A simple, accurate reversed-phase high-performance liquid chromatography (HPLC) method was developed and validated for simultaneous determination of gliclazide (GLZ) and fluoroquinolone antibacterial levofloxacin (LVO). The method was developed by using a stainless steel analytical column C18 (250 × 4.6 mm, 5 μm). The system was operated using a mobile phase consisting of methanol and phosphate buffer (pH 3.0) at a flow rate of 0.8 mL/min with ultraviolet (UV) detection at 228 nm wavelength. The proposed method was validated using ICH analytical method validation guidelines. Utilizing the HPLC technique, an assay was intended to determine in vitro effects of levofloxacin on sulphonyl urea based anti-diabetic drug gliclazide. The obtained results were further verified with UV spectrophotometric method. Availability of gliclazide was reduced in the presence of levofloxacin. These in vitro analyses confirmed that the co-administartion of gliclazide and levofloxacin may serve the foundation for designing further in vivo studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
Diabetes mellitus (DM) as a major lifestyle disease is undoubtedly among the most challenging public health problems of 21st century. Diabetes is a chronic metabolic disease that occurs when the human body is not able to produce enough of the hormone insulin. Gliclazide (GLZ) is a wellknown antidiabetic agent prescribed frequently for the treatment of DM. GLZ is known to act by its selective binding with sulfonylurea receptor (SUR-1) on the surface of pancreatic beta-cells, which in turn leads to exocytosis of insulin vesicles and insulin release. Long time high blood glucose level leads to complications in diabetic patients. Levofloxacin (LVO), as a fluoroquinolone class of antimicrobial agents is used for the treatment of various infections. LVO is active against both Gram-positive and Gram-negative bacteria and it acts by inhibiting the two type enzymes, namely DNA gyrase and topoisomerase IV [1].

Classically, diabetes is associated with complications like ketoacidosis, lactic acidosis, retinopathy and nephropathy. In addition to the typical complications of DM, it has been to associated with reduced response of T cells, neutrophil function, and disorders of humoral immunity [2]. Consequently, DM increases the susceptibility to infections, both the most common ones as well as those that almost always affect only people with DM, like rhinocerebral mucormycosis). As a result individual with diabetes are more adversely affected when they get infections than individual without the disease [3]. Controlling blood sugar levels are crucial for diabetics having several infections is more prevalent in diabetic patients than the general population. Co-administration of anti-diabetic drugs with antibacterial agent is common. At the same time, this co-administration of drugs increases the risk for momentous drug–drug interactions. In vitro drug interaction studies of antibacterial agents have also been reported in the literature [4,5,6,7], but these studies contain lacks of physiological conditions and other bewildering physiological processes.
The sensitive and robust sophisticated methods have an imperative application in the pharmaceutical industry associated with drug interactions processes such that strong, dependable information can be put into a clinical framework. These assays must be able to clinically examine for suitable drug interaction studies. As in combination therapies, co-administration of drugs increases the risk for drug–drug interactions [8]. Currently, it is important to ponder drug–drug interactions using appropriate in vitro studies to guide clinical interaction studies. The goal of designing a method for in vitro interaction studies is the prediction of clinical parameters. In vitro techniques offer a complete means to create a huge amount of data using minimum resources. These suggested that in vitro approaches should be properly characterized; validated and proper controls should be incorporated in routine use.
Literature survey reveals various reported methods for the analysis of GLZ and LVO by UV spectrophotometry and high performance liquid chromatography (HPLC) either alone or in combination with other drugs in pharmaceutical preparation. To the best of our knowledge, there is no analytical HPLC method reported for simultaneous estimation of these two co-prescription drugs. Levofloxacin, though known to exhibit wide spectrum of antimicrobial potential, has been known to precipitate few side effects including hypoglycemia in the diabetic peoples. Drug interaction occurs in between Levofloxacin and sulfonylurea class of anti-diabetic agents which leads to rare fatal side effects. Few deaths are also known to be reported of absorption interaction between above mentioned drugs. Hence it is necessary to study absorption interactions occurring between these drugs. With this important objective, in the present investigation, an attempt has been made to develop a sensitive, simple, accurate, rapid and reproducible reverse-phase HPLC method for simultaneous determination of LVO and GLZ, a representative sulphonylurea class of drug followed by its validation, in accordance with the ICH guidelines. In order to assess absorption interactions between these two drugs if any, permeability studies were undertaken by using in-vitro model. The present study has not been made previously and therefore it provides a potential explanation for the observed effect. The method was validated for the parameters like linearity, specificity, accuracy and intermediate precision, limit of detection and quantitation.
