Abstract
Background
Inhalational corticosteroids (ICS) were observed to increase the pneumonia risk in chronic obstructive pulmonary airway disorder (COPD). However, it is unknown whether any differences exist between the drugs within the ICS class.
Aim
This study aimed to evaluate the risk of pneumonia associated with different ICS and identify factors that predict pneumonia in patients with moderate-to-severe COPD using a network meta-analysis.
Method
Electronic databases (Medline, Cochrane CENTRAL and Google Scholar) were searched for trials comparing ICS in COPD patients. The outcomes were pneumonia and serious pneumonia. Odds ratios (OR) with 95% confidence interval (95% CI) were estimated. Meta-regression was used to identify the predictors. The strength of evidence was graded using the Grading of Recommendations, Assessment, Development, and Evaluations approach.
Results
Sixty-six studies (103,347 participants) were included. Fluticasone (OR: 1.46; 95% CI: 1.26, 1.7), mometasone (OR: 2.2; 95% CI: 1.05, 4.6), and beclometasone (OR: 1.7; 95% CI: 1.1, 2.6) were observed with an increased pneumonia risk compared to placebo. Fluticasone (OR: 1.5; 95% CI: 1.3, 1.7) was observed with an increased risk of serious pneumonia. High doses (OR: 1.2; 95% CI: 1.03, 1.4), BMI ≥ 25 kg/m2 (OR: 1.6; 95% CI: 1.1, 2.2), and history of exacerbations in the preceding year predicted the pneumonia risk. Evidence strength was moderate.
Conclusion
ICS class differences in pneumonia risk were observed in terms of pooled effect estimates but it is unlikely that any clinically relevant differences exist. Risk–benefit analysis supports ICS use in moderate-severe COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
Clinicians should consider dosage, body mass index, and exacerbation history while prescribing inhalational corticosteroids to mitigate the risk of pneumonia.
-
Pharmacists should educate patients about the risk of pneumonia while dispensing inhalational corticosteroids.
-
Protocols should be implemented in the healthcare delivery system emphasizing the need for regular assessment of patients on inhalational corticosteroids on the pneumonia risk.
Introduction
Chronic obstructive pulmonary airway disorder (COPD) is one of the leading causes of mortality, primarily due to acute exacerbations [1]. Inhaled corticosteroids (ICS) are one of the effective drug classes to curtail acute exacerbations in COPD, especially in moderate to very severe stages [2]. Nearly 50–80% of such patients were estimated to use ICS of which approximately 25% demonstrate a reduction in the incidence and severity of acute exacerbation in COPD [3, 4]. As per the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 recommendations, ICS therapy is indicated in patients with history of moderate exacerbations in the preceding year, hospitalization for COPD exacerbations, blood eosinophil count > 300/µl or those with concurrent asthma [5]. ICS therapy is preferably not to be administered alone as they have not been shown to modify either the decline in lung function or mortality on long term use [6]. Further, ICS alone has shown an increased risk of mortality compared to the combination with long-acting beta-2 agonist [7]. Depending on the definitions of the endpoint related to reduction in the incidence of COPD exacerbation, the number needed to treat (NNT) for ICS has been observed to range between 3 and 7 [8].
Long-term use of ICS was observed to result in altered glycemic control, osteoporosis, cataract, and oropharyngeal side effects [9]. Several studies have observed a strong association between ICS use and pneumonia with advanced age, male gender, low body mass index, and decreased airflow were identified as risk factors [10]. Several direct comparison meta-analyses were carried out evaluating the risk of pneumonia with ICS therapies and had contradictory conclusions [11,12,13]. A recent direct comparison meta-analysis included 59 randomized clinical trials but was observed with a lot of drawbacks such as the inclusion of erroneous data, publication bias was not assessed, and grading of the evidence was not carried out [14]. Lodise et al. [15] examined the variations within a single class of drugs, specifically fluticasone and budesonide, concerning the risk of pneumonia in individuals with chronic obstructive pulmonary disease (COPD). However, this study had some limitations. Firstly, it only collected data from five studies that directly compared fluticasone and budesonide. Additionally, out of these five studies, only one was a randomized trial, while the others were observational studies. Furthermore, the authors mistakenly combined data from both study designs to generate overall estimates, which was not appropriate. Hence, it is unclear whether any difference exists in terms of pneumonia risk within the ICS class of drugs, between fluticasone, budesonide, mometasone, and beclometasone. This is mainly limited by the presence of very few studies comparing the occurrence of pneumonia in clinical trials using head-to-head ICS therapies. Unfortunately, such head-to-head comparison trials in the absence of non-ICS comparators could not be included in the previous meta-analysis due to the limitations of the traditional direct comparison meta-analysis. A network meta‐analysis (NMA) provides pooled estimates using direct and indirect comparisons through a common comparator [16]. Through NMA, effect estimates can be generated between the interventions even in the absence (or limited number) of head-to-head comparison clinical trials. Moreover, previous meta-analyses have failed to identify any risk factors associated with pneumonia risk that can only be evaluated using meta-regression techniques.
