Abstract
Purpose
The present study aims to prepare poly(D,L-lactic acid) (PLA) nanofibers loaded by the immunosuppressant cyclosporine A (CsA, 10 wt%). Amphiphilic poly(ethylene glycol)s (PEG) additives were used to modify the hydrophobic drug release kinetics.
Methods
Four types of CsA-loaded PLA nanofibrous carriers varying in the presence and molecular weight (MW) of PEG (6, 20 and 35 kDa) were prepared by needleless electrospinning. The samples were extracted for 144 h in phosphate buffer saline or tissue culture medium. A newly developed and validated LC-MS/MS method was utilized to quantify the amount of released CsA from the carriers. In vitro cell experiments were used to evaluate biological activity.
Results
Nanofibers containing 15 wt% of PEG showed improved drug release characteristics; significantly higher release rates were achieved in initial part of experiment (24 h). The highest released doses of CsA were obtained from the nanofibers with PEG of the lowest MW (6 kDa). In vitro experiments on ConA-stimulated spleen cells revealed the biological activity of the released CsA for the whole study period of 144 h and nanofibers containing PEG with the lowest MW exhibited the highest impact (inhibition).
Conclusions
The addition of PEG of a particular MW enables to control CsA release from PLA nanofibrous carriers. The biological activity of CsA-loaded PLA nanofibers with PEG persists even after 144 h of previous extraction. Prepared materials are promising for local immunosuppression in various medical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyclosporine A (CsA) is a widely-used immunosuppressive drug prescribed particularly for patients undergoing organ transplantation. However, systemic CsA therapy is connected with serious side effects, such as renal dysfunction, hypertension, malignancies or liver impairment. The effects are dose-dependent. Thus, transplant patients are highly susceptible to the side effects, as their treatment requires high doses that are administered for long time periods (1). The local administration of CsA has the potential to reduce the incidence of adverse reactions connected with its systemic administration. CsA encapsulated in liposomes has been studied by several authors (2–4) to enhance its therapeutic effect on ocular diseases, such as dry eye syndrome. To control CsA release and enhance its oral bioavailability, self-nanoemulsifying drug delivery systems based on osmotic pump tablets have been proposed (3). Other studies have been focused on the cellular toxicity, release kinetics and tissue penetration in rats using CsA-loaded poly(lactide-co-glycolide) (PLGA) nanoparticles (4,5).
CsA can be quantified by immunoassays and high-performance liquid chromatography (HPLC). The main disadvantage of immunoassays is the cross-reaction of monoclonal antibodies with CsA metabolites, which leads to positive bias in CsA blood levels that may increase the risk for the patient because of the narrow therapeutic index of CsA (6,7). Therefore, HPLC is widely applied in the analyses of CsA using UV (8–10) or mass spectrometric detection (11–13). Recently liquid chromatography methods in combination with tandem mass spectrometric detection (LC-MS/MS) have been applied for the determination of CsA in human and animal biological samples (14–16).
Nanofibers formed from natural and synthetic polymers are perspective materials for use in various biological fields (17). In medicine, nanofibers can be used for the facilitation of wound healing (18,19), drug delivery systems (20–22) or scaffolds for tissue engineering or cell-based therapy (23–26). The application of nanofibers based on poly(D,L-lactic acid) (PLA), poly(ε-caprolactone), polyurethane, or PLGA as drug carriers for various antibiotics has been tested (27–29). Polymeric materials are particularly useful because of their biocompatibility and ability to adjust the degradation rate, physical, and mechanical properties over a wide range by varying the morphology and chemical composition of the co(polymers).
PLA nanofibers were loaded with CsA to study their pharmacological activity, release profile in culture medium and the ability of these nanofibers to serve as scaffolds in cell-based therapy (25). The release experiments proved that these nanofibers with CsA had pharmacological activity for at least 96 h, with the highest amount released during the first 12 h. However, the total amount of released CsA was relatively low because of the hydrophobic character of the drug.
It has been shown that the properties of drug-loaded nanofibers and the release profile of hydrophobic drugs can be modified by varying the composition of the polymer solution during nanofiber preparation, particularly through the addition of the amphiphilic polymer poly(ethylene glycol) (PEG) (30–32). The influence of PEG added to paclitaxel-loaded PLGA thin films on the release kinetics of paclitaxel was observed (31). The development of biocompatible nanofibers serving as a scaffold and carrier of drugs with adjustable release kinetics controlled by additive molecules represents a promising approach for local drug administration, especially in the case of hydrophobic compounds.
