Abstract
Alkamides are natural products formed by connecting straight-chain, mostly unsaturated, aliphatic acids with various amines by an amide linkage. More than 300 derivatives are known from eight plant families consisting of various combinations of 200 acids with 23 amines. Apart from a few saturated derivatives alkamides with unsaturated acid parts are grouped into compounds with purely olefinic patterns and those with olefinic and acetylenic linkages. Derived from C18 oleic acid the acid parts are modified either by chain elongations to C28 or by oxidative shortenings to C4 acid residues. Substrate and regiospecific desaturases and acetylenases are responsible for their characteristic patterns of unsaturation. Amine parts are derived from various amino acids by decarboxylation. Beside the widespread isobutylamines alkamides with six- and five-membered ring amines and those with phenylalanine derived amines are characteristic for the Asteraceae and Piperaceae while benzylamines are restricted to the Brassicaceae. Within the Asteraceae 2-methylbutylamine distinguishes the tribe Heliantheae from Anthemideae characterized by ring amines. Alkamides with elongated olefinic acid parts are mainly found in Piperaceae and Brassicaceae while acetylenic acid parts are typical for Asteraceae. A wide variety of biological activities ranges from the characteristic pungent/tingling property and high insecticidal toxicity to significant antifungal, antibacterial, antiprotozoal, molluscicidal, cercaricidal, and acaricidal activity. They also act as plant growth-promoting substances. Position and stereochemistry of the double bonds are essential for the different qualities of the pungent taste. Medically alkamides possess anti-inflammatory and analgesic properties and are responsible for immuno-modulatory and cannabinomimetic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, the interest in alkamides comes from the unique form of tingling pungency of some derivatives that is distinct from that produced by capsaicin from chili peppers, Capsicum annuum L., and piperine from black pepper, Piper nigrum L., and is usually accompanied by local anesthesia and salivation. Thus, plants producing these compounds, mainly species of the genera Acmella (Spilanthes), Anacyclus, Echinacea and Heliopsis of the Asteraceae, and Zanthoxylum of the Rutaceae family were used medicinally since ancient times, particularly in cases of toothache. The active material was first obtained in the crude state from the flower heads of Acmella oleracea (L.) R. K. Jansen (published as Spilanthes oleracea Jacquin) by Gerber (1903), who designated it spilanthol (92a). The pungent principle from the roots of Anacyclus pyrethrum (L.) Link, used medicinally under the names pellitory root or Pyrethri radix, was extracted by Gulland and Hopton (1930) and named pellitorine (101a). Moreover, insecticidal activities were reported for all these plant species which were attributed to the accumulation of alkamides (for ref. Greger 1984, 1988; Boonen et al. 2012a; Rios 2012). In Asia the fruits of various Zanthoxylum species, known as “hua jiao” in China or “sansho” in Japan, are widely used as spice because of the distinctive tingling taste caused by alkamides (Yang 2008; Menozzi-Smarrito et al. 2009). This particular sensation also contributes to the use of A. oleracea as vegetable, known as “pará cress” or “jambú” in Brazil. Later, the interest in alkamides was greatly inspired by their anti-inflammatory and immuno-modulatory effects investigated in Echinacea species (Gertsch et al. 2004, 2006; Woelkart and Bauer 2007; Ardjomand-Woelkart and Bauer 2014). In addition, various promising pharmacological activities of the lipophilic fraction of the methanolic extract of Lepidium meyeni Walp. of the Brassicaceae family, known as Peruvian ginseng or “maca”, may at least partly be attributed to the presence of species-specific alkamides and related olefinic fatty acids (Zhao et al. 2005; Wang et al. 2007).
As reviewed previously alkamides are regarded as a distinct class of natural products formed by connecting straight-chain, mostly unsaturated, aliphatic acids with various amines by an amide linkage (Greger 1984, 1988). As derivatives of the straight-chain fatty acid synthesis the acid parts differ biosynthetically from structurally similar but branched carbon chains from amides of the genera Capsicum of the Solanaceae (Curry et al. 1999; Aza-González et al. 2011; Kehie et al. 2015) or Ipomoea and Merremia of the Convolvulaceae family (Tofern et al. 1999). In Piperaceae the alkamides are only known from the genus Piper, where they are frequently accompanied by so-called piperamides, another class of amides, differing by an aromatic ring in the acid moiety (Parmar et al. 1997; Do Nascimento et al. 2012; Dawid et al. 2012). From the various amides of the Rutaceae fatty acid derived alkamides were mostly found in the genus Zanthoxylum, but were also reported for Tetradium daniellii (Reisch et al. 1985) and Pilocarpus trachylophus (Andrade-Neto et al. 1996). The other amides of this family are characterized by different acid parts derived from cinnamic acid (Mester 1983; Kubo et al. 1984; Riemer et al. 1997), cysteine, anthranilic or isovalerianic acid (Hofer et al. 1995; Greger et al. 1996). The biogenetic origin of the different amine parts of the alkamides was not clarified fully to date, but they are most likely derived from amino acids by decarboxylation and, sometimes, by additional transformation processes (Greger 1984, 1988; Minto and Blacklock 2008; Cortez-Espinosa et al. 2011). Other straight-chain fatty acid amides, such as the physiologically active N-acylethanolamines (Kim et al. 2010) or derivatives with N-unsubstituted amino groups (Sittie et al. 1998; Dembitsky et al. 2000) as well as those with more complex amine rests, e.g. in the malyngamides (Ainslie et al. 1985) and conioidines (Chan et al. 1993), are derived from different biosynthetic routes and, thus, are not included in the present survey (Fig. 1).
Following these biosynthetic considerations structures of more than 300 alkamides have been reported consisting of various combinations of 200 acid parts (Tables 1, 2, 3; Figs. 4, 6, 7, 8) with 23 amine parts (a–w) (Fig. 2). Apart from a few derivatives with fully saturated acid parts, mainly isolated from Piper species and L. meyeni, alkamides with unsaturated acids fall into two groups: derivatives with olefinic patterns (Tables 1, 2) and those with olefinic and acetylenic linkages (Table 3). So far 180 alkamides with purely olefinic patterns were isolated from eight plant families, namely the Asteraceae, Piperaceae, Rutaceae, Brassicaceae, Euphorbiaceae, Aristolochiaceae, Menispermaceae, and Poaceae. The majority of the 135 acetylenic alkamides is known from the Asteraceae, where they are accumulated solely in the two tribes Anthemideae and Heliantheae (Greger 1984, 1988; Christensen and Lam 1991; Christensen 1992). Apart from the plant-derived alkamides the C17 acetylenic alkamide callyspongamide A (128c) was isolated from the Red Sea sponge Callyspongia fistularis (Youssef et al. 2003) and an acetylenic C9 amide linked with the amino acid valine from the fungus Poria sinuosa (Cambie et al. 1963).
The wide range of biological activities of alkamides and their distribution has recently been summarized in three reviews, where, however, various classes of amides have been combined which are derived from different biosynthetic pathways (Boonen et al. 2012a; Rios 2012; Rios and Olivo 2014). Although these overviews provide a plethora of interesting data, they do not offer a biologically orientated evaluation of the structural diversity of the straight-chain fatty acid derived alkamides and its chemotaxonomic significance. A simple schematic of the many chemical structures of these amides would greatly help to grasp the biogenetic background of the various differentiation processes. However, their long carbon chains combined with different patterns of double and triple bonds complicate a structural overview of a greater number of derivatives. Here, simple formula depictions are presented to comprehend many derivatives and their possible structural relationships at a glance. Particular attention was drawn to the different carbon chain lengths of the acid residues as well as the characteristic clustering of olefinic and acetylenic linkages, whereas the different amine parts are indicated by small letters only (Fig. 2; Tables 1, 2, 3). This comprehensive overview of more than 300 different structures should serve as a broad-based summary to establish specific biogenetic trends and as a guide for exploiting these compounds for further pharmaceutical development, pest control, or uses in taste and food chemistry.
Structural relationships
Amine parts
The amine parts of the alkamides are most likely derived from various amino acids by decarboxylation (Bohlmann and Zdero 1973; Greger 1984, 1988; Cortez-Espinosa et al. 2011). On the basis of the present overview isobutylamine (a) is shown to be the most widespread amine part, followed by piperidine (b) and pyrrolidine (k), and, less frequently, phenethylamine (c), hydroxyisobutylamine (d), 2-methylbutylamine (g), isopentylamine (m), and benzylamine (t). The remaining amines shown in Fig. 2 are known only in a few, sometimes even only in a single amide: i.e. 37p, 58w, 101h, 101i, and 101s (Tables 1, 2, 3). Biosynthetically, the amino acid valine was regarded as precursor of isobutylamine (a), dihydroxyisobutylamine (v), and dehydroisobutylamine (w). A possible biosynthetic intermediate was found in the Basidiomycete fungus Poria sinuosa Fr., where a nona 2E-en-4,6,8-triynoic acid is linked with valine without decarboxylation (Cambie et al. 1963). The formation of piperideine (e) was suggested to be derived from lysine (Bohlmann and Zdero 1973) and a similar way may be expected for the other six-membered ring amines piperidine (b) and hydroxypiperideine (h). Correspondingly, ornithine can be regarded as precursor of the five-membered ring of pyrrolidine (k), pyrrolideine (n), and pyrroline (q). However, decarboxylation of a proline derivative also leads to pyrrolidines (Strunz 2000). Isopentylamine (m) and 2-methylbutylamine (g) are most likely derived from leucine and isoleucine, and the aromatic amines, at least those with a phenylpropanoid unit (c, f, i, l, o, r), can be interpreted as derivatives of phenylalanine or tyrosine, respectively (Cortez-Espinosa et al. 2011). The biosynthetic origin of the rare isohexylamine (p), found in P. nigrum (Siddiqui et al. 2004), remains unclear, whereas the only sulfur-containing 2-(sulfinylmethyl)-ethylamine (s), detected in P. boehmeriifolium (Tang et al. 2011), suggests to be derived from cysteine.
Regarding the wide distribution of isobutylamine (a) as part of the alkamides of seven from eight different plant families its absence in L. meyeni of the Brassicaeae deserves special attention. The divergent position of Lepidium alkamides, known as macamides, is also indicated by the specific formation of benzylamines (t, u) (Wang et al. 2007). The alkamides isolated from the Aristolochiaceae, Menispermaceae, and Poaceae are exclusively characterized by an isobutylamine residue (a). With the exception of a tyramine residue (f) in three amides of Zanthoxylum gilletii (Wansi et al. 2009), isobutylamine (a) is also predominating in the Rutaceae. However, the fruits of Zanthoxylum species deviate by an additional hydroxylation leading to an accumulation of hydroxyisobutylamine (d) and sometimes even to dihydroxyisobutylamine (v). The greatest diversity of different amine parts was shown in the Asteraceae and Piperaceae, generated primarily by the additional presence of six- (b, e, h) or five-membered ring amines (k, n, q), respectively. In addition, both families were shown to contain aromatic amine parts with phenylpropanoid structures (c, f, i, l, o, r). Within the Asteraceae the presence of 2-methylbutylamine (g) distinguishes the tribe Heliantheae from the Anthemideae. The latter, by contrast, is characterized by the frequent formation of piperidine (b, e, h) and pyrrolidine (k, n, q) derived amines, which were not found so far in the Heliantheae (Greger 1984, 1988; Christensen and Lam 1991; Christensen 1992). Especially some species of the genus Achillea were shown to be a rich source of amides containing these ring amines, from which the formation of pyrrolideine (n) even appears to be restricted to the genus (Bohlmann and Zdero 1973; Greger et al. 1982, 1983, 1987b; Hofer et al. 1986; Greger and Hofer 1989) (Fig. 9).
Acid parts
The acid parts are grouped together in Tables 1, 2, 3 in order of different carbon chain lengths. Most of them are regarded as derivatives of C18 oleic acid formed by various oxidative degradations leading to chain shortenings at the carboxyl site as well as at the terminal methyl end (Bohlmann and Dallwitz 1974) (Tables 2, 3). By contrast, fatty acid elongations up to C26 Footnote 1 lead to the alkamides listed in Table 1. Apart from different chain lengths the acid parts are characterized by different patterns of double and triple bonds formed by successive desaturase and acetylenase activities, respectively, frequently accompanied by isomerization (Minto and Blacklock 2008). Only a few amides are known with fully saturated acid parts (Tables 1, 2, 3). Generally, double bonds are frequently formed in conjugation with the carboxyl group mostly showing E-configuration. Further patterns of unsaturation along the carbon chain are often separated by a dimethylene bridge. Whereas most of the 117 saturated and olefinic acid parts, listed in Tables 1 and 2, are terminated with a methyl end, some deviate by an oxidation of the terminal carbon atom: i.e. the C10 derivatives 93, 94, 97, 98, the C6 derivatives 115, 116, and the C4 derivative 117. Insertion of oxygen at other positions results in the formation of keto-acids, frequently found in the C18 series of saturated and olefinic alkamides, and various hydroxylations mainly in the olefinic C12 and C10 series. Oxygenation also leads to cyclisation products (83, 100, 189) and endoperoxide rings (80, 81) (Devkota et al. 2013) (Table 2), as well as the unique pipercycliamide shown in Fig. 4 (Wei et al. 2004).
Most of the acetylenic alkamides listed in Table 3 are derived from oleic acid by chain shortening from C18 to C9 (Greger 1984, 1988). In this group successive oxidative degradations at the methyl end frequently lead to a terminal acetylenic hydrogen, a widespread feature of acetylenic alkamides, which can easily be detected in IR-analysis by strong signals at 3.306–3.308 cm−1 in CCl4. A graphical comparison of IR-spectra from various alkamides was presented previously (Greger 1985). Although the thiophenes 174 and 184 do not show acetylenic linkages, they are included in Table 3 due to biosynthetic considerations. As indicated by feeding experiments with 14C labelled compounds thiophene 174, named otanthic acid, is formed by incorporation of H2S into the C11 diyne 167 (Bohlmann et al. 1974). An acetylenic C13 precursor was suggested for the formation of thiophene 184 (Bohlmann et al. 1973).
Biosynthesis of the acid parts
The acid parts of the alkamides are regarded as products of fatty acid synthases, which append in a head-to-tail fashion malonyl units to a growing acyl chain (Minto and Blacklock 2008). Although limited studies exist on the biosynthesis of alkamides, the double and triple bonds of the acyl chains are most likely formed by similar enzymatic activities known from naturally occurring olefinic and acetylenic fatty acids. In this case substrate and regiospecific desaturases and acetylenases, respectively, were shown to be responsible for the characteristic patterns of unsaturation (Cahoon et al. 1997, 2001; Shanklin and Cahoon 1998; Uttaro 2006; Minto and Blacklock 2008). On the basis of feeding experiments with Echinacea purpurea Bohlmann and Dallwitz (1974) already showed the biosynthetic connection between C18 oleic acid and acetylenic alkamides of the C14, C12, and C11 series: starting with the C18 acetylenic crepenynate pathway two β-oxidations at the carboxyl site lead to anacyclin (141a), which is further shortened to 160a and 167a by successive oxidative degradations at the methyl end. The co-existence of various crepenynic acid derived C18 amides in Achillea lycaonica and A. chamaemelifolia suggested possible biosynthetic connections (Greger et al. 1982, 1987a), indicating successive desaturase (118b) and acetylenase activity (119b), as well as the elimination of the terminal propyl group by oxidative degradations (136b) (Fig. 3). The insertion of the Δ2-double bond in 120b, 122b and 136b can be interpreted as a result of oxidation and dehydration. The formation of the conjugated 8,10-diene-12-yne system in 122b, interpreted as allylic oxidation and rearrangement by Bohlmann et al. (1973), is possibly catalyzed by a specific conjugase activity (Cahoon et al. 1999) (Fig. 3). This characteristic group of unsaturation was also reported for C18 isobutylamides from Heliopsis buphthalmoides and H. helianthoides (124a–127a) (Bohlmann et al. 1983; Jakupovic et al. 1986), and a series of C16 amides with different amine parts (132k, 133a,k,n,q; 134a) from Achillea ageratifolia (Greger et al. 1983, 1987b). Besides acetylenic C18 piperidides, A. lycaonica was shown to accumulate also olefinic and saturated C18 piperidides (30b, 31b,k; 32b) and pyrrolidides (20b,k) as major compounds. Biosynthetically, they were interpreted either as more primitive or, with regard to the keto-acids 20b,k and 31b,k as hydrogenation products of formerly more unsaturated derivatives (Greger et al. 1982, 1987a). With respect to the co-existence of a series of amides with the same pattern of unsaturation in Acmella ciliata, Martin and Becker (1985) suggested a common biosynthetic sequence for C12, C10, and C8 olefinic amides derived from linolenic acid. On the basis of the present overview some general conclusions can be drawn as to the major biosynthetic trends of alkamide formation and their chemotaxonomic significance.
