Abstract
Purpose
Ephedra herb (Mao) exerts potent anti-allergic effects. This study aimed to examine the underlying mechanisms of Mao on allergic inflammation using in vitro cultured mast cells (MCs) and an in vivo model of MC-dependent anaphylaxis.
Methods
Bone marrow-derived MCs (BMMCs) were presensitized with anti-2,4-dinitrophenol (DNP) immunoglobulin E (IgE) and challenged with antigens (Ag; DNP-human serum albumin). Degranulation responses and cell surface high-affinity receptor for IgE (FcεRI) expression were assessed with/without Mao treatment. Passive systemic anaphylaxis (PSA)-treated mice were administered Mao and the pathophysiological responses were evaluated.
Results
Mao inhibited Ag-induced BMMC degranulation, but not polyclonal activation with phorbol 12-myristate 13-acetate (PMA) and ionomycin, indicating that Mao inhibits IgE-dependent activation of BMMCs. Mao-treated BMMCs exhibited significant reductions in expression of surface IgE and its receptor FcεRI. Analysis of subcellular localization revealed that Mao induces FcεRI internalization in BMMCs without degranulation. In the PSA mouse model, Mao administration prevented antigen-induced hypothermia. Mao administration significantly reduced cell surface expression of IgE-bound FcεRI on peritoneal MCs.
Conclusions
Mao induced FcεRI internalization in MCs, thereby inhibiting Ag-induced IgE-dependent degranulation. The inhibitory effects of Mao on MC degranulation may offer a novel therapeutic approach for allergic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antigen (Ag)-induced mast cell (MC) degranulation is the basis of anaphylaxis and other severe allergic reactions. Ag binding to immunoglobulin E (IgE) on the high-affinity IgE receptor (FcεRI) causes crosslinking of IgE-FcεRI complexes, resulting in the release of biologically active mediators such as histamine, serotonin, and leukotrienes within minutes. Cytokines, chemokines, and growth factors that are transcriptionally upregulated in MCs are secreted over a period of hours after initial MC activation, which leads to prolonged inflammation (1).
Regulation of MC degranulation is a potential target of anti-allergic therapeutics (2,3,4). Clinical approaches for suppressing IgE-dependent MC activation have emerged, in which FcεRI is targeted to inhibit or attenuate MC degranulation (4). For example, omalizumab, an anti-IgE monoclonal antibody, has been developed to prevent the binding of circulating IgE to FcεRI, thereby disrupting FcεRI-IgE complex formation and decreasing FcεRI expression (5). Omalizumab is the frequently used and preferred treatment for chronic urticaria and shows efficacy for the treatment of asthma (6, 7). As another example, anti-IgE DARPins (designed ankyrin repeat proteins), genetically engineered proteins comprised of a varying number of stacked ankyrin repeat domains, block IgE binding to FcεRI (8). Additionally, fusion protein-dependent coaggregation of FcεRI with its inhibitory receptor FcγRIIb blocks FcεRI signaling (9). All of these strategies are designed to inhibit FcεRI-induced mediator release and IgE-mediated anaphylaxis, which effectively downregulates Ag-induced MC degranulation.
Natural products and nutritional supplements also have been reported to inhibit MC degranulation. Their underlying mechanisms appear to involve the blockade of IgE-FcεRI binding or IgE-mediated FcεRI signaling. For example, carotenoids, a class of widespread natural pigments, suppress Ag-induced aggregation of FcεRI and inhibit FcεRI-mediated intracellular signaling (10). Procyanidin-enriched extracts from apples inhibit the binding of IgE to FcεRI and subsequent intracellular signaling (11, 12). Likewise, medicinal herbs, such as Rubia cordifolia and Dianthus superbus in China (13); KOTMIN13, composed of Inula japonica flowers, Trichosanthes kirilowii semen, Peucedanum praeruptorum radix, and Allium macrostemon bulbs, in Korea (14); and Paeonia radix and Zanthoxylum fructus in Japan (15, 16), suppress MC degranulation and allergic inflammation. These observations highlight the potential of natural plants and herbs as sources of bioactive compounds to mitigate MC activation and allergies. However, the inhibitory mechanisms of these natural products are not fully understood, especially in the context of IgE-FcεRI dynamics during the time course of inhibition. In addition, the precise mechanisms may vary depending on the plant of origin.
Ephedra sinica is an important medicinal plant, which is rich in ephedrine alkaloids. Its terrestrial stem, ephedra herb (Japanese name: “Mao”), is listed in the Japanese Pharmacopeia as a medicinal herb used in Japanese traditional medicine called “Kampo.” Mao is found in several Kampo formulas, especially those prescribed for allergic diseases, including Kakkonto, Shoseiryuto, Daiseiryuto, and Maoto. Basically, each formula is composed of multiple crude drugs that act synergistically and cooperatively with the constituent herbs; therefore, the anti-allergic effects of these formulas may involve more than Mao. However, recent studies have indicated that Mao alone is able to suppress allergic responses both in vivo and in vitro (17, 18). Mao has been reported to suppress pathophysiological allergic reactions by inhibiting cell migration mediated by Th2 cells via CCR3, CCR4, and CCR8 chemokine receptors (17). In addition, Mao inhibits IgE-mediated histamine release and increases cAMP content in rat basophilic leukemia cells (RBL-2H3) (18). Although Mao has potent anti-allergic and anti-inflammatory effects and stimulates both sympathetic and parasympathetic nerves due to its main ephedrine alkaloids (l-ephedrine, d-pseudoephedrine, l-methylephedrine, l-norephedrine), it produces some adverse effects including hypertension, palpitations, insomnia, and dysuria. Although Mao is indeed one of the most important natural medicines in Kampo, these potentially hazardous effects are taken into consideration during its clinical use. Besides ephedrine alkaloids, it is notable that recent studies have emphasized other active ingredients such as polyphenols (e.g., flavonoids and tannins) (19, 20). Some studies have indeed found that Mao extract shows alkaloid-independent pharmacological activity (21, 22). These observations indicate that there are undefined aspects of Mao that cannot be explained by ephedrine alkaloids and may be involved in the anti-allergic effects of Mao. In addition, the impact of ephedrine on MC activity is not clearly defined, although it is generally accepted that β2 adrenergic stimulation inhibits MC degranulation (23). A systematic review has also revealed that there are differences in the degree of inhibitory activity against MC degranulation that can be attained with a given agonist (23). These observations suggest unknown roles of Mao in the regulation of allergic inflammation. In addition, the study of Mao, especially investigations of its alkaloid-independent effects, might be valuable for the use of Ephedra herb not only in Asia but also in Europe and the US.