The aim of this study was to assess the modification of the release profile of the hydrophobic immunosuppressive drug CsA from electrospun PLA nanofibers produced by needleless electrospinning. First, a HPLC-MS/MS method suitable for the quantitation of CsA in release medium was developed and validated. Secondly, the extraction of CsA from PLA nanofibers was performed with nanofibers with and without PEG with different MWs, and the kinetics of CsA release was a) monitored by HPLC-MS/MS method and b) detected by a bioassay.
Materials and Methods
Materials
PLA was purchased from Nature Works, LLC (Minnetonka, MN, USA). PEGs with MW 6 and 20 kDa were purchased from Rapp Polymere GmbH (Tuebingen, Germany), and 35 kDa PEG was purchased from Sigma-Aldrich (Darmstadt, Germany). CsA was purchased from TEVA Czech Industries (Opava, Czech Republic). The chemical structures of used components are shown in Fig. 1. Chloroform, 1,2-dichloroethane and ethyl acetate were purchased from PENTA (Prague, Czech Republic). Methanol (LC-MS grade), ammonium acetate (p.a.), and phosphate buffered saline (PBS) were purchased from Sigma-Aldrich (Darmstad, Germany).
Preparation and Characterization of Nanofibers
The nanofibers were prepared by needleless electrospinning (Nanovia Ltd., Litvinov, Czech Republic) using Nanospider™ technology (33) (see illustrative photo on Fig. 2). Polymer PLA (7 wt%) was dissolved in chloroform (54 wt%), and then, two other solvents, 1,2-dichloroethane (29 wt%) and ethyl acetate (10 wt%), were added to this solution. The CsA and PEG were added to the polymer solution at a concentration of 10 wt% and 15 wt%, respectively. Both, PEG and CsA were soluble in polymer solution and homogeneous mixtures were obtained before electrospinning. The electrospinning parameters were as follows: the distance between the electrode and collector 22 cm, voltage 20–60 kV, relative humidity 25–30% and temperature 20°C. The following five nanofibrous materials were prepared: blank PLA, CsA-loaded PLA, and three CsA-loaded PLA with 15 wt% PEG of MWs 6, 20, and 35 kDa. The area weight of nanofibrous materials was ranging from 8.5 to 11.5 g/m2. The morphology of the nanofibers was analyzed using scanning electron microscopy (SEM), microscope TS 5130 VEGA (TESCAN, Brno, Czech Republic). The samples were dried at laboratory temperature under vacuum overnight and platinum sputtered. The fiber diameters were determined by measuring the diameter of at least 30 fibers from each SEM micrograph at a magnification of 1000x using software ImageJ. The fiber distribution was calculated as frequency of the fiber diameters and expressed in percentage.
The chemical structure of the nanofibers was analyzed by FT-IR spectroscopy. FT-IR spectra were recorded on a Perkin – Elmer Paragon 1000PC FT-IR spectrometer using the reflective ATR (Attenuated total reflection) technique Specac MKII Golden Gate Single Reflection ATR System with a diamond crystal with the angle of incidence 45°. All spectra were measured at wavenumber range 4400–450 cm−1 with resolution 4 cm−1 and with 32 scans. Software Spectrum v2.00 was used for processing the spectra. The samples were directly put on the diamond crystal and measured.
Loading Experiments
The CsA was added to the polymer solution in concentration of 10 wt% (relative to the PLA polymer) before electrospinning. To determine CsA amounts loaded to the prepared nanofibrous materials the extraction experiments using methanol (in which CsA is soluble) were done. CsA-loaded nanofibers were immersed into methanol for 48 h. The concentration of extracted CsA was quantified using HPLC method. The HPLC conditions were similar to those used for the determination of CsA release kinetics to PBS solution which are described in detail below (with exception of m/z 1202.9 → m/z 155.9 fragmentation used for methanolic extract of CsA). The concentration of CsA in nanofibrous materials quantified by HPLC was calculated to be 10 wt%, i.e. it is in agreement with the amount of CsA present in the initial electrospun mixture.