Major biosynthetic trends
Olefinic alkamides
Elongated saturated and olefinic C19–C28 alkamides
The acyl parts of the very long-chain amides ranging from C19 to C28 (Table 1) can be regarded as products of specific desaturase/elongase activities (Leonard et al. 2004; Uttaro 2006). Apart from the C28 and C26 tyramides (1f) from Z. gilletii (Wansi et al. 2009), the C25 tyramide 2f from Senecio erechtitoides (Ndom et al. 2010), the C24 benzylamide 3t from L. meyeni (Zhao et al. 2005), and the C20 isobutylamide 11a from Mallotus lianus (Jiang et al. 2009), the remaining elongated alkamides were isolated from Piper species. In this case they are mostly characterized by olefinic C20 acyl chains frequently accompanied by derivatives of the C18, C16, and C10 series. Compounds with longer acyl chains show a restricted distribution each known so far from single species only: the C26 longamide (1a) from P. longum (Koul et al. 1988), the C22 filfiline (4a) from P. retrofractum (=P. officinarum) (Gupta et al. 1976), and the C19 brachystine (18k) from P. brachystachyum (Koul et al. 1988). The patterns of unsaturation in the elongated acid parts are mostly characterized by one or two E-configurated double bonds conjugated with the carboxyl group together with a further single double bond located at different positions along the acyl chain. As shown in Table 1 no acetylenic linkages are formed in this group.
Saturated and olefinic C18 alkamides
The C18 alkamides are separated into two groups comprising saturated and olefinic derivatives in the first (Table 2), and acetylenic derivatives in the second (Table 3). Many derivatives of the first group were isolated from Piper species (for ref.: Parmar et al. 1997; Do Nascimento et al. 2012) (Table 4). Similar patterns of unsaturation suggest close biosynthetic connections with the elongated amides mentioned above, but also with those of the C16 series. Octadeca-2E,4E,12Z-trienoic acid derived amides were isolated from P. retrofractum (37a) (Kikuzaki et al. 1993), P. nigrum (37a,k,p) (Siddiqui et al. 2004), and P. longum (37?) (Sun et al. 2007), but were also detected in M. lianus of the Euphorbiaceae (37a,b) (Jiang et al. 2009). By contrast, C18 alkamides of L. meyeni clearly deviate by different patterns of unsaturation in the acid parts, lacking Δ2-, Δ4-double bonds (19, 21, 24–29), as well as by specific combinations with the benzylamines t and u. Otherwise, olefinic acid parts containing only these two Δ2-, Δ4-double bonds are widespread occurring with various chain lengths in different plant families and genera (Table 2). However, further patterns of unsaturation along the acyl chains, mostly separated by a dimethylene interruption, represent characteristic biosynthetic trends of restricted distribution.
Saturated and olefinic C16 alkamides
Apart from the two saturated hexadecanoic acid amides 41t and 41u from L. meyeni (Zhao et al. 2005), most of the C16 amides listed in Table 2 were found in Piper amalago (41k, 42k, 43k) and P. retrofractum (46a, 47a, 48a). It is interesting to note that all twelve alkamides detected in the root extract of the former were shown to be pyrrolidides (Achenbach et al. 1986), whereas in the fruit extract of the latter isobutylamides prevail (Kubo et al. 2013). The hexadeca-2E,7Z-dienoic acid pyrrolide (44q) and hexadeca-2E,9Z,12Z,14E-tetraenoic acid isobutylamide (45a) show an erratic distribution known so far only from the roots of A. ageratifolia (Greger et al. 1987b) and flower heads of E. purpurea (Christensen et al. 2009), respectively.
Olefinic C14 alkamides
Olefinic C14 alkamides with Δ2-, Δ4-double bonds (51a,b,d,k,f) are widespread in different plant families. In contrast, higher desaturated derivatives were mainly found in Zanthoxylum species of the Rutaceae family. The co-existence of the highly unsaturated tetradecapentaenoic acid isobutylamides γ- (58a) and δ-sanshool (59a) with a series of less desaturated derivatives, ranging from dien- (51a, 52a) to trien- (53a, 54a), and tetraenoic acid isobutylamides (55a,d, 56a,d) in Z. bungeanum (Xiong et al. 1997) and Z. integrifolium (Chen et al. 1999), suggests a common biosynthetic sequence. Alkamides with the acid residues 53 and 56 were also isolated from the propably closely related Asteraceae genera Leucocyclus (53a, 56a) (Greger et al. 1981), Achillea (53a, 56ak) (Greger et al. 1984, 1987b), and Otanthus (53a,b, 56a,b) (Christodoulopoulou et al. 2005). However, the conjugated triene system in 58 and 59, separated from the Δ2-, Δ4-double bonds by a dimethylene bridge, can be regarded as a characteristic chemical feature of Zanthoxylum. Another common chemical character of the genus is the hydroxylation of the isobutylamine part (d) in the fruits (Yasuda et al. 1982; Mizutani et al. 1988; Kashiwada et al. 1997) (Table 2). In Sanvitalia ocymoides (Asteraceae–Heliantheae) the co-existence of the unique tetradeca-2E,4E,8Z,10Z,12E-pentaenoic acid isobutylamide (60a) with a corresponding olefinic C14 methylester, showing the same configurations of the five conjugated double bonds, points to a common biosynthetic origin (Domínguez et al. 1987).
Olefinic C12 alkamides
The accumulation of olefinic C12 alkamides (Table 2) represents another major biosynthetic trend of Zanthoxylum (Menozzi-Smarrito et al. 2009), and particularly of Echinacea species of the Asteraceae tribe Heliantheae (Woelkart and Bauer 2007). Moreover, they were also shown to accumulated in the genera Asiasarum (Yasuda et al. 1981a) and Asarum (Zhang et al. 2005) of the Aristolochiaceae. Like the C14 amides, the C12 derivatives of Zanthoxylum are characterized by a conjugated triene group near the methyl end, but deviate by possessing only one Δ2-double bond (72–74). Their formation can be explained by an elimination of a C2-unit from corresponding C14 amides by β-oxidation. This relationship is also indicated by the common vernacular names α- (72a) and β-sanshool (74a) of the C12 series, and γ- (58a) and δ-sanshool (=γ-isosanshool) (59a) of the C14 series (Fig. 7). In view of their high instability (Crombie and Tayler 1957; Yasuda et al. 1981b) derivatives with oxygen functions, isolated from the fruits of Z. piperitum (75d–78d) (Hatano et al. 2004), Z. bungeanum (77d, 79d) (Huang et al. 2012), and Z. armatum (75d, 80d, 81d) (Devkota et al. 2013), can be regarded as products of successive oxidation and hydration processes. However, it remains unknown, whether these processes take place in the plant or during extraction procedures.
The accumulation of dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide (66a), with only two conjugated double bonds near the methyl end, is typical for the related genera Echinacea, Salmea, and Acmella (Spilanthes), but is also known from Asiasarum and Asarum. The co-occurrence of the isomeric pair 65a/66a with dodeca-2E,4E-dien- (62a) and 2E,4E,8Z-trienoic acid derived amides (63a) in E. purpurea (Bauer et al. 1988), E. angustifolia (Hinz et al. 2007), and E. atrorubens (Dietz and Bauer 2001), suggests a common biosynthetic sequence. From the other C12 isomers (67a–69a) dodeca-2E,4E,8E,10Z-tetraenoic acid isobutylamide (67a) was isolated from the first two Echinacea species (Matovic et al. 2011) and Acmella mauritiana (Jondiko 1986), but was also detected in Leucanthemum hosmariense of the tribe Anthemideae (Bohlmann et al. 1980a). The dodeca-2E,4Z,8Z,10E-tetraenoic acid isobutylamide (68a) was reported for A. ciliata (Martin and Becker 1985), A. radicans (Rios-Chavez et al. 2003), and for Heliopsis longipes, H. procumbens, and H. ex aff. novogaliciana (García-Chávez et al. 2004). Together with the 2E,4Z,8Z,10Z-isomer (69a) it was also isolated from Asarum forbesii (Zhang et al. 2005). A significant chemical character of Echinacea and Acmella amides is the frequent combination with 2-methylbutylamine (g). Due to the unusual terminal vinyl end a different biosynthetic pathway was suggested for 64a (Table 2). It was isolated from the aerial parts of the Australian Brachycome ciliocarpa (Asteraceae–Astereae) (Zdero et al. 1988), and, with uncertain configuration, from the toothache grass Ctenium aromaticum (Poaceae) (Gamboa-Leon and Chilton 2000).
Olefinic C10 alkamides
The well-known C10 amide pellitorine (101a) (Crombie 1955b) is the most widespread olefinic alkamide reported for Asteraceae, Piperaceae, Rutaceae, Aristolochiaceae, Menispermaceae, and Poaceae. In the first two families the deca-2E,4E-dienoic acid (101) was shown to be combined with twelve different amines (Table 2). In the roots of Cissampelos glaberrima of the Menispermaceae the predominating 101a was accompanied by octa-2E,4E-dienoic isobutylamide (112a) and traces of the more saturated 2E-decenoic (89a) and decanoic acid isobutylamide (86a) (Loureiro-Rosario et al. 1996). Pellitorine (101a) was also isolated from Ctenium aromaticum of the Poaceae, where it is accompanied by deca-2E,4E,8Z-trienoic acid isobutylamide (102a), as yet only known from Achillea species (Gamboa-Leon and Chilton 2000). In view of the wide distribution of pellitorine (101a) its restricted occurrence within the Asteraceae is noteworthy. Here, it was reported so far only for the tribe Anthemideae, while in the Heliantheae olefinic C10 amides are mainly represented by the well-known deca-2E,6Z,8E-trienoic acid isobutylamide spilanthol (=affinin) (92a) (Crombie et al. 1963; Yasuda et al. 1980). With respect to the co-existence of 92a with the C12 amide 66a in Acmella species, showing a similar pattern of unsaturation (Martin and Becker 1985; Keipert and Melzig 2009; Bae et al. 2010; Sharma et al. 2011), the formation of spilanthol (92a) may be explained by an elimination of C2 from 66a by β-oxidation. Derivatives with oxygen functions in A. ciliata (88a, 97a, 98a) (Martin and Becker 1985; Keipert 2009), H. longipes (López-Martínez et al. 2011), and A. oleracea (Simas et al. 2013) (95a, 96a) can be regarded as oxidation products of spilanthol (92a). The detection of a bornylester of 92 in H. longipes and H. novogaliciana is of biogenetic interest (García-Chávez et al. 2004). Another group of oxidized C10 amides (99a,b, 100a, 103b) and the unique cyclisation product pipercycliamide was reported for the roots of P. nigrum together with pellitorine (101a) and related derivatives (101b,k, 89b) (Wei et al. 2004). In this series the diol 99b was separated into the two erythro and threo configurated isomers (Fig. 4). The erythro isomer of 99a, named sylvamide, was isolated from the seeds of P. sylvaticum Roxb. (Banerji and Pal 1983).
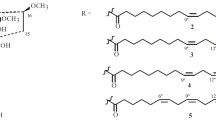
Various oxidized C10 amides from roots of P. nigrum (Wei et al. 2004)
The accumulation of various olefinic C10 amides represents a major biogenetic trend of the genus Achillea of the tribe Anthemideae. Apart from the many combinations of the widespread deca-2E,4E-dienoic acid residue (101) with different amine parts (a,b,c,e,f,h,i,m), deca-2E,4E,8Z-trienoic acid derived amides (102a,b,e) were shown to be typical for some Achillea species (Bohlmann et al. 1974; Greger 1988). However, of special chemotaxonomic significance is the formation of C10 amides with three and even four conjugated double bonds in the acid moiety (105e–108e) (Fig. 9). Beside a single report on the unique sencolaminic acid derived amides (104c,m) from Senecio colaminus (Asteraceae–Senecioneae) (Bohlmann and Zdero 1979), these highly conjugated system is typical for the taxonomically complex Achillea millefolium group: besides the predominating deca-2E,4E,6Z-trienoic acid piperideide 105e (Bohlmann and Zdero 1973), the underground parts of different species were shown to be characterized by various accumulation trends towards the two isomeric decatetraenoic acid piperideides 107e and 108e (Greger and Hofer 1989; Greger and Werner 1990) (Fig. 9).
Olefinic C8–C4 alkamides
As products of extensive oxidative degradations the short-chain olefinic alkamides of the C8 to C4 series represent a small group in the Asteraceae, Piperaceae, Rutaceae, and Menispermaceae. The oxidation of the terminal carbon atom in 115c of Salmea scandens (Bohlmann et al. 1985), 116a of A. ciliata (Keipert 2009), 116d (timuramide D) of Zanthoxylum armatum (Devkota et al. 2013), and the shortest alkamide 117a of Piper hancei (Narui et al. 1995) suggests chain shortening from the methyl end. However, it should be pointed out that a large amount of fumaric acid was found together with the fumarylisobutylamide 117a in A. ciliata (Keipert 2009), possibly indicating a different biogenetic origin of this acid moiety.
Acetylenic alkamides
Acetylenic C18–C15 alkamides
Acetylenic C18 alkamides are only known so far from the two genera Heliopsis (Fig. 6) (Bohlmann et al. 1983; Ramírez-Noya et al. 2011) and Achillea (Greger et al. 1987a). As shown in Fig. 3 the acid parts can be derived from crepenynic acid demonstrating basic steps of desaturase and acetylenase activities as well as the formation of conjugated diene-yne sytems (122–127). The insertion of Δ2-, Δ4-double bonds (120–127) appears to be the result of independent biosynthetic activity. A similar pattern of unsaturation was reported for a series of acetylenic C16 alkamides from A. ageratifolia (129–134) (Greger et al. 1983, 1987b) and A. tomentosa (134) (Greger et al. 1981), suggesting chain shortening by β-oxidation at the carboxyl end. By contrast, shortening at the methyl end possibly leads to the C16 amides 135a in Echinacea angustifolia (Bauer et al. 1989), E. purpurea (Binns et al. 2002) and in the four derivatives detected by GC/MS analysis in Heliopsis suffruticosa (Fig. 6) (Ramírez-Noya et al. 2011). The elimination of the terminal propyl group results in the formation of acetylenic C15 alkamides (136a,b,d) found in Achillea as well as in Echinacea species (Fig. 3). The unusual pattern of unsaturation in the C17 callyspongamide A (128c), isolated from the sponge C. fistularis (Youssef et al. 2003), clearly deviates, indicating a different biosynthetic origin (Table 3).
Acetylenic C14–C11 alkamides
Two different accumulation trends either towards acetylenic C14 or C12 alkamides represent important chemotaxonomic criteria differentiating between the tribes Anthemideae and Heliantheae. Derived from C18 crepenynic acid, the C14 amides are formed by two β-oxidations at the carboxyl site, whereas the C12 amides are additionally shortened at the methyl end. In the C13 acyl chains 150–154 the patterns of unsaturation suggest a cleavage of a terminal propyl group from corresponding C16 precursors. They were exclusively reported for the Heliantheae genera Acmella and Echinacea (Nakatami and Nagashima 1992) (Table 4). Apart from two isomeric tetradeca-2,4,10-triene-8-ynoic acid isobutylamides (138a, 139a) from H. buphthalmoides (Bohlmann et al. 1983; Jakupovic et al. 1986) and tetradeca-2-en-10,12-diynoic acid isobutylamide (145a), isolated in small amounts from E. angustifolia (Woelkart et al. 2005), the accumulation of acetylenic C14 alkamides listed in Table 3 was detected solely in the tribe Anthemideae. They were mainly reported for Achillea species (Table 4), where they are characterized by highly desaturated acid moieties, sometimes even leading to fully conjugated systems (147–149). Whereas most derivatives have two triple bonds, the piperidide 144b, isolated from Achillea spinulifolia, possesses three acetylenic linkages, the highest number of triple bonds found in the alkamides (Greger et al. 1982). By contrast, acetylenic C12 acid moieties (155–165) were mostly isolated from Echinacea species, where they are usually linked to isobutylamine (a), and frequently also to 2-methylbutylamine (g) (Table 4).
