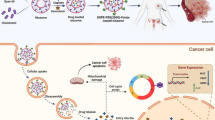
Abstract
Purpose
Folic acid-doxorubicin-double-functionalized-lipid-core nanocapsules (LNC-CS-L-Zn+2-DOX-FA) were prepared, characterized, and evaluated in vitro against ovarian and bladder cancer cell lines (OVCAR-3 and T24).
Methods
LNC-CS-L-Zn+2-DOX-FA was prepared by self-assembly and interfacial reactions, and characterized using liquid chromatography, particle sizing, transmission electron microscopy, and infrared spectroscopy. Cell viability and cellular uptake were studied using MTT assay and confocal microscopy.
Results
The presence of lecithin allows the formation of nanocapsules with a lower tendency of agglomeration, narrower size distributions, and smaller diameters due to an increase in hydrogen bonds at the surface. LNC-L-CS-Zn+2-DOX-FA, containing 98.00 ± 2.34 μg mL−1 of DOX and 105.00 ± 2.05 μg mL−1 of FA, had a mean diameter of 123 ± 4 nm and zeta potential of +12.0 ± 1.3 mV. After treatment with LNC-L-CS-Zn+2-DOX-FA (15 μmol L−1 of DOX), T24 cells had inhibition rates above 80% (24 h) and 90% (48 h), whereas OVCAR-3 cells showed inhibition rates of 68% (24 h) and 93% (48 h), showing higher cytotoxicity than DOX.HCl. The fluorescent-labeled formulation showed a higher capacity of internalization in OVCAR-3 compared to T24 cancer cells.
Conclusion
Lecithin favored the increase of hydrogen bonds at the surface, leading to a lower tendency of agglomeration for nanocapsules. LNC-CS-L-Zn+2-DOX-FA is a promising therapeutic agent against tumor-overexpressing folate receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The science of nanomedicine has ushered a new era for improving the efficacy of pharmaceutical therapeutic agents (1,2). In this context, nanotechnology has been described as the science involved in the design, synthesis, characterization, and application of materials and devices on a nanometer scale (1,2,3,4). In recent decades, nanoparticles have been considered a promising tool for the development of therapeutic agents for tumors. However, to increase the half-life of the drug in systemic circulation, direct the drug to the specific target, and obtain efficiency in treatment, nanoparticles have been functionalized with ligands (5,6).
Covalent and non-covalent conjugations have been used to functionalize various polymer systems (6). Particular peptides and antibodies are often used as targets for the surface functionalization of nanoparticles (7). In this context, folic acid (FA) has often been used as a ligand for the selective targeting and delivery of drugs in tumor cells due to folate receptors (FRs), which are widely expressed in several tumor cell lines but are highly restricted in most normal tissues (8,9). Additionally, FA has high stability, low cost, and can be conjugated to nanocarriers (10).
Ovarian cancer is considered the fourth most common cause of death due to cancer, affecting approximately 150,000 women annually worldwide (11). Meanwhile, bladder cancer is considered a common malignancy of the urinary tract and its development often occurs in the epithelial lining (12). Many chemotherapeutic agents, such as doxorubicin (DOX), cisplatin, paclitaxel (PTX), and carboplatin (13), are used in the treatment of ovarian cancer. The most commonly used anticancer drugs for the treatment of bladder cancer are DOX and PTX (14).
Chitosan (CS), a versatile and cationic polysaccharide derived from chitin, is a biocompatible and biodegradable biopolymer (15,16). In cancer therapy, CS can be tailored to provide derivatives with enhanced solubility by modifying the amino, hydroxyl, and acetamido groups and inhibiting the growth of cancer cells. It has been reported that CS and its derivatives selectively permeate through the membranes of cancer cells, showing anticancer activity involving antioxidant and apoptotic defense mechanisms and anti-angiogenic, enzymatic, and immuno-enhancing cellular pathways (17). Although the mechanisms of CS interaction with cancer cells have yet to be elucidated (15,17), the positive zeta potential of this biopolymer permits high bonding to the cell surface. This is followed by a great cellular uptake of particles, as the electrostatic interactions between the anionic cell membrane and the cationic polysaccharide facilitate uptake (18,19).
In the past few years, our group has developed a novel strategy to functionalize the surface of nanocapsules using lecithin and polysorbate 80 as a surfactant system and CS coating (20). After the nanocapsules are formed and the organic solvent evaporates, all the subsequent steps are interfacial reactions conducted in water without the need for purification. First, CS is used to coat the surface of the nanocapsules; next, the metal ion solution and the ligand solution are added in sequence. The functionalization process is based on the formation of an organometallic complex on the surface of the nanoparticles (21). The metal ion, for instance zinc-II, forms a covalent bond with the amine group of CS and a chelate with the carboxylic group of the ligand and a hydroxyl of CS (20,21). Studies have demonstrated the versatility of these complexes using ligands such as bromelain (21), laronidase (22), single-chain variable fragment anti-LDL(−) (20,23), arginylglycylaspartic acid (24), and methotrexate (25).
As DOX is an antitumor drug used in cancer chemotherapy (26) and FA can bind selectively to FRs (27), which participate in the cellular replication process being present at the cell membrane (28), we hypothesized that the FA-DOX-double-functionalized-Zn+2-complex-CS-coated lipid-core nanocapsule formulation (LNC-L-CS-Zn+2-DOX-FA) could present a higher antitumor activity than an aqueous solution of DOX hydrochloride (DOX.HCl). In this context, our objective was to obtain the LNC-L-CS-Zn+2-DOX-FA and to evaluate its activity against ovarian and bladder cancer cell lines (OVCAR-3 and T24, respectively). A pre-formulation study was conducted to determine the need for using lecithin in the composition of the nanocapsules. The OVCAR-3 cell line was selected because it is a highly metastatic and drug-resistant human ovarian carcinoma cell line (29), whereas the T24 cell line is representative of invasive bladder tumor with metastatic profile (30).
Material and Methods
Materials
FA (≥97% content), zinc acetate (99.99% content, metal bases), poly(ε-caprolactone) (PCL) (α,ω-dihydroxyl, Mw = 14,000 g mol−1), sorbitan monostearate (Span 60®), DOX.HCl, and QuantiPro™ BCA Assay Kit were obtained from Sigma-Aldrich (USA). CS with low viscosity (9 cP) was kindly gifted by Prof. Fabio Sonvico (University of Parma, Italy) - (ChitoClear®, viscosity - 9 cP, and deacetylation degree of 95%, Siglufjordur, Iceland). Caprylic/capric triglyceride and polysorbate 80 were purchased from Delaware (Brazil). Lecithin (Lipoid® S75) was obtained from Gerbras (Brazil). All other chemicals and solvents used in this study were of analytical grade.
Methods
Pre-Formulation Study to Determine the Influence of Lecithin in the Nanocapsule Composition
Preparation of Lipid-Core Nanocapsules by Self-Assembly
The LNC (without lecithin) and LNC-L (with lecithin) formulations were developed by self-assembly as previously described (31,32). An organic phase was composed of PCL (100 mg), sorbitan monostearate (40 mg), and caprylic/capric triglyceride (120 mg) dissolved in acetone (30 mL) at 40°C. In addition, ethanol (20 mL) containing Lipoid® S75 (60 mg) was poured into the organic phase. This resultant organic phase (acetone and ethanol) was added to an aqueous solution (80 mL) containing polysorbate 80 (80 mg) at 40°C, using a funnel (Hands spet, 25 mL, Eppendorf, Brazil). The organic solvents were removed using a rotary evaporator (Büchi, Switzerland) under reduced pressure, and the translucent solution was concentrated to a volume of 9 mL (40°C). The final volume was adjusted to 10 mL in a volumetric flask. The formulations were named LNC and LNC-L identifying the presence or absence of lecithin (L, Lipoid® S75).
CS Coating
CS was dispersed at 1% (w/v) in a 1% (v/v) acetic acid aqueous solution. Interfacial reactions were conducted by adding CS solution into each formulation (LNC and LNC-L) and stirring for 4 h at room temperature (22–25°C). The formulations were named LNC-CS and LNC-L-CS, respectively.
FA-Functionalized-Zn+2-Complex-CS-Coated Lipid-Core Nanocapsules
A solution was prepared by dissolving 28 mg of zinc acetate in water (10 mL). In parallel, FA was solubilized in 1 mol L−1 NaOH. The Zn+2 solution (80 μg mL−1) was slowly added to each formulation (LNC-CS and LNC-L-CS). Subsequently, an aqueous solution of FA (160 μg mL−1) was added in a ratio of 0.3:1 (mol/mol, ligand/metal ion) to passivate the surface of the nanocapsules. The formulations were named LNC-CS-Zn+2-FA and LNC-L-CS- Zn+2-FA.
