Abstract
Background
Extracellular vesicles (EVs) are a group of cell-derived membrane vesicles that carry a variety of cargo such as protein, nucleic acids, and lipids, and are secreted by almost all cell types. Functionally, EVs play important roles in physiological and pathological processes such as immune responses and tumor growth through intercellular communication by transferring this molecular information between cells. Therefore, they have potential versatile clinical applications as disease biomarkers and drug delivery carriers.
Problem
Notably, subpopulations of EVs exhibit distinct characteristics depending on their cell of origin, including the expression of surface glycans, which have been implicated in a variety of cellular processes such as field cancerization, cell recognition, and signal transduction. However, these are features have not been fully exploited because of the difficulty in analyzing these proteins.
Approach
In this paper, we summarize the advancements in glycoengineering and high-performance lectin microarray for high-throughput analysis of EV glycans to generate an index of heterogeneity to identify disease biomarkers, and describe how understanding the function of EVs in disease can enhance their potential application in the clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EVs) are assemblies of nano-/micro-lipid bilayer vesicles that are released from almost all cell types. EVs contain biomolecules (DNA, RNA, and proteins) that are derived from their donor cells, and they are mediators of intercellular communication [1]. It is becoming clear that EVs play a wide range of roles in processes such as immune responses, viral infections, tumor growth, and metastasis [2]. For example, we reported that the pathogenic protein CagA, which is one of the main causes of gastric cancer in Helicobacter pylori infection, was encapsulated in EVs and transported to sites distant from the stomach, leading to various systemic diseases [3]. In relation to cancer, EVs released from mouse CD8 + T cells were selectively taken up by cancer stromal mesenchymal stem cells (MSCs) when administered to tumor sites, and the EVs showed mesenchymal cytotoxicity. They were also found to have anti-metastasis effects on cancer stroma [4].
Recently, EVs have received great attention as disease biomarkers for diagnosis and prognosis, and as biological nanocarriers for drug delivery systems (DDSs). To improve the potential of EVs for DDSs, biological and chemical modifications of EVs have been reported [2]. We developed hybrid EVs through fusion of EVs with liposomes [5] and by coating of EVs with functional polymers such as amphiphilic polysaccharide nanogels [6] and magnetic nanogels [7].
While EVs have advantages in that they are highly effective, specific, and widely applicable, their heterogeneity makes it challenging to provide efficient quality management in clinical research [2]. In accordance with the increase in EV studies, the International Society for Extracellular Vesicles has released a new guide to initiating EV research, the Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018) [8]. Most importantly, EV is a generic term for various types of vesicles with different characteristics including their origin, size, composition, biological function, and formation mechanism.
Regarding the biogenesis of EVs, there are three subtypes of EVs: apoptotic bodies, exosomes, and microvesicles (MVs) [1, 2]. Apoptotic bodies are large vesicles released from apoptotic cells, and have generally been considered as “trash bags” for eliminating excess proteins. However, recent studies showed that apoptotic cells release nanometer-sized vesicles (apoptotic EVs) containing critical substances for immune systems. Endosome-derived vesicles, commonly referred to as exosomes, are the most extensively studied EVs that can be utilized in clinical applications. The term “exosomes” is often used for small EVs (< 200 nm) that are relatively enriched in some proteins such as tetraspanins (CD9, CD63, and CD81), essential factors of multivesicular bodies (ALIX and TSG101), heat shock proteins, and integrins [8]. MVs are formed by direct budding from the plasma membrane, and it is hard to distinguish them from exosomes because they are similar in size and composition. In addition, EVs isolated from the same cells have varying characteristics, indicating that it is necessary to establish a system that can classify EV assemblies according to their composition, morphology, and biological functions.
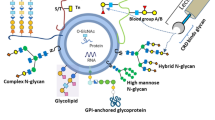
Several unique characteristics of EV subpopulations have been clarified through the identification of their lipids and proteins; however, it is difficult to make defined classification using a single indicator, they remain incompletely defined. Cells are composed of the following four elements: nucleic acids, proteins, lipids, and glycans. Cell-surface glycans are known to be involved in various biological responses such as immunity, microbial infection, cell recognition, signal transduction, and cancerization [9], and thus EV surface glycans are also thought to play an important role in these phenomena. However, compared with proteins, nucleic acids, and lipids, research on the structure and function of EV glycans has not progressed sufficiently because of the difficulty in their analysis. We proposed that the profile of the surface glycans of EVs should be an index of heterogeneity using high performance lectin microarray. In this review, we describe how the method has been useful in exploring biomarkers for diagnosis and in understanding glycan-mediated interactions between EVs and cells (Fig. 1).