With the exception of 173a from Artemisia dracunculus (Saadali et al. 2001) and the thiophenes 174a from Anacyclus clavatus (=A. tomentosus) and 174a,b,e from Otanthus maritimus (Bohlmann et al. 1974), the acetylenic C11 alkamides, listed in Table 3, are uniformly characterized by a terminal acetylenic hydrogen. Biosynthetically, they can be regarded as a result of oxidative chain shortening at the methyl end. The thiophenes (174a,b,e) are known to be formed by an incorporation of H2S into the C11-diynoic acid 167 (Bohlmann et al. 1974). Most of the C11 amides were isolated from Echinacea and Acmella species and are characterized by a 8,10-diyne group (166–172). Only derivatives of the undeca-2E,4E-dien-8,10-diynoic acid (167) are more widely distributed: they were also isolated from the Anthemideae genera Achillea, Anacyclus, Otanthus, Argyranthemum, and Chamaemelum. In this case the acyl rest 167 was shown to be linked to seven different amine parts (a,b,c,e,g,j,m) (Table 4). An unusual pattern of unsaturation was reported for decumbine (175a), an undeca-4E,6E-dien-10-ynoic acid isobutylamide, which is only known so far from Acmella decumbens (Casado et al. 2009). The unique C11 amide 173a, isolated from A. dracunculus, deviates by a terminal methyl group. It is possibly derived from a C13 precursor by an elimination of C2 at the methyl end (Bohlmann et al. 1973).
Acetylenic C10 and C9 alkamides
With respect to their various patterns of unsaturation acetylenic C10 amides can be derived from two major biosynthetic pathways (Table 3). The formation of highly unsaturated acid parts, leading even to fully conjugated systems (179–182), represents a characteristic trend of the Anthemideae genera Achillea, Anacyclus, and Cladanthus (Table 4). They are biosynthesized by direct oxidative cleavage of a C8-group from an octadec-9Z-en-12,14,16-triynoic acid precursor (Jente and Richter 1976). By contrast, the C10 acyl parts 177 and 178 from the Heliantheae genera Acmella and Salmea are most likely formed by an elimination of C2 from corresponding C12 derivatives by β-oxidation. This hypothesis is supported by the predominant formation of similar acetylenic C12 amides in the related genus Echinacea. The biosynthetic origin of the C10 tyramide acmelline (183f), isolated from A. decumbens (Casado et al. 2009), remains unclear. Like the C11 amide decumbine (175a), mentioned above, the patterns of unsaturation clearly deviate from those known from other Acmella species (Table 3). According to Bohlmann et al. (1973) the biosynthesis of thiophene 184a, isolated from Argyranthemum (=Chrysanthemum) frutescens (Bohlmann and Zdero 1967), can be explained by a terminal cleavage of a C2-unit from a trideca-2,4-dien-7,9-diynoic acid precursor, followed by incorporation of H2S and elimination of the terminal methyl group. The acetylenic C9 derivatives were isolated only from the two closely related genera Acmella and Salmea (Table 3). They are characterized by a terminal acetylenic hydrogen (185–189), apparently derived from corresponding C12 (160–162) or C10 alkamides (177, 178), respectively, by an oxidative chain shortening at the methyl end. Another dominant biogenetic trend in both genera is the combination with aromatic amine parts (c,o,r) (Table 4).
Biological activities
Pungent and tingling properties
Pungent and tingling alkamides were mainly reported for species of Acmella (Spilanthes), Heliopsis, and Anacyclus of the Asteraceae, and Zanthoxylum of the Rutaceae family. Similar taste profiles caused by alkamides are also known from Echinacea, Argyranthemum, Salmea, Aaronsohnia, Piper, and the grass C. aromaticum. The most prominent representatives are the four olefinic isobutylamides spilanthol (=affinin) (92a), pellitorine (101a), α-sanshool (=neoherculin) (72a), and γ-sanshool (58a). Due to their high instability isolation of pure compounds and structure elucidation turned out to be difficult (Fig. 5).
Spilanthol (=affinin)
Spilanthol (92a) was first obtained in the crude state from the flower heads of pará cress, A. oleracea, by Gerber (1903), who already supposed the existence of a fatty acid amide. Asano and Kanematsu (1927) initially concluded an unsaturated C10 isobutylamide with an allenic structure, and later (1932), a deca-4,6-dienoic acid isobutylamide. The correct structure of spilanthol, isolated from the roots of H. longipes, was suggested to be either a deca-2,6,8- or 2,5,7-trienoic acid isobutylamide (Acree et al. 1945). Since this plant was originally wrongly identified as Erigeron affinis, the compound was named affinin. Later, spilanthol was confirmed to be identical with affinin (Jacobson 1957a), and its stereochemistry finally assigned as 2E,6Z,8E (92a) by Crombie et al. (1963).
Pellitorine
The pungent constituent of pellitory root, A. pyrethrum, a plant native to North Africa, was first examined by Buchheim (1876), who named it “pyrethrin” and classified it erroneously as an amide related to piperine. Later, Dunstan and Garnett (1895) renamed the active material as pellitorine without any progress in elucidating the structure. Gulland and Hopton (1930) regarded it as a decadienoic isobutylamide with indefinite position of the double bonds. They retained the name pellitorine in order to avoid confusion with the insecticidal pyrethrins of Pyrethrum flowers (Tanacetum cinerariifolium). In order to determine the positions of the double bonds Jacobson (1949) investigated 6 kg of ground dry pellitory roots and concluded that pellitorine is an N-isobutyl-2,6-decadienamide. However, a re-examination by Crombie (1955b) has shown, that pellitorine contained at least three compounds with deca-2E,4E-dienoic acid isobutylamide (101a) as major component.
α-Sanshool (=neoherculin)
The pungent principle of the fruits of Japanese pepper Zanthoxylum piperitum (“Asakura Sansho”) was first investigated by Murayama and Shinozaki (1931) and named sanshool. After hydrogenation they already supposed the presence of a C12 lauric acid amide. In a subsequent study Asano and Kanematsu (1931) regarded sanshool as a dodecadienoic acid isobutylamide, which was later shown to be a mixture of two homologous substances (Aihara 1950). One of which, named sanshoöl I, had a strong pungent taste and was very unstable in pure state when exposed to air. Aihara (1950) concluded a dodecatrienoic acid isobutylamide with the double bonds located at 2,4,8-position. The bark of Z. clava-herculis, commonly known as southern prickly ash, Hercules´club, or toothache tree in the southern United States, also gives a persistent burning and paralyzing effect on the lips and tongue. The active substance was first isolated by Jacobson (1948) and named herculin. He concluded the presence of an N-isobutylamide of a C12 acid containing two double bonds at 2- and 8-position. Crombie (1954) disproved the structure by syntheses and isolated a highly unstable but pure compound, named neoherculin. It was shown to be a dodeca-2,6,8,10-tetraenoic isobutylamide, whose stereochemistry was finally assigned as 2E,6Z,8E,10E (72a) (Crombie 1955a). In a synthetical investigation Crombie and Tayler (1957) showed that sanshoöl I has not the gross structure assigned to it by Aihara (1950). They isolated a very unstable amide from Z. piperitum, named α-sanshoöl (later referred to as α-sanshool). Its structure was confirmed to be the same as that of neoherculin (Crombie and Tayler 1957).
γ-Sanshool and related isobutylamides
From the fruits of Z. ailanthoides (“Karasu Shansho”) two pungent compounds were isolated, named γ-sanshoöl and hydroxy γ-sanshoöl (later referred to as γ-sanshools). Their structures were determined as tetradeca-2E,4E,8Z,10E,12E-pentaenoic acid isobutylamide (58a) and the corresponding hydroxyisobutylamide (58d), respectively. The pungent taste of 58a was shown to be stronger than that of 58d (Yasuda et al. 1981b). In addition, two pungent C14 tetraenoic acid hydroxyisobutylamides were reported for the fruits of the Chinese Z. bungeanum (“hua jiao”) (Mizutani et al. 1988), which were later designated as bungeanool (56d) and isobungeanool (55d) (Xiong et al. 1997). The corresponding pungent isobutylamide, named hazaleamide (56a), was isolated from the bark of the Indonesian Z. rhetsa (“hazalea”) (Shibuya et al. 1992).
Dodeca-2,4,8,10-tetraenoic acid isobutylamides (“echinacein”)
The numbing and pungent principle of the roots of the American coneflower, E. angustifolia, was first isolated by Jacobson (1954), who named it echinacein. He supposed an isobutylamide of a highly unsaturated C12 straight-chain acid which might be identical with neoherculin (=α-sanshool). Twelve years later a mixture of isomeric dodeca-2,4,8,10-tetraenoic acid isobutylamides was reported for the roots of E. angustifolia and E. purpurea, which was supposed to represent Jacobson´s echinacein (Bohlmann and Grenz 1966). One of the isomers was isolated from Acmella alba and its stereochemistry was assigned as 2E,4E,8Z,10E (66a) (Bohlmann et al. 1980b). Its co-occurrence with the isomeric 2E,4E,8Z,10Z-tetraene (65a) was then reported for Asiasarum heterotropoides (Yasuda et al. 1981a) and later for E. purpurea (Bauer et al. 1988). This pair of isomers was suggested to be responsible for the numbing effect of the root extract of S. scandens (Herz and Kulanthaivel 1985).
Pungent C18 isobutylamides (scabrin, heliopsin)
Special interest deserves the discovery of the pungent principle in the roots of Heliopsis helianthoides var. scabra, which was thought to consist of two highly unsaturated C18 isobutylamides. One of which was described as a pale yellow, viscous oil which could not be distilled without decomposition, even under high vacuum. It was named scabrin, for which analysis and molecular weight determination indicated the formula C22H35NO (Jacobson 1951). Similar characteristics were found in the second compound with the molecular formula C22H33NO, designated as heliopsin (Jacobson 1957b). Both compounds exhibited an intense burning and paralytic effect which was produced only after an induction period of approximately 10 min with scabrin, and 20 min with heliopsin. The correct structures of both amides were not elucidated so far. Regarding the investigations of H. helianthoides var. scabra and H. buphthalmoides by Bohlmann et al. (1983) and Jakupovic et al. (1986), it is tempting to assume that the four acetylenic C18 isobutylamides 124a–127a (Fig. 6) isolated from the aerial parts and roots, respectively, are closely related or even identical with scabrin and heliopsin. Further experiments will have to show whether the delayed perception of the pungent taste can be explained by an enzymatic transformation or even a cleavage of these long-chained C18 alkamides. In this connection it would be interesting to confirm the structures of the acetylenic C18 and C16 alkamides of H. suffruticosa deduced from GC/MS analysis and test them for their pungent properties (Fig. 6) (Ramírez-Noya et al. 2011).
Structure–activity relationships
Active alkamides were shown to excite different populations of sensory neurons than does capsaicin or other similar pungent spices (Bryant and Mezine 1999; Bautista et al. 2008). In addition to pungency they are known to create a characteristic tingling sensation. Human judgments of 25–50 μg of α-hydroxysanshool (72d) applied directly to the tongue indicated that this sensation was more similar to a mild electric shock or a weakly carbonated solution. At higher concentrations (>100 μg), the sensation was painful. While ε-hydroxysanshool (73d), having one more Z-double bond, was as active as 72d at the same concentration, the all-E isomer β-hydroxysanshool (74d) was inactive even at 100 μg (Bryant and Mezine 1999). The fact that Z/E isomerism influences the perception of pungency was also reported for the fruits of Zanthoxylum bungeanum containing a series of related hydroxyisobutylamides. While those having a Z-double bond (55d, 56d, 58d, 72d) exhibited a strong pungency, the corresponding all-E isomers (59d, 74d) were tasteless (Mizutani et al. 1988). Shibuya et al. (1992) prepared four derivatives related to hazaleamide (56a) and found that those with a fully saturated acid part or having only the 2E-double bond swap the pungent with a bitter sensation. In order to provide more information about structure–activity relationships of the pungent taste for the sanshool-related compounds (Fig. 7), a variety of derivatives was synthesized by Galopin et al. (2004). These results confirmed that a Z-double bond in the acyl chain is a key element for the sensory properties, but it is not the only one. Chain length and a certain pattern of unsaturation, sharing a common motif, were also shown to be required for activity. Detection threshold and taste characteristics of six sanshools were examined by sensory evaluation. In these studies pungent qualities of each derivative were shown to be different: e.g. the stimulus perceived as burning and tingling was predominantly detected with α-sanshool (72a) which lasted for the longest time. γ-Sanshool (58a) was perceived as burning and fresh, and α-hydroxysanshool (72d) as tingling and numbing (Sugai et al. 2005a, b). In a more recent psychophysical half-tongue experiments using filter paper rectangles as vehicle it was confirmed that alkamides of the sanshool-group possessing at least one Z-configurated double bond (55d, 56d, 58d, 72d, 73d, ζ-hydroxysanshool) (Fig. 7) elicited the well-known tingling and paresthetic orosensation above threshold levels of 3.5–8.3 nmol/cm2. In contrast, the all-E configurated derivatives 59d and 74d induced a numbing and anesthetic sensation above thresholds of 3.9 and 7.1 nmol/cm2, respectively (Bader et al. 2014).
To elucidate the cellular and molecular basis of the action of 72d, a series of specific receptors was identified and cloned. Thus, 72d binds to and inhibits the two-pore potassium channels KCNK3 (TASK-1), KCNK9 (TASK-3), and KCNK18 (TRESK), a class of pH- and general anestethics-sensitive ion channel (Bautista et al. 2008). Ex vivo skin-nerve preparation was used to examine the pattern and intensity with which the sensory terminals of cutaneous neurons respond to 72d. It was found that it excited virtually all D-hair afferents, a distinct subset of ultrasensitive light-touch receptors in the skin, and targeted novel populations of Aβ and C fiber nerve afferents. Thus, 72d was shown to provide a novel pharmacological tool for discriminating functional subtypes of cutaneous mechanoreceptors (Lennertz et al. 2010).
Various trigeminal effects for artificial and naturally occurring alkamides related to spilanthol (92a) were reported (Ley et al. 2006a). From 26 derivatives it was shown that 92a, having one Z-double bond, was the most active tingling and saliva-inducing compound. Pellitorine (101a), lacking a Z-double bond, exhibited mainly the same sialogogic activity but without the strong tingling effect. Interestingly, the structurally corresponding 2-ketol ester acmellonate, possessing the two conjugated Z/E double bonds near the methyl end as 92a, showed similar numbing and tingling effects (Ley et al. 2006b). In order to elucidate the hypothesis that saliva induction may be correlated with a reduction of astringency sensation the potential masking effect of ten structurally related olefinic alkamides were evaluated. Among the selected compounds, the saliva-inducing 101a significantly lowered the intensity of the astringency of epigallocatechin-3-gallate (Obst et al. 2013).
In a taste dilution analysis Dawid et al. (2012) tested the active alkamides of black pepper, P. nigrum, to what extent they contribute to the pungent impression of the well-known aromatic amide piperine. While eight aromatic piperine analogues exhibited a clear pungent sensory profile, twelve long-chain C20- and C18-2E,4E-dienoic acid derived alkamides, possessing an additional isolated Z-double bond, induced a pungent impression as well as a long-lasting tingling sensation. In accordance with the sanshools (Fig. 7) the Z-double bond was shown again to be a key element for the tingling effect: when compared to the tingling activity of octadeca-2E,4E,12Z- (37a) and 2E,4E,13Z-trienoic acid isobutylamide (38a), lacking of the Z-double bond in octadeca-2E,4E-dienoic acid isobutylamide (33a) induced a complete loss of that sensation. Moreover, amides with an isobutyl (a) or pyrrolidine part (k) were less pungent and tingling than the corresponding derivatives with a piperidine part (b). For example, eicosa-2E,4E,14Z- (13b) and 2E,4E,15Z-trienoic acid piperidide (14b) showed lowest threshold concentrations for pungency when compared to the corresponding pyrrolidides (13k, 14k) and isobutylamides (13a, 14a) (Dawid et al. 2012).
Insecticidal activities
LaForge et al. (1942) reported on the presence of an insecticidal principle in the bark of Zanthoxylum clava-herculis which was later isolated and identified as α-sanshool (=neoherculin) (72a) (Crombie 1955a). It was proved to have approximately the same order of paralyzing action and toxicity to house flies (Musca domestica) as the pyrethrins (Jacobson 1948). Similar insecticidal activities are known from the roots of H. longipes traded in Mexico under the Nahuatl names of “chilcuague” and “chilmecatl” (Molina-Torres et al. 1996). Here, the major insecticidal compound was identified as spilanthol (92a) (Crombie et al. 1963), originally published as “affinin” (Acree et al. 1945). Toxicity to house flies was also observed in petroleum ether extracts of three further American Heliopsis species (Gersdorff and Mitlin 1950). Especially the roots of H. helianthoides var. scabra were shown to be very toxic. In contrast to H. longipes two highly unsaturated C18 isobutylamides were isolated and named scabrin and heliopsin, from which the former proved to be appreciably more toxic than pyrethrins to house flies. Their structures were published only tentatively as C18-pentaenoic and -hexaenoic acid isobutylamides, respectively (Jacobson 1951, 1957b). As mentioned above they are probably closely related to or even identical with the acetylenic C18 isobutylamides 124a–127a isolated from that species by Bohlmann et al. (1983) and Jakupovic et al. (1986).