Optimization and Validation of HPLC-MS/MS Method
The HPLC-MS/MS analyses were performed on an Agilent 6400 Series Triple Quadrupole LC/MS System (Agilent Technologies, Waldbronn, Germany) containing a quaternary pump coupled with a Triple Quad 6460 tandem mass spectrometer. For data acquisition, the Mass Hunter Workstation software was used. The HPLC-MS/MS method was optimized for the quantitation of CsA released into PBS solution. An XSelect CSH Phenyl-Hexyl XP column (100 x 4.6 mm, particle size 2.5 μm) from Waters (Milford, MA, USA) was used with the mobile phase containing methanol/5 mM ammonium acetate (pH 4.5). The run consisted of a fast gradient from 90–100% methanol over 0 to 2 min followed by 100% methanol from 2 to 5.5 min with a flow rate of 0.7 ml/min. The temperature of the column was kept at 45°C, the samples were maintained at 20°C and the injection volume was 5 μL.
The mass spectrometric detection was performed in selected reaction monitoring mode using electrospray ionization in positive mode. Nitrogen was used as the collision, nebulizing and desolvating gas. The optimized values were performed with a fragmentor voltage of 300 V, collision energy of 60 V, drying gas flow rate of 10 L/min, drying gas temperature of 350°C, and nebulizer gas flow rate of 50 psi. The recorded fragmentation was m/z 1224.8 → m/z 1112.8. The molecular ion was detected as a sodium adduct of CsA [M + Na]+, which confirms the stability of CsA during its incorporation into the nanofibers and the product ion was detected as a molecular ion with loss of the side chain MeBmt [MNa–C7H13O]+. The optimized HPLC-MS/MS system provided satisfactory ionization of CsA, acceptable retention time (3.0 min) and favorable peak shape. Compared to the C18 stationary phase, which has frequently been used for the analysis of CsA (12,13) these conditions showed better results.
The stock solution of CsA standard was prepared by dissolving 10 mg of CsA in 10 mL of methanol. Due to necessity to measure calibration dependence of CsA in PBS and poor solubility of CsA in polar solvents, the working solution at concentration of 20 μg/mL was prepared by diluting the stock solution in the mixture of methanol and water (6/4, v/v). After that it was possible to dilute working solution solely with PBS to obtain calibration standards and standard solutions for assessment of validation parameters.
The data obtained for the dependence of peak areas versus concentrations showed linearity (y = 2.92x - 1.71, R 2 = 0.9998) in the whole measured calibration range from 10 ng/mL to 1 μg/mL. Relative standard deviations (n = 5) varied from 0.41 to 5.71%. The limit of detection (determined as three times ratio signal to noise) was 3 ng/mL, the limit of quantitation (determined as ten times ratio signal to noise) was 10 ng/mL. The accuracy, precision and repeatability were measured at three concentration levels (10, 100 and 1000 ng/mL). Intra-day and inter-day accuracy and precision (each, n = 5) were determined by repeatedly assaying samples on the same day and on two consecutive days, respectively. The results are summarized in Table I. The data obtained confirm that the method developed is selective, precise, linear, sensitive, and hence stands validated. Selectivity of the method was conducted by comparing chromatograms of i) the blank obtained by extraction of pure PLA nanofibers, ii) the blank spiked with CsA and iii) the sample obtained by extraction of PLA nanofibers containing CsA with addition of PEG (35 kDa) and no substances in extract interfered with CsA.
Cyclosporine A Release Experiments in PBS
CsA release experiments were conducted in a tempered shaker, an Orbital Shaker-Incubator ES-20 (Biosan, Riga, Latvia) at 37°C and 140 rpm. Five round targets (diameter 12.5 mm) were cut from the CsA-loaded nanofibrous materials with approximately 10 g/m2 area weight for each experiment. All release experiments were performed in triplicate. Kinetic studies of CsA release were conducted in a mode with continuous exchange of a part of the solution as follows: five targets were put in 10 mL of PBS. At the given time period, 4 mL of the solution were removed for the analysis of CsA content, and 4 mL of fresh PBS were added into the extraction bottles. The aliquots were collected at 0.5, 1, 2, 3.5, 6.5, 10, 24, 72 and 144 h after immersion. The kinetic studies of the CsA release were performed for four different types of CsA-loaded PLA nanofibers varying in presence and MW of the added PEGs. Blank PLA nanofibers were used as a reference.
Cyclosporine A Release in Tissue Culture Medium
Samples of nanofibers (3 x 3 mm size, 10 g/m2 area weight, 10 wt% CsA, with or without PEG) were soaked in wells in a 24-well tissue culture plate (Corning, Inc., Corning, NY, USA) with 600 μL of RPMI 1640 medium (Sigma, St. Louis, MO, USA) with antibiotics (100 U/mL of penicillin and 100 μg/mL of streptomycin) at laboratory temperature. The nanofibers were repeatedly transferred after 0.5, 1, 2, 3.5, 6.5, 10, 24, 48, 72 and 144 h into new wells containing fresh medium. The supernatants from the individual wells after each transfer were harvested, stored at −20°C and tested with a bioassay for the inhibition of interleukin-2 (IL-2) production by Concanavalin (ConA)-activated mouse spleen cells.