Synthesis of LNC-L-CS-Zn+2-DOX-FA
A formulation containing lecithin and CS (LNC-L-CS) was used to prepare the double-functionalized nanocapsules with DOX and FA. In this case, we functionalized the surface in three steps: i) an aqueous solution of Zn+2 (80 μg mL−1) was slowly added to the formulation (LNC-L-CS); ii) an aqueous solution of DOX.HCl (100 μg mL−1) was slowly added to the formulation (LNC-L-CS-Zn+2); and iii) an aqueous solution of FA (106 μg mL−1) in a ratio of 0.15:0.2:1 (mol/mol/mol, DOX/FA/metal ion) was slowly added to the reaction medium (LNC-L-CS-Zn+2-DOX) to functionalize the surface of the nanocapsules, rendering nanocapsules with double functionalization (LNC-L-CS-Zn+2-DOX-FA). The reaction was maintained under stirring for 15 min at room temperature (22–25°C). To conduct the cellular uptake experiments, the nanoformulation was fluorescently labeled using a PCL conjugate, which has been previously reported (33). The conjugate was prepared via esterification of PCL with rhodamine B (PCL-RhoB) using an acid activating agent (carbodiimide). The labeled formulation was prepared as described above by substituting 10% (w/w) of PCL with the conjugate, i.e., using a mixture of PCL (0.090 g) and PCL-RhoB (0.010 g).
Physicochemical Characterization of Formulations
Laser Diffraction (LD), Photon Correlation Spectroscopy (PCS), Nanoparticle Tracking Analysis (NTA), Zeta Potential, and pH Measurements (Suppl Info., S1)
LD (Mastersizer® 2000, Malvern, UK) was used to determine the distribution of diameters for each formulation in the range from 40 nm to 2 mm. PCS (Zetasizer® Nano ZS, Malvern, UK) was used to determine the hydrodynamic diameters in the range from 1 nm to 2000 nm. NTA (NTA 3.2 Analytical Software, LM10 & NanoSight Ltd., Wiltshire, UK) was used to determine the particle number density (PND) (concentration of nanocapsules per mL of formulation), the mean diameter, D10 (particle diameter at percentile 10 of the particle size distribution curve), D50 (median diameter), and D90 (particle diameter at percentile 90 of the particle size distribution curve). Zeta potential was determined by electrophoretic mobility (Zetasizer® Nano ZEN 2600, Malvern, UK). The pH and potential difference measurements were carried out in a potentiometer (Denver Instrument, UB-10 pH/mV Meter) at 25°C, which was previously calibrated with phosphate buffer (pH 4 and pH 7).
Percentage of Zn+2 Coordinated at the Surface of the Nanocapsules (Suppl Info., S1)
The percentage of Zn+2 coordinated at the surface of the nanocapsule was determined via dialysis, followed by a reaction with dithizone, a colorimetric complexing agent, as previously validated (21). The samples were analyzed via spectrophotometry at a wavelength of 514 nm (UV-1800 PC, Spectrophotometer, Pro-Tools) with a 2.0 mm optical path.
DOX and FA Quantifications
The DOX and FA were quantified via high performance liquid chromatography (Shimadzu HPLC; composed of LC-20AT pump, SIL-20AHT injector, UV/Vis SPD-20A detector, and CBM-20A controller) using a LiChroCART® 250–4 column and LiChrospher® 100 RP - 18 (5 μm). The DOX and FA in the formulations were extracted (30 min of sonication) with acetonitrile (DOX) or methanol/water 30:70 (FA).
DOX concentration was quantified using a previously validated method: mobile phase consisting of acetonitrile/water/trifluoroacetic acid (50:50:0.1, v/v/v), injection volume of 50 μL, and flow rate of 1 mL min−1. DOX was detected at 254 nm with a retention time of 8 min, as previously reported (24,34).
FA was quantified using a mobile phase containing water:acetic acid:methanol (65:5:30, v/v/v), injection volume of 50 μL, flow rate of 0.5 mL min−1, and detection at 277 nm. The retention time was 10 min. The linearity was determined by three different curves (n = 3), ranging from 0.1 to 2.5 μg mL−1 with a coefficient of determination (R2) = 0.9937 ± 0.001. The limit of detection (LD) and limit of quantification (LQ) were 0.04 and 0.12 μg mL−1, respectively. This methodology was validated according to Resolution number 166 of the Brazilian Health Regulatory Agency (35) and the International Conference on Harmonization (36) in terms of specificity, linearity, precision, accuracy, limit of detection, and limit of quantification of the analytical method.
Detection of FA Via Colorimetric Method
The FA on the surface of the lipid-core nanocapsules was also determined using a colorimetric method (QuantiPro™ BCA Assay Kit, Sigma-Aldrich, St. Louis, MO, USA). A calibrated linear curve (n = 3) (3 to 27 μg mL−1) was prepared using dilutions of bovine serum albumin according to the manufacturer’s instructions and analyzed at 562 nm on a plate reader (SpectraMax, Molecular Devices, Sunnyvale, CA, USA).
Percentage of the DOX and FA Complexed on the Surface of the Nanocapsules
The percentages of FA or DOX complexed (Eq. 1) on the nanocapsule surfaces were calculated considering the total concentration and the concentration of FA or DOX soluble in the aqueous phase, which was determined in the ultrafiltrate after ultrafiltration-centrifugation using ultrafiltration units (30 kDa, Amicon® Ultra – 0.5 mL, Ireland) and centrifugation for 5 min (1870×g) in a centrifuge (Sigma, 1–14, Germany) (37).
where CT is the total concentration of FA or DOX in the formulation (μg mL−1) determined after extraction using organic solvents and Cultrafiltrate is the concentration in the ultrafiltrate (μg mL−1), i.e., the soluble portion of FA or DOX in the continuous phase.
Statistical Analysis of the Size Distribution Curves
The size distribution curves of the formulations obtained using both techniques: laser diffraction or photon correlation spectroscopy, were analyzed considering their symmetry and peakedness. Unimodal monodispersed curves provide symmetrical and narrow size distributions. In this way, we determined the third (Eq. 2) and the fourth moments (Eq. 3) (38), respectively, skewness and kurtosis to describe for each size distribution curve, the degree of distortion from the normal distribution (symmetrical bell curve), and the measure of outliers in that distribution.
Third moment (a3) - skewness
Fourth moment (a4) – kurtosis = (a4–3.0)
where mean (M1) is the 1st moment about the origin, variance (m2) is the 2nd moment about the mean, and skewness (a3) is the 3rd standardized moment.
-
a3 = 0 (symmetrical)
-
a3 > 0 (positively skewed)
-
a3 < 0 (negatively skewed)
The distribution can be considered reasonable if the value of a3 is between ±0.2.
Kurtosis (a4–3.0) – 4th standardized moment)
-
a4–3 = 0 same peakedness as normal curve.
-
a4–3 > 0 more peakedness than normal curve.
-
a4–3 < flatter than normal curve.
Thus, we can consider a normal distribution curve with a4–3 between ±0.5.
Infrared Spectrometry
The spectra of the nanocapsule formulations were obtained using the attenuated transmission reflectance (ATR) technique (Perkin Elmer, BX/RX with the ATR MIRacle, ZnSe accessory, USA). The measurements were performed by adding an aliquot of each sample (10–20 μL) in the equipment unit with the following parameters: measurements ranged from 4000 to 600 cm−1 and the number of scans was 32 with a resolution of 4.0 cm−1.
Transmission Electron Microscopy (TEM)
TEM analysis of the LNC-L-CS-Zn+2-DOX-FA formulation was performed at the Microscopy and Microanalysis Center of the Federal University of Rio Grande do Sul (CMM-UFRGS, Porto Alegre, Brazil) using a JEOL JEM 1200 ExII instrument (JEM 1200, Japan) operating at 80 kV. Each sample was diluted in water (1:10, v/v), deposited on 400 mesh copper grids (Formvar/Carbon film), and negatively stained with 2% uranyl acetate solution.
