producing biofuels from microalgae has gained considerable interest among leading energy experts and researchers due to the burgeoning global issues stemming from the depletion of fossil fuel reserves, future energy security, increasing greenhouse gas emissions, and the competition for limited resources between food crops and conventional biomass feedstock. This paper aims to present the recent advances in biofuel production from microalgae and the potential benefits of microalgae in the energy and environmental sectors, as well as sustainable development. Besides, bottlenecks and challenges mainly relating to techniques of cultivation and harvesting, as well as downstream processes are completely presented. Promising solutions and novel trends for realizing strategies of producing biofuels from microalgae on an industrial and commercial scale are also discussed in detail. Alternatively, the role of microalgae in the circular economy is thoroughly analyzed, indicating that the potential of scaling up current microalgae-based production could benefit from the waste-to-energy strategy with microalgae as a key intermediate. In the future, further research into combining different microalgae biomass pretreatment techniques, separating the microalgae feedstock from the cultured media, developing new species, and optimizing the biofuel production process should be carried out to reduce the prices of microalgae biofuels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Along with the extraordinary pace of technological advances, the twenty-first century also faces incredibly difficult global challenges such as overpopulation, climate change, food scarcity, depletion of fossil fuels, etc., (Hoang et al. 2021c; Bhushan et al. 2020; Nayak and Mishra 2016). Indeed, the growing reliance on nonrenewable sources of energy has prompted a growing interest in finding more sustainable solutions to meet the increasing global energy demands; this lesson could be observed more clearly from the shifting progress to clean energy and energy crisis after the COVID19 pandemic (Steffen et al. 2020; Chen et al. 2021). Among the renewable and clean energy sources, solar power presents a vast amount of renewable energy harnessed from the sun; through photosynthesis, plants use sunlight to grow and mature (Udayan et al. 2022; Hariskos and Posten 2014). Therefore, plant biomass-derived carbonaceous materials present a promising and widely available source of feedstock for biofuel production such as microalgae, crop residues, and organic wastes (Hoang et al. 2022; Papathoti et al. 2021; Alami et al. 2021). The conversion of widely available biomass into promising sources of renewable energy such as biofuels has been a popular topic among current scientists and energy researchers (Chen et al. 2022; Aissi et al. 2021; Hoang and Pham 2021). Biofuels are commonly classified into three different categories, including first, second, and third generation. Biofuels produced from food crops are considered first-generation such as bio-ethanol made from starch. For biofuels that are derived from non-food crops such as lignocellulosic biomass can be grouped into the second-generation category. Last but not least, biofuels derived from microalgae and microbes are considered the third generation category (Sirohi et al. 2022a; SB et al. 2022).
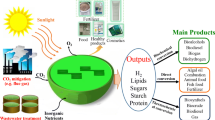
One of the major challenges facing the production of biofuels is the inadequate and inconsistent supply of biomass feedstock (Koyande et al. 2019). Furthermore, the conversion of first-generation biofuels from food crops also raises the controversial food versus fuel debate as they compete for the same amount of arable land and freshwater resources. Recently, different species of microalgae have received much attention for their potential of being a promising feedstock for the production of third-generation biofuels (Mishra et al. 2017). Due to the incredibly fast growth rate and high carbon efficiency, microalgae present major benefits as a substrate for biofuel production. Examining the structure of microalgae, one can find the presence of several bioactive compounds such as lipids, proteins, carbohydrates, and fractions of anti-oxidants (Kusmayadi et al. 2021; Yu et al. 2021a). Besides the potential as a biofuel feedstock, microalgae are packed with a significant amount of nutrients that can be utilized in food supplements and animal feed. Microalgae also have a special role in dye products, cosmetics, and the pharmaceutical industry. Furthermore, studies also have found the benefit of microalgae as an effective pollution control agent (Aliyu et al. 2021). Among major industries such as biochemical engineering, food production, and pharmaceutical, there has been an increasing trend in the use of microalgae as a solution to address the burgeoning issues of sustainable production and consumption of food, feed, and fuel, as shown in Fig. 1. Compared to other biofuel alternatives, microalgae-derived biofuels demonstrate a higher potential and practicality (Kazemi Shariat Panahi et al. 2019b). Depending on the species of microalgae, the efficiency of bioethanol can be significantly increased when there is a higher content of carbohydrates (e.g., up to 30% of dry weight) (Kazemi Shariat Panahi et al. 2019a).
Using sunlight via photosynthesis, microalgae are capable of converting nutrients in the water to bioactive compounds that make up the primary cell structure (Verma et al. 2020). As microalgae, they feed off the nutrients (e.g., nitrogen, phosphates, and trace elements) when converting CO2 into organic compounds with the energy provided from sunlight. Considering this characteristic alone, microalgae present a likely solution for wastewater treatment and CO2 bioremediation in industrial processes (Cunha et al. 2020), suggesting that microalgae could be a key and critical link in the progress of converting waste to energy. Another significant advantage in the farming of microalgae is that it does not compete for the same resources required for the cultivation of traditional food crops. Furthermore, provided with high concentrations of CO2, microalgae can grow at a rapid pace while utilizing and offsetting the CO2 source such as flue gas from thermal power plants (Singh et al. 2019a; Sirohi et al. 2021). Furthermore, the technologies utilized in the production of biofuels from microalgae also have a lower carbon footprint and lower negative impacts on the ecosystem because the equipment used in microalgae-based biofuel technologies has a low-energy consumption level (Bwapwa et al. 2017; Khoo et al. 2020). Due to this reason, promoting the use of microalgae-derived biofuels can yield a positive sustainability effect on the environment since it is considered carbon neutral; thus, there is a significant benefit in pursuing this promising source on the path toward a higher sustainable energy future based on the circular economy and carbon neutrality (Javed et al. 2019).
Despite extensive progress in the development of microalgae technology, significant challenges remain surrounding the techno-economic viability of microalgae-derived biofuels. Among the current obstacles, cultivation techniques need further research to improve the biomass concentration of microalgae grown in suspended culture, as well as find an adequate supply of CO2 and affordable growth medium for industrial-scale farming (Suparmaniam et al. 2019; MT et al. 2022). Notably, high concentrations of nutrients and different variety of salts are needed in the culture medium to support the growth of microalgae (Baicha et al. 2016). Moreover, increasing cost is a significant issue in the operation of microalgae cultivation systems in suspended cultures (Tan et al. 2015). Upon harvesting, the next step is to decide which conversion method that depends significantly on the conditions of the farmed biomass and the desired biofuel products (Kumar et al. 2020). Overall, there are reasons and gaps to have further studies of the development of biofuels production from microalgae due to several advantages and value-added products. Indeed, this review paper explores existing practices while reviewing the bio-processing of microalgae, also known as bio-refinery of microalgae. This current work also attempts to provide an overview of the current development of third-generation biofuels derived from microalgae. Furthermore, this review paper also addresses the current issues facing the implementation of microalgae bio-refinery and future opportunities for the industry. On the one hand, the paper summarizes the recent development in terms of strategies employed in the production of biofuels from microalgae. On the other hand, it also focuses prospects, opportunities, and challenges on the path toward scaling up microalgae-based biofuel production and improving widespread market penetration. More importantly, this review paper aims to provide insights and inputs in the strategic decisions by various stakeholders such as researchers, legislators, officials, environmental organizations, private sectors in advancing the progress of the biofuel industry overall and improve the viability and cost-effectiveness of the microalgae-based biofuel products specifically.
Microalgae: properties and CO2 fixation capacity
Compared to various land-dwelling species of flora, microalgae have a significantly higher concentration of light-harvesting pigment known as chlorophyll which results in more effective photosynthesis (Kumar et al. 2021b). Several factors can influence the cellular structure and make-ups of different species of microalgae. Among these, inherent conditions such as species, growth media, environmental factors, and the coexistence of multiple strains within the same culture are examples of factors that can affect the nature and characteristics of microalgae (Kim et al. 2014).
Provided with metabolic versatility, microalgae are capable of being converted into a wide range of value-added products via various processing pathways. For certain species of microalgae with high concentrations of carbohydrates (e.g. 37–55%) such as Chlorella, Chlamydomonas, Dunaliella, Scenedesmus, and Tetraselmis, the starch-rich components are mainly found in the chloroplasts and cell walls made of cellulose (Dragone et al. 2011). Besides, the lipid contents in microalgae can range between 2 and 77% of the biomass volume for certain species under favorable growth conditions. Lipids in microalgae are grouped based on the end-product from the conversion process, namely biofuel and nutrient supplements (Mimouni et al. 2018; Ho et al. 2020). For the latter category, microalgae present a potentially good source for the manufacturing of omega-3 fatty acids, in which common microalgae species used in the production of omega-3 polyunsaturated fatty acids include Bacillariophyta, Chlorophyta, Cryptophyta, Haptophyta, Haptophyta, and Rhodophyta (Ryckebosch et al. 2012, 2014). Last but not least, proteins which are considered an essential product in the biorefinery process of microalgae make up a significant component. Depending on the species, they can range anywhere between 50–70% of the biomass volume in microalgae. Proteins extracted from microalgae have different uses such as in food supplements and animal feeds (e.g., aquaculture and husbandry) (Bertsch et al. 2021). Furthermore, pigments and other bioactive compounds can also be extracted from microalgae. For example, several chemical processes often utilize chlorophylls, phycobilins, and carotenoids that are extracted from several microalgae species such as Porphyridium cruentum, Synechococcus sp., and Chlorella (Ummalyma et al. 2020).
Given the high concentration of biological molecules contained in the biomass, microalgae present an attractive option to be used as a substrate in energy conversion. The production of specific biofuel relies heavily on the biochemical make-up of the microalgae substrate. For example, various species of microalgae with a higher mass fraction of lipid content provide optimal feedstock for biodiesel production (Brindhadevi et al. 2021). On the other hand, microalgae that have higher carbohydrate content are more suitable for the production of fermentative alcohol (Phwan et al. 2019). Notably, researchers have found ways to modify the biochemical structure of microalgae species with the help of genetic engineering techniques to improve the characteristics of certain strains. Furthermore, the growth rate of selected species of microalgae can be significantly enhanced with the development and application of photobioreactors in the cultivation process (Sirohi et al. 2022b; Ranganathan et al. 2022). Moreover, microalgae species with high concentrations of fatty acids have been regarded as potential sources to supplement conventional petroleum-based fuels and fish oils. Depending on the strains of microalgae, the productivity of biomass and lipid content can vary significantly when they are used as inputs for edible oil production (Shin et al. 2018b). Despite the fact that the investigation into optimal culture conditions to increase the lipid content from microalgae has been conducted, it is unlikely to achieve equally desirable results if the chosen strains are not suitable for the conversion to edible oil (Piligaev et al. 2015). Hence, it is important to consider the microalgae species as it is the major factor affecting the productivity and composition of major components such as fatty acids, carbohydrates, lipids, and proteins (Xue et al. 2021). Figure 2 provides nutrients of different microalgae species for the purpose of biofuel production.
Nutrients of different microalgae species (Singh and Gu 2010)
Previous studies have examined different species of microalgae for biofuel production, such as Chlamydomonas sp. (Scranton et al. 2015), Chlorella sp. (Guccione et al. 2014), Senedesmus sp., Nannochloropsis sp., and a combination of various cultures included both fresh or wastewater strains (Sajjadi et al. 2018). Biological molecules found in microalgae biomass, namely lipid, protein, and carbohydrates, as well as the entire cell structure, can be converted into biofuels through proven techniques. All in all, the development of microalgae as a potential biomass feedstock for third-generation biofuel production presents a significant opportunity and viable solution for achieving a more sustainable energy future.
As reported, microalgae are found to have a higher growth rate, as well as they could fix CO2 with higher capacity compared to aquatic and agricultural plants and traditional forests. More interestingly, without any evolution beyond the cells, microalgae could adapt to popular conditions of the environment and have a strong growth in the long term (Singh and Ahluwalia 2013). In the growing process, photosynthesis is known as the critical basis in the fixation and storage of CO2 of microalgae. Many studies indicated that the efficiency of the photosynthesis process for microalgae could reach 10–20% (Li et al. 2008), even microalgae could perform 50% of the photosynthesis process on the Earth (Singh and Singh 2014). However, the level of CO2 fixation depends much on the types of microalgae and the environmental conditions of cultivation (Ho et al. 2011). For example, Chlorella species were found to have strong CO2 fixation capacity when CO2 concentration ranged from 5 to 20% (Tang et al. 2011). In another case, Spirulina species could fix CO2 at a high rate value of 37.9% (De Morais and Costa 2007). Besides, it was reported that the use of N. oculata for CO2 fixation in flue gas produced from a coal-fired power plant could offer a high efficiency that around 1/3 of CO2 could be removed from flue gas (Cheng et al. 2015b). In addition to traditional microalgae, many novel microalgae have been developed to provide excellent capacities in fixing CO2. In a study by Aghaalipour et al. (2020), they found that Psammothidium and Monoraphidium contortum species could show a good capacity of CO2 biofixation at concentration < 10%. In another report based on the experimental results obtained from 20 microalgae species, Gleocystis ampula was observed to have the highest growth rate and CO2 fixation capacity (0.281 g/l.d) (Derakhshandeh et al. 2021). Also, many studies reported that a large number of unfamiliar microalgae could offer excellent capabilities in fixing CO2 and producing biomass (Song et al. 2020; Ding et al. 2020; Tamil Selvan et al. 2020; Jin et al. 2021; Rodas-Zuluaga et al. 2021; Sung et al. 2021). In general, a critical route from the fixation process of CO2 by microalgae to biofuel production could be depicted in Fig. 3.
Critical route for biofuel production from microalgae
The existing biorefinery process of microalgae has the potential to yield many high-value products that can be grouped into fuel and non-fuel categories (Chandra et al. 2019), in which common biofuels include bioethanol (or biobutanol), biodiesel, biogas, and bio-hydrogen. In general, Fig. 4 provides examples of advanced methods utilized in the conversion of microalgae biomass to different end-products.
Different routes for biofuels/bioenergy production from microalgae (Abo et al. 2019)
Thermochemical conversion
Thermochemical liquefaction is a technique that can be utilized at low temperature and high-pressure conditions to convert wet microalgae biomass to bio-oil. In this method, the use of a catalyst is optional (Arvindnarayan et al. 2017a). Such an approach benefits from the elimination of the energy-intensive drying process. Through liquefaction, biomolecules such as lipids, carbohydrates, and proteins in microalgae biomass are processed into crude bio-oil. Several factors can affect the efficiency of the process, such as temperature, pressure, reaction time, catalyst, and the natural structure and composition of the microalgal biomass (Hong et al. 2021). Pyrolysis is another commonly used technique to yield bio-oil, syngas, and charcoal from the combustion of biomass at medium to high temperatures (350–700 °C) without the presence of oxygen (Yang et al. 2019). For fast pyrolysis, the process occurs at a medium temperature range of approximately 500 °C with a really short hot vapor residence time of 1 s. The process has the potential to obtain up to 95,5% bio-oil yield (Venderbosch 2019). In contrast to slow pyrolysis, fast pyrolysis has a lower overall energy consumption due to its fast reaction while yielding a higher amount of bio-oil outputs. Furthermore, bio-oil obtained from the fast pyrolysis process has a lower viscosity compared to its counterpart obtained from slow pyrolysis (Tan et al. 2015). Hence, the fast pyrolysis process is more appropriate for the production of liquid bio-fuel in general and much larger industrial scales specifically (Hoang et al. 2021b).
After microalgae biomass undergoes either the pyrolysis or hydrothermal liquefaction (HTL) process, the dark brown liquid output is determined as bio-oil. The main difference between these two methods is that the former can only occur under dry conditions (i.e., less the 5% of moisture contained in the biomass feedstock) while HTL can be conducted using any type of microalgae biomass (Toro-Trochez et al. 2019). Within these methods, depolymerization of organic compounds takes place without the presence of oxygen (Sun et al. 2020). The microalgae biomass feedstock is first broken down into smaller molecules through various thermophysical processes such as dehydration, dehydrogenation, deoxygenation, and decarboxylation. One of the most significant factors driving the difference in the yield and quality of bio-crude is microalgae biomass constituents. According to Arvindnarayan et al. (2017b), there is a high potential in obtaining high energy content from the pyrolysis of Chlorella species of microalgae. Furthermore, bio-oil obtained from microalgae biomass can be easily blended with conventional transportation fuel without the need for major modifications to existing engines. According to the experiment reported by Jena and Das (2011), the pyrolysis of Spirulina platensis at 350 °C yielded 39.7, 23.8, and 19.2wt.% of biochar, bio-oil, and gases, respectively. According to Shakya et al. (2017), an experiment was conducted when subjecting nine species of microalgae to hydrothermal liquefaction at 280 °C and 320 °C in operating temperature and 0.7 MPa of pressure. The study confirmed the highest yield of bio-crude output from the conversion of lipid-rich Nannochloropsis at 320 °C. Furthermore, a series of investigations by Zhu et al. (2016) utilized microalgae biomass of two different species, namely Scenedesmus and Spirulina to produce bio-oil. The authors compared the results from the application of hydrothermal liquefaction (300 °C and 10–12 MPa) and the pyrolysis method (heating to 450 °C at a rate of 50 °C/min) to the above microalgae strains. As a result, both approaches yielded comparable results in terms of bio-oil output. Notably, the experiments obtained 24–45% of bio-oil with a high heating value (35–37 MJ/kg). Hence, the outputs of the pyrolysis of microalgae biomass depend on the species and the pyrolysis conditions. Moreover, the researchers also confirmed the advantage of hydrothermal liquefaction over the pyrolysis method in terms of energy consumption ratio. When the moisture content of wet biomass exceeded 80%, the ECR values of hydrothermal liquefaction and pyrolysis were measured in the range of 0.44–0.63 and 0.92–1.24, respectively. Recently, researchers have made significant progress on the development of microalgae biomass pyrolysis to convert microalgae into biofuels. For example, one study has successfully obtained nearly 54.4% (wt./wt.) yield of bio-oil from microwave-enhanced pyrolysis subjected to a CO2 environment when using freshwater microalgae blooms naturally occurring in lakes as the main feedstock (Zhang et al. 2016). In another instance, the hydropyrolysis of the native microalgae consortium was observed to yield up to 31% of bio-oil under 200 °C operating temperature (Choudhary et al. 2017). Recent studies have explored the use of heterogeneous catalysts to enhance the conversion efficiency in the hydrothermal liquefaction of microalgae (Xu et al. 2019a; Kohansal et al. 2019). In their study, Saber et al. (2016) conducted low-temperature hydrothermal liquefaction using Nannochloropsis as the primary biomass feedstock. The experiments were conducted using three different catalysts, including nano-Ni/SiO2, synthesized zeolite, and Na2CO3, under three different temperatures (210, 230, and 250 °C). Among these catalysts, nano-Ni/SiO2 yielded the highest amount of bio-oil (30 wt.%), followed by Na2CO3 (24.2 wt.%) and zeolite (24 wt.%). In another comparable work, the hydrothermal liquefaction at 300 °C of Nannochlopsis in the presence of Ni supported TiO2 obtained a 48.23% yield of bio-crude (Wang et al. 2018).