Inhibition of IL-2 Production by CsA Released from Nanofibers
Single cell suspensions of spleen cells from BALB/c mice were prepared in RPMI 1640 medium (Sigma) containing 10% fetal calf serum (Sigma-Aldrich, Darmstadt, Germany), antibiotics (100 U/mL of penicillin and 100 μg/mL of streptomycin), 10 mmol/L HEPES buffer and 5 x 10−5 mol/L 2-mercaptoethanol. The cells (0.6 x 106/mL) were cultured in a volume of 500 μL of complete RPMI 1640 medium in 48-well tissue culture plates (Corning, NY), and they were left unstimulated or stimulated with 1.0 μg/mL of ConA (Sigma-Aldrich, Darmstadt, Germany). To determine pharmacological activity of CsA released from the nanofibers, the supernatants obtained after the extraction of the nanofibers were added to the cell cultures at a final concentration of 25 vol%. The concentrations of IL-2 in the cell culture supernatants after 24-h incubation were assessed by an enzyme-linked immunosorbent assay (ELISA) using capture and detection anti-cytokine antibodies purchased from PharMingen (San Diego, CA, USA) following the instructions of the manufacturer.
Determination of Immunosuppressive Properties of CsA-Loaded Nanofibers after their Extraction in PBS or Culture Medium
Nanofibers containing 0 wt% CsA, 10 wt% CsA and 10 wt% CsA with PEGs with a MW of 6, 20 or 35 kDa were extracted in PBS or culture medium for 144 h as described above. The samples of the extracted nanofiber material were transferred into wells of spleen cells (0.6 x 106/mL) stimulated with ConA (1.0 μg/mL). The concentrations of IL-2 in the cell culture supernatants were assessed after a 24-h incubation period using an ELISA.
Statistical Analysis
The results are expressed as the mean values ± SD. Comparisons between two groups were analyzed by Student’s t test, and multiple comparisons were analyzed by an ANOVA. A value of p < 0.05 was considered statistically significant.
Results and Discussion
Characterization of CsA-Loaded and PEG-Containing Nanofibers
Needleless electrospinning is a versatile method for the formation of fibers from tens of nanometers to tens of micrometers in diameter and with thicknesses (area weight) ranging from 1 to 100 g per m2. In this study, PLA nanofibrous materials with a mass per unit area of ca. 10 g/m2 were prepared.
Scannning Electron Microscopy Measurements
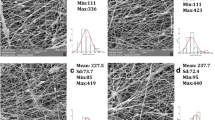
SEM measurements revealed that after the proper optimization of the electrospinning process parameters, PLA nanofibrous materials with reproducible morphologies were obtained. SEM images representing pure PLA nanofibers did not show any inhomogeneities or artifacts in the nanofibrous structures (see Fig. 3a). Figure 3b-e show the structure of CsA-loaded PLA nanofibers with or without the addition of PEG with different MWs. No considerable differences in the morphologies; i.e., no effect on nanofiber density, porosity or architecture; were observed. The homogeneous incorporation of the hydrophobic drug and auxiliary PEG molecules into the PLA nanofibers was confirmed.
The determination of fiber diameters from SEM images was processed within the distribution curves as shown in the graphs below the corresponding SEM images (see Fig. 3a-e). The overall range of fiber diameters was from 50 to 700 nm with the average fiber diameter from 165 to 300 nm. Within the connotation of nanotechnology, nanomaterials generally refer to a dimension less than 100 nm. However, in scientific literature dealing with fibers particularly for biomedical applications are fibers with diameters in the range of hundreds of nanometers (up to 1000 nm) referred as nanofibers (34,35). Quite narrow diameter distributions (from 100 to 300 nm, see Fig. 3a-c) exhibited the pure PLA nanofibers as well as CsA-loaded PLA and CsA-loaded PLA with PEG of the lowest MW (6 kDa). The slightly broader diameter distribution was found for nanofibers containing higher MW PEGs (20 and 35 kDa) (see Fig. 3d-e) which can be attributed to the changes of electrospinning conditions, i.e. the addition of these higher MW compounds may cause an increase of viscosity which is crucial parameter influencing the electrospinning process and final properties of nanofibers such as diameter.
