Abstract
Background
Previous studies on medication therapy management services, e.g. medication reconciliation and medication review, do not show consistent improvements in patient’s health-related quality of life. However, these services can reduce adverse drug events.
Aim
To evaluate the correlation between health-related quality of life and adverse events/adverse drug events reported by patients.
Method
Older patients (≥ 65 years) with polypharmacy (≥ 5 medicines) admitted to orthopaedic or surgical wards were included. Patients were contacted post-discharge to evaluate patient-reported adverse events, health-related quality of life using the EuroQol questionnaire and self-perceived health status on a 5-point Likert scale. The outcomes were the correlation between health-related quality of life and the number of adverse events/adverse drug events, and potential predictors for these events. Spearman correlation and Poisson regression were used for data analysis.
Results
102 patients were included. The correlation between health-related quality of life and adverse events was weak but significant (Spearman correlation coefficient: − 0.328, p = 0.001). No correlation was found for adverse drug events (− 0.064, p = 0.521). Self-perceived health status was a predictor for adverse events, not for adverse drug events. Health-related quality of life was neither a predictor for adverse events, nor for adverse drug events.
Conclusion
The correlation between the number of patient-reported adverse events, adverse drug events and health-related quality of life measured by the EuroQol was weak. There is a need for a questionnaire that includes the impact of medication use and is sensitive to outcomes that are affected by medication therapy management services.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
The EuroQol questionnaire (EQ-5D) is often used in clinical and health economic research to evaluate the effect of medication therapy management services. However, this questionnaire only includes questions that generally are not impacted by medication.
-
This study showed a weak correlation between the number of patient-reported adverse (drug) events and health-related quality of life measured by the EQ-5D. This result provides further support for the hypothesis that the EuroQol questionnaire is not sensitive enough to evaluate medication therapy management services.
Introduction
The use of polypharmacy has been linked to negative outcomes such as adverse drug events (ADEs) [1]. ADEs are any injuries resulting from medication use, including physical harm, mental harm or loss of function [2]. ADEs have an important effect on patient’s health status [3]. Medication therapy management services, such as medication review and medication reconciliation, can reduce ADEs [4,5,6,7,8]. Consequently, it has been suggested that these services may also improve the patient’s health-related quality of life (HRQoL) [9,10,11].
The EuroQol five-dimensional (EQ-5D) questionnaire is the most commonly used generic questionnaire to measure HRQoL [12]. This questionnaire is used in clinical and health economic research. The EQ-5D is validated and considers five dimensions including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [13]. Also in medication therapy management studies the EQ-5D is a frequently used questionnaire [14, 15].
Generic HRQoL measures have a very broad approach to health-related quality of life and are often not sensitive to changes in humanistic outcomes linked to changes in medication regimens for patients [16]. A review of Mohammed et al., discussed that HRQoL questionnaires provide a limited coverage of themes related to the burden of medication use because they mainly address somatic problems [14]. This was also highlighted in the DREAMER-study [10]. This study showed that a medication review focused on personal goals decreased the number of health problems with impact on daily life, but it did not significantly affect HRQoL measured with the EQ-5D index score [10]. Similarly, a recent randomized controlled trial incorporating pharmaceutical care did not show an effect on EQ-5D index score, while a significantly higher proportion of drug-related problems was resolved, including adverse drug events [17].
Previous studies have not evaluated the correlation between HRQoL and the number of patient-reported ADEs which are impacted by medication therapy management services.
Aim
Therefore, the aim of this study is to evaluate the correlation between HRQoL measured by EQ-5D and adverse (drug) events reported by patients.
Ethics approval
The original study was approved by the local ethics committee of OLVG hospital (Adviescommissie Wetenschappelijk Onderzoek-Medisch-Ethische Commissie, i.e. ACWO-MEC, ID WO: 17.040). Written informed consent was obtained from all patients.
Method
The present study is a sub-analysis of a previous implementation study that evaluated the effect of a geriatric stewardship program on drug‑related problems reported by patients after discharge [6]. The pre-group received usual care (no program), the post-group received the geriatric stewardship program. The program entails an inpatient medication review by a hospital pharmacist and geriatrician based on (I) clinical records to draft initial recommendations, (II) consultations with primary care providers (general practitioner and community pharmacist) to discuss the hospital-based recommendations, (III) patient interviews to assess their needs, and (IV) a multidisciplinary evaluation of all previous steps to draft final recommendations. Details regarding the methods, results and sample size calculation of the original study have been reported elsewhere [6].