2. Experimental
2.1. Chemicals and Reagents
Pure drugs levofloxacin (LVO) and gliclazide (GLZ) were provided as gift samples by Blue Cross Private Ltd, Ambad, Nashik, India. Methanol and phosphoric acid used in analysis were of HPLC grade. All other chemicals used were of analytical reagent grade. Doubly distilled water was freshly prepared by all-glass double distillation assembly purchased from Borosil, Mumbai, Maharashtra.
2.2. HPLC Instrumentation and Chromatographic Conditions
RP-HPLC instrument (Jasco) equipped with a UV-2077 detector (JASCO Corporation, Tokyo, Japan) and pu- 2080 plus(JASCO Corporation, Tokyo, Japan) Pump, manual Rheodyne injector (7725i) with 20 μL loop and Borwin Chromatography software (Version 1.50). GLZ and LVO were less soluble in water and freely soluble in selected organic solvents such as methanol (MeOH) and acetonitrile (ACN). The chromatographic conditions were optimized by different means (using different buffers and different organic phases). Early chromatographic work was performed with various combinations of buffer phase with pH in the range of 2.8 – 3.2, organic phases (ACN and/or MeOH). The flow rate of mobile phase was varied within 0.8 – 1.0 mL/min. Wavelength for monitoring the eluent was selected by scanning standard solution of drugs within 200 – 400 nm using a double-beam UV–Vis spectrophotometer (JASCO v-630). Isocratic elution with a mobile phase methanol:phosphate buffer pH 3.0 (70:30 v/v) was carried out on phenomenex kinetex C18 column (250 _ 4.6 mm, 5 μm) at a flow rate of 0.8 mL/min and the detector wavelength was set at 228 nm.
Preparation of mobile phase. About 2.7218 g of potassium dihydrogen orthophosphate was taken in 250 mL capacity beaker, 100 mL of doubly distilled water was added and stirred so as pH of the resultant solution was adjusted at 3.0 with ortho-phosphoric acid, followed by degassing of the buffer. To 70 volumes of methanol, 30 volumes of phosphate buffer (0.02 M) were added and transferred to 100 volumes of mobile phase bottle and mixed. Finally, the mixture was sonicated for 15 min for degassing the mobile phase.
Preparation of standard solution. Quantity equivalent 10 mg of GLZ was transferred into a 10.0 mL volumetric flask. To this about 5 mL of HPLC grade methanol was added, the mixture was sonicated, and the volume of solution in the flask was made to the mark by adding methanol to form a 1000 μg/L standard solution of GLZ. The standard stock solution of 1000 μg/L LVO in methanol was prepared in a similar manner
2.3. Method Development and Validation
Assessment of linearity and construction of calibration curves. Assessment of linearity of the peak area response was undertaken by two sets of five standard solutions of LVO and GLZ in the range of 5 – 25 μg/L and 1 – 5 μg/L, respectively. The solutions were injected into the HPLC system for analysis. Average peak area at each concentration level was subjected to linear regression analysis with the least square method (Fig. 1). Linearity was described in terms of the slope, intercept and R2 coefficient obtained from the regression equations (Figs. 2 and 3).
Limits of detection and quantitation. Limit of detection (LOD) and limit for quantitation (LOQ) of GLZ and LVO were determined by the following equations
where and S are the standard deviation and slope of the corresponding calibration curve, respectively (Table 1).
Precision. The intra-day precision was assessed by performing six analyses using standard stock solution containing analytes of interest. Similarly inter-day precision was assessed by performing replicate analysis using same concentration of all the analytes for three consecutive days under the same experimental conditions and the % RSD was calculated (Table 2).
Accuracy. The accuracy of the method was determined by standard addition technique. Three different levels (80,100 and 120%) of standards were added to formulation containing LVO and GLZ. The percentage recoveries of all the compounds at each level and each replicate were determined. The mean of percentage recoveries and % RSD was calculated. Results are summarized in Table 3.
Robustness. The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage. Robustness was determined by analyzing the sample solution containing 10 μg/L each of LVO and GLZ under variety of conditions of the method parameters, such as flow rate, mobile phase composition. Results obtained from robustness studies are summarized in Tables 4 and 5.