Under low-oxygen conditions, biomass can be heated at relatively high temperatures (800–1000 °C) to yield combustible gas mixtures in a process known as gasification (Clark and Deswarte 2014). The output of syngas typically contains several common gases such as CO, H2, CH4, and CO2 (Hoang et al. 2021a). Although syngas has a low energy density (4–6 MJ/m3), it is highly suitable for domestic heating and cooking by combustion in gas engines or gas turbines (McKendry 2002). In a study by Zhu et al. (2016), they conducted an experiment utilizing both wood biomass and microalgae with a 9:1 ratio in a co-gasification process. Compared to only using wood biomass, the co-gasification of microalgae and wood yielded higher H2, CO, and CH4 by 3–20%, 6 -31%, and 9–20%, respectively. Likewise, Raheem et al. (2015) performed the gasification of Chlorella biomass at 950 °C yielding H2, CO, and CH4 up to 2.9, 22.8, and 10.1 wt.% of biomass, respectively. In a study by Liu et al. (2017), they found that the gasification process exhibited an overall 81.6% efficiency with a combustion gas yield of 1.05 Nm3/kg. The authors also confirmed the factors influencing the cold gas efficiency, such as steam and moisture content in the feedstocks, as well as the mixture of the microalgae substrate to a lesser degree. More importantly, the most significant factor is the moisture content in the gasification and biomass feedstock that affects the composition of the syngas output (Azadi et al. 2014). A study conducted by Diaz-Rey et al. (2015) also has shown the potential to obtain hydrogen-rich precursors with measured HHV up to 25 MJ Nm via gasification of microalgae. Such a process still faces major obstacles in dealing with the high concentration of nitrogen and minerals found in microalgae feedstock. One way to reduce the ash content in the output has been suggested by Liu et al. (2019b) that could eliminate the amount of ash by 67.6% when acid washing the microalgae feedstock with HCL. Other studies also pointed out that the elevated concentration of potassium in microalga ash could enhance gas yield, while the presence of calcium could activate carbon capture and deamination (Liu et al. 2020a; López-González et al. 2014). Table 1 provides a summary of different studies on thermochemical conversion of microalgae into biofuels.
Biochemical conversion
Anaerobic digestion is considered to be a popular biochemical conversion of biomass into biofuels. Biogas, which makes up mainly of CH4 and CO2 along with a small amount of H2S, is the output of the anaerobic digestion process in which microbes break down and convert the organic biomass of microalgae. Typically, a constant temperature is maintained throughout the anaerobic digestion process to ensure the optimal condition for the growth of the microbes. The temperature range can be classified as mesophilic (30–42 °C) and thermophilic (43–55 °C). Most anaerobic digesters are constructed based on the characteristics of the former operating conditions, given their prevalence and stability in the treatment of different types of biomass (Gonzalez-Fernandez and Muñoz 2017). Commonly, there are three main stages of anaerobic digestion, including hydrolysis, fermentation, and methanogenesis. Initially, simple sugars are obtained from the hydrolysis of complex polysaccharides in biomass. Then, the fermentation process takes place to transform sugars into alcohols, acetic acid, volatile fatty acids, and traces of H2 and CO2. The gas mixture is further transformed into CH4 (60–70%) and CO2 (30–40%) in the presence of methanogens (Cantrell et al. 2008). The measured energy content of the biogas product is 20–40% of the lower heating value of the biomass feedstock. Brennan and Owende (Brennan and Owende 2010) have observed the suitability of wet biomass containing 80–90% of moisture as feedstock in an anaerobic digestion process. Several studies have attempted to use microalgae as the primary feedstock for the production of bio-methane via anaerobic digestion. Nevertheless, the process achieves very low yields due to the inability of bacteria to break down the outer cell structure of microalgae. Furthermore, the presence of free ammonia due to the low carbon to nitrogen ratio further inhibits the formation of methane (Chandra et al. 2019). In a study by Zamalloa et al. (2011), they confirmed relatively lower increases in methane output from the digestion of Scenedemus obliquus and Phaeodactylum tricornutum under mesophilic (0.296 L CH4/g) and thermophilic (0.462 L CH4/g). According to Trivedi et al. (2015), higher biomass yields are observed in microalgae biomass compared to biomass obtained from terrestrial plants (e.g., Jatropha curcas). However, the latter has a lower feedstock cost which improves its economic viability.
To convert microalgae to bio-alcohol, major biomass constituents, such as sugars, starch, and cellulose, are first degraded in the presence of yeast or bacteria. Further distillation is often required to achieve higher concentrations of diluted alcohol (Alves et al. 2020). Carbohydrates are mainly found in starch reserves in cell bodies as well as cellulose/polysaccharides in the cell walls. Jönsson et al. (2016) have observed a high degree of difficulty in fermenting carbohydrates found in biomass in the presence of microbes. Hence, it is necessary to break down these carbohydrates into monomeric sugars via hydrolysis using either chemical or biological agents (enzymes) before further processes (Lin et al. 2019). Nevertheless, enzymatic hydrolysis is less favorable due to the high costs associated with enzyme procurement and the required pre-treatment of microalgal biomass (Tan et al. 2015).
As part of the alcoholic fermentation process, the monomeric sugars derived from initial treatments of biomass are further processed into the desired outputs. De Farias Silva and Bertucco (de Farias Silva and Bertucco 2016) have observed the conversion of sugars found in Saccharomyces and Zymomonas into bioethanol under an oxygen-free environment. Furthermore, the fermentation process can occur in two different routes in which hydrolysis and fermentation can take place separately or simultaneously in the same reaction chamber. According to Brennan and Owende (Brennan and Owende 2010), the conversion of Chlorella vulgaris which contains 37% of starch per dry cell weight results in nearly 65% of ethanol output. In another study, Choi et al. (2010) were able to produce 0.235 g of bioethanol from each gram of C. reinhardtii biomass via the separated hydrolysis and fermentation method. Compared to other conventional fermentation methods, the use of anaerobic fermentation to convert microalgae biomass into bioethanol is considered a relatively more simple process. In one study, Daroch et al. (2013) investigated the use of genetically modified species of microalgae (e.g., Chlamydomonas perigranulata) that revealed positive results in terms of bioethanol production due to the presence of self-ferment carbohydrates. To improve the yield of ethanol, starch-rich microalgae such as Chlorella vulgaris is one of the most popular strains with the potential to achieve up to 65% conversion efficiency, and a high bioethanol yield can be obtained from dark fermentation of microalgae at 30 °C (Javed et al. 2019).
To produce biobutanol, the conventional method typically involves the conversion of sugars into a combination of acetone, butanol, and ethanol (ABE) (Veza et al. 2021). Theoretically, under a controlled fermentation process, the optimal ABE yield is 0.41 g per gram of sugar which is slightly lower than 0:5 g of bioethanol per gram of sugar. A mixture of CO2 and H2 gas is also obtained as by-products in the process. The common product ratio of these components is 3:6:1, respectively. Hence, butanol accounts for the largest portion of the outputs (Bellido et al. 2014). According to Chen et al. (2015), there is lower productivity in the production of biobutanol due to the strong resistance of degraded substances resulting from the initial steps of the fermentation process. Furthermore, there is only a single species of microalgae (i.e., Clostridium spp.) that can be converted to biobutanol leading to the lower efficiency of the conversion process. Given the fact that Clostridium spp. is saccharolytic, the conversion process of starch-rich microalgae into biobutanol is relatively easy as that of bioethanol. In another instance, Ellis et al. (2012) utilized biomass obtained from microalgae grown in wastewater with Clostridium saccharoperbutylacetonicum N1,4 in an ABE fermentation process. In this experiment, microalgae that were pretreated with acid and basic chemicals resulted in 2.74 g/L of total ABE from the fermentation process. On the other hand, a much higher yield of 9.74 g ABE/L when enzymatic hydrolysis (xylanase and cellulases) was applied. As reported, ABE biofuel has more beneficial physicochemical properties than those of fossil fuel, indicating that it could be used as the potential biofuel for engine applications (Veza et al. 2019, 2020).
Biohydrogen is produced biologically as a result of the metabolic reactions of microbes. Renewable biohydrogen presents a more attractive option compared to other biofuel productions. Regarded as a potential alternative fuel, biohydrogen is regarded to be cleaner and more sustainable while having a relatively high energy content (142 MJ/kg). Biohydrogen can be produced via different biological techniques, including photo-fermentation, dark fermentation, and electro-bio-hydrogenation. There are major pros and cons associated with each of these methods in terms of feasibility, sustainability, and energy efficiency (Kadier et al. 2018; Sivagurunathan et al. 2017). Microalgae have recently gained the interest of researchers as the potential third-generation biomass feedstock for the production of renewable biohydrogen. Studies have reported the use of different species of microalgae in the generation of biohydrogen, such as Scenedesmus, Chlorella, Synechocystis, Anabaena, Nostoc, and Tetraspora harbor hydrogenase (Eroglu and Melis 2011; Anwar et al. 2019). Naturally, some microalgae strains can use sunlight and water as the source of electrons and energy respectively to produce photo-biological biohydrogen. Moreover, hydrogen and carbon dioxide can be obtained when subjecting microalgae biomass as the organic substrate in the photo-fermentation process with the help of photosynthetic micro-organisms (Arun et al. 2020b). In another study, a 200 ml/L.h hydrogen production rate was observed when using Clostridium butyricum in the dark fermentation of C.vulgaris biomass which contains a high level of carbohydrate, in which biohydrogen also accounted for 66% of the total volume of biogas produced in the above process (Liu et al. 2013). In addition, biological hydrogen is also a potential power source for electricity produced from fuel cells (Ban et al. 2019; Eroglu and Melis 2016; He et al. 2017). In general, biofuel yield achieved from the biochemical conversion of microalgae is summarized in Table 2.
Chemical conversion
Microalgae-derived bio-oils inherently have a higher viscosity compared to diesel oils, it is thus necessary to perform a transesterification process on the microalgae oils to lower their overall viscosity before applying to engines (Akubude et al. 2019). To improve the efficiency of biodiesel from microalgae, the direct transesterification method is deemed more favorable. Notably, alcohol acts as both the reactant and solvent in a transesterification process. Due to its affordability and easy access, it is common to use methanol in this process over other types of alcohols (e.g., methanol, ethanol, propanol, butanol, and amyl alcohol). Given that alcohol is hardly soluble in different kinds of oils, the presence of a catalyst is also important due to its enhancing the solubility of alcohol. The direct application of lipid contained in wet microalgae biomass is another cost-effective strategy to reduce the cost associated with drying and extracting moisture content from the biomass feedstock. Therefore, researchers have explored the development of advanced wet lipid extraction techniques (Lakshmikandan et al. 2020). Moreover, to penetrate and break down the cell wall of microalgae within the lipid extraction process, researchers have applied different pretreatment techniques to the biomass feedstock, such as high-pressure homogenization, ultrasound sonication, microwave irradiation (Howlader and French 2020), osmotic shock, liquid hot water, Triton-X-100 application, shake mill (Ramola et al. 2019). According to Singh et al. (2019b), solvothermal methods were applied to obtain lipids from Spirulina in a microwave environment with a capacity of 750 W at 60 °C for 30 min. Furthermore, mixing an equal amount of hexane and methanol has been proven to be effective in large-scale operations (Shin et al. 2018a). The application of ionic liquids can also improve the efficiency of lipid extraction from microalgae.
Presently, microalgae are gradually gaining a favorable assessment from experts as the potential sources of biodiesel production, considering the rapid growth rates and lipid composition (50–70%) (Satputaley et al. 2017). Compared to petroleum-based diesel with 46 MJ/kg caloric values, microalgae-derived biodiesel has a comparable energy content ranging between 39 and 41 MJ/kg (Goh et al. 2019). Relatively high conversion efficiency (70–75 wt%) can be achieved in the production of biodiesel via hexane or supercritical CO2 extraction method. According to Umdu et al. (2009), a very high conversion efficiency rate of 97.5% was obtained in the transesterification of Nannochloropsis oculate with the support of CaO and Al2O3 catalysts at 50 °C. Furthermore, the addition of Zn, Ti, and Al-based catalysts in the transesterification of green microalgae was detected to yield a 90.2% conversion efficiency under 350–400 °C and 2500 psi operating conditions (McNeff et al. 2008). In another example, the transesterification of C. protothecoides along with 75% of lipase (Candida sp.) in a methanol-containing medium at 38 °C resulted in the conversion efficiency of 98.15% after 12 h (Cheng et al. 2009). According to Cheng et al. (2020), a reduction in biodiesel yield from lipids extracted with chloroform and n-hexane by as much as 41% and 65%, respectively. On the other hand, the same authors observed up to a 9% increase in FAME yield from lipids resulting from the transesterification process in the presence of TEPDA. Furthermore, the application of n-hexane/formic acid has been shown to improve the biodiesel yield compared to chloroform/methanol extraction enhanced by ultrasound, microwave, hydrothermal, and dilute nitric acid pretreatments. Particularly, Xia et al. (2020) observed an increase of biodiesel yield in the range of 79–99% by applying the mixture of n-hexane/formic acid at the ratio of 9:2 by volume at 80 °C for 2 h. Recently, the in-situ transesterification method has been gaining traction as an alternative for the conversion of biodiesel from microalgae due to the overall lower production cost resulting from the elimination of expensive biomass drying and lipid extraction steps (Ghosh et al. 2017; Ma et al. 2019; Mandik et al. 2020).
Currently, there is a significant amount of interest among the scientific research community in the generation of biodiesel from microalgae due to several advantages, such as the rapid growth rate and enhanced lipid accumulation of microalgae. Furthermore, microalgae-based biodiesel also exhibits higher energy content (i.e., up to 34% increase) compared to conventional ethanol (Moshood et al. 2021). Despite its potential, further research is needed to examine the practicality of scaling up biodiesel production via the wet lipid extraction method. The alkaline-catalyzed process is among the most prevalent and well-developed methods. However, this method is not without its setbacks associated with the high energy requirement, the challenge in glycerin and catalyst post-process extraction. Considering the negative impacts from the use of alkaline-based catalysts, researchers have proposed alternative biodiesel production methods without the need for a catalyst known as the supercritical fluid method (Ortiz-Martínez et al. 2019). This new process utilizes a single reactor to convert microalgae biomass into biodiesel under operating temperatures between 250 °C and 350°. Compared to traditional transesterification and lipid extraction pathways supported by co-solvent, the supercritical method requires a significantly lower amount of energy inputs (Dickinson et al. 2017).
Potential benefits of microalgae-based biofuels
The rapid rate of anthropogenic-related environmental degradation in the latter part of the twentieth century has resulted in unprecedented destruction of natural habitats and depletion of natural resources compared to all previous parts of human history (Zaimes 2016). Increasingly stringent regulations are putting pressure on the current trend of global consumption of fossil-based energy sources. Furthermore, producers are also facing the challenge of designing products with lower environmental footprints and incorporating more sustainable components into the manufacturing process. Diverse factors are driving sustainable development progress, namely human, cultural, political, and socio-economic issues (Xu and Chen 2020). These adverse and irreversible consequences are apparent on a global scale that not only affects ecosystems on earth but also human inhabitants and their livelihoods on this planet. Considering this multifaceted sustainability perspective, advances in the fields of science and engineering could potentially offer powerful solutions to address issues related to the satisfaction of current demands without compromising the needs of future generations. Specifically with chemical production, incorporating sustainability elements are of utmost importance in enhancing the current manufacturing processes. Compared to conventional fossil fuels, biomass-derived biofuels present a more attractive alternative that is considerably cleaner and more sustainable (Cai et al. 2019).