Setting and study population
The original study was performed in a general teaching hospital in Amsterdam, the Netherlands.
Data were collected from February 2017 until September 2018. Because this current study focussed explicitly on the correlation between HRQoL and adverse (drug) events—and a geriatric stewardship program will not impact the correlation itself between HRQoL and adverse (drug) events—data from the control and intervention group were combined for this study.
Inclusion criteria were age ≥ 65 years old, polypharmacy (chronic use of ≥ 5 medications), one or more risk factors for frailty as previsouly described in Ponjee et al. and admitted to orthopaedic or surgical wards [6]. The participants were recruited from orthopaedic or surgical wards, since these wards are classified as high risk for ADEs by the Dutch national guideline Polypharmacy in the older patients [18].
Exclusion criteria were logistical reasons (e.g. short length of stay ≤ 48 h), no informed consent, patients with a language barrier or cognitive impairment, tourists (no permanent residency in the Netherlands), patients that already had their medications reviewed in the past three months and patients who lived in a nursing home (as they do not manage their medication independently) or who received palliative care (due to the short life expectancy). In the current study we also excluded patients from whom questionnaires could not be obtained.
Data collection and classification
Patient-reported AEs, ADEs, HRQoL and self-rated health status were assessed two weeks after discharge by a telephone interview as most ADEs are known to occur in the first weeks after discharge [19]. Patients were contacted during office hours and with a maximum of three attempts in total. A validated questionnaire was used to evaluate the patient-reported events [20]. In this questionnaire a patient could indicate if he or she suffered from one or more complaints of a predetermined list of AEs or from other complaints which were not listed. If a patient reported a complaint, the patient was asked whether he or she thought it was caused by medication (ADEs) [4, 5, 20,21,22]. Previous studies addressed that healthcare professionals underestimate the prevalence of ADEs among their patients [21, 22]. Therefore, it is important to ask patients for ADEs. The most reported ADEs in the current study were stomach ache, nausea, obstipation, fatigue and pain [6].
Patients could be unsure whether their medication was causing an ADE. Also, elderly patients are known to link their medication use less often to ADEs as they think it is part of aging or due to their illness [22, 23]. Furthermore, for healthcare professionals it is difficult to clinically adjudicate the likelihood that a patient-reported complaint is an ADE. For example head ache, nausea or dizziness could be related to many medication. Therefore, we collected data on both patient-reported ADEs and patient-reported AEs. ADEs and AEs will be addressed as A(D)Es in the rest of this paper.
The EQ-5D-3L was used to evaluate HRQoL. The five dimensions, i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression, have each three severity levels that are described by statements. The EQ-5D-3L statements were scored with the Dutch value set to obtain EQ-5D-3L summary index scores that represents the patient-reported state of health [24]. The summary index score ranges from 0 (representing death) to 1 (representing full health), with negative values representing states worse than death [25].
The self-rated health status was measured with a 5-point Likert scale (very poor, poor, fair, good, excellent) on which higher scores indicated better self-rated health [25].
Patient characteristics were extracted from the medical records in the hospital information system (e.g. gender, age, living situation, using home health care, having help with medication use, education level, health literacy, ward type, length of hospital stay, risk factors of frailty, number of prescription drugs per patient and received the geriatric stewardship intervention). Health literacy was measured using the Set of Brief Screening Questions (SBSQ) [26]. This questionnaire was used as it addresses self-perceived skills concerning understanding medical information and filling out medical forms. It contains three questions making it more feasible for daily practice than many extensive health literacy questionnaires.
Outcome measures
The primary outcome was the correlation between HRQoL and the number of patient-reported AEs and ADEs. Secondary outcomes were potential predictors of AEs and ADEs.
Data analysis
Spearman’s rank correlation coefficient was used to assess the correlations between the HRQoL and the number of patient-reported AEs and ADEs, because both HRQoL and the number of reported A(D)Es were not normally distributed. To assess a possible dissimilarity in gender and age, Spearman correlations were calculated for men and women separately. The two age groups used in this study were defined as age < 75 years and 75 year or higher. A Spearman coefficient of 0.20–0.35 was considered weak, between 0.35–0.50 moderate, and > 0.5 indicates a strong correlation [27].