In Vitro Release Experiments
The drug solution and test formulation were prepared at 100 μg mL−1 DOX.HCl. To perform the dialysis, DOX.HCl solution (2 mL) or LNC-L-CS-Zn+2-DOX-FA (2 mL) was placed inside a dialysis membrane (cutoff 14,000 Da) and immersed in 30 mL of a release medium composed of phosphate buffer saline (PBS, pH 7.4) at 37°C under moderate magnetic stirring. The experiment was carried out at sink condition for 180 min. At each time interval, a sample (0.25 mL) was withdrawn from the release medium and analyzed using HPLC. All measurements were carried out in triplicate (n = 3).
Semi-empirical mathematical models were fitted to the experimental drug release data (39). In this case, zero-order (Eq. 4), and first-order, monoexponential model (Eq. 5) or the biexponential model (Eq. 6), were evaluated using Scientist® 2.0 – (MicroMath®, USA). The selection of the kinetic model was performed using the correlation coefficient (r). The evaluation was carried out to determine the apparent rate constant and half-life of drug dialysis.
where C is the concentration dissolved at time t, Co is the total drug concentration (corresponding to 100% of release at infinite time), A and B are the fractions of drug concentration (varying from 0 to 1) that contributed to the burst and sustained phases, and k, α, and β represent the apparent dissolution rate constants. The results were plotted as cumulative dialyzed drug in percentage relative to the content of drug in each case (total concentration) as a function of time.
According to Fick’s First Law, flux J (mg cm−2 min−1) was established from the slope of the linear region of the plot (cumulative amount of drug Mt as a function of t time) (Eq. 7). The model was used to compare the flux of soluble DOX after dialyzing a DOX.HCl solution or a nanoformulation containing complexed DOX.
where S is the surface area (cm3) of the dialysis bag, and M0 (mg) corresponds to the total amount of drug (40).
Additionally, the Korsmeyer-Peppas equation (Eq. 8) (Scientist® 2.0 Software - MicroMath®, USA) was used to determine the mechanism of drug release from the nanocapsules.
where ft is the fraction of drug dialyzed in t time, a is a constant incorporating structural and geometric parameters of the device, and n characterizes different drug release mechanisms. The value of the diffusional exponent (n) defines the release mechanism. For spherical shape-controlled release systems, the mechanisms are classified as Fickian diffusion when n = 0.43, anomalous (non-Fickian) transport when 0.43 < n < 1.0, and zero-order release when n = 1.0 (41). With respect to particulate systems, the size distribution influences the release rate, with drug release accelerating initially and then decreasing over longer periods. Next, a Fickian diffusion for non-monodisperse particulate systems is characterized by n = 0.30 (41).
Biological Assays
Cell Culture
Bladder transitional cell carcinomas (T24 line) and ovarian adenocarcinoma cell line (OVCAR-3 line) were obtained from the cell bank of Rio de Janeiro (PABCAM, Universidade Federal of Rio de Janeiro, Brazil). Cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, respectively, obtained from Vitrocell Embriolife (Campinas, Brazil) and Gibco (Grand Island, NY, USA). The cells were cultured in a controlled atmosphere at 37°C, 95% humidity, and 5% CO2.
Cell Treatment
The test groups in the cytotoxicity evaluation were DOX in solution (DOX.HCl) and the LNC-L-CS-Zn+2-DOX-FA formulation. The former was prepared by solubilizing DOX.HCl (100 μg) in water (1 mL), and the latter was obtained as described in section “Synthesis of LNC-L-CS-Zn+2-DOX-FA.”
After cell adhesion to the microwell plates (24 h), the cells were treated with different concentrations of drug using both test groups (1.0, 1.5, 3.5, 6.5, and 15 μmol L−1 of DOX), which corresponded to 6.99 × 10−9 – 1.04 × 10−7 μmol of nanocapsules per liter of well in the case of the LNC-L-CS-Zn+2-DOX-FA formulation). Additionally, to determine the selectivity of the ligand model, in this case FA, we used concentrations ranging from 1.3, 2.0, 4.8, 8.9, and 20 μmol L−1 of folate acid. Proliferation assays were performed after 24 and 48 h to determine cell viability. Experiments were performed using cells in the logarithmic growth phase. The results were obtained from the average of three independent experiments performed in triplicate. The median maximal inhibitory concentration (IC50) was calculated using GraphPad Prism 7.0.
Cell Viability Assay
T24 and OVCAR-3 cells were seeded in 96-well culture plates at a density of 2 × 104 mL (200 μL/well). The negative control was performed using culture medium (200 μL/well) and vehicle control was performed using water (200 μL/well). Cell proliferation was evaluated after 24 and 48 h. After each incubation period, tetrazolium salt [3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide] (MTT) was added to each well (5 mg MTT/mL solution). Thereafter, the absorbance was determined using a spectrophotometer (Thermo Plate TP-Reader) at 495 nm. The percentage of growth inhibition was determined using eq. 9.
Confocal Microscopy and Cellular Uptake
For cellular uptake evaluation, 4,6-diamidino-2-phenylindole (DAPI) staining was used to form a fluorescent complex with double-stranded DNA (staining the cell nucleus). Additionally, we delimited the actin cytoskeleton with Alexa Fluor® 633 where WGA binds to sialic acid and N-acetylglucosaminyl residues as well as the identification of rhodamine within the treated cells with the nanoformulation. Cells were seeded in a 96-well plate and treated with fluorescently-labeled nanoformulation and the control containing only DMEM at IC50 concentrations of cell lines (OVCAR-3 and T24). After treatment, cells were washed three times in PBS, fixed, and stained according to the manufacturer’s protocol. Cell morphology was examined via confocal microscopy SP8 (Leica Microsystems ©).
DAPI emission: ~ 460 nm. Red emission from Alexa: ~ 632 nm. Rhodamine emission: ~ 566 nm. Cell morphology was examined via confocal microscopy at 200x magnification.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s method with a significance level of P < 0.05 (GraphPad Prism 7.0). Data are presented as the mean and standard deviation (SD). Cell proliferation was compared via analysis of two-way ANOVA with different concentrations and times as variables. Bonferroni’s post-hoc method was employed for multiple comparisons. All statistical analyses were performed using GraphPad Prism, and P < 0.05 was considered statistically significant.
Results
Pre-Formulation Study to Determine the Influence of Lecithin in the Nanocapsule Composition
The surface functionalization of lipid-core nanocapsules was carried out in three steps after the self-assembly of materials: CS coating, Zn+2 coordination, and ligand chelate formation (Fig. 1). All formulations showed unimodal size distribution curves using LD and PCS (Fig. 2). The volume-weighted mean diameter (D [3,4]) ranged from 135 to 166 nm for formulations prepared without lecithin (LNC, LNC-CS, and LNC-CS-Zn+2-FA) and from 126 to 128 nm for formulations prepared with lecithin (LNC-L, LNC-L-CS, and LNC-L-CS-Zn+2-FA) (Table I). In parallel, the diameters at percentiles 10 [d(0.1)], 50 [d(0.5)], and 90 [d(0.9)] under the size distribution curve as well as the polydispersity (SPAN), are also described in Table I. Meanwhile, the hydrodynamic z-average diameter (Dz) ranged from 152 to 172 nm for formulations prepared without lecithin (LNC, LNC-CS, and LNC-CS-Zn+2-FA) and from 109 to 120 nm for formulations prepared with lecithin (LNC-L, LNC-L-CS, and LNC-L-CS-Zn+2-FA) with polydispersity indexes (PDI) lower than 0.21 (Table II). Both techniques (LD and PCS) showed that formulations containing lecithin presented smaller diameters (p > 0.05) when compared to formulations prepared without lecithin (Tables I and II).
Size distribution curves by Laser Diffraction (DL): (a and b) and by Photon Correlation Spectroscopy (PCS) (c and d). (a and c) - formulations prepared without lecithin and; (b and d) - formulations prepared with lecithin. LNC: lipid-core nanocapsule; LNC-CS: chitosan-coated lipid-core nanocapsule; LNC-CS-Zn+2-FA: folic acid-Zn+2-complex–chitosan-coated lipid-core nanocapsule. The presence of lecithin (Lipoid® S75) in the formulation is indicated using (L).
The zeta potential of the nanocapsule formulations just after self-assembly [LNC and LNC-L] was inverted to positive values after coating with the cationic polysaccharide, CS, (LNC-CS and LNC-L-CS) (Table III). After an organometallic complexation with Zn+2 and surface-functionalization with FA, the zeta potential of the formulations was +4.1 ± 0.6 (LNC-CS-Zn+2-FA) and + 8.9 ± 0.3 (LNC-L-CS-Zn+2-FA) (Table III). In addition, the zeta potential distribution curves showed unimodal distribution peaks (Suppl Info., S2). The conductivity of the nanoformulations (Table III) was determined prior to the determination of zeta potential values. Furthermore, the pH measurements ranged from 3.9 ± 0.0 to 5.6 ± 0.2, whereas the potential difference varied from 107 ± 7.8 to 144 ± 2.4 (mV) for all formulations (Table III).