Among various sources of biomass feedstock, microalgae are becoming a more popular choice in the production of third-generation biofuels. Microalgae serves as a promising feedstock in the production of biofuels and other value-added byproducts. Cultivated in aquatic environments, microalgae, which comprise either single-cell or multi-cellular photosynthetic microorganisms, are capable of using water and solar energy to fix atmospheric CO2 to chemical energy into the forms of lipids, carbohydrates, and proteins contained in the structure of the microalgae biomass. Similar to other plant species, microalgae are major sources of carbon dioxide sequestration that accounts for up to 40% of global CO2 (Sydney et al. 2019; Kholssi et al. 2021). As shown in Fig. 5, autotrophic microalgae absorb sunlight during photosynthesis to convert carbon dioxide into chemical energy. As a result, biomass and oxygen are the outputs of this natural process. To produce each ton of microalgae biomass, nearly two tons of CO2 along with 0.1 tN, 0.010 tP, and 0.015 tK are needed. Besides, up to two tons of O2 are emitted in the process. Microalgal biomass production has several advantages in terms of wastewater treatment and biofuel production. Several families of microalgae are suitable for the above purposes, including Spirulina (Arthrospira), Chlorella, Dunaliella, and Haematococcus (Draaisma et al. 2013).
The relationship between inputs and outputs in microalgae production (Fernández et al. 2021)
To ensure the optimal growth of autotrophic microalgae, it is important to ensure plenty amount of sunlight and CO2. Previous studies have concluded that 8–10 mol of photons are needed to obtain one mole of carbohydrates. On the other hand, the generation of each kilo of biomass necessitates nearly 2 kg of input CO2 (Kumar et al. 2011). The sustainability of microalgae production is supported by the use of natural sunlight and atmospheric CO2 (or from flue gas) (Vo Hoang Nhat et al. 2018). Because of their capability to fix atmospheric CO2 via the photosynthesis process, microalgae farming can help to reduce the current carbon stock in the atmosphere (Acién et al. 2012). In their experiment, Chiu et al. (2008) investigated the conversion efficiency of CO2 fixation in the farming of Chlorella sp. in a photobioreactor. As a result, the authors observed various rates of CO2 reduction and removal efficiency given different concentrations of CO2. Specifically, the following rate of reduction (g/h) and removal efficiency (%) are provided with the following CO2 concentrations at 2% (0.261 g/h and 58%), 5% (0.316 g/h and 27%), 10% (0.466 g/h and 20%) and 15% (0.573 g/h and 16%). Besides, earlier studies have shown that increasing the level of CO2 concentration from 5 to 15% could enhance the lipid composition and productivity in microalgae biomass (Jiang et al. 2011). In another study, the cultivation of Spirulina sp. and Scenedesmus obliquus was carried out in a three-stage serial tubular photobioreactor. The daily amounts of CO2 fixed in the process were recorded at 0.413 and 0.260 g/L for the above microalgae strains, respectively (Chisti 2007). In general, the capability of microalgae in removing atmospheric CO2 via photosynthesis can further be enhanced upon scaling up the existing microalgae-based biofuel production (Table 3).
Presently, it has been estimated to require a significantly large sum of money to startup a carbon credit economy. For example, if it was assumed a price tag of US$100 per credit of carbon, close to one trillion dollars would be needed (Bird et al. 2011). Furthermore, the annual emissions of carbon from fossil fuel sources are estimated to be around 8 Gt which also equals 8 billion carbon credits each year (Bird et al. 2011). Hence, fuels that do not contribute to the carbon emissions stock in the atmosphere can be considered carbon negative. In this sense, biofuels can be seen as more advantageous than conventional fossil fuels due to their negative carbon association. On the one hand, it would still require the consumption of fossil fuels in the cultivation of microalgae. On the other hand, the microalgae biomass itself used as the feedstock for biofuel production is considered carbon negative. In a separate study, the application of 15 g of A. fragilissima biomass was observed to produce up to 34.1% and 29.5% of carbon dioxide from the HTL and pyrolysis process, respectively (Arun et al. 2020c). As the CO2 obtained from the valorization process of plant-based biomass is fed back into the natural carbon cycle, microalgae biomass can be deemed carbon–neutral due to the prior photosynthesis process during its growth. Thus, the consumption of microalgae biomass can lead to a negative flow of CO2 as it is taken out of the natural cycle. On the other hand, if we compare to the CO2 emitted from the process of burning coal will increase the carbon stock in the atmosphere unless it can be subsequently captured and sequestered (Arun et al. 2020b). To ensure that microalgae-based biofuels are carbon negative, the cultivation methods should prioritize the reduction of carbon positive sources of energy. Furthermore, microalgae are capable of withstanding the presence of NOx and SOx in flue gas as long as the amount of SOx does not go above 400 ppm (De Bhowmick et al. 2019). Considering this dilemma, microalgae present an attractive biomass feedstock for a biofuel future (Fernández et al. 2021).
As mentioned above, common biofuels obtained from microalgae include bioethanol, biodiesel, bio-oil, biomethane, bio-hydrogen, and bio-butanol that could be used as alternative fuels for vehicle and transportation means with lower carbon emissions (Karthikeyan et al. 2018; Rajak et al. 2019; Hoang et al. 2020). Moreover, the sustainability of biofuel production from microalgae on an industrial scale was also confirmed (Hossain et al. 2019). Several benefits of this approach to produce biofuels include a relatively simple adaptation of natural habitat in the cultivation of microalgae without the need of competing with precious arable lands for farming of food and cash crops. Furthermore, microalgae also demonstrate the capability to achieve high photosynthesis efficiency and high lipid content. Notably, the integrated process of generating different types of biofuel, including biodiesel, bioethanol, and biogas, from a single feedstock has been shown to significantly improve the conversion efficiency of the microalgae biomass. Hence, it could lead to the improvement in the economic efficiency of the overall microalgae biorefinery process. Current progress made in the area of genetic and metabolic engineering promises to further enhance the cost-effectiveness and environmental sustainability of biofuel production from microalgae biomass. Indeed, Carbohydrate-rich microalgae such as Zymomonas mobilis, Saccharomyces cerevisiae., Chlorella sp., Dunaliella sp., Spirogyra sp., and Scenedesmus sp. present as promising biomass feedstock for the generation of biofuels (Özçimen and Inan 2015; Özçimen et al. 2020; Culaba et al. 2020). In addition, the potential of certain strains of microalgae (e.g., Chlorella protothecoides) could hold up to 55% of lipid in their biomass structure under a nitrogen-deprived cultivation environment, facilitating biodiesel production. (Xu et al. 2006). Hence, the development of microalgae biorefinery processing is integral to the realization of a zero-emission and sustainable circular economic model that can utilize wastewater-cultivated microalgae as a source of renewable bio-energy and bio-fuel (Serrà et al. 2020). Furthermore, experts believe that biomass can be the single renewable source of carbon-based fuel that is capable of supplying future energy demand. Due to the characteristics and composition of different microalgae species, it is possible to determine the desired product outputs as part of the conversion process. Within an integrated process of sustainable microalgae biofuel production, several stages are highly dependent and interconnected, requiring careful planning and monitoring of environmental, operational, and socio-economic conditions as well as externalities (De Bhowmick et al. 2019).
Taking sustainable development into account, the shifting of arable land and valuable resources such as freshwater from growing food to energy crops further drives the intense food versus fuel debate. Progress made in the current production of third-generation biofuels from microalgae presents a promising solution to the food versus fuel dilemma. Strong drivers for the transition to microalgae-biofuels are the positive impact on greenhouse gas reduction and saving of valuable arable land and freshwater resource. Indeed, the global consumption of freshwater in farming worldwide is estimated to be around 2700 km3 and is projected to rise close to 4000 km3 by the year 2050 (Rao et al. 2000). The significant requirements of freshwater inputs in growing energy crops face considerable pushback from the wider public considering the lack of safe drinking water in impoverished communities worldwide. Another adverse impact of farming energy crops is the potential abuse of fertilizers and pesticides that would cause surface water pollution leading to eutrophication and ecological contamination (Deknock et al. 2019). In contrast to energy crops, the outstanding advantage of microalgae is the ability to be cultivated on marginal lands, wastewater, and seawaters that are unsuitable for conventional food and energy crops. Besides, the conversion of microalgae into biofuels can also gain additional economic benefits from the generation of value-added byproducts as part of the process.
To sustain the growth of microalgae, nutrients are needed besides sunlight. Due to this reason, options to utilize wastewater or seawater that are unsuitable for drinking and farming of food crops could further improve the overall sustainability to improve the carbon footprints of microalgae-based biofuel production. Provided that wastewater has a considerably higher level of individual amino acids, microalgae can be supported in such a medium. Cultivation of microalgae can benefit from using nutrients present in wastewater such as common contaminants (e.g., NH4+, NO3−, and PO43−) (Ahmed et al. 2021; Vargas-Estrada et al. 2021). The growth rate of microalgae is less influenced by the changes in seasonal weather during the year, ensuring an uninterrupted growth cycle. Moreover, microalgae can be cultivated in wastewater, and microalgae farming has a lower environmental footprint because it does not consume freshwater (Lu et al. 2020). Studies have confirmed the capability of microalgae in lowering both the chemical oxygen demand and biochemical oxygen demand in wastewater. Common species that have been demonstrated to be effective in treating wastewater include Scendesmus sp., Chlorella sp., and Chlamydomonas reinhardtii (Wang et al. 2010; Kong et al. 2010; Park et al. 2011). Depending on the microalgae species, the extraction rate of metal ions (e.g., aluminum, calcium, ferum, mangan, and magnesium) can fluctuate between 50 and 99% (Wang et al. 2010; Woertz et al. 2009). However, Mathews (Mathews 2008) noted the optimal thresholds of nickel (1.0 ppm) and vanadium (0.1 ppm) contained in the flue gas have on the overall productivity of microalgae. Considering all these factors, Singh et al. (2011a) have concluded that microalgae are the leading candidate among existing energy crops having an energy and biofuel yield measured at 793–4457 GJ/ha and 24,355–136,886 L/ha, respectively (Rao et al. 2000). Furthermore, the current economics of biofuel production from microalgae can be enhanced by utilizing unconventional sources of nutrients such as fermented liquid (biogas liquor), animal manure, and other agricultural residues (Markou and Georgakakis 2011; Samorì et al. 2013). Beyond the positive economic benefits of microalgae cultivation using wastewater, this method also enhances the overall sustainability of the entire microalgae-based biofuel supply chain. Recently conducted Life Cycle Assessment (LCA) studies on the use of wastewater for microalgae-biofuel production have also highlighted the improved sustainability attribute. Compared with convention fuel crops such as corn, grass, and canola, the LCA of microalgae grown using wastewater resulted in improving sustainability and lowering the ecological footprints and subsequent biofuel production (Clarens et al. 2010). In another study, Yang et al. (2011) concluded that the cultivation of microalgae using wastewater could save up to 90% of freshwater. Besides, it could also eliminate the need for up to 94% of nitrogen consumption and close to 100% requirements of other common nutrients such as S, K, and Mg. If these strategies are effectively utilized, it could avoid the use of a large amount of freshwater and fertilizer while enabling the sequestration of atmospheric CO2. Grown in wastewater, microalgae can break down inorganic and organic matters via physical and biological pathways offering an innovative approach to wastewater treatment (Yin et al. 2020). Therefore, to realize the double advantages of microalgae on top of wastewater treatment, significant progress must be made in the current technology to ensure the downstream processing of microalgae can produce biofuel and other valuable biological compounds (Ubando et al. 2021). Overall, the practice of microalgae farming is considered much more sustainable compared to conventional agriculture due to the nonrequirement of arable land and freshwater.
Microalgae-based biofuel production presents several positive socioeconomic and sustainability benefits. Indeed, the value-added byproducts obtained from processing microalgae can make up for the lower capital cost of the overall production. Among these include mineralized carbon and high-value biological compounds. Either one or both of these byproducts can be obtained depending on the selection of microalgae species as the biomass feedstock. In terms of bioactive compounds, they can range from fine chemicals to compounds produced in large quantities such as fats, polyunsaturated fatty acids, oil, natural dyes, sugars, pigments, antioxidants, etc. (Sharma et al. 2019; Tang et al. 2020). These compounds can yield high economic value due to their diverse commercial uses. Hence, microalgae have the potential to contribute to significant breakthroughs in different sectors within biotechnology, such as biofuel production, nutrition, and food supplements, cosmetics, aquaculture, pharmaceuticals, and pollution control (Ranganathan et al. 2022; Suganya et al. 2016). Among the key advantages, the development of this sector can be an important economic driver for both urban and rural communities while contributing toward improving livelihoods and advancing social sustainability (Pachapur et al. 2020). In these instances, local biofuel industries can be supported by various public and private funding that would benefit the local economy of rural communities (El Semary 2020). The continued progress made in the development of microalgae-based biofuel offers potential employment opportunities for those that already have relevant skills in the conventional oil and fossil fuel industry on their transition to a more sustainable sector (Correa et al. 2019). As existing biofuels continue to face increasing obstacles associated with surging food prices and capital constraints, its prospect remains in question, which begs the need for a more sustainable alternative.
The consumption of microalgae-biofuel can contribute to reducing the amount of CO2 emitted into the atmosphere, which is an important advantage to the current exploitation of fossil fuels. Continued research and development of various species of microalgae that are suitable for biofuel production can significantly contribute toward lowering the carbon footprints and more sustainable energy production and consumption in the future. Future advances in technology associated with microalgae farming and harvesting have the potential to lower fossil fuel consumption and overall carbon footprints. All in all, the development of microalgae cultivation and microalgae biomass feedstock for biofuel production have significant advantages to the socio-economic and long-term sustainability of communities around the world.
Bottlenecks, challenges, and prospects in biofuel production progress from microalgae
Bottlenecks and challenges
Over the past decade, there has been a growing interest in the development of third-generation biofuels from microalgae biomass. Research projects on different aspects of microalgae cultivation and biofuel production have been conducted among private enterprises, academics, government agencies, and public research institutions. Nevertheless, it still requires a significant amount of time before reaching widespread commercial adoption of microalgae-based biofuels as the cost of third-generation biofuels is still much higher than conventional alternatives (Chowdhury and Loganathan 2019). Based on recent estimates, the cost of microalgae bio-oil fluctuates in the range of US $300–2600. Another study also has confirmed that the cost of microalgae-derived oil is twice as much as that of petroleum oil. According to Chisti et al. (2007), a liter of microalgae oil is estimated to be around $2.80, not including the cost of distribution, marketing, sale, and other taxes. Among the current literature on microalgae-based biofuel production, there have been a number of Techno-Economic Analysis (TEA) studies examining the practicality of different technological approaches (Vo Hoang Nhat et al. 2018). Current projections of biofuel production cost are either obtained from past research or small-scale experimental studies. More than often, the modeling of these cost estimates has not been supported and validated by actual data, while several input assumptions still need to be taken into account, such as growth rate, nutrient requirement, lipid productivity, and energy consumption (Chia et al. 2018). There are more studies on the cultivation of microalgae in open raceway ponds relative to the photobioreactor method, which costs between 2 to 2.5 times more than the former process (Barry et al. 2016). Based on current cost estimates, prices of microalgae-based biofuel would fluctuate in the range of $0.44/L and $8.76/L (Richardson et al. 2012; CARD 2018). Given these price calculations, microalgae oil is not cost-competitive against other convention fuel options. Hence, it is necessary to drive down the cost of microalgae biofuels through process optimization and advancement in cultivation methods. In addition, further systematic optimization of both production and conversion processes has the potential to drive down these costs in the near future (Chowdhury et al. 2019).
Even though a wide range of research studies have been conducted on biofuel production from microalgae over the last several decades, in reality, only a small number of pilot projects have been tested for real-world applications, while commercialization has yet to achieve any meaningful results (Su et al. 2017). Currently, there has not been an actual case study of continuous microalgae-based biofuel production on an industrial scale. Indeed, several obstacles still face the scaling up of production and product commercialization associated with microalgae-based biofuels, such as high operation and capital investment cost. Up to date, there have been several investigations into microalgae cultivation using an open system and photobioreactors. In one example, Davis et al. (2011) has conducted an overall economic analysis to identify potential cost-saving strategies. In the open system, the authors obtained $8.52/gal for 25 g/m2.day, which yielded a cost of $9.84/gal of biodiesel. On the other hand, the estimated cost for the photobioreactor approach was recorded at $18.10/gal for 1.25 kg/m3.day resulting in the biodiesel cost of $20.53/gal (Davis et al. 2011). Hence, it is time-consuming to find sufficient solutions to resolve potential technical challenges concerning the development of species suitable for sustainable biofuel production within a plausible timeframe. Among these concerns, the structure of the microbial community in microalgae cultures raises the question of the conversion efficiency of biofuel production (Zabed et al. 2020). The main issue facing the cultivation of microalgae is the propagation of biomass in the raceway pond and photobioreactor. Additional pretreatment steps and an effective yeast fermentation process are necessary for the conversion of microalgae-based bioethanol. Further research into the cultivation of engineer-modified species of microalgae with fast-growing properties is still needed to advance existing bioethanol production. In several case studies, the use of automation technology has been cited to significantly improve microalgae yield and biofuel productivity. Furthermore, only selected strains of microalgae containing a high concentration of fatty acids or lipids are deemed suitable for biodiesel production. Hence, it significantly affects the overall production costs (Branco-Vieira et al. 2020). Because of the varying environmental conditions and regeneration of microalgae after successive subcultures, the quantity and quality of lipid that can be obtained can differ significantly. Furthermore, the biggest issues with microalgae biofuel production are the relatively low biomass yield and low level of volatile solid compounds in biomass. In the case of cultivating microalgae by wastewater, several recent studies have presented innovative solutions to improve microalgae biorefinery processes by combining bioenergy production and wastewater treatment (De Bhowmick et al. 2019; Chandra et al. 2019). In this approach, wastewater streams can be utilized as the nutrient source in the cultivation of microalgae (Hemalatha et al. 2019a, 2019b). However, there are still several challenges facing the cultivation of microalgae given the different characteristics of various effluent streams such as origin, pretreatment processes, and nutrient contents resulting in uncontrolled variables in the propagation of microalgae biomass. Even though microalgae can process common nutrients present in wastewater streams, there are still certain nutrients and inhibitors found in wastewater that negatively affect microalgae growth. Furthermore, incompatible carbon to nitrogen ratio could reduce the optimal growth rate leading to lower biomass productivity (Li et al. 2020). Provided the increase in contaminants in microalgae biomass, the additional downstream processing steps would increase the overall production cost. These issues are further tied to other factors such as fertilizer costs, harvesting, moisture extraction, and biomass concentration.
