The objective of the present research is to develop a reliable, sensitive, and robust stability-indicating RP-HPLC method to quantify doravirine along with its impurities (IMP-A, IMP-B). The separation was accomplished on a Inertsil ODS C18 (250 × 4.6 mm, 5 μm) column at 216 nm using 0.1% orthophosphoric acid and acetonitrile at a flow rate of 1 mL/min eluted with a retention time of IMP-A, IMP-B, and doravirine of 2.52 min, 3.96 min, and 8.74 min respectively. The optimized method was validated in terms of accuracy, precision, linearity, specificity, system suitability, and robustness as per International Council for Harmonization guidelines. The optimized method showed linearity within the concentration range 10–200 μg/mL, 0.7–14 μg/mL, and 0.5–10 μg/mL for doravirine, IMP-A, and IMP-B respectively. The method was found to be precise at %RSD < 2 and accurate with percentage recovery for doravirine, IMP-A, and IMP-B of 100.46%, 100.2%, and 99.2% respectively. The optimized method proved to be sensitive, robust, and specific. This optimized method can be routinely employed in quality control laboratories for the quantification of doravirine along with its impurities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
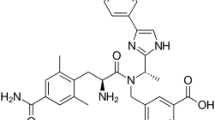
Doravirine, a pyridinone non-nucleoside transcriptase inhibitor chemically called 3-chloro-5-[1-[(4-methyl-5-oxo-1H-1,2,4-triazol-3-yl)methyl]-2-oxo-4-(trifluoromethyl)pyridin-3-yl]oxybenzonitrile (Fig. 1). Doravirine is used alone or along with other antiretroviral drugs for human immunodeficiency virus (HIV) infection in older patients with no history of prior antiretroviral treatment [1]. Doravirine exhibits action against HIV by inhibiting non-nucleoside reverse transcriptase (NNRT) enzyme. HIV generates complementary DNA (cDNA) to its RNA genome with the aid of a NNRT enzyme and inserts this cDNA into the host cell genome, where it can be transcribed into viral RNA for replication purposes [2, 3].
The development and production of quality drugs is a major challenge for pharmaceutical industries. During the formulation of various dosage forms, there may be a chance for the presence of unwanted chemicals entering through raw material or forming during production, or developing during storage, which may influence the quality, safety, and efficacy of pharmaceutical products [4,5,6,7,8]. Thus, it always requires the development of various analytical methods to determine and quantify the various impurities, even at trace level, using various analytical instruments such as spectroscopic, electrochemical, and chromatographic such as HPLC, LC-MS, and GC-MS [9].
The International Council for Harmonization (ICH) has developed various guidelines on impurities in new drug substances (ICH Q3A), new drug products (ICH Q3B), and in residual solvents (ICH Q3C) [10,11,12]. The US FDA has classified impurities and recommended the guidance prepared according to ICH guidelines. In the literature review, few RP-HPLC methods are available for the quantification of doravirine alone as a single entity [13, 14], combination with other antiretroviral agents [15,16,17], and an LC-MS method for the quantification of doravirine along with its impurities [18]. However, until now, there has been no RP-HPLC method available for quantification of doravirine along with its impurities. Two process-related impurities were found in doravirine and the present study includes the spiking of the doravirine pure drug with two of its impurities at a certain level, named IMP-A and IMP-B. The chemical names of impurity A (IMP-A) is 4-(trifluoromethyl)-1-((4,5-dihydro-4-methyl-5-oxo-1H-1,2,4-triazol-3-yl)methyl)-3-hydroxypyr idin-2(1H)-one and impurity B (IMP-B) is 3-chloro-benzonitrile. The chemical structures are shown in Fig. 1.
The main aim of the present work is to develop a simple, economical, rapid, reproducible, and sensitive RP-HPLC method to quantify doravirine along with impurities even at low concentrations in the bulk and pharmaceutical dosage form.
Experimental
Chemical and Reagents
Doravirine drug (99.98%) and reference standards of IMP-A (98.97%) and IMP-B (99.12%) were supplied by Merck Life Science pvt lmtd, Mumbai, India. Orthophosphoric acid (OPA; HPLC grade, Merck), acetonitrile (HPLC grade, Merck), and Milli Q water were also obtained.
Instrumentation
The present study was conducted on an HPLC system consisting of a Waters 2695 separation module (Waters Corporation, USA) furnished with a column thermostat, autosampler, and a Waters 2998 PDA detector. Separation and quantification of doravirine along with its impurities were done using an Inertsil ODS C18 (250 × 4.6 mm, 5 μm) column at 216 nm. Empower 2 software was employed for data collection and integration.