The aim of the present study was to examine the underlying mechanisms of Mao on allergic inflammation using in vitro cultured MCs and an in vivo model of MC-dependent anaphylaxis.
Materials and Methods
Mice
C57BL/6 N mice, female, aged 6–8 weeks were utilized for this study and housed in the animal facility of Kanazawa University under controlled conditions of temperature (23 ± 2°C), humidity (50 ± 10%), and light (12/12 h light/dark cycle). All animal studies were approved by the Animal Experiment Committee of Kanazawa University and were conducted according to the “Guide for the Care and Use of Laboratory Animals” (NIH publication No 85–23, revised 1996).
Crude Extract Preparation
The crude drugs of Kampo formulas were obtained from Uchida Wakanyaku Ltd. (Tokyo, Japan) as commercially available products, and all are currently approved as ethical drugs by the MHLWJ (Ministry of Health, Labour and Welfare of Japan). The Mao strains were grown in the cultivation station in the Ishikawa Prefecture, which is controlled by Kanazawa University. The crude extracts of the Kampo formulas and Mao were prepared as follows. Briefly, the pulverized drugs were extracted with hot water. The extract solution was separated from the insoluble waste and then concentrated by removing water under reduced pressure. Freeze-drying was used to produce a dried extract powder.
Cell Culture and Activation
Cultured bone marrow-derived MCs (BMMCs) were generated from the C57BL/6 N mice according to an established method (24). Differentiation was confirmed by flow cytometry with anti-c-Kit-Alexa Fluor 647 (BioLegend, San Diego, CA, USA) and anti-FcεRIα-Alexa Fluor 488 (BioLegend) antibodies. The cells were more than 90% c-Kit+ FcεRI+. The BMMCs were sensitized with anti-2,4-dinitrophenol (DNP)-IgE antibody (1 μg/ml) and then challenged with DNP-human serum albumin (HSA) or phorbol 12-myristate 13-acetate (PMA) plus ionomycin, with/without Mao (100 μg/ml, 0.1% water). When Mao was used, the cells were pretreated with it for the indicated time periods and Mao was maintained in the medium throughout the subsequent Ag stimulation. Degranulation was determined using a β-hexosaminidase release assay according to an established method (24). The concentrations indicated in this study are the final concentrations after experimental dilution.
In Vitro Rapid Desensitization
The IgE-sensitized BMMCs were subjected to Ag-specific desensitization with increasing doses of DNP-HSA at 10 min intervals according to the method of Oka et al. (26) with modification. The desensitization methods are described in the Supplemental Materials and Methods. The desensitization of the BMMCs was confirmed by surface expression of FcεRI and degranulation responses.
Flow Cytometry
The stimulated BMMCs were fixed with 4% paraformaldehyde. Anti-IgE-fluorescein isothiocyanate (FITC, BD Biosciences, San Jose, CA, USA), anti-c-Kit-Alexa Fluor 647, anti-FcεRIα, and anti-CD63 (MBL, Nagoya, Japan) followed by anti-rat-Alexa Fluor 647 (Abcam, Cambridge, UK) were used for labeling the fixed/non-permeabilized cells. Flow cytometry was then performed on a FACSVerse flow cytometer (BD Biosciences).
Enzyme-Linked Immunosorbent Assay (ELISA)
Cell culture medium was collected 1 h after stimulation with DNP-HSA. The release of tumor necrosis factor-α (TNF-α) was determined by an ELISA kit (R&D Systems, Minneapolis, USA) according to the manufacturer’s instructions.
Immunocytochemistry
The stimulated BMMCs were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and labeled with anti-IgE-FITC. Wheat germ agglutinin (WGA)-Alexa Fluor 647 was added before fixation/permeabilization. Images were acquired on an LSM710 confocal laser scanning microscope (CLSM, Carl Zeiss, Oberkochen, Germany) using a × 63 magnification objective. The analysis of fluorescence intensity was performed using ImageJ software (NIH Image, Bethesda, MD, USA). The extent of ruffling was evaluated by visual inspection according to the method of Kuo et al. (25) with modification. Briefly, the cells were scored using a scale of 0 to 2, with 0 = no ruffles present, 1 = ruffling confined to one area of the cell (no more than 25% of the cell circumference), and 2 = two or more discrete areas of the cell containing ruffles (25). We distinguished the areas of ruffling by their decreased fluorescence of WGA-Alexa Fluor 647.
Immunoblotting
The stimulated BMMCs were lysed and used for western blot analysis. Anti-phosphotyrosine (clone: 4G10), anti-actin (Santa Cruz Biotechnology, Dallas, TX, USA), anti-phospho-FcεRIγ Y47 (clone: γ-pY47), anti-FcεRIβ (clone: JRK), anti-phospho-Lyn Y396 (Cell Signaling Technology, Danvers, MA, USA), and anti-phospho-Syk Y519/520 (Cell Signaling Technology) antibodies were used. Images were visualized using a LiCor Odyssey Scanner and software (LiCor, Lincoln, NE, USA).