Despite the major advantages offered by the consumption of bioenergy converted from microalgae, the widespread deployment of this clean and sustainable source of energy still faces significant obstacles, such as identifying suitable strains of microalgae, input resource requirement, biomass harvesting, seasonal variability in feedstock supply, and appropriate conversion method. Among these obstacles, the low rate of sunlight distribution negatively affects the growth and biomass yield of microalgae culture. Due to the small size of microalgae cells, the low biomass concentration further raises the cost of microalgae biomass harvest. High moisture content in biomass also poses another major challenge that would require additional energy-intensive drying stages. Compared to traditional farming, the cultivation of microalgae requires significantly higher capital investment in facility construction and maintenance to ensure the proper and suitable growing conditions for microalgae. Concerning the obstacles facing biomass cultivation and the application of lipid and protein extraction methods to produce biofuels, there have been proposals on the development of biorefinery processing that could eliminate the cultivation and harvesting steps within the existing production cycle. In one instance, the investigation into lipid-extracted biomass obtained only a third of the amount of biofuel output (Quinn et al. 2014). Currently, there are several stages involved in the conversion of microalgae-extracted lipids to biodiesel. On top of that, additional use of other chemicals is also necessary, as well as extra refining steps are also taken to ensure the quality of biodiesel output is suitable for operating engines (Stengel and Connan 2015). In another example, dark fermentation for biohydrogen production is a more complex process that requires the removal of CO2 prior to the start of the treatment. This method also yields a lower hydrogen output. To achieve a higher energy density, further processing of the gas output is necessary, including drying and compressing to less than a few thousand PSI. However, the gas compression method is energy-intensive, and such expensed energy cannot be recovered. Besides, many other issues concerning bio-hydrogen production have to mention the fluctuation of production yield that is caused by the metabolic variation among hydrogen-emitting organisms (Kumar et al. 2021a). Besides, the challenge of reducing furfural and 5-hydroxymethylfurfural involved in the pre-processing stage has not yet been sufficiently addressed. The requirement to remove these unwanted toxic contaminants necessitates the use of specific adsorbent materials (Sudhakar et al. 2016).
Important socioeconomic advantages, such as energy security and independence from fossil energy sources, could be realized from the continued development of advanced biofuels production (Gasparatos et al. 2013). There have been instances in which biofuels have received significant funding from public and private sources in several countries, indicating evidence among the supportive regulatory framework and the role of policies in advancing the biofuel industries (Sun et al. 2019). Hence, there are strong reasons to identify the lack of supportive policies and effective funding mechanisms as major obstacles in maintaining the momentum of the biofuel sectors. Furthermore, the inadequate funding also has slowed the transition away from capital-intensive first-generation towards second and third-generation biofuels (Mathimani and Mallick 2018). This is a major obstacle that hinders the progress toward long-term energy security and long-term sustainability of the society as long as the reliance on non-renewable, carbon-emitting fossil fuels persists (Balsalobre-Lorente et al. 2018).
Considering the positive benefits offered by this sustainable fuel resource, microalgae are believed to act as an important source of sustainable and carbon–neutral biomass feedstock. Several methods can be used to convert microalgae into third-generation biofuels, including widely used techniques such as transesterification, pyrolysis, anaerobic digestion, hydro-treatment, and fermentation (Adeniyi et al. 2018; Pourkarimi et al. 2019; Solé-Bundó et al. 2019). However, the current slow pace of development has hindered the progress made in advancing the cost-effectiveness of biofuel production from microalgae biomass. Future successes in promoting wider commercial adoption of microalgae-based biofuel production will depend on the effective solutions to these existing challenges (Wang and Yin 2018).
Prospects and perspectives
Compared to current fossil fuel prices, the production cost of microalgae-based biodiesel is significantly higher than conventional fuel options. One of the main factors driving up the production cost of advanced biofuels is the high capital requirement associated with nutrients (e.g., nitrate and potassium) and freshwater supply for the cultivation of microalgae. Studies have shown that these expenses can account for between 20 and 30% of the total production cost of microalgae-based biodiesel (Clarens et al. 2010; Chen et al. 2011). Therefore, the cultivation of microalgae plays an integral part in the biofuel supply chain and biofuel cost. Conventional farming also runs the risk of runoff from fertilizers and pesticides, leading to increased surface water contamination and eutrophication of lakes and streams. These environmental issues should be at the forefront of future development of microalgae-based fuel to avoid undermining the environmental and social sustainability aspects of the overall production. Among current research efforts into this sector, a credible foundation has been established based on a thorough analysis of technological feasibility and production cost optimization that could serve as the initial steps toward more advanced research and development. Particularly, the operation cost should take into account the cost of land, production infrastructure, harvesting equipment, downstream processing, fixed and variable operational expenses (Beal et al. 2012; Daroch et al. 2013; Javed et al. 2019). Continued research should focus on lowering these costs. A potential strategy to reduce this cost is to cultivate microalgae cultures in wastewater that has the potential to be more cost-effective and sustainable on a large production scale (Ahmed et al. 2021; Hena et al. 2021). With the use of wastewater as the growing medium, microalgae can utilize the nutrients available in the waste effluents. Hence, such a process can produce microalgae biomass while treating the wastewater at the same time (Yu et al. 2021b). Furthermore, a higher level of biomass and lipid productivity can be obtained in the case of inorganic wastewater via mixotrophic cultivation. Indeed, carbon is the main source of nutrients that drives the growth and lipid production of microalgae. The use of organic and inorganic carbon can lead to different overall cultivation costs. According to Suali et al. (2012), an innovative approach has been proposed on the use of sweet sorghum as a carbon source for farming microalgae. It is estimated to cost approximately from $0.027 to $0.48 in terms of sweet sorghum cultivation (Gao et al. 2010; Singh and Ahluwalia 2013). Studies have reported the lipid production of up to 73% of microalgae biomass when supplying 25–50 g/L of sweet sorghum in the cultivation of microalgae. Another cheap and highly accessible source of carbon is flue gases (Suali and Sarbatly 2012). Considering the fact that microalgae can reach a 10% threshold of CO2 fixation, continued development to improve these positive characteristics will contribute positively to driving down the cultivation cost and lowering global greenhouse gas emissions (Rawat et al. 2013). In their studies, Mu et al. (2014) investigated different factors involving the production of biofuels from wastewater-grown microalgae, including (1)—open pond versus photobioreactor-based cultivation methods; (2)—multiple biomass conversion techniques; (3)—nutrient supply (Mu et al. 2014). The authors confirmed significant advantages from using wastewater over freshwater in the cultivation of microalgae biomass for biofuel production. Hence, applying the wastewater microalgae cultivation method can potentially lower the production cost of biofuels up to nearly 50% improving their cost-competitiveness with conventional petroleum-based alternatives. Nevertheless, variables such as the nutrient profile of wastewater streams and subsequent biomass processing stages still have a significant influence on the overall efficiency of the production. Due to this reason, the potential to effectively scale up current operations further relies on the accessibility and adequate supply of suitable wastewater streams. Currently, there is still significant room for continued improvements made to the existing production technologies before feasible commercialization.
The fact shows that low-conversion efficiency and large input resource requirements (e.g., land, freshwater) hinder the potential of scaling up biofuel production from conventional sources of biomass. Current advances and potential breakthrough discoveries in genomic research of microalgae hold the key to increasing the outlook of biofuel production from cellulosic biomass (Brar et al. 2021). By employing mutagenesis or transgenesis technique, the cellular structure and mechanics of an organism can be targeted and altered based on the desired output (Gan and Maggs 2017; Spicer and Molnar 2018). With advances made in this innovative research front, new strains with enhanced lipid productivity and concentration can be developed through molecular breeding. Besides, there are also ongoing efforts in discovering new species of microalgae that offer a higher capability for biofuel production, although these newer strains are yet ready for cultivation and production on a commercial scale. Selected species with demonstrated resistance against potential harm caused by viruses, fungi, and microzooplanktonic grazers are suitable for cultivation in wastewater. Besides, advanced genetic engineering methods can be explored in enhancing the photosynthetic efficiency in microalgae domestication. There has been a great deal of research interest looking into the potential of increasing the lipid content through altering the metabolic activities of microalgae and down-regulate phosphoenolpyruvate carboxylase to inhibit the breakdown of free fatty acids in microalgae (Wang et al. 2017; Aratboni et al. 2019). Besides altering the genetic makeup of selected microalgae species, the application of metabolic engineering techniques promises to improve the biofuel conversion efficiency. For example, the use of the carbon partitioning method, along with increasing the inhibition light threshold, has been observed to significantly enhance the growth and lipid productivity of microalgae (Huang et al. 2020; Bamary and Einali 2021). According to Zaslavskaia et al. (2001), photoautotrophic diatom Phaeodactylum tricornutum was detected to sustained growth in complete lack of sunlight after being interserted with glucose transporter genes from Chlorella. However, researchers are cautioned by the high environmental sensitivity of the genetically modified organism when exposed to external factors that could result in unpredictable metabolic pathways, in which environmental stress can prompt metabolic changes in selected species of microalgae that could imbue the biomass with additional metabolite (Singh et al. 2011b). As reported, fifty thousand different species of microalgae have been currently identified and sampled that offers a wealth of potential resources and knowledge base for continued research in postgenomic technologies concerning microalgae cultivation for biofuel production (Elisabeth et al. 2021). Continued developments in this research direction promise advancement in several different aspects related to the cultivation of microalgae including hydrocarbon productivity and storage in microalgae biomass, fast growth rate, improved metabolic activities, and enhanced nutrient conversion efficiency via photosynthesis (Khan et al. 2017). Future research should focus on examining the effectiveness of cultivating genetically modified strains of microalgae in an actual culture environment along with advanced methods of metabolic modifications of microalgae. Furthermore, further research is still required to improve the potential to scale up existing operations and successfully implement industrial modules in the cultivation of relevant species of microalgae. Besides, there are also significant obstacles facing the harvesting of microalgae biomass. Currently, the commonly used technique, namely self-flocculation or bio-flocculation, has demonstrated its effectiveness while being fairly affordable (Li et al. 2021; Ray et al. 2021). Given its maturity in the practice of wastewater treatment, its application in microalgae cultivation has yet to be fully explored. Potential areas of research can be of interest when examining the self-flocculation ability of different microalgae species. Furthermore, the self-flocculation process can be significantly improved upon the enhancement of mixed culture cultivation and modifying existing environmental factors affecting the growth of microalgae, these interesting observations should be further examined.
One of the major goals of the transition toward a bio-based circular economy is to ensure equal and fair access to the energy supply of all people while promoting the long-term sustainability of the planet. Compared to other conventional first and second-generation biofuels, microalgae-based biofuels have a certain important edge in terms of ethical quality because the cultivation of microalgae does not compete for valuable arable land and freshwater resource that can be used for growing food crops. However, the production of biofuels from microalgae still faces moral dilemmas similar to other types of fuels to a certain extent. Thus, in the future, one should take into account several elements such as considering human rights, justice, solidarity, sustainability, and stewardship in attempting to formulate an ethical argument in supporting or discounting the development of new energy sources or specifically microalgae-based biofuels in this case (Oncel 2013; Zhu et al. 2014; Lima 2021). Indeed, the development of an integrated biorefinery is an effective strategy in lowering the cost of microalgae-based biofuel production because the integrated system allows for more efficient operations of the different stages, including breaking down of microalgae biomass, isolation and extraction of bioactive components, and conversion to biofuels. Moreover, improvements in pretreatment processes of integrated biorefinery such as harvesting and drying biomass could result in far greater energy and cost savings. The integrated biorefinery can exploit the active bio components of microalgae besides lipid such as carbohydrates and proteins that can be converted into value-added byproducts, showing that the increased economics gained from utilizing high value-added by-products from the microalgae-based conversion process can potentially help to offer a solution to address labor and income inequality issues while building a stronger resilience in local communities (Atabani et al. 2021; Banu et al. 2020; Goswami et al. 2021). As part of the integrated biorefinery system, the economic value of biomass utilization and conversion is maximized to yield different products such as biofuels, chemicals, and feeds (Stiles et al. 2018; Reno et al. 2020; Morseletto 2020). Nevertheless, the biorefinery process yielding biofuels and chemicals does necessitate the need for large-scale operations to meet the current market demand for these biomaterials. Hence, the biorefinery process should be constructed based on the industrial perspective to ensure the integration of different liquid biofuels output as part of the large-scale operation. To save on operational costs, all major components of microalgae biomass should be taken into account in producing various types of liquid fuel due to their different properties. Recent studies have shown the successive production of different liquid biofuels, namely biodiesel, bioethanol, and bio-oil, from microalgae biomass (Fernández-Acero et al. 2019; Devadas et al. 2021; Bolognesi et al. 2021). It was found that the extraction of lipid from microalgae serves as an important ingredient in the conversion of biodiesel and bio-oil. Upon the extraction of lipids from microalgae biomass, the residues can be subjected to saccharification and bioethanol fermentation processes which take advantage of the available polysaccharides. To maximize the economic values of microalgae biomass, the residual biomass can subsequently be put through pyrolysis to yield bio-oils (Lee et al. 2015). These studies have demonstrated the successful capability of yielding multiple liquid biofuel products from the integrated microalgae biorefinery process. By integrating co-production and decarbonization of electricity as part of the entire process, the obtained biofuel is more comparable to the conventional counterpart in terms of financial feasibility and long-term sustainability (Adeniyi et al. 2018). Additional cost savings can be realized from the ability to procure input resources (e.g., CO2, nutrients, water) from less expensive sources (Reis and Gouveia 2016). However, in the future, new extraction techniques should be further investigated to offer a more efficient way of removing lipid from microalgae biomass, even in the absence of lignin. Besides, advanced techniques should be applied to allow for the extraction to occur under high-moisture conditions can eliminate the need for a high energy-intensive water extraction process. On a global scale, a smooth transition under cooperation and coordination among various stakeholders will ensure the gradual maturation of the sector to avoid any potential market instability. To reduce the chance of market monopolization, R&D results should be made available to the public while the practice of fair trade should be incorporate to uphold the industry’s ethical standards (Tait et al. 2011; Oncel 2013). Moreover, further investments for R&D studies are needed to improve the output yield while also lowering the overall production cost (Fernández et al. 2021). In general, applying microalgae biomass as a feedstock in the biorefinery process has the potential to achieve added benefits from various value-added by-products.
The integration of advanced microalgae biorefinery with wastewater treatment also has positive effects on greenhouse gas reduction through effective sequestration of carbon dioxide. Several studies have also proposed the use of mixed-biomass feedstocks, such as plant-based waste, food discard, agricultural residues, sewage effluents, to sustain the continuous production of the biorefinery (Brilman et al. 2017; Chen et al. 2014; Wang et al. 2019). Besides, the presence of auto-sedimentation traits in selected species of microalgae can eliminate the need for using flocculants and other energy-intensive harvesting techniques. This will improve the recyclability of the used effluents in microalgae cultivation and biofuel production as well as enhance the yield of the biofuel output of the microalgae biomass feedstock. In addition, the economic feasibility of microalgae-based biofuel production cannot be realized without the integration of other available technologies. In the future, the application of microalgae as the anodic feedstock for the case of microbial fuel cells should be further investigated and exploited. Particularly, both the extracted oil content and the carbohydrates and protein-containing in residual microalgae biomass can serve as the anode in the fuel cell system. Particularly, both the extracted oil content and the carbohydrates and protein-containing in residual microalgae biomass can serve as the anode in the fuel cell system (Ndayisenga et al. 2018; Mekuto et al. 2020). According to a close-circuit microbial fuel cell system developed by Kakarla and Min (Kakarla and Min 2019), the CO2 produced from the residual microalgae biomass at the anode is transferred to the cathode chamber where the CO2 can be fixed by the fresh microalgae. In this instance, the microalgae grown in the cathode chamber can be propagated by fixing on the supplied CO2. However, issues related to the practical application and commercialization should be examined through TEA and LCA, despite TEA and LCA can only be conducted on pilot-scale demonstration projects on the use of mixed biomass feedstock in an integrated closed-loop biorefinery with a multi-product recovery scheme (Mishra et al. 2019), resulting in limiting the potential assessment and prediction of issues facing larger scale commercial production. Above the feedstock supply challenge, other obstacles facing large-scale conversion of biomass to biofuels have to mention the ability to recover waste after each stage of the process as well as the impact of biomass harvesting methods on wastewater effluents valorization. To address these challenges, an innovative solution to overcome these current obstacles has been proposed toward improving energy efficiency and sustainability of the microalgae biorefinery process, as shown in Fig. 6. This new approach also emphasizes the capability of reducing both liquid and solid waste streams through recycling and reusing materials as part of the circular economy. Furthermore, the integration of advanced microalgae biorefinery with wastewater treatment also has positive effects on greenhouse gas reduction through effective sequestration of carbon dioxide.