The percentage of Zn+2 complexed on the surface of the lipid-core nanocapsules was similar (p > 0.05) for both formulations (76 ± 1.2% for LNC-CS-Zn+2-FA and 78 ± 1.0% for LNC-L-CS-Zn+2-FA). These values are in agreement with the efficiency of Zn2+ binding, as previously described for other nanoformulations (21). Furthermore, the complexation efficiency of FA varied from 60 ± 2% (using HPLC) to 69 ± 1.8% (using colorimetric method) (Suppl Info., S3), corresponding to an average FA content of 124 ± 3 μg mL−1.
All formulations showed slightly positively skewed curves (Table IV). The PCS technique showed similar skewness for all formulations (p > 0.05). Nevertheless, comparing each series of formulations to determine the influence of each step on the asymmetry of the size distribution curve via LD, those containing lecithin (LNC-L, LNC-L-CS, and LNC-L-CS-Zn+2-FA) showed similar (p > 0.05) skewness, whereas the formulations prepared without lecithin (LNC, LNC-CS, and LNC-CS-Zn+2-FA) showed higher skewness after CS coating (Table IV) (p < 0.05). All formulations analyzed via LD and PCS showed (p < 0.05) kurtosis (Table IV), with a tendency for platykurtic distributions.
To determine the molecular interactions among the materials and justify the use of lecithin in the composition, we analyzed the formulation using infrared spectroscopy. We observed the presence of very weak absorption intensity peaks at 2959 and 2853 cm−1 for ν(CH3) and ν(CH2) stretching modes on LNC-L, respectively, which confirmed the derivative function in the spectra. As a result, we observed stretching of the CH3 group in the LNC/LNC-L samples. In addition, LNC-L-CS and LNC-L-CS-Zn+2-FA samples showed entropy levels of the hydrocarbon group. Additionally, we observed an increase in the number of peaks when lecithin was present in the formulation (LNC-L/LNC-L-CS/LNC-L-Cs/Zn+2/FA) and a downshift in the ν (C=O) mode for these samples, showing a hydrogen bonding environment effect on the nanocapsules. A more detailed analysis is shown in Suppl Info., S4.
Synthesis of LNC-L-CS-Zn+2-DOX-FA
The LD and PCS techniques showed that the LNC-L-CS-Zn+2-DOX-FA formulation had unimodal distribution curves (Fig. 3a and b). The mean diameters of LNC-L-CS-Zn+2-DOX-FA were 124 ± 2 nm (LD) and 123 ± 4 nm (PCS). The variance via PCS (polydispersity index, PDI) was 0.22 ± 0.0. In addition, the diameters corresponding to the percentiles 10, 50, and 90 were 72 ± 3.0 [d(0.1)], 116 ± 0.5 [d(0.5)], and 188 ± 10.3 [d(0.9)] nm by LD, with a polydispersity (SPAN) of 0.99 ± 0.11. The size distribution curves by NTA were unimodal for LNC-L-CS-Zn+2-DOX-FA at three different dilutions (Fig. 8S, 9S, and 10S) (Suppl Info., S5). The particle concentrations varied from 3.47 × 1012 to 4.23 × 1012 mL−1, the mean diameter and diameters corresponding to percentiles 10, 50, and 90 under the size distribution curve showed to be suitable for LNC-L-CS-Zn+2-DOX-FA formulation (Suppl Info., S5). According to the statistical analysis, there was no significant difference (p > 0.05) in the particle number density (concentration of particles per mL of formulation without dilution) after diluting the samples from 8000× to 12,000×. The results indicated that the nanocapsules were kinetically stable with very low (not detectable) aggregation or deagglomeration after dilution.
The zeta potential was positive at +12 ± 1.32 mV and the pH value was 5.13 ± 0.03. The zeta potential analysis showed unimodal distribution peaks (Fig. 3c). Moreover, the DOX and FA contents were 98 ± 2.34 μg mL−1 and 105 ± 2.05 μg mL−1, respectively, and the complexation efficiencies [%] were 80 ± 1.89% (DOX) and 74 ± 6.64% (FA), respectively. Additionally, the standardized moments indicated a positive asymmetry (skewness) with a small tendency of agglomeration by both techniques (LD) +0.78 ± 0.18 and (PCS) +0.61 ± 0.23 (LNC-L-CS-Zn+2-DOX-FA). Meanwhile, the kurtosis values indicated a tendency for a platykurtic distribution by LD (−1.03 ± 0.32) and PCS (−1.22 ± 0.25) (LNC-L-CS-Zn+2-DOX-FA).
The drug release profile was determined via a direct dialysis experiment (Fig. 4). We compared the dialysis profiles of DOX by assaying the DOX.HCl aqueous solution and LNC-L-CS-Zn+2-DOX-FA. DOX.HCl solution presented 100% of the drug dialyzed after 30 min of experiment (Fig. 4), whereas LNC-L-CS-Zn+2-DOX-FA showed the total release of the drug in 180 min. The selected model to describe the dialysis profile of DOX (LNC-L-CS-Zn+2-DOX-FA and DOX.HCI solution) was a first-order monoexponential equation (Eq. 5). This was chosen as the correlation coefficient was higher for the solution and the biexponential model for the nanoformulation showed very similar half-lives for the burst and sustained phases. The apparent kinetic rate constants (k, α, β), correlation coefficients (r), MSC, and half-lives of drug dialysis are shown in Table V. Comparing the DOX.HCl solution and the LNC-L-CS-Zn+2-DOX-FA formulation, the half-life of drug dialysis increased by a few minutes (p < 0.05), indicating that the drug can be released from the nanoformulation, and that the chemical interaction of the drug with the nanocapsules delayed DOX diffusion through the dialysis bag. According to Fick’s first law of diffusion, DOX dialysis from LNC-L-CS-Zn+2-DOX-FA showed a flux (J) of (3.37 ± 0.47) × 10−3 mg cm−2 min−1, while the drug solution had a J of 2.32 ± 0.22 × 10−2 mg cm−2 min−1 (p < 0.05). The flux of the drug was 6.9 times smaller when LNC-L-CS-Zn+2-DOX-FA was assayed compared to DOX.HCl solution. Additionally, the Korsmeyer-Peppas equation showed a value of 0.30 ± 0.06 for the DOX release from LNC-L-CS-Zn+2-DOX-FA, indicating that the mechanism of release is Fickian diffusion (41).
The infrared spectrum (1800–1480 cm−1) of LNC-L-CS-Zn+2-DOX-FA showed a broad band with a maximum at 1643 cm−1 (Fig. 5a). After applying the second derivative function on this interval, we observed peaks at 1643, 1628, and a small shoulder intensity peak at 1650 cm−1, which are associated with the ν(COO−) group. We observed an upshift in the wave number of the ν(C = O) mode from 1638 cm−1 to 1643 cm−1 in the LNC-L-CS-Zn+2-DOX-FA formulation (Fig. 5b). Consequently, this effect shows a conformational dynamic process in the structure. Finally, we observed the derived peak at 1739 cm−1 for the LNC-L-CS-Zn+2-DOX-FA sample related to the ν(C = O) mode of ester groups that is not present in the DOX spectrum.
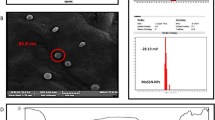
Additionally, we observed via TEM that LNC-L-CS-Zn+2-DOX-FA has spherical structures (Fig. 6a and b). In addition, we visualized the presence of micellar and cylindrical structures surrounding the nanocapsules constituting the hydrophilic corona (Fig. 6b).