Intracellular Ca2+ Measurements
IgE-loaded BMMCs were incubated with Fluo-4 acetoxymethyl ester (AM, 2 μM) and Fura Red AM (10 μM) for 30 min. The cells were washed and resuspended in Tyrode’s buffer (1.8 mM CaCl2). An LSM710 CLSM was used for ratiometric measurements with 488 nm wavelength light from an argon laser. The fluorescence of Fluo-4 and Fura Red was detected through a bandpass filter (505–530 nm) and longpass filter (>560 nm), respectively. Calcium responses were calculated as the ratio of Fluo-4 to Fura Red normalized as a fold increase relative to the signal in unstimulated cells. Fluorescence images were collected every 5 s, and the fluorescence intensity was quantified using LSM710 software.
Passive Systemic Anaphylaxis Induction
The mice were sensitized by intraperitoneal injection with 300 μg/kg anti-DNP IgE. Twenty-four hours later, the mice were administered (intraperitoneally) 50 mg/kg Mao or vehicle (phosphate buffered saline (PBS)). Six hours later, DNP-HSA was administered (intravenously) at 10 mg/kg after anesthesia with isoflurane. Rectal body temperature (normalized to naïve control) was measured at 5–10 min intervals. Mouse peritoneal mast cells (PMCs) were prepared according to the outline in a previous report (26) with modification. Whole peritoneal cells from mouse peritoneal cavities were collected in RPMI medium (Nacalai Tesque, Kyoto, Japan). The cells were washed and filtered through 40-μm nylon mesh, subsequently stained with anti-IgE-Alexa Fluor 488 and anti-c-Kit-Alexa Fluor 647, and then subjected to flow cytometric analysis.
Statistical Analysis
Statistical significance was assessed using GraphPad Prism, version 7.0d (Graphpad Software, San Diego, CA, USA). Statistical differences were determined by Student’s t tests (between two groups), one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s multiple-comparison test as appropriate (for comparing multiple groups), or two-way ANOVA (for curves). A P value of less than 0.05 was considered statistically significant.
Results
Mao Inhibits IgE-Dependent Degranulation of BMMCs
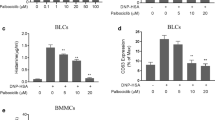
Ag stimulation induces degranulation in IgE-sensitized BMMCs. To evaluate the anti-allergic properties of the candidate medicinal herbs, four Japanese Kampo formulas containing Mao and a single crude extract of Mao (their compositions and compound profiles are described in Supplemental Figs. 1A and B) were applied to cultured BMMCs followed by Ag stimulation. Ag-induced degranulation was significantly inhibited by Mao (Fig. 1a), and the other Kampo crude extracts showed a tendency to inhibit degranulation. Mao inhibited Ag-induced degranulation in a concentration-dependent manner (Fig. 1b). Mao could have cytotoxic effects that resulted in reduced MC degranulation; we examined whether Mao treatment had a negative impact on MC functions. The viability of the Mao-treated cells was assessed by flow cytometry using a 7-aminoactinomycin D (7-AAD) dye exclusion test. Mao did not affect the viability of the BMMCs at the concentration used in this study (Supplemental Fig. 2). The BMMCs were also analyzed by flow cytometry for surface expression of CD63, a degranulation marker present on the membranes of secretory granules. The upregulation of CD63 after stimulation with Ag was markedly attenuated in the Mao-treated cells compared to that in the vehicle-treated cells, confirming that Mao extracts have an inhibitory effect on degranulation (Fig. 1c). The degranulation response of BMMCs by stimulation with PMA/ionomycin was not inhibited by Mao (Fig. 1d). These data indicate that Mao inhibits IgE-dependent, but not IgE-independent activation of BMMCs. In addition, we observed TNF-α release in Ag-treated cells (Fig. 1e), which was attenuated by Mao treatment, consistent with the degranulation responses. We confirmed that the suppression of the degranulation response by Mao still occurred if the cells were washed before Ag stimulation. Both conditions, with and without washing after Mao-treatment, induced significant inhibition of the Ag-induced degranulation response, and there was no significant difference in the extent of inhibition between them (76.5 ± 7.9% and 80.4 ± 5.0%, respectively).
Mao inhibits Ag-induced degranulation of BMMCs. IgE-sensitized BMMCs were treated with Mao for 30 min without washout and stimulated with Ag (a), (c), and (e): 50 ng/ml, (b): 0, 1, 10, and 100 ng/ml) or PMA/ionomycin for 30 min (a-d) or 60 min (e). Fixed/non-permeabilized samples were stained for CD63. Cell supernatants were analyzed for degranulation % and TNF-α release. (a) and (b) Degranulation responses to Ag stimulation. (c) Representative histogram of flow cytometric analysis (left panel) and fold changes in gMFI (right panel). Isotype control is shown in gray. (d) Degranulation responses to PMA (0, 2, and 10 nM) and ionomycin (0, 0.5, and 2 μM). (e) TNF-α concentration in the medium. Data are means ± standard errors. n = 5 (a) or n = 3 (b)-(e) independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. gMFI, geometric mean fluorescence intensity.