Conclusions
In this paper, the latest update on the current landscape of microalgae-based biofuel research, including the benefits and existing challenges for the multitude of biofuels that can be derived from microalgae such as biodiesel, bioethanol, biohydrogen, biomethane, was comprehensively analyzed. Regarding the benefits of microalgae biofuels, it was indicated that the high ability to fix CO2 in the atmosphere, a significant reduction of environmental pollution as using wastewater as a nutrient in microalgae cultivation, and contribution to sustainable development are considered as the main advantages of microalgae biofuels. However, it was found that the biggest obstacle remains given the costly energy requirements in the cultivation, harvesting, and pre-processing treatment of microalgae feedstock, resulting in a further increase in the overall processing cost. Therefore, possible future research directions should investigate the application of recombinant gene technology to enhance the metabolic pathways aiming to result in the creation of biofuel-grade chemicals. Furthermore, interdisciplinary research should be carried out aiming to improve the productivity of biofuel production. Last but not least, the integration of microalgae biorefinery processing and the economic practicality of microalgae-based biofuel production should be further explored in the context of sustainable development and circular economy.
References
Abo BO, Odey EA, Bakayoko M, Kalakodio L (2019) Microalgae to biofuels production: a review on cultivation, application and renewable energy. Rev Environ Health 34:91–99
Acién FG, Fernández JM, Magán JJ, Molina E (2012) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Adv 30:1344–1353. https://doi.org/10.1016/j.biotechadv.2012.02.005
Adeniyi OM, Azimov U, Burluka A (2018) Algae biofuel: Current status and future applications. Renew Sustain Energy Rev 90:316–335. https://doi.org/10.1016/j.rser.2018.03.067
Adnan MA, Xiong Q, Muraza O, Hossain MM (2020) Gasification of wet microalgae to produce H2-rich syngas and electricity: a thermodynamic study considering exergy analysis. Renew Energy 147:2195–2205
Aghaalipour E, Akbulut A, Güllü G (2020) Carbon dioxide capture with microalgae species in continuous gas-supplied closed cultivation systems. Biochem Eng J 163:107741
Ahmed SF, Mofijur M, Parisa TA, et al (2021) Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 131656
Aissi FZ, El Hadi D, Megateli S, Ketfi S (2021) Statistical optimization of pretreatment of orange processing waste using response surface methodology for bioethanol production. Energy Sources Part A Recover Util Environ Eff 1–15
Akubude VC, Nwaigwe KN, Dintwa E (2019) Production of biodiesel from microalgae via nanocatalyzed transesterification process: a review. Mater Sci Energy Technol 2:216–225
Alami AH, Tawalbeh M, Alasad S et al (2021) Cultivation of Nannochloropsis algae for simultaneous biomass applications and carbon dioxide capture. Energy Sources Part A Recover Util Environ Eff 1:1–12
Aliyu A, Lee JGM, Harvey AP (2021) Microalgae for biofuels: a review of thermochemical conversion processes and associated opportunities and challenges. Bioresour Technol Reports 100694
Alves JLF, da Silva Filho VF, Machado RAF, Marangoni C (2020) Ethanol enrichment from an aqueous stream using an innovative multi-tube falling film distillation column equipped with a biphasic thermosiphon. Process Saf Environ Prot 139:69–75
Anwar M, Lou S, Chen L et al (2019) Recent advancement and strategy on bio-hydrogen production from photosynthetic microalgae. Bioresour Technol 292:121972
Aramkitphotha S, Tanatavikorn H, Yenyuak C, Vitidsant T (2019) Low sulfur fuel oil from blends of microalgae pyrolysis oil and used lubricating oil: properties and economic evaluation. Sustain Energy Technol Assessments 31:339–346. https://doi.org/10.1016/j.seta.2018.12.019
Aratboni HA, Rafiei N, Garcia-Granados R et al (2019) Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Fact 18:1–17
Arun J, Gopinath KP, SundarRajan P et al (2020a) Hydrothermal liquefaction of Scenedesmus obliquus using a novel catalyst derived from clam shells: Solid residue as catalyst for hydrogen production. Bioresour Technol 310:123443. https://doi.org/10.1016/j.biortech.2020.123443
Arun J, Gopinath KP, SundarRajan P et al (2020b) A conceptual review on microalgae biorefinery through thermochemical and biological pathways: bio-circular approach on carbon capture and wastewater treatment. Bioresour Technol Reports 11:100477. https://doi.org/10.1016/j.biteb.2020.100477
Arun J, Gopinath KP, SundarRajan P et al (2020c) Hydrothermal liquefaction and pyrolysis of Amphiroa fragilissima biomass: Comparative study on oxygen content and storage stability parameters of bio-oil. Bioresour Technol Rep 11:100465. https://doi.org/10.1016/j.biteb.2020.100465
Arvindnarayan S, Prabhu KKS, Shobana S et al (2017a) Upgrading of micro algal derived bio-fuels in thermochemical liquefaction path and its perspectives: a review. Int Biodeterior Biodegrad 119:260–272
Arvindnarayan S, Sivagnana Prabhu KK, Shobana S et al (2017b) Potential assessment of micro algal lipids: A renewable source of energy. J Energy Inst 90:431–440. https://doi.org/10.1016/j.joei.2016.03.006
Atabani AE, Tyagi VK, Fongaro G, et al (2021) Integrated biorefineries, circular bio-economy, and valorization of organic waste streams with respect to bio-products. 1
Azadi P, Brownbridge GPE, Mosbach S et al (2014) Production of biorenewable hydrogen and syngas via algae gasification: a sensitivity analysis. Energy Procedia 61:2767–2770. https://doi.org/10.1016/j.egypro.2014.12.302
Bagnoud-Velásquez M, Brandenberger M, Vogel F, Ludwig C (2014) Continuous catalytic hydrothermal gasification of algal biomass and case study on toxicity of aluminum as a step toward effluents recycling. Catal Today 223:35–43. https://doi.org/10.1016/j.cattod.2013.12.001
Baicha Z, Salar-García MJ, Ortiz-Martínez VM et al (2016) A critical review on microalgae as an alternative source for bioenergy production: a promising low cost substrate for microbial fuel cells. Fuel Process Technol 154:104–116. https://doi.org/10.1016/j.fuproc.2016.08.017
Balsalobre-Lorente D, Shahbaz M, Roubaud D, Farhani S (2018) How economic growth, renewable electricity and natural resources contribute to CO2 emissions? Energy Policy 113:356–367. https://doi.org/10.1016/j.enpol.2017.10.050
Bamary Z, Einali A (2021) Changes in carbon partitioning and pattern of antioxidant enzyme activity induced by arginine treatment in the green microalga dunaliella salina under long-term salinity. Microb Ecol 1:1–15
Ban S, Lin W, Luo Z, Luo J (2019) Improving hydrogen production of Chlamydomonas reinhardtii by reducing chlorophyll content via atmospheric and room temperature plasma. Bioresour Technol 275:425–429. https://doi.org/10.1016/j.biortech.2018.12.062
Banu JR, Kavitha S, Gunasekaran M, Kumar G (2020) Microalgae based biorefinery promoting circular bioeconomy-techno economic and life-cycle analysis. Bioresour Technol 302:1222
Barry A, Wolfe A, English C, et al (2016) 2016 National algal biofuels technology review
Batista AP, Ambrosano L, Graça S et al (2015) Combining urban wastewater treatment with biohydrogen production – An integrated microalgae-based approach. Bioresour Technol 184:230–235. https://doi.org/10.1016/j.biortech.2014.10.064
Beal CM, Hebner RE, Webber ME et al (2012) Comprehensive evaluation of algal biofuel production: experimental and target results. Energies 5:1943–1981. https://doi.org/10.3390/en5061943
Bellido C, Loureiro Pinto M, Coca M et al (2014) Acetone–butanol–ethanol (ABE) production by Clostridium beijerinckii from wheat straw hydrolysates: efficient use of penta and hexa carbohydrates. Bioresour Technol 167:198–205. https://doi.org/10.1016/j.biortech.2014.06.020
Beneroso D, Bermúdez JM, Arenillas A, Menéndez JA (2013) Microwave pyrolysis of microalgae for high syngas production. Bioresour Technol 144:240–246. https://doi.org/10.1016/j.biortech.2013.06.102
Bertsch P, Böcker L, Mathys A, Fischer P (2021) Proteins from microalgae for the stabilization of fluid interfaces, emulsions, and foams. Trends Food Sci Technol 108:326–342
Bhushan S, Kalra A, Simsek H et al (2020) Current trends and prospects in microalgae-based bioenergy production. J Environ Chem Eng 8:104025
Bibi F, Yasmin H, Jamal A, et al (2021) Deciphering role of technical bioprocess parameters for bioethanol production using microalgae. Saudi J Biol Sci
Bird MI, Wurster CM, de Paula Silva PH et al (2011) Algal biochar—production and properties. Bioresour Technol 102:1886–1891. https://doi.org/10.1016/j.biortech.2010.07.106
Biswas B, Kumar A, Fernandes AC et al (2020) Solid base catalytic hydrothermal liquefaction of macroalgae: effects of process parameter on product yield and characterization. Bioresour Technol 307:123232. https://doi.org/10.1016/j.biortech.2020.123232
Bolognesi S, Bernardi G, Callegari A et al (2021) Biochar production from sewage sludge and microalgae mixtures: properties, sustainability and possible role in circular economy. Biomass Convers Biorefinery 11:289–299
Borges FC, Xie Q, Min M et al (2014) Fast microwave-assisted pyrolysis of microalgae using microwave absorbent and HZSM-5 catalyst. Bioresour Technol 166:518–526
Branco-Vieira M, Mata TM, Martins AA et al (2020) Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep 6:325–332
Brar A, Kumar M, Soni T, et al (2021) Insights into the genetic and metabolic engineering approaches to enhance the competence of microalgae as biofuel resource: a review. Bioresour Technol 125597
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577. https://doi.org/10.1016/j.rser.2009.10.009
Brilman DWF, Drabik N, Wądrzyk M (2017) Hydrothermal co-liquefaction of microalgae, wood, and sugar beet pulp. Biomass Convers Biorefinery 7:445–454. https://doi.org/10.1007/s13399-017-0241-2
Brindhadevi K, Mathimani T, Rene ER et al (2021) Impact of cultivation conditions on the biomass and lipid in microalgae with an emphasis on biodiesel. Fuel 284:119058
Brown TM, Duan P, Savage PE (2010) Hydrothermal Liquefaction and Gasification of Nannochloropsis sp. Energy Fuels 24:3639–3646. https://doi.org/10.1021/ef100203u
Bwapwa JK, Anandraj A, Trois C (2017) Possibilities for conversion of microalgae oil into aviation fuel: A review. Renew Sustain Energy Rev 80:1345–1354. https://doi.org/10.1016/j.rser.2017.05.224
Cai W, Lai K, Liu C et al (2019) Promoting sustainability of manufacturing industry through the lean energy-saving and emission-reduction strategy. Sci Total Environ 665:23–32. https://doi.org/10.1016/j.scitotenv.2019.02.069
Cantrell KB, Ducey T, Ro KS, Hunt PG (2008) Livestock waste-to-bioenergy generation opportunities. Bioresour Technol 99:7941–7953. https://doi.org/10.1016/j.biortech.2008.02.061
Caporgno MP, Olkiewicz M, Torras C et al (2016) Effect of pre-treatments on the production of biofuels from Phaeodactylum tricornutum. J Environ Manag 177:240–246
CARD (2018) Prices for Ethanol, Corn, and Natural Gas
Chandra R, Iqbal HMN, Vishal G et al (2019) Algal biorefinery: a sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour Technol 278:346–359. https://doi.org/10.1016/j.biortech.2019.01.104
Chen C-Y, Yeh K-L, Aisyah R et al (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81. https://doi.org/10.1016/j.biortech.2010.06.159
Chen H, Qiu T, Rong J et al (2015) Microalgal biofuel revisited: an informatics-based analysis of developments to date and future prospects. Appl Energy 155:585–598. https://doi.org/10.1016/j.apenergy.2015.06.055
Chen W-H, Chong CT, Thomas S et al (2021) Impacts of COVID-19 pandemic on the global energy system and the shift progress to renewable energy: Opportunities, challenges, and policy implications. Energy Policy 154:112322
Chen W-H, Nižetić S, Sirohi R et al (2022) Liquid hot water as sustainable biomass pretreatment technique for bioenergy production: a review. Bioresour Technol 344:1207. https://doi.org/10.1016/j.biortech.2021.126207
Chen W-T, Zhang Y, Zhang J et al (2014) Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crude oil. Appl Energy 128:209–216. https://doi.org/10.1016/j.apenergy.2014.04.068
Cheng H-H, Whang L-M, Chan K-C et al (2015a) Biological butanol production from microalgae-based biodiesel residues by Clostridium acetobutylicum. Bioresour Technol 184:379–385
Cheng J, Guo H, Qiu Y et al (2020) Switchable solvent N, N, N′, N′-tetraethyl-1, 3-propanediamine was dissociated into cationic surfactant to promote cell disruption and lipid extraction from wet microalgae for biodiesel production. Bioresour Technol 312:123607. https://doi.org/10.1016/j.biortech.2020.123607
Cheng J, Yang Z, Huang Y et al (2015b) Improving growth rate of microalgae in a 1191 m2 raceway pond to fix CO2 from flue gas in a coal-fired power plant. Bioresour Technol 190:235–241
Cheng Y, Zhou W, Gao C et al (2009) Biodiesel production from Jerusalem artichoke (Helianthus Tuberosus L. ) tuber by heterotrophic microalgae Chlorella protothecoides. J Chem Technol Biotechnol 84:777–781. https://doi.org/10.1002/jctb.2111
Chia SR, Ong HC, Chew KW et al (2018) Sustainable approaches for algae utilisation in bioenergy production. Renew Energy 129:838–852. https://doi.org/10.1016/j.renene.2017.04.001
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Chiu S-Y, Kao C-Y, Chen C-H et al (2008) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396. https://doi.org/10.1016/j.biortech.2007.08.013
Choi SP, Nguyen MT, Sim SJ (2010) Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour Technol 101:5330–5336. https://doi.org/10.1016/j.biortech.2010.02.026
Choudhary P, Malik A, Pant KK (2017) Mass-scale algal biomass production using algal biofilm reactor and conversion to energy and chemical precursors by hydropyrolysis. ACS Sustain Chem Eng 5:4234–4242. https://doi.org/10.1021/acssuschemeng.7b00233
Chowdhury H, Loganathan B (2019) Third-generation biofuels from microalgae: a review. Curr Opin Green Sustain Chem 20:39–44
Chowdhury H, Loganathan B, Mustary I, et al (2019) Algae for biofuels: the third generation of feedstock. In: Second and third generation of feedstocks. Elsevier, pp 323–344
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44:1813–1819. https://doi.org/10.1021/es902838n
Clark JH, Deswarte F (2014) Introduction to chemicals from biomass. Wiley
Constantino A, Rodrigues B, Leon R et al (2021) Alternative chemo-enzymatic hydrolysis strategy applied to different microalgae species for bioethanol production. Algal Res 56:102329
Correa DF, Beyer HL, Possingham HP et al (2019) Global mapping of cost-effective microalgal biofuel production areas with minimal environmental impact. GCB Bioenergy 11:914–929. https://doi.org/10.1111/gcbb.12619
Culaba AB, Ubando AT, Ching PML et al (2020) Biofuel from microalgae: sustainable pathways. Sustainability 12:8009. https://doi.org/10.3390/su12198009
Cunha C, Silva L, Paulo J et al (2020) Microalgal-based biopolymer for nano-and microplastic removal: a possible biosolution for wastewater treatment. Environ Pollut 263:114385
Daroch M, Geng S, Wang G (2013) Recent advances in liquid biofuel production from algal feedstocks. Appl Energy 102:1371–1381. https://doi.org/10.1016/j.apenergy.2012.07.031
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531. https://doi.org/10.1016/j.apenergy.2011.04.018
De Bhowmick G, Sarmah AK, Sen R (2019) Zero-waste algal biorefinery for bioenergy and biochar: a green leap towards achieving energy and environmental sustainability. Sci Total Environ 650:2467–2482. https://doi.org/10.1016/j.scitotenv.2018.10.002
de Farias Silva CE, Bertucco A (2016) Bioethanol from microalgae and cyanobacteria: a review and technological outlook. Process Biochem 51:1833–1842. https://doi.org/10.1016/j.procbio.2016.02.016
De Morais MG, Costa JAV (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445
Deknock A, De Troyer N, Houbraken M et al (2019) Distribution of agricultural pesticides in the freshwater environment of the Guayas river basin (Ecuador). Sci Total Environ 646:996–1008