Preparation of Solution
The 0.1% OPA was prepared by dissolving 1 mL of OPA in 1 L of HPLC grade water and the resulting solution was filtered through a 0.45-μm membrane filter.
Preparation of Standard Solution
One milligram per milliliter, 0.07 mg/mL, and 0.05 mg/mL stock solutions of doravirine, IMP-A, and IMP-B were prepared respectively by weighing 10 mg of doravirine, 0.7 mg of IMP-A, and 0.5 mg of IMP-B in three 10-mL volumetric flasks separately and the volume was made up by using a mixture of acetonitrile and 0.1% OPA (40:60, v/v) as diluent. One milliliter of each solution from the above solutions was taken into a 10-mL volumetric flask and diluted to get a final concentration of 100 μg/mL, 7 μg/mL, and 5 μg/mL of doravirine, IMP-A, and IMP-B.
Preparation of Sample Solution (Doravirine)
Twenty tablets of Pifeltro were crushed to a fine powder form and powder equivalent to 10 mg of doravirine was taken in a 10-mL volumetric flask containing 7 mL of diluent, sonicated for 30 min to dissolve the contents, and made up to the mark with diluent.
Preparation of Spiked Sample Solution
One milliliter of unspiked sample solution and 1 mL of impurity solution containing 0.07 mg and 0.05 mg of IMP-A and IMP-B respectively were taken in a 10-mL volumetric flask, dissolved, and made up to the mark with diluent.
Method Validation
Validation of the optimized method was carried out by analyzing parameters such as system suitability, linearity, robustness, accuracy, precision, limit of detection, and limit of quantification as per ICH guidelines [19].
System Suitability
A system suitability test determines and ensures the suitability of the chromatographic system preceding use. System suitability was analyzed by six injections of a standard solution containing 100 μg/mL of doravirine, 7 μg/mL of IMP-A, and 5 μg/mL of IMP-B and was assessed in terms of theoretical plates, tailing factor, and selectivity.
Specificity
Specificity was analyzed by injecting the standard solution, sample solution, and spiked sample solution into the chromatography system. The resulting chromatograms were compared to check for any interference at the retention times of doravirine, IMP-A, and IMP-B.
Linearity and Range
Linearity was checked for standard solutions of doravirine and respective impurities at concentrations of 10% to 200% of standard concentration. Relevant aliquots of doravirine, IMP-A, IMP-B are prepared within the range 10 to 200 μg/mL, 0.7 to 14 μg/mL, and 0.5 to 10 μg/mL respectively. Linearity was analyzed by plotting a graph of peak area vs concentration. Intercept, slope, and correlation coefficient were calculated to confirm the linearity.
Precision
Precision is measured in terms of method precision and intermediate precision. Precision is determined by injecting the six spiked sample solutions containing doravirine (100 μg/mL), IMP-A (7 μg/mL), and IMP-B (5 μg/mL) and the response was recorded. Intermediate precision was checked by injecting six solutions of standard concentration for 3 successive days. Precision was checked at each level by calculating assay, standard deviation, and % relative standard deviation (RSD).
Accuracy
Accuracy was measured by preparing and injecting triplicates of three determinations at standard concentration levels of 50%, 100%, and 150%. Percentage recovery and %RSD were calculated for all nine determinations.
Limit of Detection and Limit of Quantification
Limit of detection (LOD) and limit of quantification (LOQ) were determined based on signal-to-noise ratio of 3:1 and 10:1 respectively.
Robustness
The robustness of the method was assessed by small, intentional changes in chromatography conditions such as flow rate and mobile phase composition. These alterations were checked for assay and %RSD. Robustness confirms that the method is reliable and stable to intentional changes in method parameters.
Forced Degradation
A forced degradation study was carried out for the spiked sample solution to confirm the stability-indicating nature of the developed method. Spiked sample solution was subjected to acidic, alkaline, oxidative, reductive, thermal, and photolytic degradation [16]. A forced degradation study was conducted for the sample solution containing 1000 μg of doravirine, 0.07 mg of IMP-A, and 0.05 mg of IMP-B under conditions 1N HCl, 1N NaOH, 30% H2O2, and 30% NaHSO4.