Biological Studies
We determined the IC50 of DOX after the application of the LNC-L-CS-Zn+2-DOX-FA formulation at different concentrations in ovarian and bladder cancer cell cultures. The IC50 values were 2.45 ± 0.50 μmol L−1 (24 h) and 0.85 ± 0.16 μmol L−1 (48 h) for the T24 cell line. Meanwhile, for the OVCAR-3 cell line, the IC50 values were 8.85 ± 11.09 μmol L−1 (24 h) and 3.15 ± 0.88 μmol L−1 (48 h). Subsequently, we evaluated the effect of LNC-L-CS-Zn+2-DOX-FA on OVCAR-3 and T24 cell line viability. It was also compared to cellular viability after treatment with DOX.HCl solution, which was administered at the same doses. OVCAR-3 and T24 cell lines were treated with drug concentrations ranging from 1 to 15 μmol L−1 of DOX using concentrations of particles ranging from 6.99 × 10−9 to 1.04 × 10−7 μmol of nanocapsules per liter of well (Fig. 7a, b, c, and d). As a selective targeting ligand, FA has several advantages (27,28). In this context, we used concentrations from 1.3 to 20 μmol L−1 of FA to determine their selectivity in OVCAR-3 and T24 cell lines (Fig. 7e and f). The results for LNC-L-CS-Zn+2-DOX-FA showed a selective decrease in cell proliferation and better results in the T24 tumor cell line over OVCAR-3. These displayed inhibition rates above 80% and 90% at a concentration of 15 μmol L−1 of DOX at 24 and 48 h (Fig. 7a, b, c, and d), respectively. Following this approach, the assays showed inhibition of cell growth after 24 h of incubation (OVCAR-3) ranging from: −0.6% to 22% for DOX.HCl, and from 9% to 68% for LNC-L-CS-Zn+2-DOX-FA. After 48 h of incubation, the inhibition range increased from 25% to 47% (DOX.HCl) and from 22% to 93% (LNC-L-CS-Zn+2-DOX-FA) (Fig. 7a and b). However, for the T24 line, the treatment showed the highest rates of cell death, ranging from 31% to 52% (DOX.HCl) and from 30% to 80% (LNC-L-CS-Zn+2-DOX-FA) after 24 h of incubation. Nevertheless, after 48 h of incubation, the nanoformulation inhibited T24 cell growth at a rate of growth inhibition ranging from 52% to 99%, whereas DOX.HCl inhibited from 44% to 87% (Fig. 7c and d).
Treatment with DOX.HCl solution and LNC-L-CS-Zn+2-DOX-FA for 24 and 48 h effect in reducing cell viability. Growth inhibition in OVCAR-3 (a, b and e) and T24 (c, d and f) were measured using MTT assay. Cell lines (OVACAR-3 and T24) were treated in concentrations (1, 1.5, 3.5, 6.5 and 15 μmol L−1 of doxorubicin and 6.99 × 10−9, 1.05 × 10−8, 2.45 × 10−8, 4.54 × 10−8 and 1.04 × 10−7 μmol L−1 of nanocapsules per well). To determine the selectivity of the folic acid, was used concentrations ranging from 1.3, 2.0, 4.8, 8.9 and 20 μmol L−1 of folate acid. Data were expressed as mean ± SEM (n = 3). * Indicate significant diferences p < 0.05, ** p < 0.003, *** p < 0.0008, **** p < 0.0001. DOX.HCl solution: doxorubicin hydrochloride. FA - Solution: folic acid. LNC-L-CS-Zn+2-DOX-FA: folic acid-doxorubicin-double-functionalized-Zn+2-complex-chitosan-coated lipid-core nanocapsules formulation.
To conduct the confocal microscopy studies (Fig. 8a, b, c, and d), we prepared a fluorescently-labeled nanoformulation with a PCL-rhodamine B conjugate. Considering that DOX presents a fluorescent emission at a wavelength similar to that of rhodamine B, we prepared a formulation with a PCL-rhodamine B conjugate without DOX (RhoB-LNC-L-CS-Zn+2-FA). After treatment of the OVCAR-3 and T24 cancer cell lines with the fluorescently labeled nanoformulation, the images obtained via confocal microscopy showed a higher fluorescence emission intensity for the OVCAR-3 cancer cell line (Fig. 8b) compared to the T24 cancer cell line (Fig. 8d). The results suggested a higher cellular uptake of the nanoformulation by the OVCAR-3 cells. Moreover, using ImageJ software, we determined the area of fluorescence emission corresponding to the nanocapsules that were in contact and/or internalized in the cells (OVCAR-3 and T24) (Fig. 8e). In both cancer cell lines (OVCAR-3 and T24), the fluorescence of the nanocapsules was observed, indicating their uptake by the cells. Nevertheless, statistical analysis indicated that the RhoB-LNC-L-CS-Zn+2-FA formulation showed a higher area of fluorescence emission related to the nanocapsules in contact and/or internalized in the OVCAR-3 cell line, when compared to T24. Based on our data, we suggest there is a better uptake capacity for OVCAR-3 compared to T24 cancer cells.
Confocal microscopy analysis of cellular uptake after 24 h of treatment with fluorescently-labeled nanoformulation. Untreated cells (control) OVCAR-3 (a) and T24 (control) (c). Treatment of cells with nanoformulation (OVCAR-3) (b) and (T24) (d). Representative graphical of the number of particles per cancer cell lines (e). To interpret the color references in this figure legend, DAPI (cell nucleus) – Blue fluorescence; Alexa Fluor® 633 (cytoskeleton) – Green fluorescence and; fluorescently-labeled nanoformulation – Red fluorescence. Data are expressed as means ± SEM from three independent experiments. Significance was considered at p < .05
Discussion
A hypothesis raised in the present study was to evaluate the need for lecithin to produce nanocapsules with unimodal narrow size distributions. When lecithin and polysorbate 80 are used in the composition, micelles of both surfactants form a hydrophilic corona at the nanocapsule surface (31). A pre-formulation study was dedicated to investigating the influence of the lecithin presence on the symmetry and peakedness of the size distribution curve. In this way, two series of formulations were prepared with one containing lecithin (LNC, LNC-CS and LNC-CS-Zn+2-FA/LNC-L, LNC-L-CS and LNC-L-CS-Zn+2-FA). Our results are in agreement with previously reported data for lipid-core nanocapsules coated with polysorbate 80 (32), lipid-core nanocapsules coated with lecithin and polysorbate 80 (31,42), and lipid-core nanocapsules coated with lecithin, polysorbate 80, and CS (31,42). In addition, the mean diameters found in our experiment for the different batches of surface-functionalized formulations (LNC-L-CS-Zn+2-FA) corroborate the mean diameters of functionalized-nanoformulations previously obtained using diverse ligands: single chain variable fragment anti-LDL(−) (20,23), bromelain (21), laronidase (22), arginylglycylaspartic acid (24), and methotrexate (25). After self-assembly of the nanocapsule formulations, the zeta potential became positive once coated with CS. The inversion of zeta potential indicates a proper coating with CS (22,31,42,43), which is slightly positive due to the presence of CS coated with polysorbate 80 at the particle-water interface. The percentage of Zn+2 complexed on the surface of the lipid-core nanocapsules is in agreement with the efficiency of Zn2+ binding, as previously described for other nanoformulations (21).
The bicinchoninic acid test produces a purple color in the presence of amines in alkaline conditions; this is caused by the reduction of Cu2+ to Cu+ (44), followed by the chelation of Cu+ with BCA (45). This colorimetric method was used as an alternative test to determine the complexation efficiency of FA on the surface of the nanocapsules, since FA has several amine groups. Additionally, considering the statistical analysis of the size distribution curves, the average size was determined by the first moment and the value of variance by the second moment (46). In the present study, we propose further evaluations of the size distribution curves, such as symmetry and peakedness. These values are accessed by calculating the third and fourth unidimensional moments of the size distribution curves, which give skewness and kurtosis. A distribution curve negatively skewed indicates deagglomeration of the particles, whereas a positively skewed curve shows agglomeration/aggregation or coalescence (47,48). When the particles are well-dispersed and uniform (normal distribution), the degree of asymmetry approaches zero (49). In this way, the analysis of the third moment indicated that the series of formulations prepared without lecithin had a higher tendency to form aggregates after the CS coating, likely due to the lack of the lecithin layer at the nanocapsule surface. Additionally, via infrared spectroscopy, LNC-L-CS and LNC-L-CS-Zn+2-FA samples showed an entropy level of the hydrocarbon group (50) that indicates the functionalization of lecithin at the nanocapsules. As an overall view of the infrared section, we see an increase in the number of peaks when lecithin is present in the formulation (LNC-L/LNC-L-CS/LNC-L-Cs-Zn+2-FA).