EV Glycan Analysis by Lectin Microarray
We proposed a method for the high-throughput analysis of EV surface glycans using a lectin microarray with evanescent field fluorescence (EFF) detection [10, 11]. Because glycan structures are complicated and diverse, glycans are released from samples, and detailed glycan structural information can be identified with a plurality of methods including capillary electrophoresis, isoelectric focusing, and mass spectrometry coupled with high performance liquid chromatography [12]. Although they are the most used approaches, skilled technicians, sufficient sample quantity, and analysis processing are required. To directly determine surface glycan patterns on EVs without destruction, we applied an EFF-assisted lectin microarray for the first time [11]. In this system, only EVs that bind to lectins can be detected without washing steps, despite the weak glycan–lectin interactions, and hence this system is capable of accurately analyzing EVs. Shimoda et al. reported that an EFF-assisted lectin microarray is useful for determining surface EV glycan patterns using a tiny amount of EV sample (< 500 ng) [11]. We showed that MSC-derived EVs specifically bind to sialic acid-binding lectins and that EVs can interact with target cells via EV surface glycans. Saito et al. found that human induced pluripotent stem cell (hiPSC)-derived EVs interact with a hiPSC-specific lectin, rBC2LCN (the recombinant N-terminal domain of BC2L-C lectin) and showed that hiPSC-EVs can be detected by sandwich assay using rBC2LCN and TIM4, the latter of which is a receptor for phosphatidylserine [13].
Classification of EV Subpopulations by their Surface Glycans
We examined whether EV surface glycan patterns varied according to their cell origin, size, and isolation method using an EFF-assisted lectin microarray. Shimoda et al. showed that glycan patterns on small EVs without destruction are dependent on their original cells by comparison with EVs from 20 different types of cells [14]. In addition to small EVs that were isolated by ultracentrifugation at 120,000 × g, we also collected medium-sized EVs and large EVs by low-speed centrifugation at 10,000 × g and 2,000 × g, respectively. The size distribution and the expression levels of tetraspanins of these three types of vesicles differed. Furthermore, some lectins with different binding affinities were identified, indicating that these lectins might be a measure of EV heterogeneity.
Ultracentrifugation is the most used method to isolate EVs, probably because it can collect the greater part of all subpopulations [15]. However, immunoaffinity methods capture part of the whole population using specific membrane proteins or lipids on EVs. Then, we isolated cancer- or MSC-derived EVs using ultracentrifugation, two different commercially available kits (lipid-based interactions), and size exclusion chromatography. Besides confirming different size distributions and protein expression levels, EVs collected by ultracentrifugation bound more strongly with sialic acid-recognizing lectins than those isolated by lipid-based immunoaffinity methods. These results indicated that EV subtypes are dependent on their isolation methods and EV surface glycan patterns can become an index for their heterogeneity. Several groups also focused on EV glycans for evaluation of their subpopulations. Matsuda et al. isolated pancreatic cancer-derived EVs using the tetraspanin (CD9, CD63, and CD81)-based immunoaffinity method and found that glycan profiles on EVs vary with the type of tetraspanin [16] using an EFF-assisted lectin microarray.
Zhang et al. separated large (90–120 nm) EVs, small (60–80 nm) EVs, and non-membranous particles referred to as exomeres (approximately 35 nm) using the asymmetric-flow field-flow fractionation method and reported that different-sized EVs have different characteristics of N-glycan profiles [17], similar to our results. In this case, information of N-glycan profiles was obtained by mass spectrometry after destruction of EVs. EFF-assisted lectin microarray should be convenient for evaluating the profiles of surface glycans of intact EVs.