Univariable Poisson regression was used to identify predictors for the number of A(D)Es as a hypothesis generating study. Predictors with a p value < 0.1 in univariable analysis were entered in a multivariable model to determine the independent effects. A model including HRQoL was analysed and a model replacing HRQoL with self-rated health status was analysed. All analyses were done using IBM SPSS Statistics version 27. p values < 0.05 were considered statistically significant.
Results
Baseline patient characteristics
A total of 127 patients participated in the original study. Twenty five patients did not complete the HRQoL questionnaire mainly because patients felt too ill to respond. Consequently, 102 patients were included in the analysis.
Patient baseline characteristics are presented in Table 1. Forty-six percent of the patients were male, the median age of patients was 77 years (IQR: 10.8). The mean number of prescription drugs per patient was 9.9 (SD 3.4). Half of the patients had received the geriatric stewardship program.
Correlation HRQoL and adverse (drug) events
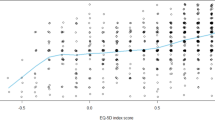
A significant weak correlation was found between HRQoL and the number of patient-reported AEs (correlation coefficient − 0.328, p = 0.001, i.e. a lower summary index score of HRQoL correlates with a higher number of AEs). No significant correlation was found between HRQoL and the number of patient-reported ADEs (correlation coefficient − 0.064, p = 0.521). Figure 1 shows that there is considerable heterogeneity between the patient-reported HRQoL and A(D)Es.
When exploring gender, a significant correlation between HRQoL and AEs was present in both males (correlation coefficient − 0.298, p = 0.042) and females (correlation coefficient − 0.323, p = 0.016). For both genders, there was no significant correlation between HRQoL and ADEs.
In patients under 75 years there was a significant correlation between HRQoL and AEs (correlation coefficient − 0.468, p = 0.002). In patients 75 years and older no significant correlation was found (correlation coefficient − 0.242, p = 0.060). For both age groups there was no significant correlation between HRQoL and patient-reported ADEs (p = 0.350 and p = 0.965, respectively).
Predictors of adverse (drug) events
In univariable Poisson regression the following characteristics were associated with the number of patient-reported AEs (i.e., p < 0.1): gender, hospital location, living alone, health literacy, HRQoL summary index score, number of prescription drugs, length of stay and receiving the geriatric stewardship intervention. In multivariable analyses only low health literacy (adjusted rate ratio (aRR) 1.29, 95% CI 1.01–1.65; p = 0.043) was significantly associated with a higher number of patient-reported AEs (Table 2a).
In univariable Poisson regression the following characteristics were associated with the number of patient-reported ADEs (i.e., a p < 0.1): ward type, living alone, health literacy, having home care, number of prescription drugs, and receiving the geriatric stewardship intervention. The multivariable Poisson regression analyses for the risk of developing an extra ADE showed four predictors that were independently associated with a higher number of ADEs (Table 2b); inadequate health literacy (aRR 1.77, 95% CI 1.13–2.77; p = 0.013), admission to the orthopaedic ward (instead of surgical, aRR 1.88, 95% CI 1.18–2.98, p = 0.008), having home care (aRR 1.53, 95% CI 1.01–2.33; p = 0.047) and having received the geriatric stewardship program (aRR 0.46, 95% CI 0.29–0.74; p = 0.001).
When replacing the HRQoL summary index score with the self-rated health status of patients, in the multivariable analysis the self-rated health status was a predictor (aRR 0.57 (95% CI 0.37–0.88) for very good/excellent health vs. bad health; overall p = 0.036) for AE but not for ADE (aRR 0.47 (95% CI 0.18–1.23) for very good/excellent health vs. bad health; overall p = 0.223).
Discussion
Key findings
This study showed a weak, but significant correlation between health-related quality of life (HRQoL) and patient-reported adverse events (AEs), and no correlation between HRQoL and patient-reported adverse drug events (ADEs) in older patients with polypharmacy. HRQoL was not a predictor for the number of patient-reported A(D)Es.