Aim
This study aimed to evaluate the risk of pneumonia associated with different ICS and identify factors that predict pneumonia in patients with moderate-to-severe COPD using a network meta-analysis.
Method
Search strategy
The protocol for this systematic review is accessible in the Open Science Framework [17]. Medline, Cochrane CENTRAL and Google Scholar were the databases searched for articles. The search strategy is provided in the Electronic Supplementary Table 1. Only randomized clinical trials meeting the PICO criteria published until 2nd November 2023 were included. No limits were placed either with the publication year or language. We also carried out a hand search of the published articles to find suitable publications.
Eligibility criteria
We included studies that met the following eligibility criteria:
-
Population: Adults of either gender diagnosed with COPD.
-
Intervention: ICS of any dose and through any device.
-
Control: Placebo/No ICS/ICS of any dose and through any device.
-
Outcome: The Medical Dictionary for Regulatory Activities (MedDRA) defined pneumonia as mentioned by the preferred terms such as pneumonia, lobar pneumonia, and bronchopneumonia as the primary outcome. Additionally, we also considered serious pneumonia as defined by the authors either as a serious adverse event or pneumonia leading to death as a secondary outcome.
Study procedure
Two authors independently searched for eligible studies and obtained the following details from each eligible study: Trial identification, site, year, participants, interventions, and outcomes. Any disagreement was resolved through discussion and a consensus was reached. The present network meta-analysis complies with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) [18]. Cochrane risk of bias tool was used for assessing the risk of bias of the included studies on the following domains: generation of random sequence; concealment of allocation; blinding of participants, study personnel, and outcome assessment; incomplete outcome reporting; and selective reporting of outcomes [19]. For comparisons with at least five studies, we assessed the publication bias using the Funnel plot and Begg and Mazumdar test [20]. We used a random-effects model for obtaining the direct, indirect, and mixed treatment comparison pooled estimates. The direct comparison pooled estimates were obtained from the data included in the head-to-head clinical trials while the indirect pooled estimates were obtained from the trials using common comparators. Mixed treatment comparison estimates were obtained from the data from studies included for both direct and indirect comparison pooled estimates. The effect estimates were represented using odds ratios [95% confidence intervals] (OR, 95% CI). We used H statistics for evaluating the inconsistency between direct and indirect pooled estimates, and the inconsistency was classified as follows: mild (< 3), modest (3–6), and large (> 6) [21]. A separate analysis was carried out to obtain the pooled estimates between various doses of ICS. The doses were classified into low, medium, and high doses according to the National Institute for Health and Care Excellence (NICE) recommendations (Electronic Supplementary Table 2). Sensitivity analysis was carried out by excluding the estimates from studies with the potential high risk of bias. Leave-one-out meta-analysis was carried out by excluding the data of one study at a time and observing its impact on the pooled estimates. Cumulative meta-analysis was carried out where the studies were added chronologically and the changes in the pooled estimates obtained before the addition were analyzed. We used MetaXL© to estimate the mixed comparison pooled estimates [22]. The final estimates were graded using the grades of recommendation, assessment, development, and evaluation (GRADE) working group approach [23]. VOSviewer© was used for constructing and analyzing the bibliometric networks for identifying common author groups involved in the included trials [24]. Litmap© was used for mapping the included articles where the most recent articles are placed on the right-most column and the most cited articles on the top and the connecting lines between them indicate the citations between the included articles [25]. Meta-regression for the pneumonia risk was carried out with the following variables as covariates: ICS intervention (fluticasone, budesonide, mometasone, and beclometasone), doses (low, medium, and high), age (< 65, and ≥ 65 years), body mass index (BMI) (< 25 and ≥ 25 kg/m2), concomitant drug classes (LABA, LAMA, LABA plus LAMA, and ICS alone), pack-years of smoking (< 10 and ≥ 10), GOLD stage (I, II, and III/IV), number of previous exacerbations (0, 1, and ≥ 2), blood eosinophilia (< 300 and ≥ 300cells/mm3), and duration of ICS treatment (< 24and ≥ 24 weeks). A few studies did not report BMI and imputed with the median values. Meta-regression was carried out using the maximum likelihood random-effects method and the output on the natural logarithm of odds (Ln[odds]) was converted to OR. We attempted to estimate the number needed to harm (NNH) with 95% CI were estimated based on the pooled risk differences across the studies. Meta-regression, leave-on-out, and cumulative meta-analysis were carried out by OpenMEE [26]. A p-value of ≤ 0.05 was considered significant for covariates obtained in meta-regression and for RR and NNH values. We carried out a Trial sequential analysis (TSA, Copenhagen, DK) for comparisons with significant pooled estimates against the control group for assessing the power of evidence that has accumulated so far in obtaining the pooled estimates [27].
