Abstract
In 2015, the United States of America (USA) Department of Health and Human Services (HHS) released an issue brief that addressed opioid addiction, opioid overdoses, and opioid-related deaths as a public health concern within the country. After collaboration with state and stakeholder organizations, the HHS identified three target initiatives aimed to mitigate the negative consequences of opioid use within the USA. One initiative included implementation of guidelines to help reduce inappropriate opioid prescribing with a goal to reduce morbidity and mortality. The aim of this commentary is to discuss the misapplication and unintended consequences of the USA CDC Guideline for Prescribing Opioids for Chronic Pain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
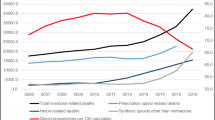
In 2015, the United States of America (USA) Department of Health and Human Services (HHS) released an issue brief that addressed illicit and prescription opioid addiction, opioid overdoses, and opioid-related deaths as a public health concern within the country [1]. The USA HHS reflected on the steady incline of opioid-related deaths over the previous two decades and the need to respond to the concerning trends [1].
After collaboration with state and stakeholder organizations, the HHS identified three target initiatives aimed to mitigate the negative consequences of opioid use within the USA [1]. The three target initiatives were 1) expanding access to medication-assisted treatment (MAT), 2) expanding use and distribution of naloxone, and 3) targeting opioid prescribing practices [1]. Of note, MAT is the combination of medication (methadone, buprenorphine, or naltrexone) and behavioral therapies used for treatment of opioid use disorders [2]. These three target initiatives were identified because they had limited, but evolving research to support their effectiveness in reducing the misuse of opioid medications [1]. The first target initiative, expanding MAT, was identified as an underutilized treatment modality with the potential to decrease morbidity and mortality. The second target initiative, expanding the use and distribution of naloxone, was identified because certain communities reported a decrease in overdose death rates where naloxone distribution programs were implemented [1]. The final target initiative, targeting opioid prescribing practices, was two-fold. The first aspect was to optimize prescription drug monitoring programs (PDMPs) [1]. PDMPs are state-run electronic databases that monitor prescribing of medications with abuse potential, such as controlled substances [1]. PDMPs enable prescribers and pharmacists to see a patient’s prescription history, which may assist health care providers to better detect patients who are potentially misusing their medications or obtaining prescriptions from multiple physicians [1]. The second aspect was the implementation of guidelines [1]. This was selected based on a previous study which showed that implementation of opioid prescribing guidelines led to a reduction in opioid prescribing, long acting opioid prescribing, and opioid-related overdose deaths [3]. The USA Centers for Disease Control and Prevention (CDC) aimed to develop opioid prescribing guidelines for providers that could potentially be incorporated into electronic health record (EHR) tools [1]. The aim of this commentary is to discuss the misapplication and unintended consequences of the subsequent USA CDC Guideline for Prescribing Opioids for Chronic Pain.
Summary of the CDC guideline for prescribing opioids for chronic pain
In 2016, The CDC released the Guideline for Prescribing Opioids for Chronic Pain. The purpose of the guideline was to provide opioid prescribing recommendations to primary care providers managing adult patients with chronic pain outside of the indications of active cancer treatment, palliative care, and end-of-life care [4]. Over 19 million adults in the United States have chronic pain, and hundreds of billions of dollars are lost annually in productivity due to employee pain [5, 6]. The foundation of the guideline was built on a systematic review that aimed to evaluate the effectiveness, benefits, and harms of long term opioid therapy for chronic pain [7]. The review was subsequently analyzed by experts in the field, key stakeholders, and the public to form recommendations [4]. Throughout the process, key themes emerged resulting in twelve recommendations from the CDC for prescribing opioids for chronic pain [4]. The guideline recommended that prescribers utilize non-pharmacologic and non-opioid pharmacologic therapy, and that opioids should only be used if the benefits outweigh the risks [4]. It was recommended that providers establish treatment goals and expectations with patients prior to initiation, and that patients should understand the risks and limitations of opioid therapy [4]. The guideline recommended utilizing immediate release preparations over extended release preparations as well as provided recommendations for follow up [4]. In addition, the guideline provided guidance on recommended maximum morphine milligram equivalents (MME) as well as tapering recommendations. Additional recommendations by the CDC from the guideline are listed below [4]:
-
“Although the clinical evidence review did not find high quality studies comparing the effectiveness of different tapering protocols for use when opioid dosage is reduced or opioids are discontinued, tapers reducing weekly dosage by 10–50% of the original dosage have been recommended by other clinical guidelines, and a rapid taper over 2–3 weeks has been recommended in the case of a severe adverse event such as overdose [4].”