Derakhshandeh M, Atici T, Tezcan Un U (2021) Evaluation of wild-type microalgae species biomass as carbon dioxide sink and renewable energy resource. Waste Biomass Valorization 12:105–121
Devadas VV, Khoo KS, Chia WY et al (2021) Algae biopolymer towards sustainable circular economy. Bioresour Technol 1:124702
Díaz-Rey MR, Cortés-Reyes M, Herrera C et al (2015) Hydrogen-rich gas production from algae-biomass by low temperature catalytic gasification. Catal Today 257:177–184. https://doi.org/10.1016/j.cattod.2014.04.035
Dickinson S, Mientus M, Frey D et al (2017) A review of biodiesel production from microalgae. Clean Technol Environ Policy 19:637–668
Ding GT, Yasin NHM, Takriff MS et al (2020) Phycoremediation of palm oil mill effluent (POME) and CO2 fixation by locally isolated microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7. J Water Process Eng 35:101202
Draaisma RB, Wijffels RH, Slegers P et al (2013) Food commodities from microalgae. Curr Opin Biotechnol 24:169–177. https://doi.org/10.1016/j.copbio.2012.09.012
Dragone G, Fernandes BD, Abreu AP et al (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335. https://doi.org/10.1016/j.apenergy.2011.03.012
Efremenko EN, Nikolskaya AB, Lyagin IV et al (2012) Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour Technol 114:342–348
El-Mekkawi SA, Abdo SM, Samhan FA, Ali GH (2019) Optimization of some fermentation conditions for bioethanol production from microalgae using response surface method. Bull Natl Res Cent 43:1–8
El Semary NAH (2020) Algae and Fishes: Benefits and Hazards. Climate change impacts on agriculture and food security in Egypt. Springer, Cham, pp 465–479
Elisabeth B, Rayen F, Behnam T (2021) Microalgae culture quality indicators: a review. Crit Rev Biotechnol 41:457–473
Ellis JT, Hengge NN, Sims RC, Miller CD (2012) Acetone, butanol, and ethanol production from wastewater algae. Bioresour Technol 111:491–495. https://doi.org/10.1016/j.biortech.2012.02.002
Eroglu E, Melis A (2011) Photobiological hydrogen production: recent advances and state of the art. Bioresour Technol 102:8403–8413. https://doi.org/10.1016/j.biortech.2011.03.026
Eroglu E, Melis A (2016) Microalgal hydrogen production research. Int J Hydrogen Energy 41:12772–12798. https://doi.org/10.1016/j.ijhydene.2016.05.115
Fernández-Acero FJ, Amil-Ruiz F, Durán-Peña MJ et al (2019) Valorisation of the microalgae Nannochloropsis gaditana biomass by proteomic approach in the context of circular economy. J Proteomics 193:239–242
Fernández FGA, Reis A, Wijffels RH et al (2021) The role of microalgae in the bioeconomy. N Biotechnol 61:99–107
Figueroa-Torres GM, Mahmood WMAW, Pittman JK, Theodoropoulos C (2020) Microalgal biomass as a biorefinery platform for biobutanol and biodiesel production. Biochem Eng J 153:107396
Gan SY, Maggs CA (2017) Random mutagenesis and precise gene editing technologies: applications in algal crop improvement and functional genomics. Eur J Phycol 52:466–481
Gao C, Zhai Y, Ding Y, Wu Q (2010) Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl Energy 87:756–761. https://doi.org/10.1016/j.apenergy.2009.09.006
Garoma T, Nguyen D (2016) Anaerobic Co-Digestion of Microalgae Scenedesmus sp. and TWAS for Biomethane Production. Water Environ Res 88:13–20
Gasparatos A, Stromberg P, Takeuchi K (2013) Sustainability impacts of first-generation biofuels. Anim Front 3:12–26. https://doi.org/10.2527/af.2013-0011
Gholkar P, Shastri Y, Tanksale A (2021) Renewable hydrogen and methane production from microalgae: A techno-economic and life cycle assessment study. J Clean Prod 279:123726
Gholkar P, Shastri Y, Tanksale A (2019) Catalytic reactive flash volatilisation of microalgae to produce hydrogen or methane-rich syngas. Appl Catal B Environ 251:326–334
Ghosh S, Banerjee S, Das D (2017) Process intensification of biodiesel production from Chlorella sp. MJ 11/11 by single step transesterification. Algal Res 27:12–20. https://doi.org/10.1016/j.algal.2017.08.021
Goh BHH, Ong HC, Cheah MY et al (2019) Sustainability of direct biodiesel synthesis from microalgae biomass: a critical review. Renew Sustain Energy Rev 107:59–74. https://doi.org/10.1016/j.rser.2019.02.012
Gonzalez-Fernandez C, Muñoz R (2017) Microalgae-based biofuels and bioproducts. United Kingdom Woodhead Publ
Goswami RK, Mehariya S, Verma P et al (2021) Microalgae-based biorefineries for sustainable resource recovery from wastewater. J Water Process Eng 40:101747
Guccione A, Biondi N, Sampietro G et al (2014) Chlorella for protein and biofuels: from strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol Biofuels 7:84. https://doi.org/10.1186/1754-6834-7-84
Guldhe A, Singh P, Ansari FA et al (2017) Biodiesel synthesis from microalgal lipids using tungstated zirconia as a heterogeneous acid catalyst and its comparison with homogeneous acid and enzyme catalysts. Fuel 187:180–188
Guldhe A, Singh P, Renuka N, Bux F (2019) Biodiesel synthesis from wastewater grown microalgal feedstock using enzymatic conversion: a greener approach. Fuel 237:1112–1118
Gumbytė M, Makareviciene V, Skorupskaite V et al (2018) Enzymatic microalgae oil transesterification with ethanol in mineral diesel fuel media. J Renew Sustain Energy 10:13105
Hariskos I, Posten C (2014) Biorefinery of microalgae - opportunities and constraints for different production scenarios. Biotechnol J 9:739–752. https://doi.org/10.1002/biot.201300142
He S, Fan X, Luo S et al (2017) Enhanced the energy outcomes from microalgal biomass by the novel biopretreatment. Energy Convers Manag 135:291–296. https://doi.org/10.1016/j.enconman.2016.12.049
Hemalatha M, Sarkar O, Venkata Mohan S (2019a) Self-sustainable azolla-biorefinery platform for valorization of biobased products with circular-cascading design. Chem Eng J 373:1042–1053. https://doi.org/10.1016/j.cej.2019.04.013
Hemalatha M, Sravan JS, Min B, Venkata Mohan S (2019b) Microalgae-biorefinery with cascading resource recovery design associated to dairy wastewater treatment. Bioresour Technol 284:424–429. https://doi.org/10.1016/j.biortech.2019.03.106
Hena S, Gutierrez L, Croue J-P (2021) Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: a review. J Hazard Mater 403:124041
Ho S-H, Chen C-Y, Lee D-J, Chang J-S (2011) Perspectives on microalgal CO2-emission mitigation systems—a review. Biotechnol Adv 29:189–198
Ho S-H, Zhang C, Tao F, et al (2020) Microalgal torrefaction for solid biofuel production. Trends Biotechnol
Hoang AT, Huang Z, Nižetić S, et al (2021a) Characteristics of hydrogen production from steam gasification of plant-originated lignocellulosic biomass and its prospects in Vietnam. Int J Hydrogen Energy
Hoang AT, Ong HC, Fattah IMR et al (2021b) Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process Technol 223:1097
Hoang AT, Pandey A, Huang Z, et al (2022) Catalyst-based synthesis of 2, 5-dimethylfuran from carbohydrates as a sustainable biofuel production route. ACS Sustain Chem Eng
Hoang AT, Pham VV (2021) 2-Methylfuran (MF) as a potential biofuel: A thorough review on the production pathway from biomass, combustion progress, and application in engines. Renew Sustain Energy Rev 148:111265
Hoang AT, Pham VV, Nguyen XP (2021c) Integrating renewable sources into energy system for smart city as a sagacious strategy towards clean and sustainable process. J Clean Prod 305:127161. https://doi.org/10.1016/j.jclepro.2021.127161
Hoang AT, Tabatabaei M, Aghbashlo M et al (2020) Rice bran oil-based biodiesel as a promising renewable fuel alternative to petrodiesel: a review. Renew Sustain Energy Rev 135:110204. https://doi.org/10.1016/j.rser.2020.110204
Hong W, Chen J, Ding Q et al (2021) Efficient thermochemical liquefaction of microalgae Haematococcus pluvialis for production of high quality biocrude with high selectivity over Fe/montmorillonite catalyst. J Energy Inst 97:73–79
Hong Y, Chen W, Luo X et al (2017) Microwave-enhanced pyrolysis of macroalgae and microalgae for syngas production. Bioresour Technol 237:47–56
Hossain N, Zaini J, Mahlia TMI, Azad AK (2019) Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew Energy 131:617–624. https://doi.org/10.1016/j.renene.2018.07.082
Howlader MS, French WT (2020) Pretreatment and lipid extraction from wet microalgae: challenges, potential, and application for industrial-scale application. In: Microalgae biotechnology for food, health and high value products. Springer, pp 469–483
Hu S, Barati B, Odey EA et al (2020) Experimental study and economic feasibility analysis on the production of bio-oil by catalytic cracking of three kinds of microalgae. J Anal Appl Pyrolysis 149:104835
Huang B, Mimouni V, Lukomska E et al (2020) Carbon partitioning and lipid remodeling during phosphorus and nitrogen starvation in the marine microalga Diacronema lutheri (Haptophyta). J Phycol 56:908–922
Ibrahim AFM, Dandamudi KPR, Deng S, Lin JYS (2020) Pyrolysis of hydrothermal liquefaction algal biochar for hydrogen production in a membrane reactor. Fuel 265:116935
Javed F, Aslam M, Rashid N et al (2019) Microalgae-based biofuels, resource recovery and wastewater treatment: a pathway towards sustainable biorefinery. Fuel 255:115826
Jehlee A, Rodjaroen S, Waewsak J et al (2019) Improvement of biohythane production from Chlorella sp. TISTR 8411 biomass by co-digestion with organic wastes in a two-stage fermentation. Int J Hydrogen Energy 44:17238–17247
Jena U, Das KC (2011) Comparative Evaluation of Thermochemical Liquefaction and Pyrolysis for Bio-Oil Production from Microalgae. Energy Fuels 25:5472–5482. https://doi.org/10.1021/ef201373m
Jiang L, Luo S, Fan X et al (2011) Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2. Appl Energy 88:3336–3341. https://doi.org/10.1016/j.apenergy.2011.03.043
Jin B, Duan P, Zhang C et al (2014) Non-catalytic liquefaction of microalgae in sub-and supercritical acetone. Chem Eng J 254:384–392. https://doi.org/10.1016/j.cej.2014.05.137
Jin X, Gong S, Chen Z et al (2021) Potential microalgal strains for converting flue gas CO2 into biomass. J Appl Phycol 33:47–55
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Kadier A, Kalil MS, Chandrasekhar K et al (2018) Surpassing the current limitations of high purity H2 production in microbial electrolysis cell (MECs): strategies for inhibiting growth of methanogens. Bioelectrochemistry 119:211–219. https://doi.org/10.1016/j.bioelechem.2017.09.014
Kakarla R, Min B (2019) Sustainable electricity generation and ammonium removal by microbial fuel cell with a microalgae assisted cathode at various environmental conditions. Bioresour Technol 284:161–167
Karimi M (2017) Exergy-based optimization of direct conversion of microalgae biomass to biodiesel. J Clean Prod 141:50–55. https://doi.org/10.1016/j.jclepro.2016.09.032
Karthikeyan S, Dharma Prabhakaran T, Prathima A (2018) Environment effect of La2O3 nano-additives on microalgae-biodiesel fueled CRDI engine with conventional diesel. Energy Sources Part A Recover Util Environ Eff 40:179–185
Kavitha S, Schikaran M, Kannah RY et al (2019) Nanoparticle induced biological disintegration: a new phase separated pretreatment strategy on microalgal biomass for profitable biomethane recovery. Bioresour Technol 289:1224
Kazemi Shariat Panahi H, Dehhaghi M, Aghbashlo M et al (2019a) Shifting fuel feedstock from oil wells to sea: Iran outlook and potential for biofuel production from brown macroalgae (ochrophyta; phaeophyceae). Renew Sustain Energy Rev 112:626–642. https://doi.org/10.1016/j.rser.2019.06.023
Kazemi Shariat Panahi H, Tabatabaei M, Aghbashlo M et al (2019b) Recent updates on the production and upgrading of bio-crude oil from microalgae. Bioresour Technol Reports 7:100216. https://doi.org/10.1016/j.biteb.2019.100216
Khan S, Siddique R, Sajjad W et al (2017) Biodiesel production from algae to overcome the energy crisis. Hayati J Biosci 24:163–167
Kholssi R, Ramos PV, Marks EAN et al (2021) 2Biotechnological uses of microalgae: a review on the state of the art and challenges for the circular economy. Biocatal Agric Biotechnol 1:102114
Khoo KS, Chew KW, Yew GY et al (2020) Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresour Technol 304:122996
Kim B-H, Ramanan R, Cho D-H et al (2014) Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenerg 69:95–105. https://doi.org/10.1016/j.biombioe.2014.07.015
Kim B, Im H, Lee JW (2015) In situ transesterification of highly wet microalgae using hydrochloric acid. Bioresour Technol 185:421–425. https://doi.org/10.1016/j.biortech.2015.02.092
Kohansal K, Tavasoli A, Bozorg A (2019) Using a hybrid-like supported catalyst to improve green fuel production through hydrothermal liquefaction of Scenedesmus obliquus microalgae. Bioresour Technol 277:136–147. https://doi.org/10.1016/j.biortech.2018.12.081
Kong Q, Li L, Martinez B et al (2010) Culture of Microalgae Chlamydomonas reinhardtii in Wastewater for Biomass Feedstock Production. Appl Biochem Biotechnol 160:9–18. https://doi.org/10.1007/s12010-009-8670-4
Koyande AK, Show P-L, Guo R et al (2019) Bio-processing of algal bio-refinery: a review on current advances and future perspectives. Bioengineered 10:574–592
Kumar BR, Mathimani T, Sudhakar MP et al (2021a) A state of the art review on the cultivation of algae for energy and other valuable products: application, challenges, and opportunities. Renew Sustain Energy Rev 138:1149
Kumar G, Zhen G, Sivagurunathan P et al (2016) Biogenic H2 production from mixed microalgae biomass: impact of pH control and methanogenic inhibitor (BESA) addition. Biofuel Res J 3:470
Kumar K, Dasgupta CN, Nayak B et al (2011) Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour Technol 102:4945–4953. https://doi.org/10.1016/j.biortech.2011.01.054
Kumar M, Sun Y, Rathour R et al (2020) Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci Total Environ 716:137116. https://doi.org/10.1016/j.scitotenv.2020.137116
Kumar V, Sharma N, Jaiswal KK et al (2021b) Microalgae with a truncated light-harvesting antenna to maximize photosynthetic efficiency and biomass productivity: Recent advances and current challenges. Process Biochem 104:83–91
Kusmayadi A, Leong YK, Yen H-W et al (2021) Microalgae as sustainable food and feed sources for animals and humans–Biotechnological and environmental aspects. Chemosphere 271:129800
Lakshmikandan M, Murugesan AG, Wang S et al (2020) Sustainable biomass production under CO2 conditions and effective wet microalgae lipid extraction for biodiesel production. J Clean Prod 247:119398
Lee OK, Seong DH, Lee CG, Lee EY (2015) Sustainable production of liquid biofuels from renewable microalgae biomass. J Ind Eng Chem 29:24–31. https://doi.org/10.1016/j.jiec.2015.04.016
Li H, Liu Z, Zhang Y et al (2014) Conversion efficiency and oil quality of low-lipid high-protein and high-lipid low-protein microalgae via hydrothermal liquefaction. Bioresour Technol 154:322–329. https://doi.org/10.1016/j.biortech.2013.12.074
Li S, Ji L, Chen C et al (2020) Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour Technol 309:123362
Li T, Hu J, Zhu L (2021) Self-flocculation as an efficient method to harvest microalgae: a mini-review. Water 13:2585
Li Y, Horsman M, Wu N et al (2008) Biofuels from microalgae. Biotechnol Prog. https://doi.org/10.1021/bp070371k
Lima MGB (2021) The politics of bioeconomy and sustainability: lessons from biofuel governance, policies and production strategies in the emerging world. Springer
Lin R, Deng C, Ding L et al (2019) Improving gaseous biofuel production from seaweed Saccharina latissima: the effect of hydrothermal pretreatment on energy efficiency. Energy Convers Manag 196:1385–1394
Liu B, Wang Z, Feng L (2021) Effects of reaction parameter on catalytic hydrothermal liquefaction of microalgae into hydrocarbon rich bio-oil. J Energy Inst 94:22–28
Liu C-H, Chang C-Y, Liao Q et al (2013) Biohydrogen production by a novel integration of dark fermentation and mixotrophic microalgae cultivation. Int J Hydrogen Energy 38:15807–15814. https://doi.org/10.1016/j.ijhydene.2013.05.104