Results
Search results
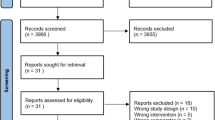
One thousand six hundred and forty-one articles were obtained with the search strategy. The PRISMA flowchart of the articles from screening until the final inclusion is depicted in Fig. 1. Sixty-six studies (103,387 participants) [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,93] were included in this systematic review. Key characteristics of the included studies in the review are mentioned in the Electronic Supplementary Table 3. Litmap© revealed that Burge et al. [35], Wedzicha et al. [87], and Aaron et al. [28] were the most cited (Electronic Supplementary Figure 1). Bibliometric analyses revealed two clusters of authors commonly involved in publishing research articles in this area (Electronic Supplementary Figure 2). Two studies [41, 69] were included in the systematic review but were excluded from the meta-analysis as they provided the estimates for the outcome in terms of rate and not in proportion. These studies compared high and medium doses of fluticasone plus salmeterol, and fluticasone plus umeclidinium plus vilanterol, respectively. Another study [48] compared medium with high doses of fluticasone plus salmeterol and was included only in the sub-group analysis. A summary of the risk of bias in included studies is depicted in the Electronic Supplementary Figure 3. Most studies had low risk while a few studies had a high risk of bias in the selection, performance, and detection bias. Details of the risk of bias for individual studies are depicted in the Electronic Supplementary Figure 4.
Pooled estimates for the pneumonia risk
Sixty-one studies (100,934 patients) reported this outcome and were included in this analysis. The network plot depicting the relationship between the interventions assessed for this outcome is depicted in Fig. 2. The comparison between budesonide/formoterol/indacaterol/tiotropium with indacaterol/tiotropium could not be analyzed as there was no common comparator with either of these medical therapies. The mixed treatment comparison estimates for each of the interventions in comparison with placebo are depicted in Fig. 3. Fluticasone (OR: 1.46; 95% CI: 1.26, 1.7), fluticasone plus salmeterol (OR: 1.74; 95% CI: 1.38, 2.19), and mometasone plus formoterol (OR: 2.2; 95% CI: 1.05, 4.6) were observed with a high risk of pneumonia compared to placebo. Mild inconsistency was observed (H value of 1.041).
Network plot of ICS interventions for the risk of pneumonia. Beclo—Beclometasone; B—Budesonide; F—Fluticasone; For—Formoterol; G—Glycopyrrolate; I—Indacaterol; M—Mometasone; O—Olodaterol; S—Salmeterol; T—Tiotropium; U—Umeclidinium; and V—Vilanterol. Nine studies compared inhalational budesonide plus formoterol with formoterol, six compared fluticasone plus vilanterol with vilanterol, and five each compared fluticasone plus salmeterol with salmeterol, fluticasone plus vilanterol with placebo, and formoterol with placebo, followed by others
Forest plot of the individual ICS for the risk of pneumonia compared to placebo. Beclo—Beclomethasone; B—Budesonide; F—Fluticasone; For—Formoterol; G—Glycopyrrolate; I—Indacaterol; M—Mometasone; O—Olodaterol; S—Salmeterol; T—Tiotropium; U—Umeclidinium; V—Vilanterol; OR—Odds ratio; and CI—Confidence interval. The X-axis indicates the odds ratio and the Y-axis on the left side indicates the various drug treatment/s and odds ratios with 95% confidence intervals are represented on the right side. The green circle indicates the point estimates, and the pink line represents the 95% confidence interval. The vertical black line indicates the line of no difference between the interventions
When the individual ICS was compared against all the control interventions, fluticasone (OR: 1.5; 95% CI: 1.3, 1.7) and beclometasone (OR: 1.7; 95% CI: 1.1, 2.6) showed a significantly increased risk of pneumonia while no significant differences were observed among the other ICS (Electronic Supplementary Figure 5). Mild inconsistency was observed (H value of 1).
Pooled estimates for the risk of serious pneumonia
Twenty-four studies (42,847 patients) were included in the analysis of this outcome. The network plot depicting the relationship between the interventions assessed for this outcome is depicted in the Electronic Supplementary Figure 6. None of the interventions were observed with any significant risk of serious pneumonia (Electronic Supplementary Figure 7). Mild inconsistency was observed (H value of 1).
When the individual ICS was compared against all the control interventions, only fluticasone (OR: 1.5; 95% CI: 1.3, 1.7) showed a significantly higher risk of serious pneumonia and no significant differences were observed among the ICS for the pneumonia risk (Electronic Supplementary Figure 8). Mild inconsistency was observed (H value of 1).
Pooled estimates comparing the various doses of ICS
Low (OR: 1.26; 95% CI: 1.12, 1.43), medium (OR: 1.42; 95% CI: 1.19, 1.68), and high doses (OR: 1.58; 95% CI: 1.32, 1.89) of ICS were associated with significantly higher risks of pneumonia compared to control group of interventions (Fig. 4). However, no significant differences were observed between the different doses (Fig. 4) albeit a trend with an increased odds ratio from low to high doses of ICS.