EV Surface Glycans as Biomarkers
Over the last decade, it has become clear that changes in EV surface glycan can potentially serve as a biomarker for evaluating cell state [18] or cancer diagnosis [19]. Our group recently discovered that EV surface glycan can be applied for evaluating MSC differentiation to osteoblasts [20]. EVs were isolated from both undifferentiated and osteogenically differentiated MSCs, and their surface glycans were analyzed by an EFF-assisted lectin microarray from which glycan patterns were compared before and after osteogenic differentiation. Several lectins that recognize Galβ1-4GlcNAc or terminal β-GalNAc specifically bind to osteogenically differentiated MSC-derived EVs. With regard to the relationship between glycan profiles and osteogenic differentiation of MSCs, some reports indicated that cell-surface glycan changes in osteogenically differentiated MSCs reflect the mineralization and differentiation potential of MSCs, but our results were the first to show that monitoring of glycan patterns on EVs as well as on the cell surface is useful for elucidation of the cell state of MSCs.
Changes in glycosylation are widely known to be associated with cancer diagnosis, for example, N-linked branching glycan, fucosylation, sialylation, and truncated O-glycans. While O-glycan truncation is frequently observed in cancer cells, it is absent in normal and benign cells, and Freitas et al. reported that the truncated gastric cancer-associated O-glycans sialyl-Tn (Neu5Acα2-6GalNAcα-O-Ser/Thr) is a potential biomarker and the EV glycan sialyl-Tn can serve as specific marker for gastric cancer [20]. Odaka et al. collected EVs from the sera of patients with Alzheimer's disease (AD) and healthy donors using the TIM4-affinity method, and their glycan profiles were compared using an EFF-assisted lectin microarray. They found that mannose-binding lectins strongly bound to EVs from AD patients, while platelet-related glycoproteins were identified as candidate AD biomarkers [22].
Role of EV Surface Glycans in Cellular Interactions and Biodistribution
Understanding the mechanisms of EV–cell interactions is of great importance for clarifying how EV functional molecules work inside and outside the recipient cells. Because many factors including EV size and surface molecules on EVs and recipient cells affect the interactions of EVs with cells, there are multiple mechanisms of EV–cell interactions [23]. EVs are attached to the cell surface and trigger intracellular signaling. EV binding by recipient cells is mediated by protein–protein, lipid–protein, lipid–lipid, and glycan–protein interactions. For example, tetraspanins, integrins, various adhesion molecules, fibronectins, and phosphatidylserine are known to be involved in the process of EV binding. In addition to EV docking, EVs can be internalized into recipient cells through different uptake pathways. Particularly well-known among the mechanisms are endocytic pathways such as receptor-mediated and clathrin-independent endocytosis, pinocytosis, and phagocytosis. Of these, receptor-mediated endocytosis is basically common to multiple types of pathways, and many glycans or glycoproteins on EVs that are related to EV internalization have been identified thus far.
Falcon–Perez’s group prepared EVs from mouse liver progenitor cell lines, and the biodistribution of EVs with or without treatment with neuraminidase was evaluated in vivo [25].
To examine whether EV glycans act as mediators, our group also demonstrated the effects of EV surface glycans on in vitro cellular uptake and in vivo biodistribution [11]. Surface glycan profiles of MSC-derived EVs were obtained by an EFF lectin array and the results showed that they strongly interacted with sialic acid-recognizing lectins. To elucidate whether sialic acids on MSC–EVs are involved in cellular uptake, MSC–EVs were added to sialic acid-binding Ig-like lectin (Siglec)-expressing cells with or without competitive inhibitors (sialic acid and anti-Siglec antibody), with the result that reduced uptake was observed according to inhibitor concentration. In addition, MSC–EVs were subcutaneously injected into mice and their internalization by antigen-presenting cells in lymph nodes was assessed. EVs preferred to interact with CD11b-expressing cells, especially Siglec-positive cells, indicating that cell-surface lectin-mediated entry is a possible route for EV uptake and that EV glycans serve as ligands for specific cell targeting.
Saunderson et al. obtained a similar result in that B cell-derived EVs were enriched in α2,3-linked sialic acids and bound Siglec-1-positive macrophages in spleen and lymph nodes [24]. These results indicated that EV surface glycans, as with various factors including EV size, cell source, and other EV surface molecules, have an important role in EV transfer to recipient cells.