FTIR Analysis
The successful incorporation of PEG, CsA, or both was checked by FT-IR spectroscopy. A set of representative FT-IR spectra are showed in Fig. 4. (The spectra for nanofibers containing PEG of 20 or 35 kDa were almost identical and therefore not shown). In all spectra the characteristic bands at 1752 and 1184 cm−1 associated with polyester PLA were observed. The presence of PEG is demonstrated by appearance of two new bands at 960 and 840 cm−1. In the case of CsA-loaded nanofibers the new band at 1640 cm−1 appeared corresponding to the amide groups in CsA structure.
The Kinetics of CsA Release in PBS Detected by HPLC-MS/MS
Four nanofibrous materials were studied. Each contained 10 wt% CsA, and three of them contained 15 wt% hydrophilic PEG with different MWs (6, 20 and 35 kDa). The kinetic release profiles of CsA were evaluated and compared. The continuous exchange mode was chosen to better simulate physiological conditions. The release rates of CsA in a particular time period were calculated according to Eq. (1):
where v is the release rate, m n is the amount of CsA (ng) in an aliquot collected at t n (h), m n-1 is the amount of CsA (ng) in an aliquot collected at t n-1 (h) reduced in regard to the exchanged part of the media and a is the square area of the nanofibrous targets (cm2).
The calculated release rates of CsA are summarized in Table II. The total cumulative amounts of released CsA in time course are shown in Fig. 5a, and the amounts of released CsA corresponding to the given time period are depicted in Fig. 5b. The results indicate that nanofibers containing PEGs significantly released higher amounts of CsA compared to the nanofibers containing CsA only. Apparently, the amount of CsA released increased, and the release profiles prolonged with the decreasing MW of the added PEGs.
In our previous work, we examined the release of PEGs with MWs of 2, 6, 10 and 20 kDa from PLA, polycaprolactone and polyvinyl alcohol fibers. With all the tested nanofibers, we observed higher release rates of PEGs with higher MW (36). This trend agrees with the results of a study by Huang et al. (32), who tested the effect of PEG of 0.4 and 10 kDa on the release of hydrophobic paclitaxel from PLGA films. Their experiments showed a higher release rate of the hydrophobic drug from nanofibers containing PEG of higher MW (10 kDa). PEG of low MW, such as 0.4 kDa, were found to be less effective in drug release modification. Steele et al. incorporated PEGs of 8 and 35 kDa into PLGA films at various contents to moderate paclitaxel release (31). Their experiments with the same percentage of added PEGs (15 wt%) as in our study showed a constant hydrophobic drug release for 30 days. In the case of PEG of 35 kDa, there was an observed burst release within the first 2 days approximately, which was explained by the higher crystallinity of PEG of 35 kDa, the phase separation of the polymer matrix and the subsequent fast release of PEG with paclitaxel into the aqueous media. However, in our system, this effect was not observed. We suppose that it can be caused by different preparation procedure, resulting in different polymer matrix morphology, i.e. polymer films versus nanofibers. PLA fibrous materials with relatively high surface areas are created by electrospinning, and faster solvent evaporation leads to lower crystalline formations. The other electrospinning parameters, such as temperature or the distance of electrodes, influence solvent evaporation and final polymer matrix behavior. Apparently, also interaction of additive molecules (PEG) with the releasing hydrophobic drug also plays an important role. With the similar weight concentration of PEGs in electrospun mixture, larger amount of molecules of PEG 6 kDa may lead to better solvation of hydrophobic drug molecules. This effect can increase and prolong the drug release.
The Kinetics of CsA Release in PBS and Culture Medium Detected by a Bioassay
To study the kinetics of CsA release from CsA-loaded nanofibers with or without PEG, samples of the nanofiber materials were soaked at laboratory temperature in PBS or RPMI 1640 medium, and at the indicated time intervals, the nanofibers were transferred into the same volume of fresh medium. The presence of pharmacologically active CsA released from the nanofibers was assessed using its ability to inhibit IL-2 production by ConA-activated spleen cells. As demonstrated in Fig. 6, a significant amount of CsA was detected in the media from nanofibers without PEG, which were harvested only at the very beginning of the extraction. However, in nanofibers containing PEGs, a biologically significant amount of CsA was released within 0.5 h, and the profound release continued for the whole observation period (144 h). These biological data agree with the results determined by HPLC-MS/MS. Thus, the higher released amount of CsA corresponds to a lower ability of IL-2 production by ConA-stimulated spleen cells (Fig. 5b versus Fig. 6a).