Forest plot comparing the ICS doses for the risk of pneumonia. Forest plots comparing the ICS therapy for the risk of pneumonia. The comparators are A—Control; B—Low doses of ICS; C—Medium doses of ICS; and D—High doses of ICS. The X-axis indicates the odds ratio and the Y-axis on the left side indicates the various drug treatment/s and odds ratios with 95% confidence intervals are represented on the right side. The green circle indicates the point estimates and the pink line represents the 95% confidence interval. The vertical black line indicates the line of no difference between the interventions
Medium (OR: 1.68; 95% CI: 1.19, 2.37) doses of ICS were associated with a significantly higher risk of serious pneumonia compared to the control group of interventions (Electronic Supplementary Figure 9). Also, no significant differences were observed between the different doses (Electronic Supplementary Figure 9).
Meta-regression analysis
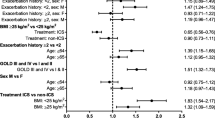
Meta-regression analysis revealed interventions and dose categories to be the significant predictors of outcomes (Table 1). All the included studies recruited patients with at least 10 smoking pack-years and only 1 study included the details on blood eosinophil count due to which they could not be assessed in this study. Compared to fluticasone, budesonide was observed with a significantly lower risk of pneumonia. Similarly, high doses of ICS were observed with a significantly greater risk of pneumonia like those with BMI ≥ 25 kg/m2. Also, those with at least one previous exacerbation in the last 12 months showed a significantly increased risk of pneumonia compared to those without.
NNH analysis
Due to the very low-risk differences observed for most comparisons, NNH could be computed only for fluticasone (NNH: 100; 95% CI: 100, 50) and high doses of ICS (NNH: 100; 95% CI: 100, 50).
Publication bias
Eleven comparisons were observed with more than five studies and the funnel plots did not reveal the presence of any publication bias (Electronic Supplementary Figure 10). Begg and Mazumdar’s test did not reveal publication bias for the following comparisons for pneumonia: fluticasone versus control (p = 0.8); budesonide versus control (p = 0.6); medium dose versus control (p = 0.8); low dose versus control (p = 0.9); high dose versus control (p = 0.5); fluticasone plus vilanterol (p = 0.9); and budesonide plus formoterol (p = 0.7). Similarly, no publication bias was detected for the following comparisons for serious pneumonia: fluticasone versus control (p = 0.9); budesonide versus control (p = 0.4); low dose versus control (p = 0.9); and medium dose versus control (p = 0.7).
Sensitivity analyses
Seven studies [35, 55, 57, 61, 65, 67, 80] were observed with a high risk of bias in at least one domain. Removal of the data from these studies showed similar results as that of the overall analysis and no significant difference was observed for the risk of pneumonia with mometasone plus formoterol (Electronic Supplementary Figure 11). The leave-one-out meta-analysis also revealed no significant changes in the pooled estimates when the results of each included study were removed from the analysis (Electronic Supplementary Figure 12).
Trial sequential analysis and cumulative meta-analysis
Trial sequential analysis was carried out between fluticasone and budesonide with control. Significant pooled estimates observed between these interventions are sufficient for concluding that these interventions were associated with an increased risk of pneumonia (Electronic Supplementary Figures 13 and 14). Cumulative meta-analysis revealed that data published later than Caverley et al. 2007 did not change the pooled estimates significantly (Electronic Supplementary Figure 15).
Grading the strength of evidence
Grading the evidence for the significant estimates is outlined in Table 2. Moderate strength of evidence was observed for fluticasone with control group comparison for the pneumonia risk while either low or very low strength was observed for other comparisons.
Discussion
Statement of key findings
The present network meta-analysis evaluated the risk of pneumonia with ICS therapies in COPD patients. Fluticasone, mometasone, and beclometasone were observed with a significantly increased risk of pneumonia compared to placebo. Fluticasone was observed to increase the risk of serious pneumonia. A trend of increased risk of pneumonia was observed with an increase in the ICS doses. Meta-regression analysis revealed a high risk of pneumonia with high ICS doses, BMI ≥ 25 kg/m2, at least one previous exacerbation, and with fluticasone compared to budesonide. The results did not change significantly in the sensitivity analysis and the publication bias was not detected. A moderate level of certainty was observed for most of the comparisons with statistically significant pooled estimates. TSA results confirmed the adequacy of evidence for pneumonia risk for fluticasone and budesonide. Cumulative meta-analysis revealed that the pooled estimates did not change significantly with the addition of data later than the year 2007. NNH was 100 for fluticasone and high doses of ICS for the risk of pneumonia.
Strengths and weaknesses
This is the first network meta-analysis comparing the differences in the risk of pneumonia between various ICS therapies. To this date, this is the largest meta-analysis carried out evaluating the risk of this outcome. Most of the included studies were high quality with a low risk of bias. Additionally, we have also carried out robust sensitivity analysis and meta-regression for identifying the predictors of pneumonia risk with ICS therapies. We have also evaluated the NNH to make an informed decision by the clinicians and regulatory authorities. However, the study is limited in imputing values for BMI for a few studies as well as there were no studies that recruited patients with low BMI due to which we could not analyze the effect of underweight as a covariate. Future studies should also consider blood eosinophil count for stratifying patients. We observed that only one trial used a cut-off limit of blood eosinophil count as 300 cells/µl. GOLD 2023 guidelines recommend < 100 and ≥ 300 cells/µl as cut-off [5] and should be considered in future trials for assessing the predictive ability of this factor.