Liu G, Liao Y, Wu Y et al (2017) Characteristics of microalgae gasification through chemical looping in the presence of steam. Int J Hydrogen Energy 42:22730–22742. https://doi.org/10.1016/j.ijhydene.2017.07.173
Liu G, Liao Y, Wu Y, Ma X (2019a) Evaluation of Sr-substituted Ca2Fe2O5 as oxygen carrier in microalgae chemical looping gasification. Fuel Process Technol 191:93–103. https://doi.org/10.1016/j.fuproc.2019.03.019
Liu H, Chen Y, Yang H et al (2019b) Hydrothermal carbonization of natural microalgae containing a high ash content. Fuel 249:441–448. https://doi.org/10.1016/j.fuel.2019.03.004
Liu H, Chen Y, Yang H et al (2020a) Conversion of high-ash microalgae through hydrothermal liquefaction. Sustain Energy Fuels 4:2782–2791. https://doi.org/10.1039/C9SE01114E
Liu H, Zhang Z, Zhang H et al (2020b) Evaluation of hydrogen yield potential from Chlorella by photo-fermentation under diverse substrate concentration and enzyme loading. Bioresour Technol 303:1256
López-González D, Fernandez-Lopez M, Valverde JL, Sanchez-Silva L (2014) Comparison of the steam gasification performance of three species of microalgae by thermogravimetric–mass spectrometric analysis. Fuel 134:1–10. https://doi.org/10.1016/j.fuel.2014.05.051
Lozano P, Bernal JM, Gómez C et al (2020) Green biocatalytic synthesis of biodiesel from microalgae in one-pot systems based on sponge-like ionic liquids. Catal Today 346:87–92
Lu D, Zhang XJ (2016) Biogas production from anaerobic codigestion of microalgae and septic sludge. J Environ Eng 142:4016049
Lu Z, Loftus S, Sha J et al (2020) Water reuse for sustainable microalgae cultivation: current knowledge and future directions. Resour Conserv Recycl 161:104975
Luiza Astolfi A, Rempel A, Cavanhi VAF et al (2020) Simultaneous saccharification and fermentation of Spirulina sp and corn starch for the production of bioethanol and obtaining biopeptides with high antioxidant activity. Bioresour Technol 301:1298. https://doi.org/10.1016/j.biortech.2019.122698
Ly HV, Choi JH, Woo HC et al (2019) Upgrading bio-oil by catalytic fast pyrolysis of acid-washed Saccharina japonica alga in a fluidized-bed reactor. Renew Energy 133:11–22. https://doi.org/10.1016/j.renene.2018.09.103
Ly HV, Kim S-S, Choi JH et al (2016) Fast pyrolysis of Saccharina japonica alga in a fixed-bed reactor for bio-oil production. Energy Convers Manag 122:526–534
Ma C, Geng J, Zhang D, Ning X (2020) Non-catalytic and catalytic pyrolysis of Ulva prolifera macroalgae for production of quality bio-oil. J Energy Inst 93:303–311. https://doi.org/10.1016/j.joei.2019.03.001
Ma Y, Liu S, Wang Y et al (2019) Direct biodiesel production from wet microalgae assisted by radio frequency heating. Fuel 256:115994. https://doi.org/10.1016/j.fuel.2019.115994
Mahdy A, Ballesteros M, González-Fernández C (2016a) Enzymatic pretreatment of Chlorella vulgaris for biogas production: influence of urban wastewater as a sole nutrient source on macromolecular profile and biocatalyst efficiency. Bioresour Technol 199:319–325
Mahdy A, Mendez L, Tomás-Pejó E et al (2016b) Influence of enzymatic hydrolysis on the biochemical methane potential of Chlorella vulgaris and Scenedesmus sp. J Chem Technol Biotechnol 91:1299–1305
Makareviciene V, Gumbyte M, Skorupskaite V, Sendzikiene E (2017) Biodiesel fuel production by enzymatic microalgae oil transesterification with ethanol. J Renew Sustain Energy 9:23101
Malekghasemi S, Kariminia H-R, Plechkova NK, Ward VCA (2021) Direct transesterification of wet microalgae to biodiesel using phosphonium carboxylate ionic liquid catalysts. Biomass Bioenerg 150:106126
Mandik YI, Cheirsilp B, Srinuanpan S et al (2020) Zero-waste biorefinery of oleaginous microalgae as promising sources of biofuels and biochemicals through direct transesterification and acid hydrolysis. Process Biochem 95:214–222. https://doi.org/10.1016/j.procbio.2020.02.011
Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: a review. Appl Energy 88:3389–3401. https://doi.org/10.1016/j.apenergy.2010.12.042
Mathews JA (2008) Carbon-negative biofuels. Energy Policy 36:940–945. https://doi.org/10.1016/j.enpol.2007.11.029
Mathimani T, Mallick N (2018) A comprehensive review on harvesting of microalgae for biodiesel—key challenges and future directions. Renew Sustain Energy Rev 91:1103–1120. https://doi.org/10.1016/j.rser.2018.04.083
McKendry P (2002) Energy production from biomass (part 3): gasification technologies. Bioresour Technol 83:55–63. https://doi.org/10.1016/S0960-8524(01)00120-1
McNeff CV, McNeff LC, Yan B et al (2008) A continuous catalytic system for biodiesel production. Appl Catal A Gen 343:39–48. https://doi.org/10.1016/j.apcata.2008.03.019
Mekuto L, Olowolafe AVA, Huberts R et al (2020) Microalgae as a biocathode and feedstock in anode chamber for a selfsustainable microbial fuel cell technology: a review. South Afr J Chem Eng 31:7–16
Mimouni V, Couzinet-Mossion A, Ulmann L, Wielgosz-Collin G (2018) Lipids from microalgae. In: Microalgae in health and disease prevention. Elsevier, pp 109–131
Min KJ, Oh DY, Park KY (2021) Pilot-scale cultivation of water-net in secondary effluent using an open pond raceway for nutrient removal and bioethanol production. Chemosphere 277:130129
Mishra S, Roy M, Mohanty K (2019) Microalgal bioenergy production under zero-waste biorefinery approach: recent advances and future perspectives. Bioresour Technol 292:122008
Mishra V, Dubey A, Prajapti SK (2017) Algal biomass pretreatment for improved biofuel production. Algal Biofuels. Springer International Publishing, Cham, pp 259–280
Morseletto P (2020) Targets for a circular economy. Resour Conserv Recycl 153:1053
Moshood TD, Nawanir G, Mahmud F (2021) Microalgae biofuels production: a systematic review on socioeconomic prospects of microalgae biofuels and policy implications. Environ Challenges 100207
MT V, H V, M J, et al (2022) Microalgae-based carbon capture and utilisation: A critical review on current system developments and biomass utilization. Crit Rev Environ Sci Technol
Mu D, Min M, Krohn B et al (2014) Life cycle environmental impacts of wastewater-based Algal biofuels. Environ Sci Technol 48:11696–11704. https://doi.org/10.1021/es5027689
Narula V, Khan MF, Negi A et al (2017) Low temperature optimization of biodiesel production from algal oil using CaO and CaO/Al2O3 as catalyst by the application of response surface methodology. Energy 140:879–884
Nayak SK, Mishra PC (2016) Application of Nagchampa biodiesel and rice husk gas as fuel. Energy Sources Part A Recover Util Environ Eff 38:2024–2030. https://doi.org/10.1080/15567036.2015.1017672
Ndayisenga F, Yu Z, Yu Y et al (2018) Bioelectricity generation using microalgal biomass as electron donor in a bio-anode microbial fuel cell. Bioresour Technol 270:286–293
Nguyen TT, Lam MK, Cheng YW et al (2021) Reaction kinetic and thermodynamics studies for in-situ transesterification of wet microalgae paste to biodiesel. Chem Eng Res Des 169:250–264
Nurdiawati A, Zaini IN, Irhamna AR et al (2019) Novel configuration of supercritical water gasification and chemical looping for highly-efficient hydrogen production from microalgae. Renew Sustain Energy Rev 112:369–381
Olsson J, Forkman T, Gentili FG et al (2018) Anaerobic co-digestion of sludge and microalgae grown in municipal wastewater–a feasibility study. Water Sci Technol 77:682–694
Onay M (2020) The effects of indole-3-acetic acid and hydrogen peroxide on Chlorella zofingiensis CCALA 944 for bio-butanol production. Fuel 273:117795
Onay M (2018) Investigation of biobutanol efficiency of Chlorella sp. cultivated in municipal wastewater. J Geosci Environ Prot 6:40–50
Oncel SS (2013) Microalgae for a macroenergy world. Renew Sustain Energy Rev 26:241–264
Onwudili JA, Lea-Langton AR, Ross AB, Williams PT (2013) Catalytic hydrothermal gasification of algae for hydrogen production: composition of reaction products and potential for nutrient recycling. Bioresour Technol 127:72–80. https://doi.org/10.1016/j.biortech.2012.10.020
Ortigueira J, Alves L, Gouveia L, Moura P (2015) Third generation biohydrogen production by Clostridium butyricum and adapted mixed cultures from Scenedesmus obliquus microalga biomass. Fuel 153:128–134. https://doi.org/10.1016/j.fuel.2015.02.093
Ortiz-Martínez VM, Andreo-Martinez P, Garcia-Martinez N et al (2019) Approach to biodiesel production from microalgae under supercritical conditions by the PRISMA method. Fuel Process Technol 191:211–222
Özçimen D, Inan B (2015) An Overview of Bioethanol Production From Algae. In: Biofuels - Status and Perspective. InTech
Özçimen D, Koçer AT, İnan B, Özer T (2020) Bioethanol production from microalgae. In: Handbook of Microalgae-Based Processes and Products. Elsevier, pp 373–389
Pachapur PK, Pachapur VL, Brar SK, et al (2020) Food Security and Sustainability. In: Sustainability. Wiley, pp 357–374
Papathoti NK, Laemchiab K, Megavath VS, et al (2021) Augmented ethanol production from alkali-assisted hydrothermal pretreated cassava peel waste. Energy Sources, Part A Recover Util Environ Eff 1–11
Park J, Kim B, Chang YK, Lee JW (2017) Wet in situ transesterification of microalgae using ethyl acetate as a co-solvent and reactant. Bioresour Technol 230:8–14. https://doi.org/10.1016/j.biortech.2017.01.027
Park JBK, Craggs RJ, Shilton AN (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol 102:35–42. https://doi.org/10.1016/j.biortech.2010.06.158
Phanduang O, Lunprom S, Salakkam A et al (2019) Improvement in energy recovery from Chlorella sp. biomass by integrated dark-photo biohydrogen production and dark fermentation-anaerobic digestion processes. Int J Hydrogen Energy 44:23899–23911
Phwan CK, Chew KW, Sebayang AH et al (2019) Effects of acids pre-treatment on the microbial fermentation process for bioethanol production from microalgae. Biotechnol Biofuels 12:1–8
Piligaev AV, Sorokina KN, Bryanskaya AV et al (2015) Isolation of prospective microalgal strains with high saturated fatty acid content for biofuel production. Algal Res 12:368–376. https://doi.org/10.1016/j.algal.2015.08.026
Pourkarimi S, Hallajisani A, Alizadehdakhel A, Nouralishahi A (2019) Biofuel production through micro- and macroalgae pyrolysis—a review of pyrolysis methods and process parameters. J Anal Appl Pyrolysis 142:104599. https://doi.org/10.1016/j.jaap.2019.04.015
Qu W, Show PL, Hasunuma T, Ho S-H (2020) Optimizing real swine wastewater treatment efficiency and carbohydrate productivity of newly microalga Chlamydomonas sp QWY37 used for cell-displayed bioethanol production. Bioresour Technol 305:123072
Quinn JC, Hanif A, Sharvelle S, Bradley TH (2014) Microalgae to biofuels: Life cycle impacts of methane production of anaerobically digested lipid extracted algae. Bioresour Technol 171:37–43. https://doi.org/10.1016/j.biortech.2014.08.037
Raheem A, Wan Azlina WAKG, Taufiq Yap YH et al (2015) Thermochemical conversion of microalgal biomass for biofuel production. Renew Sustain Energy Rev 49:990–999. https://doi.org/10.1016/j.rser.2015.04.186
Rajak U, Nashine P, Verma TN (2019) Assessment of diesel engine performance using spirulina microalgae biodiesel. Energy 166:1025–1036
Ramola B, Kumar V, Nanda M et al (2019) Evaluation, comparison of different solvent extraction, cell disruption methods and hydrothermal liquefaction of Oedogonium macroalgae for biofuel production. Biotechnol Reports 22:e00340. https://doi.org/10.1016/j.btre.2019.e00340
Ranganathan P, Pandey AK, Sirohi R, et al (2022) Recent advances in computational fluid dynamics (CFD) modelling of photobioreactors: Design and applications. Bioresour Technol 126920
Rao MS, Singh SP, Singh AK, Sodha MS (2000) Bioenergy conversion studies of the organic fraction of MSW: assessment of ultimate bioenergy production potential of municipal garbage. Appl Energy 66:75–87. https://doi.org/10.1016/S0306-2619(99)00056-2
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2013) Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl Energy 103:444–467. https://doi.org/10.1016/j.apenergy.2012.10.004
Ray A, Banerjee S, Das D (2021) Microalgal bio-flocculation: present scenario and prospects for commercialization. Environ Sci Pollut Res 1–19
Reis A, Gouveia L (2016) Low cost microalgal production for biofuels: A review. Curr Biotechnol 5:266–276. https://doi.org/10.2174/2211550105666160712223225
Rempel A, de Souza SF, Margarites AC et al (2019) Bioethanol from Spirulina platensis biomass and the use of residuals to produce biomethane: an energy efficient approach. Bioresour Technol 288:121588. https://doi.org/10.1016/j.biortech.2019.121588
Reno U, Regaldo L, Gagneten AM (2020) Circular Economy and Agro-Industrial Wastewater: Potential of Microalgae in Bioremediation Processes. Valoris Agro-industrial Residues–Volume I Biol Approaches 111
Richardson JW, Johnson MD, Outlaw JL (2012) Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res 1:93–100. https://doi.org/10.1016/j.algal.2012.04.001
Rocha DN, Barbosa EG, dos Santos RN et al (2020) Improving biofuel production by thermochemical conversion of defatted Scenedesmus obliquus biomass. J Clean Prod 275:124090
Rodas-Zuluaga LI, Castaneda-Hernandez L, Castillo-Vacas EI, et al (2021) Bio-capture and influence of CO2 on the growth rate and biomass composition of the microalgae Botryococcus braunii and Scenedesmus sp. J CO2 Util 43:101371
Ryckebosch E, Bruneel C, Muylaert K, Foubert I (2012) Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technol 24:128–130. https://doi.org/10.1002/lite.201200197
Ryckebosch E, Bruneel C, Termote-Verhalle R et al (2014) Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem 160:393–400. https://doi.org/10.1016/j.foodchem.2014.03.087
Sen TJ, Lee SY, Chew KW et al (2020) A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 11:116–129. https://doi.org/10.1080/21655979.2020.1711626
Saber M, Golzary A, Hosseinpour M et al (2016) Catalytic hydrothermal liquefaction of microalgae using nanocatalyst. Appl Energy 183:566–576. https://doi.org/10.1016/j.apenergy.2016.09.017
Sajjadi B, Chen W-Y, Raman AAA, Ibrahim S (2018) Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew Sustain Energy Rev 97:200–232. https://doi.org/10.1016/j.rser.2018.07.050
Samorì G, Samorì C, Guerrini F, Pistocchi R (2013) Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res 47:791–801. https://doi.org/10.1016/j.watres.2012.11.006
Satputaley SS, Zodpe DB, Deshpande NV (2017) Performance, combustion and emission study on CI engine using microalgae oil and microalgae oil methyl esters. J Energy Inst 90:513–521. https://doi.org/10.1016/j.joei.2016.05.011
SB U, R S, A U, et al (2022) Sustainable microalgal biomass production in food industry wastewater for low-cost biorefinery products: A review. Photochem Rev
Scranton MA, Ostrand JT, Fields FJ, Mayfield SP (2015) Chlamydomonas as a model for biofuels and bio-products production. Plant J 82:523–531. https://doi.org/10.1111/tpj.12780
Serrà A, Artal R, García-Amorós J et al (2020) Circular zero-residue process using microalgae for efficient water decontamination, biofuel production, and carbon dioxide fixation. Chem Eng J 388:124278. https://doi.org/10.1016/j.cej.2020.124278
Shahi T, Beheshti B, Zenouzi A, Almasi M (2020) Bio-oil production from residual biomass of microalgae after lipid extraction: The case of Dunaliella Sp. Biocatal Agric Biotechnol 23:1094
Shakya R, Adhikari S, Mahadevan R et al (2017) Influence of biochemical composition during hydrothermal liquefaction of algae on product yields and fuel properties. Bioresour Technol 243:1112–1120. https://doi.org/10.1016/j.biortech.2017.07.046
Sharma P, Slathia PS, Raina N, Bhagat D (2019) Microbial diversity in freshwater ecosystems and its industrial potential. In: Freshwater Microbiology. Elsevier, pp 341–392
Shin H-Y, Shim S-H, Ryu Y-J et al (2018a) Lipid Extraction from Tetraselmis sp. microalgae for biodiesel production using hexane-based solvent mixtures. Biotechnol Bioprocess Eng 23:16–22. https://doi.org/10.1007/s12257-017-0392-9