Glycoengineering of EV Surface for Control of Cellular Interactions
Glycoengineering of cells and EVs has been applied for enhancing cellular uptake and modulating biodistribution. Falcon–Perez’s group prepared EVs from mouse liver progenitor cell lines, and the biodistribution of EVs with or without treatment with neuraminidase was evaluated in vivo [25]. First, they analyzed their accumulation in various organs after intravenous injection with radiolabeled EVs. After 72 h, higher lung accumulation of EVs after trimming of surface sialic acids with neuraminidase was observed. Furthermore, EV accumulation was dependent on injection routes. EVs via hock injection were mainly delivered to lymph nodes, and neuraminidase-treated EVs particularly accumulated in the axillary region, suggesting that surface sialic acids on EVs are associated with their biodistribution according to some previous studies. The group further investigated the role of EV surface glycans in cellular uptake using EVs from two murine hepatic cell lines and neuraminidase and PNGaseF (removing N-glycans) glycosidases [26]. The EV-uptake efficiency of 28 human cell lines relied on both cell types and glycosidases, probably because of cell-surface characteristics and changes in EV charge.
Dusoswa et al. engineered EVs with glycan ligands for dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) to increase the cell-targeting efficiency [27]. DC-SIGN is a c-type lectin that is expressed on antigen-presenting cells such as macrophages and dendritic cells and is known to bind Lewis antigens. To modify Lewis Y (Le Y) to surface EVs, they synthesized palmitoyl-Le Y and incorporated it onto EVs using the post-insertion method. Le Y-modified EVs were more efficiently internalized into monocyte-derived dendritic cells, indicating that EV surface glycan can be a candidate for specific cell targeting.
Nishida-Aoki et al. assessed the influence of deglycosylation of EVs on their cellular uptake efficiency using both N- and O-glycosidases [28]. They found that N- and/or O-glycan-depleted EVs from breast cancer cell lines were more effectively internalized into human umbilical vein endothelial cell lines in vitro. Furthermore, in vivo biodistribution of EVs with or without deglycosylation after intravenous injection into mice showed that O-glycosidase-treated EVs significantly accumulated in the lungs, whereas little difference was observed in the accumulation of EVs in other organs such as liver, spleen, and brain. The authors considered that EV glycans have some role in preventing non-selective biodistribution to undesired organs.
Glycan-modified EVs by glycoengineering have mainly been characterized by lectin blotting analysis. Because this analysis destroyed EVs to measure them, it does not accurately reflect the state of sugar chains on the surface of intact EVs. The EFF-assisted lectin microarray that we developed can directly detect the surface glycans of modified EVs without destruction. We prepared various glycosidases and glycosyltransferases for glycan engineering to obtain EVs with four types of surface glycan patterns: sialic acid, galactose, N-acetylglucosamine, and mannose. An EFF-assisted lectin microarray analysis demonstrated for the first time that most EV surface glycans were successfully modified by the enzyme treatment. In addition, we evaluated whether EV surface glycans could affect their cellular uptake efficiency in vitro and in vivo [14]. First, four types of glycoengineered EVs were added to three cell lines, and then their internalization into these cells was evaluated, with the result that the cellular uptake efficiency of EVs was dependent on both EV surface glycans and recipient cells. Moreover, the biodistribution of four different glycoengineered EVs after intravenous injection into mice was investigated. Although all types of EVs tended to accumulate mainly in the liver and spleen, more effective accumulation of sialic acid-bearing EVs in the spleen, lung, and heart was observed. Various kinds of carbohydrate receptors including Siglec, mannose receptors, and C-type lectins are expressed on cells and they are known to play important roles in cell surface glycan-lectin interactions [29]. It is considered that cell surface lectins are involved in the tissue distribution of EVs, and our data suggest that modification or removal of EV glycans would be important for determining EV cellular fate.
Conclusion
Glycoconjugates are the main components of EVs, but the functions of glycan itself when attached to lipids and proteins are not yet fully clarified because of the complexity of their structures and the analysis. Understanding the role of EV surface glycans in biological events can widen the potential clinical application of EVs.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977.
Shimoda A, Ueda K, Nishiumi S, Murata-Kamiya N, Mukai SA, Sawada S, et al. Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci Rep. 2016;6:18346.
Seo N, Shirakura Y, Tahara Y, Momose F, Harada N, Ikeda H, et al. Activated CD8+ T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9:435.
Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933.
Sawada S, Sato YT, Kawasaki R, Yasuoka J, Mizuta R, Sasaki Y, et al. Nanogel hybrid assembly for exosome intracellular delivery: effects on endocytosis and fusion by exosome surface polymer engineering. Biomater Sci. 2020;8:619–30.
