The features of extracting macroelements from medicinal plant raw materials (pharmacopoeial types) into infusions and decoctions were studied. The richest mineral compositions were found in infusions of Urtica dioica L. leaves, Leonurus quinquelobatus Gilib. herb, and Plantago major L. leaves and the decoction of Arctium lappa L. roots. The transfer of potassium into the extracts was relatively low (from 35.69% to 67.49%) although its content in all studied infusions and decoctions ranged from 62.72% to 75.99% of the entire elemental composition. High concentrations were noted for calcium (from 4.82 to 23.27% of the elemental complex of infusions and decoctions) and phosphorus (from 2.35% to 14.76%). The degree of calcium extraction varied from 7.11% to 32.30%; phosphorus, from 12.73% to 53.39%; magnesium, from 22.11% to 33.00%. Sodium amounted to 0.23 – 1.12% of the elemental profile of the aqueous extracts while the degree of sodium extraction was the highest among all determined macroelements (77.69 – 99.39%). The dependences of the macroelement content in infusions and decoctions on their content in the medicinal plants showed that their concentrations in the water extracts increased as the concentration of all determined elements in the raw material increased although the efficiency of their transfer into the aqueous extracts decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
According to the RF State Drug Registry, herbal medicines (HMs) numbered over 2,000 in September 2022 [1]. HMs were always in great demand on the domestic market because of their effectiveness and relative safety [2,3,4]. The various types of biologically active compounds contained in them exert a complex effect in vivo in humans [5,6,7].
Increasing attention is now being paid to studies of not only organic biologically active substances occurring in medicinal plant raw material (MPM) but also minerals, which affect the course of vitally important in vivo processes and participate in various biochemical reactions [8, 9]. A deficit of biologically significant elements is responsible for many diseases. In this instance, MPMs and HMs based on them can be used as sources of these elements in humans because they occur in the optimal ratios in MPM and in the most bioavailable organically bound forms [10,11,12,13,14,15].
Macroelements are especially interesting in this respect. Their contents in the human body exceeds 0.1% and varies from tens of grams [e.g., magnesium (Mg)] to kilograms [e.g., oxygen (O)] [16,17,18]. MPMs and HMs can act as important sources of macroelements such as calcium (Ca), phosphorus (P), potassium (K), sodium (Na), and Mg. Therefore, aqueous extracts from MPMs, i.e., infusions and decoctions, are especially interesting as the most available and frequently used dosage forms in medical and pharmaceutical practice and folk medicine. Currently, they are insufficiently studied as supplemental sources of macroelements in vivo in humans [5, 7].
The goal of the research was to study features of the extraction of macroelements from MPMs into infusions and decoctions.
Experimental Part
The studied subjects were the pharmacopoeial MPMs nettle leaves (Urtica dioica L.), plantain leaves (Plantago major L.), common tansy flowers (Tanacetum vulgare L.), linden flowers (Tilia cordata Mill.), wormwood herb (Artemisia absinthium L.), quinquelobate motherwort (Leonurus quinquelobatus Gilib.), yarrow herb (Achillea millefolium L.), knotgrass herb (Polygonum aviculare L.), burdock roots (Arctium lappa L.), and dandelion roots (Taraxacum officinale F. H. Wigg). Various types of MPM, including various plant organs or groups of organs (leaves, flowers, herbs, roots) from various forms of producing plants (herbaceous and woody plant forms), were represented in the research. MPM was collected according to pharmacopoeial rules [19, 20] at an ecologically clean site in natural growths in Peskov Voronezh State Nature Biosphere Reserve in Ramonsky district, Voronezh Region. Decoctions were prepared from the studied plant roots; infusions, from the other MPM species, according to requirements of GPM.1.4.1.0018.15 “Infusions and decoctions.” The ratio of MPM and purified H2O was 1:10 [20].