The kinetics of the CsA release from nanofibers detected by a bioassay. CsA-free and CsA-loaded nanofibers with or without PEGs were soaked in (a) PBS or (b) culture medium for the indicated time intervals. The samples with extracted CsA were harvested and tested for their ability to inhibit IL-2 production by Con A-stimulated spleen cells.
To demonstrate the ability of PEG-containing nanofibers to release the drug, even after a 6-day extraction in PBS or in culture medium (with a regular exchange of the solution), the samples of CsA-free nanofibers and nanofibers with 10 wt% CsA or with 10 wt% CsA and PEGs of different MWs were extracted for 144 h and then added to the cultures of ConA-stimulated spleen cells. The production of IL-2 was determined by an ELISA. As shown in Fig. 7, CsA-free samples or CsA-loaded nanofibers without PEGs had not a considerable effect on IL-2 production, while the samples of nanofibers containing CsA and PEGs significantly inhibited the production of IL-2. It was confirmed by the values with asterisks which are significantly different (*P˂ 0.001) from the control represented by ConA-stimulated spleen cells. In accordance with the results above, the highest inhibition of IL-2 production (corresponding to the highest CsA release) was detected in samples containing PEG with the lowest MW (6 kDa). These results clearly demonstrate that nanofibers extracted for 144 h still retain, and continue to release a sufficient amount of CsA to inhibit production of IL-2 by ConA-activated T cells. It is important that CsA-loaded nanofibers containing PEG with the lowest MW, which have the highest release of CsA at the beginning of extraction, preserve the highest release also after the prolonged (144 h) incubation period.
The persistence of a significant drug release from PEG-containing nanofibers after the extraction of nanofibers (containing 0 wt% CsA, 10 wt% CsA or 10 wt% CsA and PEG of 6 kDa, 20 kDa or 35 kDa) in PBS or culture medium. Each bar represents the mean ± SD from 3 experiments. The values with an asterisk represent the significant inhibition of IL-2 production (*P < 0.001).
It has been demonstrated that concentrations of the drug as low as 50–200 ng/g of tissue are sufficient to suppress T-cell reactivity and inflammation (37). We have recently shown that the amount of CsA released from CsA-loaded nanofibers without PEG, applying onto damage ocular surface, is sufficient to suppress a rapid inflammatory reaction developing shortly after tissue damage (38). A rapid CsA release can be beneficial to attenuate rapidly developing immune response after allogeneic transplantation as it was demonstrated in mouse model. Covering skin allografts with nanofibers containing CsA attenuates a local rejection reaction and modifies mechanisms of transplantation reaction (39). Thus, the advantages of described CsA-loaded nanofibers containing PEG additives can be seen in the enhanced drug release efficacy and the kinetic of drug release might be useful for suppression of rapid inflammatory or transplantation reaction.
Conclusions
The release kinetics of cyclosporine A (CsA) were studied with four different types of CsA-loaded poly(D,L-lactide) (PLA) nanofibers. A newly developed HPLC-MS/MS method was proven to be simple, sensitive, reproducible, and suitable for the detection and quantitation of CsA in PBS release solution. The results showed that the release of drug from CsA-loaded nanofibers containing PEG was significantly higher in comparison with the PEG-free nanofibers. Particularly, the addition of PEG with the lowest MW (6 kDa) yielded promising results, significantly increased amounts of CsA and prolonged the release of CsA. It was found that the interaction of CsA with PEG plays an important role for moderating drug release. In vitro experiments on ConA-stimulated spleen cells revealed the biological activity of the PLA nanofibers with incorporated CsA, even after 144 h of previous extraction.
To the best of our knowledge, there are no studies concerning nanofibrous carriers of hydrophobic CsA using PEG molecules to moderate drug release. Our findings suggest possibilities for the application of CsA-loaded and PEG-containing nanofibers as an effective scaffold for cell-based therapies using allogeneic cells and are a promising tool for the local suppression of inflammatory reaction or immune response after transplantation.
Abbreviations
- ConA:
-
Concanavalin
- CsA:
-
Cyclosporine A
- HPLC:
-
High-performance liquid chromatography
- IL-2:
-
Interleukin-2
- LC-MS/MS:
-
Liquid chromatography tandem mass spectrometry
- MW:
-
Molecular weight
- PBS:
-
Phosphate buffer saline
- PEG:
-
Poly(ethylene glycol)
- PLA:
-
Poly(D,L-lactic acid)
- PLGA:
-
Poly(lactide-co-glycolide)
- SEM:
-
Scanning electron microscopy
References
Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993;24(6):472–95.