The pronounced insecticidal activity of spilanthol (92a) was also detected in bioassays against the American cockroach, Periplaneta americana. The acute toxicity became apparent when compared to three conventional insecticides. It was found to be 1.3, 2.6, and 3.8 times more toxic than carbaryl, bioresmethrin, and lindan, respectively. Electrophysiological studies suggested a neurotoxic action indicating immediate hyperexcitation followed by complete inhibition of the cercal nerve activity (Kadir et al. 1989). In addition to 92a the structurally similar undeca-2E,7Z,9E-trienoic acid isobutylamide (85a) and the acetylenic undeca-2E-en-8,10-diynoic acid isobutylamide (169a), isolated from Acmella paniculata (=Spilanthes acmella), were shown to be very active against larvae of the mosquito Aedes aegypti (=Stegomyia aegypti) and neonates of the moth Helicoverpa zea, a major agricultural pest. While the two olefinic alkamides 92a and 85a showed 50 % mortality at 6.25 μg/ml, the co-occurring acetylenic derivative 169a showed only 30 % mortality at the same concentration. However, in antifeedant tests 169a showed significantly higher weight reduction of the larvae of H. zea compared to 92a and 85a (Ramsewak et al. 1999). In search of ecofriendly and easily biodegradable naturally-occurring insecticides against Tuta absoluta (Lepidoptera), one of the key pests of tomato crops, hexane and ethanol extracts from 23 mostly South American plant species out of 16 different families were tested (Moreno et al. 2012). The hexane extract from aerial parts of A. oleracea exhibited by far the highest activity. In the bioactive fractions 92a was identified as major component together with the two acetylenic C11 alkamides 169a and 169g. In accordance with previous findings (Kadir et al. 1989) 92a was shown to be the most active compound being approximately 5 times more toxic than the commercial permethrin and a good deal more potent than the seed extract from Neem-tree, Azadirachta indica. The two acetylenic derivatives 169a and 169g exhibited activities similar to that of permethrin (Moreno et al. 2012). Bioassay-guided chromatographic fractionation of the leaf extract of A. oleracea led to a mixture of active alkamides consisting of nona-2Z-en-6,8-diynoic (187c) and deca-2Z-en-6,8-diynoic acid phenethylamide (178c). This mixture was shown to be active against A. aegypti larvae at LC50 = 7.6 ppm (Simas et al. 2013).
Pellitorine (101a) was reported to be a major contributor to the antilarval properties of the methanolic extract of A. millefolium showing 100 % mortality at 5 ppm against 24-h -old Aedes triseriatus larvae. However, it could not be ascertained if 101a is the sole active compound. To gain information regarding the minimum molecular structural requirement for anitlarval activity three analog isobutylamides of 2E-decenoic (89a), decanoic (86a), and 2E,4E-hexadienoic (sorbic) acid were prepared. While the first two derivatives showed 96 and 59 % mortality, respectively, at 20 ppm, the sorbamide was inactive at the same concentration (LaLonde et al. 1980). In an artificial diet feeding assay 101a and the closely related C8 isobutylamide 112a together with three cinnamic acid derived isobutylamides from the bark of Z. gilletii (=Fagara macrophylla) were tested against four species of lepidopteran larvae of agricultural importance. Concerning growth-inhibitory activity 101a, the second most abundant amide, was shown to be the most active compound, especially against Pectinophora gossypiella (ED50 = 15 ppm). It also caused death (LD = 25 ppm) to P. gossypiella larvae, but not to those of Heliothis virescens, H. zea, and Spodoptera frugiperda. The closely related 2E,4E-octadienoic acid isobutylamide (112a) also caused mortality to P. gossypiella only (LD90 = 100 ppm). 101a also proved to be the most toxic amide against the house mosquito Culex pipiens with a LD100 value at 5 ppm (Kubo et al. 1984).
The two isomeric C12-tetraenoic isobutylamides 65a and 66a, isolated from E. purpurea roots, were reported to exhibit mosquito larvicidal activity against A. aegypti. They proved to be most effective with 87.5 % mortality within 15 min at a concentration of 100 µg/ml. A significant activity was still shown at 10 µg/ml, with 63 % mortality in 1 h. Slightly less active was the co-occurring acetylenic undeca-2E,4Z-dien-8,10-diynoic acid isobutylamide (168a) with 71 and 100 % mortality by 2 and 9 h, respectively, while the related C11 and C12 isobutylamides 166a and 161a showed activity at the end of 9 h, with 78 and 50 % mortality, respectively. Interestingly, the corresponding 2-methylbutylamides 168g and 161g demonstrated the lowest effects (Clifford et al. 2002). Mosquitocidal activity was also reported for the isomeric C12-tetraenoic isobutylamide 67a, isolated from the aerial parts of A. mauritiana (Jondiko 1986). While most of the insecticidal alkamides mentioned above are characterized by olefinic C10-, C12- or acetylenic C11-acyl chains, the mosquitocidal activity of Piper nigrum fruits was ascribed to the C18 isohexylamide pipnoohine (37p) (Siddiqui et al. 2004). Insecticidal activity with longer acyl chains was also shown in Chrysanthemum varieties, Dendranthema morifolium, where concentration of the acetylenic C14 isobutylamide 140a was positively correlated with the degree of resistance against the western flower thrips, Frankliniella occidentalis, a major insect pest in the greenhouse industry (Tsao et al. 2003). Six olefinic C14 alkamides, isolated from Otanthus maritimus, were shown to be toxic against the ant Crematogaster scutellaris and the termite Reticulitermes balkanensis. Among them tetradeca-2E,4E-dienoic acid piperidide (51b) exhibited the highest activity, whereas the corresponding isobutylamide (51a) showed the lowest response (Christodoulopoulou et al. 2005). Bioassays of fifty mostly synthetic 2,4-dienamides against house flies (M. domestica) and mustard beetles (Phaedon cochleariae) showed that insecticidal activity was influenced by structural differences at the non-amide end of the acyl chain. These results presented clear indications concerning the necessity for a functional group containing unsaturation at the non-amide end and the strong dependence of the relationships detected on test species. Thus, pellitorine (101a) and the whole series of methyl-terminated amides displayed a much lower activity against M. domestica and P. cochleariae than phenyl- or vinyl-terminated derivatives (Elliott et al. 1987). Stimulated by the insecticidal activities of 2E,4E-dienamides derived from isobutylamine, piperidine, and pyrrolidine an efficient synthesis of corresponding derivatives was stereoselectively achieved by Abarbri et al. (1998).
Antibacterial and antifungal activities
Spilanthol (92a) was also shown to exhibit pronounced antibacterial and antifungal properties. Growth of Escherichia coli and Saccharomyces cerevisiae was inhibited at concentrations as low as 25 µg/ml. However, higher concentrations were necessary to inhibit growth of Pseudomonas solanacearum and Bacillus subtilis (Molina-Torres et al. 1999). In order to evaluate the importance of unsaturated bonds in the in vitro bacteriostatic and fungistatic properties the two less desaturated 2E-decenoic (89a) and decanoic acid isobutylamide (86a) were prepared from spilanthol (92a) by catalytic hydrogenation and tested against a number of different fungi and bacteria. While 92a was very active against Sclerotium rolfsii, S. cepivorum, Phytophthora infestans, S. cerevisiae, and Rhizoctonia groups AG3 and A-5, displaying a growth inhibition around 100 %, the more saturated derivatives 89a and 86a showed no fungitoxic activity. 92a was also shown to have a definite negative effect on the growth of the bacteria E. coli and B. subtilis, but Erwinia carotovora was not sensitive even at the highest dose. By contrast, 89a was more potent against E. coli and E. carotovora, than 92a, and only the saturated 86a displayed a significant activity on the growth of B. subtilis. These data suggested a different mechanism of action of alkamides against fungi and bacteria, indicating that the 2E-unsaturation is insufficient for the fungitoxic action. It requires further unsaturation in conjunction with the unsaturation in either positions 6Z, 8E or both (Molina-Torres et al. 2004). UV light-mediated antifungal activity was reported for extracts of some Echinacea species. In this case the combination of high levels of the C12 tetraene amides 65a/66a together with various polyacetylenes exhibited a very effective phototoxic action against a variety of clinically isolated humanpathogenic fungi (Merali et al. 2003). In a more recent study the hypothesis was tested, that alkamides from Echinacea exert antifungal activity by disrupting the fungal cell wall/membrane complex (Cruz et al. 2014). The results showed that S. cerevisiae cells exposed to sub-inhibitory concentrations of each of seven synthetic alkamides found in Echinacea extracts exhibited increased frequencies of cell wall damage and death that were comparable to the positive control caspofungin, a lipopeptide antifungal drug of the new class of echinocandins. Among the alkamides tested, the acetylenic C11 derivatives 166a and 169a showed the greatest antifungal and cell wall disruption activities, as opposed to the five remaining less active C12 derivatives, suggesting that the length of the acyl chain has an effect on the biological activity. The presence of a diynoic moiety in 166a and 169a enhanced cell wall disruption activity while an opposite trend was observed in the membrane disruption assay. In the latter case the dienoic group in the olefinic C12 derivatives 62a, 63a, 65a, and 66a was shown to be more effective. Based on these findings the authors proposed that alkamides found in Echinacea act synergistically to disrupt the fungal cell wall/membrane complex (Cruz et al. 2014).
Antiprotozoal activities
Acmella species are used as traditional herbal medicines in Africa and India to treat malaria. Spilanthol (92a) and the acetylenic C11 alkamide 169a, isolated from A. paniculata (=Spilanthes acmella), were tested against the chloroquine-resistent strain K1 of Plasmodium falciparum, originating from Thailand, and the mildly chloroquine-resistent strain PFB, originating from Brazil. For the Brazilian strain the IC50 (half maximal inhibiting concentration) for 92a and 169a were 16.5 and 41.4 µg/ml, respectively, while for the Thai strain the effect was significantly greater, with 5.8 and 16.3 µg/ml, respectively. Moreover, a comparison of fresh plant water extract with an ethanol extract, containing ten times the concentration of 92a, was performed in vivo on Plasmodium yoellii yoellii-infected mice. Surprisingly, the water extract exhibited a higher activity with 53 % reduction in parasitemia than the ethanol extract with 36 % reduction. This suggested that in addition to 92a, there may be water soluble constituents that are also active against Plasmodium, or the treatment could have induced immunological activity (Spelman et al. 2011). In the course of an ongoing screening of plants of the family Asteraceae for antiprotozoal activity, a CH2Cl2-extract from the flowering aerial parts of Achillea ptarmica was found to be active in vitro against Trypanosoma brucei rhodesiense with IC50 = 0.67 µg/ml and P. falciparum with IC50 = 6.6 µg/ml. From the bioactive fractions six alkamides were isolated from which pellitorine (101a) and 8,9-Z-dehydropellitorine (102a) were shown to be the major components accompanied by the acetylenic C11 derivatives 167a,b,c and C14 anacyclin (141a). Pellitorine (101a) exhibited the highest activity against P. falciparum with an IC50 value at 3.26 µg/ml. The antiplasmodial activity was thus about twofold higher than that of the crude extract. Although 8,9-Z-dehydropellitorine (102a) was shown to be the most active compound against T. brucei with an IC50 = 2.0 µg/ml, it corresponded only to a threefold lower activity in comparison with the crude extract. Since the promising activity of the crude extract could not be attributed to any of the isolated alkamides on its own it was hence conceivable that either some minor, not as yet isolated, constituents were responsible for the high activity, or a synergistic effect was at work (Althaus et al. 2014).
Miscellaneous properties
Spilanthol (92a) was reported to be also a potential agent to control schistosomiasis. It was tested against the freshwater snail Physa occidentalis and the cercariae released by the mollusc. Above 50 mg/l in H2O snails were shown to be inactive after 60 min and dead within 18 h. At 150 mg/l, the solubility limit for 92a, cercarial emergence ceased and the snails showed immobility after 30 min. The cercariae ceased to move after 5 s and convulsed after 1 min (Johns et al. 1982). Acaricidal activity was detected in the hexane extract of the aerial parts of A. oleracea which most likely can be attributed to the presence of the major constituent 92a. It was highly effective against larvae of the cattle tick Rhipicephalus microplus with an LC50 = 0.8 mg/ml, and against engorged females, where it reduced oviposition and hatchability of eggs with an LC50 = 79.7 mg/ml (Castro et al. 2014). 92a was also shown to act as plant growth-promoting substance. Together with the two more saturated alkamides dec-2E-enoic (89a) and decanoic acid isobutylamide (86a) it was found to alter the architecture of the root system and regulate cell division and differentiation processes in Arabidopsis thaliana (Ramírez-Chávez et al. 2004; López-Bucio et al. 2006; Morquecho-Contreras et al. 2010).
Medical properties
Anti-inflammatory and analgesic activities
The roots of H. longipes are claimed to be effective for the alleviation of toothache pain and are used extensively in the rural areas of Mexico. Spilanthol (92a) was identified as the active principle and its analgesic activity was first evaluated by Ogura et al. (1982). The topical anti-inflammatory effects of 92a and its saturated analog 86a were evaluated in the mouse ear edema test using arachidonic acid (AA) and phorbol myristate acetate (PMA) as irritating agents. 92a was shown to inhibit the AA-induced edema in a dose-dependent manner with an ED50 value at 1.2 mg/ear, while 86a displayed the same effect with an ED50 at 0.9 mg/ear. The acute PMA-induced inflammation was inhibited with ED50 values at 1.3 mg/ear with 92a and 1.1 mg/ear with 86a (Hernández et al. 2009). Various pharmacological experiments were carried out to study the mechanism of action of the antinociceptive and analgesic effects of H. longipes root extract and specifically of its main alkamide 92a (Rios et al. 2007; Cilia-López et al. 2010; Cariño-Cortés et al. 2010; Déciga-Campos et al. 2010, 2012).
Spilanthol (92a) was reported to exhibit a dose-dependent inhibition of 5-lipoxygenase with an IC50 value at 50 µmol, and was assumed to be the anti-inflammatory principle of the extract of A. oleracea, topically used in anti-rheumatic therapy. In contrast, it did not show an inhibiting effect on prostaglandin synthase. A similar inhibitory effect on 5-lipoxygenase was found for an alkamide fraction of the root extract of E. purpurea, consisting of ten different polyunsaturated isobutylamides. This activity contributed to the antiphlogistic activity of the drug formerly attributed to the water extract and the polysaccharides (Wagner et al. 1989). Since Echinacea and Achillea species were used in traditional medicine in North America and Europe for anti-inflammatory purposes, various alkamides from both genera were tested for in vitro inhibition of 5-lipoxygenase and cyclooxygenase (Müller-Jakic et al. 1994). The isomeric C12-tetraenoic isobutylamides 65a/66a were shown to be the major constituents of the root extract of E. angustifolia and a 1:1 mixture inhibited both, cyclooxygenase 54.7 % and 5-lipoxygenase 62.2 % at 50 µmol. However, in comparison to the n-hexan extract with 62.4 and 81.8 % inhibition, respectively, the mixture 65a/66a was shown to be less potent suggesting the existence of other active compounds or synergistic effects. In order to determine the main active principle, and to perform structure–activity relationship studies eight alkamides from E. angustifolia and ten from Achillea species together with one each from Anacyclus pyrethrum and Aaronsohnia factorovskyi (=Matricaria pubescens) were tested. In summary, all compounds appeared to be more or less potent inhibitors of cyclooxygenase, but only few inhibited 5-lipoxygenase. For the latter the mixture 65a/66a from E. angustifolia showed the highest activity followed by the C14 acetylenic piperidide 144b from Achillea spinulifolia and the C16 acetylenic pyrrolide 130q from A. ageratifolia. For cyclooxygenase the two closely related C16 and C15 acetylenic isobutylamides 135a and 136a from E. angustifolia, the thiophene 184a from A. factorovskyi, and the C14 pyrrolidide 139k from Achillea nana exhibited higher inhibition than the dominating isomers 65a/66a (Müller-Jakic et al. 1994). In the study of Clifford et al. (2002) the latter did not show activity at 100 µg/ml for either cyclooxygenase (COX)-1 or -2. Here, the two isomers undeca-2E,4Z-dien-8,10-diynoic (168a) and undeca-2Z,4E-dien-8,10-diynoic isobutylamide (166a) exhibited 36 and 60 % inhibition, respectively, of COX-1 activity. 166a exhibited the highest inhibition to both COX-1 and -2 enzymes. The two 2-methylbutylamides 161g and 168g, differing by one methyl group in the amine part, demonstrated higher activity against the two enzymes when compared to the corresponding isobutylamides (161a, 168a). The two latter, by contrast, differing among each other by one terminal methyl group in the acid chain, exhibited equal inhibitory activity (Clifford et al. 2002).