Mao Induces Internalization of IgE on the Surface of BMMCs
To examine the cellular mechanism by which Mao inhibits IgE-dependent activation of BMMCs, we assessed the presence of IgE, which is bound to FcεRI, on the cells. Flow cytometry revealed that the levels of cell-surface IgE were decreased in the BMMCs after Ag stimulation. Mao-treated BMMCs showed decreased levels of the surface IgE-FcεRI complex (Fig. 2a), although degranulation responses were significantly attenuated in Mao-treated BMMCs compared to vehicle-treated cells (Fig. 1). We next evaluated the effects of Mao alone on surface IgE (FcεRI internalization) and found that there was a significant reduction in the cell-surface IgE-bound FcεRI in Mao-treated BMMCs without detectable degranulation (Supplemental Figs. 3A and B). To evaluate internalization of the IgE-FcεRI complex in Mao-treated BMMCs, we observed its subcellular localization by CLSM. Figures 2b and c show that surface IgE-FcεRI complexes were significantly internalized after Mao treatment without marked membrane ruffling (a characteristic behavior of MC activation). The culture medium of these cells showed decreased degranulation (data not shown) with the same pattern observed in Fig. 1a and b. Collectively, these results indicate that Mao can induce IgE-bound FcεRI internalization in BMMCs without cell activation.
Mao induces IgE-FcεRI internalization from the surface of BMMCs. IgE-sensitized BMMCs were treated with Mao for 30 min without washout and stimulated with Ag (50 ng/ml) for 30 min. Non-permeabilized cells were labeled with anti-IgE alone (a). Permeabilized cells were labeled with anti-IgE and wheat germ agglutinin (WGA) to identify the plasma membrane (b) and (c). (a) Representative histogram of flow cytometric analysis (left panel) and fold changes in gMFI (right panel). Isotype control is shown in gray. (b) CLSM images (magnification ×63, scale bar = 20 μm) and line profiles of fluorescence intensity (green: IgE, red: WGA). (c) Left panel: The rate of FcεRI (IgE-bound) internalization is expressed as a ratio of surface to intracellular FITC-IgE fluorescence intensity. Right panel: The extent of ruffling of each cell. Each column represents the mean ± standard error from three independent experiments that were derived from 10 to 40 cells in each single experiment. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. gMFI, geometric mean fluorescence intensity; DIC, differential interference contrast.
Mao Induces Internalization of Unsensitized-FcεRI on the Surface of BMMCs
To examine further whether Mao directly regulates FcεRI expression on BMMCs, unsensitized-FcεRI on their surface was analyzed. A significant reduction in FcεRI was observed on the Mao-treated BMMCs without affecting the cell-surface expression of c-Kit, a marker of mature MCs. These data suggest that Mao specifically regulates FcεRI internalization in BMMCs (Fig. 3a). The magnitude of this downregulation showed a time- and concentration-dependent response (Fig. 3b). These findings are consistent with the regulation of surface IgE by Mao as shown in Fig. 2. These findings show that Mao induces IgE-FcεRI internalization in BMMCs, which could lead to a failure to respond to an optimal dose of Ag.
Mao reduces FcεRI on the surface of BMMCs. BMMCs were treated with Mao and compared to untreated control cells. (a) 100 μg/ml Mao for 6 h. Representative histogram of flow cytometric analysis (upper panel) and the percentage surface expression levels (lower panel). Isotype control is shown in gray. (b) Time- (left panel; 100 μg/ml of Mao) and concentration- (right panel; 6 h treatment with Mao) dependent responses to Mao treatment in cells. BMMCs were treated with Mao at concentrations of 0, 0.3, 1, 3.3, 10, 33, and 100 μg/ml for a maximum of 6 h. Isotype control is shown in gray. Data are means ± standard errors. n = 3 independent experiments. *p < 0.01, **p < 0.01, ***p < 0.001; ns, not significant.
Activation of FcεRI-Dependent Signaling Is Attenuated in Mao-Treated BMMCs
We next evaluated the signaling steps in Mao-treated BMMCs. Aggregation of the IgE-FcεRI complex with multivalent Ag triggers a series of protein phosphorylation reactions. Rapid tyrosine phosphorylation of FcεRI by Lyn kinase followed by activation of Syk elicits sequential protein phosphorylation and dynamically regulates downstream signaling. BMMCs pretreated with Mao for 1 h were stimulated with Ag and subjected to immunoblot analysis. The phosphotyrosine levels and phosphorylation of Lyn, Syk, and the γ subunit of FcεRI, which contains the immunoreceptor tyrosine-based activation motif (ITAM) that is essential for FcεRI-mediated signal transduction, were significantly attenuated (Fig. 4a). Thirty minutes of Mao pretreatment also prevented Ag-induced tyrosine phosphorylation, but the effect was less pronounced (Supplemental Fig. 4). However, while Mao alone could enhance the phosphorylation, the cells did not show marked degranulation (Supplemental Fig. 3B). Previous reports have described that IgE-FcεRI internalization, even in the case of suboptimal doses of Ag, can facilitate the phosphorylation of signaling molecules in the FcεRI pathway. Additionally, FcεRI-mediated Ca2+ mobilization monitored using fluorescent calcium indicators was impaired in Mao-treated cells (Fig. 4b). These observations are consistent with the interpretation that Mao-pretreated BMMCs fail to respond to Ag and elicit FcεRI signaling, possibly because of the Mao-regulated constitutive internalization of IgE-FcεRI.
Attenuation of Ag-induced FcεRI signaling in Mao-treated cells. IgE-loaded BMMCs were treated with 100 μg/ml Mao for 1 h and then stimulated with Ag (50 ng/ml). (a) Protein phosphorylation in Ag-stimulated cells (2 min). One representative blot is shown of three independent experiments. The band density normalized to that of unstimulated cells is shown below each band. (b) Calcium responses. Each line represents the mean ± standard error of four independent experiments that were derived from 100 cells/group in each single experiment. *p < 0.05.