Specificity. Specificity is the method ability to assess the analyte unequivocally in the presence of components that may be expected to occur in the sample matrix (ICH, 2005). In order to determine specificity of the method in the presence of excipients (i.e. microcrystalline cellulose, croscarmellose sodium, magnesium stearate, lactose and hydroxypropylmethyl cellulose), no peak of excipients was found in chromatogram (Fig. 4) which proved that the method can be applied successfully to the given dosage formulation (Table 6).
Drug interaction studies. Accurately weighed 10 mg of standard drugs GLZ and LVO each, was transferred to separate 100 mL volumetric flasks using buffer solution as diluents. 50 μg/L of GLZ and 250 μg/L of LVO was mixed in donor compartment. The donor compartment was maintained in water bath at 37°C with constant stirring. Sample aliquots (20 μL) were taken and chromatographed at zero minute and then periodically every 15 min over continuous two hours (Tables 7 and 8).
3. Results and Discussion
Simple and reliable HPLC method for simultaneous estimation of GLZ and LVO drugs in the active statehave been developed for the first time. Method reported In the present work is known for several advantages including its being simple, rapid and devoid of any widespread sample preparation or extraction processes. In order to establish the potential for clinical drug–drug interactions, a number of in vitro drug interaction models will be required to study. Reported methods are lacking in approach, robust quality standards, and not designed to provide information of clinical importance. The present investigation provided consistent and relevant in vitro parameters which could be used to recognize latent drug–drug interactions. Significant change in availability of gliclazide was observed in the presence of levofloxacin
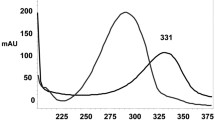
The concentration of the methanol and buffer were optimized to give symmetric peak with short run time based on the asymmetry factor and peak area obtained. Various mobile phases were tried and satisfactory separation, well resolved and good symmetrical peaks were obtained with a mobile phase of methanol:phosphate buffer ratio of 70:30 (v/v). The retention times of LVO and GLZ were found to be 2.8 and 5.8 min, respectively. The RSD values for accuracy and precision studies were less than 2%, which revealed that the developed method was accurate and precise. A typical chromatogram showing the separation of GLZ and LVO is shown in Fig. 5.
The outcome of the developed HPLC method demonstrates that simultaneous determination of gliclazide and levofloxacin is very useful for pharmaceutical manufacturers, physicians and clinicists. The proposed method is simple and suitable for the analysis of bulk drug as well their formulations. Note that this study does not support co-administration of gliclazide and levofloxacin for diabetic health management regimen.
The present investigation also suggests a suitable and relevant clinical study to investigate drug-drug interaction. The developed method was used to understand the interaction of gliclazide and fluroquinolone antibacterial levofloxacin with significant precision and robustness. From the present studies it is evident that the availability of gliclazide is significantly reduced in the presence of levofloxacin as compared to the availability of gliclazide alone. So it is suggested that co-prescription of these drugs may be unsafe in diabetic patients. In vivo studies should be carried out to further confirm drug interactions by co-prescription of these drugs.
Acknowledments
The authors are thankful to the Principal and management of the Mumbai Education Trust’s Institute of Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik (India) for providing necessary facility to undertake this research.
References
S. E. Geerlings, A. I. Hoepelman, Immunol. Med. Microbiol., 26, 256 – 265 (1999).
A. I. Hoepelman, E. Hak, K. J. Gorter, et al., Clin. Infect Dis., 41, 281 – 288 (2005).
K. J. Gorter, W. L. Goudzwaard, A. Y. Peleg, et al., Diabetes Metab.. Res. Rev., 23(3), 3 – 13 (2007).
R. Dougherty and L. V. Friedrich, J. Human Pharmacol. Drug Therapy, 24(1), 1807 – 1812 (2004).
E. Canseco and T. Kelesidis, Am. J. Med., 31(3), 122 – 134 (2009).
C. Aguirre, F. Cibrian, U. Lertxundi, and R. Hernandez, Eur. Geriatr. Med., 30(3), 181 – 183 (2012).
M. W. Pound and S. M. Miller, Am. J. Health-System Pharm., 1, 66 – 75 (2009).
L. Micheli, M. Sbrilli, and C. S. Nencini, Int. J. Clin. Pharmacol. Ther., 1, 50 – 55 (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chhajed, S.S., Sonawnae, S.S., More, P.K. et al. HPLC Method Development, Validation and Application to Determining In-Vitro Effect of Levofloxacin on the Availability of Gliclazide. Pharm Chem J 57, 445–450 (2023). https://doi.org/10.1007/s11094-023-02903-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02903-3