Since inhibition of COX-2 was proven as an effective strategy to suppress pain and inflammation, the impact of several alkamides, isolated from the roots of E. angustifolia, on both activity and expression of this enzyme was investigated. A 48-h treatment of H4 human neuroglioma cells with the CO2 extract led to a significant suppression of prostaglandin E2 (PGE2) formation, the major product of the COX-2 pathway. From eight different alkamides the three acetylenic derivatives undeca-2Z-ene-8,10-diynoic isobutylamide (170a), dodeca-2E-en-8,10-diynoic isobutylamide (163a), and dodeca-2E,4Z-dien-8,10-diynoic 2-methylbutylamide (161g) were shown to contribute to this response and interfere with COX-2 activity (Hinz et al. 2007). Moreover, inhibition of PGE2 formation in lipopolysaccharide (LPS)-stimulated RAW264.7 mouse macrophage cells was assessed with an enzyme immunoassay following treatments with Echinacea extracts or synthesized alkamides. All of the 13 alkamides screened significantly inhibited the production of PGE2 at 50 µmol. The C12-tetraene 66a and the acetylenic C12 derivatives 162a and 163a were shown to reduce the PGE2 levels at 25 µmol. Only 163a significantly inhibited PGE2 production at 10 µmolar. Because the innate concentrations of individual alkamides found in crude extracts did not reach concentrations shown to have significant PGE2 inhibition, it was assumed that they might have contributed toward the anti-inflammatory activity in a synergistic or additive manner (LaLone et al. 2007). Similar results were obtained in a previous study where Echinacea alkamides showed anti-inflammatory activity as measured by inhibition of nitric oxide (NO) production in LPS-stimulated RAW264.7 cells. As a pro-inflammatory mediator NO was significantly reduced by a mixture of several alkamides ranging from 1.6 to 30 µg/ml (Chen et al. 2005). On the basis of a mitogen-induced murine skin inflammation study a comparative metabolomics approach coupled with cell- and gene-based assays was used to evaluate the anti-inflammatory activity of three Echinacea species. The order of efficacy was E. angustifolia > E. purpurea > E. pallida (Hou et al. 2010). In order to characterize the anti-inflammatory activity of the specific alkamides the authors compared an alkamide-enriched fraction with the predominant C12 isomers 65a/66a. Immunoblotting analysis of COX-2 protein expression in LPS-stimulated macrophages showed better suppression for the alkamide fraction of the E. purpurea root extract than the isolated isomers 65a/66a. In accordance with previous findings (Müller-Jakic et al. 1994; LaLone et al. 2007) it was suggested, that the other alkamide(s) present in the mixture, apart from 65a/66a, also contributed to the inhibition of COX-2 activity. The anti-inflammatory activity of 65a/66a in mice was shown to be directly associated with the protective effect in LPS/D-GalN-induced acute hepatitis and liver injury (Hou et al. 2011).
Immunomodulation and cannabinomimetic effects
In the prevention and treatment of common cold Echinacea plant preparations are widely used in North America and Europe. The standardized tincture Echinaforce™ was analyzed and found that it induced de novo synthesis of tumor necrosis factor α (TNF-α) mRNA in primary human monocytes/macrophages, but not TNF-α protein. Moreover, LPS-stimulated TNF-α protein was potently inhibited in the early phase but prolonged in the late phase. Among the main constituents of the tincture the olefinic C12 isobutylamides 65a/66a, 62a, and 63a were shown to be responsible for this effect which was ascribed to their interactions with the cannabinoid type 2 receptor (CB2) on monocytes (Gertsch et al. 2004). These findings were independently confirmed by Woelkart et al. (2005). Due to the structural similarity of alkamides with the endogenous cannabinoid ligand anandamide (N-arachidonoyl ethanolamide) (Fig. 8) they investigated the receptor binding of twelve Echinacea alkamides to rodent cannabinoid receptors CB1 and CB2. Concerning selectivity the acetylenic pentadeca-2E,9Z-dien-12,14-diynoic acid isobutylamide (136a) showed the highest affinity for CB1 with an inhibitory constant (Ki) of 2.0 µmol, followed by dodeca-2E-en-8,10-diynoic acid 2-methylbutylamide (163g) with a Ki of 4.1 µmol, while tetradeca-2E-en-10,12-diynoic acid isobutylamide (145a) with a Ki of 1.9 µmol was shown to be the most selective and most affine ligand for CB2. A further evidence of CB-receptor-binding of alkamides was demonstrated by Raduner et al. (2006). At concentrations below 100 nmol, the two olefinic C12-isobutylamides 65a and 62a potently displaced the radioligand from membrane recombinantly overexpressing CB2 receptors with Ki values of 57 ± 14 and 60 ± 13 nmol, respectively. Immunomodulatory effects of 65a and 62a were also investigated by Sasagawa et al. (2006) who reported on their inhibitory effect to interleukin-2 formation on human Jurkat T cells. In the review of Gertsch et al. (2006) Echinacea alkamides were described as a new class of cannabinomimetics which specifically engage and activate the CB2 receptors and are likely to provide novel lead structures for the development of CB2-directed drugs. The affinity of alkamides to cannabinoid receptors was shown to depend on their solubility and thus it is important to understand their physicochemical behavior in an aqueous environment. Raduner et al. (2007) discovered that 62a and 65a form micelles in aqueous medium which could not be observed for the acetylenic C11 derivative 169a (which has no affinity to cannabinoid receptors) or for structurally related endogenous cannabinoids, such as anandamide (Fig. 8). Depending on concentration microscopy images showed that 62a formed globular and rod-like supermicelles which did not bind to CB2. While anandamide did not aggregate and thus freely interacted with CB2, 62a exhibited differential receptor affinity competing with self-aggregation as a function of concentration. Woelkart and Bauer (2007) summarized the results of pharmacological experiments with Echinacea extracts and demonstrated the significant anti-inflammatory and immunomodulatory properties of individual alkamides. Gertsch (2008) reported on the increasing evidence that fatty acid derived alkamides can modulate the action of endogenous lipid signals. In a more recent study the functional interaction of alkamides from Heliopsis helianthoides var. scabra and Lepidium meyeni (“maca”) with the endocannabinoid system was investigated. The newly described hexadeca-2,4,9,12-tetraenoic acid 2-methylbutylamide (Alk 3 in Fig. 8) from H. helianthoides var. scabra and 27t from L. meyeni showed submicromolar and selective binding affinities for CB1 with Ki values of 0.31 and 0.48 µmol, respectively. Due to the structural similarities of the 9,12 double-bond system of the lineleoyl group in 27t with anandamide the results provided additional strong evidence of the endocannabinoid substrate mimicking of some alkamides (Fig. 8) (Hajdu et al. 2014).
C18 and C16 alkamides isolated from H. helianthoides var. scabra and L. meyeni compared with the C20 endocannabinoid anandamide. Compounds in the boxes showed submicromolecular binding affinities for the CB1 recepetor (Hajdu et al. 2014). The newly described alkamides Alk 1–3 are not listed in Tables 2, 3 and 4
On the basis of a newly developed plate-based CB1/CB2 receptor functional assay the extracts of several Zanthoxylum species were screened for active compounds. The objective was to identify novel antagonists of CB1, which simultaneously display agonist activity against CB2. Compounds matching this criterion could be potential candidates for the treatment of type-1 diabetes. The extract from Z. bungeanum was deemed active, leading to the identification of eight alkamides of the sanshool group (Fig. 7). One of them, δ-sanshool (59a) (erroneously published as γ-sanshool), was obtained as a promising lead compound (Dossou et al. 2013).
Pharmacokinetics and bioavailability
The bioavailability of the isomeric C12 tetraenes 65a/66a in human blood after oral administration was reported by Dietz et al. (2001), and their permeability through Caco-2 monolayers described by Jager et al. (2002). The isomers were found to be nearly completely transported from the apical to the basolateral side of the monolayers in 6 h by passive diffusion and that no significant metabolism occurred. The apparent permeability of twelve Echinacea alkamides was investigated by Matthias et al. (2004). They found that most of the alkamides readily cross the Caco-2 monolayer with many more than 50 % transported after 90 min and that increasing unsaturation gave rise to increases in the apparent permeability: e.g. the 2,4-dienes 161g and 168a appeared to be more readily transported than the equivalent 2-enes 163g and 169a. Methylation at the terminal diyne group, e.g. in 161a, caused a reduction of permeability compared to the corresponding structure of 168a with a terminal acetylenic hydrogen. Woelkart et al. (2009) showed that the tetraenes 65a/66a were available in plasma and rat tissues with a rapid passage across the blood–brain barrier. For these compounds an LC–MS/MS assay was developed and validated for quantification in human plasma. The results showed that they can be accurately and precisely quantified and are chemically stable under relevant conditions (Goey et al. 2012). Furthermore, the interaction of eight Echinacea alkamides with P-glycoprotein, a major constituent of the blood–brain barrier, showed that four derivatives inhibited P-glycoprotein in primary endothelial cells freshly isolated from porcine brain blood vessels (Mahringer et al. 2013). The transdermal permeation behavior of spilanthol (92a) from Acmella paniculata (=Spilanthes acmella) extracts was investigated by Boonen et al. (2010a, b), who reported on the influence of ethanol (65 %) and propylene glycol (10 %) as solvents. Similar permeation properties were recently also described for the Anacyclus pyrethrum extract and the main alkamide pellitorine (101a) (Veryser et al. 2014).
Miscellaneous properties
As part of the crude drug Dai-kenchu-to, a Chinese prescription frequently used in recent Japan to treat paralytic ileus after laparotomy and severe constipation, the fruits of Zanthoxylum piperitum are known to relax the gastric body as well as contract the ileum and distal colon. Using the gastrointestinal tract isolated from guinea pig it was shown that the alkamides γ- (58a), β-sanshool (74a), and especially β-hydroxysanshool (74d) isolated from Z. piperitum potently induced ileal contraction and acted directly on smooth muscle of the gastric body (Hashimoto et al. 2001).
The nuclear factor peroxisome proliferator-activated receptor (PPAR) γ is predominantly found in adipose tissue and is known to regulate adipocyte differentiation as well as glucose homeostasis. A hexane extract of the flowers of Echinacea purpurea was found to significantly activate PPARγ without stimulating adipocyte differentiation. Bioassay-guided fractionations yielded the C12 alkamides 62a, 65a, 66a, 157a, and the newly described hexadeca-2E,9Z,12Z,14E-tetraenoic acid isobutylamide (45a) together with three fatty acids that all activated PPARγ. The C16 alkamide 45a exhibited a significant activation of more than tenfold at 30 μmol without a concurrent stimulation of adipocyte differentiation, but still retained the insulin-sensitizing effects. This makes it a potential beneficial PPARγ partial agonist (Christensen et al. 2009).
The subterranean parts (hypocotyl tubers) of Lepidium meyeni (“maca”) are used as foodstuff in the central Andes of Peru and are also known for its aphrodisiac properties (Zhao et al. 2005). The effect of a lipid extract on sexual behavior in mice and rats was investigated by Zheng et al. (2000). They proposed that alkamides (“macamides”) and the structurally corresponding 5-oxo-6E,8E-octadecadienoic acid (“macaene”) (24) are involved in improving sexual performance. However, in spite of the many studies already carried out on this drug, the effective substances and the mechanism of action were not fully elucidated so far (Wang et al. 2007). The roots of Anacyclus pyrethrum, commonly referred to as “Akarkara” in Ayurvedic system of Indian medicine are also considered aphrodisiac and sexual stimulant (Sharma et al. 2009; Annalakshmi et al. 2012). Administration of alkamide-rich extracts of A. pyrethrum showed improvement in sexual behavior of male rats. With respect to the similar properties observed in L. meyeni the authors suspected the presence of pellitorine (101a) as possible contributor to these effects and hypothesized that alkamides may mimic the action of testosterone or stimulate secretion of testosterone that improves sexual function (Sharma et al. 2010, 2013). Similar effects on sexual behavior in male rats were detected in the ethanolic flower extract of Acmella paniculata. In this case spilanthol (92a) and other alkamides of the extract were supposed as causative agents (Sharma et al. 2011).
The lipophilic extract from the fruit husks of Z. bungeanum (Zanthalene®), mainly consisting of α-(72d) and β-hydroxysanshool (74d), was validated as an anti-itching cosmetic ingredient and was shown to potently inhibit synaptic transmission. Thus, its capacity to relax subcutaneous muscles and to act as topical lifting agent for wrinkles was investigated. The results of this study fully qualified the extract as a functional cosmetic ingredient for the temporary improvement of skin wrinkles (Artaria et al. 2011).
Chemotaxonomic significance
Due to the absence of Δ2-, Δ4-double bonds in the unsaturated acid parts, and the combination with benzylamines (t, u), the “macamides” from Lepidium meyeni of the Brassicaceae can be clearly distinguished from other plant-derived alkamides. The accumulation of alkamides with elongated saturated and olefinic acid moieties (Table 1) represents a typical biogenetic trend of the Piperaceae and Brassicaceae, while the formation of acetylenic derivatives (Table 3) is a characteristic chemical feature of the Asteraceae solely known from the two tribes Anthemideae and Heliantheae. Compounds with six- (b, e, h) or five-membered ring amines (k, n, q) and those with phenylalanine derived residues (c, f, i, l, o, r) typify the two families Asteraceae and Piperaceae. Within the Asteraceae derivatives with a 2-methylbutylamine residue (g) distinguishes the tribe Heliantheae from Anthemideae which is characterized by alkamides with dominating ring amines. Specific desaturase activity in the acid part leads to the conjugated triene system of the sanshool group characteristic for Zanthoxylum species comprising C14-pentaene and C12-tetraene derivatives (Fig. 7; Table 2). By contrast, the isomeric C12-tetraenes 65a/66a, containing a conjugated diene group near the methyl end, represent a typical chemical character of the related Heliantheae genera Echinacea, Acmella, and Salmea.
Broad-based phytochemical comparisons within the tribe Anhemideae exhibited a predominant alkamide formation in many Achillea species, while most of the other genera were characterized by a variety of polyacetylenes (Greger 1977, 1988; Christensen 1992). Alkamide formation was also reported for the genera Anacyclus, Leucocyclus and Otanthus (Table 4) whose close relationships to Achillea were already suggested previously (Greger 1978; Greger et al. 1981). Meanwhile multidisciplinary studies presented a new circumscription of Achillea including the former unispecific genera Leucocyclus (as Achillea formosa (Boiss.) Sch. Bip.) and Otanthus (as Achillea maritima (L.) Ehrend. and Y.-P. Guo). Extended DNA sequences also suggested Anacyclus to be the sister genus of Achillea (Ehrendorfer and Guo 2005, 2006). The European species/cytotypes of the taxonomically complex A. millefolium group can be distinguished from the Asian and North-American representatives by the predominance of the C10-2E,4E,6Z-trienoic acid piperideide (105e) (Greger and Hofer 1989; Greger and Werner 1990) (Fig. 9). With regard to the enormous capacity for vegetative reproduction and gregarious clonal growth due to the abundant formation of rootsuckers in many Achillea species, e.g. in A. wilhelmsii, the accumulation of alkamides in these parts deserves also ecological attention (Greger and Hofer 1987). Taking into account that unsaturated alkamides were considered to be unstable when heated and to be non-volatile with water vapour it is noteworthy that the three piperideides 101e, 105e, 106e together with 101a and 101b were detected after distillation in a GC/MS analyses of the root oil of A. distans ssp. distans, a member to the A. millefolium group (Lazarević et al. 2010).
Notes
In addition, a saturated C28 tyramide (f) has been isolated from Zanthoxylum gilletii (Wansi et al. 2009).