Taking the correlation coefficient for patient-reported AEs (− 0.328) into account a weak correlation was found. Furthermore, there was considerable heterogeneity between the patient-reported AEs as we observed patients with one AE reporting a low HRQoL versus patients with ≥ 5 AEs reporting an excellent HRQoL. The EuroQol questionnaire is limited to five dimensions, of which pain and anxiety/depression can be the result of potential adverse events. Complaints such as nausea, obstipation and fatigue will less likely influence the other dimensions measuring functioning such as mobility, self-care and usual activities. These complaints are expected to reduce HRQoL compared to patients who do not have these complaints. Therefore, it might be difficult to impact HRQoL measured with questionnaires that also focus on somatic problems. In a larger study population the EQ-5D subscales may be more useful in measuring elements of quality of life such as pain or anxiety/depression. This study was too small to perform subscale analysis.
To our knowledge, this is the first study that evaluated the correlation between a frequently used HRQoL questionnaire, i.e. the EuroQol questionnaire (EQ-5D), and A(D)Es. The EQ-5D is often used in clinical and health economic research to evaluate the effect of medication therapy management services [27]. A systematic review by Payakachat et al. studied the responsiveness of the EQ-5D to detect meaningful clinical change in health states. They found that the EQ-5D was responsive in only half of the studies, as an effect of the use of the five domains which did not cover disease-specific measures. They also reported that the EQ-5D is less sensitive when it comes to small changes or less severe conditions [28].
In this study the EQ-5D-3L was used. A newer version the EQ-5D-5L, with five instead of three answer options, is widely researched and generally accepted as more sensitive, but conflicting results have been published [29]. A recent European study showed similar measurement properties between EQ-5D-3L and EQ-5D-5L in older patients with polypharmacy [27]. EQ-5D-5L uses the same five dimensions and thus has the same shortcoming as the EQ-5D-3L. To evaluate the impact of pharmaceutical interventions, such as medication reconciliation, patient education and medication review, there is a need for a HRQoL questionnaire that includes the impact of medication use and that is validated in multiple countries. New tools have been proposed such as the Medication-Related Burden Quality of Life (MRBQoL), the Medication-related Quality of Life (MRQoL) and the Living with Medicines Questionnaire (LMQ) [30,31,32,33]. The correlation with patient-reported outcomes that are impacted by medication management services need to be further explored.
Strengths and weaknesses and further research
A strength of this study is the use of patient-reported outcomes, as A(D)Es found in patient records can be incomplete [21, 22]. However, there are also limitations to this study. First, we did not assess whether there was a causal association between the patient-reported ADEs and the medication use of patients. This would have required medication reconciliation after discharge to assess the exact medication use of patients. But even then, distinguishing ADEs from the impact of the patient’s disease is difficult, especially for ADEs such as nausea or headache. Therefore, we reported the correlation for AEs and ADEs. Previous studies have shown that the sensitivity of patient-reported ADEs was lower (29–70%) than the specificity (85–93%) [22, 34]. This means that patients more often have difficulty in linking their complaints to their medication use. For this study, this would result in an underestimation of patient-reported ADEs compared to clinically adjudicated ADEs. Nevertheless, we think the correlation with HRQoL would still be weak as the overall correlation with AEs was weak.
Second, poor HRQoL could influence the perceptions of patients on A(D)Es as they might accept certain complaints as part of their disease and would not report them. However, to reduce this patients were questioned on a predetermined list of adverse events and patients addressed also other complaints which were not listed.
Third, the sample size in this study was limited and selection bias can be present as several patient groups were excluded due to the difficulty in obtaining the questionnaires (e.g. patients with a language barrier or cognitive impairment). There is a possibility that in other study populations better correlations are seen. Also, in larger study populations the HRQoL might be a predictor for the number of A(D)Es. It must be kept in mind, however, that medication therapy management services can only impact preventable ADEs which occur less often and probably would require very large sample sizes to show an effect on a generic HRQoL. Third, this study was performed in one hospital limiting the generalizability. Finally, there are many HRQoL measures of which some might better correlate. In this study the visual analogue scale (EQ-VAS) was not used due to the phone interview. Therefore, the self-rated health status was used. As the EQ-VAS is on a scale of 0–100 it might better reflect how patients perceive their HRQoL. In two randomized controlled trials, the DREAMER study and the study of Sakthong et al., no effect was seen on the EQ5D index score but there was an effect on EQ-VAS [10, 17]. Future studies should evaluate whether better correlations are found between other HRQoL measures and A(D)Es. Also, future studies should evaluate and validate for multiple patient groups what patients regard a good HRQoL in relation to their medication use.