Comparison of Mao-Treated Cells to Antigen-Induced Desensitized Cells
To compare the extent of the FcεRI internalization induced by Mao, we utilized a rapid desensitization model. This model utilizes sequential applications of suboptimal concentrations of Ag to MCs to facilitate FcεRI internalization. As a result, the subsequent activation of these cells is diminished. We conducted the desensitization model experiments as described by Oka et al. with some modifications. The suppressive efficacy against BMMC degranulation (Fig. 5a) and the reduction in surface FcεRI that was observed before Ag stimulation (Fig. 5b) were comparable and not significantly different between the Mao-treated and FcεRI-desensitized cells. Microscopic observation also revealed similar FcεRI internalization between the Mao-treated cells and the cells with rapid desensitization (Fig. 5c). In addition, the levels of total tyrosine phosphorylation were comparable between the groups (Fig. 5d). These data may provide insight into the extent that Mao treatment effects the internalization of FcεRI.
Validation of the suppressive efficacy of Mao by comparison with rapidly desensitized cells. Sensitized BMMCs were treated with Mao or subjected to Ag-specific desensitization with increasing suboptimal concentrations of Ag. To evaluate their suppressive efficacy in the cells, the degranulation responses were observed under Ag stimulation with or without desensitization. (a) Degranulation responses were observed 30 min after Ag stimulation. (b) Surface IgE-FcεRI expressions were observed after desensitization. (c) Subcellular localizations of FcεRI were observed in Mao-treated cells and desensitized cells (magnification ×63, scale bar = 20 μm). (d) Levels of total tyrosine phosphorylation in BMMCs. The sensitized cells were treated with 100 μg/ml Mao for 30 min or subjected to the desensitization protocol (DS). The final Ag stimulations were performed with 100 ng/ml of DNP-HSA. CTL: untreated and unstimulated control, Ag: untreated and stimulated control. n = 4 independent experiments (a), (b). *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant.
Mao Prevents Allergic Response by Regulating IgE-FcεRI Internalization of MCs
To examine whether Mao-induced FcεRI internalization in MCs relates to allergic responses in vivo, we utilized a passive systemic anaphylaxis (PSA) mouse model. As shown in Fig. 6a, 6–8-week-old C57BL/6 N female mice were sensitized by intraperitoneal administration of Ag-specific IgE and then intraperitoneally injected with Mao. After 6 h, the mice were intravenously challenged with Ag. Figure 6b shows that Mao administration prevented Ag-induced hypothermia in the PSA mice. To test whether MCs are involved in the ameliorating effects of Mao on the PSA reaction, peritoneal MCs (PMCs) were collected before Ag stimulation. The PMCs from the Mao-administered mice exhibited significantly lower cell-surface IgE levels than the vehicle-administered mice (Fig. 6c), indicating that Mao induces IgE-FcεRI internalization in the PMCs and therefore could prevent anaphylactic reactions in the mice.
Mao prevents Ag-induced hypothermia in PSA mice. PSA was induced by sensitization with 300 μg/kg of anti-DNP IgE and by challenging 24 h later with 10 mg/kg Ag. (a) Experimental protocol. (b) Change in body temperature. (c) IgE+ c-Kit+ PMCs were analyzed at pre-DNP challenge. Representative dot plot of flow cytometric analysis (left panel) and the quantitative result (right panel). Data are means ± standard errors. n = 3 mice per group. *p < 0.05. i.p., intraperitoneal; i.v., intravenous.
Discussion
We demonstrated that Mao inhibits Ag-induced IgE-dependent MC activation by induction of IgE-FcεRI internalization in MCs, thereby ameliorating IgE-dependent allergic inflammation.
IgE-FcεRI internalization in MCs without cell activation has long been recognized as an essential process in the regulation of MCs and subsequent allergic inflammation. Especially in recent decades, IgE-FcεRI internalization in MCs and basophils is believed to be an initial and critical event in the sequential immune responses of Ag-specific immunotherapy (AIT), the only current treatment for allergic disease that builds immune tolerance through the administration of specific Ags (3). During the course of AIT, IgE-FcεRI internalization in MCs is rapidly induced, which then initiates prolonged immune responses. This rapid IgE-FcεRI internalization is also known as Ag-induced rapid desensitization of MCs (27, 28). It is important to avoid unnecessary inflammation in AIT, and therefore effective strategies to induce MC desensitization have been investigated during establishment of this therapy. In addition to AIT, the application of omalizumab and a component of other strategies aimed to block IgE-FcεRI binding are reported to induce IgE-FcεRI internalization (4, 29), although the precise mechanisms of the connections between constitutive IgE-FcεRI internalization and IgE-FcεRI blockade remain to be determined. Thus, IgE-FcεRI internalization in MCs is indeed an essential event to establish safe and effective approaches to inhibit the release of inflammatory mediators and allergic responses. In this study, we found that Mao induced IgE-FcεRI internalization without triggering BMMC activation in vitro. The inactivation state of the BMMCs was confirmed by several observations, such as diminished degranulation responses (Fig. 1ba and b) and CD63 expression (Fig. 1c), lack of morphological changes in the cell membrane (Fig. 2b and c) that are indicative of degranulation response (30, 31), and attenuated FcεRI signaling (Fig. 4). These observations in the Mao-treated BMMCs may mimic the Ag-induced rapid desensitization states reported by Oka et al. (26) and those in the present study (Fig. 5). Therefore, we will next discuss the therapeutic potential of Mao as an anti-allergic treatment.