Acidic degradation was performed by transferring 1 mL of the above sample solution into a round bottom flask of 50-mL capacity. Then, 1 mL of 1N HCL was added and refluxed for 1 h at 60°C and neutralized with 1N NaOH. Basic degradation was performed by adding 1 mL of 1N NaOH separately to a round bottom flask containing 1 mL of sample solution. The resulting solution was refluxed for 1 h at 60°C and neutralized with 0.1N HCL. Oxidative degradation was conducted by adding 1 mL 30% H2O2 to a round bottom flask containing 1 mL sample solution and refluxed for 30 min at 60°C. Reductive degradation was conducted by adding 30% of NaHSO4 to 1 mL sample solution contained in a round bottom flask and refluxed for 30 min at 60°C. After the reflux resulting refluxed solutions are transferred into separate volumetric flasks of 10 mL capacity and made up to mark with diluent. A photolytic degradation study was conducted by exposing sample to sunlight for 3 h. Sample solution (100 μg of doravirine 7 μg of IMP-A, and 5 μg of IMP-B) was prepared from that solution. The resulting solution was injected at 12 h, 18 h, and 24 h into the chromatographic system.
Stability
To assess the stability of the solution, the sample solution was held at room temperature, 2 – 8°C and injected at 6 h, 12 h, 18 h, and 24 h. The resulting chromatograms were calculated for percentage assay. The assay results were compared with the calibrated results.
Results and Discussion
Various columns including X-bridge C18 (150 × 4.6 mm, 5 μm), Waters X-Terra C18 (250 × 4.6 mm, 5 μm), Inertsil ODS C18 (250 × 4.6 mm, 5 μm) and various mobile phases such as acetonitrile and 0.1% formic acid at (80:20, v/v) and (70:30, v/v), 0.1% TFA:ACN (50:50, V/V), (40:60, v/v), (30:70, v/v), and 0.1% OPA:ACN at (40:60, v/v), (45:55, v/v), (50:50, v/v), and (60:40, v/v) were tried to determine doravirine and its related impurities in bulk and in pharmaceutical dosage form. Finally, a mixture of 0.1% OPA:ACN (40:60, v/v) on an Inertsil ODS C18 (250 × 4.6 mm, 5 μm) at a flow rate of 1 mL/min with a runtime of 12 min at 216 nm, produced symmetrical peaks with good resolution. The retention times of doravirine, IMP-A, and IMP-B were 2.52 min, 3.96 min, and 8.74 min respectively. A chromatogram for the optimized method is shown in Fig. 2 and the developed conditions are tabulated in Table 1.
Method Validation
System Suitability
System suitability was analyzed in terms of resolution, theoretical plates, and tailing factor. All the parameters were found to be within the acceptance criteria (resolution 2, tailing factor <2, plate count >2000) as shown in Table 2.
Linearity and Range
The optimized method was found to be linear for doravirine, IMP-A, and IMP-B within the range 10–200 μg/mL (r2=0.999), 0.7–14 μg/mL (r2=0.999), and 0.5–10 μg/mL (r2=0.999) respectively (Fig. 3).The method was found to be sensitive. LOD and LOQ values of IMP-A, IMP-B, and doravirine are tabulated in Table 2.
Precision
Percentage RSD for intraday precision was found to be 0.77, 0.32, and 0.44 for doravirine, IMP-A, and IMP-B respectively. %RSD calculated for interday precision on 3 consecutive days was found to be within acceptable limits, which proves that the method is precise (Tables 3, 4).
Accuracy
Percentage recovery calculated by applying the standard addition method for doravirine, IMP-A, and IMP-B was 100.46 %, 100.2%, and 99.2% respectively. All experimental results are within the acceptable criteria. Hence, the developed method proved to be accurate. (Table 5)
Robustness
The robustness of the method was assessed by applying a ±0.2 mL/min change in flow rate and a ±5% change in the composition of the mobile phase. The consequent effect of variation was found by determining the assay and %RSD for the recovery of triplicate injections and comparing the tailing factor and theoretical plates with those of the optimized method. All results were found to be within acceptable limits. Hence, the optimized method proved to be robust and susceptible to deliberate variations in parameters (Table 6).
Specificity
Specificity was analyzed by peak purity analysis (Fig. 4) by comparing the chromatograms of standard solution, spiked and unspiked samples. It was observed that the purity angle of doravirine, IMP-A, IMP-B were less than the respective purity threshold, which shows that the peak purity test was passed (Table 7). The developed method was proved to be specific as no eluting peaks at the retention times of drugs and impurities in the spiked sample chromatogram.