Interpretation and further research
The European Medicine Agency (EMA) reviewed the data available until 2016 and concluded the risk of pneumonia with ICS therapies [94]. However, EMA has concluded that there is no conclusive evidence of any intra-class differences and the dose of ICS with pneumonia. In the present study, we observed that budesonide had a significantly lower risk of pneumonia compared to fluticasone. A similar conclusion was made from a direct comparison meta-analysis where fluticasone users were observed with a 13.5% increase in the risk of pneumonia compared to budesonide [15]. We observed an OR of 0.7 (20% decrease) for pneumonia with budesonide compared to fluticasone with a NNH difference of 92.8. Hence, it takes 90 additional patients to observe one pneumonia with budesonide compared to fluticasone. Similarly, Yang et al. carried out a direct comparison meta-analysis with 25 RCTs with 49,982 participants and concluded a significant difference between fluticasone doses and pneumonia risk but not with budesonide therapy [9]. A nationwide observational study in Korea in a total of 47,473 patients revealed that 14% of 14,518 patients receiving fluticasone developed pneumonia as like 10.66% of 14,518 patients on budesonide users [95]. A record-linked 10-year data from Sweden revealed a number needed to harm as 22 patients treated with fluticasone developed pneumonia as against one additional patient with budesonide [96]. This difference could potentially be explained by increased lipophilicity coupled with a sustained and more potent immunosuppressive effect with fluticasone [97]. Further, the deposition of fluticasone in the lungs was observed to be five times more than budesonide, and a larger fraction was excreted in the sputum [98]. The authors of that study did not include any data regarding other ICS therapies such as mometasone and beclometasone. In the present study, we have included all the ICS therapies and budesonide was observed with the least risk of developing pneumonia. However, the NNH values of fluticasone and high ICS doses were relatively higher, so it is likely that the risk–benefit balance favors the continued use of ICS in COPD patients with moderate-to-severe exacerbations. Further, due to the very low-risk differences between other ICS therapies, it is unlikely that any clinically relevant differences might exist within the ICS class.
We observed a gradient increase in the risk of pneumonia with an increase in the ICS dose. Further, high doses of ICS were observed with a 10% additional risk of pneumonia compared to low doses in the present study. A recent study revealed that the addition of low-to-moderate doses of ICS could be observed with a net clinical benefit in patients with baseline exacerbation to an extent of 54–83% [99]. As recommended in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 guidelines, de-escalation of ICS should be considered wherever possible [5,6,7,5]. Frequent episodes of pneumonia are a contraindication for receiving ICS [100]. Despite these, the GOLD 2023 guidelines do not provide any recommendations on the preference of either budesonide over fluticasone or on the doses of ICS to be initiated and titrated. It is high time the clinical differences in the key outcomes between inhalational fluticasone and budesonide are recognized in the standard treatment guidelines and recommendations emerge regarding the dosing strategies.
Body mass index was observed to be a significant predictor in the present study. Chen et al. carried out a sub-group analysis on the population from similar studies and observed that an increase in the risk of pneumonia was observed in both the groups with BMI < 25 and with ≥ 25 kg/m2 [14]. However, in the present study, we carried out meta-regression and observed that the latter category poses a significant risk for pneumonia. Although a greater BMI was observed with an increased risk of pneumonia, those in the overweight category were observed with a reduced risk of mortality [101]. Future trials should consider personalizing the ICS therapy based on BMI values and shed more light on this predictive factor in COPD patients.
Conclusion
ICS class differences in pneumonia risk were observed in terms of pooled effect estimates but it is unlikely that any clinically relevant differences exist. Risk–benefit analysis generally supports ICS use in moderate-severe COPD. Guidelines should consider ICS type and dose. Further studies are needed to confirm the link between BMI and pneumonia risk in COPD patients receiving ICS.
References
Park SC, Kim DW, Park EC, et al. Mortality of patients with chronic obstructive pulmonary disease: a nationwide population based cohort study. Korean J Intern Med. 2019;34(6):1272–8.
Global Initiative for Asthma 2022. Global strategy for asthma management and prevention. Available at: https://ginasthma.org/wp-content/uploads/2022/05/GINA-Main-Report-2022-FINAL-22-05-03-WMS.pdf. Accessed 9 Nov 2023.
Miravitlles M, Roman-Rodríguez M, Ribera X, et al. Inhaled corticosteroid use among COPD patients in primary care in Spain. Int J Chronic Obstr Pulm Dis. 2022;17:245–58.