References
Abarbri M, Parrain JL, Duchêne A (1998) A synthetic approach to natural dienamides of insecticidal interest. Synth Commun 28:239–249
Achenbach H, Fietz W, Wörth J, Waibel R, Portecop J (1986) Constituents of tropical medicinal plants, IXX. GC/MS-investigations of the constituents of Piper amalago—30 new amides of the piperine-type. Planta Med 52:12–18
Acree F Jr, Jacobson M, Haller HL (1945) An amide possessing insecticidal properties from the roots of Erigeron affinis DC. J Org Chem 10:236–242
Adesina SK, Reisch J (1988) Arnottianamide and other constituents of Zanthoxylum gillettii root. J Nat Prod 51:601–602
Ahn JW, Ahn MJ, Zee OP, Kim EJ, Lee SG, Kim HJ, Kubo I (1992) Piperidine alkaloids from Piper retrofractum fruits. Phytochemistry 31:3609–3612
Aihara T (1950) On the principles of Xanthoxylum piperitum DC. II. The isolation of sanshoöls and the structure of sanshoöl I. J Pharm Soc Jpn 70:405–409
Ainslie RD, Barchi JJ Jr, Kuniyoshi M, Moore RE, Mynderse JS (1985) Structure of malyngamide C. J Org Chem 50:2859–2862
Althaus JB, Kaiser M, Brun R, Schmidt TJ (2014) Antiprotozoal activity of Achillea ptarmica (Asteraceae) and its main alkamide constituents. Molecules 19:6428–6438
Andrade-Neto M, Mendes PH, Silveira ER (1996) An imidazole alkaloid and other constituents from Pilocarpus trachyllophus. Phytochemistry 42:885–887
Annalakshmi R, Uma R, Subashchandran G, Muneeswaran A (2012) A treasure of medicinal herb—Anacyclus pyrethrum, a review. Indian J Drugs Dis 1:59–67
Ardjomand-Woelkart K, Bauer R (2014) Echinacea: a survey of current literature [Echinacea: Eine Bestandsaufnahme der neueren Literatur]. Z Phytother 35:128–134
Artaria C, Maramaldi G, Bonfigli A, Rigano L, Appendino G (2011) Lifting properties of the alkamide fraction from the fruit husks of Zanthoxylum bungeanum. Int J Cosmet Sci 33:328–333
Asano M, Kanematsu T (1927) Ueber die Bestandteile von Spilanthes acmella L. f. fusca Makino. J Pharm Soc Jpn 544:521–525
Asano M, Kanematsu T (1931) Über Sanshool, einen Bestandteil von Xanthoxylum piperitum D. C. J Pharm Soc Jpn 51:384–390
Asano M, Kanematsu T (1932) Über die Konstitution des Spilanthols, des scharfen Prinzips der Parakresse. Ber Dtsch Chem Ges 65:1602–1604
Aza-González C, Núñez-Palenius HG, Ochoa-Alejo N (2011) Molecular biology of capsaicinoid biosynthesis in chili pepper (Capscicum spp.). Plant Cell Rep 30:695–706
Bader M, Stark TD, Dawid C, Lösch S, Hofmann T (2014) All-trans-configuration in Zanthoxylum alkylamides swaps the tingling with a numbing sensation and diminishes salivation. J Agric Food Chem 62:2479–2488
Bae SS, Ehrmann BM, Ettefagh KA, Cech NB (2010) A validated liquid chromatography-electrospray-ionization-mass spectrometry method for quantification of spilanthol in Spilanthes acmella (L.) Murr. Phytochem Anal 21:438–443
Banerji A, Pal SC (1982) A new alkamide from Piper sylvaticum. Phytochemistry 21:1321–1323
Banerji A, Pal SC (1983) Total synthesis of sylvamide, a Piper alkamide. Phytochemistry 22:1028–1030
Bauer R, Remiger P, Wagner H (1988) Alkamides from the roots of Echinacea purpurea. Phytochemistry 27:2339–2342
Bauer R, Remiger P, Wagner H (1989) Alkamides from the roots of Echinacea angustifolia. Phytochemistry 28:505–508
Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D (2008) Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci 11:772–779
Binns SE, Livesey JF, Arnason JT, Baum BR (2002) Phytochemical variation in Echinacea from roots and flowerheads of wild and cultivated populations. J Agric Food Chem 50:3673–3687
Bohlmann F, Dallwitz E (1974) Notiz über die Biogenese von Polyinamiden. Chem Ber 107:2120–2122
Bohlmann F, Grenz M (1966) Über die Inhaltsstoffe aus Echinacea-Arten. Chem Ber 99:3197–3200
Bohlmann F, Hoffmann H (1983) Further amides from Echinacea purpurea. Phytochemistry 22:1173–1175
Bohlmann F, Zdero C (1967) Über zwei neue Isobutylamide aus Chrysanthemum frutescens L. Chem Ber 100:104–106
Bohlmann F, Zdero C (1970) Die Inhaltsstoffe aus Anthemis fuscata Brot. Chem Ber 103:2856–2859
Bohlmann F, Zdero C (1973) Neue Inhaltsstoffe aus Achillea-Arten. Chem Ber 106:1328–1336
Bohlmann F, Zdero C (1979) Neue C10-Säureamide, Furaneremophilane und andere Inhaltsstoffe aus bolivianischen Senecio-Arten. Phytochemistry 18:125–128
Bohlmann F, Burkhardt T, Zdero C (1973) Naturally occurring acetylenes. Academic Press, London
Bohlmann F, Zdero C, Suwita A (1974) Weitere Amide aus der Tribus Anthemideae. Chem Ber 107:1038–1043
Bohlmann F, Fritz U, Dutta L (1980a) Neue Acetylenverbindungen aus Leucanthemum-Arten und Revision der Struktur eines Germacranolids. Phytochemistry 19:841–844
Bohlmann F, Ziesche J, Robinson H, King RM (1980b) Neue Amide aus Spilanthes alba. Phytochemistry 19:1535–1537
Bohlmann F, Gerke T, Ahmed M, King RM, Robinson H (1983) Neue N-Isobutylamide aus Heliopsis-Arten. Liebigs Ann Chem 1202–1206
Bohlmann F, Hartono L, Jakupovic J (1985) Highly unsaturated amides from Salmea scandens. Phytochemistry 24:595–596
Boonen J, Baert B, Burvenich C, Blondeel P, De Saeger S, De Spiegeleer B (2010a) LC-MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bio-active spilanthol. J Pharm Biomed Anal 53:243–249
Boonen J, Baert B, Roche N, Burvenich C, De Spiegeleer B (2010b) Transdermal behaviour of the N-alkylamide spilanthol (affinin) from Spilanthes acmella (Compositae) extracts. J Ethnopharmacol 127:77–84
Boonen J, Bronselaer A, Nielandt J, Veryser L, De Tré G, De Spiegeleer B (2012a) Alkamid database: chemistry, occurrence and functionality of plant N-alkylamides. J Ethnopharmacol 142:563–590
Boonen J, Sharma V, Dixit VK, Burvenich C, De Spiegeleer B (2012b) LC-MS N-alkylamide profiling of an ethanolic Anacyclus pyrethrum root extract. Planta Med 78:1787–1795
Bowden K, Ross WJ (1963) The local anaesthetic in Fagara xanthoxyloides. J Chem Soc 3503–3505
Bryant BP, Mezine I (1999) Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res 842:452–460
Buchheim R (1876) Ueber die pharmakologische Gruppe des Piperins. Arch Exp Pathol Pharm 5:455–462
Burden RS, Crombie L (1969) Amides of vegetable origin Part XII. A new series of alka-2,4-dienoic tyramin-amides from Anacyclus pyrethrum D.C. (Compositae). J Chem Soc C Org 19:2477–2481
Cahoon EB, Lindqvist Y, Schneider G, Shanklin J (1997) Redesign of soluble fatty acid desaturases from plants for altered substrate specificity and double bond position. Proc Natl Acad Sci USA 94:4872–4877
Cahoon EB, Carlson TJ, Ripp KG, Schweiger BJ, Cook GA, Hall SE, Kinney AJ (1999) Biosynthetic origin of conjugated double bonds: production of fatty acid components of high-value drying oils in transgenic soybean embryos. Proc Natl Acad Sci USA 96:12935–12940
Cahoon EB, Ripp KG, Hall SE, Kinney AJ (2001) Formation of conjugated Δ8, Δ10-double bonds by Δ12-oleic-acid desaturase-related enzymes. J Biol Chem 276:2637–2643
Cambie RC, Gardner JN, Jones ERH, Lowe G, Read G (1963) Chemistry of the higher fungi. Part XIV. Polyacetylenic metabolites of Poria sinuosa Fr. J Chem Soc 2056–2064
Cariño-Cortés R, Gayosso-De-Lucio JA, Ortiz MI, Sánchez-Gutiérrez M, García-Reyna PB, Cilia-López VG, Pérez-Hernández N, Moreno E, Ponce-Monter H (2010) Antinociceptive, genotoxic and histopathological study of Heliopsis longipes S. F. Blake in mice. J Ethnopharmacol 130:216–221
Casado M, Ortega MG, Peralta M, Agnese AM, Caprera JL (2009) Two new alkamides from roots of Acmella decumbens. Nat Prod Res 23:1298–1303
Castro KNC, Lima DF, Vasconcelos LC, Leite JRSA, Santos RC, Paz Neto AA, Costa-Júnior LM (2014) Acaricide activity in vitro of Acmella oleracea against Rhipicephalus microplus. Parasitol Res 113:3697–3701
Chaaib F, Queiroz EF, Ndjoko K, Diallo D, Hostettmann K (2003) Antifungal and antioxidant compounds from the root bark of Fagara zanthoxyloides. Planta Med 69:316–320
Chan GW, Berry D, DeBrosse CW, Hemling ME, MacKenzie-LoCasto L, Offen PH, Westley JW (1993) Conioidines A and B, novel DNA-interacting pyrrolidines from Chamaesaracha conioides. J Nat Prod 56:708–713
Chen IS, Chen TL, Lin WY, Tsai IL, Chen YC (1999) Isobutylamides from the fruit of Zanthoxylum integrifoliolum. Phytochemistry 52:357–360
Chen Y, Fu T, Tao T, Yang J, Chang Y, Wang M, Kim L, Qu L, Cassady J, Scalzo R, Wang X (2005) Macrophage activating effects of new alkamides from the roots of Echinacea species. J Nat Prod 68:773–776
Chen JJ, Chung CY, Hwang TL, Chen JF (2009) Amides and benzenoids from Zanthoxylum ailanthoides with inhibitory activity on superoxide generation and elastase release by neutrophils. J Nat Prod 72:107–111
Christensen LP (1992) Acetylenes and related compounds in Anthemideae. Phytochemistry 31:7–49
Christensen LP, Lam J (1991) Acetylenes and related compounds in Heliantheae. Phytochemistry 30:11–49
Christensen KB, Petersen RK, Petersen S, Kristiansen K, Christensen LP (2009) Activation of PPARγ by metabolites from the flowers of purple coneflower (Echinacea purpurea). J Nat Prod 72:933–937
Christodoulopoulou L, Tsoukatou M, Tziveleka LA, Vagias C, Petrakis PV, Roussis V (2005) Piperidinyl amides with insecticidal activity from the maritime plant Otanthus maritimus. J Agric Food Chem 53:1435–1439
Cilia-López VG, Juárez-Flores BI, Aguirre-Rivera JR, Reyes-Agüero JA (2010) Analgesic activity of Heliopsis longipes and its effect on the nervous system. Pharm Biol 48:195–200
Clifford LJ, Nair MG, Rana J, Dewitt DL (2002) Bioactivity of alkamides isolated from Echinacea purpurea (L.) Moench. Phytomedicine 9:249–253
Cortez-Espinosa N, Aviña-Verduzco JA, Ramírez-Chávez E, Molina-Torres J, Ríos-Chávez P (2011) Valine and phenylalanine as precursors in the biosynthesis of alkamides in Acmella radicans. Nat Prod Commun 6:857–861
Crombie L (1954) Isolation and structure of neo-herculin from Zanthoxylum clava-herculis L. Nature 174:833
Crombie L (1955a) Amides of vegetable origin. Part III. Structure and stereochemistry of neoherculin. J Chem Soc 995–998
Crombie L (1955b) Amides of vegetable origin. Part IV. The nature of pellitorine and anacyclin. J Chem Soc 999–1006
Crombie L, Tayler JL (1957) Amides of vegetable origin. Part VIII. The constitution and configuration of the sanshoöls. J Chem Soc 2760–2766
Crombie L, Krasinski AHA, Manzoor-i-Khuda M (1963) Amides of vegetable origin. Part X. The stereochemistry and synthesis of affinin. J Chem Soc 4970–4976
Cruz I, Cheetham JJ, Arnason JT, Yack JE, Smith ML (2014) Alkamides from Echinacea disrupt the fungal cell wall-membrane complex. Phytomedicine 21:435–442
Curry J, Aluru M, Mendoza M, Nevarez J, Melendrez M, O´Connell MA (1999) Transcripts for possible capsaicinoid biosynthetic genes are differentially accumulated in pungent and non-pungent Capsicum spp. Plant Sci 148:47–57
Dawid C, Henze A, Frank O, Glabasnia A, Rupp M, Büning K, Orlikowski D, Bader M, Hofmann T (2012) Structural and sensory characterization of key pungent and tingling compounds from black pepper (Piper nigrum L.). J Agric Food Chem 60:2884–2895
Déciga-Campos M, Rios MY, Aguilar-Guadarrama AB (2010) Antinociceptive effect of Heliopsis longipes extract and affinin in mice. Planta Med 76:665–670
Déciga-Campos M, Arriaga-Alba M, Ventura-Martínez R, Aguilar-Guadarrama B, Rios MY (2012) Pharmacological and toxicological profile of extract from Heliopsis longipes and affinin. Drug Dev Res 73:130–137
Dembitsky VM, Shkrob I, Rozentsvet OA (2000) Fatty acid amides from freshwater green alga Rhizoclonium hieroglyphicum. Phytochemistry 54:965–967
Devkota KP, Wilson J, Henrich CJ, McMahon JB, Reilly KM, Beutler JA (2013) Isobutylhydroxyamides from the pericarp of Nepalese Zanthoxylum armatum inhibit NF1-defective tumor cell line growth. J Nat Prod 76:59–63
Dhar KL, Atal CK (1967) Occurrence of N-isobutyldeca-trans-2-trans-4-dienamide in Piper longum Linn. and Piper peepuloides Royle. Indian J Chem 5:588–589
Dietz B (2002) Untersuchungen zu den Inhaltsstoffen von Echinacea atrorubens sowie zur Wirkung und Bioverfügbarkeit von Alkamiden. Dissertation, Heinrich-Heine-Universität Düsseldorf
Dietz B, Bauer R (2001) The constituents of Echinacea atrorubens roots and aerial parts. Pharm Biol 39:11–15
Dietz B, Heilmann J, Bauer R (2001) Absorption of dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides after oral application of Echinacea purpurea tincture. Planta Med 67:863–864
Do Nascimento JC, De Paula VF, David JM, David JP (2012) Occurrence, biological activities and 13C NMR data of amides from Piper (Piperaceae). Quim Nova 35:2288–2311
Domínguez XA, Sánchez VH, Slim SJ, Jakupovic J, Lehmann L, Bohlmann F (1987) Highly unsaturated amides from Sanvitalia ocymoides. Rev Latinoamer Quim 18:114–115
Doskotch RW, Beal JL (1970) The isolation and identification of the numbing principle in Chrysanthemum anethifolium. Lloydia 33:393–394
Dossou KSS, Devkota KP, Morton C, Egan JM, Lu G, Beutler JA, Moaddel R (2013) Identification of CB1/CB2 ligands from Zanthoxylum bungeanum. J Nat Prod 76:2060–2064
Dunstan WR, Garnett H (1895) Note on the active constituent of the pellitory of medicine. J Chem Soc 67:100–102
Ee GCL, Lim CM, Rahman M, Shaari K, Bong CFJ (2010) Pellitorine, a potential anti-cancer lead compound against HL60 and MCT-7 cell lines and microbial transformation of piperine from Piper nigrum. Molecules 15:2398–2404
Ehrendorfer F, Guo YP (2005) Changes in the circumscription of the genus Achillea (Compositae–Anthemideae) and its subdivision. Willdenowia 35:49–54
Ehrendorfer F, Guo YP (2006) Multidisciplinary studies on Achillea sensu lato (Compositae–Anthemideae): new data on systematics and phylogeography. Willdenowia 36:69–87
Elliott M, Farnham AW, Janes NF, Johnson DM, Pulman DA (1987) Synthesis and insecticidal activity of lipophilic amides. Part 3: influence of chain length and terminal group in N-(2-methylpropyl)-2,4-dienamides. Pestic Sci 18:211–221