Mao is traditionally and widely used in Kampo medicine and is the terrestrial stem of E. sinica. Its main component is ephedrine, although it contains other active compounds, such as flavonoids and tannins, as shown in Supplemental Fig. 1B. The contents of its ingredients vary slightly depending on its origin. Ephedrine stimulates both sympathetic and parasympathetic neurons due to its structural similarity to adrenaline. As generally accepted, β-adrenergic stimulation ameliorates allergic reactions through direct and indirect activation of the sympathetic nervous system. However, β-adrenergic stimulation is also known to affect MC function (32), and it downregulates MC degranulation responses through cAMP induction (33). The effects of β-adrenergic stimulation with different agonists are known to be variable (32); however, the reasons for the differences have remained unclear. Furthermore, the effects of β-adrenergic agonists on Ag-dependent MC activation need to be elucidated. We tested the effects of isoprenaline, a full agonist for β-adrenergic receptors, on allergic responses. However we observed no detectable changes in both the degranulation responses of BMMCs and their surface IgE expression levels. In addition, propranolol, a β-adrenergic receptor antagonist, failed to block the inhibitory effects of Mao on the Ag-induced degranulation of BMMCs (Supplemental Figs. 5A and B). We further tested the contribution of ephedrine using six types of Mao originating from different strains of E. sinica with varying contents of ephedrine alkaloids (Supplemental Figs. 6A and B). All of these Mao showed inhibitory effects on degranulation responses in MCs, and no significant differences were observed among them (Supplemental Fig. 6C). Even more unexpectedly, recent studies have indicated that ephedrine does not possess the ability to directly inhibit Ag-induced MC degranulation, although it suppresses allergic reactions in a PCA (passive cutaneous anaphylaxis) mouse model (34, 35). With respect to the anti-allergic effect on ephedrine, Shibata et al. (35) have suggested that there is an unidentified mechanism(s) in addition to the classical direct or indirect mechanism acting through the sympathetic system. To our knowledge, there have been no systematic studies addressing the relationship between IgE-FcεRI internalization and ephedrine or β-adrenergic stimulation. All these observations combined with our findings suggest that the effect of IgE-FcεRI internalization induced by Mao is unlikely attributable to ephedrine, but rather may be due to other active components.
Mao contains a variety of polyphenols that are known to mediate the activities of Mao and are suspected to be active compounds in Mao (21). Some polyphenols are known to regulate FcεRI (36, 37), but these studies were limited to the polyphenols contained in tea. Polyphenols are a diverse group of molecules, and the effects of polyphenols vary according to their chemical structures. In the present study, we could detect six polyphenols (catechin, epicatechin, kaempferol 3-O-rhamnoside 7-O-glucoside, herbacetin 7-O-glucoside, herbacetin, and kaempferol) from the six different Mao strains (Supplemental Figs. 6A and B). As shown in Supplemental Fig. 6, Strains C and F contain more polyphenols than the other strains. These two strains tend to suppress degranulation responses more than the other strains. However, all strains, with varying contents of ephedrine alkaloids and polyphenols, could significantly suppress MC degranulation (Supplemental Fig. 6C). We could not detect a clear correlation between the amount of total polyphenols and the suppressive effect on MC activation. The polyphenols contained in Mao remain incompletely investigated, indicating that unknown candidates that are responsible for the suppression of MC activation and allergic inflammation may exist. Further experiments are needed to identify the active compounds in Mao for induction of IgE-FcεRI internalization.
One of the limitations of our study is that the mechanism by which Mao induces IgE-FcεRI internalization has not been fully determined. As shown in Fig. 3, our data suggest that Mao directly affects FcεRI internalization without binding IgE. Mao induced unsensitized-FcεRI internalization to a similar degree as IgE-sensitized FcεRI internalization when we compared differences 30 min or 1 h after Mao treatment (data not shown and Fig. 2), although longer Mao treatments would be preferable to accentuate differences. Since surface FcεRI complex stability, which is linked to complex internalization and degradation, varies according to the presence or absence of IgE (38,39,40), Mao-induced FcεRI internalization may also exhibit different patterns by the binding of IgE. To elucidate the precise mechanism by which Mao induces FcεRI internalization, further studies are needed to understand the differences involved. The effect of Mao on FcεRI internalization was time- and concentration-dependent. The IgE-FcεRI internalization induced by Mao was comparable to that induced by Ag stimulation. Ag stimulation leads to crosslinking of IgE-FcεRI complexes, which obviously induces IgE-FcεRI internalization along with MC activation. In contrast, Mao may directly influence FcεRI and then the internalized IgE-FcεRI complex, or there may be different types of FcεRI aggregation and/or IgE-FcεRI internalization mechanisms specifically induced by Mao, compared to those induced by Ag stimulation. Indeed, FcεRI aggregation initiates intracellular signaling and activation of MCs. Therefore, we suggest here that the conformational changes in IgE-FcεRI induced by Mao serve as a key attribute that drives successful internalization without MC activation.
PSA is recognized to result from increased vascular permeability, which mediates leakage of intravascular fluid into the extravascular space within minutes, thereby inducing hypotension and hemoconcentration. Oka et al. (26) did not observe PSA in MC-deficient C57BL/6-Kit W-sh/W-sh mice. In the present study, we used a model of MC-dependent anaphylaxis (PSA mouse model) to assess the effects of Mao on pathophysiological allergic responses and anti-allergic effects. We generated this PSA model by intraperitoneal sensitization to analyze PMCs, especially their surface IgE expression. As expected, Mao treatment of the PSA model mice prevented Ag-induced hypothermia, and their PMCs, which were collected before Ag stimulation, exhibited significant reductions in surface IgE expression. These data support the hypothesis that Mao administration induces IgE internalization in the PSA mouse model. Herbal crude extracts, including Mao crude extract, are generally administered to mice as a single oral dose of approximately 500 mg/kg based on human studies. Here, we injected Mao intraperitoneally at 50 mg/kg. This dose and delivery of Mao did not induce pyrexia, although it slightly reduced body temperature (Supplemental Fig. 7). Given this effect of Mao on the thermal stability of mice, the attenuation of anaphylactic hypothermia observed with Mao treatment may be explained by Mao exerting an anti-allergic effect on the PSA mice. The PMCs in the Mao-treated mice showed IgE internalization without affecting c-Kit expression, consistent with the effect of Mao on BMMCs (Fig. 6c). We also observed that the internalization in PMCs continued 3 h after Ag exposure (data not shown). Previous reports on the PSA mice and our findings suggest that physiological changes in the Mao-treated PSA mice depend on PMC functions, supporting the in vitro findings with BMMCs and our interpretation that IgE-FcεRI internalization by Mao exerts anti-allergic effects under physiological situations.