Quint JK, Ariel A, Barnes PJ. Rational use of inhaled corticosteroids for the treatment of COPD. NPJ Prim Care Respir Med. 2023;33(1):27.
GOLD 2023 guidelines. Available at: https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf. Accessed 12 March 2024.
Celli BR, Anderson JA, Cowans NJ, et al. Pharmacotherapy and lung function decline in patients with chronic obstructive pulmonary disease: a systematic review. Am J Respir Crit Care Med. 2021;203:689-98. https://doi.org/10.1164/rccm.202005-1854OC.
Calverley P. Reigniting the TORCH: chronic obstructive pulmonary disease mortality and inhaled corticosteroids revisited. Am J Respir Crit Care Med. 2021;203(5):531–2.
Cates C. Inhaled corticosteroids in COPD: quantifying risks and benefits. Thorax. 2013;68(6):499–500.
Pandya D, Puttanna A, Balagopal V. Systemic effects of inhaled corticosteroids: an overview. Open Respir Med J. 2014;8:59–65.
Mkorombindo T, Dransfield MT. Inhaled corticosteroids in chronic obstructive pulmonary disease: benefits and risks. Clin Chest Med. 2020;41(3):475–84.
Zhang Q, Li S, Zhou W, et al. Risk of pneumonia with different inhaled corticosteroids in COPD patients: a meta-analysis. COPD. 2020;17(4):462–9.
Yang M, Du Y, Chen H, et al. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019;77: 105950.
Suissa S. Inhaled corticosteroids and pneumonia mortality in COPD patients. Eur Respir J. 2019;54(3):1901276.
Chen H, Sun J, Huang Q, et al. Inhaled corticosteroids and the pneumonia risk in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12: 691621.
Lodise TP, Li J, Gandhi HN, et al. Intraclass difference in pneumonia risk with fluticasone and budesonide in COPD: a systematic review of evidence from direct-comparison studies. Int J Chronic Obstr Pulm Dis. 2020;15:2889–900.
Sridharan K, Sequeira RP. Drugs for treating severe hypertension in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(9):1906–16.
Proposal for pneumonia risk with ICS. Available at: https://osf.io/tegpk. Accessed 1 Jan 2024.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Higgins JP, Altman DG, Gøtzsche PC, et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Gjerdevik M, Heuch I. Improving the error rates of the Begg and Mazumdar test for publication bias in fixed effects meta-analysis. BMC Med Res Methodol. 2014;14:109.
Asthma: diagnosis, monitoring and chronic asthma management. Available at: https://www.nice.org.uk/guidance/ng80/resources/asthma-diagnosis-monitoring-and-chronic-asthma-management-pdf-1837687975621. Accessed 2 Nov 2023.
EpiGear International. Available at: https://www.epigear.com/index.html. Accessed 6 Nov 2023.
GRADE handbook. Introduction to GRADE handbook. Available at: https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 6 Nov 2023.
VOSviewer version 1.6.20. Available at: https://www.vosviewer.com/. Accessed 4 Nov 2023.
Litmaps (2023). Available at: https://www.litmaps.com/. Accessed 4 Nov 2023.
Wallace BC, Lajeunesse MJ, Schmid CH, et al. OpenMEE: Intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol Evol. 2017;8(8):941–7.
Thorlund K, Engstrom J, Wetterslev J, et al. Trial sequential analysis. Copenhagen trial unit. Available at: https://ctu.dk/tsa/. Accessed 4 Nov 2023.
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone–salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007;146:45-55.
Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD J Chronic Obstr Pulm Dis. 2009;6(5):320–9.
Bansal S, Anderson M, Anzueto A, et al. Single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) triple therapy versus tiotropium monotherapy in patients with COPD. Prim Care Respir Med. 2021;31(1):29.
Beeh KM, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat(®) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler(®) (ENERGITO(®) study). Int J Chronic Obstr Pulm Dis. 2016;4(11):193–205.
Beeh KM, Kuna P, Corradi M, et al. Comparison of Dry-powder inhaler and pressurized metered-dose inhaler formulations of extrafine beclomethasone dipropionate/formoterol fumarate/glycopyrronium in patients with COPD: the TRI-D randomized controlled trial. COPD. 2021;16:79–89.
Betsuyaku T, Kato M, Fujimoto K, et al. A randomized trial of symptom-based management in Japanese patients with COPD. COPD. 2018;13:2409–23.
Bhatt S, Dransfield M, Cockcroft J, et al. A randomized trial of once-daily fluticasone furoate/vilanterol or vilanterol versus placebo to determine effects on arterial stiffness in COPD. COPD. 2017;12:351–65.
Burge PS, Calverley PM, Jones PW et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–303.
Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–9.
Calverley PM, Rennard S, Nelson HS, et al. One-year treatment with mometasone furoate in chronic obstructive pulmonary disease. Respir Res. 2008;9(1):73.
Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89.
Calverley PMA, Kuna P, Monsó E, et al. Beclomethasone/formoterol in the management of COPD: A randomised controlled trial. Respir Med. 2010;104(12):1858–68.
Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–39.
Cheng SL, Su KC, Wang HC, et al. Chronic obstructive pulmonary disease treated with inhaled medium- or high-dose corticosteroids: a prospective and randomized study focusing on clinical efficacy and the risk of pneumonia. Drug Des. 2014:601-7.
Covelli H, Pek B, Schenkenberger I, et al. Efficacy and safety of fluticasone furoate/vilanterol or tiotropium in subjects with COPD at cardiovascular risk. COPD. 2015;1.
Doherty D, Tashkin D, Kerwin, et al. Effects of mometasone furoate/formoterol fumarate fixed-dose combination formulation on chronic obstructive pulmonary disease (COPD): results from a 52-week Phase III trial in subjects with moderate-to-very severe COPD. COPD. 2012;57.
Donohue JF, Worsley S, Zhu CQ, et al. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109(7):870–81.
Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–23.
Dransfield MT, Cockcroft JR, Townsend RR, et al. Effect of fluticasone propionate/salmeterol on arterial stiffness in patients with COPD. Respir Med. 2011;105(9):1322–30.
Dransfield MT, Crim C, Criner GJ, et al. Risk of exacerbation and pneumonia with single-inhaler triple versus dual therapy in IMPACT. Annals ATS. 2021;18(5):788–98.
Fatima S, Tariq F, Saqib Ur Rehman M, et al. Risk of pneumonia with inhaled corticosteroid/long-acting β2 agonist therapy in chronic obstructive pulmonary disease. PJMHS. 2023;17(4):12–6.
Ferguson GT, Anzueto A, Fei R, et al. Effect of fluticasone propionate/salmeterol (250/50 μg) or salmeterol (50 μg) on COPD exacerbations. Respir Med. 2008;102(8):1099–108.
Ferguson GT, Brown N, Compton C, et al. Once-daily single-inhaler versus twice-daily multiple-inhaler triple therapy in patients with COPD: lung function and health status results from two replicate randomized controlled trials. Respir Res. 2020;21(1):131.
Ferguson GT, Papi A, Anzueto A, et al. Budesonide/formoterol MDI with co-suspension delivery technology in COPD: the TELOS study. Eur Respir J. 2018;52(3):1801334.
Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–58.
Ferguson GT, Tashkin DP, Skärby T, et al. Effect of budesonide/formoterol pressurized metered-dose inhaler on exacerbations versus formoterol in chronic obstructive pulmonary disease: the 6-month, randomized RISE (Revealing the Impact of Symbicort in reducing Exacerbations in COPD) study. Respir Med. 2017;132:31–41.
Frith PA, Ashmawi S, Krishnamurthy S, et al. Efficacy and safety of the direct switch to indacaterol/glycopyrronium from salmeterol/fluticasone in non-frequently exacerbating COPD patients: the FLASH randomized controlled trial. Respirology. 2018;23(12):1152–9.
Fukuchi Y, Samoro R, Fassakhov R, et al. Budesonide/formoterol via Turbuhaler® versus formoterol via T urbuhaler® in patients with moderate to severe chronic obstructive pulmonary disease: phase III multinational study results. Respirology. 2013;18(5):866–73.
Hanania NA, Crater GD, Morris AN, et al. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106(1):91–101.
Hanania NA, Papi A, Anzueto A, et al. Efficacy and safety of two doses of budesonide/formoterol fumarate metered dose inhaler in COPD. ERJ Open Res. 2020;6(2):00187–2019.
Huang K, Guo Y, Kang J, et al. The efficacy of adding budesonide/formoterol to ipratropium plus theophylline in managing severe chronic obstructive pulmonary disease: an open-label, randomized study in China. Ther Adv Respir Dis. 2019;13:175346661985350.
Ichinose M, Fukushima Y, Inoue Y, et al. Long-term safety and efficacy of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler formulated using co-suspension delivery technology in Japanese patients with COPD. COPD. 2019;14:2993–3002.
Jung KS, Park HY, Park SY et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir med. 2012;106:382-9.
Kardos P, Wencker M, Glaab T, et al. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(2):144–9.
Kerwin EM, Ferguson GT, Mo M, et al. Bone and ocular safety of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a 52-week randomized study. Respir Res. 2019;20(1):167.
Kerwin EM, Scott-Wilson C, Sanford L, et al. A randomised trial of fluticasone furoate/vilanterol (50/25 μg; 100/25 μg) on lung function in COPD. Respir Med. 2013;107(4):560–9.
Lee S, Xie C, Yunus F, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a randomized, multicentre study in East Asia. Respirology. 2016;21(1):119–27.
Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–80.
Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–94.
Martinez FJ, Boscia J, Feldman G, et al. Fluticasone furoate/vilanterol (100/25; 200/25 μg) improves lung function in COPD: a randomised trial. Respir Med. 2013;107(4):550–9.
Muiser S, Imkamp K, Seigers D, et al. Budesonide/formoterol maintenance and reliever therapy versus fluticasone/salmeterol fixed-dose treatment in patients with COPD. Thorax. 2023;78(5):451–8.