Forced Degradation Study
To assess the stability-indicating nature, the method was further utilized for forced degradation study by subjecting the dosage form to various stress conditions to discover the percentage degradation. Both the drug and impurities have shown degradation under stress conditions (Table 8). In acidic and basic degradations, two small peaks were observed at 24 h with retention times of 5.117 min and 6.76 min. In the peroxide degradation, one degradant peak was observed at a retention time of 4.279 min. Another degradant peak was observed at 7.061 min in photo degradation. No degradant peak was observed in reductive and hydrolytic degradation. All the degradant peaks well resolved from the drug and impurity peaks confirm the stability-indicating nature of the method. Chromatograms of the forced degradation study are shown in Fig. 5.
Stability
Results of the stability studies were compared with the calibrated results. The results proved that the sample solution was stable at room temperature and at 2–8°C for 24 h (Table 9).
Conclusion
To quantify doravirine along with IMP-A and IMP-B, a RP-HPLC method was successfully developed with remarkable resolution. This method helps in the easy separation of impurities from the parent drug, doravirine. The optimized method was validated as per ICH guidelines and was found to be precise, accurate, robust, sensitive, and reproducible. As there are to our knowledge no RP-HPLC methods available for the quantification of doravirine along with its impurities, this developed method can be practiced successfully for the determination of doravirine and its related impurities in bulk and pharmaceutical dosage forms.
Acknowledgement
The authors are thankful to the Raghu College of Pharmacy, Visakhapatnam, India, and the GITAM Institute of
Pharmacy, Visakhapatnam, India, for supporting and providing necessary facilities for the research work.
Declarations:
Availability of data and material
All data are available upon request.
Funding
This work was not supported by any funding agencies.
Authors’ contributions
Gollu Gowri (corresponding author) was a major contributor in developing and validating the optimized method and Sowjanya Gummadi (co-author) was a major contributor in calculating validation parameters and writing the manuscript. All authors have read and approved the manuscript.
Conflicts of interest statement
The authors declare that there are no conflicts of interest.
References
K. John Wilby and N. Ahmed Eissa, Eur. J. Drug Metab. Pharmacokinet., 43, 637 – 644 (2018).
N. Sluis-Cremer and G. Tachedjian, Virus Research, 134(1 – 2), 147 – 56 (2008).
K. Andries, H. Azijn, T. Thielemans, et al., Antimicrob. Agents & Chemother., 48, 4680 – 4686 (2004).
R. Solank, IJDRT, 2, 231 – 238 (2017).
J. Roy, AAPs Pharm. Sci. Tech., 3, 1 – 8 (2002).
S. B. Bari, B. R. Kadam, Y. S. Jaiswal, A. A. Shirkhedkar, Eurasian J. Anal. Chem., 2, 32 – 53 (2007).
S. Ahuja and K. M. Alsante, Academic Press, 75 – 88 (2003).
F. Qiu and D. L. Norwood, J. Liq. Chromatogr. Relat. Technol., 30, 877 – 93 (2007).
M. Raza Siddiqui, A. A. Zeid, N. Rahman, Arab. J. Chem., 10, S1409 – S1421 (2017).
Guideline, I. H. T. Impurities in new drug products. Q3B (R2), Current Step. 4, 1 – 5 (2006).
Guideline, I. H. T. Impurities in new drug substances. Q3A (R2), Current Step. 4, 1 – 6 (2006).
Guideline, I. H. T. Impurities in residual solvents. Q3A (R2), Current Step. 4, 1 – 6 (2019).
T. Hanuman, T. Sivakumar, S. Sridhar, Indo Am. J. Pharm., 7, 33 – 39 (2020).
B. Bhadru, V. Venkata Rao, S. Vidyadhara, IJLPR., 10, 29 – 35 (2020).
V. L. N. B. G. Tiruveedhi, V. R. Battula, K. B. Bonige, Int. J. App. Pharm., 13, 153–159 (2021).
G. Gowri and G. Sowjanya, Pharm. Chem. J., 54, 526 – 535 (2020).
S. Marakatham and P. Shanmugapandiyan, RJPT, 14, 4087 – 1 (2021).
L. K. Zhang, R. Yang, H. Sheng, et al., J. Mass Spectrom., 51, 959 – 968 (2016).
ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology, Q2 (R1): International Conference on Harmonization, IFPMA, Geneva, Switzerland (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gollu, G., Gummadi, S. Development and Validation of LC-PDA Method for the Estimation of Doravirine and Related Impurities. Pharm Chem J 57, 1130–1137 (2023). https://doi.org/10.1007/s11094-023-02993-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02993-z