-
“Avoid increasing dosage to ≥ 90 MME/day or carefully justify a decision to titrate dosage to ≥ 90 MME/day [4].”
-
The guideline notes that “long-term opioid use often begins with treatment of acute pain” and as a result recommends “When opioids are used for acute pain, clinicians should prescribe the lowest effective dose of immediate-release opioids and should prescribe no greater quantity than needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than seven days will rarely be needed [4].”
We highlight the above recommendations because they provide explicit dosing guidance, despite the guideline not intending to be prescriptive [8]. The CDC emphasized to readers that the evidence forming the recommendations within the guideline was of low quality and that additional data would be critical to ensure safe and effective medication practices [4].
Consequences of the CDC guideline for prescribing opioids for chronic pain
The guideline received several critiques, with a major focus surrounding the recommended maximum opioid medication dosages in MME [6]. MME is a standardized way to compare one opioid dose to another opioid dose by converting to equivalent dosages in milligrams of morphine. However, while MME appears to be a useful tool to convert between opioid prescriptions, conversions are not standardized and in practice different providers may determine different MME values for the same medication [6]. Furthermore, inappropriate conversions may cause patients to experience withdrawal. In addition, a maximum dose of 90 MME may not be appropriate for all patients and can create barriers when a provider takes over the care of a new patient with long-term chronic pain coming from a different clinic [6]. Thus, the recommendation provided a blanket statement that did not encompass all clinical situations.
Second, several states took action to reduce inappropriate prescribing of prescription opioid medications such as mandating legal limitations on the quantity or duration of opioids that may be prescribed or dispensed to patients with acute pain. A review of state laws that imposed mandatory limits on opioid prescribing and dispensing for acute pain showed that by the end of 2017, 26 states within the United States had implemented laws surrounding initial opioid prescriptions for acute pain [9]. The authors of the review note that some of the laws may have been driven by the CDC guidelines; however, no direct correlations can be made. On March 18, 2016, the CDC guideline was posted online as a Morbidity and Mortality Weekly Report (MMWR) Early Release [4]. Five of the 26 states had implemented opioid limit laws prior to the guideline release. Three of the 26 states passed laws later in 2016 after the guideline release, and 17 of the 26 states passed laws in the year 2017. The laws lacked standardization and varied widely [9]. It appeared some of the laws attempted to align themselves with the recommendations within the 2016 CDC guidelines, which was not the intent of the document [9]. Following the implementation of the CDC’s guideline, in December of 2017, the American Academy of Pain Medicine Foundation assembled a multidisciplinary panel of physician experts to review the recommendations as well as navigate obstacles to guideline implementation [8]. The panel reflected on the clinical and regulatory overstep as a result of the guideline. The panel emphasized that the guideline was “not meant to be prescriptive” and in some instances, had been rigidly implemented by regulatory bodies [8]. The panel identified several unintended consequences, one being the guideline’s recommendation for daily dosage thresholds which were interpreted as rigid maximum daily dose limits by a number of states and private insurers [8]. Additionally, the panel expressed concern surrounding opioid taper recommendations, as they appeared to be inappropriately applied by some clinicians, policy-makers, and health insurance organizations [8]. The panel also expressed concern about the potential seven day limit for immediate acting opioid prescriptions [8]. The panel noted that if this recommendation were to be rigidly implemented, patients with severe pain requiring longer duration of therapy may not have access to the medications that they need [8].