Conclusion
In conclusion, HRQoL measured by the EQ-5D showed a weak correlation with patient-reported adverse events (AEs) and no correlation with adverse drug events (ADEs). HRQoL was not a predictor for the number of patient-reported A(D)Es. There is a need for a questionnaire that includes the impact of medication use and is sensitive to outcomes that are affected by medication therapy management services.
References
Pellegrino AN, Martin MT, Tilton JJ, et al. Medication therapy management services: definitions and outcomes. Drugs. 2009;69(4):393–406. https://doi.org/10.2165/00003495-200969040-00001.
Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34.
Forster AJ, Murff HJ, Peterson JF, et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–7. https://doi.org/10.7326/0003-4819-138-3-200302040-00007.
Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counselling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565–71. https://doi.org/10.1001/archinte.166.5.565.
Stuijt CCM, Bekker CL, van den Bemt BJF, et al. Effect of medication reconciliation on patient reported potential adverse events after hospital discharge. Res Soc Adm Pharm. 2021;17(8):1426–32. https://doi.org/10.1016/j.sapharm.2020.10.012.
Ponjee GHM, van de Meerendonk HWPC, Janssen MJA, et al. The effect of an inpatient geriatric stewardship on drug-related problems reported by patients after discharge. Int J Clin Pharm. 2021;43(1):191–202. https://doi.org/10.1007/s11096-020-01133-x.
Daliri S, Hugtenburg JG, Ter Riet G, et al. The effect of a pharmacy-led transitional care program on medication-related problems post-discharge: a before–after prospective study. PLoS ONE. 2019;14(3): e0213593. https://doi.org/10.1371/journal.pone.0213593.
Al-Hashar A, Al-Zakwani I, Eriksson T, et al. Impact of medication reconciliation and review and counselling, on adverse drug events and healthcare resource use. Int J Clin Pharm. 2018;40(5):1154–64. https://doi.org/10.1007/s11096-018-0650-8.
Bladh L, Ottosson E, Karlsson J, et al. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Qual Saf. 2011;20(9):738–46. https://doi.org/10.1136/bmjqs.2009.039693.
Verdoorn S, Kwint HF, Blom JW, et al. Effects of a clinical medication review focused on personal goals, quality of life, and health problems in older persons with polypharmacy: a randomised controlled trial (DREAMeR-study). PLoS Med. 2019;16(5): e1002798. https://doi.org/10.1371/journal.pmed.1002798.
Oonk NGM, Movig KLL, Munster EM, et al. The effect of a structured medication review on quality of life in Parkinson’s disease: the study protocol. Contemp Clin Trials Commun. 2019;13: 100308. https://doi.org/10.1016/j.conctc.2018.100308.
Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34(7):645–9. https://doi.org/10.1007/s40273-016-0389-9.
The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. https://doi.org/10.1016/0168-8510(90)90421-9.
Mohammed MA, Moles RJ, Chen TF. Pharmaceutical care and health related quality of life outcomes over the past 25 years: have we measured dimensions that really matter? Int J Clin Pharm. 2018;40(1):3–14. https://doi.org/10.1007/s11096-017-0582-8.
Pickard AS, Hung SY. An update on evidence of clinical pharmacy services’ impact on health-related quality of life. Ann Pharmacother. 2006;40(9):1623–34. https://doi.org/10.1345/aph.1G653.
Lech LVJ, Jónsdóttir ED, Niclasen J, et al. Translation and psychometric validation of a Danish version of the medication-related quality of life scale. Int J Clin Pharm. 2020;42(2):667–76. https://doi.org/10.1007/s11096-020-00979-5.
Sakthong P, Jaisue P. Impact of a drug-related patient-reported outcome measure on drug-related problem identification, physicians’ acceptance, and clinical and quality of life outcomes: a randomized controlled trial. Int J Clin Pharm. 2022;44(2):320–9. https://doi.org/10.1007/s11096-021-01341-z.
Addendum Polyfarmacie bij ouderen in de tweede lijn [in Dutch]. 2018. Available at https://richtlijnendatabase.nl/nieuws/addendumpolyfarmacie_bij_ouderen_in_de_tweede_l.html. Accessed 07 Mar 2022.