Karn PR, Kim HD, Kang H, Sun BK, Jin SE, Hwang SJ. Supercritical fluid-mediated liposomes containing cyclosporin A for the treatment of dry eye syndrome in a rabbit model: comparative study with the conventional cyclosporin A emulsion. Int J Nanomedicine. 2014;9:3791–800.
Zhang X, Yi Y, Qi J, et al. Controlled release of cyclosporine A self-nanoemulsifying systems from osmotic pump tablets: near zero-order release and pharmacokinetics in dogs. Int J Pharm. 2013;452(1–2):233–40.
Aksungur P, Demirbilek M, Denkbas EB, Vandervoort JTP, Ludwig A, Unlu N. Development and characterization of cyclosporine A loaded nanoparticles for ocular drug delivery: cellular toxicity, uptake, and kinetic studies. J Control Release. 2011;151(3):286–94.
Di Tommaso C, Bourges JL, Valamanesh F, et al. Novel micelle carriers for cyclosporin A topical ocular delivery: in vivo cornea penetration, ocular distribution and efficacy studies. Eur J Pharm Biopharm. 2012;81(2):257–64.
Ansermot N, Fathi M, Veuthey JL, Desmeules J, Rudaz S, Hochstrasser D. Quantification of cyclosporine and tacrolimus in whole blood. Comparison of liquid chromatography-electrospray mass spectrometry with the enzyme multiplied immunoassay technique. Clin Biochem. 2008;41(10–11):910–3.
Tszyrsznic W, Borowiec A, Pawlowska E, et al. Two rapid ultra performance liquid chromatography/tandem mass spectrometry (UPLC/MS/MS) methods with common sample pretreatment for therapeutic drug monitoring of immunosuppressants compared to immunoassay. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;928:9–15.
Ouyang J, Baeyens WR, Duan J, Delanghe J. Improvement of cyclosporin A determination in whole blood by reversed-phase high-performance liquid chromatography. Biomed Chromatogr. 2003;17(6):404–10.
Bonifacio FN, Giocanti M, Reynier JP, Lacarelle B, Nicolay A. Development and validation of HPLC method for the determination of Cyclosporin A and its impurities in Neoral capsules and its generic versions. J Pharm Biomed Anal. 2009;49(2):540–6.
Khoschsorur G, Semmelrock HJ, Rodl S, et al. Rapid, sensitive high-performance liquid chromatographic method for the determination of cyclosporin A and its metabolites M1, M17 and M21. J Chromatogr B Biomed Sci Appl. 1997;690(1–2):367–72.
Magni F, Pereira S, Leoni M, Grisenti G, Galli KM. Quantitation of cyclosporin A in whole blood by liquid chromatography/stable isotope dilution electrospray ionization mass spectrometry. J Mass Spectrom. 2001;36(6):670–6.
Zaater MF, Tahboub YR, Najib NM. Liquid chromatographic-electrospray mass spectrometric determination of cyclosporin A in human plasma. Anal Bioanal Chem. 2005;382(1):223–30.
Vollenbroeker B, Koch JH, Fobker M, Suwelack B, Hohage H, Muller U. Determination of cyclosporine and its metabolites in blood via HPLC-MS and correlation to clinically important parameters. Transplant Proc. 2005;37(4):1741–4.
Muller A, Jungen H, Iwersen-Bergmann S, Sterneck M, Andresen-Streichert H. Analysis of cyclosporin a in hair samples from liver transplanted patients. Ther Drug Monit. 2013;35(4):450–8.
Fang ZG, You BG, Chen YG, et al. Analysis of cyclosporine A and its metabolites in rat urine and feces by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(15–16):1153–62.
Li AC, Li Y, Guirguis MS, Caldwell RG, Shou WZ. Advantages of using tetrahydrofuran-water as mobile phases in the quantitation of cyclosporin A in monkey and rat plasma by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2007;43(1):277–84.
Kai D, Liow SS, Loh XJ. Biodegradable polymers for electrospinning: towards biomedical applications. Mater Sci Eng C. 2014;45:659–70.
Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322–37.
Dubsky M, Kubinova S, Sirc J, et al. Nanofibers prepared by needleless electrospinning technology as scaffolds for wound healing. J Mater Sci Mater Med. 2012;23(4):931–41.
Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12–21.