In April 2019, the Food and Drug Administration (FDA) received reports of “serious harm in patients” who were “physically dependent on opioid pain medicines suddenly having these medicines discontinued or the dose rapidly decreased [10].” Reports of adverse events included but were not limited to uncontrolled pain, mental distress, severe withdrawal, and suicide [10]. In some cases, withdrawal symptoms can be so uncomfortable that they drive patients with pain to self-medicate with heroin or other illicit substances [10]. As a result of these safety concerns, the FDA warned against tapering patients too rapidly, as this could result in uncontrolled pain and withdrawal symptoms [10]. The FDA recommended to execute patient-specific plans with gradual dose reduction schedules, and to appreciate that there is no one size fits all approach to opioid management and therapy de-escalation [10]. In addition to the guidance by the FDA, a cohort study by Fenton, et al. illustrated that rates of dose tapering increased after the publication of the CDC guideline [11]. Of course, fluctuations in opioid prescribing are likely multifactorial and may have also been impacted by federal policy changes, news and social media influence, personal and clinical experience.
In May 2019, the CDC identified that the guideline was being misinterpreted and being applied inappropriately [12]. Dr. Robert “Chuck” Rich, who represents the American Academy of Family Physicians (AAFP) on the American Medical Association (AMA) Task Force, was part of the initial panel of experts that assisted in creating the guideline [12]. Dr. Rich emphasized that the recommendations had been inappropriately implemented by insurers and regulatory agencies, leading to inappropriate management of patient for certain patients [12]. The panel identified that the available literature was limited at the time of guideline development and that the intent was to guide providers through a joint decision-making process with their patients, as opposed to setting set standards and regulations [12].
Viewpoint
In the United States, 96% of hospitals use computerized prescriber order entry (CPOE) systems with clinical decision support in their EHR, which replaces physical paper charts [13]. The use of order sets is a component of CPOE clinical decision support. An order set allows a prescriber to select a pre-built collection of orders and medications, instead of selecting and requesting them individually. An order set could include all the required medications, consult service requests, and nursing communications that should be ordered for a patient admitted with a certain diagnosis. For example, a post-surgical order set may contain pre-selected medications for pain management as well as nursing orders for the assessment of the patient’s pain scores. The patient’s pain score will dictate the medication and dose the patient will receive for pain management. Since the provider does not have to enter each order individually, this should save time and reduce the number of errors [14]. Order sets shape prescribing patterns within an institution because it promotes providers to order similar pre-approved medication dosages and frequencies. Hospitals have the opportunity to create their own order sets, or choose the default order sets created by their EHR vendors. At our institution, order sets are reviewed and approved by hospital leadership prior to development and implementation. Some EHR vendors have aligned their default opioid prescribing settings in response to the CDC guidelines [15]. As a result, we encourage pharmacists to evaluate opioid prescribing practices within their organizations. Pharmacist should consider the following steps in evaluating opioid prescribing practices within their institutions:
-
Review of all default opioid prescribing settings within an EHR, including all order sets containing opioids. Pharmacists should collaborate with interdisciplinary committees to determine if the preset drug choice, dosing, and frequency are appropriate for their patient populations.
-
When evaluating surgical patients, consider expanding from a single “post-operative” order set to order sets categorized based on the anticipated pain severity after surgery.
-
Consider categorizing pain management order sets based on patient baseline opioid use, with categories for opioid naïve and opioid tolerant.
-
Evaluate current best practice alerts (BPA) to ensure they are not promoting non-ideal prescribing practices.
-
Consider implementation of BPAs for rapid opioid dose de-escalation or percent change in total daily dose prescribing in order to prevent risk of opioid withdrawal.
-
Review all institutional protocols and guidelines that contain opioid prescribing recommendations. Again, choice of drug, dosing, and frequency should be evaluated and use in specific practice areas should be defined and described.
It is essential that institutions re-evaluate their internal prescribing practices. For example, a large academic medical center with the United States identified opportunities to optimize their opioid prescribing defaults [16]. Additionally, one institution showed that customized opioid clinical decision support reduced opioid prescribing without negatively impacting patient satisfaction [17]. We encourage pharmacists to provide education to prescribers about the risks of rapid tapering and abrupt opioid cessation. This can include but is not limited to continuing education presentations, grand rounds presentations, and informal in-service presentations. Finally, we encourage pharmacists to be actively involved within their local and national governments as well as pharmacy organizations in order to influence change. In the USA, each state is comprised of multiple local pharmacy organizations that make up larger national organizations. The local state organizations have the opportunity to escalate ideas and issues to the national level as warranted. It is our job as pharmacists to practice a questioning attitude and understand the data that influences our practice.