Shin YS, Il CH, Choi JW et al (2018b) Multilateral approach on enhancing economic viability of lipid production from microalgae: A review. Bioresour Technol 258:335–344. https://doi.org/10.1016/j.biortech.2018.03.002
Silitonga AS, Masjuki HH, Ong HC et al (2017) Optimization of extraction of lipid from Isochrysis galbana microalgae species for biodiesel synthesis. Energy Sources Part A Recover Util Environ Eff 39:1167–1175
Singh A, Nigam PS, Murphy JD (2011a) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102:10–16. https://doi.org/10.1016/j.biortech.2010.06.032
Singh A, Nigam PS, Murphy JD (2011b) Mechanism and challenges in commercialisation of algal biofuels. Bioresour Technol 102:26–34. https://doi.org/10.1016/j.biortech.2010.06.057
Singh HM, Kothari R, Gupta R, Tyagi VV (2019a) Bio-fixation of flue gas from thermal power plants with algal biomass: Overview and research perspectives. J Environ Manage 245:519–539
Singh J, Gu S (2010) Commercialization potential of microalgae for biofuels production. Renew Sustain Energy Rev 14:2596–2610
Singh R, Bux F, Sharma YC (2020) Optimization of biodiesel synthesis from microalgal (Spirulina platensis) oil by using a novel heterogeneous catalyst, β-strontium silicate (β-Sr2SiO4). Fuel 280:118312
Singh R, Kumar A, Chandra Sharma Y (2019b) Biodiesel production from microalgal oil using barium–calcium–zinc mixed oxide base catalyst: optimization and kinetic studies. Energy Fuels 33:1175–1184. https://doi.org/10.1021/acs.energyfuels.8b03461
Singh R, Kumar A, Sharma YC (2019c) Biodiesel synthesis from microalgae (Anabaena PCC 7120) by using barium titanium oxide (Ba2TiO4) solid base catalyst. Bioresour Technol 287:121357
Singh SP, Singh P (2014) Effect of CO2 concentration on algal growth: a review. Renew Sustain Energy Rev 38:172–179
Singh UB, Ahluwalia AS (2013) Microalgae: a promising tool for carbon sequestration. Mitig Adapt Strateg Glob Chang 18:73–95. https://doi.org/10.1007/s11027-012-9393-3
Sirohi R, Choi H Il, Sim SJ (2022a) Microalgal fuels: Promising energy reserves for the future. Fuel 312:122841
Sirohi R, Lee JS, Yu BS, et al (2021) Sustainable production of polyhydroxybutyrate from autotrophs using CO2 as feedstock: challenges and opportunities. Bioresour Technol 341:125751
Sirohi R, Pandey AK, Ranganathan P, et al (2022b) Design and applications of photobioreactors-A review. Bioresour Technol 126858
Sivagurunathan P, Kumar G, Kobayashi T et al (2018) Co-digestion of untreated macro and microalgal biomass for biohydrogen production: Impact of inoculum augmentation and microbial insights. Int J Hydrogen Energy 43:11484–11492
Sivagurunathan P, Kumar G, Mudhoo A et al (2017) Fermentative hydrogen production using lignocellulose biomass: an overview of pre-treatment methods, inhibitor effects and detoxification experiences. Renew Sustain Energy Rev 77:28–42. https://doi.org/10.1016/j.rser.2017.03.091
Sivarathnakumar S, Jayamuthunagai J, Baskar G et al (2019) Bioethanol production from woody stem Prosopis juliflora using thermo tolerant yeast Kluyveromyces marxianus and its kinetics studies. Bioresour Technol 293:122060. https://doi.org/10.1016/j.biortech.2019.122060
Solé-Bundó M, Passos F, Romero-Güiza MS et al (2019) Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew Sustain Energy Rev 112:471–482
Song C, Hu X, Liu Z et al (2020) Combination of brewery wastewater purification and CO2 fixation with potential value-added ingredients production via different microalgae strains cultivation. J Clean Prod 268:122332
Sotoudehniakarani F, Alayat A, McDonald AG (2019) Characterization and comparison of pyrolysis products from fast pyrolysis of commercial Chlorella vulgaris and cultivated microalgae. J Anal Appl Pyrolysis 139:258–273. https://doi.org/10.1016/j.jaap.2019.02.014
Spicer A, Molnar A (2018) Gene editing of microalgae: scientific progress and regulatory challenges in Europe. Biology (basel) 7:21
Steffen B, Egli F, Pahle M, Schmidt TS (2020) Navigating the Clean Energy Transition in the COVID-19 Crisis. Joule
Stengel DB, Connan S (eds) (2015) Natural products from Marine Algae. Springer
Stiles WAV, Styles D, Chapman SP et al (2018) Using microalgae in the circular economy to valorise anaerobic digestate: challenges and opportunities. Bioresour Technol 267:732–742
Su Y, Song K, Zhang P et al (2017) Progress of microalgae biofuel’s commercialization. Renew Sustain Energy Rev 74:402–411. https://doi.org/10.1016/j.rser.2016.12.078
Suali E, Sarbatly R (2012) Conversion of microalgae to biofuel. Renew Sustain Energy Rev 16:4316–4342. https://doi.org/10.1016/j.rser.2012.03.047
Sudhakar MP, Merlyn R, Arunkumar K, Perumal K (2016) Characterization, pretreatment and saccharification of spent seaweed biomass for bioethanol production using baker’s yeast. Biomass Bioenerg 90:148–154. https://doi.org/10.1016/j.biombioe.2016.03.031
Suganya T, Varman M, Masjuki HH, Renganathan S (2016) Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: a biorefinery approach. Renew Sustain Energy Rev 55:909–941
Sun J, Xiong X, Wang M et al (2019) Microalgae biodiesel production in China: a preliminary economic analysis. Renew Sustain Energy Rev 104:296–306. https://doi.org/10.1016/j.rser.2019.01.021
Sun K, Li Q, Zhang L et al (2020) Impacts of water-organic solvents on polymerization of the sugars and furans in bio-oil. Bioresour Technol Reports 10:100419. https://doi.org/10.1016/j.biteb.2020.100419
Sundar Rajan P, Gopinath KP, Arun J, Grace Pavithra K (2019) Hydrothermal liquefaction of Scenedesmus abundans biomass spent for sorption of petroleum residues from wastewater and studies on recycling of post hydrothermal liquefaction wastewater. Bioresour Technol 283:36–44. https://doi.org/10.1016/j.biortech.2019.03.077
Sung YJ, Lee JS, Yoon HK et al (2021) Outdoor cultivation of microalgae in a coal-fired power plant for conversion of flue gas CO2 into microalgal direct combustion fuels. Syst Microbiol Biomanuf. 1:90–99
Suparmaniam U, Lam MK, Uemura Y et al (2019) Insights into the microalgae cultivation technology and harvesting process for biofuel production: a review. Renew Sustain Energy Rev 115:109361. https://doi.org/10.1016/j.rser.2019.109361
Sydney EB, Sydney ACN, de Carvalho JC, Soccol CR (2019) Potential carbon fixation of industrially important microalgae. In: Biofuels from Algae. Elsevier, pp 67–88
Tait J, Adcock M, Barker GC, et al (2011) Biofuels: ethical issues
Tamil Selvan S, Velramar B, Ramamurthy D et al (2020) Pilot scale wastewater treatment, CO2 sequestration and lipid production using microalga, Neochloris aquatica RDS02. Int J Phytoremediation 22:1462–1479
Tan CH, Show PL, Chang J-S et al (2015) Novel approaches of producing bioenergies from microalgae: a recent review. Biotechnol Adv 33:1219–1227
Tang D, Han W, Li P et al (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076
Tang DYY, Khoo KS, Chew KW, et al (2020) Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour Technol 304:122997
Toro-Trochez JL, Carrillo-Pedraza ES, Bustos-Martínez D et al (2019) Thermogravimetric characterization and pyrolysis of soybean hulls. Bioresour Technol Rep 6:183–189. https://doi.org/10.1016/j.biteb.2019.02.009
Torres S, Acien G, García-Cuadra F, Navia R (2017) Direct transesterification of microalgae biomass and biodiesel refining with vacuum distillation. Algal Res 28:30–38. https://doi.org/10.1016/j.algal.2017.10.001
Trivedi J, Aila M, Bangwal DP et al (2015) Algae based biorefinery—How to make sense? Renew Sustain Energy Rev 47:295–307. https://doi.org/10.1016/j.rser.2015.03.052
Tsai T-Y, Lo Y-C, Dong C-D et al (2020) Biobutanol production from lignocellulosic biomass using immobilized Clostridium acetobutylicum. Appl Energy 277:115531
Ubando AT, Africa ADM, Maniquiz-Redillas MC et al (2021) Microalgal biosorption of heavy metals: a comprehensive bibliometric review. J Hazard Mater 402:123431
Udayan A, Sirohi R, Sreekumar N et al (2022) Mass cultivation and harvesting of microalgal biomass: Current trends and future perspectives. Bioresour Technol 344:126406
Umdu ES, Tuncer M, Seker E (2009) Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour Technol 100:2828–2831. https://doi.org/10.1016/j.biortech.2008.12.027
Ummalyma SB, Sahoo D, Pandey A (2020) Microalgal biorefineries for industrial products. In: Microalgae Cultivation for Biofuels Production. Elsevier, pp 187–195
Vargas-Estrada L, Longoria A, Okoye PU, Sebastian PJ (2021) Energy and nutrients recovery from wastewater cultivated microalgae: Assessment of the impact of wastewater dilution on biogas yield. Bioresour Technol 341:125755
Venderbosch RH (2019) Fast pyrolysis. Thermochem Process biomass Convers into fuels, Chem power 175–206
Verma K, Kumar PK, Krishna SV, Himabindu V (2020) Phycoremediation of sewage-contaminated lake water using microalgae-bacteria co-culture. Water Air Soil Pollut 231:1–16
Veza I, Roslan MF, Muhamad Said MF et al (2020) Cetane index prediction of ABE-diesel blends using empirical and artificial neural network models. Energy Sources Part A Recover Util Environ Eff 1:1–18
Veza I, Said MFM, Latiff ZA (2021) Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 144:105919
Veza I, Said MFM, Latiff ZA (2019) Progress of acetone-butanol-ethanol (ABE) as biofuel in gasoline and diesel engine: a review. Fuel Process Technol 196:106179
Vo Hoang Nhat P, Ngo HH, Guo WS et al (2018) Can algae-based technologies be an affordable green process for biofuel production and wastewater remediation? Bioresour Technol 256:491–501. https://doi.org/10.1016/j.biortech.2018.02.031
Wágner DS, Radovici M, Smets BF et al (2016) Harvesting microalgae using activated sludge can decrease polymer dosing and enhance methane production via co-digestion in a bacterial-microalgal process. Algal Res 20:197–204
Wang C, Chen X, Li H et al (2017) Artificial miRNA inhibition of phosphoenolpyruvate carboxylase increases fatty acid production in a green microalga Chlamydomonas reinhardtii. Biotechnol Biofuels 10:1–11
Wang J, Peng X, Chen X, Ma X (2019) Co-liquefaction of low-lipid microalgae and starch-rich biomass waste: The interaction effect on product distribution and composition. J Anal Appl Pyrolysis 139:250–257. https://doi.org/10.1016/j.jaap.2019.02.013
Wang J, Yin Y (2018) Fermentative hydrogen production using pretreated microalgal biomass as feedstock. Microb Cell Fact 17:22. https://doi.org/10.1186/s12934-018-0871-5
Wang L, Min M, Li Y et al (2010) Cultivation of Green Algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162:1174–1186. https://doi.org/10.1007/s12010-009-8866-7
Wang W, Xu Y, Wang X et al (2018) Hydrothermal liquefaction of microalgae over transition metal supported TiO2 catalyst. Bioresour Technol 250:474–480. https://doi.org/10.1016/j.biortech.2017.11.051
Wang Y, Guo W, Cheng C-L et al (2016) Enhancing bio-butanol production from biomass of Chlorella vulgaris JSC-6 with sequential alkali pretreatment and acid hydrolysis. Bioresour Technol 200:557–564
Wieczorek N, Kucuker MA, Kuchta K (2014) Fermentative hydrogen and methane production from microalgal biomass (Chlorella vulgaris) in a two-stage combined process. Appl Energy 132:108–117. https://doi.org/10.1016/j.apenergy.2014.07.003
Woertz I, Feffer A, Lundquist T, Nelson Y (2009) Algae grown on dairy and municipal wastewater for simultaneous nutrient removal and lipid production for biofuel feedstock. J Environ Eng 135:1115–1122. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000129
Wu S, Song L, Sommerfeld M et al (2017) Optimization of an effective method for the conversion of crude algal lipids into biodiesel. Fuel 197:467–473
Xia A, Jacob A, Tabassum MR et al (2016) Production of hydrogen, ethanol and volatile fatty acids through co-fermentation of macro-and micro-algae. Bioresour Technol 205:118–125
Xia A, Sun C, Fu Q et al (2020) Biofuel production from wet microalgae biomass: comparison of physicochemical properties and extraction performance. Energy 212:118581. https://doi.org/10.1016/j.energy.2020.118581
Xu D, Guo S, Liu L et al (2019a) Water-soluble and -insoluble biocrude production from hydrothermal liquefaction of microalgae with catalyst. Energy Procedia 158:97–102. https://doi.org/10.1016/j.egypro.2019.01.052
Xu D, Savage PE (2017) Effect of temperature, water loading, and Ru/C catalyst on water-insoluble and water-soluble biocrude fractions from hydrothermal liquefaction of algae. Bioresour Technol 239:1–6. https://doi.org/10.1016/j.biortech.2017.04.127
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507. https://doi.org/10.1016/j.jbiotec.2006.05.002
Xu J, Upcraft T, Tang Q et al (2019b) Hydrogen generation performance from Taihu algae and food waste by anaerobic codigestion. Energy Fuels 33:1279–1289
Xu X-L, Chen HH (2020) Exploring the relationships between environmental management and financial sustainability in the energy industry: linear and nonlinear effects. Energy Environ 31:1281–1300. https://doi.org/10.1177/0958305X19882406
Xue Z, Li S, Yu W, et al (2021) Research advancement and commercialization of microalgae edible oil: a review. J Sci Food Agric
Yang C, Li R, Zhang B et al (2019) Pyrolysis of microalgae: a critical review. Fuel Process Technol 186:53–72
Yang J, Xu M, Zhang X et al (2011) Life-cycle analysis on biodiesel production from microalgae: water footprint and nutrients balance. Bioresour Technol 102:159–165. https://doi.org/10.1016/j.biortech.2010.07.017
Yin Z, Zhu L, Li S et al (2020) A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour Technol 301:122804
Yu BS, Sung YJ, Il CH et al (2021a) Concurrent enhancement of CO2 fixation and productivities of omega-3 fatty acids and astaxanthin in Haematococcus pluvialis culture via calcium-mediated homeoviscous adaptation and biomineralization. Bioresour Technol 340:125720
Yu KL, Chen W-H, Sheen H-K et al (2020) Bioethanol production from acid pretreated microalgal hydrolysate using microwave-assisted heating wet torrefaction. Fuel 279:118435
Yu KL, Lee XJ, Ong HC et al (2021b) Adsorptive removal of cationic methylene blue and anionic Congo red dyes using wet-torrefied microalgal biochar: Equilibrium, kinetic and mechanism modeling. Environ Pollut 272:115986
Yuan T, Tahmasebi A, Yu J (2015) Comparative study on pyrolysis of lignocellulosic and algal biomass using a thermogravimetric and a fixed-bed reactor. Bioresour Technol 175:333–341
Zabed HM, Akter S, Yun J et al (2020) Biogas from microalgae: Technologies, challenges and opportunities. Renew Sustain Energy Rev 117:109503
Zaimes GG (2016) Integrated Life Cycle Framework for Evaluating the Sustainability of Emerging Drop-In Replacement Biofuels. University of Pittsburgh
Zamalloa C, Vulsteke E, Albrecht J, Verstraete W (2011) The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour Technol 102:1149–1158. https://doi.org/10.1016/j.biortech.2010.09.017
Zaslavskaia LA, Lippmeier JC, Shih C et al (2001) Trophic conversion of an obligate photoautotrophic organism through metabolic engineering. Science 292:2073–2075. https://doi.org/10.1126/science.160015
Zhang R, Li L, Tong D, Hu C (2016) Microwave-enhanced pyrolysis of natural algae from water blooms. Bioresour Technol 212:311–317. https://doi.org/10.1016/j.biortech.2016.04.053
Zhang S, Liu Z (2021) Advances in the biological fixation of carbon dioxide by microalgae. J Chem Technol Biotechnol 96:1475–1495
Zhao B, Su Y (2014) Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renew Sustain Energy Rev 31:121–132
Zhu LD, Hiltunen E, Antila E et al (2014) Microalgal biofuels: flexible bioenergies for sustainable development. Renew Sustain Energy Rev 30:1035–1046
Zhu Y, Piotrowska P, van Eyk PJ et al (2016) Fluidized Bed Co-gasification of Algae and Wood Pellets: Gas Yields and Bed Agglomeration Analysis. Energy Fuels 30:1800–1809. https://doi.org/10.1021/acs.energyfuels.5b02291
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoang, A.T., Sirohi, R., Pandey, A. et al. Biofuel production from microalgae: challenges and chances. Phytochem Rev 22, 1089–1126 (2023). https://doi.org/10.1007/s11101-022-09819-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-022-09819-y