MPM samples were decomposed by a mixture of HF and HNO3 using a microwave treatment system. The resulting samples were quantitatively transferred to 10-mL tubes, rinsing three times with deionized H2O. The volume was adjusted to the mark. An aliquot (1 mL) was taken with an automated pipette and diluted to 10 mL with HNO3 (0.5%). The aqueous extract (1 mL) in a volumetric tube was treated with deionized H2O (7 mL) and conc. HNO3 (0.5 mL) and adjusted to 10 mL with deionized H2O. The elemental composition of the MPM was determined by inductively coupled plasma mass spectroscopy on an ELAN-DRC instrument (PerkinElmer Life and Analytical Sciences, USA) according to MUK 4.1.1483(03 “Determination of the content of chemical elements in diagnosable biosubstrates, preparations and biologically active additives by mass spectrometry with inductively coupled argon plasma.” The accuracy of determination was monitored using the method of additions. Each determination was made in triplicate. The results were statistically processed for confidence probability 95%.
Results and Discussion
TABLEs 1 – 5 present the results obtained from the study of the elemental composition of the MPMs and aqueous extracts.
All studied MPMs contained mostly (>84%) macroelements, which was explained by the high biological demand for them in the plants to supply their own metabolic processes (Table 1). The highest concentration of K in the analyzed MPMs was found in motherwort herb and burdock roots; of Ca, in plantain and nettle leaves; of Na, in burdock roots. The leaders for Mg accumulation were nettle leaves; for P, wormwood and motherwort herbs and nettle leaves.
An analysis of the relative content of macroelements in the total mineral complex (Table 2) showed the greatest fraction of K in burdock roots and tansy flowers; of Ca, in linden flowers and plantain leaves; of Na, in dandelion and burdock roots. The greatest fraction of Mg in the studied MPMs was noted for nettle leaves; of P, for dandelion roots.
Elemental analyses of the infusions and decoctions obtained from a given MPM found that macroelements also had the highest fraction (94.95 – 98.99%) in the sum of the elemental complex of the aqueous extracts (Tables 3 and 4). The leader in content in all studied MPMs was K (62.72 – 75.99% of the elemental complex or 5.1 – 15.7 mg/mL). However, the transfer of K into the aqueous extract was relatively low at 35.69 – 67.49% (Table 5). High concentrations were noted for Ca (74.1 – 590 μg/mL or 4.82 – 23.27% of the total elemental composition of the aqueous extracts) and P (59.7 – 204 μg/mL or 2.35 – 14.76% of the mineral complex). The extraction efficiency for Ca was 7.11 – 32.30%; for P, 12.73 – 53.39%. The greatest contents of Ca were found in the infusions of plantain and nettle leaves. Infusions of motherwort and wormwood herbs and tansy flowers were richest in P. The Mg contents in the studied MPMs varied from 33.9 μg/mL (dandelion root decoction) to 204 μg/mL (nettle leaves infusion). The degree of extraction ranged from 22.11 to 33.00%. Mg made up 2.16 – 7.29% of the mineral complex of the aqueous extracts. The highest Na concentrations were found in decoctions of dandelion and burdock roots and in knotgrass infusion (>60 μg/mL). This parameter was 3.5 – 23.5 μg/mL for the other extracts, i.e., 0.23 – 1.12% of the elemental profile of the aqueous extracts. The extraction efficiency for Na from the MPMs was the highest of the determined macroelements at 77.69 – 99.39%.
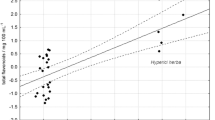
Plots of the dependences of the macroelement contents in the infusions and decoctions on their contents in the MPMs (Figs. 1 – 5) showed that their concentrations in the aqueous extracts increased with increasing concentrations of all determined elements in the MPM. However, the extraction efficiency into the aqueous extracts decreased for K, Ca, Mg, P, and Na as their contents in the MPMs increased (Figs. 6 – 10). This was probably related to the specifics of the pharmacopoeial method for obtaining the infusions and decoctions, which did not call for periodic addition of pure extractant and led to a gradual attainment of the equilibrium concentrations between the MPM and the obtained extract. Then, the transfer rate of the elements into the extract dropped sharply. The degree of reduction of the transfer efficiency of the elements into the aqueous extracts was different. The dependences for K, Na, and Mg appeared to be the most consistent. The degree of extraction of K decreased by an average of 0.001%; of Na and Mg, by 0.003%, as the concentration in the MPM increased by 1 μg/g.