Conclusion
Chronic pain is a widespread, disabling, and expensive condition [8]. There is an opportunity to learn from the USA CDC Guideline for Prescribing Opioids for Chronic Pain. While not intended to provide prescriptive recommendations, the guideline resulted in a number of downstream effects on institutional behaviors, insurers, and individual prescribers [12]. We encourage leaders in healthcare to navigate solutions outside of traditional legislative and regulatory initiatives as well as encourage pharmacists to critically evaluate the practices within their own institutions.
References
Opioid Abuse in the U.S. and HHS Actions to address opioid-drug related overdoses and deaths. Department of Health & Human Services USA. https://aspe.hhs.gov/system/files/pdf/107956/ib_OpioidInitiative.pdf. March 26, 2015. Accessed February 25, 2020.
Information about Medication-Assisted Treatment (MAT). US food and drug administration. https://www.fda.gov/drugs/information-drug-class/information-about-medication-assisted-treatment-mat. February 14, 2019. Accessed April 14, 2020.
Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. Bending the prescription opioid dosing and mortality curves: impactof the Washington State opioid dosing guideline. Am J Ind Med. 2012;55(4):325–31.
Dowell D, Haegerich TM, Chou R. 2016 CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 65(No. RR-1):1–49. https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed April 14, 2020.
Bonnie R, Schumacher M. Pain management and opioid regulation: continuing public health challenges. Am J Public Health. 2019;109(1):31–4.
Pergolizzi JV, Rosenblatt M, Lequang JA. Three years down the road: the aftermath of the cdc guideline for prescribing opioids for chronic pain. Adv Ther. 2019;36(6):1235–40.
Clinical Evidence Review for the CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. CDC Stacks Public Health Publications. https://stacks.cdc.gov/view/cdc/38026. Accessed April 14, 2020.
Kroenke K, Alford DP, Argoff C, Canlas B, Covington E, Frank JW, et al. Challenges with implementing the centers for disease control and prevention opioid guideline: a consensus panel report. Pain Med. 2019;20(4):724–35.
Davis CS, Lieberman AJ, Hernandez-delgado H, Suba C. Laws limiting the prescribing or dispensing of opioids for acute pain in the United States: a national systematic legal review. Drug Alcohol Depend. 2019;194:166–72.
FDA Drug Safety Communication: FDA Identified harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes. May 9, 2019. Accessed on November 25, 2019.
Fenton JJ, Agnoli AL, Xing G, Hang L, Altan AE, Tancredi DJ et al. Trends and rapidity of dose tapering among patients prescribed long-term opioid therapy, 2008–2017. JAMA Netw Open. 2019;2(11):e1916271.
CDC Warns of Misapplication of its Opioid Guideline. American Academy of Family Physicians. https://www.aafp.org/news/health-of-the-public/20190509cdcopioidgdln.html. May 9, 2019. Accessed February 25, 2020.
Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: prescribing and transcribing-2016. Am J Health Syst Pharm. 2017;74(17):1336–522.
Connelly TP, Korvek SJ. Computer Provider Order Entry (CPOE) [Updated 2020 Feb 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470273/
The Epic Problem with the CDC Opioid Guideline. Orthopedics This Week. https://ryortho.com/breaking/the-epic-problem-with-the-cdc-opioid-guideline/. November 21, 2018. Accessed April 14, 2020.
Overton HN, Hanna MN, Bruhn WE, Hutfless S, Bicket MC, Makary MA et al. Opioid-prescribing guidelines for common surgical procedures: an expert panel consensus. J Am Coll Surg. 2018;227(4):411–8.
Linder BJ, Occhino JA, Wiest SR, Klingele CJ, Trabuco EC, Gebhart JB. Assessing the impact of procedure-specific opioid prescribing recommendations on opioid stewardship following pelvic organ prolapse surgery. Am J Obstet Gynecol. 2019;221(5):515.e1–.e8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Author Kathleen Adams, PharmD, BCPS and Michael Guerra, PharmD, BCPS declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adams, K., Guerra, M. Unintended consequences of United States chronic pain guidelines. Int J Clin Pharm 43, 313–317 (2021). https://doi.org/10.1007/s11096-020-01129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-020-01129-7