The pre-formulation study showed that the formulation containing lecithin produces a hydrogen bonding environment on the nanocapsule surface. Therefore, we selected LNC-L as a building block to obtain double-functionalized nanocapsules (LNC-L-CS-Zn+2-DOX-FA). The values observed for this formulation (LNC-L-CS-Zn+2-DOX-FA) (Diameter, SPAN, and PDI) correspond with narrow or moderate polydispersity, appropriate for this kind of drug delivery system, when compared to other formulations of nanocapsules containing ligands on their surface (21,22,23,24,25). Infrared analysis of the LNC-L-CS-Zn+2-DOX-FA formulation showed that interactions can be attributed to a hydrogen-bonded carbonyl (-C=O H-bonded) group, as the authors observed for a silica-polycaprolactone system (51). In this context, this shift could be related to an increase in hyperconjugation in the carbonyl groups, as previously observed (52), and also associated with lipid packing behavior together with a resonance effect (53). This finding shows that the presence of PCL and CS should be responsible for the stability of the dispersed phase (the nanocapsules). In addition, we previously observed the presence of micelles surrounding lipid-core nanocapsules (LNC-L) using TEM (31). The cylindrical structures can be attributed to the presence of CS on the nanocapsule surface as the zeta potential changed from negative to positive after the interfacial reaction of the nanocapsules with CS.
FA is a promising ligand for selective delivery. Studies indicate that polymeric nanoparticles using FA functionalization demonstrated a more effective antitumor activity and better cellular uptake (27,54,55,56,57) since FA promotes cellular uptake of nanoparticles through FR-mediated endocytosis (27). For confocal microscopy studies, the fluorescently-labeled nanoformulation with a PCL-Rhodamine B conjugate was prepared (33). Our results showed a similar correlation between cellular uptake and viability and the importance of FA functionalization in decreasing OVCAR-3 cell viability.
Our research group evaluated the antiproliferative activity of DOX-loaded lipid-core nanocapsules (58) and arginylglycylaspartic acid-surface-functionalized DOX-loaded lipid-core nanocapsules (24) in cell lines. These findings showed satisfactory inhibition of cell growth. Although the antiproliferative activity of DOX has been reported in several studies (11,24,58,59,60,61,62,63), here we demonstrate the antiproliferative activity of DOX functionalized on the surface of lipid-core nanocapsules. Here, we also used FA as a ligand for selective cellular targeting. The use of FA as a ligand for cell targeting has been reported in several studies, showing increased therapeutic efficacy (8,9,27). In parallel, FA has been reported to play various roles in different types of cancer. In this context, studies have shown that FA inhibits cancer cell proliferation by activating FRα (64), FRα/c-SRC (65), and FRα/ERK1/2/TSLC1 (66).
In our study, the OVCAR-3 and T24 lineages were used for driving experimentation. We used these cell lines because of the presence of FRs in their cell membrane (27,28) and FA binds selectively to FRs (27). LNC-L-CS-Zn+2-DOX-FA showed higher cytotoxicity than DOX.HCl at treatment concentrations of 1.5, 3.5, 6.5, and 15 μmol L−1 of DOX (OVCAR-3 and T24) (p < 0.05). In other words, this nanotechnology-based formulation (LNC-L-CS-Zn+2-DOX-FA) showed a higher rate of induced cell death in ovarian (OVCAR-3) and bladder (T24) cancer cell lines than the DOX.HCl solution.
The use of nanomaterials and anticancer agents can promote better therapeutic efficacy, which can lead to more selective and effective treatments for ovarian (67) and bladder cancer (68). Comparative studies to determine the effectiveness of diverse nanomaterials, such as graphene oxide (GO), reduced GO (rGO), silver nanoparticles (AgNPs), and nanocomposites (GO-AgNPs) containing two different nanomaterials, have been conducted to inhibit ovarian cancer growth (69,70). In particular, among the nanomaterials tested, the rGOAgNP nanocomposite demonstrated high cytotoxicity against ovarian cancer cells. In another study, PTX-loaded cationic nanostructured lipid nanoparticles (PTX-NLCs) decreased the IC50 value by 10 times compared to PTX in solution; thus, the PTX-NLC formulation showed greater cytotoxic effect against ovarian cancer cells (71). Promising for the treatment of ovarian cancer, curcumin nanoparticle (72) conjugates of the multiblock-GEM N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer and HPMA-PTX copolymer (73), and QU/MPEG-PCL micelles (74) inhibited ovarian cancer cell growth in vitro.
Furthermore, poly(ethyl 2-cyanoacrylate) nanoparticles containing epirubicin showed increased cytotoxicity in bladder cancer (75). In vitro studies with bladder cancer cells have shown the cellular uptake of nanoparticle-loaded ZnPc (76). In addition, nanocapsule-like PLGA containing gemcitabine monophosphate and cisplatin exhibited synergistic anti-cancer effects in a stroma-rich bladder tumor model (77). In another study, tetrahydropyranyl-adriamycin (THP)-loaded functional single-walled carbon nanotubes (SWNTs) increased the inhibition of bladder cancer cell proliferation compared to THP in solution. Nevertheless, SWNT-THP has great potential as a therapy for bladder cancer (78). Additionally, lapatinib-loaded nanocapsules showed cytotoxic effects in HER-positive bladder cells and reduced cell viability (79).
Conclusion
The pre-formulation study showed the need to use lecithin in the formulation. Even though both series of formulations (one containing lecithin) presented unimodal size distribution curves, we carried out statistical analysis to determine their tendency to aggregate and have a broad size distribution. Our results showed that formulations containing lecithin presented smaller diameters with a lower tendency to aggregate with a narrower size distribution, when compared to formulations prepared without lecithin. Our results indicated that beyond the electrostatic interactions, lecithin increases hydrogen bond formation at the nanocapsule surface, furnishing lipid-core nanocapsules with narrower size distributions and smaller mean diameters. Furthermore, specific spectral and distribution characteristics were determined, such as interactions and conformational effects of constituent materials, using infrared spectroscopy. The determination of skewness and kurtosis allied to infrared spectroscopy data represent a novel approach for the pre-formulation studies considering this kind of formulation, since in the past we used to compare only the laser diffraction data: mean diameters and diameters at percentiles 10, 50, and 90.
Additionally, we proposed for the first time double functionalization at the nanocapsule surface. We selected FA to interact with FRs and DOX as an antineoplastic agent. Indeed, our hypothesis was validated since the cell growth inhibition assays revealed that the LNC-L-CS-Zn+2-DOX-FA formulation showed a higher rate of induced cell death in ovarian (OVCAR-3) and bladder (T24) lines when compared to the DOX.HCl solution. Furthermore, based on the cellular uptake study, we observed a higher uptake capacity of OVCAR-3 compared to T24 cancer cells. Overall, our findings suggest that this innovative nanoformulation may have promising applications in nanomedicine considering tumor-overexpressing FRs.
Acknowledgements and Disclosures
The authors thank the Brazilian Agencies: Coordination for the Improvement of Higher Education Personnel (CAPES), National Council of Technological and Scientific Development (CNPq) and Research Support Foundation of the State of Rio Grande do Sul (FAPERGS: PRONEX FAPERGS/CNPq 12/2014 #16/2551–0000467-6 and Pesquisador Gaúcho 17/2551–0001 002–7). “This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001”. This study is part of the National Institute of Science and Technology in Pharmaceutical Nanotechnology: a transdisciplinary approach INCT-NANOFARMA, which is supported by São Paulo Research Foundation (FAPESP, Brazil) Grant #2014/50928–2, and by National Council of Technological and Scientific Development (CNPq, Brazil) Grant # 465687/2014–8. We are thanking Prof. João Henrique Z. dos Santos (IQ-UFRGS) for facilities provided on the use of Zetasizer Nano ZEN 2600 equipment and to Prof. Fabio Sonvico (University of Parma) for having kindly gifted the chitosan used in this study. We would like to thank Editage (www.editage.com) for English language editing. The authors have no conflict of interest to declare.
References
Saini R, Saini S, Sharma S. Nanotechnology: the future medicine. J Cutan Aesthet Surg. 2010;3(1):32–3.
Tran S, DeGiovanni PJ, Piel B, Rai P. Cancer nanomedicine: a review of recente success in drug delivery. Clin Trans Med. 2010;6(1):1–21.
Jurj A, Braicu C, Pop LA, Tomuleasa C, Gherman CD, Berindan-Neagoe I. The new era of nanotechnology, an alternative to change cancer treatment. Drug design Development and Therapy. 2017;11:2871–90.
Dawidczyk CM, Russell LM, Searson PC. Nanomedicines for cancer therapy: state-of-the-art and limitations to pre-clinical studies that hinder future developments. Frontiers in Chemistry. 2014;2(69):1–13.
Abd Ellah NH, Abouelmagd SA. Surface functionalization of polymeric nanoparticles for tumor drug delivery: approaches and challenges. Expert Opinion on Drug Deliver. 2017;14(2):201–14.