Cua YM, Kripalani S. Medication use in the transition from hospital to home. Ann Acad Med Singap. 2008;37(2):136–136.
Willeboordse F, Grundeken LH, van den Eijkel LP, et al. Information on actual medication use and drug-related problems in older patients: questionnaire or interview? Int J Clin Pharm. 2016;38(2):380–7. https://doi.org/10.1007/s11096-016-02SBS58-9.
Blenkinsopp A, Wilkie P, Wang M, et al. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007;63(2):148–56. https://doi.org/10.1111/j.1365-2125.2006.02746.x.
Cahir C, Wallace E, Cummins A, et al. Identifying adverse drug events in older community-dwelling patients. Ann Fam Med. 2019;17(2):133–40. https://doi.org/10.1370/afm.2359.
Dijkstra NE, Sino CGM, Schuurmans MJ, et al. Medication self-management: considerations and decisions by older people living at home. Res Social Adm Pharm. 2022;18(3):2410–23. https://doi.org/10.1016/j.sapharm.2020.09.004.
EuroHRQoL Reasearch Foundation. EQ-5D-3L. User Guide, 2018. Available from: https://euroHRQoL.oorg/publications/user-guides. Accessed 07 Mar 2022.
Mangen MJ, Bolkenbaas M, Huijts SM, et al. Quality of life in community-dwelling Dutch elderly measured by EQ-5D-3L. Health Qual Life Outcomes. 2017;15(1):3. https://doi.org/10.1186/s12955-016-0577-5.
Fransen MP, Van Schaik TM, Twickler TB, et al. Applicability of internationally available health literacy measures in the Netherlands. J Health Commun. 2011;16(Suppl 3):134–49. https://doi.org/10.1080/10810730.2011.604383.
Alldred DP, Kennedy MC, Hughes C, et al. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2016;2(2):CD009095. https://doi.org/10.1002/14651858.CD009095.pub3.
Payakachat N, Ali MM, Tilford JM. Can the EQ-5D detect meaningful change? A systematic review. Pharmacoeconomics. 2015;33(11):1137–54. https://doi.org/10.1007/s40273-015-0295-6.
Bhadhuri A, Kind P, Salari P, et al. Measurement properties of EQ-5D-3L and EQ-5D-5L in recording self-reported health status in older patients with substantial multimorbidity and polypharmacy. Health Qual Life Outcomes. 2020;18(1):317. https://doi.org/10.1186/s12955-020-01564-0.
Katusiime B, Corlett SA, Krska J. Development and validation of a revised instrument to measure burden of long-term medicines use: the Living with Medicines Questionnaire version 3. Patient Relat Outcome Meas. 2018;28(9):155–68. https://doi.org/10.2147/PROM.S151143.
Mohammed MA, Moles RJ, Hilmer SN, et al. Development and validation of an instrument for measuring the burden of medicine on functioning and well-being: the Medication-Related Burden Quality of Life (MRB-QoL) tool. BMJ Open. 2018;8(1): e018880. https://doi.org/10.1136/bmjopen-2017-018880.
Jennings ELM, O’Mahony D, Gallagher PF. Medication-related quality of life (MRQoL) in ambulatory older adults with multi-morbidity and polypharmacy. Eur Geriatr Med. 2022;13(3):579–83. https://doi.org/10.1007/s41999-021-00573-6.
Sakthong P. Pharmacotherapy related quality of life in Thai patients with chronic diseases. Int J Clin Pharm. 2019;41(4):1004–11. https://doi.org/10.1007/s11096-019-00857-9.
Mannesse CK, Derkx FH, de Ridder MA, et al. Do older hospital patients recognize adverse drug reactions? Age Ageing. 2000;29(1):79–81. https://doi.org/10.1093/ageing/29.1.79.
Acknowledgements
We would like to express our gratitude to the patients involved in this study.
Funding
No funding was received for performing this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beerlage-Davids, C.J., Ponjee, G.H.M., Vanhommerig, J.W. et al. Correlation between the number of patient-reported adverse events, adverse drug events, and quality of life in older patients: an observational study. Int J Clin Pharm 44, 1434–1441 (2022). https://doi.org/10.1007/s11096-022-01481-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01481-w