Mizuta R, Sasaki Y, Kawasaki R, Katagiri K, Sawada SI, Mukai SA, et al. Magnetically navigated intracellular delivery of extracellular vesicles using amphiphilic nanogels. Bioconjug Chem. 2019;30:2150–5.
Théry C, Witwer KW, Aikawa EE, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15:346–66.
Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M, et al. Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling. Nat Methods. 2005;2:851–6.
Shimoda A, Tahara Y, Sawada SI, Sasaki Y, Akiyoshi K. Glycan profiling analysis using evanescent-field fluorescence-assisted lectin array: Importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem Biophys Res Commun. 2017;491:701–7.
Delafield DG, Li L. Recent advances in analytical approaches for glycan and glycopeptide quantitation. Mol Cell Proteomics. 2021;20: 100054.
Saito S, Hiemori K, Kiyoi K, Tateno H. Glycome analysis of extracellular vesicles derived from human induced pluripotent stem cells using lectin microarray. Sci Rep. 2018;8:3997.
Shimoda A, Miura R, Tateno H, Seo N, Shiku H, Sawada SI, et al. Assessment of surface glycan diversity on extracellular vesicles by lectin microarray and glycoengineering strategies for drug delivery applications. Small Methods. 2022;6: e2100785.
Liu WZ, Ma ZJ, Kang XW. Current status and outlook of advances in exosome isolation. Anal Bioanal Chem. 2022;414:7123–41.
Matsuda A, Kuno A, Yoshida M, Wagatsuma T, Sato T, Miyagishi M, et al. Comparative glycomic analysis of exosome subpopulations derived from pancreatic cancer cell lines. J Proteome Res. 2020;19:2516–24.
Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–43.
Wilson KM, Thomas-Oates JE, Genever PG, Ungar D. Glycan profiling shows unvaried N-Glycomes in MSC clones with distinct differentiation potentials. Front Cell Dev Biol. 2016;4:52.
Costa AF, Campos D, Reis CA, Gomes C. Targeting glycosylation: a new road for cancer drug discovery trends. Cancer. 2020;6:757–66.
Shimoda A, Sawada SI, Sasaki Y, Akiyoshi K. Exosome surface glycans reflect osteogenic differentiation of mesenchymal stem cells: profiling by an evanescent field fluorescence-assisted lectin array system. Sci Rep. 2019;9:11497.
Freitas D, Balmaña M, Poças J, Campos D, Osório H, Konstantinidi A, et al. Different isolation approaches lead to diverse glycosylated extracellular vesicle populations. J Extracell Vesicles. 2019;8:1621131.
Odaka H, Hiemori K, Shimoda A, Akiyoshi K, Tateno H. Platelet-derived extracellular vesicles are increased in sera of Alzheimer’s disease patients, as revealed by Tim4-based assays. FEBS Open Bio. 2021;11:741–52.
Li M, Li S, Du C, Zhang Y, Li Y, Chu L, et al. Exosomes from different cells: characteristics, modifications, and therapeutic applications. Eur J Med Chem. 2020;207: 112784.
Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–16.
Royo F, Cossío U, Ruiz de Angulo A, Llop J, Falcon-Perez JM. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale. 2019;11:1531–7.
Williams C, Pazos R, Royo F, González E, Roura-Ferrer M, Martinez A, et al. Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci Rep. 2019;9:11920.
Dusoswa SA, Horrevorts SK, Ambrosini M, Kalay H, Paauw NJ, Nieuwland R, et al. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J Extracell Vesicles. 2019;8:1648995.
Nishida-Aoki N, Tominaga N, Kosaka N, Ochiya T. Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake. J Extracell Vesicles. 2020;9:1713527.
Smith BAH, Bertozzi CR. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat Rev Drug Discov. 2021;20:217–43.
Funding
This study was supported in part by CREST, Japan Science and Technology Agency (Grant No. JPMJCR17H2) and a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (Grant No. 20K12645).
Author information
Authors and Affiliations
Contributions
A.S. and K.A. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest directly relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimoda, A., Akiyoshi, K. Surface Glycan Profiling of Extracellular Vesicles by Lectin Microarray and Glycoengineering for Control of Cellular Interactions. Pharm Res 40, 795–800 (2023). https://doi.org/10.1007/s11095-023-03511-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03511-2