The active compounds in Mao have not been revealed, and the precise mechanisms of FcεRI internalization are still unclear. The present study demonstrated that Mao regulates FcεRI internalization, possibly in an ephedrine-independent manner. Furthermore, the present study may provide novel information to understand the mechanisms of Mao in treating allergic diseases and may prove valuable for the usage of ephedrine-free Ephedra in Europe and the US. Our findings may indicate that Mao induces IgE-FcεRI internalization in MCs, which not only supports the therapeutic potential of Mao but may contribute to the development of promising approaches for allergy treatments based on Mao activity.
Conclusion
Mao significantly induced IgE-FcεRI internalization in MCs without activation in vitro and in vivo. This inhibitory effect of Mao on MC activation may offer a novel approach for allergy therapeutics.
Abbreviations
- Ag:
-
Antigen
- AIT:
-
Antigen specific immunotherapy
- BMMC:
-
Bone marrow-derived mast cell
- CLSM:
-
Confocal laser scanning microscope
- DNP:
-
2,4-dinitrophenol
- FcεRI:
-
High-affinity receptor for immunoglobulin E
- HSA:
-
Human serum albumin
- IgE:
-
Immunoglobulin E
- MC:
-
Mast cell
- PBS:
-
Phosphate buffered saline
- PMA:
-
Phorbol 12-myristate 13-acetate
- PMC:
-
Peritoneal mast cell
- TNF-α:
-
Tumor necrosis factor-α
References
Elieh Ali Komi D, Bjermer L. Mast cell-mediated orchestration of the immune responses in human allergic asthma: current insights. Clin Rev Allergy Immunol. 2019;56(2):234–47.
Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704.
Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133(3):621–31.
Gomez G. Current strategies to inhibit high affinity FcepsilonRI-mediated signaling for the treatment of allergic disease. Front Immunol. 2019;10:175.
Gomez G, Jogie-Brahim S, Shima M, Schwartz LB. Omalizumab reverses the phenotypic and functional effects of IgE-enhanced Fc epsilonRI on human skin mast cells. J Immunol (Baltimore, Md : 1950). 2007;179(2):1353–61.
Tonacci A, Billeci L, Pioggia G, Navarra M, Gangemi S. Omalizumab for the treatment of chronic idiopathic Urticaria: systematic review of the literature. Pharmacotherapy. 2017;37(4):464–80.
Pennington LF, Tarchevskaya S, Brigger D, Sathiyamoorthy K, Graham MT, Nadeau KC, et al. Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nat Commun. 2016;7:11610.
Baumann MJ, Eggel A, Amstutz P, Stadler BM, Vogel M. DARPins against a functional IgE epitope. Immunol Lett. 2010;133(2):78–84.
Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin fc gamma fc epsilon bifunctional fusion protein inhibits fc epsilon RI-mediated degranulation. Nat Med. 2002;8(5):518–21.
Sakai S, Sugawara T, Matsubara K, Hirata T. Inhibitory effect of carotenoids on the degranulation of mast cells via suppression of antigen-induced aggregation of high affinity IgE receptors. J Biol Chem. 2009;284(41):28172–9.
Tokura T, Nakano N, Ito T, Matsuda H, Nagasako-Akazome Y, Kanda T, et al. Inhibitory effect of polyphenol-enriched apple extracts on mast cell degranulation in vitro targeting the binding between IgE and FcepsilonRI. Biosci Biotechnol Biochem. 2005;69(10):1974–7.
Nakano N, Nishiyama C, Tokura T, Nagasako-Akazome Y, Ohtake Y, Okumura K, et al. Procyanidin C1 from apple extracts inhibits fc epsilon RI-mediated mast cell activation. Int Arch Allergy Immunol. 2008;147(3):213–21.
Lopez-Exposito I, Castillo A, Yang N, Liang B, Li XM. Chinese herbal extracts of Rubia cordifolia and Dianthus superbus suppress IgE production and prevent peanut-induced anaphylaxis. Chin Med. 2011;6:35.
Lee E, Kim SG, Park NY, Park HH, Jeong KT, Choi J, et al. KOTMIN13, a Korean herbal medicine alleviates allergic inflammation in vivo and in vitro. BMC Complement Altern Med. 2016;16:169.
Kageyama-Yahara N, Suehiro Y, Maeda F, Kageyama S, Fukuoka J, Katagiri T, et al. Pentagalloylglucose down-regulates mast cell surface FcepsilonRI expression in vitro and in vivo. FEBS Lett. 2010;584(1):111–8.
Wang X, Kageyama-Yahara N, Hayashi S, Yamamoto T, Kadowaki M. Sphingosine kinase-1-dependent and -independent inhibitory effects of zanthoxyli fructus to attenuate the activation of mucosal mast cells and ameliorate food allergies in mice. Evid Based Complement Alternat Med. 2012;2012:862743.
Matsuo K, Koizumi K, Fujita M, Morikawa T, Jo M, Shibahara N, et al. Efficient use of a crude drug/herb library reveals Ephedra herb as a specific antagonist for TH2-specific chemokine receptors CCR3, CCR4, and CCR8. Front Cell Dev Biol. 2016;4:54.