Galopin CC, Furrer SM, Goeke A (2004) Pungent and tingling compounds in Asian cuisine. In: Hofmann T, Ho CT, Pickenhagen W (eds) Challenges in taste chemistry and biology, ACS symposium series 867. American Chemical Society, Washington DC
Gamboa-Leon R, Chilton WS (2000) Isobutylamide numbing agents of the toothache grass, Ctenium aromaticum. Biochem Syst Ecol 28:1019–1021
García-Chávez A, Ramírez-Cháves E, Molina-Torres J (2004) El genero Heliopsis (Heliantheae, Asteraceae) en Mexico y las alcamidas presents en sus raíces. Acta Bot Mex 69:115–131
Gerber E (1903) Ueber die chemischen Bestandteile der Parakresse (Spilanthes oleracea, Jacquin). Arch Pharm 241:270–289
Gersdorff WA, Mitlin N (1950) Insecticidal action of American species of Heliopsis. J Econ Entom 43:554–555
Gertsch J (2008) Immunomodulatory lipids in plants: plant fatty acid amides and the human endocannabinoid system. Planta Med 74:638–650
Gertsch J, Schoop R, Kuenzle U, Suter A (2004) Echinacea alkylamides modulate TNF-α gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett 577:563–569
Gertsch J, Raduner S, Altmann KH (2006) New natural noncannabinoid ligands for cannabinoid type-2 (CB2) receptors. J Recept Signal Transduct Res 26:709–730
Goey AKL, Rosing H, Meijerman I, Sparidans RW, Schellens JHM, Beijnen JH (2012) The bioanalysis of the major Echinacea purpurea constituents dodeca-2E,4E,8Z,10E/Z-tetraenoic isobutylamides in human plasma using LC–MS/MS. J Chromatogr B 902:151–156
Greger H (1977) Anthemideae-chemical review. In: Heywood VH, Harborne JB, Turner BL (eds) The biology and chemistry of the Compositae, vol 2. Academic Press, London
Greger H (1978) Comparative phytochemistry and systematics of Anacyclus. Biochem Syst Ecol 6:11–17
Greger H (1984) Alkamides: structural relationships, distribution and biological activity. Planta Med 50:366–375
Greger H (1985) Vergleichende Phytochemie als biologische Disziplin. Plant Syst Evol 150:1–13
Greger H (1988) Comparative phytochemistry of the alkamides. In: Lam J, Breteler H, Arnason T, Hansen L (eds) Chemistry and biology of naturally-occurring acetylenes and related compounds (NOARC). Elsevier, Amsterdam
Greger H, Hofer O (1984) On the pungent principle of Matricaria pubescens. Phytochemistry 23:1173–1174
Greger H, Hofer O (1987) Highly unsaturated isopentyl amides from Achillea wilhelmsii. J Nat Prod 50:1100–1107
Greger H, Hofer O (1989) Polyenoic acid piperideides and other alkamides from Achillea millefolium. Phytochemistry 28:2363–2368
Greger H, Werner A (1990) Comparative HPLC analyses of alkamides within the Achillea millefolium group. Planta Med 56:482–486
Greger H, Grenz M, Bohlmann F (1981) Amides from Achillea species and Leucocyclus formosus. Phytochemistry 20:2579–2581
Greger H, Grenz M, Bohlmann F (1982) Piperidides and other amides from Achillea species. Phytochemistry 21:1071–1074
Greger H, Zdero C, Bohlmann F (1983) Weitere ungesättige Amide aus Achillea-Arten. Liebigs Ann Chem 1194–1201
Greger H, Zdero C, Bohlmann F (1984) Pyrrolidine and piperidine amides from Achillea. Phytochemistry 23:1503–1505
Greger H, Hofer O, Werner A (1985) New amides from Spilanthes oleracea. Monatsh Chem 116:273–277
Greger H, Hofer O, Werner A (1987a) Biosynthetically simple C18-alkamides from Achillea species. Phytochemistry 26:2235–2242
Greger H, Zdero C, Bohlmann F (1987b) Pyrrole amides from Achillea ageratifolia. Phytochemistry 26:2289–2291
Greger H, Zechner G, Hofer O, Vajrodaya S (1996) Bioactive amides from Glycosmis species. J Nat Prod 59:1163–1168
Gulland JM, Hopton GU (1930) Pellitorine, the pungent principle of Anacyclus pyrethrum. J Chem Soc (resumed) 6–11
Gupta OP, Gupta SC, Dhar KL, Atal CK (1976) Structure of a new amide, filfiline, isolated from Piper officinarum. Indian J Chem 14B:912–913
Hajdu Z, Nicolussi S, Rau M, Lorántfy L, Forgo P, Hohmann J, Csupor D, Gertsch J (2014) Identification of endocannabinoid system-modulating N-alkylamides from Heliopsis helianthoides var. scabra and Lepidium meyenii. J Nat Prod 77:1663–1669
Hashimoto K, Satoh K, Kase Y, Ishige A, Kubo M, Sasaki H, Nishikawa S, Kurosawa S, Yakabi K, Nakamura T (2001) Modulatory effect of aliphatic acid amides from Zanthoxylum piperitum on isolated gastrointestinal tract. Planta Med 67:179–181
Hatano T, Inada K, Ogawa T, Ito H, Yoshida T (2004) Aliphatic acid amides of the fruits of Zanthoxylum piperitum. Phytochemistry 65:2599–2604
Hernández I, Márquez L, Martínez I, Dieguez R, Delporte C, Prieto S, Molino-Torres J, Garrido G (2009) Anti-inflammatory effects of ethanolic extract and alkamides-derived from Heliopsis longipes roots. J Ethnopharmacol 124:649–652
Herz W, Kulanthaivel P (1985) An amide from Salmea scandens. Phytochemistry 24:173–174
Hinz B, Woelkart K, Bauer R (2007) Alkamides from Echinacea inhibit cyclooxygenase-2 activity in human neuroglioma cells. Biochem Biophys Res Commun 360:441–446
Hofer O, Greger H, Robien W, Werner A (1986) 13C NMR and 1H lanthanide induced shifts of naturally occurring alkamides with cyclic amide moieties—amides from Achillea falcata. Tetrahedron 42:2707–2716
Hofer O, Zechner G, Vajrodaya S, Lutz G, Greger H (1995) New anthranilic and methylsulfonylpropenoic acid amides from Thai Glycosmis species. Liebigs Ann 1789–1794
Hohmann J, Rédei D, Forgo P, Szabó P, Freund TF, Haller J, Bojnik E, Benyhe, S (2011) Alkamides and a neolignan from Echinacea purpurea and the interaction of alkamides with G-protein-coupled cannabinoid receptors. Phytochemistry 72:1848–1853
Hou CC, Chen CH, Yang NS, Chen YP, Lo CP, Wang SY, Tien YJ, Tsai PW, Shyur LF (2010) Comparative metabolomics approach coupled with cell- and gene-based assays for species classification and anti-inflammatory bioactivity validation of Echinacea plants. J Nutr Biochem 21:1045–1059
Hou CC, Huang CC, Shyur LF (2011) Echinacea alkamides prevent lipopolysaccharide/d-galactosamine-induced acute hepatic injury through JNK pathway-mediated HO-1 expression. J Agric Food Chem 59:11966–11974
Huang S, Zhao L, Zhou XL, Ying M, Wang CJ, Weng J (2012) New alkylamides from pericarps of Zanthoxylum bungeanum. Chin Chem Lett 23:1247–1250
Iseli V, Potterat O, Hagmann L, Egli J, Hamburger M (2007) Characterization of the pungent principles and the essential oil of Zanthoxylum schinifolium pericarp. Pharmazie 62:396–400
Jacobson M (1948) Herculin, a pungent insecticidal constituent of southern prickly ash bark. J Amer Chem Soc 70:4234–4237
Jacobson M (1949) The structure of pellitorine. J Amer Chem Soc 71:366–367
Jacobson M (1951) Constituents of Heliopsis species. I. Scabrin, an insecticidal amide from the roots of H. scabra Dunal. J Amer Chem Soc 73:100–103
Jacobson M (1954) Occurrence of a pungent insecticidal principle in American coneflower roots. Science 120:1028–1029
Jacobson M (1957a) The structure of spilanthol. Chem Ind (London) 50–51
Jacobson M (1957b) Constituents of Heliopsis species. V. Heliopsin, a second insecticidal amide from the roots of H. helianthoides var. scabra. J Amer Chem Soc 79:356–358
Jager H, Meinel L, Dietz B, Lapke C, Bauer R, Merkle HP, Heilmann J (2002) Transport of alkamides from Echinacea species through Caco-2 monolayers. Planta Med 68:469–471
Jakupovic J, Schuster A, Bohlmann F, King RM, Robinson H (1986) New lignans and isobutylamides from Heliopsis buphthalmoides. Planta Med 52:18–20
Jang KH, Chang YH, Kim DD, Oh KB, Oh U, Shin J (2008) New polyunsaturated fatty acid amides isolated from the seeds of Zanthoxylum piperitum. Arch Pharm Res 31:569–572
Jente R, Richter E (1976) Zur Biosynthese des Dehydromatricariaesters. Phytochemistry 15:1673–1679
Jente R, Bonnet PH, Bohlmann F (1972) Über die Inhaltsstoffe von Anacyclus pyrethrum DC. Chem Ber 105:1694–1700
Jiang LY, Chen JJ, He S, Sun CR (2009) High-throughput structural elucidation of amides in Mallotus lianus Croiz plant materials by LC–ESI–MS–MS. Chromatographia 70:439–445
Johns T, Graham K, Towers GHN (1982) Molluscicidal activity of affinin and other isobutylamides from the Asteraceae. Phytochemistry 21:2737–2738
Jondiko IJ (1986) A mosquito larvicide in Spilanthes mauritiana. Phytochemistry 25:2289–2290
Kadir HA, Zakaria MB, Kechil AA, Azirun MS (1989) Toxicity and electrophysiological effects of Spilanthes acmella Murr. extracts on Periplaneta americana. Pestic Sci 25:329–335
Kashiwada Y, Ito C, Katagiri H, Mase I, Komatsu K, Namba T, Ikeshiro Y (1997) Amides of the fruit of Zanthoxylum ssp. Phytochemistry 44:1125–1127
Kehie M, Kumaria S, Tandon P, Ramchiary N (2015) Biotechnological advances on in vitro capsaicinoids biosynthesis in Capsicum: a review. Phytochem Rev 14:189–201
Keipert R (2009) Acmella ciliata (H.B.K.) Cassini. Phytochemische und enzymatische Untersuchungen, galenische Präformulierungen. Dissertation, Freie Universität Berlin
Keipert R, Melzig MF (2009) Acmella ciliata (H.B.K.) Cassini. Z Phytother 30:44–50
Kikuzaki H, Kawabata M, Ishida E, Akazawa Y, Takei Y, Nakatani N (1993) LC–MS Analysis and structural determination of new amides from Javanese long pepper (Piper retrofractum). Biosci Biotech Biochem 57:1329–1333
Kim SC, Chapman KD, Blancaflor EB (2010) Fatty acid amide lipid mediators in plants. Plant Sci 178:411–419
Koul SK, Taneja SC, Agarwal VK, Dhar KL (1988) Minor amides of Piper species. Phytochemistry 27:3523–3527
Kubo I, Matsumoto T, Klocke JA, Kamikawa T (1984) Molluscicidal and insecticidal activities of isobutylamides isolated from Fagara macrophylla. Experientia 40:340–341
Kubo M, Ishii R, Ishino Y, Harada K, Matsui N, Akagi M, Kato E, Hosoda S, Fukuyama Y (2013) Evaluation of constituents of Piper retrofractum fruits on neurotrophic activity. J Nat Prod 76:769–773
Kuropka G, Glombitza KW (1987) Further polyenic and polyynic carboxamides and sesamin from Achillea ptarmica. Planta Med 53:440–442
Kuropka G, Koch M, Glombitza KW (1986) Säureamide aus Achillea ptarmica. Planta Med 52:244–245
LaForge FB, Haller HL, Sullivan WN (1942) The presence of an insecticidal principle in the bark of southern prickly ash. J Am Chem Soc 64:187
LaLonde RT, Wong CF, Hofstead SJ, Morris CD, Gardner LC (1980) N-(2-Methylpropyl)-(E, E)-2,4-decadienamide. A mosquito larvicide from Achillea millefolium L. J Chem Ecol 6:35–48
LaLone CA, Hammer KDP, Wu L, Bae J, Leyva N, Liu Y, Solco AKS, Kraus GA, Murphy PA, Wurtele ES, Kim OK, Seo KII, Widrlechner MP, Birt DF (2007) Echinacea species and alkamides inhibit prostaglandin E2 production in RAW264.7 mouse macrophage cells. J Agric Food Chem 55:7314–7322
Lazarević J, Radulović N, Zlatković B, Palić R (2010) Composition of Achillea distans Willd. subsp. distans root essential oil. Nat Prod Res 24:718–731
Leitão da-Cunha EV, de Oliveira Chaves MC (2001) Two amides from Piper tuberculatum fruits. Fitoterapia 72:197–199
Lennertz RC, Tsunozaki M, Bautista DM, Stucky CL (2010) Physiological basis of tingling paresthesia evoked by hydroxy-alpha-sanshool. J Neurosci 30:4353–4361
Leonard AE, Pereira SL, Sprecher H, Huang YS (2004) Elongation of long-chain fatty acids. Prog Lipid Res 43:36–54
Ley JP, Krammer G, Looft J, Reinders G, Bertram HJ (2006a) Structure-activity relationships of trigeminal effects for artificial and naturally occurring alkamides related to spilanthol. In: Bredie WLP, Petersen MA (eds) Flavour science: recent advances and trends. Elsevier, Amsterdam
Ley JP, Blings M, Krammer G, Reinders G, Schmidt CO, Bertram HJ (2006b) Isolation and synthesis of acmellonate, a new unsaturated long chain 2-ketolester from Spilanthes acmella. Nat Prod Res 20:798–804
Li GP, Shen BC, Zhao JF, Yang XD, Li L (2007) Two new alkamides from Spilanthes callimorpha. J Integr Plant Biol 49:1608–1610
Li K, Zhu W, Fu Q, Ke Y, Jin Y, Liang X (2013) Purification of amide alkaloids from Piper longum L. using preparative two-dimensional normal-phase liquid chromatography × reversed-phase liquid chromatography. Analyst 138:3313–3320
Likhitwitayawuid K, Ruangrungsi N, Lange GL, Decicco CP (1987) Structural elucidation and synthesis of new components isolated from Piper sarmentosum (Piperaceae). Tetrahedron 43:3689–3694
Loder JW, Moorhouse A, Russell GB (1969) Tumour inhibitory plants. Amides of Piper novae-hollandiae (Piperaceae). Aust J Chem 22:1531–1538
López-Bucio J, Acevedo-Hernández G, Ramírez-Chávez E, Molina-Torres J, Herrera-Estrella L (2006) Novel signals for plant development. Curr Opin Plant Biol 9:523–529
López-Martínez S, Aguilar-Guadarrama B, Rios MY (2011) Minor alkamides from Heliopsis longipes S.F. Blake (Asteraceae) fresh roots. Phytochem Lett 4:275–279
Loureiro-Rosario S, da Silva AJR, Parente JP (1996) Alkamides from Cissampelos glaberrima. Planta Med 62:376–377
Mahringer A, Ardjomand-Woelkart K, Bauer R, Fricker G, Efferth T (2013) Alkamides from Echinacea angustifolia interact with P-glycoprotein of primary brain capillary endothelial cells isolated from porcine brain blood vessels. Planta Med 79:214–218
Martin R, Becker H (1984) Spilanthol-related amides from Acmella ciliata. Phytochemistry 23:1781–1783
Martin R, Becker H (1985) Amides and other constituents from Acmella ciliata. Phytochemistry 24:2295–2300
Matovic NJ, Hayes PY, Penman K, Lehmann RP, DeVoss JJ (2011) Polyunsaturated alkyl amides from Echinacea: synthesis of diynes, enynes, and dienes. J Org Chem 76:4467–4481
Matthias A, Blanchfield JT, Penman KG, Toth I, Lang CS, DeVoss JJ, Lehmann RP (2004) Permeability studies of alkylamides and caffeic acid conjugates from echinacea using a Caco-2-cell monolayer model. J Clin Pharm Ther 29:7–13
Menozzi-Smarrito C, Riera CE, Munari C, Le Coutre J, Robert F (2009) Synthesis and evaluation of new alkylamides derived from α-hydroxysanshool, the pungent molecule in Szechuan pepper. J Agric Food Chem 57:1982–1989