Ohar JA, Crater GD, Emmett A, et al. Fluticasone propionate/salmeterol 250/50 μg versus salmeterol 50 μg after chronic obstructive pulmonary disease exacerbation. Respir Res. 2014;15(1):105.
Panettieri RA Jr, Camargo CA Jr, Cheema T, et al. Effect of recent exacerbation history on the efficacy of once-daily single-inhaler fluticasone furoate/umeclidinium/vilanterol triple therapy in patients with chronic obstructive pulmonary disease in the FULFIL trial. Int J Chronic Obstr Pulm Dis. 2022;1(17):2043–52.
Papi A, Dokic D, Tzimas W, et al. Fluticasone propionate/formoterol for COPD management: a randomized controlled trial. COPD. 2017;12:1961–71.
Pepin JL, Cockcroft JR, Midwinter D, et al. Long-acting bronchodilators and arterial stiffness in patients with COPD. Chest. 2014;146(6):1521–30.
Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48.
Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–65.
Rossi A, Wehbe L, Quinn M, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44:1548-56.
Sharafkhaneh A, Southard JG, Goldman M, et al. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106(2):257–68.
Siler TM, Nagai A, Scott-Wilson CA, et al. A randomised, phase III trial of once-daily fluticasone furoate/vilanterol 100/25 μg versus once-daily vilanterol 25 μg to evaluate the contribution on lung function of fluticasone furoate in the combination in patients with COPD. Respir Med. 2017;123:8–17.
Singh D, Worsley S, Zhu CQ, et al. Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm Med. 2015;15(1):91.
Tashkin DP, Rennard SI, Martin P, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs. 2008;68(14):1975–2000.
Tashkin DP, Doherty DE, Kerwin E, et al. Efficacy and safety of a fixed-dose combination of mometasone furoate and formoterol fumarate in subjects with moderate to very severe COPD: results from a 52-week Phase III trial. Int J Chronic Obstr Pulm Dis. 2012;7:43–55.
Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. The Lancet. 2016;387(10030):1817–26.
Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–60.
Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. The Lancet. 2017;389(10082):1919–29.
Vestbo J, Søorensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. The Lancet. 1999;353(9167):1819–23.
Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48(4):1030–9.
Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol–fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60.
Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–34.
Wedzicha JA, Calverley PMA, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26.
Wedzicha JA, Singh D, Vestbo J, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108(8):1153–62.
Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–50.
Zheng J, Baldi S, Zhao L, et al. Efficacy and safety of single-inhaler extrafine triple therapy versus inhaled corticosteroid plus long-acting beta2 agonist in eastern Asian patients with COPD: the TRIVERSYTI randomised controlled trial. Respir Res. 2021;22(1):90.
Zheng J, De Guia T, Wang-Jairaj J, et al. Efficacy and safety of fluticasone furoate/vilanterol (50/25 mcg; 100/25 mcg; 200/25 mcg) in Asian patients with chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Curr Med Res Opin. 2015;31(6):1191–200.
Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. COPD. 2015;10:1015–26.
EMA completes review of inhaled corticosteroids for chronic obstructive pulmonary disease. Available at: https://www.ema.europa.eu/en/news/ema-completes-review-inhaled-corticosteroids-chronic-obstructive-pulmonary-disease. Accessed 5 Nov 2023
Choi JH, Jeong KB, Park YH, et al. Comparison of risk of pneumonia caused by fluticasone propionate versus budesonide in chronic obstructive pulmonary disease: a nationwide retrospective cohort study. Int J Chronic Obstr Pulm Dis. 2021;16:3229–37.
Janson C, Larsson K, Lisspers KH, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS). BMJ. 2013;346: f3306.
Maassen van den Brink KI, Boorsma M, Staal-van den Brekel AJ, et al. Evidence of the in vivo esterification of budesonide in human airways. Br J Clin Pharmacol. 2008;66(1):27–35.
Dalby C, Polanowski T, Larsson T, et al. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10(1):104.
Yebyo HG, Braun J, Menges D, et al. Personalising add-on treatment with inhaled corticosteroids in patients with chronic obstructive pulmonary disease: a benefit-harm modelling study. Lancet Digit Health. 2021;3(10):e644–53.
Sibila O, Soto-Gomez N, Restrepo MI. The risk and outcomes of pneumonia in patients on inhaled corticosteroids. Pulm Pharmacol Ther. 2015;32:130–6.
Kim RY, Glick C, Furmanek S, et al. Association between body mass index and mortality in hospitalised patients with community-acquired pneumonia. ERJ Open Res. 2021;7(1):00736–2020.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sridharan, K., Sivaramakrishnan, G. Intraclass comparison of inhaled corticosteroids for the risk of pneumonia in chronic obstructive pulmonary airway disorder: a network meta-analysis and meta-regression. Int J Clin Pharm 46, 831–842 (2024). https://doi.org/10.1007/s11096-024-01736-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-024-01736-8