Chou S-F, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. J Control Release. 2015;220(Part B):584–91.
Sebe I, Kallai-Szabo B, Zelko R, Szabo D. Polymers and formulation strategies of nanofibrous systems for drug delivery application and tissue engineering. Curr Med Chem. 2015;22(5):604–17.
Pelipenko J, Kocbek P, Kristl J. Critical attributes of nanofibers: preparation, drug loading, and tissue regeneration. Int J Pharm. 2015;484(1–2):57–74.
James R, Toti U, Laurencin C, Kumbar S. Electrospun Nanofibrous scaffolds for engineering soft connective tissues. Biomed Nanotechnol. 2011;726:243–58.
Holan V, Chudickova M, Trosan P, et al. Cyclosporine A-loaded and stem cell-seeded electrospun nanofibers for cell-based therapy and local immunosuppression. J Control Release. 2011;156(3):406–12.
Zajicova A, Pokorna K, Lencova A, et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transplant. 2010;19(10):1281–90.
Buschle-Diller G, Cooper J, Xie ZW, Wu Y, Waldrup J, Ren XH. Release of antibiotics from electrospun bicomponent fibers. Cellulose. 2007;14(6):553–62.
Chen DW, Liao JY, Liu SJ, Chan EC. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: an in vitro and in vivo study. Int J Nanomedicine. 2012;7:763–71.
Sirc J, Kubinova S, Hobzova R, et al. Controlled gentamicin release from multi-layered electrospun nanofibrous structures of various thicknesses. Int J Nanomedicine. 2012;7:5315–25.
Herrmann S, Winter G, Mohl S, Siepmann F, Siepmann J. Mechanisms controlling protein release from lipidic implants: effects of PEG addition. J Control Release. 2007;118(2):161–8.
Steele TW, Huang CL, Widjaja E, Boey FY, Loo JS, Venkatraman SS. The effect of polyethylene glycol structure on paclitaxel drug release and mechanical properties of PLGA thin films. Acta Biomater. 2011;7(5):1973–83.
Huang CL, Steele TWJ, Widjaja E, Boey FYC, Venkatraman SS, Loo JSC. The influence of additives in modulating drug delivery and degradation of PLGA thin films. NPG Asia Mater. 2013;5:e54.
Jirsák O, inventor, Lukáš D, Kotek V, Martinová L, Chaloupek J, assignees. A method of nanofibres production from a polymer solution using electrostatic spinning and a device for carrying out the method. United States patent 20060290031. 2004 Sept 8.
Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS. Electrospinning of nanofibers. J Appl Polym Sci. 2005;96:557–69.
Zhang Y, Lim CT, Ramakrishna S, Huang ZM. Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mater Sci-Mater Med. 2005;16:933–46.
Hrib J, Sirc J, Hobzova R, et al. Nanofibers for drug delivery - incorporation and release of model molecules, influence of molecular weight and polymer structure. Beilstein J Nanotechnol. 2015;6:1939–45.
Kaswan RL. Intraocular penetration of topically applied cyclosporine. Transplant Proc. 1988;20(2 Suppl 2):650–5.
Cejkova J, Cejka C, Trosan P, Zajicova A, Sykova E, Holan V. Treatment of alkali-injured cornea by cyclosporine A-loaded electrospun nanofibers - an alternative mode of therapy. Exp Eye Res. 2016;147:128–37.
Hajkova M, Javorkova E, Zajicova A, Trosan P, Holan V, Krulova M. A local application of mesenchymal stem cells and cyclosporine A attenuates immune response by a switch in macrophage phenotype. J Tissue Eng Regen Med. 2015;29 doi:10.1002/term.2044.
Acknowledgments and Disclosures
This work was supported by the Charles University in Prague [project number 307115 and SVV260440], the Grant Agency of the Czech Republic [project number 16-04863S] and the Ministry of Education, Youth and Sports of the Czech Republic within the National Sustainability Program II [Project BIOCEV-FAR LQ1604] and by the project “BIOCEV” [CZ.1.05/1.1.00/02.0109]. The authors thank to Nanovia Ltd. for cooperation in needleless electrospinning.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirc, J., Hampejsova, Z., Trnovska, J. et al. Cyclosporine A Loaded Electrospun Poly(D,L-Lactic Acid)/Poly(Ethylene Glycol) Nanofibers: Drug Carriers Utilizable in Local Immunosuppression. Pharm Res 34, 1391–1401 (2017). https://doi.org/10.1007/s11095-017-2155-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2155-x