Merali S, Binns S, Paulin-Levasseur M, Ficker C, Smith M, Baum B, Brovelli E, Arnason JT (2003) Antifungal and anti-inflammatory activity oft he genus Echinacea. Pharm Biol 41:412–420
Mester I (1983) Structural diversity and distribution of alkaloids in the Rutales. In: Waterman PG, Grundon MF (eds) Chemistry and chemical taxonomy of the Rutales. Academic Press, London
Minto RE, Blacklock BJ (2008) Biosynthesis and function of polyacetylenes and allied natural products. Prog Lipid Res 47:233–306
Mizutani K, Fukunaga Y, Tanaka O, Takasugi N, Saruwatari YI, Fuwa T, Yamauchi T, Wang J, Jia MR, Li FY, Ling YK (1988) Amides from Huajiao, pericarps of Zanthoxylum bungeanum MAXIM. Chem Pharm Bull 36:2362–2365
Molina-Torres J, Salgado-Garciglia R, Ramírez-Chávez E, Del Rio RE (1996) Purely oleofinic alkamides in Heliopsis longipes and Acmella (Spilanthes oppositifolia). Biochem Syst Ecol 24:43–47
Molina-Torres J, García-Chávez A, Ramírez-Chávez E (1999) Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: affinin and capsaicin. J Ethnopharmacol 64:241–248
Molina-Torres J, Salazar-Cabrera CJ, Armenta-Salinas C, Ramírez-Chávez E (2004) Fungistatic and bacteriostatic activities of alkamides from Heliopsis longipes roots: affinin and reduced amides. J Agric Food Chem 52:4700–4704
Moreno SC, Carvalho GA, Picanço MC, Morais EGF, Pereira RM (2012) Bioactivity of compounds from Acmella oleracea against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and selectivity to two non-target species. Pest Manag Sci 68:386–393
Morikawa T, Matsuda H, Yamaguchi I, Pongpiriyadacha Y, Yoshikawa M (2004) New amides and gastroprotective constituents from the fruit of Piper chaba. Planta Med 70:152–159
Morquecho-Contreras A, Méndez-Bravo A, Pelagio-Flores R, Raya-González J, Ortíz-Castro R, López-Bucio J (2010) Characterization of drr1, an alkamide-resistant mutant of Arabidopsis, reveals an important role for small lipid amides in lateral root development and plant senescence. Plant Physiol 152:1659–1673
Muhammad I, Zhao J, Dunbar DC, Khan IA (2002) Constituents of Lepidium meyenii ‘maca’. Phytochemistry 59:105–110
Müller-Jakic B, Breu W, Pröbstle A, Redl K, Greger H, Bauer R (1994) In vitro inhibition of cylclooxygenase and 5-lipoxygenase by alkamides from Echinacea and Achillea species. Planta Med 60:37–40
Murayama Y, Shinozaki K (1931) Über den scharfen Bestandteil von Xanthoxylum piperitum D. C. J Pharm Soc Jpn 51:379–384
Nakatami N, Nagashima M (1992) Pungent alkamides from Spilanthes acmella L. var. oleracea Clarke. Biosci Biotech Biochem 56:759–762
Narui T, Takeuchi M, Ishii R, Ishida T, Okuyama T (1995) Studies on the constituents of Piper hancei of spice from Okinawa. Nat Med 49:438–441
Navarrete A, Hong E (1996) Anthelminthic properties of alpha-sanshool from Zanthoxylum liebmannianum. Planta Med 62:250–251
Ndom JC, Mbafor JT, Meva´a LM, Kakam Z, Phanuel AS, Ndongo E, Harwood LM, Mpondo TN (2010) New alkamide and ent-kaurane diterpenoid derivatives from Senecio erechtitoides (Asteraceae). Phytochem Lett 3:201–206
Obst K, Paetz S, Backes M, Reichelt KV, Ley JP, Engel KH (2013) Evaluation of unsaturated alkanoic acid amides as maskers of epigallocatechin gallate astringency. J Agric Food Cem 61:4242–4249
Ogura M, Cordell GA, Quinn ML, Leon C, Benoit PS, Soejarto DD, Farnsworth NR (1982) Ethnopharmacologic studies I: rapid solution to a problem—oral use of Heliopsis longipes—by means of a multidisciplinary approach. J Ethnopharmacol 64:215–219
Ospina de Nigrinis LS, Olarte Caro J, Nuñez Olarte E (1986) Estudio fitofarmacologico de la fraccion liposoluble de las flores de la Spilanthes americana (Mutis) Parte I: Estudio fitoquimico. Rev Colomb Cienc Quim-Farmac 15:37–47
Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OD, Prasad AK, Wengel J, Olsen CE, Boll PM (1997) Phytochemistry of the genus Piper. Phytochemistry 46:597–673
Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, Altmann KH, Gertsch J (2006) Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J Biol Chem 281:14192–14206
Raduner S, Bisson W, Abagyan R, Altmann KH, Gertsch J (2007) Self-assembling cannabinomimetics: supramolecular structures of N-alkyl amides. J Nat Prod 70:1010–1015
Rahman MAA, Cho SC, Song J, Mun HT, Moon SS (2007) Dendrazawaynes A and B, antifungal polyacetylenes from Dendranthema zawadskii (Asteraceae). Planta Med 73:1089–1094
Ramírez-Chávez E, López-Bucio J, Herrera-Estrella L, Molina-Torres J (2004) Alkamides isolated from plants promote growth and alter root development in Arabidopsis. Plant Physiol 134:1058–1068
Ramírez-Noya D, González-Elizondo MS, Molina-Torres J (2011) Heliopsis suffruticosa (Compositae, Heliantheae), una nueva especie des occidente de Zacatecas. Acta Bot Mex 97:39–47
Ramsewak RS, Erickson AJ, Nair MG (1999) Bioactive N-isobutylamides from the flower buds of Spilanthes acmella. Phytochemistry 51:729–732
Reisch J, Hussain RA, Adesina SK, Szendrei K (1985) A new amide from Evodia hupehensis fruit hull. J Nat Prod 48:862–863
Riemer B, Hofer O, Greger H (1997) Tryptamine derived amides from Clausena indica. Phytochemistry 45:337–341
Rios MY (2012) Natural alkamides: pharmacology, chemistry and distribution. In: Vallisuta O (ed) Drug discovery research in pharmacognosy. InTech, Rijeka
Rios MY, Olivo HF (2014) Natural and synthetic alkamides: applications in pain therapy. In: Atta-ur-Rahman (ed) Studies in natural products chemistry, vol 43. Elsevier, Amsterdam
Rios MY, Aguilar-Guadarrama AB, Gutiérrez MdC (2007) Analgesic activity of affinin, an alkamide from Heliopsis longipes (Compositae). J Ethnopharmacol 110:364–367
Rios-Chavez P, Ramirez-Chavez E, Armenta-Salinas C, Molina-Torres L (2003) Acmella radicans var. radicans: in vitro culture establishment and alkamide content. In Vitro Cell Dev Biol Plant 39:37–41
Saadali B, Boriky D, Blaghen M, Vanhaelen M, Talbi M (2001) Alkamides from Artemisia dracunculus. Phytochemistry 58:1083–1086
Sasagawa M, Cech NB, Gray DE, Elmer GW, Wenner CA (2006) Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. Int Immunopharmacol 6:1214–1221
Shanklin J, Cahoon EB (1998) Desaturation and related modifications of fatty acids. Annu Rev Plant Physiol Plant Mol Biol 49:611–641
Sharma V, Thakur M, Chauhan NS, Dixit VK (2009) Evaluation of the anabolic, aphrodisiac and reproductive activity of Anacyclus pyrethrum DC in male rats. Sci Pharm 77:97–110
Sharma V, Thakur M, Chauhan NS, Dixit VK (2010) Effects of petroleum ether extract of Anacyclus pyrethrum DC. on sexual behavior in male rats. J Chin Integr Med 8:767–773
Sharma V, Boonen J, Chauhan NS, Thakur M, DeSpiegeleer B, Dixit VK (2011) Spilanthes acmella ethanolic extract: LC-MS alkylamide profiling and its effects on sexual behavior in male rats. Phytomedicine 18:1161–1169
Sharma V, Boonen J, De Spiegeleer B, Dixit VK (2013) Androgenic and spermatogenic activity of alkamide-rich ethanol solution extract of Anacyclus pyrethrum DC. Phytother Res 27:99–106
Shibuya H, Takeda Y, Zhang RS, Tong RX, Kitagawa I (1992) Indonesian medicinal plants III. On the constituents of the bark of Fagara rhetza (Rutaceae). (1): alkaloids, phenylpropanoids, and acid amide. Chem Pharm Bull 40:2325–2330
Siddiqui BS, Gulzar T, Mahmood A, Begum S, Khan B, Afshan F (2004) New insecticidal amides from petroleum ether extract of dried Piper nigrum L. whole fruits. Chem Pharm Bull 52:1349–1352
Simas NK, Da Dellamora ECL, Schripsema J, Salgueiro Lage CL, de Oliveira Filho AM, Wessjohann L, Porzel A, Kuster RM (2013) Acetylenic 2- phenylethylamides and new isobutylamides from Acmella oleracea (L.) R. K. Jansen, a Brazilian spice with larvicidal activity on Aedes aegypti. Phytochem Lett 6:67–72
Singh J, Dhar KL, Atal CK (1971) Studies on the genus Piper-XII. Structure of trichonine, a new N-pyrrolidinyl eicosa-trans-2, trans-4 dienamide. Tetrahedron Lett 2119–2120
Sittie AA, Lemmich E, Olsen CE, Hviid L, Christensen SB (1998) Alkamides from Phyllanthus fraternus. Planta Med 64:192–193
Spelman K, Depoix D, McCray M, Mouray E, Grellier P (2011) The traditional medicine Spilanthes acmella, and the alkylamides spilanthol and undeca-2E-ene-8,10-diynoic acid isobutylamide, demonstrate in vitro and in vivo antimalarial activity. Phytother Res 25:1098–1101
Strunz GM (2000) Unsaturated amides from Piper species (Piperaceae). In: Atta-ur-Rahman (ed) Studies in natural products chemistry, vol 24. Elsevier, Amsterdam
Sugai E, Morimitsu Y, Iwasaki Y, Morita A, Watanabe T, Kubota K (2005a) Pungent qualities of sanshool-related compounds evaluated by a sensory test and activation of rat TRPV1. Biosci Biotechnol Biochem 69:1951–1957
Sugai E, Morimitsu Y, Kubota K (2005b) Quantitative analysis of sanshool compounds in Japanese pepper (Xanthoxylum piperitum DC.) and their pungent characteristics. Biosci Biotechnol Biochem 69:1958–1962
Sun C, Pei S, Pan Y, Shen Z (2007) Rapid structural determination of amides in Piper longum by high-performance liquid chromatography combined with ion trap mass spectrometry. Rapid Commun Mass Spectrom 21:1497–1503
Tackie AN, Dwuma-Badu D, Ayim JSK, ElSohly HN, Knapp JE, Slatkin DJ, Schiff PL Jr (1975) N-Isobutyloctadeca-trans-2-trans-4-dienamide: a new constituent of Piper guineense. Phytochemistry 14:1888–1889
Tang GH, Chen DM, Qiu BY, Sheng L, Wang YH, Hu GW, Zhao FW, Ma LJ, Wang H, Huang QQ, Xu JJ, Long CL, Li J (2011) Cytotoxic amide alkaloids from Piper boehmeriaefolium. J Nat Prod 74:45–49
Thomsen MO, Fretté XC, Christensen KB, Christensen LB, Grevsen K (2012) Seasonal variations in the concentrations of lipophilic compounds and phenolic acids in the roots of Echinacea purpurea and Echinacea pallida. J Agric Food Chem 60:12131–12141
Tofern B, Mann P, Kaloga M, Jenett-Siems K, Witte L, Eich E (1999) Aliphatic pyrrolidine amides from two tropical convolvulaceous species. Phytochemistry 52:1437–1441
Tsao R, Attygalle AB, Schroeder FC, Marvin CH, McGarvey BD (2003) Isobutylamides of unsaturated fatty acids from Chrysanthemum morifolium associated with host-plant resistance against the western flower thrips. J Nat Prod 66:1229–1231
Uttaro AD (2006) Biosynthesis of polyunsaturated fatty acids in lower eukaryotes. IUBMB Life 58:563–571
Veryser L, Taevernier L, Roche N, Peremans K, Burvenich C, De Spiegeleer B (2014) Quantitative transdermal behavior of pellitorine from Anacyclus pyrethrum extract. Phytomedicine 21:1801–1807
Wagner H, Breu W, Willer F, Wierer M, Remiger P, Schwenker G (1989) In vitro inhibition of arachidonate metabolism by some alkamides and prenylated phenols. Planta Med 55:566–567
Wang Y, Wang Y, McNeil B, Harvey LM (2007) Maca: an Andean crop with multi-pharmacological functions. Food Res Int 40:783–792
Wansi JD, Nwozo SO, Mbaze LM, Devkota KP, Moladje SMD, Fomum ZT, Sewald N (2009) Amides from the stem bark of Fagara macrophylla. Planta Med 75:517–521
Wei K, Li W, Koike K, Pei Y, Chen Y, Nikaido T (2004) New amide alkaloids from the roots of Piper nigrum. J Nat Prod 67:1005–1009
Winterfeldt E (1963) Strukturaufklärung und Synthese einer Thiophenverbindung aus Chrysanthemum frutescens L. Chem Ber 96:3349–3358
Woelkart K, Bauer R (2007) The role of alkamides as an active principle of Echinacea. Planta Med 73:615–623
Woelkart K, Xu W, Pei Y, Makriyannis A, Picone RP, Bauer R (2005) The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Med 71:701–705
Woelkart K, Frye RF, Derendorf H, Bauer R, Butterweck V (2009) Pharmacokinetics and tissue distribution of dodeca-2E,4E,8E,10E/Z-tetraenoic acid isobutylamides after oral administration in rats. Planta Med 75:1306–1313
Xiong QB, Shi DW, Yamamoto H, Mizuno M (1997) Alkylamides from pericarps of Zanthoxylum bungeanum. Phytochemistry 46:1123–1126
Yang X (2008) Aroma constituents and alkylamides of red and green Huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J Agric Food Chem 56:1689–1696
Yasuda I, Takeya K, Itokawa H (1980) The geometric structure of spilanthol. Chem Pharm Bull 28:2251–2253
Yasuda I, Takeya K, Itokawa H (1981a) Structures of amides from Asiasarum heterotropoides Maek. var. mandshuricum Maek. Chem Pharm Bull 29:564–566
Yasuda I, Takeya K, Itokawa H (1981b) Two new pungent principles isolated from the pericarps of Zanthoxylum ailanthoides. Chem Pharm Bull 29:1791–1793
Yasuda I, Takeya K, Itokawa H (1982) Distribution of unsaturated aliphatic acid amides in Japanese Zanthoxylum species. Phytochemistry 21:1295–1298
Youssef DTA, van Soest RWM, Fusetani N (2003) Callyspongamide A, a new cytotoxic polyacetylenic amide from the Red Sea sponge Callyspongia fistularis. J Nat Prod 66:861–862
Zdero C, Bohlmann F, King RM, Lander NS (1988) An isobutylamide and beyerene derivatives from Brachycome species. Phytochemistry 27:2984–2985
Zhang F, Chu CH, Xu Q, Fu SP, Hu JH, Xiao HB, Liang XM (2005) A new amide from Asarum forbesii Maxim. J Asian Nat Prod Res 7:1–5
Zhao JP, Muhammad I, Dunbar DC, Mustafa J, Khan IA (2005) New alkamides from Maca (Lepidium meyenii). J Agric Food Chem 53:690–693
Zheng BL, He K, Kim CH, Rogers L, Shao Y, Huang ZY, Lu Y, Yan SJ, Qien LC, Zheng QY (2000) Effect of a lipid extract from Lepidium meyenii on sexual behavior in mice and rats. Urology 55:598–602
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to my teacher and friend the late Professor Robert Hegnauer, University of Leiden, The Netherlands.
Rights and permissions
About this article
Cite this article
Greger, H. Alkamides: a critical reconsideration of a multifunctional class of unsaturated fatty acid amides. Phytochem Rev 15, 729–770 (2016). https://doi.org/10.1007/s11101-015-9418-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-015-9418-0