Saito SY, Maruyama Y, Kamiyama S, Nakahata N, Ohizumi Y. Ephedrae herba in Mao-Bushi-Saishin-to inhibits IgE-mediated histamine release and increases cAMP content in RBL-2H3 cells. J Pharmacol Sci. 2004;95(1):41–6.
Amakura Y, Yoshimura M, Yamakami S, Yoshida T, Wakana D, Hyuga M, et al. Characterization of phenolic constituents from ephedra herb extract. Molecules. 2013;18(5):5326–34.
Amakura Y. Characterization of phenolic constituents from Ephedra herb extract. Yakugaku Zasshi. 2017;137(2):167–71.
Hyuga S, Hyuga M, Oshima N, Maruyama T, Kamakura H, Yamashita T, et al. Ephedrine alkaloids-free Ephedra herb extract: a safer alternative to ephedra with comparable analgesic, anticancer, and anti-influenza activities. J Nat Med. 2016;70(3):571–83.
Nakamori S, Takahashi J, Hyuga S, Yang J, Takemoto H, Maruyama T, et al. Analgesic effects of Ephedra herb extract, ephedrine alkaloids-free Ephedra herb extract, ephedrine, and pseudoephedrine on formalin-induced pain. Biol Pharm Bull. 2019;42(9):1538–44.
Kay LJ, Peachell PT. Mast cell beta2-adrenoceptors. Chem Immunol Allergy. 2005;87:145–53.
Suzuki R, Leach S, Liu W, Ralston E, Scheffel J, Zhang W, et al. Molecular editing of cellular responses by the high-affinity receptor for IgE. Science. 2014;343(6174):1021–5.
Kuo CH, Collins AM, Boettner DR, Yang Y, Ono SJ. Role of CCL7 in Type I hypersensitivity reactions in murine experimental allergic conjunctivitis. J Immunol (Baltimore, Md : 1950). 2017;198(2):645–56.
Oka T, Rios EJ, Tsai M, Kalesnikoff J, Galli SJ. Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells. J Allergy Clin Immunol. 2013;132(4):922–32.e1–16.
Berings M, Karaaslan C, Altunbulakli C, Gevaert P, Akdis M, Bachert C, et al. Advances and highlights in allergen immunotherapy: on the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol. 2017;140(5):1250–67.
Kulis MD, Patil SU, Wambre E, Vickery BP. Immune mechanisms of oral immunotherapy. J Allergy Clin Immunol. 2018;141(2):491–8.
Gasser P, Tarchevskaya SS, Guntern P, Brigger D, Ruppli R, Zbären N, et al. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun. 2020;11(1):165.
Yokawa S, Suzuki T, Hayashi A, Inouye S, Inoh Y, Furuno T. Video-rate bioluminescence imaging of degranulation of mast cells attached to the extracellular matrix. Front Cell Dev Biol. 2018;6:74.
Pfeiffer JR, Seagrave JC, Davis BH, Deanin GG, Oliver JM. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 1985;101(6):2145–55.
Barnes PJ. Effect of beta-agonists on inflammatory cells. J Allergy Clin Immunol. 1999;104(2 Pt 2):S10–7.
Weston MC, Peachell PT. Regulation of human mast cell and basophil function by cAMP. Gen Pharmacol. 1998;31(5):715–9.
Shibata H, Nabe T, Yamamura H, Kohno S. L-ephedrine is a major constituent of Mao-Bushi-Saishin-to, one of the formulas of Chinese medicine, which shows immediate inhibition after oral administration of passive cutaneous anaphylaxis in rats. Inflamm Res. 2000;49(8):398–403.
Shibata H, Minami E, Hirata R, Mizutani N, Nabe T, Kohno S. Immediate inhibition by oral l-ephedrine of passive cutaneous anaphylaxis of rats: indirect inhibition of anaphylactic chemical mediator release from the mast cell. Inflamm Res. 2000;49(10):553–9.
Fujimura Y, Tachibana H, Maeda-Yamamoto M, Miyase T, Sano M, Yamada K. Antiallergic tea catechin, (−)-epigallocatechin-3-O-(3-O-methyl)-gallate, suppresses FcepsilonRI expression in human basophilic KU812 cells. J Agric Food Chem. 2002;50(20):5729–34.
Yano S, Tachibana H, Yamada K. Flavones suppress the expression of the high-affinity IgE receptor FcepsilonRI in human basophilic KU812 cells. J Agric Food Chem. 2005;53(5):1812–7.
Kawakami T, Blank U. From IgE to omalizumab. J Immunol (Baltimore, Md : 1950). 2016;197(11):4187–92.
Kubo S, Matsuoka K, Taya C, Kitamura F, Takai T, Yonekawa H, et al. Drastic up-regulation of Fcepsilonri on mast cells is induced by IgE binding through stabilization and accumulation of Fcepsilonri on the cell surface. J Immunol (Baltimore, Md : 1950). 2001;167(6):3427–34.
Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol (Baltimore, Md : 1950). 2001;167(3):1290–6.
Author information
Authors and Affiliations
Contributions
YN, HA, YS, and RS contributed by conducting experiments, summarizing data, and/or generating necessary reagents. YN and RS conceived and directed this study. YN, HA, YS, and RS participated in the writing of the manuscript.
Corresponding author
Ethics declarations
Research Involving Human Participants and/or Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed Consent
No informed consent was required to prepare the manuscript.
Conflict of Interest
This research was supported by JSPS KAKENHI Grant Number 16H05082. The authors have no conflicting financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 44127 kb)
Rights and permissions
About this article
Cite this article
Nagata, Y., Ando, H., Sasaki, Y. et al. Ephedra Herb, Mao, Inhibits Antigen-Induced Mast Cell Degranulation by Induction of the Affinity Receptor for IgE Internalization. Pharm Res 38, 569–581 (2021). https://doi.org/10.1007/s11095-021-03020-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03020-0