Crampton HL, Simanek EE. Dendrimers as drug delivery vehicles: non-covalent interactions of bioactive compounds with dendrimers. Polym Int. 2007;56(4):489–96.
Spencer DS, Puranik AS, Peppas NA. Intelligent nanoparticles for advanced drug delivery in Cancer treatment. Curr Opin Chem Eng. 2015;7:84–92.
Zhang H, Li F, Yi J, Gu C, Fan L, Qiao Y, et al. Folate-decorated maleilated pullulan-doxorubicin conjugate for active tumor-targeted drug delivery. Eur J Pharm Sci. 2011;42(5):517–26.
Zhang Y, Li J, Lang M, Tang X, Li L, Shen X. Folate-functionalized nanoparticles for controlled 5-fluorouracil delivery. J Colloid Interface Sci. 2011;354(1):202–9.
Scarano W, Duong HTT, Lu H, De Souza PL, Stenzel MH. Folate conjugation to polymeric micelles via Boronic acid Ester to deliver platinum drugs to ovarian Cancer cell lines. Biomacromolecules. 2013;14(4):962–75.
Di Lorenzo G, Ricci G, Severini GM, Romano F, Biffi S. Imaging and therapy of ovarian cancer: clinical application of nanoparticles and future perspectives. Theranostics. 2018;8(16):4279–94.
Chen CH, Chan TM, Wu YJ, Chen JJ. Review: application of nanoparticles in Urothelial Cancer of the urinary bladder. J Med Biol Eng. 2015;35:419–27.
Das J, Choi YJ, Han JW, Reza AMT, Kim JH. Nanoceria-mediated delivery of doxorubicin enhances the antitumour efficiency in ovarian cancer cells via apoptosis. Sci Rep. 2017;9513(7):1–12.
Wei Y, Gao L, Wang L, Shi L, Wei E, Zhou B, et al. Polydopamine and peptide decorated doxorubicin loaded mesoporous silica nanoparticles as a targeted drug delivery system for bladder cancer therapy. Drug Delivery. 2017;24(1):681–91.
Chang PH, Sekine K, Chao HM, Hsu SH, Chern E. Chitosan promotes cancer progression and stem cell properties in association with Wnt signaling in colon and hepatocellular carcinoma cells. Sci Rep. 2017;45751(7):1–14.
Babu A, Ramesh R. Multifaceted applications of chitosan in Cancer drug delivery and therapy. Mar Drugs. 2017;15(96):1–19.
Adhikari HS, Yadav PN. Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. International Journal of Biomaterials. 2018;2952085:1–29.
Zhang Y, Yang M, Park JH, Singelyn J, Ma H, Sailor MJ, et al. A surface-charge study on cellular-uptake behavior of F3-peptide-conjugated Iron oxide nanoparticles. Small. 2009;5(17):1–15.
Honary S, Zahir F. Effect of zeta potential on the properties of Nano-drug delivery systems - a review (part 1). Trop J Pharm Res. 2013;12(2):255–64.
Bender EA, Cavalcante MF, Adorne MD, Colomé LM, Guterres SS, Abdalla DSP, et al. New strategy to surface functionalization of polymeric nanoparticles: one-pot synthesis of scFv anti-LDL(−)-functionalized nanocapsules. Pharm Res. 2014;31(11):2975–87.
Oliveira CP, Prado WA, Lavayen V, Büttenbender SL, Beckenkamp A, Martins BS, et al. Bromelain-functionalized Multiple-Wall lipid-Core Nanocapsules: formulation, chemical structure and Antiproliferative effect against human breast Cancer cells (MCF-7). Pharm Res. 2017;34(2):438–52.
Mayer FQ, Adorne MD, Bender EA, De Carvalho TG, Dilda AC, Beck RCR, et al. Laronidase-functionalized multiple-wall lipid-core nanocapsules: promising formulation for a more effective treatment of mucopolysaccharidosis type I. Pharm Res. 2015;32(3):941–54.
Cavalcante MF, Kazuma SM, Bender EA, Adorne MD, Ullian M, Veras MM, et al. A nanoformulation containing a scFv reactive to electronegative LDL inhibits atherosclerosis in LDL receptor knockout mice. Eur J Pharm Biopharm. 2016;107:120–9.
Antonow MB, Franco C, Prado W, Beckenkamp A, Silveira GP, Buffon A, et al. Arginylglycylaspartic acid-surface-functionalized doxorubicin-loaded lipid-Core nanocapsules as a strategy to target alpha(V) Beta(3) integrin expressed on tumor cells. Nanomaterials. 2018;8(1):1–18.
De Oliveira CP, Büttenbender SL, Prado WA, Beckenkamp A, Asbahr AC, Buffon A, et al. Enhanced and selective Antiproliferative activity of methotrexate-functionalized-Nanocapsules to human breast Cancer cells (MCF-7). Nanomaterials. 2018;8(1):1–19.
Blum RH, Carter SK. Adriamycin. A new anticancer drug with significant clinical activity. Ann. Intern. Med. 1974;80(2):249–59.
Wang J, Liu W, Tu Q, Wang J, Song N, Zhang Y, et al. Folate-decorated hybrid polymeric nanoparticles for chemically and physically combined paclitaxel loading and targeted delivery. Biomacromolecules. 2011;12(1):228–34.
Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J Pharm Sci. 2005;94(10):2135–46.
Vaskivuo L, Rysä J, Koivuperä J, Myllynen P, Vaskivuo T, Chvalova K, et al. Azidothymidine and cisplatin increase p14ARF expression in OVCAR-3 ovarian cancer cell line. Toxicol Appl Pharmacol. 2006;216(1):89–97.
Zhao ZF, Wang K, Guo FF, Lu H. Inhibition of T24 and RT4 human bladder Cancer cell lines by heterocyclic molecules. Med Sci Monit. 2017;23:1156–64.
Cé R, Marchi JG, Bergamo VZ, Fuentefria AM, Lavayen V, Guterres SS, et al. Chitosan-coated dapsone-loaded lipid-core nanocapsules: growth inhibition of clinical isolates, multidrug-resistant Staphylococcus aureus and Aspergillus ssp. colloids and surfaces a: Physicochem. Eng. Aspects. 2016;511(20):153–61.
Bulcao RP, De Freitas FA, Venturini CG, Dallegrave E, Durgante J, Goethel G, et al. Acute and subchronic toxicity evaluation of poly(ɛ-Caprolactone) lipid-Core Nanocapsules in rats. Toxicol Sci. 2013;132(1):162–76.
Poletto FS, Fiel LA, Lopes MV, Schaab G, Gomes AMO, Guterres SS, et al. Fluorescent-labeled poly(ε-caprolactone) lipid-core nanocapsules: synthesis, physicochemical properties and macrophage uptake. J Colloid Sci Biotechnol. 2012;1(1):89–98.
Missirlis D, Kawamura R, Tirelli N, Hubbell JA. Doxorubicin encapsulation and diffusional release from stable, polymeric, hydrogel nanoparticles. Eur J Pharm Sci. 2006;29(2):120–9.
BRAZIL. Anvisa. Resolução RDC n°166, de 24 de julho de 2017. Dipõe sobre a validação de métodos analíticos e de outras providências. Publicada no DOU n°141, de 25 de julho de 2017.
ICH. Q2B (R1), 2005. Validation of Analytical Procedures: Methodology.
Oliveira CP, Venturini CG, Donida B, Poletto FS, Guterres SS, Pohlmann AR. An algorithm to determine the mechanism of drug distribution in lipid-core nanocapsule formulations. Soft Matter. 2013;9(4):1141–50.
Econ 2370, 2000 - Statistics and Probability.http://www.uh.edu/~odonnell/econ2370/moment.pdf
Cruz L, Soares LU, Costa TD, Mezzalira G, Silveira NP, Guterres SS, et al. Diffusion and mathematical modeling of release profiles from nanocarriers. Int J Pharm. 2006;313(1–2):198–205.
Poletto FS, Jäger E, Cruz L, Pohlmann AR, Guterres SS. The effect of polymeric wall on the permeability of drug-loaded nanocapsules. Mater Sci Eng C. 2008;28(4):472–8.
Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36.
Bender EA, Adorne MD, Colomé LM, Abdalla DSP, Guterres SS, Pohlmann AR. Hemocompatibility of poly(ɛ-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int J Pharm. 2012;426(1–2):271–9.