It was also noteworthy that the degree of extraction of the macroelements into the aqueous extracts could vary for comparable contents of them in the MPM. For example, the extraction of K into infusions of tansy flowers and motherwort herb was 38.49 and 51.32%, respectively, if the K contents in them was about 30 mg/g. Many factors are known to affect the extraction of elements. However, the histological structure of the MPM and features of the intracellular chemical binding of the elements apparently had the greatest influence because the extractions were made at the same temperature by the same method with finely ground MPM with the same MPM-to-extractant ratio of 1:10. For example, Ca in plants can exist as phosphates, sulfates, carbonates, and as salts of pectic and oxalic acids, which have different solubilities in H2O. A significant part of it in plants is soluble in H2O (from 20 to 65%, varying individually for each species). The remainder can be extracted from the MPM only by treatment with acids [18, 19].
Thus, the research results demonstrated that the studied MPMs had rich macroelement compositions and could be used in medical and pharmaceutical practice to adjust physiological standards for element contents in vivo in humans. The derived dependences of the macroelement contents in infusions and decoctions on their contents in the MPMs showed that the concentrations of all determined elements in the aqueous extracts increased as their concentrations in the MPMs increased. However, the transfer efficiency of K, Ca, Mg, P, and Na into the aqueous extracts decreased
References
State Registry of Medicines [in Russian]; https: //www.grls.rosminzdrav.ru.
I. V. Gravel’, N. V. Ivashchenko, and I. A. Samylina, Farmatsiya, No. 1, 9 – 11 (2011).
A. S. Korolev, A. A. Gladyshev, and I. S. Yutkina, Izv. Orenburg. Gos. Agrar. Univ., No. 5, 159 – 161 (2014).
N. A. D’yakova, Tr. Karel. Nauchn. Tsentra Ross. Akad. Nauk, No. 5, 70 – 79 (2020).
N. A. D’yakova, A. I. Slivkin, and S. P. Gaponov, Khim.-farm. Zh., 54(6), 68 – 72 (2020).
N. A. D’yakova, A. I. Slivkin, E. E. Chupandina, and S. P. Gaponov, Khim. Rastit. Syr?ya, No. 4, 5 – 13 (2020).
N. A. D’yakova, I. A. Samylina, A. I. Slivkin, et al., 49(6), 25 – 28 (2015).
A. A. Gudkova, A. S. Chistyakova, A. I. Slivkin, and A. A. Sorokina, Mikroelem. Med., No. 1, 35 – 42 (2019).
M. A. Rudaya, O. V. Trineeva, and A. I. Slivkin, Mikroelem Med., No. 3, 49 – 59 (2018).
A. I. Popov, Farmatsiya, No. 1, 51 – 53 (1993).
A. I. Popov, Khim.-farm. Zh., No. 11, 50 – 52 (1993).
A. I. Popov, Vopr. Pitan., No. 1, 38 – 39 (1994).
C. H. Hill and G. Matrone, Fed. Proc., No. 4, 1474 – 1481 (1970).
Li Jiaxu and M. Sarah, Plant Physiol., No. 123, 807 – 809 (2000).
O. Wada and H. Yanagisawa, Med. Drug J., No. 12, 126 – 134 (1997).
A. V. Skal’nyi and I. A. Rudakov, Vestn. Orenburg. Gos. Univ., No. 2, 4 – 8 (2005).
A. V. Skal’nyi, Macroelements: Alertness, Health, Longevity [in Russian], Pero, Moscow (2019).
A. V. Skal’nyi, M. G. Skal?naya, A. A. Kirichuk, and A. A. Tin’kov, Medical Elementology [in Russian], Nauka, Moscow (2021).
V. A. Kurkin, Pharmacology [in Russian], SamGMU, Samara (2004), pp. 473 – 476.
State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 4, FEMB, Moscow (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 12, pp. 47 – 52, December 2022
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dyakova, N.A. Features of Extracting Macroelements from Medicinal Plant Raw Materials Into Infusions and Decoctions. Pharm Chem J 56, 1633–1638 (2023). https://doi.org/10.1007/s11094-023-02838-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02838-9