Mosqueira VCF, Legrand P, Pinto-alphandary H, Puisieux F, Barratt G. Poly(D,L-lactide) nanocapsules prepared by a solvent displacement process: Influence of the composition on physicochemical and structural properties. J Pharm Sci 2000;89(5):614–626, Poly(D,L‐Lactide) Nanocapsules Prepared by a Solvent Displacement Process: Influence of the Composition on Physicochemical and Structural Properties.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Slocum TL, Deupree JD. Interference of biogenic amines with the measurement of proteins using bicinchoninic acid. Anal Biochem. 1991;195(1):14–7.
Peng X, Wickham J, Alivisatos AP. Kinetics of II-VI and III-V colloidal semiconductor Nanocrystal growth: “focusing” of size distributions. J, am. Chem Soc. 1998;120(21):5343–4.
Granqvist C, Schiavone L. Ultrafine metal particles. Appl Phys Lett. 1976;47(5):2200.
Cárdenas G, Oliva R. Ni–cu bimetallic colloids prepared in nonaqueous solvents. Colloid Polym Sci. 2003;281(6):497–504.
Karnezis PA, Durrant G, Cantor B. Characterization of reinforcement distribution in cast Al-alloy/SiCp composites. Mater Charact. 1998;40(2):97–109.
Qi G, Wang Y, Estevez L, Duan X, Anako N, Park AHA, et al. High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy Environ Sci. 2011;4(2):444–52.
Wiens M, Elkhooly TA, Schröder HC, Mohamed THA, Müller WEG. Characterization and osteogenic activity of a silicatein/biosilica-coated chitosan-graft-polycaprolactone. Acta Biomater. 2014;10(10):4456–64.
Falahat R, Wiranowska M, Toomey R, Alcantar N. ATR-FTIR analysis of spectral and biochemical changes in glioma cells induced by chlorotoxin. Vib Spectrosc. 2016;87:164–72.
Barth A. Infrared spectroscopy of proteins. Biochim Biophys Acta. 2007;1767(9):1073–101.
Gong YC, Xiong XY, Ge XJ, Li ZL, Li YP. Effect of the Folate ligand density on the targeting property of Folated-conjugated polymeric nanoparticles. Macromol Biosci. 2018;19(2):1–11.
Roger E, Kalscheuer S, Kirtane A, Guru BR, Grill AE, Whittum-Hudson J, et al. Folic acid functionalized nanoparticles for enhanced Oral drug delivery. Mol Pharm. 2012;9(7):2103–10.
Butzbach K, Konhäuser M, Fach M, Bamberger DN, Breitenbach B, Epe B, et al. Receptor-mediated uptake of folic acid-functionalized dextran nanoparticles for applications in photodynamic therapy. Polymers. 2019;11(5):1–12.
Li X, Szewczuk MR, Malardier-Jugroot C. Folic acid-conjugated amphiphilic alternating copolymer as a new active tumor targeting drug delivery platform. Drug Design, Development and Therapy. 2016;15(10):4101–10.
Antonow MB, Asbahr ACC, Raddatz P, Beckenkamp A, Buffon A, Guterres SS, et al. Liquid formulation containing doxorubicin-loaded lipid-core nanocapsules: cytotoxicity in human breast cancer cell line and in vitro uptake mechanism. Mater Sci Eng C. 2017;1(76):374–82.
Ak G, Yilmaz H, Güneş A, Sanlier SH. In vitro and in vivo evaluation of folate receptor targeted a novel magnetic drug delivery system for ovarian cancer therapy. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46(1):S926–37.
Fazilati M. Folate decorated magnetite nanoparticles: synthesis and targeted therapy against ovarian cancer. Cell Biol Int. 2014;38(2):154–63.
Zhou DH, Zhang G, Yu QS, Gan ZH. Folic acid modified polymeric micelles for Intravesical instilled chemotherapy. Chinese J Polym Sci. 2017;36(1):479–87.
Yu Q, Zhang J, Zhang G, Gan Z. Synthesis and functions of well-defined polymer-drug conjugates as efficient Nanocarriers for Intravesical chemotherapy of bladder Cancer. Macromol Biosci. 2015;15(4):509–20.
Schultze E, Buss J, Coradini K, Begnini KR, Guterres SS, Collares T, et al. Tretinoin-loaded lipid-Core Nanocapsules overcome the triple-negative breast Cancer cell resistance to Tretinoin and show synergistic effect on cytotoxicity induced by doxorubicin and 5-fluororacil. Biomed Pharmacother. 2017;96:404–9.
Lin SY, Lee WR, Su YF, Hsu SP, Lin HC, Ho PY, et al. Folic acid inhibits endothelial cell proliferation through activating the cSrc/ERK 2/NF-kappaB/p53 pathway mediated by folic acid receptor. Angiogenesis. 2012;15(4):671–83.
Kuo CT, Chang C, Lee WS. Folic acid inhibits COLO-205 colon cancer cell proliferation through activating the FRalpha/c-SRC/ERK1/2/NFkappaB/TP53 pathway: in vitro and in vivo studies. Sci Rep. 2015;9(5):1–13.
Liu Z, Jin X, Pi W, Liu S. Folic acid inhibits nasopharyngeal cancer cell proliferation and invasion via activation of FRα/ERK1/2/TSLC1 pathway. Biosci Rep. 2017;37(6):1–11.
Gurunathan S, Kang MH, Qasim M, Kim JH. Nanoparticle-mediated combination therapy: two-in-one approach for Cancer. Int J Mol Sci. 2018;19(10):1–37.
Tomlinson B, Lin TY, Dall'Era M, Pan CX. Nanotechnology in bladder cancer: current state of development and clinical practice. Nanomedicine (Lond). 2015;10(7):1189–201.
Gurunathan S, Han JW, Kim ES, Park JH, Kim JH. Reduction of graphene oxide by resveratrol: a novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int J Nanomedicine. 2015;15(10):2951–69.
Gurunathan S, Kim JH. Graphene oxide–silver nanoparticles Nanocomposite stimulates differentiation in human Neuroblastoma Cancer cells (SH-SY5Y). Int J Mol Sci. 2017;18(12):1–23.
Wang L, Jia E. Ovarian cancer targeted hyaluronic acidbased nanoparticle system for paclitaxel delivery to overcome drug resistance. Drug Delivery. 2016;23(5):1810–7.
Yallapu MM, Maher DM, Sundram V, Bell MC, Jaggi M, Chauhan SC. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. Journal of Ovarian Research. 2010;3(11):1–12.
Larson N, Yang J, Ray A, Cheney DL, Ghandehari H, Kopecek J. Biodegradable multiblock poly(N-2-hydroxypropyl)methacrylamide gemcitabine and paclitaxel conjugates for ovarian cancer cell combination treatment. Int J Pharm. 2013;454(1):435–43.
Gao X, Wang B, Wei XW, Men K, Zheng F, Zhou Y, et al. Anticancer effect and mechanism of polymer micelle encapsulated quercetin on ovarian cancer. Nanoscale. 2012;4(22):7021–30.
Chang LC, Wu SC, Tsai JW, Yu TJ, Tsai TR. Optimization of epirubicin nanoparticles using experimental design for enhanced intravesical drug delivery. Int J Pharm. 2009;376(1–2):195–203.
Guo H, Qian H, Idris NM, Zhang Y. Singlet oxygen-induced apoptosis of cancer cells using upconversion fluorescent nanoparticles as a carrier of photosensitizer. Nanomedicine. 2010;6(3):486–95.
Miao L, Guo S, Zhang J, Kimb WY, Huang L. Nanoparticles with precise Ratiometric co-loading and co-delivery of gemcitabine monophosphate and Cisplatin for treatment of bladder Cancer. Adv Funct Mater. 2014;24(42):1–11.
Chen G, He Y, Wu X, Zhang Y, Luo C, Jing P. In vitro and in vivo studies of pirarubicin-loaded SWNT for the treatment of bladder cancer. Braz J Med Biol Res. 2012;45(8):771–6.
Buss JH, Begnini KR, Bruinsmann FA, Ceolin T, Sonego MS, Pohlmann AR, et al. Lapatinib-loaded Nanocapsules enhances Antitumoral effect in human bladder Cancer cell. Front Oncol. 2019;9(9):1–10.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1389 kb)
Rights and permissions
About this article
Cite this article
Cé, R., Lavayen, V., Couto, G.K. et al. Folic Acid-Doxorubicin-Double-Functionalized-Lipid-Core Nanocapsules: Synthesis, Chemical Structure Elucidation, and Cytotoxicity Evaluation on Ovarian (OVCAR-3) and Bladder (T24) Cancer Cell Lines. Pharm Res 38, 301–317 (2021). https://doi.org/10.1007/s11095-021-02989-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-02989-y